Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Brown KL, Wray J, Knowles RL, et al. Infant deaths in the UK community following successful cardiac surgery: building the evidence base for optimal surveillance, a mixed-methods study. Southampton (UK): NIHR Journals Library; 2016 May. (Health Services and Delivery Research, No. 4.19.)

Infant deaths in the UK community following successful cardiac surgery: building the evidence base for optimal surveillance, a mixed-methods study.
Show detailsSome of the text of this chapter has been published previously as Crowe et al., 2016.67 © 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell. This is an open access article under the terms of the Creative Commons Attribution-Non Commercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Introduction
The main focus for the audit of paediatric cardiac surgery outcomes in registries or multi-institutional databases to date has been operative mortality, expressed as either 30-day11 or discharge outcome.68 These early mortality outcomes have improved over time to the current, historically low, levels.4 However, although important, these outcome measures are relatively limited in scope, and longer-term measures of outcome and metrics such as morbidity or complications are also essential to consider in quality assurance and improvement. A challenge for the audit of longer-term events for patients with CHD at a population level, in national or international registries outside the UK, such as the European Association of Cardiac Surgery database and the Society of Thoracic and Cardiovascular Surgery database in North America, is the reliable capture of appropriate data. The UK has a unique resource in mandatory national audit data sets for both paediatric cardiac procedures, represented by NCHDA,69 and paediatric intensive care unit (PICU) admissions, represented by PICANet,70 as well as life status tracking that enables late deaths to be reliably and independently ascertained. In respect of life status ascertainment, the NCHDA submits regular requests to the Central Register of NHS patients, as approved by the National Health Research Authority, in order to ascertain the survival or life status of patients. This information is reliably forthcoming for all patients possessing an NHS number who are based in England and Wales. Unfortunately, life status tracking is not currently available in Scotland or in Northern Ireland, where the two relevant specialist centres are responsible for ascertaining the life status of their own patients.
These UK national audit data sources enabled a national study addressing the following aims.
- To explore patient-level risk factors for postdischarge death outside a planned PICU admission (outcome 1) and for postdischarge death or emergency readmission to PICU (outcome 2) in infants with CHD undergoing interventions in the UK.
- To develop a clinically meaningful classification of patients in terms of the level and nature of their risk of outcome 2, with a view to informing improvements to the services provided for infants discharged alive following an initial major intervention for CHD.
Methods
Constructing the data set for analyses
Data sources and patient population
Data submissions to NCHDA have been mandatory since 2000 for all hospitals performing cardiac surgery in the UK, with data validated and subject to a quality assurance processes (all hospitals are inspected annually with local records examined to ensure every case has been submitted and a random sample of case notes examined to assess data quality).71 All UK PICUs submit data to PICANet, which is also validated and subject to a quality assurance processes (PICUs receive annual visits from a PICANet team, during which submitted data are verified against hospital notes and admissions numbers are checked).72
For this study, all children who received their first recorded interventional catheterisation or cardiac surgery procedure for a congenital heart defect at < 1 year of age and in the period 1 January 2005 to 31 December 2010 were identified by NCHDA. (Data were requested for the period 1 January 2005 to 31 March 2011 but were supplied for the time period specified above because of reporting restrictions within NCHDA.) In accordance with governance approvals, records within PICANet for these patients were then identified by a trusted third party by matching of unique patient NHS number. Data extracts fulfilling these criteria from each audit were provided to the study team as two separate data sets with the same patient-level identifier (pseudonymised NHS number). A single patient potentially had multiple procedure-based records in the NCHDA extract and multiple admission-based records in the PICANet extract. A flow diagram indicating the number of records present for each stage of this process is provided in Appendix 4.
The study team then constructed a single patient-based analysis data set by linking events pertaining to the same patient within each of the extracts and between the two using the patient-level identifier. This enabled information from both sources to be used in the analysis, with cardiac-related details and life status available in NCHDA and rich comorbidity and emergency PICU admission information available in PICANet.
