Introduction
Changes in fertility and life expectancy are leading to major changes in the structure of the global population and, in turn, in the scale of the cancer problem worldwide and at every resource level (WHO 2011b). In addition to the increasing burden of cancer is a changing spectrum of common cancers that is in different regions correlated with levels of human development (Bray and others 2012). The ongoing cancer transition includes a reduction in infection-related cancers (for example, stomach and cervical cancer) that is offset by increases in cancers linked to a Westernization of lifestyle (for example, breast, prostate, and colorectal cancer). The transition also encompasses changes in risk behavior, including tobacco uptake, with a delayed but large impact on the burden from lung and other tobacco-related cancers (Bray and others 2012). The cancer transition is not uniform, however: in Sub-Saharan Africa, recent increases in cervical cancer are observed in Uganda and Zimbabwe; in many countries, a residual burden of cancers associated with infectious agents accompanies the increasing burden of cancers associated with economic transition (Parkin and others 2014).
This chapter presents a global overview of the cancer burden, patterns and profiles, recent trends in common cancers, and the expected future scale of the disease by 2030.
We link geographical and temporal patterns of cancer to corresponding levels of economic progress to provide an overview of the key characteristics of the global cancer transition. We use gross national income (GNI) per capita as a national indicator of societal as well as economic development (http://data.worldbank.org/news/new-country-classifications), and corresponding rates of cancer incidence and mortality as markers of the extent of the global cancer transition. We draw attention to geographical variations and trends in cancer-specific rates according to differing economic profiles and in each of the world’s regions. In addition, we provide a global, trends-based projection of the likely cancer burden in 2030, based on historical trends refined by incorporating an indicator of level of development.
We also examine the number of potentially avoidable new cases and cancer deaths, assuming a reduction in risk factors (Hanley 2001). Even today, tobacco smoking is by far the most important risk factor for cancer (Lim and others 2012). Although the smoking habit is in decline in many high-income countries (HICs), tobacco consumption is still rising in many low- and middle-income countries (LMICs) (Thun and others 2012). As part of the global socioeconomic transition, many countries presently classified as low- or middle-income are increasingly adopting Westernized diets and more sedentary and less physically active lifestyles, leading to a rapid shift in the profile of common cancers in these populations (Bray and others 2012). In view of these developments, this chapter also reviews the main causes of cancer, with an emphasis on the sources of disparities that contribute to an increasingly greater proportional burden from cancer in LMICs, and the prospects for cancer control in different settings.
We conclude by pointing to how the high-level political commitment to reduce the rising burden of cancer and other noncommunicable diseases (NCDs) can advance the measurement of cancer to inform cancer control action. There remains an overwhelming need to improve the quality and coverage of population-based cancer registration in most LMICs, as an essential component in planning and evaluating national cancer control activities.
This chapter uses the World Health Organization’s (WHO) geographical regions: Africa, the Americas, South-East Asia, Europe, Eastern Mediterranean, and Western Pacific.
Cancer in Context: Comparisons with Other Noncommunicable Diseases by Development Level
Worldwide, an estimated 38 million deaths—almost two-thirds of the annual 56 million total deaths—are caused by NCDs, principally from cardiovascular disease, diabetes, chronic respiratory disease, and cancer (WHO 2011b). In 2012, nearly 80 percent of all NCD deaths (28 million) occurred in LMICs; almost 30 percent of deaths occurred before age 60 years in these countries. Among NCDs, cancer is a leading cause of death ().
Top 10 Causes of Death Worldwide, 2012. (millions of deaths)
This observation is consistent with the global socioeconomic transitions. Between 1990 and 2010, there was a 17 percent reduction in communicable maternal and neonatal and nutritional deaths, offset by a 30 percent increase in NCD-related deaths (Lozano and others 2012). This increase was greater in LMICs, compared with HICs (39 percent versus 15 percent, respectively). The shift is largely driven by population growth and improved longevity. Although the number of NCD-related deaths increased from 27 million to 34 million between 1990 and 2010, death rates declined by 19 percent (646 to 520 per 100,000) over the same period (Lozano and others 2012).
The overall statistics conceal variations among countries. Cancer is an even larger component of the NCD burden in HICs. In Japan, cancer represents over half of all NCD deaths combined; in approximately 40 countries, age-adjusted cancer mortality rates are equal to or above those of cardiovascular disease in premature mortality at ages under 70 years (WHO 2013).
Key Indicators of the Global Cancer Burden
Comparisons of incidence rates can be used to investigate cancer risk factors, aid planning and prioritizing of cancer control resources, as well as facilitate monitoring and evaluating of the impact of specific primary prevention interventions. Mortality has often been considered the best means of evaluating overall success in reducing the cancer burden. An assessment of cancer-specific mortality rates is particularly useful in evaluating the effectiveness of secondary prevention, particularly where the goal is early detection of malignant tumors; it is also useful in tertiary prevention in determining the impact of cancer management and treatment. Combined successes in cancer prevention, early detection, screening, and treatment have resulted in a reduction in overall cancer mortality rates in some more developed countries, predominantly as a result of declines in the incidence and/or mortality from a number of specific types of common cancer (Doll 1990, Karim-Kos and others 2008). The broad spectrum of interventions and their tendency to produce real or artifactual changes in cancer incidence, mortality, or survival, however, lends support to combining analyses of all three measures.
Cancer is complex to monitor accurately because of its biological diversity; the underlying coding and classification issues are considerably more intricate than the other NCD categories. There has been a long history of cancer registration in many areas of the world, however, and a tradition of maintaining comparable accurate and complete global cancer incidence data that spans half a century. The serial publication, Cancer Incidence in Five Continents (CI5, http://ci5.iarc.fr), published by the International Agency for Research on Cancer (IARC) and the International Association of Cancer Registries, is regarded as the definitive source of local cancer data. The 10th volume of CI5, published in 2013, compiles incidence data from high-quality population-based cancer registries (PBCRs) for 2003–07.
The IARC is also the key reference source for statistics on the global, regional, and national burden of cancer; it publishes estimates of the core indicators for cancer at the country level as part of the GLOBOCAN series (http://globocan.iarc.fr). The most recent database contains estimates of the cancer incidence, mortality, prevalence, and disability-adjusted life years for all cancers combined for 2012, as well as for 27 cancer sites in 184 countries and 20 regions (Ferlay and others 2013).
The methods used to compile the GLOBOCAN estimates have evolved, but the underlying principle remains the use of the best available observed data within a country to build up the global picture. Details are provided by Ferlay and others (2015). Estimation of cancer-specific incidence and mortality rates are dependent on local data sources, and the cancer registry data from CI5 provide a key input into the compilation. Unfortunately, there remains a paucity of high-quality cancer incidence and mortality data in low-resource and medium-resource areas, and hence the accuracy of the estimates from these regions is generally lower; an alphanumeric scoring system has been introduced to provide a broad indication of the robustness of the estimation within each country (Ferlay and others 2015).
The definitions, sources, and mode of estimation of the key indicators used in this chapter are provided. Counts and rates of cancer incidence and mortality are presented; rates per 100,000 were age-standardized using the world standard population (Doll, Payne, and Waterhouse 1966).
Incidence
Cancer incidence is defined as the frequency of occurrence of new cases of cancer in a specific population over a given period of time. It can be expressed as the absolute number of cases or as a rate per unit-time, with new cancer cases the numerator and the corresponding person-time at risk the denominator.
PBCRs are the essential institutions enabling these activities (Bray 2014; Parkin 2006). They collect and classify information on all new cases of cancer within a well-defined population and provide statistics on occurrence for the purposes of assessing and controlling the impact of cancer in the community. Registries are either national or regional in their coverage, with a high degree of completeness, accuracy, and comparability of the collected data essential for making reliable inferences regarding geographical and temporal variations in the underlying rates. Although incidence trends are unaffected by the impact of changes in treatment and consequently survival, changes in registration practices, definitions of malignancy, and the International Classification of Diseases can all impact recorded incidence (Muir, Fraumeni, and Doll 1994). Inclusion in CI5 is a reliable marker of the quality of a given registry’s data, in that the editorial process includes numerous assessments of the quality of the submitted data set. A substantial disparity exists in the availability of high-quality cancer registration data between HICs and LMICs. While 99 percent of the North American population is covered in the 10th volume of CI5, only 7 percent, 5 percent, and 2 percent of the respective South American, Asian, and African countries have registries that were accepted for inclusion.
Mortality
Mortality provides a unique measure of the outcome or impact of cancer and is expressed as either the number of deaths occurring or a mortality rate per unit-time. Mortality is a product of the incidence and the case-fatality of a given cancer, and fatality (1-survival) represents the probability that an individual with cancer will die from it. Mortality rates then measure average risk to the population of dying from a specific cancer. If case fatality is constant across populations or in a specified population over time, geographic or temporal comparisons of incidence can be inferred from mortality. It is evident, however, most notably in HICs and for cancers where treatment and cancer management have markedly improved, that incidence and mortality trends can be at considerable variance.
