NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chesnut RM, Carney N, Maynard H, et al. Rehabilitation for Traumatic Brain Injury. Rockville (MD): Agency for Health Care Policy and Research (US); 1999 Feb. (Evidence Reports/Technology Assessments, No. 2.)
This publication is provided for historical reference only and the information may be out of date.
An estimated 4.5 million people in the United States are disabled as a result of traumatic brain injury (Centers for Disease Control and Prevention [CDC], 1998). Advances in medical technology and improvements in regional trauma services have increased the number of survivors1 of traumatic brain injury (TBI), producing the social consequences and medical challenges of a growing pool of people with disabilities (Annoni, Beer, and Kesselring, 1992; Ewing, Thomas, Sansces, et al., 1983). Lifelong disability is the consequence for 80,000 to 90,000 individuals each year (CDC, 1998). As a result, answers to questions about recovery are being pursued through a multitude of research projects by various communities with distinct objectives.
Wider awareness of the scope of the problem and its consequences for society has led to rapid growth in the rehabilitation industry. Because of this growth, and particularly because clinical rehabilitation strategies vary widely, many groups are interested in the effectiveness of rehabilitation for TBI.
Rehabilitation experts have recognized the inadequacy of applying traditional models, effective with broken arms or legs, to the task of recovery from brain injury and cerebral tissue damage.
Payers have begun to recognize the inadequacy of current standards for funding rehabilitation from brain injury; they want to know what long-term outpatient programs are most likely to return a person to functional independence; when specific types of rehabilitation should start and when they should end; and which components of complex, multidisciplinary rehabilitation programs are effective.
Congress has raised questions about unmet needs for rehabilitation services, the adequacy of care in existing facilities, and the relative costs and effectiveness of the wide variety of rehabilitation services offered to survivors of TBI.
A strong and growing advocacy movement of survivors of TBI and their families has a research agenda that includes defining recovery and functional status in terms of quality of life as well as financial independence.
Three questions about the status of brain injury research underlie uncertainty about the effectiveness of rehabilitation services. First, how should fundamental concepts such as recovery, functional status, and disability be defined? Because brain function is highly complex, TBI has an extremely wide range of potential outcomes, including, for example, cognitive deficits, motor disabilities, changes in emotional and social function, personality changes, and changes in appearance. As a result, therapeutic aims and perspectives vary widely among studies, as do definitions of outcomes, making valid comparisons across studies difficult.
Second, how should the type and severity of the injury itself be measured? Variation in methods to assess the severity of injury in people entering rehabilitation make it difficult to estimate the effectiveness of different methods of rehabilitation.
Third, which therapies are effective, and how can patients best be matched to treatment approaches likely to be effective for them? Today, a person's path to a rehabilitation program after sustaining brain injury may be determined by the mechanism of injury, the resources of the community, the person's employment or financial status, the consent of the family, and/or the accuracy of emergency department diagnosis. While a few metropolitan areas have organized referral systems that connect people with TBI with resources and rehabilitation programs, systematic methods for evaluating the needs of those who have sustained brain injury and referring them to appropriate programs are unusual. Without knowing the efficacy of rehabilitation methods in their specific applications, systematic referral that produces the desired result is not possible.
Another major theme in the literature and in public discourse concerns the costs associated with traumatic brain injury and the cost-effectiveness of its treatment. The clinical economic problem posed by people with TBI is how much to invest in their rehabilitation after it is clear they will survive their injuries. Our ability to maximize the return on this investment is limited by a lack of accurate information about the costs of TBI and the costs and benefits associated with various treatments.
Given the current interest in the effectiveness of rehabilitation for TBI, the Agency for Health Care Policy and Research (AHCPR) selected this topic as one to be investigated by an Evidence-based Practice Center (EPC). AHCPR selected Oregon Health Sciences University (OHSU) to produce the evidence report. The OHSU EPC found a partner in the planning committee for the Consensus Development Conference on Rehabilitation of Persons with Traumatic Brain Injury, sponsored by the National Institutes of Health and held in October 1998. In addition, the Brain Injury Association, Inc., an organization with a mission to support research leading to better outcomes for people who sustain a brain injury, indicated their willingness to serve as a partner with the OHSU EPC in the development of this evidence report.
In this report, we examine available evidence about the effectiveness of rehabilitation for adult survivors of TBI. Specifically, we report the results of a systematic effort to identify the best available evidence about the various strategies to improve the outcome of traumatic brain injury in the most common rehabilitation settings. The main attribute of a systematic review is the application of methods designed to avoid the biases inherent in less formal approaches to reviewing the literature. For example, to avoid bias in the identification and selection of articles, a systematic review uses predefined search strategies and explicit criteria for inclusion or exclusion of studies. This approach can uncover published evidence that might be ignored in an informal review in which studies that are widely known or that support a particular viewpoint are more likely to be identified. Similarly, a systematic review applies methods to reduce bias in interpreting studies, such as review by more than one investigator and the use of a data abstraction form.