Defining the index admission period and index procedure
The index admission period for each child was defined as the continuous period as an in-patient within a specialist paediatric cardiac hospital or PICU that started with or included their first surgical or interventional catheter procedure (see Appendix 5 for the list of procedures and their subgroupings; we included interventional cardiology procedures applicable to neonates or small infants that are undertaken with the aim of either primary correction or primary palliation such that a child can be discharged home). This period defined the index LOS within the specialist hospital (and associated PICU care).
Within the NCHDA data set, each procedure is described by a combination of up to eight individual procedural International Pediatric and Congenital Cardiac Codes (IPCCCs).73 An algorithm developed by the NCHDA Steering Committee (which includes experienced paediatric cardiac surgeons and cardiologists) links the individual IPCCCs for a given record to one of 57 specific procedures, that is recognisable surgical operations or catheter procedures (see Appendix 5). The algorithm imposes a hierarchy, with the record assigned the most complex specific procedure consistent with the collection of codes recorded. Approximately 85% of procedures fall into one of these 57 specific procedures.
As some children undergo more than one procedure within a single index admission period, an individual’s index procedure was defined by applying the NCHDA-specific procedure hierarchy across all of the interventions carried out during the index admission. Therefore, the index procedure for each patient was defined as the most complex (specific) procedure (either surgery or interventional cardiology procedure) undergone during the index admission period. In a small subset of patients, an interventional catheter procedure was logged in NCHDA before the index admission. Based on clinical knowledge of the patient histories the research team considered that these early interventional catheters, which in most case were balloon atrial septostomy for a neonatal transposition of the great arteries (TGA), should not represent the index procedure. Instead the index procedure was considered to be an arterial switch operation carried out during a subsequent admission.
Exclusions
Children undergoing an excluded catheter procedure only (see Appendix 5 for the list of catheters included and excluded; rationale stated above), and therefore without an index admission, were excluded from the analysis. Premature babies who underwent only ligation of patent ductus arteriosus were also excluded because, unlike other infants with disease, the majority of these premature infants would have been subject to discharge and follow-up processes run mainly from neonatal intensive care units. Similarly, transplant patients have their own specific pathway of care and so were removed from the analysis. Finally, patients with unknown life status were removed (this included all patients within two UK cardiac units outside England whose life status was not linked to the NHS Central Register; see comment in Introduction).
Patient variables
Potential risk factors available in the patient-based analysis data set that would be known at the point of discharge were prespecified and are listed in Table 7, grouped according to whether they were non-medical, preoperative or postoperative factors. As detailed in the following sections, some variables were simplified prior to the statistical analyses in order to reduce the number of values (degrees of freedom) in the model and hence the risk of overfitting (see Harrell et al.76). An appropriate power calculation was performed. The origin of each risk factor (either the PICANet or the NCHDA data set) is noted in Table 2, along with whether it relates to the index procedure or index admission period or is a characteristic of the child. Where both audits contained information on a particular risk factor, the risk factor was taken from the source that was most descriptive and complete or, in the case of ethnicity, a combination of the two (see Table 7). Some post-procedural factors originating from different audits overlap but were considered to provide sufficiently different information to include within multivariate analyses. In what follows, we briefly describe each of the patient variables considered in the analysis.
Non-medical variables
Deprivation was defined using quintiles of the English Index of Multiple Deprivation (IMD) 2010,74 which is calculated at the level of small (≈ 1500 people) geographic areas covering England and combines deprivation indicators across income, employment, health and disability, education skills and training, barriers to housing and other services, crime and living environment.77
Ethnicity information is recorded in both audits, with NCHDA using a bespoke classification scheme and PICANet using the Census 2001 classification used by the Office for National Statistics.78 PICANet was therefore used as the primary source for our ethnicity variable for the purposes of potential comparability with the Office for National Statistics population statistics, with further collapsing of codes into seven groups for considerations of model stability: white, mixed, Asian, black, Chinese, other and not stated. The most frequently recorded ethnic group was assigned to the child if they had multiple admission records.
Preprocedural variables
Cardiac diagnoses and procedural information
Each NCHDA record contains up to six IPCCC diagnostic codes, the combination of which can be mapped to 1 of 24 primary cardiac diagnoses using a hierarchical scheme developed by Brown et al.75 For the purposes of this study, this mapping scheme was implemented with two minor adjustments (see Appendix 6 for details). In addition to their overall primary cardiac diagnosis, a child was identified as having an acquired cardiac diagnosis if any of his or her records included an IPCCC diagnostic code mapping to this category, for example ventricular dysfunction and ventricular hypoplasia. Some non-cardiac IPCCC diagnostic codes were identified as post-procedural morbidities (see Non-cardiac diagnosis and comorbidity information and Appendix 7 for details).