Data derive from vital registration systems, where usually a clinical practitioner certifies the fact and cause of death. The International Classification of Diseases provides the uniform system for nomenclature and coding and a suggested format for the death certificate. Mortality data are affected by the accuracy of the recorded cause of death and the completeness of registration. Errors of death certification are well documented (Doll and Peto 1981); patients diagnosed with cancer, for example, may die of the disease without this being written on the death certificate, and such inaccuracies are known to vary considerably between countries and over time.
The WHO mortality databank hosted at IARC (http://www-dep.iarc.fr/WHOdb/WHOdb.htm) contains national cancer mortality data on more than 80 countries; for many, the data cover decades. The greater availability by country and over time is confined mainly to higher-income countries; at least 15 years of data are available for 78 countries (35 in Europe, 25 in the Americas, 15 in Asia, 2 in Oceania, and 1 in Africa), but may explain its usage as a surrogate for incidence in geographic and temporal studies of cancer. In South America, mortality data are more comprehensive than incidence data, while both incidence and mortality are limited in Asia and Africa. In such settings, where deaths are often not attended by physicians, alternative methods, such as verbal autopsy, may be used to complement data collected from death certificates.
Income as a Proxy for Human Development
The remainder of this section examines cancer profiles with respect to the current burden, recent changes, and the future burden at the global, regional, and national levels. It is important to understand these patterns and trends in relation to the rapid increments in human development over recent decades. Tracking these changes helps to clarify how demographic and epidemiologic transitions impact the overall burden and the changing distribution of common cancers in different populations. In keeping with previous DCP volumes, we utilized national income level (GNI per capita) as a proxy for human development (UNDP 2009).
In examining aspects of the cancer burden according to resource levels, we used predefined categories of the distribution according to the World Bank classification of countries by income-group (see endnote for per capita income cut-offs). The estimated burden of cancer in 2030 is presented by gender for all cancers combined for all levels of income on the basis of the rates in 2008, assuming population growth and aging in 2030 occur according to the United Nations World Population Prospects medium-fertility variant.
Proportion of the Burden Attributable to Risk Factors
The population attributable fraction (PAF) (Hanley 2001) is a standard measure used to quantify the proportion of disease burden attributable to a risk factor. It is calculated using information on the prevalence of a risk factor in a population and the relative risk that the risk factor poses for development of disease, compared with those where the risk factor is absent. The derived proportion is indicative of the reduction in cancer incidence or mortality that would be expected if exposure to the risk factor were absent. For risk factors where zero exposure is inapplicable, such as for body weight, PAF is calculated by comparing population exposure to reported exposures at the lowest level of cancer risk.
The results presented in this chapter are largely based on a systematic search of published and unpublished reviews from major databases, censuses, health and nutrition surveys, and epidemiological studies (Lim and others 2012). Wherever possible, the data are supplemented by national, regional, or global studies with a focus on cancer (Agudo and others 2012; Boffetta and others 2006; Boffetta and others 2009; de Martel and others 2012; Ezzati and others 2005; Forman and others 2012; Goldie and others 2008; Goldstein and others 2005; McCormack and others 2012; McCormack and Schuz 2012; Renehan and others 2010). Because of the known lag time between the inception of smoking and cancer development, lung cancer mortality rates were converted into smoking impact ratios and as a proxy for exposure to smoking, thereby taking into account the duration and intensity of the exposure. To calculate PAF linked to infection, the prevalence of infection among cancer cases was used instead of the prevalence of infection in the population, because of the unreliability of population surveys on infection prevalence (de Martel and others 2012). As for effect sizes relating exposure to disease, wherever possible, the results were as reported in meta-analysis or were derived from the most up-to-date epidemiological findings.
The quality of the estimation depends largely on data availability, and a large gap remains in data availability and data quality in LMICs relative to HICs. The estimation of PAF also assumes that the association between a risk factor and cancer is causal and that a reduction in exposure to a specific risk factor will lead to a decline in the incidence of the cancer in question (Rockhill, Newman, and Weinberg 1998). To avoid an overestimation of the role of certain risk factors, we only report results for factors that have been confirmed as having sufficient evidence in causing cancer (Cogliano and others 2011). Epidemiological studies that report risk associations between exposure and cancer are prone to several limitations. In estimating PAF, studies have tried to overcome this issue by exclusively using risk estimates based on large meta-analyses that included only high-quality studies and, wherever possible, only cohort studies.
Global Cancer Burden and Distribution According to Income Group
For 2012, GLOBOCAN estimates indicate that there were 9.1 million new cases of cancer and 4.4 million cancer deaths between the ages of 0 and 69 years (). Female breast cancer is the most frequently diagnosed cancer globally (1.3 million new cases, 14.7 percent of all cancer cases), but it ranks third as the most common cause of cancer death (358,000 deaths, 8.2 percent of all cancer deaths). Lung cancer is the leading cause of death (767,000 deaths, 17.5 percent) and ranks second in incidence (1 million deaths, 10.8 percent). In terms of incidence, the most frequent cancer types thereafter are colorectal (744,000 cases, 174,000 deaths), prostate (556,000 cases, 56,000 deaths), stomach (539,000 cases, 242,000 deaths), and liver (503,000 cases, 336,000 deaths).
Global Burden of Cancer: Estimated Counts of Cancer Incidence and Mortality, by Region, 2012.
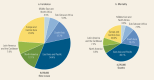
Four cancers constitute nearly 50 percent of the total cancer burden in HICs; for cancers of the prostate, lung, and colorectum, comparable numbers (between 350,000 and 400,000 new cases) of each type are estimated in 2012, with female breast cancer accounting for 550,000 cases (). Female breast cancer is also the most common form of cancer in LMICs, with almost 770,000 estimated cases in 2012. In combination, eight cancers (breast, lung, liver, cervix, stomach, colorectum, esophagus, and leukemia) account for almost two-thirds of the estimated cancer burden in LMICs. It is noteworthy that several types of cancer, more commonly associated with infection and poverty, less commonly diagnosed in HICs, are major cancers in LMICs; they include stomach and liver cancer—which are more frequently diagnosed than colorectal or prostate cancer in lower-income economies—as well as cancers of the cervix and esophagus.
Global Burden of Cancer: Cancer Incidence and Mortality by Income Level, 2012.
In terms of risk of developing specific neoplasms worldwide, the lifetime incidence of breast cancer is highest (2.8 percent), with the lifetime risk of the next four most common cancers varying from 1 to 1.5 percent. In HICs, however, the lifetime risk exceeds 2 percent for cancers of the breast (female), prostate, lung, and colorectum.
In terms of mortality, the most frequent causes of cancer death in HICs are lung, colorectum, female breast, and pancreas, which together account for about 48 percent of the total cancer deaths in 2012. Relative to incidence, a greater proportion of the mortality burden occurs in LMICs, particularly for those cancers more frequently diagnosed in LMICs, including liver and stomach (). Together with lung, female breast, and colorectal cancer, these neoplasms constitute half of the total cancer mortality burden in these areas. In terms of risk of death, the lifetime cumulative mortality from lung cancer and female breast cancer ranks highest in HICs (1.8 and 1.2 percent, respectively) and LMICs (1.3 and 1 percent, respectively). Colorectal cancer ranks third in HICs, and liver and cervical cancer rank third and fourth, respectively, in LMICs.
Diversity of Cancer by Type: Geographical Variations by Region
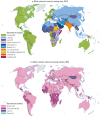
The classification of cancer patterns by broad levels of economic development draws attention to some of the key macroeconomic determinants of inequality and the variations in the risk of developing cancer globally. To underscore the diversity of cancer, it is important to show the extent to which cancer-specific patterns vary. , panel a, outlines the most common form of cancer among men in 184 countries in 2012. Worldwide, 10 types of cancer rank highest, with prostate cancer the most frequent cancer form in 68 countries, chiefly in HICs, but also in parts of Latin America and the Caribbean and in the southern regions of the African continent. Lung cancer is the most common neoplasm in 42 countries, mainly in Eastern Europe, Northern Africa, and Asia, including China and Indonesia. Liver cancer is the most common form in 27 countries, particularly in Western Africa and South-East Asia.
Most Common Cancers Diagnosed in Men and Women in 184 Countries, 2012, Ages 0–69 Years.
Among men, the incidence and mortality patterns vary most markedly by region in Africa (Jemal and others 2012), with liver cancer the most common cancer in 18 of the 47 Sub-Saharan African countries. Clusters of very high incidence of Kaposi sarcoma are observed in 13 countries in Eastern Africa; prostate cancer is the most frequently observed tumor in nine countries (Parkin and others 2014). In women—and in some contrast to the diversity of the highest ranking cancers in men—either breast cancer (143 countries) or cervical cancer (39 countries) ranks as the most frequent cancer diagnosed in almost all countries worldwide; exceptions include the very high female incidence rates of thyroid cancer in the Republic of Korea and liver cancer in Mongolia (, panel b).