In addition to being systematic, we employed methods to assess the methodologic strength of individual studies and the strength of evidence supporting assertions about the effectiveness of interventions. The strongest evidence for effectiveness comes from experimental studies, in which subjects are randomly assigned to alternative interventions. In many cases, inferences about effectiveness are drawn from the results of uncontrolled, or poorly controlled, cohort studies. In these observational studies, a group of subjects is followed over time. Such studies are particularly useful for describing the incidence of certain outcomes over time and for analyzing the relationship between risk factors and those outcomes. However, observational studies often provide weak or flawed evidence about effectiveness, because it is not clear if the observed outcomes resulted from specific interventions or if they would have occurred anyway in the absence of the intervention.
How should the information in this report be used? Our main goal is to provide a guide to the strengths and limitations of the evidence about these interventions that organizations can use to develop evidence-based practice guidelines and other tools for rehabilitation. Another goal is to identify information gaps and controversies that can be addressed in future research studies. A finding that a particular treatment is proven effective or proven ineffective may dominate a discussion about what should be done. Most findings, however, are in between--i.e., "not proven" rather than "proven not." In these situations, factors other than the strength of evidence should be considered in deciding on a clinical recommendation. Patient and societal preferences and values such as equity, attitudes about risk, and evidence about the relative benefits and harms should be considered in making a recommendation about practice.
The Course and Lifetime Burden of TBI
Incidence and Costs of TBI
TBI is the leading cause of death and disability among children and young adults in the United States (CDC, 1998). An estimated 56,000 lives are lost in the United States each year to TBI (Kraus and McArthur, 1996). Motor vehicle accidents, followed by injuries due to firearms and falls, are the leading causes of death from TBI (Sosin, Sniezek, and Waxweiler, 1995). Males are 3.4 times as likely as females to die of TBI (National Institute of Neurological Disorders and Stroke). About 50 percent of people who sustain TBI are intoxicated at the time of injury (Ruff, Marshall, Klauber, et al., 1990; Kreutzer, Doherty, Harris, et al., 1990).
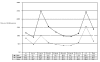
In a recent analysis based on hospital discharge data and vital statistics, the annual incidence of TBI in the United States was estimated as 102.8 per 100,000 (CDC, 1997). Figure 1 shows TBI incidence rates by age group and sex. In males, the incidence peaks between the ages of 15 and 24 (248.3 per 100,000) and again above 75 years of age (243.4 per 100,000). The incidence in females peaks in the same age range, but the absolute rates are lower (101.6 and 154.9, respectively). These rates may underestimate the true incidence of head trauma because people with milder symptoms at the time of injury are usually not hospitalized.
Figure
Figure 1. Incidence of traumatic brain injury per 100,000 U.S. population. Source: MMWR - Morbidity and Mortality Weekly Report. 46(1):8-11, 1997 Jan 10.
About three-quarters of traumatic brain injuries that require hospitalization are nonfatal. Each year, about 80,000 survivors of TBI will incur some disability or require increased medical care (Kraus and Sorenson, 1994). From an economic viewpoint, the problem posed by survivors of TBI is how much to invest in their rehabilitation after it becomes clear they will survive. The type, intensity, and duration of rehabilitation services affect the total economic impact associated with TBI. For example, investing in rehabilitation services to help a survivor become independent or return to work may reduce the lifetime economic burden of illness. To date, no study has comprehensively examined the lifetime costs of TBI. As shown in Table 1, the total economic impact includes many types of costs besides direct medical costs. A starting point for estimating these costs would be information about the prevalence of chronic TBI in the general population, but such data are lacking.
Table
Table 1. Direct medical care and rehabilitation costs.
Although it is difficult to precisely quantify costs, useful information can be gleaned by studying charge data from published studies in various settings. The total cost of traumatic brain injuries in the United States is estimated to be $48.3 billion annually. Hospitalization accounts for an estimated $31.7 billion, whereas fatal brain injuries cost the Nation approximately $16.6 billion (Lewin, 1992).
In recent years, LOS and inpatient costs for rehabilitation have decreased. In the years 1988-1992, reports of average LOS for the initial admission for inpatient rehabilitation range from 40 to 165 days (Blackerby, 1990; Carey, Seibert, and Posavac, 1988; Giacino, Kezmarsky, DeLuca, et al., 1991; Mackay, Bernstein, Chapman, et al., 1992; McMordie and Barker, 1988; Rappaport, Herrero-Backe, Rappaport, et al., 1989). In one older multicenter study (the Model Systems study), the average rehabilitation length of stay (LOS) was 61 days, and the average charge was $64,648 exclusive of physician fees. Total charges averaged $154,256 (Lehmkulh, Hall, Mann, et al., 1993). In more recent studies performed in the early 1990s, rehabilitation LOS and charges were lower, ranging from 19 days and $24,000 for patients with milder injuries to 27 days and $38,000 for those with severe injuries (Cowen, Meythaler, DeVivo, et al., 1995). In the Medicare population in 1994, mean charges for patients admitted for brain injury (excluding stroke) were $42,056 (Chan, Koepsell, Deyo, et al., 1997).