For reasons of model reliability and validity of predictive discrimination (i.e. to reduce the risk of overfitting), we grouped the diagnostic categories into four cardiac diagnosis groups considered clinically meaningful to the study focus (see Appendix 6 for mappings, including the relevant references underpinning our selection of the groupings). The choice of groupings was informed by the literature reviews in that the majority of the included studies referred to HLHS as being at higher risk and of special interest for late mortality. Other studies, although a smaller number, noted higher risk with other types of SV conditions and palliated circulations (see procedural groupings that follow):
- HLHS
- functionally univentricular heart (UVH) or pulmonary atresia (PA) (including PA with an intact ventricular septum)
- ventricular septal defect (VSD)
- ‘other’ (the remaining 22 diagnosis categories).
Alongside this, the index (specific) procedures were aggregated into three procedural groups (see Appendix 5 for mappings including the relevant references underpinning our selection of the groupings); again this grouping was based on the findings of the literature review in Chapter 2, which noted a higher risk in babies with palliated circulations:
- palliative (e.g. Norwood, bidirectional cavopulmonary shunt, arterial shunt)
- corrective [e.g. truncus arteriosus repair, atrioventricular septal defect (AVSD) complete repair, tetralogy repair]
- ‘ungrouped’ if not a specific procedure.
Non-cardiac diagnosis and comorbidity information
Non-cardiac diagnosis and comorbidity information was primarily sourced from PICANet, in which any given PICU admission can record up to 24 clinical Read Codes (a clinical coding system used as standard in general practice in the UK and maintained by the Health and Social Care Information Centre). In total 3325 discrete Read Codes were present in the data set and so to explore the potential for this information to add discriminatory power to the risk model, we developed a new clinically intuitive scheme linking each code to at most 1 of 17 system-based categories (see Appendix 7).
If any PICU admission within the index admission period contained a Read Code in the congenital anomaly category, then the child was assigned this attribute. This included syndromes such as Down and DiGeorge and non-syndromic congenital anomalies such as urogenital/renal malformations, tracheal/tracheo-oesophageal malformations, vision/hearing deficits and exomphalos/gastrointestinal malformations. Similarly, if any Read Code within the index admission period was linked to the neurodevelopmental category, then the child was assigned this attribute. These comprised a range of acquired and congenital conditions including epilepsy/seizures, developmental delay, sleep apnoea, hydrocephalus, retinopathy of prematurity, stroke, hemiparesis/hemiplegia, anoxic encephalopathy, cerebral venous sinus thrombosis and cerebral palsy. Finally, a child was assigned the attribute of prematurity (< 37 completed weeks’ gestation) if a Read Code linked to this category appeared within any of his or her recorded PICU admissions.
Additional preprocedural patient variables
Additional preprocedural factors considered on the basis of potential clinical relevance and availability within the NCHDA data set were patient sex, age at procedure, weight-for-age at procedure (calculated using World Health Organization reference standards) and whether or not there was an antenatal diagnosis. From the PICANet data set it was determined if the patient had clinically deteriorated prior to the index intervention (assigned as such if the index admission or any prior PICU admissions were urgent and unplanned).
Post-procedural variables
The following post-procedural patient variables known at the point of discharge were considered: LOS for the index admission period; whether or not any surgical or catheter procedures were performed during the index admission in addition to the index procedure; whether or not renal support was required during the index admission (including dialysis and haemofiltration); whether or not extracorporeal membrane oxygenation (ECMO) support was required during the index admission; whether or not the index admission was associated with any intensive care unit events (assigned as such if any admission to PICU during the index admission period contained a Read Code category for collapse or cardiac arrest, acquired injury or complications or a non-cardiac operation in PICU); whether or not the index admission was associated with any acquired comorbidities [assigned if any admission to PICU during the index admission period contained a Read Code acquired category (1–8 in Appendix 7)]; and whether or not the index admission was associated with post-procedural morbidity (assigned if any NCHDA record during the index admission period contained an IPCCC code corresponding to a post-procedural morbidity).