Global Cancer Transitions: Recent Trends and Future Burden Circa 2030
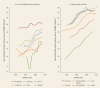
The high-quality cancer registry data from CI5 (http://ci5.iarc.fr) illustrate temporal aspects of the global cancer transition: increases in breast and prostate cancer are apparent in countries that have transitioned from low-income to middle-income or middle-income to high-income ( and ); for colorectal cancer, the increases are more evident in registries transitioning from middle-income countries; those countries that have reached very high levels of income tend to have also attained very high incidence rates of this cancer, and a recent peak in incidence is observed in several countries (). Nevertheless, the trends point to a Westernization effect in countries undergoing transition. Increasing average levels of economic progress serve as a proxy for a changing population prevalence of reproductive, dietary, metabolic, and hormonal risk factors (toward levels more akin to those more commonly observed in the West) that correspondingly increase the risk of these cancers (Bray 2014).
Trends in Age-Standardized (World) Incidence Rates of Female Breast Cancer, by Income Level, 1980–2010.
Trends in Age-Standardized (World) Incidence Rates of Prostate Cancer, by Income Level, 1980–2010.
Trends in Age-Standardized (World) Incidence Rates of Male Colorectal Cancer, by Income Level, 1980–2010.
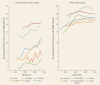
Evidently, better diagnosis, earlier detection, and screening may have inflated the incidence burden in many settings, particularly where precursor lesions are not the target of the intervention. This includes prostate and breast cancers where prostate-specific antigen testing and mammographic screening have impacted the increasing incidence of the respective cancers in many populations (Bleyer and Welch 2012; Bray and others 2010; Bray, McCarron, and Parkin 2004). Nevertheless, such increases are countered by another hallmark of cancer transition: a reduction in infection-based neoplasms (Forman and others 2012; Vaccarella and others 2013). Most of the registries record declines in cervical and stomach cancer incidence (Bray and others 2012) ( and ). There have been rapid increases in the incidence of breast, prostate, and colorectal cancer documented in countries in Sub-Saharan Africa with good quality incidence data; for example, rates of breast cancer have increased by 3.6 percent annually in Kampala, Uganda, and by 4.9 percent in the black population of Harare, Zimbabwe. There is little evidence of another hallmark of cancer transition—declining cervical cancer—in these populations: rather, rates exhibit persistent increases in incidence in recent years (Chokunonga and others 2013; Wabinga and others 2014).
Trends in Age-Standardized (World) Incidence Rates of Cervical Cancer, by Income Level, 1980–2010.
Trends in Age-Standardized (World) Incidence Rates of Men with Stomach Cancer, by Income Level, 1980–2010.
The importance of lung cancer in the overall global burden from cancer is a reflection of the prevalence of tobacco smoking. The differences in lung cancer incidence by national income level reflect the historical and current variations of smoking habits in the underlying populations. Lung cancer rates are decreasing in men in most countries, notably in HICs and in countries where the habit has long been established, but they are increasing in women (Jemal and others 2010). Such contrasting male and female trends are consistent with the population-specific rise and fall in adult cigarette utilization over past decades (Lortet-Tieulent and others 2013). The correlation between income and male smoking prevalence has shown a curvilinear trend (Talley 2010) that mimics the smoking epidemic curve (Thun and others 2012). Smoking prevalence tends to be low in countries of low average income but rises as income increases, until it stabilizes and begins to decline as countries attain high mean income levels.
The prevalence of smoking in men has increased sharply in many middle-income countries, including China and Indonesia (Jha 2009). The future burden of lung cancer and other smoking-related cancers (IARC 2004), such as bladder, esophageal, and oral cavity in different regions and countries, will largely depend on gender-specific smoking patterns at the population level, including the duration of smoking, extent of cessation, and types of tobacco smoked (Doll and others 2004; Pirie and others 2013; Thun and others 2012).
A recent study of incidence trends in 1988–2002 for the seven most frequently diagnosed cancers worldwide (constituting more than 58 percent of the global burden) estimated the annual percentage change of trends in age-adjusted incidence using data from 101 cancer registries (Bray and others 2012). The trends were stratified by medium, high, or very high levels of the Human Development Index (HDI), a composite measure of life expectancy at birth, income as GNI per capita, and access to education. Although the HDI is highly correlated with GNI, the latter reflects average national income, whereas the former additionally provides some indication as to how income is spent, at least in the areas of health and education (UNDP 2009).
An estimated 21.6 million new cancer cases are predicted for 2030 (an increase of 53 percent from 2012), based solely on projected demographic changes and unchanged cancer incidence rates (). If the cancer-specific incidence trends up to 2002 continue, an absolute increase of 62 percent in incidence is expected, representing an overall increase of 1.2 million new cases per year in 2012–30. The increases in cancer incidence are proportionally greatest in low HDI settings, with a predicted 77 percent increase in both genders.
Cancer Incidence Projected to 2030 with and without Incorporating Recent Changes in Rates by Gender, Income Level, and Age Group.
Although such analyses appear to present a rather pessimistic view of the future burden of cancer, targeted interventions can significantly reduce the projected rise in the number of cancer patients through resource-appropriate interventions. Such actions include primary prevention strategies to control lifestyle factors, including tobacco avoidance and cessation foremost, a reduction in alcohol consumption and obesity, and the promotion of increased levels of physical activity. Vaccination programs for liver and cervical cancer and early detection programs for breast and cervical cancer are important.
Risk Factors, Interventions and Policies, and Potential Impacts on the Burden of Cancer
Approximately 30 percent of all cancers worldwide are considered preventable by modification of the predominant risk factors (Danaei and others 2005; Martin-Moreno, Soerjomataram, and Magnusson 2008). Smoking and dietary risk factors have particularly important roles in potentially reducing the cancer burden in HICs and LMICs (Ezzati and others 2005; Jha 2009; Lim and others 2012; WCRF and AICR 2007). For cancers with strong risk modifiers, trends in risk factors are followed by trends of cancer incidence with a lag of 20–30 years (Doll and others 2004; Jha 2009). Illustrative examples include the temporal patterns of cigarette smoking and lung cancer, and age at first childbirth and breast cancer (Jha 2009; Soerjomataram and others 2008). Unfortunately, for most risk factors, the relation to specific cancer forms is weaker than these associations. In addition, risk factors often interact, making it difficult to link directly the changing prevalence of a single risk factor to the observed trends of specific cancer types.
Tobacco
Tobacco smoking is the largest single avoidable cause of premature death. Worldwide, 70 percent of lung cancer deaths; 42 percent of cancers of the esophagus and oral cavity (Ezzati and others 2005; Lim and others 2012); a significant proportion of cancers of the larynx, urinary bladder, and pancreas; a smaller proportion of cancers of the kidney, stomach, and cervix; and myeloid leukemia are attributable to cigarette smoking (Agudo and others 2012). Between 1990 and 2010, mortality rates from cancers related to smoking in higher resource settings decreased (Lim and others 2012), reflecting a mean decrease of smoking prevalence, especially among men. However, in countries undergoing transition, the proportion of cancer deaths attributable to smoking has increased from 12 to 14 percent (Lim and others 2012), reflecting increasing cigarette consumption (Ng and others 2014). Efforts to reduce tobacco consumption are central to preventing deaths from cancer and other diseases.
Smoking prevalence has decreased for many decades in HICs; in LMICs, it is stable or showing only limited signs of a decline very recently. In China, for example, with one of the highest smoking rates in the world, prevalence among men dropped from 57 to 53 percent between 2002 and 2007 (Ng and others 2014). This change may represent the first impact of increasing tobacco control measures in the country. The WHO MPOWER report on the global tobacco epidemic indicated that, of the 48 countries that had implemented at least one tobacco control measure since 2007, four-fifths were LMICs (WHO 2008). The impact of such measures will not be evident in lung cancer trends for some decades, and increasing rates may continue to rise in many LMICs (McCormack and Boffetta 2011). The prevalence of women in LMICs who smoke has remained rather low, but it may be expected to rise as social and cultural prohibitions erode (Thun and others 2012). It is debatable whether the impact of tobacco on the cancer burden in some LMICs will reach the level observed in many HICs (McCormack and Boffetta 2011).
