This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.98.1
1. Introduction
Embryoid bodies (EB) are the three-dimensional aggregates formed in suspension by pluripotent stem cells (PSC), including embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC). EB differentiation is a common platform to generate specific cell lineages from PSCs. However, most EB formation protocols contain undefined components, such as Fetal Bovine Serum (FBS), Knock-Out Serum Replacement (KOSR) or albumin product. These animal-sourced components significantly limit the application of EB formation to generate potentially clinically relevant cell products. At the same time, the undefined composition of the above components can lead to inconsistent outcomes in experiments due to batch differences in their production. This inconsistency also severely affects researchers’ ability to further improve procedure and its final products. Meanwhile, unlike their mouse counterparts, human PSCs usually cannot survive in suspension unless in aggregates or under ROCK inhibitor treatment. As such, it is essential that EB formation and further differentiation can be conducted in chemically defined, animal product-free conditions. This will allow better consistency as well as an easier route to translate into clinically relevant production. We previously developed a fully chemically defined medium Essential 8 (or E8) for the maintenance and expansion of human pluripotent stem cells in the clinical grade environment. In this protocol, we describe a set of optimized procedures to produce EBs from human PSCs in E8 or E8-based media.
2. Protocol
2.1. Reagent and material preparation
- EDTA/PBS solution: dilute 0.5 ml 0.5M EDTA (pH 8.0) in 500 ml PBS (calcium and magnesium free), add 0.9g NaCl, mix and filtrate. The final EDTA concentration is 0.5 mM.
- Matrigel coated plate/dish: Pour 30 ml cold DMEM/F12 in conical tube, use 1.5 ml to resuspend 4 mg frozen Matrigel with 5 ml pipet. Mix the Matrigel with the media well, plate 6 ml in one six-well plate (1 ml per well) or 6 ml in one 10 cm dish and shake well to cover the entire surface. 4 mg Matrigel is used to coat 5 six-well plates or 5 10 cm dishes. Leave the plates/dishes at room temperature for at least 30 minutes or at 4°C overnight. The Matrigel-coated plates/dishes can be sealed and stored at 4°C for more than one week before use.
- Vitronectin coated plate/dish: Apply the same procedure of Matrigel coating to coat vitronectin, 2 mg vitronectin is used to coat 10 six-well plates or 10 10 cm dishes.
- E8/Essential 8 media: The medium could be prepared based on the formula in previous publication (Chen et al., 2011). As an alternative, the medium is also available from Thermal Fisher/Life Technologies Inc. as Essential 8, or from Stem Cell Technologies as TeSR-E8. In this protocol, we simplify all such hPSC maintenance media as E8.
- E6/Essential 6 medium: E6 is similar to E8 medium but without FGF2 and TGF-β. The medium could be prepared based on the formula in previous publication (Chen et al., 2011). Similar medium is available from Thermal Fisher/Life Technologies Inc. as Essential 6, or from Stem Cell Technologies as TeSR-E6.
- PVA containing media: Polyvinyl Alcohol (PVA) powder is added into specific medium (E8 or E6) at 4 mg/ml, mixed and stirred for one hour at room temperature, filtered and then stored at 4°C. E8/PVA is used for EB formation (Ng et al., 2008).
- Poly-HEMA coated petri dish: Poly-HEMA solution is made at 20 mg/ml by dissolve 1g Poly-HEMA in 50 ml 95% ethanol on rock overnight in 37°C. To coat the petri dishes, pour the Poly-HEMA/ethanol mixture to cover the dishes and let it dry out overnight at room temperature (Kuroda et al., 2013).
2.2. Flow Chart
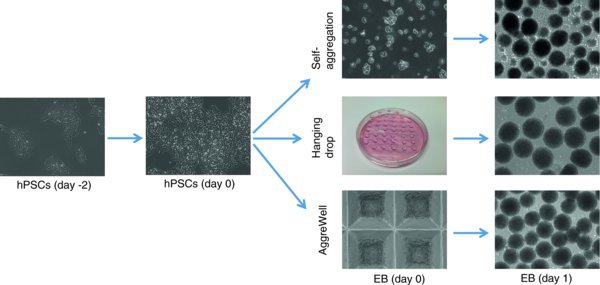
Figure 1.
Overview of three different EB formation methods.
2.3. Procedures
- hPSCs are cultured in E8 medium on Matrigel or vitronectin coated plates, and medium is changed daily.
