Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kiru G, Bicknell C, Falaschetti E, et al.; on behalf of the AARDVARK collaborators. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomised placebo-controlled trial (AARDVARK). Southampton (UK): NIHR Journals Library; 2016 Jul. (Health Technology Assessment, No. 20.59.)

An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomised placebo-controlled trial (AARDVARK).
Show details
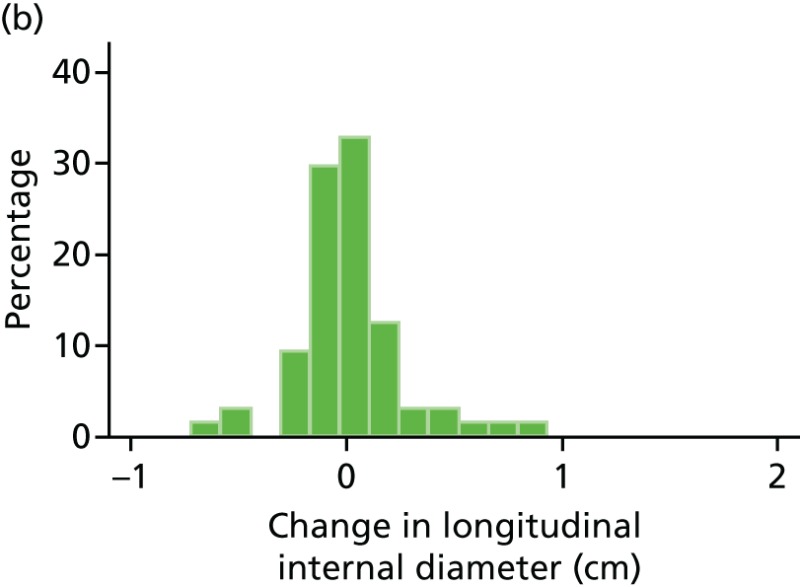
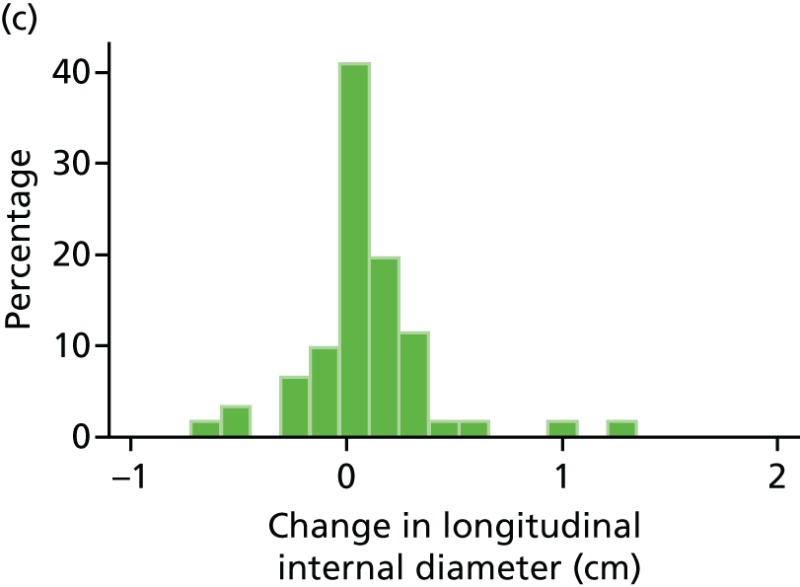

FIGURE 44
Histograms of change in AAA longitudinal internal diameter from baseline to month 3. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
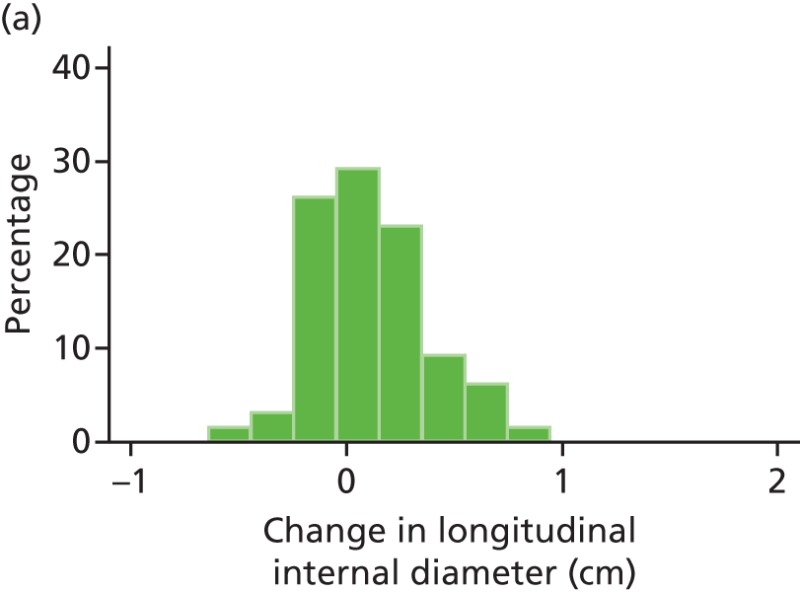


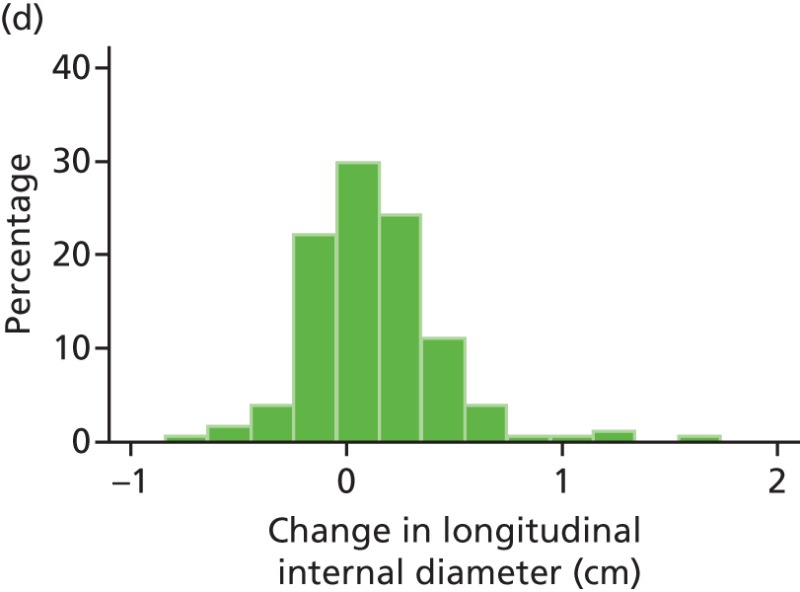
FIGURE 45