Persistent Infections
The most recent estimates state that at least 16.1 percent or two million new cases of human malignancies worldwide are attributable to persistent infections with bacteria, viruses, or parasites (de Martel and others 2012). This fraction is higher in LMICs (22.9 percent or 1.6 million new cases) than in HICs (7.4 percent or 410,000 new cases). Helicobacter pylori, hepatitis B and C viruses, and human papilloma viruses (HPVs) are responsible for 1.9 million cases, mainly gastric, liver, and cervical cancers. The remaining 163,000 cases are attributed to Epstein-Barr virus, human herpes virus type 8, human T-cell lymphotropic virus type 1, Opisthorchis viverrini, Clonorchis sinensis, and Schistosoma haematobium. By region, the attributable fraction shows marked variation (), from 33.2 percent of all cancers in Sub-Saharan Africa to 3.3 percent in Australia and New Zealand. The proportion of cancers associated with chronic infections ranges from 1 in 3 in Sub-Saharan Africa and 1 in 4 in China to 1 in 30 in Australia and New Zealand and 1 in 25 in North America. Awareness of the role of infectious agents in cancer has expanded rapidly, leading to the formulation of new strategies, including vaccines against hepatitis B to prevent liver cancer, and against high-risk strains of HPV to prevent cervical and other HPV-related cancer types (Beutels 2001; Harper and others 2004; Herrero and others 2013).
Cancer and Infection: Estimated Attributable Fraction, by Region or Country, 2008.
Increasing coverage of hepatitis B virus vaccination has been related to a decrease in the prevalence of chronic hepatitis B virus throughout the world (Goldstein and others 2005). For example, in Taiwan, China, a 75 percent decrease in the incidence of hepatocellular carcinoma in children was reported since the initiation of routine infant hepatitis B vaccination (Chang and others 1997). Gavi, the Vaccine Alliance, has subsidized vaccines in poor countries, leading to a rapid increase in coverage of hepatitis B vaccination, with a similar effort underway in implementing HPV vaccination (Kane and others 2012). Based on current epidemiologic data in 72 countries, it is predicted that we will observe a mean reduction in lifetime risk of cervical cancer of between 31 percent (for example, in Guinea and Senegal) and 60 percent (for example, in Ethiopia), assuming an increase in vaccination coverage to 70 percent (Goldie and others 2008). Differences in the results of vaccination stem from variation in risk factor prevalence and the background incidence of the cancer being targeted. Safe and effective vaccines have proven highly important in reducing infection-related cancers.
Alcohol
Excess alcohol consumption is an important risk factor for several cancers, most notably, of the oral cavity, pharynx, larynx, and esophagus, as well as for cancers of the breast, liver, and colorectum (IARC 2010). Up to 4 percent of cancer cases worldwide are attributable to alcohol intake (Boffetta and others 2006), with similar distributions in HICs and LMICs (Rehm and Shield 2013). Global alcohol consumption has been stable in recent decades, with a small decrease in countries where intake was historically high (for example, in Europe) and a small increase in countries where intake has been relatively low (for example, in South-East Asia) (Shield and others 2013). In HICs, a notable exception is an increase in consumption in the Russian Federation between 1986 and 2000, which has subsequently stabilized. Among LMICs, Uganda has the heaviest alcohol intake level (10.9 liters of pure alcohol per capita, compared with 11.3 in the Russian Federation) (WHO 2011a). This is in contrast to traditionally Muslim countries (for example, the Arab Republic of Egypt and Indonesia), where alcohol consumption is very low (0.66 and 0.67 liters of pure alcohol per capita, respectively) (WHO 2011a). Sweden is one example of a successful government policy geared toward effectively reducing alcohol intake at the population level. After restricting beer sales based on alcohol content in 1977, consumption decreased by approximately 15 percent (Makela, Tryggvesson, and Rossow 2002). Such an example points to the importance of nationwide population interventions as an effective way to reduce alcohol consumption.
Diet
Recent estimates report that about 15 percent of all cancer deaths are related to unhealthy diets, including high intake of red meat, processed meat, and sodium, as well as low intake of fruits and vegetables (Lim and others 2012). Yet, most research is still inconclusive about specific dietary items (WCRF and AICR 2007). The most recent comprehensive review of diet and cancer risks confirms an elevated risk related to red and processed meat but finds less evidence supporting the benefit of the consumption of fruits and vegetables in reducing risk (WCRF and AICR 2007). Variations in the strength of the evidence in different studies over time make the measurement of dietary intake problematic in epidemiological studies; if only well-established risk factors are included in the estimation, approximately 5 percent of cancer deaths worldwide (387,000) would be attributable to dietary risk factors (Lim and others 2012).
Consumption of red meat and processed meat increases the risk of colorectal cancer by an estimated 43 percent (WCRF and AICR 2007). Consumption of red meat and processed meat is generally rising in LMICs but is stable in HICs. Another major dietary risk factor is high salt intake, which increases the risk of stomach cancer. Globally, salt intake has declined, and the associated cancer burden has declined as a result (Brown and others 2009); currently, 1.5 percent and 2 percent of all cancer deaths in HICs and LMICs, respectively, are attributed to high salt intake, compared with 2.2 percent and 2.7 percent, respectively, in 1990 (Lim and others 2012). In the United Kingdom, 10 percent of new stomach cancer cases diagnosed in 2010 may be attributable to high salt intake (Parkin 2011).
Although many of the underlying mechanisms are not yet established, food and diet are clearly important determinants of cancer risk. Over the past century, better food preservation methods, reducing the need for salt, have probably been a key factor in the decline in stomach cancer incidence. The levels of red meat consumption in recent decades may explain the low colorectal cancer rates in southern Asia (WCRF and AICR 2007), as well as the high rates in many western European countries (Center, Jemal, and Ward 2009; Center and others 2009).
Evidence linking obesity and overweight to cancer is firmer than the dietary evidence, indicating that 3 and 6 percent of cancer deaths in LMICs and HICs, respectively, can be attributed to excess weight, as quantified by the body mass index (BMI) (Bergstrom and others 2001; Lim and others 2012; Renehan and others 2010). BMI is already an important correlate of cancer incidence in HICs, and it is likely to continue increasing in importance in some LMICs, as average BMI has increased in most Central and South American countries and also in southern Africa (Stevens and others 2012). BMI has remained low in Sub-Saharan Africa (except in southern Africa) and South and Southeast Asia. High BMI has increased as a cause of cancer deaths more substantively in LMICs, a 54 percent increase over the past 30 years, compared with a 26 percent increase in HICs over the same period (Lim and others 2012; Stevens and others 2012).
Physical Inactivity
Regular physical activity is associated with a reduction in the risk of developing colon cancer (Harriss and others 2009) and breast cancer (Monninkhof and others 2007). Many studies have suggested that regular physical activity additionally reduces the risk of cancers of the endometrium and prostate (WCRF and AICR 2007). Although this effect seems to be strongly linked to the impact of physical activity on body weight, the preventive effect of regular exercise for some cancers seems to act independently of weight control (Friedenreich and others 2006; Giovannucci and others 1995; Pischon and others 2006). In 2010, global physical inactivity was estimated to be related to 3.2 and 5.5 percent of cancer deaths in LMICs and HICs, respectively (Lim and others 2012). Motivating people to increase physical activity or reduce their body weight has proven difficult; hence, there has been a more recent emphasis on altering the built environment (Diez Roux and others 2007; Giles-Corti and others 2005). In Northern Ireland, a greenways urbanization project was considered very cost-effective at reducing major chronic diseases, including breast and colon cancer (Dallat and others 2013).
Environmental Factors
Other environmental risk factors, such as household air pollution, may have a very minor role in causing cancer in HICs, but these factors are much more important in LMICs, where they may account for about 2.4 percent of all cancer deaths (Evans and others 2013; Lim and others 2012). In China, the risk of lung cancer among smokers is three times that of nonsmokers, a risk ratio that is much lower than in other countries (Lin and others 2008), probably as a result of the common practice of cooking indoors with solid fuel—which significantly increases lung cancer risk. Some improvements in cooking conditions have already taken place and further improvements could potentially prevent a substantial number of lung cancer deaths in the future.
Many outdoor air pollutants may increase the risk of neoplasms, most notably lung cancer (Raaschou-Nielsen and others 2013). Increasing air pollution in large cities in LMICs has caused concern about links to several chronic diseases, including cancer (Evans and others 2013). Currently 3.6 percent of cancer deaths in LMICs are attributed to ambient air pollution, compared with 2.4 percent in 1990 (Lim and others 2012).
Protective measures in the workplace have led to the prevention of many cancers. A small proportion of cancer deaths are attributed to occupational risk factors (1.6 percent in LMICs and 1.3 percent in HICs). Despite the small numbers, there is cause for concern regarding occupational exposures in certain countries undergoing development, including higher industrial exposures, as well as a longer duration of exposure due to the common practice of child labor (McCormack and Schuz 2012).
Asbestos is among the best known occupational exposures and has been strongly linked to mesothelioma and lung cancer (IARC 1987). Because of past exposure, the current proportion of lung cancer attributable to asbestos is highest in the United States and the United Kingdom, ranging from 12 to 18 percent, but the future burden is likely to increase in China, India, and Russia, where current exposure to and use of asbestos are at the highest levels worldwide (McCormack and others 2012).
