Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Tylee A, Barley EA, Walters P, et al.; on behalf of the UPBEAT-UK team. UPBEAT-UK: a programme of research into the relationship between coronary heart disease and depression in primary care patients. Southampton (UK): NIHR Journals Library; 2016 May. (Programme Grants for Applied Research, No. 4.8.)

UPBEAT-UK: a programme of research into the relationship between coronary heart disease and depression in primary care patients.
Show detailsPlan
The previous chapters detail how we gathered the views of patients, GPs and PNs as well as findings from published studies concerning requirements and preferences for a future intervention for people with sCHD and comorbid depressive symptoms. In this work package of the UPBEAT-UK programme, our aim was to develop and evaluate an intervention that would be feasible to deliver in UK primary care.
We conducted an iterative evidence review and synthesised data from previous work packages to develop the intervention, which we then modified informed by findings of a focus group study with people with sCHD and depressive symptoms and further literature review.
We then conducted an exploratory randomised controlled study to examine the acceptability, feasibility and potential costs of the new intervention, and to test the methods for a definitive trial.
Developing the intervention
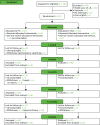
Barley et al.2 published a full account of the process that we used to develop the intervention in an open access journal in 2012. The key steps of the process, which followed MRC guidelines for developing complex interventions,30 are summarised here. This process is also depicted in Figure 3.
FIGURE 3
The UPBEAT-UK study intervention development stages. Reproduced from Barley et al. © 2012 Barley et al.; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), (more...)
Phase 1: gathering evidence
This phase included the qualitative studies of patients and primary care clinicians, and the metasynthesis of published literature detailed in Chapters 2 and 3. Essentially, all three studies led to the conclusion that the needs of patients with CHD and depression are diverse and include psychosocial problems involving interpersonal and health/control losses that primary care staff are uncertain how to manage.
Phase 2: synthesis of findings from previous work packages and iterative literature review
Multidisciplinary team discussions involving GPs, psychiatrists, psychologists, nurses and a cardiologist, of our earlier studies led to agreement that the UPBEAT-UK intervention should:
- improve depression, quality of life and cardiac outcomes in patients with CHD
- help patients and clinicians to manage an individualised range of problems, including social problems
- be nurse-led and feasible for delivery in primary care.
These conclusions were used to guide literature searches focused on identifying high-quality systematic reviews and evidence-based guidelines: we searched the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effectiveness and the NICE website. We also looked for evidence published subsequently to the reviews and guidelines using MEDLINE, EMBASE and PsycINFO. Findings from the evidence reviews were discussed and used to support choices for the intervention content. This is detailed in Table 5 (first published in our RCT development paper).2
TABLE 5
Iterative evidence review to guide development of an empirically based intervention to improve mood and cardiac outcomes in patients with CHD
We discussed the meaning of these findings in the context of UK primary care, where an established component of chronic disease management is the provision of self-management support; this means enabling patients to take better care of themselves, for instance by providing information and helping them to change unhealthy behaviours.
Two important factors for behaviour change are known to be belief in the importance of an outcome and belief in capacity to succeed (self-efficacy).105 This suggested to us that instead of focusing on generic CHD or depression risk factors, the new intervention should enable patients to specify their own goals, for instance stopping smoking or increasing social contact, so that work is directed towards outcomes important to patients.
Informed by our evidence review a ‘toolkit’ of behaviour change skills and existing local resources was developed to facilitate nurses, acting as case managers, to help the patient to increase their self-efficacy and achieve their desired outcomes.
The intervention we developed therefore was a PN-led PC intervention. It comprises the following:
Personalised care planning: the nurse acting as case manager conducts a standardised biopsychosocial assessment (including physical and mental health, difficulties with current treatment regimens, problems with daily activities and social problems) face to face with the patient either at the patient’s GP surgery or at their home, according to patient preference. Patients are helped to identify up to three problems that they consider contribute to their depression and which they most want to address. The nurse case managers, as applicable, provide information, signpost patients to existing resources (e.g. leisure centres, social clubs, IAPT services) and use evidence-based behaviour change techniques to help patients set and achieve goals. The underlying intention of the intervention is to increase the patient’s self-efficacy to achieve their desired goals (as opposed to goals determined by others such as symptom management or reduction of cardiac risk factors, a primary aim of many previous collaborative care projects). Details of the assessment and action plan are recorded in a ‘personalised health plan’, which the patient holds.
Follow-up care: follow-up interviews to determine progress and/or set new goals are conducted via telephone to conserve nurse time. This is initially weekly and then the frequency of contact is agreed between the patient and nurse case manager. Calls are planned to last 15 minutes.
The intervention process is detailed in Figures 4 and 5 (first published in our RCT development paper).2
Phase 3: modelling
Having developed the intervention, and following MRC guidance,30 we now wanted to explore its acceptability among people with sCHD and depressive symptoms. Using maximum variation sampling to select patients varying by sex, age, ethnicity and borough of residence, we invited participants of the UPBEAT-UK cohort study to attend two focus groups.
In a short presentation, two researchers (RS and Zoe Fortune) explained the proposed intervention and the intervention materials (patient information leaflet, assessment form, personalised health plan) were provided. All participants gave informed consent and ethical approval was granted, along with that for the other qualitative studies, by Bexley and Greenwich Research Ethics Committee (07/H0809/38).
Sixteen patients agreed to take part. Thirteen actually participated; five out of six came for the first group and eight out of 10 came for the second group. Reasons for not attending were not elicited. Those who participated (53% male) were aged between 48 and 86 years (mean 71 years). All but two, who were African Caribbean, described themselves as white British.
Discussions were tape-recorded and transcribed by RS. Transcripts were entered into NVivo 8 qualitative software. Coding was informed by the aims of the study. The coding frame and themes were discussed within the research team; we used notes of key discussion points (verified by participants) to guide our analysis.
Key findings of the discussion and modifications to the intervention
The participants were very focused on their need for someone to confide in and felt that a case manager would help with this.
The concept of self-management, in which the case manager would help them to solve their own problems, did not seem to be well understood. We planned to examine this in our exploratory study.
Participants agreed that PNs were the correct people to act as case managers because of their understanding of heart disease and its associated problems. Nurses should also be able to provide the continuity of care that participants emphasised as important.
Some participants disliked the planned use of goal-setting. Rather, participants were focused on wanting case managers to be a source of social contact. Based on our work with PNs, who complained of lack of time to manage these patients, this did not seem feasible. Instead, we felt that goal-setting and action-planning could be used to help patients obtain social contact. Furthermore, our literature searches identified good evidence for the feasibility of goal-setting in primary care.106 This highlighted to us the importance, when designing interventions, of utilising multiple sources of evidence. We planned to explore the acceptability of goal-setting further in our exploratory study.
Finally, a minor point was that some of the wording used on the assessment form was not clear. We had developed our care plan format from the framework proposed by the Department of Health as the standard assessment for adults;107,108 this grouped health-related domains such as ‘activities of daily living and mobility’ under headings such as ‘improved personal dignity and autonomy’. These higher-level headings appeared to confuse patients, so they were removed from intervention documents.