Histograms of change in AAA longitudinal internal diameter from baseline to month 6. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

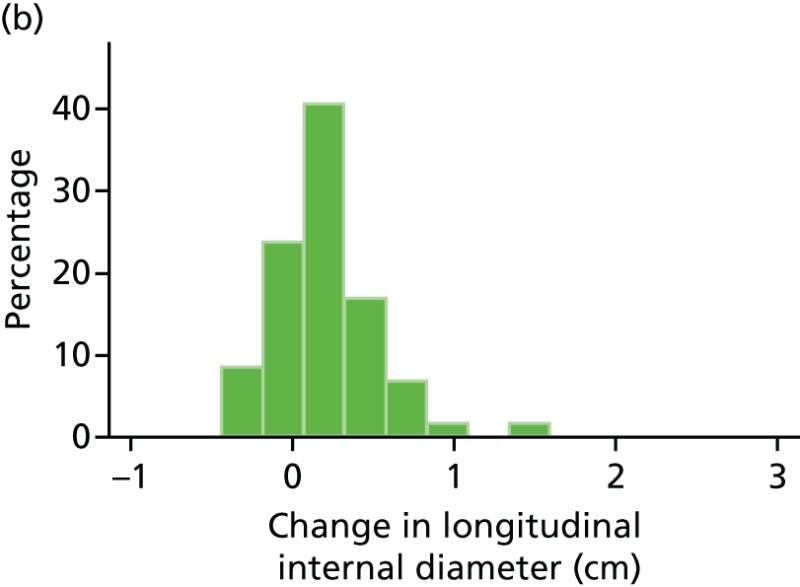


FIGURE 46
Histograms of change in AAA longitudinal internal diameter from baseline to month 12. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
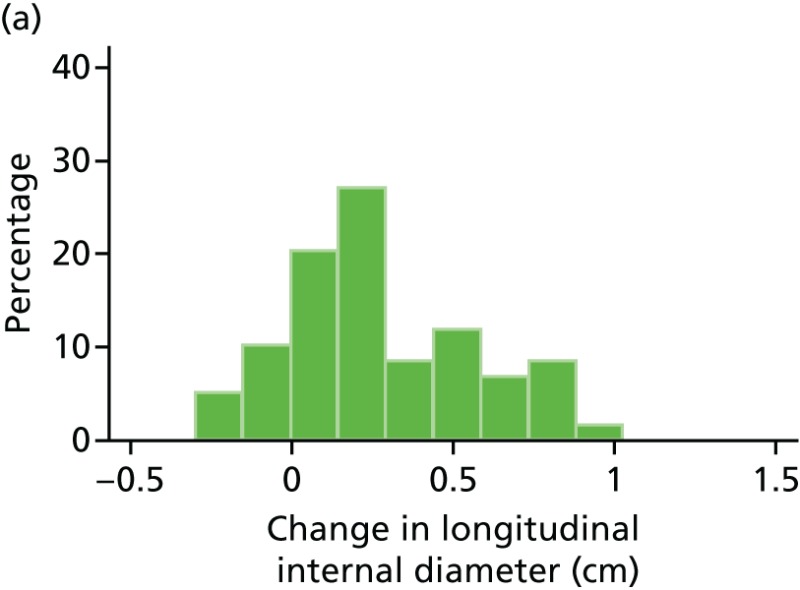
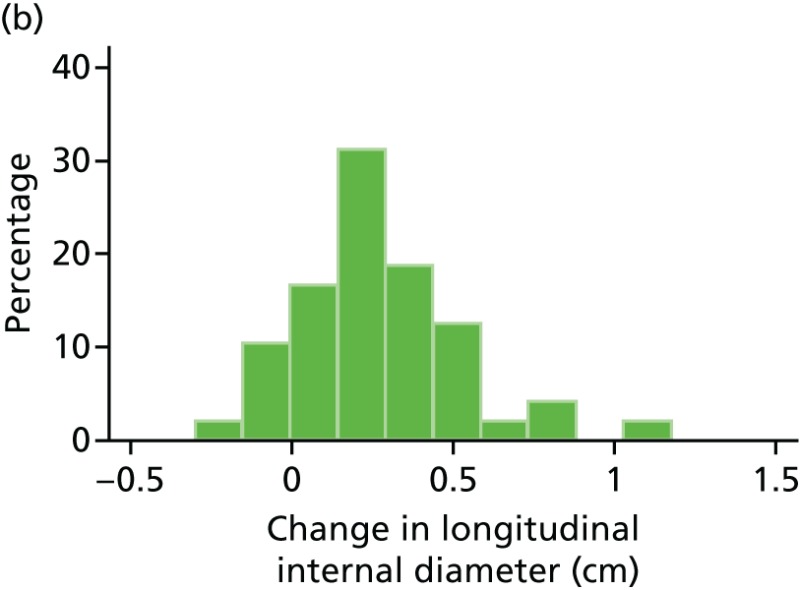
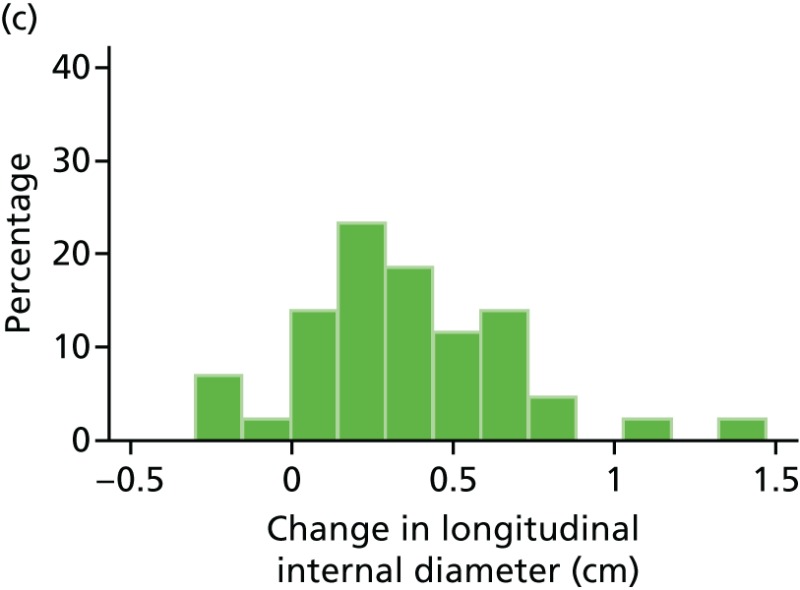