Conceptual Model of the Lifetime Burden of Illness
To focus attention on important questions, we characterized the life of a person with TBI in terms of five phases, as presented in Figure 2. The first phase is "pre-injury." "Medical treatment" is divided into the acute (or immediate) treatment phase, and the intensive treatment phase, lasting days to weeks. The "rehabilitation" phase may last months to years. The "survivor" phase signifies the remaining life of the person with TBI and involves continual development and adjustment. This division into phases clarifies the three challenges to assessing the efficacy of rehabilitation discussed above. Patient populations are defined generally in terms of the phases, and interventions and outcomes must be specific to the phase being evaluated.

Figure
Figure 2. The career of a TBI survivor.
Mechanisms of Brain Injury
In TBI, the disease process begins at the moment of impact and extends thereafter for a protracted period of time. In strictly biological aspects, the brain injury disease process can be divided into primary and secondary insults. The primary insult initiates with the physical trauma to the brain. The secondary insults occur thereafter and, in many cases, are the primary determinants of outcome.
Beginning at impact and continuing for a generally brief period, primary insults to the brain include formation of intracranial hematomas (subdural or epidural), intracerebral hematoma, cerebral parenchymal contusions, cerebral swelling, and/or diffuse axonal injury. Intracranial hematomas such as subdurals and epidurals occur outside of the brain parenchyma and exert much of their pathological effects via increasing pressure on the brain (elevation of intracranial pressure [ICP]). Particularly with respect to epidural hematomas, if this pressure is avoided or rapidly reversed through expedient surgery, there may be no damage to the underlying brain and no residual effects of the hematoma. When there is a period of protracted intracranial hypertension, the ICP elevation may result in herniation of the brain tissue through orifices of the skull and/or cerebral ischemia due to interference with cerebral blood flow. Depending on magnitude and duration, such insults can produce deficits that vary from subtle to mortal.
Subdural hematomas also can cause significant intracranial hypertension and have a high likelihood of damaging underlying brain. This damage is often the primary determinant of long-term recovery.
Similar concerns about ICP are also relevant to cerebral swelling. Intracranial processes such as increased extracellular or intracellular fluid or elevated intracranial blood volume can raise ICP and impair adequate blood flow to the brain. In addition, some of these processes can cause primary cellular damage or ischemia by interfering with oxygen transport between vessels and cells.
Injuries such as intracerebral hematomas and cerebral contusions result in blood intermixed with brain. In the case of contusions, the pathology includes primary damage to neuronal cells. In both instances, the presence of blood within brain tissue appears to have significant toxic effects which can produce profound secondary insults to the injured tissue.
Diffuse axonal injury is a somewhat different disease entity. In this injury type, the physical forces the brain sustains during an injury characteristically consist of linear as well as angular acceleration or deceleration. These forces disrupt axons within the cerebral white matter, and these are called shear injuries. In this injury type, there may be no herniation or intracranial hypertension and, indeed, computer tomographic (CT) studies often may be normal or remarkably benign in appearance. Unfortunately, the widespread damage to the white matter will produce a recovery that is characteristically slow and incomplete.
Primary brain injuries occur at the time of injury and, by definition, must be treated post hoc. Secondary brain injuries are initiated sometime after injury and are often due to systemic factors. Their generally delayed onset allows them to be treated when they occur and also to be forestalled. This aspect of prevention has stimulated many of our trauma protocols and drives significant ongoing pharmaceutical research efforts.
The best known and possibly most devastating secondary brain insult is that of cerebral ischemia due to systemic hypotension. Systemic hypotension is extremely common, occurring in about one-third of patients with TBI during the period of injury through the end of resuscitation. A single hypotensive episode is associated with a doubling of mortality (Chesnut, Marshall, Klauber et al., 1993). Systemic hypotension illustrates the profound influence secondary brain insults can have on recovery and strongly supports the potential benefits of optimal trauma care.
Secondary brain insults may be due to intracranial processes that are initiated by:
- Primary brain injury.
- Secondary systemic insults such as hypoxia or hypotension.
- Injuries to extracranial organ systems.
- Systemic complications directly or indirectly related to the initial traumatic incident.
Secondary brain insults arising from the initial trauma include a number of toxic cellular and subcellular biologic processes, including:
- Cerebral edema.