Missing data
When ethnic group was not available from PICANet, the NCHDA ethnic code was used to assign white, Asian or black ethnicity (which showed strong concordance across the two audits) but not to assign Chinese, other or mixed ethnicity (which showed poorer concordance). Sensitivity analyses were performed excluding records without PICANet-derived ethnicity. Index procedure weights recorded as zero (i.e. missing) were replaced by weight recorded within the same index admission (if available). Weight-for-age z-scores outside the range ± 5 z-scores were assumed erroneous and treated as missing.
Outcomes
Two nested outcomes of interest were defined.
- Outcome 1: death within 1 year following discharge from the index admission and not during a planned admission.
- Outcome 2: either death (outside a planned admission) or an emergency unplanned readmission to PICU within 1 year following discharge from the index admission. Outcome 2 was viewed as including ‘near misses’ for death, given that all patients experiencing emergency intensive care unit admission are gravely ill. A decision was made to combine deaths and near misses for this section of the analyses in order to increase the number of events, and to form a useful basis for classification and regression tree (CART) analysis was used to identify patient groups with different ‘profiles of risk’ that could usefully inform the development and prioritisation of interventions aimed at improving outcomes within this patient population.
In order to ascertain these outcomes the following information was used: age at death (if applicable) and life status, which were available in NCHDA; emergency unplanned admissions to PICU were extracted from PICANet (defined as admission type ‘unplanned’ or ‘unplanned after surgery’ AND identified as a retrieval from another unit as an emergency transfer AND not defined as an elective admission). Note that death within 1 year of discharge from index admission that occurred during a planned readmission to intensive care was not considered an adverse outcome in this analysis designed to inform improvements in postdischarge services.
Statistical methods
Descriptive and univariate analyses
Descriptive analyses were performed to characterise the data set and univariate logistic regression analysis was used to assess the relationship of each candidate predictor with each outcome using fractional polynomials to investigate departure from linearity. This informed which variables were considered in two further, complementary, strands of analysis: the development of risk models for outcomes 1 and 2; and the identification of patient groups differentiated by risk of outcome 2.
Developing risk models for outcomes 1 and 2
The significant variables from the univariate analysis were investigated in a multivariable model for each outcome in turn. Initially, models for outcomes 1 and 2 were developed in which the continuous predictors were used in their original form. For the final model development, however, the continuous predictors were categorised through a process of discussion between clinicians and analysts influenced by considerations of model interpretability as well as statistical performance.
Multiple imputation assuming data were missing at random was used to investigate missing data when fitting the models. We generated 20 data sets and ran a backward stepwise logistic regression (factors where p < 0.05 remained in the model), implementing a bootstrap (200 samples) for each imputed data set. This was done for preoperative and postoperative factors only. Factors were selected based on the inclusion frequency of each predictor, over the imputed data sets, that is the proportion of times that the factor appeared in the model.79 A threshold of 50% was set and estimates were combined using the Rubin rules.80 The final models were estimated by taking up the factors whose inclusion frequency exceeded the threshold, and estimating the regression weights of this predictor set on the imputed data.
Sensitivity analysis for the multiple models derived on the complete case data was also performed to explore the effect of including and excluding prematurity from the set of potential predictors (using Akaike criteria to assess model fit). Sensitivity of the results to adjustment for clustering at both the hospital level and regional level (English Primary Care Trusts81) was assessed for each model. The Hosmer–Lemeshow statistic was used to test calibration (goodness of fit)82 and discrimination was described using the c-statistic (area under the receiver-operator curve), corrected for overfitting using bootstrapping (an internal validation method that is an alternative to data-splitting and cross-validation83).
All analyses were performed in Stata statistical software (Version 12.1 StataCorp LP, College Station, TX, USA) and a p-value of < 0.05 was considered statistically significant.