- Two days before EB formation, 60–70% confluent hPSCs are passaged onto Matrigel or vitronectin coated plates/dishes with EDTA/PBS as previously described (Beers et al., 2012). The passage ratio is usually 1:3 or 1:4, in E8 media with 10 μM ROCK inhibitor (Y-27632) to increase cell survival.
- The next day, change media to E8 medium without ROCK inhibitor.
- On the day of EB formation when the cells grow to 60-80% confluence, cells are washed once and then incubated in EDTA/PBS for 3–15 minutes to dissociate colonies to cell clumps or single cells according to EB formation methods.
- 3–5 minutes incubation – Self-aggregated EB formation.
- 10–15 minutes incubation – Forced aggregation by hanging drop or using AggreWell.
- Cell Harvest. Two methods are used to harvest the cells according to EDTA treatment time.
- 3–5 minutes EDTA incubation where most cells are still attached to plate surface. Aspirate EDTA/PBS, wash cell clumps off the plate by E8/PVA (5 ml/dish) with ROCK inhibitor. This method is suitable for Self-aggregated EB formation.
- 10–15 minutes EDTA incubation where most cells detach from the plate surface to become single cells or small aggregates. Gently break the aggregates with PBS/EDTA (5 ml/dish) by pipetting, and then transfer the cells into 15 ml tube, neutralize with equal volume of E8/PVA medium with ROCK inhibitor, count cell number, and spin the cells down at 1,000 RPM for 5 minutes. Finally, re-suspend the cells in E8/PVA medium with ROCK inhibitor. This method is suitable for forced aggregation, such as hanging drop or using AggreWell (Mohr et al., 2010; Watanabe et al., 2007).
- EB formation
- To form self-aggregated EBs, suspend cell clumps into Corning low attachment dishes or poly-HEMA coated petri dishes via 1:1 passage (suspend the cells from one 10 cm dish in 5 ml E8/PVA media with Rock inhibitor to one 10 cm low attachment dish to allow self-aggregation in 37°C incubator overnight. On the second day, EBs should form in various sizes.
- To form hanging drop EBs, single cell drops (2000 cells/20 uL) are hanging cultured on the lid of Petri dishes. Incubate the dishes in 37°C incubator overnight. On the second day EBs should form with uniform size. The aggregated EBs can be washed off into Corning low attachment dishes or poly-HEMA coated petri dishes (Lin et al., 2014).
- To form EBs in AggreWells, rinse each well of AggreWell-800 by DMEM/F12 before use. Add 0.5 mL E8/PVA medium to each well that will be used and centrifuge to remove air bubbles. Add 1.5 × 106 cells/1.5 mL into each well (5000 cells/microwell) to generate the desired size of EBs. Centrifuge the AggreWell plate at 100 × g, 3 mins to capture cells in the microwells. Incubate the plate in 37°C incubator overnight. On the second day EB should form with uniform size. Gently pipet the medium up and down in AggreWell to remove the EBs from microwells and transfer them into Corning low attached dishes or poly-HEMA coated petri dishes (Stem Cell Technologies technical manual).
- After EBs are formed and transferred to Corning low attachment dishes or poly-HEMA coated petri dishes, tilt the plate at 30°–45° angle to allow the EBs to gather at the bottle of well. Gently remove most medium with pipette, and change media to desired differentiation conditions for specific cell type differentiation. For example, in spontaneous differentiation, the medium is switched to E6 medium to culture 9 to 14 days. The medium can be changed every 2 days.
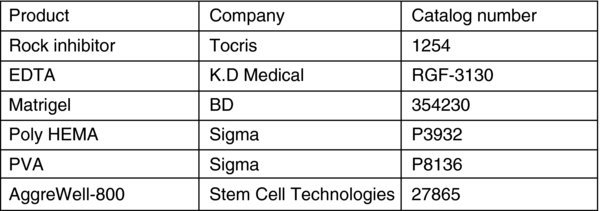
3. Materials
- Human PSCs

- E8 and related Media

- Reagent
4. Notes and troubleshooting
- For EDTA dissociation, different lines may have various treatment times.
- Self-aggregation forms heterogeneous size and irregularly shape EBs while hanging drops or using AggreWell form uniform size and shape EBs.
- To improve cell survival and EB formation, the cells need to be freshly passaged; we usually pass the cells (1:3 or 1:4) 2 days before EB formation.