Discussion
Informed by the MRC framework for the design of complex interventions,30 we conducted empirical studies and iterative literature searching to identify evidence and theory to develop and model a new PN-led PC intervention to improve mood and cardiac outcomes in sCHD patients with depressive symptoms.
Our approach is only one possible approach to developing a complex intervention using the MRC framework.30 For instance, others109 have drafted an intervention around a specific psychological theory and have tested theory-related hypotheses. However, we feel that a strength of our approach is that the intervention development was driven by the patients who will receive it and by the clinicians who will deliver it, with theory (i.e. self-efficacy theory and behaviour change theory) and evidence used to support choices concerning its content. The fact that all the studies reported here were funded by a single programme grant facilitated access to patients and allowed subsequent work to build on earlier findings in a timely fashion.
Furthermore, our approach led us to change our initial plans, which, as we have explained in Chapter 1, were to develop a nurse-led stepped care intervention. Our intervention development work clearly demonstrated a need for an intervention that could be tailored to the differing needs of individual patients; this could be included within a stepped care approach. It was also clear from this work that some PNs will need support to deliver an intervention for depression and that, since their workload is already very high, they would need convincing that any new intervention is feasible for them to deliver as well as effective for patients. Hence, with so much uncertainty around the feasibility of PN-led interventions for depression, we changed our plan to conduct a definitive RCT and decided to conduct an exploratory study which would provide this evidence and inform the best methods of a future definitive RCT.
The exploratory randomised controlled trial
In our exploratory study, two nurse researchers, independent of the participating practices, acted as case managers; one was a general and mental health nurse with experience of working in primary care, and the other was a general nurse who had subsequently qualified as a health psychologist. We also knew, from our cohort study, that CHD patients have high levels of comorbidity and that for some any cardiac event would have been several years ago; we therefore recruited only those patients with sCHD (i.e. reporting current chest pain) in order to ensure that they would understand the intervention in terms of their CHD. The study explored the acceptability, feasibility and potential costs of PN-led PC for primary care CHD patients who have depressive symptoms and current chest pain with the overarching aim to test the methods for a definitive trial. The full details of this study have been published in a peer reviewed paper by Barley et al.110 Here we summarise our methods and findings and report additional exploratory analyses.
Aims and objectives
- To examine the rate of participant recruitment and reasons for non-participation.
- To examine research procedures such as consent, randomisation/blinding and data collection.
- To explore possible differences between primary outcome measurements and explore the most appropriate secondary outcome measures in relation to patients’ reported problems.
- To identify any trends between the groups in changes in self-efficacy and the impact of this on depression outcomes.
- To determine the acceptability and feasibility of the intervention to practices and participants.
- To explore whether or not the intervention can be standardised and whether or not therapist effects are likely to be important.
- To explore potential costs of the intervention.
Methods
Design
The design was a patient-randomised pilot study with a control condition. We compared PN-led PC plus TAU for 6 months with TAU alone. The protocol for the study has been published;111 deviations from the protocol are explained in the published report of this study.110 The study was reviewed and approved by the south-east London Research Ethics Committee (reference 10/H0808/5) and is registered with Current Controlled Trials International Standard Randomised Controlled Trial Number 21615909. The UPBEAT-UK Programme Grant Steering Committee, who decided that a data monitoring committee would not be necessary, oversaw the study.
Study setting
Practices in south London were recruited via the Greater London Primary Care Research Network (PCRN-GL). To be included, the practice had to keep a register of patients with CHD for the QOF112 and be willing to liaise over patients in the PC arm when necessary.
Participant eligibility and recruitment
Inclusion criteria were sCHD (registered on GP CHD QOF register and reporting chest pain), reporting depression symptoms. All patients on practice case registers for CHD were asked by their GP for consent to contact from a researcher. Those consenting were contacted by a researcher and assessed for depressive symptoms using the PHQ-280 and for symptoms of current chest pain using the modified Rose angina questionnaire.113 Patients scoring ≥ 3 on the PHQ-2, and who reported currently experiencing chest pain (using the modified Rose angina questionnaire) were assessed further using the HADS.114 If they scored ≥ 8 on the depression scale of HADS (HADS-D) they were eligible to participate. Those consenting to participate were then randomised to either the intervention (PN-led PC and general practice TAU) or control (general practice TAU). Patients who were temporary registrants or currently hospitalised, or who a GP from the practice deemed actively suicidal, suffering from psychotic depression or non-English speaking were excluded.
As estimation of an effect size was not the focus of this pilot study, we used only a preliminary sample size calculation. An end-of-study mean difference between intervention and control score of ≥ 3 on the HADS-D, assuming a pooled SD around mean scores of 3.5, would require 30 participants per group for 90% power at the 5% significance level. To allow for loss to follow-up, estimated at 25%, our plan was to recruit 80 participants (40 per arm) into the study. The target figure of 3 was based on consensus discussion among clinicians at planning meetings and the assumed SD of 3.5 was obtained from the baseline cohort study. The estimate of attrition of 25% was considered reasonably conservative; it was felt that higher levels would have indicated lack of feasibility for implementing and testing the intervention. We estimated from the results of the UPBEAT-UK cohort study1 that 10–15 practices each with around 10,000 patients would be needed to recruit 40 persons per arm.
Randomisation and blinding
Randomisation at patient level was conducted independently by the Mental Health and Neurosciences Clinical Trials Unit at King’s College London. A random permuted block design was used to balance the numbers between groups. PC group participants were randomly allocated to one of two nurse researchers acting as case managers.
To ensure that those responsible for outcome data collection were blind to participants’ allocation status, participants were asked at the beginning of each follow-up interview not to mention if they had been in contact with other study staff. The statistician was also kept uninformed of allocation status.
Outcome data collection
Research assistants who were blind to allocation collected data at baseline and at 1, 6 and 12 months post randomisation. Data were collected face to face at baseline and at follow-up via telephone.
The intervention: personalised care
This was delivered over 6 months, as detailed above.
Usual care
All patients received primary care TAU from their GP and/or PN; this may or may not include specific depression intervention such as antidepressant prescription or referral to talking therapy. The nature of TAU may vary between practices; we assumed that important differences in care delivery between the participating practices would be randomised between the groups.
Outcomes
Baseline demographic variables
All participants provided baseline demographic data including sex, age, ethnicity, socioeconomic status,115 employment and relationship status, living arrangement and lifestyle factors (e.g. smoking status, alcohol consumption, body mass index).
Aim 1: to examine participant recruitment
We made detailed records of recruitment rates. The number of participants at each stage of the study was documented and reasons for attrition were recorded.
Aim 2: to examine the study procedures
We recorded the number of randomisation errors [e.g. numbers of participants randomised despite being ineligible or who were randomised to the intervention (PC) but who did not receive it], and recorded rates and reasons for attrition and missing data for outcome measure at each time point.