Identifying patient groups differentiated by risk of outcome 2
Classification and regression tree analysis was used to identify patient groups with different ‘profiles of risk’ that could usefully inform the development and prioritisation of interventions aimed at improving outcomes within this patient population. The analysis was designed to fulfil this purpose rather than, for example, the development of a prediction tool (which was not our intention). For other examples of CART applications in health care, see Ridley et al.,84 Garbe et al.85 and Fonarow et al.86 Given our aim here was to inform service improvement, we focused on outcome 2 so as to include the ‘near misses’ as well as deaths (with the combined higher incidence rate also providing greater model stability). The technique recursively partitioned the data into subsets that were as homogeneous as possible with respect to outcome 2, that is into subsets of increasing ‘purity’.87 This was implemented using a Gini impurity measure with minimum change in improvement of 0.0001. All variables significantly associated with outcome 2 in univariate analysis (and weight-for-age z-score) were included in the CART analysis. The continuous variables were entered in their categorised form used in the risk model development (see Table 9). To prevent overfitting, the CART groups were developed in a random 60% of the data. In order to limit the number of groups created (for reasons of statistical robustness and potential usability), we restricted the tree depth to 4 and required a minimum of 100 cases for branching to continue, with at least 50 cases in either branch. The resulting classification tree was applied to the patients in the remaining 40% of the data set and the occurrence of outcome 2 among patients at each node was compared with the corresponding group in the development set to assess model stability. All analysis was performed in SPSS 22 (IBM SPSS statistical software 2013, Armonk, NY, USA).
Results
Data set and headline outcomes
Approximately 20% of records in NCHDA did not have a valid NHS number (the unique patient identifier used in this study to match across the two audits); many of these records pertain to overseas patients as only patients resident in England or Wales are allocated an NHS number. A total of 12,390 infants with a valid patient identifier and meeting the inclusion criteria for the study were identified in NCHDA, of whom 9385 (76%) were linked to at least one record in PICANet. Failure to link a record from NCHDA with one in PICANet could be accounted for by a procedure (in general a catheter) which occurred without an intensive care admission or the lack of an NHS number for a given patient in PICANet. A total of 115 children who underwent only an excluded catheter procedure were excluded from the analysis, as were 765 premature babies who underwent ligation of patent ductus arteriosus (PDA) only and 24 transplant patients. A further 505 patients with unknown life status were also removed.
Of the remaining 7976 patients, 333 [4.2%, 95% confidence interval (CI) 3.7 to 4.6] died during their index admission period and were excluded from our analyses, leaving a final data set comprising 7643 infants discharged alive from their index admission. Of these, 246 (3.2%, 95% CI 2.8 to 3.6) died within 1 year following discharge from the index admission and not during a planned admission (outcome 1). A total of 514 children (6.7%, 95% CI 6.2 to 7.3) either died or were admitted unplanned, as an emergency, to a PICU within 1 year following discharge from the index admission (outcome 2). Finally, 115 children (1.5%, 95% CI 1.2% to 1.8%) died during a planned admission to PICU within 1 year following discharge from the index admission (which was not considered an outcome in this analysis), giving an overall mortality within the year following discharge from index admission of 4.7% (95% CI 4.2% to 5.2%).
Ethnic group was not available from PICANet for 1703 children in the final data set. Of these, the NCHDA ethnic code was used to assign white (n = 1001), Asian (n = 243), black (n = 113) or missing (n = 346) ethnicity. Excluding records without PICANet-derived ethnicity from the analyses did not significantly affect the results. In a total of 528 children weight-for-age was missing or anomalous (assumed erroneous and treated as missing). The variable for which the level of missing data was highest, markedly so, was prematurity [n = 2101 (27.5%)]; sensitivity analysis comparing models with and without prematurity showed a marginally better fit if it was included (based on Akaike criteria) and very little difference in the odds ratio coefficients for all other factors.
Descriptive and univariate analyses
The following variables showed no univariate association with either outcome and so were not considered in further analyses: sex, and whether or not any catheter procedures were performed during the index admission in addition to the index procedure. Both outcome 1 and outcome 2 were significantly associated with all of the other candidate variables in univariate analysis (p-value < 0.05). Table 8 shows the observed numbers of patients and rate of outcomes 1 and 2 in the data set for those parameters significant in univariate analysis (and weight-for-age), along with the results of the univariate analysis.