Exposure to the ultraviolet component of sunlight is the predominant environmental cause of skin cancer, affecting mainly fair skinned persons who live predominantly in HICs (de Vries and Coebergh 2004). A recent study predicted that protection from ultraviolet rays may reduce the incidence of melanoma by 13 to 21 percent in various Western populations over a 40-year period up to 2050 (de Vries and others 2012).
Hormonal and Reproductive Factors
Hormonal and reproductive factors play an important role in the etiology of several cancers among women, in particular, breast cancer. Established risk factors include early age at menarche, older age at first birth, low parity, and late age at menopause (Beral, Bull, and Reeves 2005; Beral and others 2007; Beral and others 2011; Reeves and others 2012; Rosner and Colditz 1996). The current changes in these factors are expected to contribute to a 25 percent increase in breast cancer incidence (McCormack and Boffetta 2011). Reversing the current trend of these factors is not commonly considered feasible or desirable; hence, breast cancer prevention must focus on other, more modifiable factors. These include reductions in BMI, physical inactivity, and alcohol consumption, and increasing the duration of breast-feeding (McCormack and Boffetta 2011).
Summary
Quantifying the totality of preventable cancers through the effects of the interventions as described above is a complicated exercise, given imprecision in the estimates and risk factor interactions that are not fully understood. One study has estimated that around 35 percent of cancer deaths are avoidable by modifying the major cancer risk factors (Danaei and others 2005).
Of seven million deaths from cancer worldwide in 2001, 0.76 million preventable deaths were in HICs and 1.67 million were in LMICs. Primary prevention would most effectively target infection and tobacco, as well as better nutrition and increased physical activity (Jemal and others 2012; Jha 2009). In more developed nations, interventions focusing on reducing smoking, improving dietary habits, and avoiding excess weight are likely to be most effective (Jha and others 2013; Murray and others 2013; Pirie and others 2013; Renehan and others 2010).
More effective early detection and better treatment will further reduce the number of deaths in LMICs (Farmer and others 2010; Sankaranarayanan and others 2011). The large burden of cancer in these regions and the great potential to prevent them call for rational and balanced investment in a broad spectrum of cancer control activities that includes primary prevention, screening, early detection, therapy, follow-up, and palliative care (Stewart 2012). In addition, surveillance of risk factor information and cancer data, particularly in LMICs, can improve global cancer risk assessment and inform national cancer control planning.
Prospects for Enhancing Cancer Surveillance Systems in LMICs
Cancer is already a leading NCD at ages under 70 years worldwide and is the most common cause of death in more than 40 HICs. Global efforts are underway at a high political level to combat the rising cancer and NCD burden. The WHO Action Plan for the Global Strategy for the Prevention and Control of Noncommunicable Diseases provides a road map for 2013–20 that includes a target for member states of a global reduction of 25 percent in premature mortality from the four main NCD groups by 2025 (“25 by 25”) (WHO 2011b). Adequate surveillance mechanisms at the country level are needed to monitor progress. With a greater number of governments adopting national cancer control plans, attention is increasingly turning to population-based cancer surveillance data as a central component in prioritizing and evaluating interventions.
Vital registration (mortality data) and population-based cancer registries (incidence and survival data) are complementary, and having the availability of both to a high standard is ideal. But for many LMICs, national vital statistics are unavailable, partial, or inaccurate. Where data are available, the coverage and completeness of the mortality data are often poor. It is thus much easier at present to quantify premature cancer mortality for higher resource countries than for those at the lower end. Efforts to develop and improve vital registration systems are being augmented by a number of verbal autopsy studies in countries and communities where many deaths occur at home and without medical attention (for example, the Million Death Study in India [Dikshit and others 2012]).
Population-based cancer registries describe the present scale and profile of cancer in the community and aid planning by assessing past and future trends. Knowing the size and nature of the cancer problem serves as an important stimulus to implement and change policy. However, although it is feasible to implement PBCRs even in the lowest income settings (Bray and others 2014), their availability is limited in most countries undergoing development, with only one in five LMICs currently predisposed with the necessary population-based data for cancer control action. This situation reflects a lack of advocacy for the value of registries historically and the subsequent lack of resources and prioritization of cancer data for cancer control among the competing demands on limited health care services (Wild 2014).
Nevertheless, positive momentum is building through the recognition of the role of cancer and other NCDs in hampering human development, changing priorities within countries at the highest political level. Importantly and as part of the global monitoring framework, WHO member states are being asked to collect cancer incidence, by type of cancer per 100,000 population, one of 25 indicators to monitor progress toward the 25 by 25 target, thereby obliging countries to establish and sustain population-based cancer registries.
Clearly, in aligning registries to cancer control action, technical guidance is needed to empower countries to improve their information systems so that they can be used for their vital purpose. Several technical and funding organizations are now working in a cooperative and coordinated manner under the auspices of the Global Initiative for Cancer Registry Development (http://gicr.iarc.fr) to bring about the needed improvement in the quantity and quality of data on cancer incidence through investment in PBCRs. There is a longstanding and ongoing commitment at IARC to support the development of such registries worldwide, particularly where they are in limited supply and most needed, in most LMICs. The presence of six recently established IARC regional hubs for cancer registration () across LMIC regions is providing the necessary localized support and training for cancer registries in the respective countries, as well as strengthening registry networking in each region to improve data standards and develop joint research activities.
Implementing the Global Initiative for Cancer Registry through Six IARC Regional Hubs for Cancer Registration.
Conclusions
This chapter has examined the increasing global importance of cancer among the major NCDs, highlighted the extent of the ongoing demographic and epidemiologic transitions, described the key risk factors involved, and highlighted the extent to which cancer deaths may be avoided.
Changing fertility patterns and increasing life expectancy are leading to major changes in the scale of the cancer problem worldwide for populations at all levels of human development. A cancer transition is clearly underway in countries transiting to high levels of income and development whereby reductions in infection-related cancers are offset by concomitant increases in cancers linked to a “Westernization” of lifestyle, including “risky” behavior. Changes in tobacco habits and the expanding tobacco markets in LMICs may have major consequences on the future burden of lung and other tobacco-related cancers in the decades that follow.
The global trends therefore place special emphasis on the need for better cancer control in LMICs and the need for expanding coverage and quality of population-based cancer registration systems in each country. Cancer statistics should also serve as a catalyst for further research on human inequality and the scale and profile of cancer from global and regional perspectives, for better determination of how and why macroeconomic determinants influence cancer indicators. It is imperative that public health clinicians and cancer control specialists are alerted to the increasing magnitude of cancer incidence and mortality worldwide. This analysis serves to highlight the need for global action to reduce the increasing incidence and mortality burden from cancer and alleviate suffering among cancer patients.
Acknowledgment
The authors thank Mathieu Laversanne, IARC, for statistical analysis and generation of the graphics.
Notes
World Bank income classifications as of July 2014 are as follows, based on estimates of gross national income (GNI) per capita for 2013:
Low-income countries (LICs): US$1,045 or less
Middle-income countries (MICs) are subdivided:
a) lower-middle-income: US$1,046–US$4,125
b) upper-middle-income (UMICs): US$4,126–US$12,745
High-income countries (HICs): US$12,746 or more
Except for and estimates presented in the section Risk Factors, Interventions and Policies, and Potential Impacts on the Burden of Cancer, the maps and figures in this chapter are based on incidence and mortality estimates for ages 0 to 69, consistent with reporting in all DCP3 volumes. Global cancer statistics are estimates for the year 2012 and have been provided by the International Agency for Research on Cancer from its GLOBOCAN 2012 database. Observable population-based data were derived from Cancer Incidence in Five Continents, 10th edition and for trends over time from CI5 Plus (http://ci5.iarc.fr/CI5plus/Default.aspx). The discussion of burden (including risk factors), however, includes all ages unless otherwise noted. Interventions also apply to all age groups, except where age ranges or cutoffs are specified.