Aim 3: to explore outcome measures
The preliminary primary outcome was the HADS-D. We observed depression status (response defined as ≥ 50% decrease in score from baseline at follow-up and remission defined as a score of < 8 at follow-up) and severity (continuous score). We also explored the PHQ-9116 as an alternative measure of depression severity and extracted the number of GP/PN consultations for depression, antidepressant prescriptions and referrals to talking therapy during the 12-month study period from participants’ medical records for both groups.
Our cohort study found that self-reported chest pain (measured using the modified Rose angina questionnaire) is associated with mood and social problems, so was also explored as a potential primary outcome for a future trial. The number of GP/PN consultations for heart-related problems during the 12-month study period was also extracted from participants’ medical records as a proxy measure of participants’ cardiac status in both groups.
Potential secondary outcomes explored were: anxiety [HADS – anxiety subscale (HADS-A)],114 well-being (Warwick–Edinburgh Mental Well-being Scale),117 quality of life [Short Form questionnaire-12 items (SF-12)],118 functional status (specific activity schedule),119 number of reported social problems120 and adherence to antidepressant medication (if relevant – adapted version of Morisky Adherence Index121). To try to capture between-patient variety in reported problems and needs, we used a validated patient-generated measure of problems, function and well-being – the Psychological Outcome Profiles Questionnaire.122
To reflect on how these outcome measures relate to the problems that patients consider important or feasible to change, we explored the types of needs and problems identified by intervention patients in collaboration with their case manager. This information was extracted from the notes made by nurse case managers during consultations. The Brief Illness Perceptions Questionnaire (BIPQ)123 asks participants to ‘Please list in rank-order the three most important factors that you believe caused your illness’ – we also explored these responses.
Aim 4: to explore changes in self-efficacy
We used the General Self Efficacy Scale;124 the scale has 12 items designed to assess perceived self-efficacy in order to predict coping with daily hassles and adaption after life events, with a high score indicating greater self-efficacy (range 10–40). We also used the BIPQ123 to assess changes in perceptions about illness along the following dimensions: consequences, timeline (anticipated duration of illness), personal control, treatment control, identity (symptoms associated with the illness), illness concern, illness coherence (understanding of CHD) and emotional representations (emotional impact of CHD). Each of these eight items is scored 1–10. We examined General Self Efficacy Scale and BIPQ total scores as mediating factors for depression symptom reduction, remission and response.
Aim 5: to determine the acceptability and feasibility of the intervention
We recorded the time taken for assessment and the number and duration of follow-up telephone calls per patient, and explored participant satisfaction using an 11-item questionnaire devised for the study that was posted to the intervention patients after their 12-month follow-up.
Aim 6: to explore whether or not the intervention can be standardised and whether or not therapist effects are likely to be important
We developed a manual for the intervention, examined differences in patient outcomes between the two nurse researchers delivering the intervention and recorded nurse actions during the intervention.
Aim 7: to explore potential costs of the intervention
We calculated quality-adjusted life-year (QALY) gain using the European Quality of Life-5 Dimensions (EQ-5D).125 The EQ-5D consists of five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and each is rated 1 (no problem), 2 (moderate problems) or 3 (major problems). UK values were applied to the distinct health states derived from the EQ-5D to estimate the utility value for each patient at each time point and area-under-the-curve methods were used to calculate the QALYs.126
Economic costs were calculated from a societal perspective. PC costs included the time spent by PNs with patients in face-to-face assessments and subsequent telephone reviews. A unit cost of £36 per hour was attached to the average intervention duration for each patient. Other service use was recorded using the Client Service Receipt Inventory127 for the 6-month period preceding baseline, 6- and 12-month follow-ups. Health services included hospital inpatient and outpatient visits, GPs, psychiatrists, psychologists, physiotherapists, counsellors, nurses and other therapists. Unit costs were applied to service-use data using the NHS reference costs in 2009–10 prices128 and the 2010 Unit Costs of Health and Social Care.129 In addition, data were collected on the weekly number of hours of help (i.e. personal or child care, help in/around and outside the house) received from friends and relatives of the patient. The unit cost of a home care worker was used as a proxy for costing informal care.
Medication use was recorded and costs were calculated based on the 2010 prices from the British National Formulary130 and the Prescription Cost Analysis.131 The basic types of medication included psychological, cardiovascular (most common type), gastrointestinal, respiratory, eye, ear, nose and oropharynx drugs. Indirect costs of productivity loss because of CHD and comorbid depression were calculated using the human capital approach. For employed patients, productivity loss was the product of the days missed from work caused by sickness and the national mean daily wage in the UK,132 adjusted for full- or part-time working. However, only resource-use costs were considered in the cost-effectiveness analysis, as data on medication and sickness absence were self-reported and available for the baseline only (regarding the past 6 months).
Analysis
We conducted exploratory analyses using Stata version 11.2 (StataCorp LP, College Station, TX, USA). The intention-to-treat principle was used for all analyses. Owing to the exploratory nature of the analysis, p-values are reported for the preliminary primary outcome (HADS-D) only. We were fortunate to have a National Institute for Health Research-funded statistical fellow attached to our programme and were therefore able to conduct detailed exploratory analyses; these are explained fully in the published report of this study.110 Essentially, we developed a single statistical model to estimate the difference in mean scores between participants randomised to PC and TAU across the three follow-up points (1, 6 and 12 months). Other exploratory analyses compared the median number of responders (≥ 50% decrease in score from baseline at follow-up) and remitters (score of < 8 at follow-up) according to the HADS-D score between groups and explored changes in self-efficacy, the effect of nurse contact time and therapist effects using t-tests and chi-squared tests.
A health economics analysis used multiple regression, incremental cost-effectiveness ratios and the net benefit approach to estimate mean differences in costs and QALYs. Non-parametric bootstrap analyses were conducted to account for the highly skewed distribution of the cost data; results were plotted on a cost-effectiveness plane and used to estimate cost-effectiveness acceptability curves.
Results
Aim 1: examining recruitment
Seventeen practices were approached by the PCRN-GL and agreed to participate. Practices were recruited between October 2010 and June 2011; practice recruitment was therefore completed in considerably less than the 12 months planned in the study proposal, indicating that recruitment of practices for a definitive trial would be feasible.
We have published patient recruitment details in a paper by Tylee et al.111 We summarise it briefly here. Of the 3325 people on the 17 GP CHD registers, 1001 consented to be contacted by returning a letter to their GP. A brief screen by telephone found that 126 were eligible for assessment (PHQ-9 score of ≥ 3 and reporting current chest pain on the modified Rose angina questionnaire). Of the 126 who were eligible, 40 had a HADS score of < 8, two had experienced hallucinations, two had no current chest pain and one did not have sufficient English, therefore, 81 were found to be eligible. These were consented and randomised (41 to intervention, 40 to control). The screening process involved minimal effort (return of a letter and a brief telephone screen). Target recruitment was achieved within 8 months, which was considerably faster than expected (we had planned a 12-month recruitment period based on experience of other studies); recruitment of patients for a definitive RCT, therefore, seems promising.