The relationship between LOS and both outcomes was non-linear and the fractional polynomial transformation was used in the subsequent risk model development analyses; for age we used a log transformation. The relationship between weight-for-age and each outcome did not depart significantly from linearity.
Developing risk models for outcomes 1 and 2
In the multivariate analysis in which continuous variables were treated as such, the significant risk factors for both outcomes were age at procedure (continuous), weight-for-age z-score (continuous), index procedure group, cardiac diagnosis group, non-cardiac congenital anomaly, prematurity, ethnicity and LOS in a specialist centre (continuous). Preprocedure clinical deterioration was additionally significant to outcome 1, whereas neurodevelopmental condition and acquired cardiac diagnoses were additionally significant to outcome 2.
In the final model development, in which ease of interpretation was considered as well as statistical performance, the continuous predictors (age at procedure, weight-for-age z-score and LOS) were categorised as shown in Table 9 (along with observed numbers of patients and rate of outcomes 1 and 2 in the data set). The final risk model for outcome 1 comprises age at procedure (categorical), weight-for-age z-score (categorical), index procedure group, cardiac diagnosis group, non-cardiac congenital anomaly, prematurity, ethnicity, LOS in specialist centre (categorical) and clinical deterioration. The final risk model for outcome 2 comprises age at procedure (categorical), weight-for-age z-score (categorical), index procedure group, cardiac diagnosis group, non-cardiac congenital anomaly, prematurity, ethnicity, LOS in specialist centre (categorical), neurodevelopmental condition and acquired cardiac diagnoses. Details of the regression models (odds ratio, standard errors and 95% CIs) are shown in Tables 10 and 11.
For each patient variable the multivariate odds ratios, standard errors and 95% CIs are presented and the reference category indicated.
We note that, although variable selection was based on > 50% inclusion frequency over the imputed data sets, in practice there was clear discrimination between those factors that were included in the model (≥ 90%) and those that were not included (< 40%). For both outcomes, sensitivity analysis of the models derived on complete case data showed a marginally better fit to the data when prematurity was included, based on Akaike criteria. However, for models with and without prematurity the odds ratios for all other factors remained very similar, indicating robustness of model variable selection. Furthermore, for all models, adjusting for clustering at either the hospital level or regional (primary care trust) level had no significant impact on results so we have presented the unadjusted results.
Risk model performance
The final (categorical) model for outcome 1 gave a combined c-index of 0.78 (95% CI 0.75 to 0.82), indicating good discrimination. This was only marginally less discriminative than the continuous model (c-index 0.80). The final (categorical) model for outcome 2 also showed good discrimination with a combined c-index of 0.78 (95% CI 0.75 to 0.80), compared with a c-index of 0.78 for the continuous model. Calibration of the final categorical and continuous models for both outcomes was also good, with Hosmer–Lemeshow p-values ranging from 0.10 to 0.75 across the models fitted on the 20 imputed data sets, indicating no statistically significant differences between observed and expected number of deaths when calculated in deciles of predicted risk for each of the imputed data sets.
Identifying patient groups differentiated by risk of outcome 2
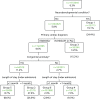
Figure 4 depicts the final tree generated by the CART analysis along with, for each node, the test set figures for the number of patients and rate of outcome 2. The rate of outcome 2 evaluated across the entire data set is shown below the box for each final patient group. The rate of outcome 2 across the entire analysis data set (total number of patients 7643) was 6.7%. The rate of outcome 2 within a 60% development subset was 7.0%, compared with 6.3% in the 40% test set.
Of the 18 variables entered in the analysis, CART identified presence/absence of a neurodevelopmental condition as the best single discriminator between patients experiencing outcome 2 or not. For those without a neurodevelopmental condition, the next best discriminator is whether the cardiac diagnosis is high risk (HLHS, UVH or PA) or low risk (VSD or ‘other’). Of the latter, the next best discriminator is presence/absence of a congenital anomaly followed by the LOS in the specialist hospital (threshold 1 month). This branching creates six discrete patient groups, details of which are set out in Table 12.