- ROCK inhibitor can be used to improve cell survival, while at the same time it promotes cell attachment to uncoated plate surface. E8 medium is albumin free by which cells are prone to attach to the dish. The low attach dishes (either ultra low cluster plates from Costar, Corning or poly-HEMA coated petri dishes) are required to avoid EB attachment.
- To promote EB formation under self-aggregation, the cell dissociation time by EDTA should be strictly monitored to avoid single cell dissociation. Aggregation of cell clumps increases the rate of EB formation.
- PVA is strongly recommended to be added to help EB formation, but can be removed from media after EBs were formed.
- Differentiation medium could be directly used in EB formation instead of E8 medium (Essential 6 Medium Manual).
Reference
- Beers J., et al. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nature Protocols. 2012;7(11):2029–40. [PMC free article: PMC3571618] [PubMed: 23099485]
- Chen G., et al. Chemically defined conditions for human iPS cell derivation and culture. Nature Methods. 2011;8(4):424–429. [PMC free article: PMC3084903] [PubMed: 21478862]
- Essential 6 Medium Manual: Embryoid Body (EB) formation using Essential 6 Medium. Life Technologies Inc. and Cellular Dynamics International.
- Kuroda Y., et al. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nature Protocols. 2013;8:1391–1415. [PubMed: 23787896]
- Lin Y., et al. Snail1-dependent control of embryonic stem cell pluripotency and lineage commitment. Nature Communications. 2014;5:3070. [PMC free article: PMC4115678] [PubMed: 24401905]
- Mohr KC., et al. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–1893. [PMC free article: PMC2813988] [PubMed: 19945747]
- Ng ES., et al. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nature Protocols. 2008;351:768–76. [PubMed: 18451785]
- Stem Cell Technologies technical manual: Reproducible and Uniform Embryoid Bodies Using AggreWell™ Plates.
- Watanabe K., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology. 2007;25(6):681–686. [PubMed: 17529971]
Last revised May 10, 2014. Published June 1, 2014. This chapter should be cited as: Lin, Y. and Chen, G., Embryoid body formation from human pluripotent stem cells in chemically defined E8 media (June 1, 2014), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.98.1.
- A High Proliferation Rate is Critical for Reproducible and Standardized Embryoid Body Formation from Laminin-521-Based Human Pluripotent Stem Cell Cultures.[Stem Cell Rev Rep. 2016]A High Proliferation Rate is Critical for Reproducible and Standardized Embryoid Body Formation from Laminin-521-Based Human Pluripotent Stem Cell Cultures.Dziedzicka D, Markouli C, Barbé L, Spits C, Sermon K, Geens M. Stem Cell Rev Rep. 2016 Dec; 12(6):721-730.
- Review Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach.[World J Stem Cells. 2020]Review Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach.Guo NN, Liu LP, Zheng YW, Li YM. World J Stem Cells. 2020 Jan 26; 12(1):25-34.
- Embryoid body formation from embryonic and induced pluripotent stem cells: Benefits of bioreactors.[World J Stem Cells. 2009]Embryoid body formation from embryonic and induced pluripotent stem cells: Benefits of bioreactors.Rungarunlert S, Techakumphu M, Pirity MK, Dinnyes A. World J Stem Cells. 2009 Dec 31; 1(1):11-21.
- Superior Red Blood Cell Generation from Human Pluripotent Stem Cells Through a Novel Microcarrier-Based Embryoid Body Platform.[Tissue Eng Part C Methods. 2016]Superior Red Blood Cell Generation from Human Pluripotent Stem Cells Through a Novel Microcarrier-Based Embryoid Body Platform.Sivalingam J, Lam AT, Chen HY, Yang BX, Chen AK, Reuveny S, Loh YH, Oh SK. Tissue Eng Part C Methods. 2016 Aug; 22(8):765-80. Epub 2016 Aug 1.
- Review Cryopreservation of human pluripotent stem cells in defined medium.[Curr Protoc Stem Cell Biol. 2014]Review Cryopreservation of human pluripotent stem cells in defined medium.Liu W, Chen G. Curr Protoc Stem Cell Biol. 2014 Nov 3; 31:1C.17.1-13. Epub 2014 Nov 3.
- Embryoid body formation from human pluripotent stem cells in chemically defined ...Embryoid body formation from human pluripotent stem cells in chemically defined E8 media - StemBook
Your browsing activity is empty.
Activity recording is turned off.
See more...

 and
and