Baseline demographic and lifestyle data are reported elsewhere.110 There were 27 (66%) males in the PC group and 25 (63%) in the TAU group; mean age was 64.2 years (SD 13.0 years) in the PC group and 64.9 years (SD 8.5 years) in the TAU group. Any differences between the groups on the recorded demographic, lifestyle and outcome variables appeared small and the randomisation process appears to have produced balanced groups.
Past depression
Forty-eight participants reported having ever been diagnosed with depression (21/41 = 51% PC; 27/40 = 67% TAU). Of these, 12 had had one episode, seven had had two episodes, four had had three episodes and 23 reported having had four or more episodes (data on number of episodes were missing for two participants). Forty-six participants had previously received treatment for depression; of these, 41 had taken antidepressants and 29 had had talking therapy. Eighteen reported having received other treatment such as ‘anger management’, seeing a psychiatrist, electroconvulsive therapy, inpatient psychiatric care and relaxation and assertiveness courses. Our participants therefore represent a chronic and severe group.
Current depression
Twenty-four participants reported that they were currently receiving treatment for depression (9 in PC, 22%; 15 in TAU, 38%). According to the medical notes data, 13 in PC (32%) and 17 in TAU (43%) were taking some form of antidepressant medication at baseline. Despite being prescribed antidepressants, these participants were still reporting depressive symptoms. Nineteen participants reported their current episode had lasted > 12 months, two said it had lasted between 6 and 12 months, and three said it had lasted < 6 months.
Mean HADS-D scores [PC 11.6 (SD 3.3); TAU 11.4 (SD 3.0)] indicated moderate depression and mean PHQ-9 scores [PC 16.0 (SD 5.3); TAU 15.4 (SD 5.5)] indicated moderately severe depression in both groups. At baseline, according to the HADS-D, 21 (51.2%) participants in the PC group could be considered mild, 14 (34.2%) moderate and six (14.6%) severe. In the TAU group there were 19 (47.5%) mild, 15 (37.5%) moderate and six (15.0%) severe. For the PHQ-9, three (7.3%) were mild, 10 (24.4%) were moderate, 14 (34.2%) were moderately severe and 12 (29.3%) were severe in the PC group. In the TAU group, there were eight (20.0%) mild, eight (20.0%) moderate, 14 (35.0%) moderately severe and nine (22.5%) severe. The correlation between baseline HADS-D and PHQ-9 was r = 0.48 (p < 0.0001).
Coronary heart disease status
Patients were recruited if they reported current chest pain. Overall, 19 were current smokers and 53 were overweight or obese (see Table 8). Participants were also asked if they had high blood pressure and cholesterol, and diabetes; 56 out of 76 who responded (74%) said yes to high blood pressure (29/37 in PC; 27/39 in TAU), 42 out of 72 who responded (58%) said yes to high cholesterol (21/34 in PC; 21/38 in TAU) and 22 out of 80 who responded (28%%) said yes to diabetes (12/40 in PC; 10/40 in TAU).
TABLE 8
Comparison of psychiatric baseline scores between completers and non-completers of follow-ups
Aim 2: examining study procedures
Randomisation
Three patients who were ineligible owing to no current chest pain were randomised in error (two in the intervention arm); reasons for this are unclear. Based on the intention-to-treat principle these were included in all analyses, however, we conducted a sensitivity analysis and found that there were no differences in our conclusions when these patients were omitted from the analyses.
Blinding
Over the course of the study, there were many staff changes, especially among the research assistants responsible for outcome data collection. It was therefore not possible to test formally whether or not those collecting data remained blinded to the patients’ allocation status. However, it was noted that some participants had reported contact with the case manager. Following the conduct of the main analyses, the statistician reported becoming unblinded; this was as a result of hearing that an additional participant had been randomised to PC.
Attrition
The Consolidated Standards of Reporting Trials diagram for the study is shown in Figure 6 and has been published.110 By 12 months, six people in the intervention group had dropped out (two because they found participation upsetting, two because they felt too physically unwell to continue and two gave no reason) and one from the control group had dropped out (because they found participation upsetting). Two intervention group participants received baseline assessment but no intervention, as the nurses were unable to contact them. Overall, attrition was low (7/81 = 9%), with data collected at one or more follow-up points for 79 people (98%).
Across the study period, completion was better for the TAU group than the PC group (Table 6). Completion was better among non-drinkers and those that drank the most (> 11 units per week), as well as those who were retired compared with being in paid employment across time points. Characteristics of completers are described in Table 7, which lists the demographics of those who completed follow-up and those who did not. HADS-D scores (Table 8) were marginally lower at 1 and 6 months among non-completers; however, they evened out by 12 months. We compared the models from our analyses with an additional model that controlled for any variables that were associated with missing follow-ups; these analyses did not give us any reason to alter any of our conclusions.
TABLE 6
Demographics of those who complete follow-up and those who do not
TABLE 7
Continuous demographics of those who complete follow-up and those who do not
Data collection
The maximum number of observations available was 81 at baseline, 77 at 1 month, 74 at 6 months and 69 at 12 months. Table 9 shows the number of (and percentage of available) missing scores for each questionnaire at each assessment point. Note: missing data for the Social Problems Questionnaire (SPQ) are not recorded because of confusion concerning whether items were missing or left out because they were not applicable (for analysis purposes no response was considered to mean ‘not applicable’ and therefore no problem in this area). Two of our outcome measures had no missing scores at any point: the modified Rose angina questionnaire and the specific activity schedule. The second question of the BIPQ (BIPQ2) had the most missing scores, with 14% missing at 6 months. For the other measures, between 5% and 10% of scores were missing at one or more assessment points for the General Self Efficacy Scale, the BIPQ (questions 3 and 4) and the Warwick–Edinburgh Mental Well-Being Scale; and < 5% of scores were missing for HADS-D, PHQ-9, BIPQ (questions 1, 5, 6, 7, 8), HADS-A and the SF-12 (mental and physical components). Therefore, our selected outcome measures appeared to be acceptable to the participants and would be feasible to use in definitive trial within a similar population.
TABLE 9
Completeness of outcome measure data collection
Depression
Depression outcomes are shown in Table 10. Both groups showed some improvement in depression symptoms (HADS-D score) at all time points, with the mean score in both groups moving from indicating moderate depression severity at baseline to mild severity at 12 months. We saw a similar pattern using the PHQ-9, with mean scores in both groups indicating moderately severe depression at baseline reducing to moderate depression at 12 months.
TABLE 10
Depression outcomes
According to the HADS-D, there was a greater percentage of remitters in the TAU group compared with the PC group at 6 and 12 months; there was also a greater percentage of responders at 6 months in the TAU compared with the PC group, but by 12 months more PC group participants had responded. However, the mixed-effects models showed no significant differences between groups over time for any measure of depression and CIs were wide so an effect in favour of either group cannot be ruled out.
