Continuing Education Activity
Superior vena cava (SVC) syndrome is a collection of clinical signs and symptoms resulting from partial or complete obstruction of blood flow through the SVC. This obstruction is most commonly a result of thrombus formation or tumor infiltration of the vessel wall. The superior vena cava is formed by the junction of the left and right innominate (brachiocephalic) veins and is tasked with returning blood from the head, neck, upper extremities, and torso to the heart. Today, this syndrome is most commonly seen secondary to malignancy, although there has been a more recent rise in benign etiologies. This activity describes the causes, pathophysiology, and presentation of superior vena cava syndrome and highlights the role of the interprofessional team in its management.
Objectives:
- Evaluate the causes of superior vena cava syndrome.
- Identify the pathophysiology of superior vena cava syndrome.
- Assess the treatment options for superior vena cava syndrome.
- Communicate the importance of improving care coordination among interprofessional team members to improve outcomes for patients affected by superior vena cava syndrome.
Introduction
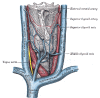
Superior vena cava (SVC) syndrome is a collection of clinical signs and symptoms resulting from partial or complete obstruction of blood flow through the SVC. This obstruction is most commonly a result of thrombus formation or tumor infiltration of the vessel wall. The SVC is formed by the junction of the left and right innominate (brachiocephalic) veins and is tasked with returning blood from the head, neck, upper extremities, and torso back to the heart (see Image. Veins and Arteries of the Neck). Today, this syndrƒome is most commonly seen secondary to malignancy, although there has been a recent rise in benign etiologies. The resulting venous congestion produces a clinical scenario of increased upper-body venous pressure. The most common signs and symptoms include face or neck swelling, upper extremity swelling, dyspnea, cough, and dilated chest vein collaterals.[1][2][3]
Etiology
SVC syndrome saw a dramatic decrease throughout the twentieth century. Today, the majority of SVC syndromes are the result of mediastinal malignancies, primarily among which are small-cell bronchogenic carcinoma. The second most commonly associated malignancy is non-Hodgkins lymphoma, followed by metastatic tumors. In addition, benign or nonmalignant causes of SVC syndrome now comprise at least 40% of cases. Iatrogenic thrombus formation or SVC stenosis is a growing etiology due to pacemaker wires and semipermanent intravascular catheters used for hemodialysis, long-term antibiotics, or chemotherapy.[4][5]
Epidemiology
An estimated 15,000 cases of SVC syndrome occur each year in the United States, with studies pointing to increasing frequency due to the concomitant rise in the use of semipermanent intravascular catheters. The incidence of SVC syndrome reported in the literature ranges from 1 in 650 to 1 in 3100 patients.
Pathophysiology
The SVC is part of the low-pressure venous system containing thin walls susceptible to damage by various pathologic mechanisms. These mechanisms can be divided into 3 categories: compromised vessel anatomy, impaired venous flow, and diminished vessel wall integrity. These mechanisms often coexist in patients presenting with SVC syndrome. Extrinsic compression and obstruction of the SVC by a mass in the mediastinum is the most common cause of SVC syndrome. This is often associated with malignancy; however, a variety of nonmalignant masses and dilation of the overlying aorta can cause compression. A growing proportion of SVC syndromes are now associated with occlusive venous thrombus formation that compromises venous flow back to the heart. The increasing use of indwelling intravascular devices such as catheters, pacemakers, and implantable cardioverter-defibrillator (ICD) leads to this growth. Resultant venous wall inflammation, fibrosis, and eventual thrombus lead to vessel stenosis.[6][7][8]
History and Physical
SVC syndrome diagnosis is largely based on a patient’s history and physical findings, which often develop over days to weeks. This insidious onset results from a collateral vascular network that diverts blood to the lower body, where it is then returned to the heart through the inferior vena cava, azygos vein, and intercostals. The clinical findings in SVC syndrome are closely linked to venous congestion and the resultant elevation in venous pressures seen in the upper body. A careful physical examination is often sufficient to rule out a cardiogenic origin of the patient’s symptoms. The most common presenting symptoms of SVC syndrome are face/neck swelling, distended neck veins, cough, dyspnea, orthopnea, upper extremity swelling, distended chest vein collaterals, and conjunctival suffusion. Other less common symptoms of SVC syndrome include stridor, hoarseness, dysphagia, pleural effusion, head plethora, headache, nausea, lightheadedness, syncope, change in vision, altered mental status, upper body edema, cyanosis, papilledema, stupor, and coma. Some rare but serious clinical consequences reported in SVC syndrome include cerebral edema and upper respiratory compromise secondary to edema of the larynx and pharynx.