References
Agudo A, Bonet C, Travier N, Gonzalez C A, Vineis P., and others. 2012. “Impact of Cigarette Smoking on Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Study.” Journal of Clinical Oncology
30 (36): 4550–57. doi:JCO.2011.41.0183 [Pii] 10.1200/JCO.2011.41.0183. [
PubMed: 23169508]
Beral V, Bull D, Green J, Reeves G. 2007. “Ovarian Cancer and Hormone Replacement Therapy in the Million Women Study.” The Lancet
369 (9574): 1703–10. doi:S0140-6736(07)60534-0 [Pii] 10.1016/S0140-6736(07)60534-0. [
PubMed: 17512855]
Beral V, Bull D, Reeves G. 2005. “Endometrial Cancer and Hormone-Replacement Therapy in the Million Women Study.” The Lancet
365 (9470): 1543–51. doi:S0140-6736(05)66455-0 [Pii] 10.1016/S0140-6736(05)66455-0. [
PubMed: 15866308]
Beral V, Reeves G, Bull D, Green J. 2011. “Breast Cancer Risk in Relation to the Interval between Menopause and Starting Hormone Therapy.” Journal of the National Cancer Institute
103 (4): 296–305. doi:Djq527 [Pii] 10.1093/Jnci/Djq527. [
PMC free article: PMC3039726] [
PubMed: 21278356]
Bergstrom A, Pisani P, Tenet V, Wolk A, Adami H O. 2001. “Overweight as an Avoidable Cause of Cancer in Europe.” International Journal of Cancer
91 (3): 421–30. doi:10.1002/1097-0215(200002)9999:9999<::AID-IJC1053>3.0.CO;2-T [Pii]. [
PubMed: 11169969]
Beutels P.
2001. “Economic Evaluations of Hepatitis B Immunization: A Global Review of Recent Studies (1994–2000).” Health Economics
10 (8): 751–74. doi:10.1002/Hec.625 [Pii]. [
PubMed: 11747055]
Bleyer A, Welch H G. 2012. “Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence.” New England Journal of Medicine
367 (21): 1998–2005. doi:10.1056/Nejmoa1206809. [
PubMed: 23171096]
Boffetta P, Hashibe M, Vecchia C La, Zatonski W, Rehm J. 2006. “The Burden of Cancer Attributable to Alcohol Drinking.” International Journal of Cancer
119 (4): 884–87. doi:10.1002/Ijc.21903. [
PubMed: 16557583]
Boffetta P, Tubiana M, Hill C, Boniol M, Aurengo A. and others. 2009. “The Causes of Cancer in France.” Annals of Oncology
20 (3): 550–55. doi:Mdn597 [Pii]10.1093/Annonc/Mdn597. [
PubMed: 18765462]
Bray F.
2014. “Transitions in Human Development and the Global Cancer Burden.” In World Cancer Report 2014, edited by Wild C P, Lyon B A Stewart, editors. . France: International Agency for Research on Cancer.
Bray F, Jemal A, Grey N, Ferlay J, Forman D. 2012. “Global Cancer Transitions According to the Human Development Index (2008–2030): A Population-Based Study.” The Lancet Oncology
13 (8): 790–801. doi:10.1016/S1470-2045(12)70211-5. [
PubMed: 22658655]
Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. 2010. “Prostate Cancer Incidence and Mortality Trends in 37 European Countries: An Overview.” European Journal of Cancer
46 (17): 3040–52. doi:S0959-8049(10)00878-6 [Pii]10.1016/J.Ejca.2010.09.013. [
PubMed: 21047585]
Bray F, McCarron P, Parkin D M. 2004. “The Changing Global Patterns of Female Breast Cancer Incidence and Mortality.” Breast Cancer Research
6 (6): 229–39. doi:Bcr932 [Pii]10.1186/Bcr932. [
PMC free article: PMC1064079] [
PubMed: 15535852]
Bray F, Znaor A, Cueva P, Korir A, Swaminathan R., and others. 2014. “Planning and Developing Population-Based Cancer Registration in Low- and Middle-Income Settings.” Technical Publication 43, International Agency for Research on Cancer, Lyon, France. [
PubMed: 33502836]
Brown I J, Tzoulaki I, Candeias V, Elliott P. 2009. “Salt Intakes around the World: Implications for Public Health.” International Journal of Epidemiology
38 (3): 791–813. doi:Dyp139 [Pii] 10.1093/Ije/Dyp139. [
PubMed: 19351697]
Center M M, Jemal A, Smith R A, Ward E. 2009. “Worldwide Variations in Colorectal Cancer.” CA: A Cancer Journal for Clinicians
59 (6): 366–78. doi:59/6/366 [Pii]10.3322/Caac.20038. [
PubMed: 19897840]
Center M M, Jemal A, Ward E. 2009. “International Trends in Colorectal Cancer Incidence Rates.” Cancer Epidemiology Biomarkers & Prevention
18 (6): 1688–94. doi:18/6/1688 [Pii]10.1158/1055-9965.EPI-09-0090. [
PubMed: 19505900]
Chang M H, Chen C J, Lai M S, Hsu H M, Wu T C. and others. 1997. “Universal Hepatitis B Vaccination in Taiwan and the Incidence of Hepatocellular Carcinoma in Children. Taiwan Childhood Hepatoma Study Group.” New England Journal of Medicine
336 (26): 1855–59. doi:10.1056/NEJM199706263362602. [
PubMed: 9197213]
Chokunonga E, Borok M Z, Chirenje Z M, Nyakabau A M, Parkin D M. 2013. “Trends in the Incidence of Cancer in the Black Population of Harare, Zimbabwe 1991–2010.” International Journal of Cancer
133 (3): 721–29. doi:10.1002/Ijc.28063. [
PubMed: 23364833]
Cogliano V J, Baan R, Straif K, Grosse Y, Lauby-Secretan B. and others. 2011. “Preventable Exposures Associated with Human Cancers.” Journal of the National Cancer Institute
103 (24): 1827–39. doi:Djr483 [Pii] 10.1093/Jnci/Djr483. [
PMC free article: PMC3243677] [
PubMed: 22158127]
Dallat M A, Soerjomataram I, Hunter R F, Tully M A, Cairns K J., and others. 2013. “Urban Greenways Have the Potential to Increase Physical Activity Levels Cost-Effectively.” European Journal of Public Health
24 (2): 190–95. doi:Ckt035[Pii]10.1093/Eurpub/Ckt035. [
PubMed: 23531527]
Danaei G, Hoorn S Vander, Lopez A D, Murray C J, Ezzati M. 2005. “Causes of Cancer in the World: Comparative Risk Assessment of Nine Behavioural and Environmental Risk Factors.” The Lancet
366 (9499): 1784–93. doi:S0140-6736(05)67725-2 [Pii]10.1016/S0140-6736(05)67725-2. [
PubMed: 16298215]
Martel de C, Ferlay J, Franceschi S, Vignat J, Bray F. and others. 2012. “Global Burden of Cancers Attributable to Infections in 2008: A Review and Synthetic Analysis.” The Lancet Oncology
13 (6): 607–15. doi:S1470-2045(12)70137-7 [Pii]10.1016/S1470-2045(12)70137-7. [
PubMed: 22575588]
de Vries E, Arnold M, Altsitsiadis E, Trakatelli M, Hinrichs B. and others. 2012. “Potential Impact of Interventions Resulting in Reduced Exposure to Ultraviolet (UV) Radiation (UVA and UVB) on Skin Cancer Incidence in Four European Countries, 2010–2050.” British Journal of Dermatology
167 (Suppl. 2):53–62. doi:10.1111/J.1365-2133.2012.11087.X. [
PubMed: 22881588]
Vries de E, Coebergh J W. 2004. “Cutaneous Malignant Melanoma in Europe.” European Journal of Cancer
40 (16): 2355–66. doi:S0959804904004678 [Pii]10.1016/J.Ejca.2004.06.003. [
PubMed: 15519506]
Diez Roux A V, Evenson K R, McGinn A P, Brown D G, Moore L., and others. 2007. “Availability of Recreational Resources and Physical Activity in Adults.” American Journal of Public Health
97 (3): 493–99. doi:AJPH.2006.087734 [Pii] 10.2105/AJPH.2006.087734. [
PMC free article: PMC1805019] [
PubMed: 17267710]
Dikshit R, Gupta P C, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L. and others. 2012. “Cancer Mortality in India: A Nationally Representative Survey.” The Lancet
379 (9828): 1807–16. doi:10.1016/S0140-6736(12)60358-4. [
PubMed: 22460346]
Doll R.
1990. “Are We Winning the Fight against Cancer? An Epidemiological Assessment. EACR-Muhlbock Memorial Lecture.” European Journal of Cancer
26 (4): 500–08. [
PubMed: 2141517]
Doll R, Payne P, Waterhouse A J H. 1966. Cancer Incidence in Five Continents: A Technical Report. New York: Springer.