From the medical notes, across the 12-month study period, in the PC group, 31 participants (76%) saw their GP or PN regarding their mental health (total of 101 consultations recorded); in the TAU group, 29 participants (73%) made a mental health consultation (total of 102 mental health consultations recorded). Of those participants who were not treated for depression at baseline (i.e. no record of antidepressant prescription or talking therapy referral), three PC participants had received a prescription for an antidepressant [Citalopram (Cipramil®, Lundbeck), n = 2; Mirtazepine (Mirtazepine®, Merck Sharp & Dohme, Corp.), n = 1; one of these participants was also referred for ‘counselling’] and one additional PC group participant had been referred to a ‘psychiatric clinic’ by 12 months; no participants in the TAU group had a new referral for depression treatment or a new prescription for an antidepressant at the end of the study.
Chest pain
The most notable difference between the PC and TAU groups was in self-reported chest pain (modified Rose angina questionnaire). At 6 months the proportion of patients who no longer reported chest pain was 37% in the PC group versus 18% in the TAU group and at 12 months it was 31% in the PC group versus 19% in the TAU group. From the medical notes across the 12-month study period, in the PC group, 34 participants (83%) saw their GP or PN regarding their CHD (total of 158 consultations recorded); in the TAU group, 29 participants (73%) made a CHD consultation (total of 170 consultations recorded). It is not clear from the notes whether these were routine or emergency visits, so we examined recorded accident and emergency (A&E) visits.
In the PC group, 10 participants (six for heart problems, two for other state reasons, two no reason recorded) visited A&E (total 13 visits: nine heart problems, two other stated reasons, two no reason recorded). In the TAU group, 15 participants (four for heart problems, five for other state reasons, six no reason recorded) visited A&E (total 26 visits: seven heart problems, six other stated reasons, 13 no reason recorded). PC participants therefore made fewer A&E visits (24% in PC vs. 38% in TAU), although missing data concerning the reason for these visits makes this information difficult to interpret.
Preliminary secondary outcomes
At 6 and 12 months both groups improved on all outcomes (Table 11); these data have been published.111 There was no evidence for an interaction between time point and study arm for any outcome, so a differential effect over time appears unlikely.
TABLE 11
Mean scores (SD) for outcomes other than depression at 1, 6 and 12 months
Aim 3: validity of outcome measures in comparison with participant-reported problems
Using the BIPQ, participants were asked to list the three most important problems that caused their illness (CHD). Sixty-one participants gave at least one reason (these data are published online as an appendix110). The most common reason given was ‘genetics or heredity’, followed by lifestyle factors such as smoking, poor diet and lack of exercise. Mood problems, especially stress and work-related stress were also mentioned, and comorbid or past health problems were also blamed. Four patients mentioned relationship problems and one mentioned financial problems.
Participants in the PC group (n = 41) identified 21 types of problem as contributing to their depression and which were addressed during the intervention (up to three problems per patient); most common were pain (chest and other pain, e.g. arthritis) (n = 18), lack of exercise (n = 17), difficulty sleeping (n = 13), anxiety (n = 11) and being overweight (n = 11). Reported problems and whether or not they were addressed during the intervention are published online as an appendix.110
Participants therefore explained both their CHD and their depression in terms of wide-ranging problems that appeared similar for the two conditions; lifestyle problems in particular were associated with both.
Within our study, mood outcomes were assessed using the HADS (depression and anxiety) and the PHQ-9 (depression); however, we had no measure of change in lifestyle-related outcomes. The PC intervention was aimed at tackling the problems that each participant felt were important rather than addressing specific cardiac risk factors; however, in view of our current finding that patients do consider lifestyle factors known to be associated with CHD as contributing to their depression, inclusion of a measure of change in cardiac risk factors should be considered for a definitive trial of PC. It will be important to select a measure that captures the variation between participants in terms of which risk factors they want to address; a validated measure of goal attainment may therefore be appropriate.
Aim 4: exploring changes in self-efficacy
Self-efficacy improved over the course of the study (see Table 11). At 12 months, the PC group had a mean increase of 2.5 points versus 0.9 points in the TAU group, suggesting a greater increase in self-efficacy in the PC group; however, the mixed-effects model indicated no difference between groups over time (adjusting for baseline self-efficacy): mean difference –0.58 (95% CI –3.05 to 1.89).
At 6 and 12 months the overall illness perceptions score and most measured dimensions showed improvement in both groups, though differences between groups were small (Table 12). The mean improvement in overall score from baseline to 12 months was greater in the PC group than in the TAU group: 7.8 points versus 2.5 points. As expected, the biggest difference in mean improvement between the PC and TAU groups in dimension scores was in personal control (mean change in BIPQ from baseline = 1.5 for PC group vs. 1.1 for TAU at 6 months; and 1.1 for PC vs. 0.1 for TAU at 12 months); however, the mixed-effects model suggested no difference between groups over time (adjusting for baseline total BIPQ score): mean difference –0.42 (95% CI –4.57 to 3.72).
TABLE 12
Brief Illness Perception Questionnaire scores
Controlling for changes in self-efficacy or overall illness perceptions had little effect on change in depression over time, whether or not considering depression severity, remission or response (Table 13). Since CIs were wide, change in favour of either PC or TAU cannot be ruled out.
TABLE 13
Effect of controlling for changes in self-efficacy and illness perceptions on depression outcomes over the study period
Post-hoc analyses
As anxiety symptoms were high at baseline, we also explored HADS-A score as mediator for improvement in depression. Controlling for anxiety slightly reduced the difference in depression symptoms between the groups over time: mean difference –0.43 (95% CI –1.48 to 0.63; p = 0.43). Controlling for anxiety considerably reduced the odds of remission in the TAU group versus PC group in favour of the PC group: odds ratio (OR) remission in TAU versus PC group 0.42, 95% CI 0.10 to 1.68; p = 0.22, which suggests that changes in anxiety symptoms may be a mediator for depression remission. The odds of depression response in the TAU group compared with the PC group were also slightly reduced when anxiety scores were controlled, although the odds were still in favour of TAU (OR 1.12, 95% CI 0.32 to 3.94; p = 0.86). None of these analyses showed a statistically significant effect; all changes in effect were small and CIs were wide so we cannot rule out benefit for PC or TAU.
Aim 5: exploring acceptability and feasibility of personalised care
Nurse time used for intervention
Intervention patients (n = 41) received a mean of 203 minutes (SD 100 minutes) of nurse time [78 minutes (SD 19 minutes) for assessment, 125 minutes (SD 91 minutes) in telephone follow-up calls over 6 months]. The mean number of follow-up calls was nine (SD five); the mean duration of calls was 14 minutes (SD 4 minutes). The nurses arranged a time to call the patient, but sometimes patients did not respond; there was considerable variation between patients in the number of failed follow-up contact attempts by nurses over the 6-month intervention period (range 0–32), but on average nurses made 2.8 calls for every successful contact.
Effect of intervention intensity
The amount of time spent talking to the nurse varied considerably between patients (range 74–406 minutes), so we used the median duration (167 minutes) to divide the participants into high (n = 20) and low (n = 19) ‘dose’ groups. There were no significant differences (p > 0.05) between the groups at baseline in depression [HADS-D mean: low-dose group 11.0 (SD 3); high-dose group 12 (SD 3.7)]. The magnitude of improvement in depression over time was greater for the high- compared with the low-dose group and fewer high-dose patients had chest pain at 6 and 12 months, although the mixed-effects models indicated little difference between the groups: depression mean difference –0.72, 95% CI –3.03 to 1.60; chest pain OR 0.34, 95% CI 0.01 to 7.53.