Evaluation
Patients with high clinical suspicion for SVC syndrome should undergo imaging of the upper body and vasculature. Ultrasound of the jugular, subclavian, and innominate veins can help to identify a thrombus within the vessel lumen. Radiographic imaging and MRI also play a critical role in providing additional information about the SVC obstruction's location, severity, and etiology. CT of the chest with collateral vessels is associated with a diagnostic sensitivity of 96% and a specificity of 92%. Venography is widely accepted as the gold standard for visualizing and diagnosing a venous obstruction. This modality should be used concomitantly with endovascular intervention for patients with a severe presentation of SVC syndrome.[9][10]
Treatment / Management
Following a clinical diagnosis, supportive therapy and medical management are commonly initiated. This involves elevating the patient’s head as a simple maneuver to decrease venous pressure. Further management is guided by the patient’s underlying SVC syndrome etiology. For patients with thrombus related to an indwelling intravascular device, removal should be considered along with anticoagulation therapy and catheter-directed thrombolysis. Multidisciplinary treatment planning for those with obstruction due to malignancy is important as tumor type and staging can help to guide appropriate chemotherapy or radiation therapy. Open surgical repair through bypass grafting with spiral saphenous vein, femoral vein, polytetrafluoroethylene (PTFE) graft, or Dacron graft has traditionally been considered to overcome SVC obstruction. However, this is now reserved for cases where recanalization through endovascular repair is either impossible or has previously failed. With expanding treatment options for both benign and malignant etiology, endovascular therapy is now widely considered the first-line treatment for SVC syndrome. Less invasive endovascular management can offer patients immediate relief of symptoms. Acute or subacute thrombus can be managed with catheter-based thrombolysis or thrombectomy before venoplasty and stent placement.[11][12][13]
Differential Diagnosis
The differential diagnosis for superior vena cava syndrome includes the following:
- Cardiac tamponade
- Mediastinitis
- Thoracic aortic aneurysm
- Tuberculosis
Pearls and Other Issues
A widely accepted and standardized set of criteria outlining presentation severity does not exist for SVC syndrome. The Kishi Scoring System for Signs and Symptoms of SVC syndrome grades severity based on the patient’s clinical presentation. This scoring system includes the patient’s neurologic symptoms, laryngopharyngeal or thoracic symptoms, nasal and facial signs or symptoms, and the presence of venous dilatation.
Enhancing Healthcare Team Outcomes
Because there are many causes of SVCS, the condition is best managed by an interprofessional team of healthcare workers, including a vascular surgeon, interventional radiologist, radiation therapist, oncologist, pain specialist, and cardiac surgeon. The nurse plays a vital role in the monitoring of these patients. Many of them have cerebral and laryngeal edema and need close monitoring. The pharmacist should be fully aware of the drugs used to manage SVCS, which include corticosteroids and diuretics. Because many of these patients have an unpredictable course, radiation is often administered as a palliative measure to shrink the tumor and relieve the symptoms. Patients with Hodgkin lymphoma are managed with chemotherapy.[14][15]
Outcomes
The prognosis of patients with SVCS depends on the cause. For patients with a benign cause of SVCS, the life expectancy is not changed, but for malignant cases, there is a significant drop in survival. Individuals who have features of cerebral and laryngeal edema can develop life-threatening symptoms and suddenly die. Patients with SVCS as a result of lung cancer usually live less than 24 months. For those who do not respond to radiation treatment, the survival is less than a year.[16][17][18]
Review Questions
References
- 1.
- Drouin L, Pistorius MA, Lafforgue A, N'Gohou C, Richard A, Connault J, Espitia O. [Upper-extremity venous thrombosis: A retrospective study about 160 cases]. Rev Med Interne. 2019 Jan;40(1):9-15. [PubMed: 30122260]
- 2.
- Zimmerman S, Davis M. Rapid Fire: Superior Vena Cava Syndrome. Emerg Med Clin North Am. 2018 Aug;36(3):577-584. [PubMed: 30037444]
- 3.
- Carmo J, Santos A. Chronic Occlusion of the Superior Vena Cava. N Engl J Med. 2018 Jul 05;379(1):e2. [PubMed: 29972752]
- 4.
- De Potter B, Huyskens J, Hiddinga B, Spinhoven M, Janssens A, van Meerbeeck JP, Parizel PM, Snoeckx A. Imaging of urgencies and emergencies in the lung cancer patient. Insights Imaging. 2018 Aug;9(4):463-476. [PMC free article: PMC6108967] [PubMed: 29644546]
- 5.