Doll R, Peto R. 1981. “The Causes of Cancer: Quantitative Estimates of Avoidable Risks of Cancer in the United States Today.” Journal of the National Cancer Institute
66 (6): 1191–308. [
PubMed: 7017215]
Doll R, Peto R, Boreham J, Sutherland I. 2004. “Mortality in Relation to Smoking: 50 Years’ Observations on Male British Doctors.” British Medical Journal
328 (7455): 1519. doi: 10.1136/Bmj.38142.554479.AE Bmj.38142.554479.AE [Pii]. [
PMC free article: PMC437139] [
PubMed: 15213107]
Evans J, Donkelaar A Van, Martin R V, Burnett R, Rainham D G., and others. 2013. “Estimates of Global Mortality Attributable to Particulate Air Pollution Using Satellite Imagery.” Environmental Research
120
33–42. doi:S0013-9351(12)00240-X[Pii]10.1016/J.Envres.2012.08.005. [
PubMed: 22959329]
Ezzati M, Henley S J, Lopez A D, Thun M J. 2005. “Role of Smoking in Global and Regional Cancer Epidemiology: Current Patterns and Data Needs.” International Journal of Cancer
116 (6): 963–71. doi:10.1002/Ijc.21100. [
PubMed: 15880414]
Farmer P, Frenk J, Knaul F M, Shulman L N, Alleyne G. and others. 2010. “Expansion of Cancer Care and Control in Countries of Low and Middle Income: A Call to Action.” The Lancet
376 (9747): 1186–93. doi:S0140-6736(10)61152-X [Pii] 10.1016/S0140-6736(10)61152-X. [
PubMed: 20709386]
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S. and others. 2013. GLOBOCAN 2012 v1.0. In Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C., and others. 2015. “Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012.” International Journal of Cancer
136 (5): E359–86. doi:10.1002/Ijc.29210. [
PubMed: 25220842]
Forman D, Martel C de, Lacey C J, Soerjomataram I, Lortet-Tieulent J., and others. 2012. “Global Burden of Human Papillomavirus and Related Diseases.” Vaccine 30 (Suppl. 5): F12–23. doi:S0264-410X(12)01080-8 [Pii] 10.1016/J.Vaccine.2012.07.055.
Friedenreich C, Norat T, Steindorf K, Boutron-Ruault M C, Pischon T. and others. 2006. “Physical Activity and Risk of Colon and Rectal Cancers: The European Prospective Investigation into Cancer and Nutrition.” Cancer Epidemiological Biomarkers & Prevention
15 (12): 2398–407. doi: 15/12/2398 [Pii] 10.1158/1055-9965.EPI-06-0595. [
PubMed: 17164362]
Giles-Corti B, Broomhall M H, Knuiman M, Collins C, Douglas K. and others. 2005. “Increasing Walking: How Important Is Distance to, Attractiveness, and Size of Public Open Space?” American Journal of Preventive Medicine 28 (2 Suppl. 2):169–76. doi:S0749-3797(04)00298-3 [Pii] 10.1016/J.Amepre.2004.10.018. [
PubMed: 15694525]
Giovannucci E, Ascherio A, Rimm E B, Colditz G A, Stampfer M J., and others. 1995. “Physical Activity, Obesity, and Risk for Colon Cancer and Adenoma in Men.” Annals of Internal Medicine
122 (5): 327–34. [
PubMed: 7847643]
Goldie S J, O’Shea M, Campos N G, Diaz M, Sweet S. and others. 2008. “Health and Economic Outcomes of HPV 16,18 Vaccination in 72 GAVI-Eligible Countries.” Vaccine
26 (32): 4080–93. doi:S0264-410X(08)00493-3[Pii]10.1016/J.Vaccine.2008.04.053. [
PubMed: 18550229]
Goldstein S T, Zhou F, Hadler S C, Bell B P, Mast E E., and others. 2005. “A Mathematical Model to Estimate Global Hepatitis B Disease Burden and Vaccination Impact.” International Journal of Epidemiology
34 (6): 1329–39. doi:Dyi206 [Pii]10.1093/Ije/Dyi206. [
PubMed: 16249217]
Harper D M, Franco E L, Wheeler C, Ferris D G, Jenkins D. and others. 2004. “Efficacy of a Bivalent L1 Virus-Like Particle Vaccine in Prevention of Infection with Human Papillomavirus Types 16 and 18 in Young Women: A Randomised Controlled Trial.” The Lancet
364 (9447): 1757–65. doi:S0140673604173984 [Pii]10.1016/S0140-6736(04)17398-4. [
PubMed: 15541448]
Harriss D J, Atkinson G, Batterham A, George K, Cable N T., and others. 2009. “Lifestyle Factors and Colorectal Cancer Risk (2): A Systematic Review and Meta-Analysis of Associations with Leisure-Time Physical Activity.” Colorectal Disease
11 (7): 689–701. doi:CDI1767 [Pii] 10.1111/J.1463-1318.2009.01767.X. [
PubMed: 19207713]
Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L. and others. 2013. “Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years after Bivalent HPV Vaccination in A Randomized Clinical Trial in Costa Rica.” Plos One
8 (7): E68329. doi:10.1371/Journal.Pone.0068329 PONE-D-12-40404 [Pii]. [
PMC free article: PMC3714284] [
PubMed: 23873171]
IARC (International Agency for Research on Cancer). 1987. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs 1–440. Monographs on the Evaluation of Carcinogenic Risks to Humans, IARC, Lyon, France. [
PubMed: 3482203]
IARC (International Agency for Research on Cancer). 2004. “Tobacco Smoke and Involuntary Smoking.” Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 83, IARC, Lyon, France. [
PMC free article: PMC4781536] [
PubMed: 15285078]
IARC (International Agency for Research on Cancer). 2010. “Alcohol Consumption and Ethyl Carbamate.” Monographs on the Evaluation of Carcinogenic Risks to Humans, IARC, Lyon, France.
Jemal A, Bray F, Forman D, O’Brien M, Ferlay J. and others. 2012. “Cancer Burden in Africa and Opportunities for Prevention.” Cancer
118 (18): 4372–84. doi:10.1002/Cncr.27410. [
PubMed: 22252462]
Jemal A, Center M M, Desantis C, Ward E M. 2010. “Global Patterns of Cancer Incidence and Mortality Rates and Trends.” Cancer Epidemiology Biomarkers & Prevention
19 (8): 1893–907. doi:1055-9965.EPI-10-0437 [Pii] 10.1158/1055-9965.EPI-10-0437. [
PubMed: 20647400]
Jha P.
2009. “Avoidable Global Cancer Deaths and Total Deaths from Smoking.” Nature Reviews Cancer
9 (9): 655–64. doi:Nrc2703 [Pii] 10.1038/Nrc2703. [
PubMed: 19693096]
Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M. and others. 2013. “21st-Century Hazards of Smoking and Benefits of Cessation in the United States.” New England Journal of Medicine
368 (4): 341–50. doi:0.1056/Nejmsa1211128. [
PubMed: 23343063]
Kane M A, Serrano B, Sanjose S De, Wittet S. 2012. “Implementation of Human Papillomavirus Immunization in the Developing World.” Vaccine
30 (Suppl. 5): F192–200. doi:S0264-410X(12)00956-5 [Pii] 10.1016/J.Vaccine.2012.06.075. [
PubMed: 23199963]
Karim-Kos H E, Vries E de, Soerjomataram I, Lemmens V, Siesling S, Coebergh J W. 2008. “Recent Trends of Cancer in Europe: A Combined Approach of Incidence, Survival and Mortality for 17 Cancer Sites Since the 1990s.” European Journal of Cancer
44 (10): 1345–89. doi: 10.1016/j.ejca.2007.12.015. [
PubMed: 18280139]
Lim S S, Vos T, Flaxman A D, Danaei G, Shibuya K. and others. 2012. “A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010.” The Lancet
380 (9859): 2224–60. doi:10.1016/S0140-6736(12)61766-8. [
PMC free article: PMC4156511] [
PubMed: 23245609]
Lin H H, Murray M, Cohen T, Colijn C, Ezzati M. 2008. “Effects of Smoking and Solid-Fuel Use on COPD, Lung Cancer, and Tuberculosis in China: A Time-Based, Multiple Risk Factor, Modelling Study.” The Lancet
372 (9648): 1473–83. doi:S0140-6736(08)61345-8 [Pii] 10.1016/S0140-6736(08)61345-8. [
PMC free article: PMC2652750] [
PubMed: 18835640]
Lortet-Tieulent J, Renteria E, Sharp L, Weiderpass E, Comber H. and others. 2013. “Convergence of Decreasing Male and Increasing Female Incidence Rates in Major Tobacco-Related Cancers in Europe in 1988–2010.” European Journal of Cancer. doi: S0959-8049(13)00952-0 [Pii] 10.1016/J.Ejca.2013.10.014. [
PubMed: 24269041]
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K. and others. 2012. “Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010.” The Lancet
380 (9859): 2095–128. doi:S0140-6736(12)61728-0 [Pii]10.1016/S0140-6736(12)61728-0. [
PubMed: 23245604]
Makela P, Tryggvesson K, Rossow I. 2002. “Who Drinks More or Less When Policies Change? The Evidence from 50 Years of Nordic Studies.” In The Effects of Nordic Alcohol Policies: Analyses of Changes in Control Systems, edited by Room R, editor. . Helsinki: Nordic Council for Alcohol and Drug Research.