Patient satisfaction with personalised care
Of the 41 PC participants, 21 completed and returned the satisfaction questionnaire. The questionnaire and responses are shown in Table 14. On the whole, patients reported finding the different elements of the intervention (assessment, care plan and follow-up calls) helpful. They also found that the intervention helped them communicate with other health professionals such as their GP. Most respondents agreed or strongly agreed that the nurse was able to answer their questions about their mood, heart or other health problems and that they could understand the information given, and that the nurse provided support and encouragement and had a courteous manner. Of the 15 patients who responded to this question, all said that they would like their GP to offer a similar service.
TABLE 14
Patient satisfaction with the PC intervention
The patients were also asked which aspect of working with their nurse they had liked best and least. Seventeen patients responded regarding what they liked best: two patients liked ‘everything’ or ‘all’; several comments referred to the patient’s pleasure in having someone pleasant to talk to:
it’s just good to talk to somebody.
sympathetic helpful and friendly approach.
[Nurse] is a nice person and very pleasant.
Other comments were more specific and referred to the nurse’s ability to treat them as an individual when offering advice and understanding:
Their personal interest in me as a patient and not just a number, like you feel in hospital sometimes. Their interest in my problems and how they could help me with my problems and difficulties and trying to help, in getting me to understand my problems and difficulties and that there was a light at the end of the tunnel and they has been so good to me in that area and I’m hoping I can do their help worthy.
They always had a nice manner (sic). Listens very well and after what I’ve been through they had a good answer and good advice. Please thank [nurse] for their time.
their advice about routine – listening to radio and when you believe somebody care about you – here I am alone, no family (except my children), no friend. We need such as this service.
Regarding what they liked least: 10 patients responded ‘none’ or ‘N/A’ [not applicable]; one patient responded ‘forms’; another, who had been positive about the intervention, appeared to indicate that they had found participation difficult:
Knowing the value of time it was so difficult to convince myself that I was not wasting both (the Nurse’s) and my time operating this plan. I fought hard against this feeling.
This patient’s comment in the ‘liked best’ section seemed to suggest that they nevertheless valued the intervention, so their earlier comment may be reflective of their depressed state:
Honestly I didn’t really like any of it. I feared that I was too set in my regimes to open my soul and shortcomings. However I understood that it was important for me to proceed and tried to give it my full attention. But it wasn’t likeable.
Aim 6: exploring standardisation and therapist effects
Intervention fidelity
As planned, the nurse case managers used a range of nursing and behaviour change techniques to help patients address their problems. Classifiable behaviour change techniques reported by nurses were: general encouragement, information linking health and behaviour, goal-setting and action-planning, barrier identification and focus on past success. Other nurse-reported actions included lifestyle advice, signposting (e.g. to relevant local resources such as leisure or day centres), promoting adherence to therapy and supportive counselling.
There was also some evidence of collaborative care. The nurse case manager contacted the patient’s GP (10 patients), the patient’s PN (four patients), social services (one patient) or another professional, for example IAPT worker or other therapist, occupational therapist, community mental health nurse, physiotherapist, housing or benefits officer (17 patients). With the patient’s permission, the nurse case managers consulted a family member of four patients.
Therapist effects
The patients of Nurse 1 had a higher mean baseline HADS-D score (12.4 vs. 10.9, Wilcoxon rank-sum test; p = 0.07). However, the random-effects model (combining data from 1, 6 and 12 months) indicated little difference in the average therapist effect on the HADS-D score across the time points (adjusting for baseline HADS depression score): mean difference –0.86 (95% CI –2.81 to 1.10).
Regarding self-reported chest pain, of Nurse 1’s (registered general nurse and health psychologist) patients, 44% continued to report chest pain at 6 months, compared with 79% of Nurse 2’s (registered general and mental health nurse) patients (p = 0.03). In the random-effects model, the odds of reporting chest pain across the study period were higher for Nurse 2 than for Nurse 1 (OR 7.80, 95% CI 0.88 to 69.40).
Aim 7: examine the potential cost of personalised care
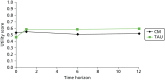
The average EQ-5D utility scores at baseline were slightly higher for the PC group (see Figure 7, which is also published elsewhere110), although the difference between groups was not statistically significant (95% CI –0.98 to 0.25; p = 0.40). By the 1-month follow-up, the TAU group had a higher utility score, and this difference was maintained up to the 12-month follow-up [95% CI –0.26 to 0.11; p = 0.422 (at 6-month follow-up: 95% CI –0.27 to 0.11, p = 0.408)]. In terms of QALYs, the TAU group showed an incremental QALY gain of 0.038 compared with PC over the 12-month treatment period. In Figure 7 the area between the two curves represents the QALY gain for the control group.
Service use and costs
Service use was fairly similar between the intervention and the control groups during the study period (Table 15, which is also published online as an appendix to our paper110). Hospital services were used more intensively by the TAU group than the PC group at all time points, with inpatient and outpatient care being the most frequently used services. The TAU group incurred higher inpatient costs than the PC group at each time point (particularly at baseline and at the 12-month follow-up). Few patients used day hospital services, but the costs incurred were high for both groups. The majority of patients received care from GPs and the costs of this were similar between the groups. Informal care was used slightly more among patients in the PC group than in the TAU group. Average total costs at each time point were lower for the PC group than for the TAU group. However, the differences were not statistically significant. For the PC group, the intervention itself accounted for only 6.7% of total costs.
TABLE 15
Service use and costs (£) at baseline, 6- and 12-month follow-ups (by randomisation group)
Cost–utility analysis
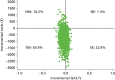
Of the total 81 participants, cost and QALY data at each time point were available for 68 patients (84%). Cost–utility results yielded an incremental cost-effectiveness ratio of £29,921 per additional QALY. Cost-effectiveness plane and cost-effectiveness acceptability curves were produced from bootstrapped resamples. The distribution of the cost-effectiveness point estimates on the cost-effectiveness plane (Figure 8, which can also be found in Barley et al.110) indicated a strong likelihood of cost savings for the PC group compared with the TAU group. The point estimate of the incremental cost-effectiveness ratio falls in the south-western quadrant, representing the situation where the PC group has reduced costs and worse outcomes. The second most likely result is that PC results in lower costs and better outcomes (south-east quadrant).
The cost-effectiveness acceptability curves (Figure 9) for the PC group compared with the TAU group was downward sloping. There is a greater likelihood of PC being the most cost-effective option up to a QALY threshold of £3035.
Discussion
We developed a PN-led PC intervention, which was designed to be easily implemented within practice in order to improve current primary care. In this patient-randomised pilot trial we explored the acceptability, feasibility and potential costs of the intervention for primary care CHD patients who have probable concurrent depression and current chest pain. We also explored the feasibility of the trial protocol to inform the methods of a definitive trial.