- Friedman T, Quencer KB, Kishore SA, Winokur RS, Madoff DC. Malignant Venous Obstruction: Superior Vena Cava Syndrome and Beyond. Semin Intervent Radiol. 2017 Dec;34(4):398-408. [PMC free article: PMC5730434] [PubMed: 29249864]
- 6.
- Kalinin RE, Suchkov IA, Shitov II, Mzhavanadze ND, Povarov VO. [Venous thromboembolic complications in patients with cardiovascular implantable electronic devices]. Angiol Sosud Khir. 2017;23(4):69-74. [PubMed: 29240058]
- 7.
- Ghorbani H, Vakili Sadeghi M, Hejazian T, Sharbatdaran M. Superior vena cava syndrome as a paraneoplastic manifestation of soft tissue sarcoma. Hematol Transfus Cell Ther. 2018 Jan-Mar;40(1):75-78. [PMC free article: PMC6001923] [PubMed: 30057975]
- 8.
- Labriola L, Seront B, Crott R, Borceux P, Hammer F, Jadoul M. Superior vena cava stenosis in haemodialysis patients with a tunnelled cuffed catheter: prevalence and risk factors. Nephrol Dial Transplant. 2018 Dec 01;33(12):2227-2233. [PubMed: 29893920]
- 9.
- Mansour A, Saadeh SS, Abdel-Razeq N, Khozouz O, Abunasser M, Taqash A. Clinical Course and Complications of Catheter and Non-Catheter-Related Upper Extremity Deep Vein Thrombosis in Patients with Cancer. Clin Appl Thromb Hemost. 2018 Nov;24(8):1234-1240. [PMC free article: PMC6714774] [PubMed: 30025472]
- 10.
- Khan UA, Shanholtz CB, McCurdy MT. Oncologic Mechanical Emergencies. Hematol Oncol Clin North Am. 2017 Dec;31(6):927-940. [PubMed: 29078930]
- 11.
- Nossair F, Schoettler P, Starr J, Chan AKC, Kirov I, Paes B, Mahajerin A. Pediatric superior vena cava syndrome: An evidence-based systematic review of the literature. Pediatr Blood Cancer. 2018 Sep;65(9):e27225. [PubMed: 29781569]
- 12.
- Ierardi AM, Jannone ML, Petrillo M, Brambillasca PM, Fumarola EM, Angileri SA, Crippa M, Carrafiello G. Treatment of venous stenosis in oncologic patients. Future Oncol. 2018 Dec;14(28):2933-2943. [PubMed: 29623736]
- 13.
- Kalra M, Sen I, Gloviczki P. Endovenous and Operative Treatment of Superior Vena Cava Syndrome. Surg Clin North Am. 2018 Apr;98(2):321-335. [PubMed: 29502774]
- 14.
- Hague J, Tippett R. Endovascular techniques in palliative care. Clin Oncol (R Coll Radiol). 2010 Nov;22(9):771-80. [PubMed: 20833516]
- 15.
- Gradishar WJ, Magid D, Bitran JD. Supportive care of the lung cancer patients. Hematol Oncol Clin North Am. 1990 Dec;4(6):1183-99. [PubMed: 1704881]
- 16.
- Lianos GD, Hasemaki N, Tzima E, Vangelis G, Tselios A, Mpailis I, Lekkas E. Superior vena cava syndrome due to central port catheter thrombosis: a real life-threatening condition. G Chir. 2018 Mar-Apr;39(2):101-106. [PubMed: 29694310]
- 17.
- Kuo TT, Chen PL, Shih CC, Chen IM. Endovascular stenting for end-stage lung cancer patients with superior vena cava syndrome post first-line treatments - A single-center experience and literature review. J Chin Med Assoc. 2017 Aug;80(8):482-486. [PubMed: 28501315]
- 18.
- Nakano T, Endo S, Kanai Y, Otani S, Tsubochi H, Yamamoto S, Tetsuka K. Surgical outcomes after superior vena cava reconstruction with expanded polytetrafluoroethylene grafts. Ann Thorac Cardiovasc Surg. 2014;20(4):310-5. [PubMed: 23801179]
Disclosure: Marc Seligson declares no relevant financial relationships with ineligible companies.
Disclosure: Scott Surowiec declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Marc T. Seligson1; Scott M. Surowiec2.Affiliations
Publication History
Last Update: September 26, 2022.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Seligson MT, Surowiec SM. Superior Vena Cava Syndrome. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.