Discussion
As far as we are aware, based on the systematic review reported in Chapter 2, this is the first study of its kind to explore adverse postdischarge outcomes for infants with CHD based on national audit data with equivalent universal coverage. Of 7643 infants discharged alive following an initial major intervention for CHD, representing the entire case load for England and Wales over a 6-year period, 246 (3.2%) died within 1 year and not during a planned readmission (outcome 1) and 514 (6.7%) either died or had an emergency unplanned readmission to PICU within 1 year (outcome 2). The following risk factors were associated with increased multivariate risk of both adverse outcomes: younger age at procedure, lower weight-for-age z-score, index procedure group (palliative procedures higher risk), cardiac diagnosis group (HLHS, UVH, PA higher risk), non-cardiac congenital anomaly, prematurity, ethnicity (‘other’ ethnicity higher risk), LOS in specialist centre (> 1 month higher risk). In addition, for outcome 1 preprocedure clinical deterioration was associated with increased multivariate risk whereas for outcome 2 neurodevelopmental conditions and acquired cardiac diagnoses were additionally associated with increased multivariate risk.
When considering the results of the two multivariable models in the context of previous evidence identified within the systematic review outlined in Chapter 2, one barrier is that very few of the previous studies presented risk factors for a large diverse group of CHD patients. There was concordance for the following risk factors being linked to adverse outcome: index procedure group (palliative),31,34 which relates to cardiac diagnosis group (HLHS was the predominant condition represented in the systematic review), non-cardiac congenital anomaly,34,36 prematurity32 and ethnicity26 (albeit in relation to different ethnic populations based in the USA).
The risk models in our study further identified the following factors: lower weight at operation, which is a factor in risk models for adverse early outcome11 and may be correlated with feeding difficulties that featured in the systematic review as a higher risk;33 acquired cardiac diagnoses and preoperative clinical deterioration, which would suggest that individual patients with more severe forms of a given CHD type are at higher late risk (as was the case for HLHS within systematic review studies26,88); and neurodevelopmental conditions, which did not feature in the systematic review, although these may have overlapped with congenital anomalies in previous studies (see previous paragraph).
One patient characteristic that featured as an adverse risk factor in the systematic review (in relation to populations from the USA)26,32 but was not linked to risk in our study was lower socioeconomic status [we included a measure of deprivation (IMD) as a potential risk factor in our analyses]. Further descriptive information on deprivation and a wider discussion of ethnicity is presented in Chapter 5, but we note that our data originate from a different health-care system to the USA (i.e. the NHS) and speculate that this may have a role in these observed differences.
Prolonged LOS in hospital was an important adverse risk factor in our study, but within the systematic review evidence in respect of this was conflicting: two studies indicated LOS to be a risk factor34,36 and one smaller single-centre study presenting the opposite viewpoint.30 Prolonged LOS may reflect numerous different aspects of a patient’s condition and journey, but generally indicates greater complexity including potentially being a surrogate measure of postprocedural complications,89 which may lead to greater fragility post discharge. Younger age at surgery was associated with higher risk in our study, and in two studies in the systematic review that included a range of different CHD types,31,34 yet the systematic review indicated older age to be associated with higher risk of HLHS and aortic stenosis.23,35 It is likely that the finding of higher risk at younger age represents a broad effect, such as neonates being generally more vulnerable than older infants post discharge.
Our results reflect outcomes within the context of recent historical provision of services for major forms of CHD requiring treatment in infancy, and are potentially insightful for ongoing quality improvement initiatives. To this end, we identified distinct patient groups using CART analysis with a view to informing the design (and potential targeting) of appropriate interventions and service improvements. The six groups that were identified span a wide range of risk of late death or emergency readmission to PICU (3–24% outcome 2). The groups are defined in terms of the following patient characteristics: neurodevelopmental conditions; cardiac diagnosis of HLHS, UVH or PA + intact ventricular septum (IVS); congenital anomalies; and LOS > 1 month. The two highest-risk groups are made up of patients with a neurodevelopmental condition (group 1: 24% outcome 2) and patients with a low-risk cardiac diagnosis who have a congenital anomaly and long LOS (but no neurodevelopmental condition) (group 2: 24% outcome 2). Group 3 comprises those patients most widely recognised as vulnerable to late death (see Chapters 2 and 3), namely patients with cardiac diagnoses of HLHS, UVH or PA + IVS (and no neurodevelopmental condition) (15% outcome 2), although we note that some patients in group 1 may also have these cardiac diagnoses.