- Alterations in intracranial ionic homeostasis (e.g., calcium, chloride).
- Free radical formation.
- Alterations at the molecular biology level.
These processes may result in ongoing and self-perpetuating brain injury. Cerebral edema will interfere with transport of nutrients, oxygen, and waste products and cause injuries to cells surrounding the area of primary injury as well as impair the healing of the initially damaged cells. Alterations in ionic homeostasis will disrupt the transmission properties of the neuronal and neighboring cells. Free radical formation can initiate a self-perpetuating cascade of toxic elements that may damage cells initially untouched by the injury. Finally, alterations at the molecular biology level will interfere with the primary genetic cellular control processes.
Secondary brain insults due to hypoxia or hypotension have become very well recognized over the past two decades and are a primary target of resuscitation protocols. The magnitude of importance of these insults is illustrated by the data on hypotension. Because we now have several tools to effectively recognize and treat such insults that are so devastating, intense interest is currently focused on managing them. Secondary insults also may occur from systemic trauma to extracranial organ systems. Although much of the influence of such extracranial trauma is mediated through hypoxia or hypotension, some aspects are unique to the individual organ system. For instance, long bone fractures may produce fat emboli to cerebral vessels that interfere with cerebral circulation. Alterations in timing and techniques of managing such fractures may improve cerebral outcome by decreasing the risk of embolism.
Finally, cerebral injury can be influenced by systemic complications occurring during the acute phase of management. The most well established of these complications as determinants of outcome include hypotension, pneumonia, sepsis, and coagulopathy. The importance of such factors in determining outcome supports the necessity of excellent critical care in managing patients with TBI.
The biologic aspects of repair or recovery are of primary importance during the early postinjury period, while the cognitive, psychological, and behavioral issues become increasingly predominant over time. The primary processes involved in biologic neuronal recovery are (1) reparative and (2) adaptive or plastic processes. Scientists believe that, except under extremely unusual circumstances, new neurons are not formed in the mature human brain, and thus when a neuron dies, its contribution to brain function is irreparably lost. Since neuronal death apparently can be induced not only by direct trauma but also by traumatic activation of inherent biological processes of programmed cell death (apoptosis), the question of cessation or reversal of such processes is an active focus of research.
Neurons have somewhat limited capabilities of self-repair, but the physiologic underpinnings of this regeneration are not completely understood. Although some clinical evidence suggests that neuronal repair can be facilitated pharmacologically in the injured spinal cord, no clinical evidence suggests that we can favorably influence such processes in the brain.
Research in animals suggests that there are critical periods during recovery wherein interventions may be particularly beneficial. The existence of similar periods has been postulated for the injured human brain, both for biological and behavioral interventions. To date, however, no clinical evidence supports the existence of such critical periods in the human.
Another biologic recovery mechanism is adaptation. In a biologic sense, this involves the formation of new circuitry to replace that lost from trauma. There is a great deal of plasticity in the nervous system, some of which continues throughout the organism's life--for example, learning of new information. At the more macroscopic level of altering neuronal circuits to directly replace those lost, however, abilities seem to be significantly limited in the adult human brain. In the immature nervous system, such plasticity is commonplace. A primary example is the alteration of laterality of language function resulting from very early injury to the left cerebral hemisphere. Unfortunately, the ability to make adaptations of such magnitude is lost early in life and the degree to which plasticity of lesser magnitude persists or can be induced in a more mature brain remains unclear. The question of such plasticity is fundamental to the concept of restorative cognitive therapy as well as to the concept of critical periods.
Social, Behavioral, and Emotional Factors in Recovery from TBI
Neither traumatic brain injury nor its recovery can be described in purely biologic terms. The sudden onset of TBI combines with extreme changes in behavior, personality, memory, and general function to produce a catastrophic perturbation in a person's social system (Goldstein,1995; Johnston and Hall, 1994). Memory deficits and inappropriate behavior limit the ability to return to work (Treadwell and Page, 1996). Personality changes and behavioral problems mimic other pathologies such as mental retardation or psychiatric disorders. These behaviors in turn elicit negative reactions from family and friends that operate to impede the recovery process. Long-term consequences include financial dependence, social isolation (Dikmen, Machamer, and Temkin, 1993), divorce (Lezak, 1995), and various forms of incarceration such as lockup care facilities, State hospitals, or prisons.
The recovery course is partially determined by the presence or absence of factors in the survivor's social context (Goldstein, 1995). Size and strength of immediate and extended family (Kozloff, 1987), access to social services, and adequate resources (both money and programs) (Johnston and Hall, 1994) contribute to the recovery process. Aspects of the survivor's psychology, such as premorbid modes of behavior, may persist after injury (Dikmen, Machamer, and Temkin, 1993), influencing how the survivor engages in the present task of recovery. Drug or alcohol abuse (Sparadeo, Strauss, and Barth, 1990) may provide an alternative to the discomfort of persistent disorientation, confusion, and physical pain.