Feasibility and acceptability of personalised care
The PC intervention appeared to be feasible and acceptable for use in current primary care: a short amount of nurse time was needed, engagement with the nurse case managers was high and most of the PC group participants who returned our questionnaire reported satisfaction with all aspects. To further understand participants’ experience of the intervention, we have interviewed a subsample of 12 PC group participants. We used purposive sampling to select participants by age [>/≤ 60 years], sex, nurse case manager, depression (HADS-D >/≤ 10) and nurse case manager impression of depression response. Owing to research assistants leaving the programme for positions with longer-term prospects, these data are yet to be analysed.
As expected, this exploratory study confirmed the findings of our earlier qualitative work that patients with CHD and depression symptoms report a wide range of problems that they consider contribute to their low mood. The PC intervention enabled patients to identify and address these problems with the nurse case managers. This is an improvement on current care for these patients since management of depression and/or psychosocial problems is not routinely addressed in this population,31,59 despite recent routine depression screening under the QOF.112 Compared with the widespread organisational change that would be needed for collaborative care interventions as trialled in the USA,12 our PC intervention appears to offer an enhanced form of TAU which could be implemented easily in current primary care practice. Evidence from the UK-based proactive care by PNs for people with depression and anxiety (ProCEED) trial133,134 that care reviews delivered by PNs acting as case managers were acceptable to patients with long-term depression supports this.134
We explored a wide range of outcomes focusing on depression, as measured by the HADS-D as a potential primary outcome. Findings tended to show slight benefit for TAU compared with PC for depression symptoms, remission and response, except that at 12 months more PC participants had responded. However, differences between groups were very small and wide CIs mean that improvement with either PC or TAU cannot be ruled out. Our mixed-effects model indicated that PC would be unlikely to do much harm compared with TAU (mean difference –0.73), but could improve symptoms up to 2.1 points on the HADS-D (95% CI –2.08 to 0.62). We see little reason to change to a different outcome.
Accepted health psychology models agree on two factors important for behaviour change: belief in the importance of an outcome and belief in capacity to succeed (self-efficacy). Our intervention facilitated participants to work on outcomes important to them, and we used techniques such as action-planning to increase self-efficacy. Our data indicated that self-efficacy and illness perceptions, especially personal control, which is closely related to self-efficacy, were increased in those receiving PC but the difference from the increase in TAU group participants was not great.
Although both groups improved on all our measured outcomes, there were no large differences between groups, except in self-reported chest pain, which was also an inclusion criterion for the study. Our cohort study indicated that chest pain has a range of negative impacts and is therefore an important outcome to study.
Potential costs of personalised care
There were no great differences in service use and costs between PC and TAU, with the exception of inpatient care, which also accounted for a substantial proportion of total costs. Overall, it appears that PC reduced costs compared with TAU, but produced slightly lower benefits in terms of QALYs. This may appear counterintuitive given the other findings, but the utility scores underlying the QALY calculations were fairly similar and did not change much over time. Costs may have been underestimated owing to reliance on patient self-report in service use, the lack of medication and sick-leave data at all time points and the approach used to quantify informal care. Informal care constitutes a major cost driver in chronically ill populations. In this analysis, the ‘proxy good method’135 and the unit cost of home care worker was used to calculate informal care. However, in a future trial, an alternative cost, such as the national minimum wage, could be used to quantify informal care in the context of a sensitivity analysis. A future trial should also test whether or not a longer time horizon is needed for this particular patient group to benefit from an intervention of this kind. It may be considered unusual to include a full economic analysis in a feasibility study and, therefore, these results should be seen as exploratory.
Implications of clinical findings for a future trial of personalised care
An implication of the lack of difference between PC and TAU and the small degree of change in depression symptoms detected is that, if depression symptoms are to be used as a primary outcome in a definitive trial, a large sample size would be required to replicate difference of the order found here [e.g. using the HADS-D mean and SD (PC: mean 10.3, SD 4.6; TAU: mean 9.2, SD 4.6) at 6 months an achieved sample size of 368 per group would be required for 90% power at a 5% significance level (two-sided)]. This would be increased considerably if a cluster design were employed, which would be necessary to reduce contamination if PC were tested using PNs based in practice, and would also need to be increased to take account of attrition. On the other hand, a clinically significant effect of 3 on HADS-D, as originally proposed, would require fewer. The main implications for planning are the somewhat higher SD than that originally assumed (4.6 compared with 3.5), and the lower attrition rate (13% compared with 25%). The recruitment of practices to achieve these figures is another factor to be considered in planning. The fact that only a small improvement in depression symptoms was found over the entire sample is consistent with systematic review evidence indicating that even intensive evidence-based psychological treatments, such as CBT, problem-solving and pharmacological intervention with selective serotonin reuptake inhibitors, have only a small effect on depression in people with CHD.96,136
Furthermore, our sample appears to represent a hard-to-treat group: the level of depression symptoms was high, more than half reported recurrent depression and more than one-quarter reported that they were receiving depression treatment at baseline and yet still reported depression symptoms. In addition, a large longitudinal cohort study (n = 1209)137 has found that pain, mediated by baseline severity of mood symptoms, was predictive of a worse course of depressive and anxiety disorders; all of our participants reported current chest pain, which was an inclusion criterion. A 3-point change in HADS-D score (which a trial of this size could have detected) after 6 months of relatively low-intensity intervention may therefore be an unrealistic expectation and a more intensive intervention for depression with engagement over a longer period may be needed for patients such as these. Our data, which suggest that receipt of more nurse time is associated with greater improvement in depression and self-reported chest pain, support this.
As well as chest pain, anxiety comorbid with depression is predictive of a worse depression outcome;138 our participants reported high anxiety levels and we found some evidence of anxiety as a mediator for depression improvement. In a future trial, more active treatment of anxiety should be tested, particularly when associated with chest pain.
The potential to detect differences between PC and TAU in our trial may have been reduced because TAU is itself an active intervention: at baseline 43% of the TAU group versus 32% of the PC group were prescribed antidepressants (according to their medical notes), and we were unable to control for this difference in our analyses. It is also possible that TAU may have been intensified during the trial: during our qualitative work GPs and PNs reported greater awareness of the problem of comorbid depression and CHD as a result of participation in our cohort study, the same may apply to participation in our RCT. This may have led to more than usual intervention in the TAU group, although from the medical notes it appeared that the number of mental health consultations were similar for both groups. In a future trial, changes in TAU during the trial should be recorded systematically.