Therefore, the groups identified by these population-based data as high risk include patients with diagnoses other than those conventionally recognised as benefiting from enhanced surveillance, that is HLHS52–54 and in small number of studies, other UVH conditions.57 Our findings suggest that as well as HLHS, infants with UVH and PA + IVS are also at significant risk of poor interstage outcome and that these patient groups are candidates for home monitoring. Our study also indicates that it is important to mitigate risks arising from patient factors beyond simply cardiac diagnosis, in particular we note the very significant risks of poor postdischarge outcome in the presence of neurodevelopmental conditions and congenital anomalies with prolonged LOS. The implications of this for service provision are discussed further in Chapter 10.
Strengths and weaknesses
The national audit data underpinning this study offer a unique opportunity for a population-based analysis, and the study was designed, conducted and reported based on the Strobe Statement for observational studies.90 First, the data are of high quality (as demonstrated by the results of a regular systematic independent validation process). Second, life-status tracking based on NHS number enables late deaths outside treatment centres to be reliably ascertained. Third, the mandatory and universal nature of the data submission means that all relevant cases are captured. Our findings may therefore be considered more generalisable than those based on single-centre studies or those from a more limited geographical area. That said, our study outcomes inevitably reflect recent historic services provision by the NHS in addition to intrinsic medical risk; hence, findings may differ to some extent between geographical regions. The study also reflects the demographics of the UK in terms of ethnicity, which may in turn have implications for the distribution of CHD types and other congenital anomalies.91
A further positive feature of the study is that the combined data set used is more valuable for the purposes of this study than either of the individual audit data sets in isolation, since each contains different types of information concerning potential risk and, when variables in the two data extracts overlapped, the item with the highest data completeness and discrimination was selected. The strength of the NCHDA data set includes the detailed cardiac-specific information as determined by IPCCC codes related to CHD and the cardiac interventions undertaken. The strengths of the PICANet data set include the rich medical coding and information pertaining to the type of admission (such as where an emergency occurred), as well as intensive-care supports and interventions.
We note, however, that our results reflect only those elements that are captured within the national audit data and so certain known risk factors, for example postprocedural weight gain33 and selected abnormalities based on echocardiography,36 could not be incorporated. Furthermore, there may be other as yet unknown risk factors that are not captured in the registry data.
It was necessary to group cardiac diagnoses and procedures in order to render them tractable for statistical analysis (to reduce the degrees of freedom in the data set). Although this was performed with a strong clinical sense of what was pertinent, based on literature and clinical experience, we note that such groupings are to an extent subjective and, as in other registry-based studies, there remained a subset of ungrouped procedures. The choice of diagnosis group ‘HLHS’ reflects the body of literature related to ‘interstage’ deaths in this population. A separate group of ‘UVH or PA + IVS’ was chosen because of the high-risk nature of these conditions, although with a view to distinguishing these from HLHS patients. Of the remaining cardiac diagnosis groups, we elected to review the VSD group separately in order for us to evaluate the face validity of comparisons between HLHS or UVH/PA + IVS and the much larger, less homogeneous group of mainly biventricular diagnoses. We note that results for the VSD group and the diverse ‘other’ group were comparable.
We note that the ethnicity group classified as ‘other’ was associated with higher risk: this is discussed in more detail in Chapter 5. The audit data will not have captured the scenario in which babies were discharged to home on a palliative care pathway, and hence our outcomes may have incorporated a subset of babies that were expected to die. However, local audit of cases at two English tertiary centres suggests this occurs in only a minority of cases.6 Finally, given its focus on the population of infants who have undergone an intervention, the analysis does not include infants awaiting intervention who are also known to be potentially vulnerable.
- Development of a risk model for death or emergency readmission within 1 year fol...Development of a risk model for death or emergency readmission within 1 year following hospital discharge from infant cardiac intervention for congenital heart disease and identification of patient risk groups for the purposes of service improvement - Infant deaths in the UK community following successful cardiac surgery: building the evidence base for optimal surveillance, a mixed-methods study
Your browsing activity is empty.
Activity recording is turned off.
See more...