During the initial phase of recovery, the patient will manifest behaviors that are an immediate attempt to become oriented (Goldstein, 1995). These behaviors are often inappropriate to the context, unexpected, and may appear to be maladaptive. They are a consequence of the patient's sudden reduction in perception of the environment and ability to respond effectively to stimuli. This phase is of primary importance in rehabilitation, in that the milieu must be designed to accommodate the behaviors and not impede them. Attempts to suppress these behaviors may operate to slow the recovery process and keep a patient from engaging a new orientation. However, resistance to odd behaviors is usually a natural response from family and friends. The newly injured patient will often focus on the familiar, usually a family member, to achieve orientation; training the family to respond appropriately is an important component in establishing a context in which the patient can recover (Rosenthal and Young, 1988).
Chronic Complications of TBI
TBI can cause severe cognitive, physical, and psychosocial/behavioral/ emotional dysfunction. The most important cognitive sequelae are memory loss; difficulties with concentration, judgment, communication and planning; and spatial disorientation. Physical problems include abnormalities of muscle tone, vision, hearing, smell, taste, and speech, as well as reduced endurance, headaches, and seizures. Frequently encountered psychosocial/behavioral/emotional problems arising from TBI include anxiety and depression, mood swings, denial, sexual difficulties, emotional lability, egocentricity, impulsiveness and disinhibition, agitation, and isolation. A recent review examined papers describing the psychosocial and emotional sequelae for survivors of TBI (Morton and Wehman, 1995). The results of those studies demonstrated that survivors of severe TBI often lose friendships and social support, have limited opportunities to develop new social contacts and friends, have few leisure activities, and have high levels of anxiety and depression for prolonged periods of time. In addition to the psychosocial problems described above, categories of functional status used to describe outcomes from TBI include memory, mobility and independence, organization and productivity, physical disabilities, and inappropriate behavior.
Memory
Brain injury can cause deficits in memory that range from mild, intermittent forgetfulness to profound inability to recall anything from the past or to integrate new information. Cognitive scientists and clinicians have made distinctions in mechanisms of memory that reflect modes of memory loss. Implicit memory records information that occurs nonconsciously; explicit memory is a function of active work such as repetition. Some brain injury depletes implicit memory but not explicit or vice versa. Semantic memory allows for understanding of the meaning of words; episodic memory records time- and place-specific experiences. Procedural memory is reflected in behavioral routines; declarative memory is reflected in the ability to explicitly report.
The burden of illness with respect to memory loss depends on the scope and degree of deficit and is also context-specific. For example, loss of procedural memory may result in devastating occupational consequences to a person whose work tasks are routine. However, if that is the only affected mechanism, other intact modes of memory may substitute for the deficit, and the person may be able to learn a new skill and regain independence.
The inability to integrate new information can result in global loss of independence, especially when accompanied by intact premorbid memory. The individual clearly remembers profession, life circumstances, and family from before the injury but nothing thereafter. Because they do not remember, they do not know that they do not remember, which leads them to insist on a daily and sometimes hourly basis that they are who they used to be. A reminder of the injury may last a minute or a day but will fade with other postmorbid information. These people cannot work, and the burden of their illness is evidenced in the consequences to family and caregivers.
Mobility and Independence
Limitations in mobility and independence may result in the inability to drive or ride a bus, work and earn a living, balance a checkbook, or prepare meals. Mobility also will be affected by physical impairment and independence by degree of memory deficit. The burden is also affected by the individual's social milieu. For example, for one person the presence of family to facilitate mobility may mediate the impact of the trauma; for someone else, dependence on family may be abhorred and may compound the burden of illness.
Organization and Productivity
Many survivors of brain injury exhibit an obsession for orderliness. Some are capable of servicing the obsession to varying degrees. For example, one person's home may be cluttered and disorganized, with one room (a computer room or tool shop) in meticulous order. The burden of illness can be observed in the amount of time and energy devoted to the orderly space and the confusion and lack of productivity experienced in the disorderly environments. Often, on a daily basis, a person never disconnects from the obsessional tasks in order to engage in other productive activities.
Physical Limitations
Physical deficits include difficulty with ambulation, hearing, vision, speaking, fatigue, and use of the hands. Such deficits may result from injuries sustained at the same time as, but distinct from, the head injury, or they may result directly from brain and spinal column nerve damage.
Inappropriate Behavior
People with traumatic brain injury often lose the ability to monitor and control behavior (Lezak, 1995). They may say whatever comes to their minds, even at inappropriate times. They may misunderstand the meaning of a situation or conversation and respond according to their misunderstanding. This problem can have profound effects on a person's life, resulting in loss of work, friends, and family.