We hypothesised that the intervention would increase self-efficacy to achieve desired outcomes which would lead to improved depression outcomes. However, the small recorded changes in self-efficacy and related illness perceptions had little effect on depression outcomes. This may have been expected: a difference between our intervention and that of others that have not found improved self-efficacy139 following self-management intervention is that our participants chose the outcomes on which to work on, that is, they identified the factors that they felt contributed to their low mood, rather than being required to work directly on their depression. A better examination of the theory behind our PC intervention would be to explore the effect of changes in self-efficacy on a measure of goal attainment, then test the effects of goal attainment on depression over the long term, although this would require a complex trial. Fewer PC compared with TAU participants visited A&E (24% vs. 38%), which may indicate increased self-efficacy in self-management in the PC group, though in a future trial a more robust measure than self-report of A&E attendance should be used to examine this, for instance Hospital Episode Statistics.140
The possibility that our PC intervention may impact on reported chest pain requires further investigation. We are unable to determine whether or not self-reported chest pain in our trial participants was of cardiac origin. It is estimated that in half of all patients presenting with chest pain, the pain is of non-cardiac origin.141 A high-quality review of psychological interventions for chest pain in patients with normal coronary anatomy (15 studies, 803 participants),142 suggests a modest to moderate benefit, especially for CBT and possibly hypnotherapy. Our trial indicates that non-pharmacological intervention may also be effective for chest pain in patients with CHD. Given the impact of chest pain on patients’ quality of life, mood and on the health service, self-reported chest pain may be an important primary outcome for a future trial. However, since determination of the causes of self-reported chest pain is complex, this should be supported by a more objective measure of cardiac status, such as heart rate variability, which is a predictor of a range of cardiac outcomes and associated with a number of psychological risk factors for CHD.143
Lessons learnt concerning delivery of personalised care
The PC intervention was designed, informed by the findings from our qualitative work, to facilitate both patient choice and clinical judgement, so variation in intervention delivery was expected. However, we produced a manual for the intervention and the nurse case managers reported using its key elements of behaviour change interventions, signposting and liaison with other professionals. In weekly study group meetings, the multidisciplinary clinical team was satisfied that the PC was delivered as planned. Exploratory investigation of therapist effects indicated no difference in depression outcomes between the patients of the two nurse case managers, but that the patients of the nurse case manager with more health psychology experience may have had a greater reduction in self-reported chest pain. There was a very small sample size for this analysis, so this finding should be interpreted with caution, but it suggests that training in the behaviour change aspects of the intervention is important for case managers. However, other studies have shown that even nurses and GPs trained in behaviour change techniques may have difficulty applying them.144,145 Further research into how best to train, or whether or not non-psychologists can be trained, to work more psychologically to increase the effectiveness of care is needed. A future trial of PC could test it as delivered by IAPT psychological well-being practitioners (PWPs).
An alternative approach would be to make greater effort to ensure that depressed patients managed using PC receive guideline-informed treatment. Multidisciplinary collaboration to ensure receipt of available, effective depression treatment has been a key element in a number of successful trials of complex interventions for depression in primary care patients.12,146,147 In this trial, four participants in the PC group, compared with none in the TAU group, received new depression treatment (antidepressant treatment or psychological treatment) by the end of the trial. The nurse case managers often contacted other professionals involved in the care of the majority of PC group participants. However, the nurses reported difficulty in accessing some of the participants’ GPs and PNs (e.g. several telephone attempts needed, lack of response to e-mails); this limitation may be overcome by using case managers based within the GP surgery. However, even when contact was made, patients may not have received guideline-informed depression treatment (e.g. one GP was reluctant to prescribe antidepressants to a severely depressed patient despite advice from the nurse case manager because of expressed anxiety concerning multipharmacy and because IAPT services were unavailable in some areas). This suggests that in a future definitive trial, nurse case managers should be embedded within study practices to increase multidisciplinary collaboration (e.g. by planned times for discussion of cases with GPs and PNs, as is often the case in collaborative care) and means of ensuring that patients can access guideline-informed depression treatment should be predetermined.
Lessons learnt concerning the study protocol
Overall, the findings suggest that the trial protocol was feasible, with high levels of compliance and acceptability. For instance, attrition was < 10% and rates of missing data for most outcomes were low, despite the large number of measures. The PCRN-GL was responsible for practice recruitment, which was achieved well within our predicted time frame. This was helped by their prior knowledge of practices willing and able to conduct such research. Patient recruitment was also as expected and was in line with other studies of depression interventions in primary care.146,148,149 Only around one-third of patients invited to participate by his or her GP provided consent to contact; this was also the case in our cohort study. Use of this ‘opt-in’ system appears to result in substantial loss of potential participants; however, this is the usual method of recruitment for studies conducted in UK primary care. All of the patients meeting our inclusion criteria at baseline agreed to be randomised. Low attrition and high acceptability suggest that people with CHD, depressive symptoms and chest pain are receptive to additional support.
Conclusions
We have developed an intervention that is acceptable to primary care CHD patients who have probable concurrent depression and current chest pain, and which is feasible for use in current practice. The PC intervention could, we think, be delivered by PNs, although training in behaviour change techniques will be necessary. IAPT PWPs may also be potential case managers, but they would need training in long-term condition management.
Depression symptoms in CHD patients who report chest pain appear difficult to treat, as evidenced by this trial and by previous work.96 Our data suggest that more nurse time was associated with improved outcomes, so more intensive follow-up should be included in future use of the PC intervention.
In this trial, collaborative working to ensure that patients received guideline-informed depression intervention was not optimal; this may be improved by using case managers based within trial practices and having agreed methods for providing access to guideline-informed care. Other trials12,146 suggest that this will improve depression outcomes.
It is uncertain which outcomes, other than depression, should be used in a future trial of PC. Our data suggest that self-reported chest pain may be important, but given the complexities in the relationship between self-reported chest pain and cardiac outcome, use of this measure should be supplemented with a more objective measure of cardiac health, for instance heart rate variability.
Our data also suggest that the underlying theory of our PC intervention, that increased self-efficacy to achieve desired outcomes would lead to improvements in depression, needs further testing. Since participants chose a wide range of problems on which to work on, it was difficult to determine how many achieved their goals; a measure of goal attainment should be included in a future trial. However, changes in self-efficacy were small and more intensive psychological intervention may be needed to achieve greater improvement. Longer follow-up is likely to be needed to see impact on depression outcomes.
The trial protocol appeared, on the whole, to be successful with high levels of compliance and acceptability. However, we found, in common with other studies, that large numbers of patients have to be approached and screened for a sufficient number of CHD patients with both chest pain and depressive symptoms to be randomised. This makes such trials costly.
In so much as patients were able to address the wide variety of problems that trouble them, the PC intervention represents enhanced care, which may also be cheaper than TAU. If a fair test of the differences between this low-intensity, quality improvement intervention and TAU is to be made, in future trials it will also be important to monitor more closely any changes in TAU over the course of the trial which may occur owing to increased awareness in participating clinicians of the need to manage depression in people with CHD.
- Development and testing of an intervention for primary care patients with sympto...Development and testing of an intervention for primary care patients with symptomatic coronary heart disease and depressive symptoms (work package 2) - UPBEAT-UK: a programme of research into the relationship between coronary heart disease and depression in primary care patients
- Topic guide for general practitioners and practice nurses - UPBEAT-UK: a program...Topic guide for general practitioners and practice nurses - UPBEAT-UK: a programme of research into the relationship between coronary heart disease and depression in primary care patients
Your browsing activity is empty.
Activity recording is turned off.
See more...