Martin-Moreno J M, Soerjomataram I, Magnusson G. 2008. “Cancer Causes and Prevention: A Condensed Appraisal in Europe in 2008.” European Journal of Cancer
44 (10): 1390–403. doi:S0959-8049(08)00090-7 [Pii] 10.1016/J.Ejca.2008.02.002. [
PubMed: 18329264]
McCormack V A, Boffetta P. 2011. “Today’s Lifestyles, Tomorrow’s Cancers: Trends in Lifestyle Risk Factors for Cancer in Low- and Middle-Income Countries.” Annals of Oncology
22 (11): 2349–57. doi:Mdq763 [Pii] 10.1093/Annonc/Mdq763. [
PubMed: 21378201]
McCormack V, Peto J, Byrnes G, Straif K, Boffetta P. 2012. “Estimating the Asbestos-Related Lung Cancer Burden from Mesothelioma Mortality.” British Journal of Cancer
106 (3): 575–84. doi:Bjc2011563 [Pii] 10.1038/Bjc.2011.563. [
PMC free article: PMC3273352] [
PubMed: 22233924]
McCormack V A, Schuz J. 2012. “Africa’s Growing Cancer Burden: Environmental and Occupational Contributions.” Cancer Epidemiology
36 (1): 1–7. doi:S1877-7821(11)00146-9 [Pii] 10.1016/J.Canep.2011.09.005. [
PubMed: 21996568]
Monninkhof E M, Elias S G, Vlems F A, Tweel I Van Der, Schuit A J. and others. 2007. “Physical Activity and Breast Cancer: A Systematic Review.” Epidemiology
18 (1): 137–57. doi:10.1097/01.Ede.0000251167.75581.98. [
PubMed: 17130685]
Muir C S, Fraumeni Jr J F, Doll R. 1994. “The Interpretation of Time Trends.” Cancer Surveys 19–20
5–21. [
PubMed: 7895222]
Murray C J, Abraham J, Ali M K, Alvarado M, Atkinson C. and others. 2013. “The State of US Health, 1990–2010: Burden of Diseases, Injuries, and Risk Factors.” Journal of the American Medical Association
310 (6): 591–606. doi:1710486 [Pii] 10.1001/Jama.2013.13805. [
PMC free article: PMC5436627] [
PubMed: 23842577]
Ng M, Freeman M K, Fleming T D, Robinson M, Dwyer-Lindgren L. and others. 2014. “Smoking Prevalence and Cigarette Consumption in 187 Countries, 1980–2012.” Journal of the American Medical Association
311 (2): 183–92. doi:10.1001/Jama.2013.284692. [
PubMed: 24399557]
Parkin D M.
2006. “The Evolution of the Population-Based Cancer Registry.” Nature Reviews Cancer
6 (8): 603–12. doi:Nrc1948 [Pii] 10.1038/Nrc1948. [
PubMed: 16862191]
Parkin D M.
2011. “Cancers Attributable to Dietary Factors in the UK in 2010. IV Salt.” British Journal of Cancer
105 (Suppl. 2): S31–33. doi:Bjc2011480 [Pii] 10.1038/Bjc.2011.480. [
PMC free article: PMC3252061] [
PubMed: 22158317]
Parkin D M, Bray F, Ferlay J, Jemal A. 2014. “Cancer in Africa 2012.” Cancer Epidemilogy Biomarkers & Prevention
23 (6): 953–66. doi:10.1158/1055-9965.EPI-14-0281. [
PubMed: 24700176]
Pirie K, Peto R, Reeves G K, Green J, Beral V. 2013. “The 21st Century Hazards of Smoking and Benefits of Stopping: A Prospective Study of One Million Women in the UK.” The Lancet
381 (9861): 133–41. doi:S0140-6736(12)61720-6 [Pii]10.1016/S0140-6736(12)61720-6. [
PMC free article: PMC3547248] [
PubMed: 23107252]
Pischon T, Lahmann P H, Boeing H, Friedenreich C, Norat T. and others. 2006. “Body Size and Risk of Colon and Rectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC).” Journal of the National Cancer Institute
98 (13): 920–31. doi:98/13/920 [Pii]10.1093/Jnci/Djj246. [
PubMed: 16818856]
Raaschou-Nielsen O, Andersen Z J, Beelen R, Samoli E, Stafoggia M. and others. 2013. “Air Pollution and Lung Cancer Incidence in 17 European Cohorts: Prospective Analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE).” The Lancet Oncology
14 (9): 813–22. doi:S1470-2045(13)70279-1 [Pii]10.1016/S1470-2045(13)70279-1. [
PubMed: 23849838]
Reeves G K, Pirie K, Green J, Bull D, Beral V. 2012. “Comparison of the Effects of Genetic and Environmental Risk Factors on In Situ and Invasive Ductal Breast Cancer.” International Journal of Cancer
131 (4): 930–37. doi:10.1002/Ijc.26460. [
PubMed: 21952983]
Renehan A G, Soerjomataram I, Tyson M, Egger M, Zwahlen M. and others. 2010. “Incident Cancer Burden Attributable to Excess Body Mass Index in 30 European Countries.” International Journal of Cancer
126 (3): 692–702. doi:10.1002/Ijc.24803. [
PubMed: 19645011]
Rosner B, Colditz G A. 1996. “Nurses’ Health Study: Log-Incidence Mathematical Model of Breast Cancer Incidence.” Journal of the National Cancer Institute
88 (6): 359–64. [
PubMed: 8609645]
Sankaranarayanan R, Swaminathan R, Jayant K, Brenner H. 2011. “An Overview of Cancer Survival in Africa, Asia, the Caribbean and Central America: The Case for Investment in Cancer Health Services.” IARC Scientific Publications
162
257–91. [
PubMed: 21675431]
Shield K D, Rylett M, Gmel G, Kehoe-Chan T A, Rehm J. 2013. “Global Alcohol Exposure Estimates by Country, Territory and Region for 2005: A Contribution to the Comparative Risk Assessment for the 2010 Global Burden of Disease Study.” Addiction
108 (5): 912–22. doi:10.1111/Add.12112. [
PubMed: 23347092]
Soerjomataram I, Pukkala E, Brenner H, Coebergh J W. 2008. “On the Avoidability of Breast Cancer in Industrialized Societies: Older Mean Age at First Birth as an Indicator of Excess Breast Cancer Risk.” Breast Cancer Research and Treatment
111 (2): 297–302. doi:10.1007/S10549-007-9778-2. [
PMC free article: PMC2491409] [
PubMed: 17932743]
Stevens G A, Singh G M, Lu Y, Danaei G, Lin J K., and others. 2012. “National, Regional, and Global Trends in Adult Overweight and Obesity Prevalences.” Population Health Metrics
10 (1): 22. doi:1478-7954-10-22 [Pii] 10.1186/1478-7954-10-22. [
PMC free article: PMC3543235] [
PubMed: 23167948]
Stewart B W.
2012. “Priorities for Cancer Prevention: Lifestyle Choices versus Unavoidable Exposures.” The Lancet Oncology
13 (3): E126–33. doi:S1470-2045(11)70221-2 [Pii]10.1016/S1470-2045(11)70221-2. [
PubMed: 22381935]
Talley M B.
2010. Examining the Impact of Development, Tobacco Taxation, and Tobacco Prices on Global Adult Male Smoking Prevalence. Altanta, GA: Georgia Institute of Technology, Georgia State University.
Thun M, Peto R, Boreham J, Lopez A D. 2012. “Stages of the Cigarette Epidemic on Entering Its Second Century.” Tobacco Control
21 (2): 96–101. doi:Tobaccocontrol-2011-050294 [Pii] 10.1136/Tobaccocontrol-2011-050294. [
PubMed: 22345230]
UNDP (United Nations Develpoment Programme). 2009. Human Development Report 2009: Overcoming Barriers: Human Mobility and Development. New York: UNDP.
Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. 2013. “Worldwide Trends in Cervical Cancer Incidence: Impact of Screening against Changes in Disease Risk Factors.” European Journal of Cancer
49 (15): 3262–73. doi:S0959-8049(13)00358-4 [Pii]10.1016/J.Ejca.2013.04.024. [
PubMed: 23751569]
WCRF (World Cancer Research Fund) and AICR (American Institute for Cancer Research). 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: WCRF and AICR.
WHO (World Health Organization). 2008. Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva: WHO.
WHO (World Health Organization). 2011a. Global Status Report on Alcohol and Health. Geneva: WHO.
WHO (World Health Organization). 2011b. Global Status Report on Non-Communicable Diseases 2010. Geneva: WHO.
Wild C P.
2014. “Foreword.” In Planning and Developing Population-Based Cancer Registration in Low- and Middle-Income Settings, edited by Bray F, Znaor A, Cueva P, Korir A, Swaminathan R, editors. and others Lyon, France: IARC. [
PubMed: 33502836]