Interventions
Rehabilitation methods differ in setting, level, and range of provided therapeutic interventions, durations of treatment, and overall expenses. Lack of standard classification and different aims of therapies are problems in evaluating rehabilitation as an intervention. Three issues complicate classification of interventions:
- 1.
General versus specific modes of classification. A very general mix of therapies constitutes the protocol of some rehabilitation programs. In contrast, other programs use intricate evaluations to identify deficits and then design interventions specific only to those deficits.
- 2.
Discipline-driven classification. Another approach to classification of intervention for TBI is to stratify according to the discipline that generates the treatment, such as cognitive rehabilitation, occupational therapy, or physical therapy.
- 3.
Comparison of intervention categories. In evaluating the effectiveness of a treatment, with what should the treatment be compared? Should inpatient rehabilitation be compared with outpatient, vocational with cognitive? Or should rehabilitation be compared with no rehabilitation?
The specific aims of therapy also vary widely, encompassing interventions aimed at an extremely wide range of potential outcomes, including, for example, cognitive deficits, motor disabilities, changes in emotional and social function, personality changes, and changes in appearance. As a result, therapeutic aims and perspectives vary widely among studies, as do definitions of outcome, making valid comparisons across studies difficult.
Another problem is variation in practice. Allocation of interventions appears to be arbitrary and not necessarily dictated by established standards of practice. A substantial minority (30 to 40 percent) of severely injured patients do not enter rehabilitation, while about 30 percent with mild head injury do. In one study (Dombovy and Olek, 1997), of 48 patients assessed 6 months after discharge from acute care, only 8 had received any postacute rehabilitation.
On a population basis, only a selected subset of patients with TBI undergo inpatient rehabilitation after discharge from the acute care hospital (Wrigley, Yoels, Webb, et al., 1994). Patients seen by a physiatrist during the acute hospitalization were more likely to be provided postacute rehabilitation. The presence or absence of a physiatrist at the hospital was a stronger determinant of referral to inpatient rehabilitation than clinical factors and patient characteristics that would seem to be reasonable criteria for referral (Wrigley, Yoels, Webb, et al., 1994).
In Table 2, interventions are mapped onto the phases from Figure 2 to illustrate which practice settings and techniques are most relevant to each specific phase of recovery.
Table
Table 2. Distribution of practice settings and techniques in TBI treatment phases.
Practice Settings
The following are practice settings. Although they are presented separately here, they often overlap to varying degrees.
- (Coma) treatment centers--A small number of people with head injuries will remain in a minimally responsive state for months or longer after injury. A few centers will accept such individuals once they are medically stable and attempt to achieve improvement by the use of various stimulation techniques. Skilled nursing care and physical therapy are also important elements of these programs.
- Acute rehabilitation programs are prepared to treat patients as soon as they are medically stable and are discharged from the acute hospital. Most of these are located in rehabilitation hospitals. Their primary emphasis is to provide intensive physical and mental restorative services in the early months after injury. Many will have specialized head injury units with an interdisciplinary team composed of physicians, nurses, speech and occupational therapists, and neuropsychologists. These programs are relatively short term, but longer stays may occur.
- Long-term rehabilitation programs provide extended rehabilitation and management services. They may provide a full range of rehabilitation services for the person with brain injury who is in need of a structured environment and is making slow improvements. Such programs generally are not for permanent placements, although they may have this service available. Usually a person may remain in the program as long as there continues to be some improvement.
- Transitional living programs--The goal of a transitional program is to prepare individuals for maximum independence, teach the skills necessary for community interaction, and work on prevocational and vocational training. Specialized programs stressing cognitive, memory, speech, and behavioral therapies are usually structured to the needs of the individual. Programs of this type are being established in a variety of settings--small group homes, special educational institutions--as part of a continuum of care in rehabilitation centers.
- Behavior management programs--Severe maladaptive or aggressive behavior will limit an individual's participation in most rehabilitation settings. While these programs treat the common behavioral problems following head injury, many of them cannot handle destructive behavior to self or others (e.g., sexual aggression).
- Day treatment programs are nonresidential facilities that emphasize services to upgrade functional skills. These services are similar to those described above under transitional living programs. Some offer day-care (supervision) services for those unable to benefit from an active program.
- Extended intensive rehabilitation--the more seriously injured person may require extended therapies in a structured program that has all the elements found in the acute rehabilitation center. Emphasis will usually be on cognitive and memory retraining, speech therapy, activities of daily living (ADLs), restructuring lost social behaviors, and continuing physical therapy. Prevocational and vocational training, recreational therapy, and community reentry are usually part of each program. Patients will remain in these programs as long as progress is being made--usually 6 to 12 months.