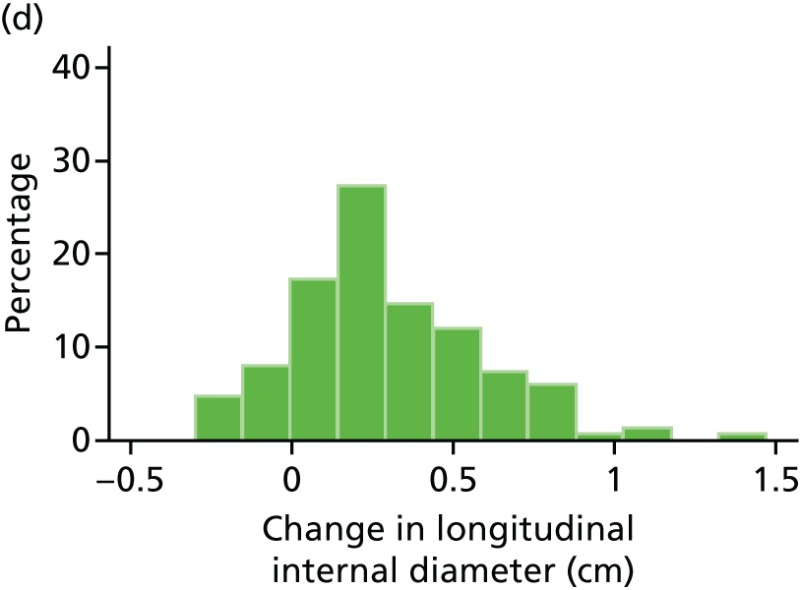
FIGURE 47
Histograms of change in AAA longitudinal internal diameter from baseline to month 18. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

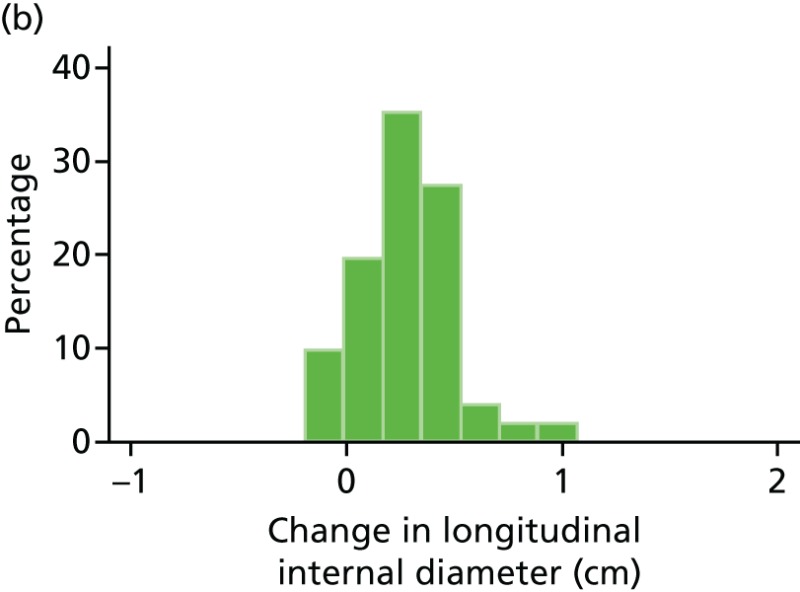
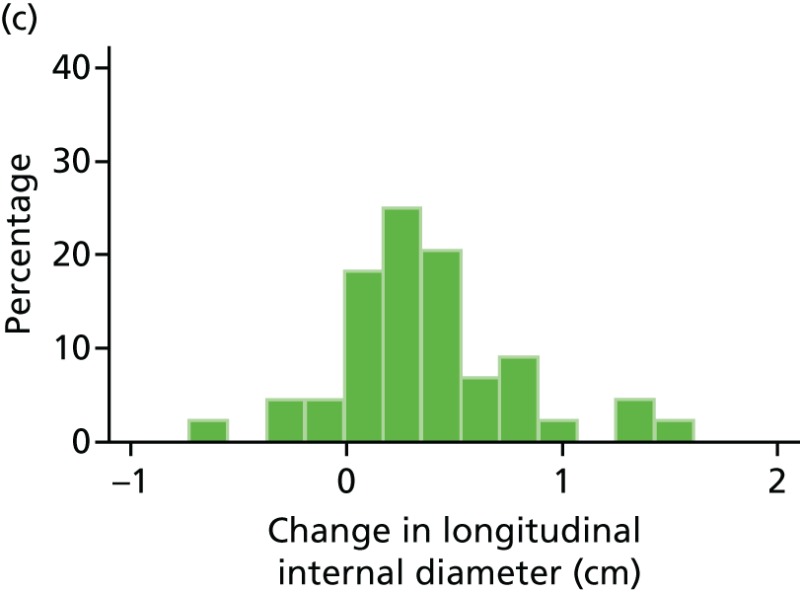
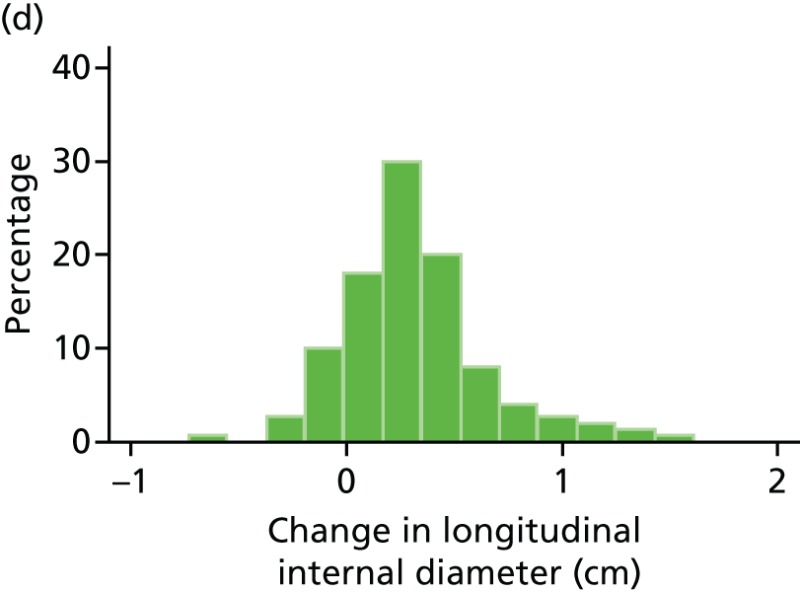
FIGURE 48
Histograms of change in AAA longitudinal internal diameter from baseline to month 24. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
- Histograms of change in abdominal aortic aneurysm longitudinal internal measurem...Histograms of change in abdominal aortic aneurysm longitudinal internal measurements - An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomised placebo-controlled trial (AARDVARK)
Your browsing activity is empty.
Activity recording is turned off.
See more...