- Late rehabilitation--After discharge from the acute rehabilitation center, many people with head injury will need extended rehabilitation either in a residential or inpatient setting or in an outpatient program. Admission requirements may vary and be defined by a specific time after head injury.
- Independent living programs (ILPs) are community-based services that assist people with severe disabilities living in their own homes to increase personal self-determination and independence. ILPs provide both direct and indirect services ranging from residential/transitional programs to resource referral.
- Life-long residential programs--For those individuals unable to live at home or independently, a residential program may be the only alternative. There are very few programs of this type specifically set up for people with head injuries. Some facilities that have had experience with other disabled populations are beginning to explore this possibility.
- The home--For some, the home environment provides the most productive setting for therapy. In addition to in-home nursing care, rehabilitation professionals may come to the client's home on a routine basis to conduct therapy sessions.
- TBI social clubs--Although not necessarily a setting for formal rehabilitation, social clubs provide an environment in which people with TBI can associate with each other, form friendships, and instruct each other in how to manage life with a disability.
Techniques
Therapies discussed here may be provided on an individual basis or in group settings.
- Physical therapy comprises treatment designed to restore normal physical function: walking, use of hands, use of arms, and so forth. A physiatrist may specify the course of treatment, integrating physical therapy with other programs.
- Therapeutic recreation focuses on resuming leisure activities, community skills, and social skills.
- Speech and language therapy--Language disruption is common with TBI and is specifically addressed in speech therapy. Speech therapy encompasses relearning appropriate methods of communication, verbal and nonverbal, as well as relearning communication of abstract thought.
- Cognitive therapy offers retraining in the ability to think, use judgment, and make decisions. The focus is on correcting deficits in memory, concentration and attention, perception, learning, planning, sequencing, and judgment. A neuropsychologist, aided by other specialists (for example, occupational therapists [OTs], speech and language pathologists [SLPs]) may be asked to evaluate the level and kind of cognitive dysfunction following TBI, and they may reassess the individual over time to measure recovery.
- Occupational therapy offers retraining to enable the person with TBI to cope with the routine demands of a work environment. Often the occupational tasks are at a level below that of preinjury status.
- Behavioral therapy involves modification of maladjusted, asocial, or socially inappropriate behaviors.
- Psychotherapy targets emotional issues, social adaptation, and self-awareness. Group psychotherapy is useful for feedback, support, and confrontation by peers. Family members may participate in therapy to help them cope with the stress of being a caregiver and to build their ability to provide appropriate in-home support.
- Social skills training may be provided as a separate program, or it may be integrated into any of the methods described above.
Measures of Injury Severity and Disability
Table 3 lists measures that are commonly used to assess or predict the outcomes of TBI. As shown in the table, the choice of outcome measure or predictor depends on the individual's phase of treatment and recovery.
Table
Table 3. Use of measures of injury and disability in phases of recovery from TBI.
The validation of TBI rehabilitation systems and the study of neurobehavioral outcomes measurement are evolving. Currently there is no consensus on which measures of outcome should be used to assess long-term recovery.
The Glasgow Outcomes Scale (GOS) has commonly been used in the acute care literature to measure outcomes from TBI. It is widely felt to be too simple to be useful as a single indicator of outcome. The GOS (Jennett and Bond, 1975; Jennett, Snoek, Bond, et al., 1981) may be used to rate outcome during any phase of recovery and is often a part of a patient's acute hospital record (Marshall, Bowers-Marshall, Klauber, et al., 1991; Braakman, Gelpke, Habbema, et al., 1980; Narayan, Anderson, et al., 1988). It has five levels: (1) death, (2) persistent vegetative state (absence of cortical function), (3) severe disability (conscious but disabled), (4) moderate disability (disabled but independent), and (5) good recovery (resumption of "normal life"). Many studies group the various levels into poor outcome (GOS 1-3) or good outcome (GOS 4 or 5). The simplicity of the scale does not lend itself to accurate prediction of future performance, particularly for people categorized as moderately disabled (Brooks, Campsie, Symington, et al., 1986).
The Rancho Los Amigos Scale (RLA) (Hagen, 1979) is also used by acute care staff to categorize a patient's status and determine placement at discharge. It consists of eight levels of cognitive functioning: (1) no response, (2) generalized response, (3) localized response, (4) confused-agitated, (5) confused-inappropriate, (6) confused-appropriate, (7) automatic-appropriate, and (8) purposeful-appropriate.
The Functional Independence Measure (FIM) is an 18-item scale that evaluates self-care, sphincter control, mobility, communication, psychosocial Choi, adjustment, and cognitive function (Granger, Hamilton, and Sherwin, 1986). Because it serves as the outcome measure in the Uniform Data System for Medical Rehabilitation, it has been applied by inpatient rehabilitation programs to a large population of patients with diverse problems, including TBI (Guide for the Uniform Data System for Medical Rehabilitation [Adult FIM], 1993; Fiedler and Granger, 1997).
The FIM is considered to be the best single outcome scale for use during inpatient rehabilitation. However, a high score on the FIM does not necessarily mean a return to full function. For example, by FIM scores, about one-quarter of people with TBI are independent at the time of discharge from inpatient rehabilitation, and one-half are independent by 1 year after injury. Followup studies, however, suggest that people with moderate or severe traumatic brain injury are unlikely to return to competitive employment (High, Boake, and Lehmkuhl, 1995).
Attempts to circumvent this "ceiling effect" by adding more cognitive/psycho-social information to the scale (FIM + FAM [functional adaptability measure]) have met with limited success (Hall, Mann, High, et al., 1996). Judging by the FIM or FIM + FAM scores, little progress in recovery is realized after the first year postinjury. However, without evidence of such a plateau in the recovery process, the utility of these scales for long-term followup is in question. This limitation of the FIM (or FIM + FAM) is not surprising, since these indexes were designed to describe progress during rehabilitation and not functional status after discharge. In fact, therapists sometime use the attainment of threshold performance on items that are contained within the FIM as criteria for discharge.
The Disability Rating Scale (DRS) was developed to improve on the GOS as a global disability outcome tool (Rappaport, 1982). Questions on the DRS span the recovery phases, so the instrument can be used beginning in acute care through outpatient rehabilitation to track individual progress. It has been shown to have validity, high reliability, and good utility (Eliason and Topp, 1984; Hall, Cope, and Rappaport, 1985; Fryer and Haffer, 1987; Gouvier, Blanton, LaPorte, et al., 1987). The DRS also has been shown to be a good predictor of employment (Rappaport, Herrero-Backe, Rappaport, et al., 1989; Rao and Kilgore, 1992; Cope, Cole, Hall, et al., 1991) and to interact with measures of severity of injury (Thatcher, Cantor, McAlister, et al., 1991). The DRS appears to have less ceiling effect than the FIM or FIM + FAM (Hall, Mann, High, et al., 1996) but probably is not as useful as the FIM for inpatient assessment.
High scores on functional measures do not necessarily predict a successful return to the community. In response, tools to evaluate reintegration have been developed. The Community Integration Questionnaire (CIQ) is a 15-item survey that evaluates home integration, social integration, and integration into productive activities. The questions are about practical, everyday tasks that are markers of independence, such as shopping, managing finances, meal preparation, and pursuing leisure time activities. Another scale used to evaluate deficits that may impede reintegration is the Portland Adaptability Inventory (PAI) (Lezak, 1987), a set of three scales that measure temperament and emotionality, activities and social behavior, and physical capabilities.
How well do these measures predict what happens after discharge from the rehabilitation unit? One study compared estimated working capacity as evaluated at discharge with actual employment at 6 months followup (N = 147) (McLaughlin and Peters, 1993). As measured by assessment at the time of discharge, 11 percent were classified as unemployed; the actual outcome at 6 months was 39 percent. Other studies (McLaughlin and Peters, 1993; Najenson, Groswasser, Mendelson, et al., 1980; Olver, Ponsford, and Curran, 1996) found that some survivors of TBI regress as a function of their transition from one phase of treatment to the next. These observations suggest that measures taken at the time of discharge from the inpatient unit may not be valid shortly thereafter. Repeating the FIM or other standard measures some time after discharge from the inpatient rehabilitation facility might improve prediction and counteract the "ceiling effect" described earlier.
Because standard measures may fail to predict outcome for a large cross-section of survivors (Sbordone, Liter, and Pettler-Jennings, 1995), many clinics and rehabilitation programs have developed their own instruments for tracking patient progress. These instruments may be useful within their specific milieu, but their use hinders comparisons among centers and among published studies.
Long-Term Outcomes of Traumatic Brain Injury
Only a few population-based studies have been done to examine the long-term outcomes of individuals who survive traumatic brain injuries (Dawson and Chipman, 1995; Edna and Cappelen, 1987; Pentland and Miller, 1986; van Balen, Mulder, and Keyser, 1996). In a Canadian study of survivors 13 years after injury, 66 percent reported the need for ongoing assistance with some ADLs, 75 percent were not working, and 90 percent reported some limitations or dissatisfaction with their social integration (Dawson and Chipman, 1995). In a study performed in the Netherlands, 67 percent of the population with major TBI had long-term situational, cognitive, and behavioral disabilities, and only 10 percent received any rehabilitation services after the acute-care period (van Balen, Mulder, and Keyser, 1996).
Comparable population-based information from the United States is sparse. In a study of 520 Vietnam War veterans 15 years after surviving penetrating head trauma, 56 percent were employed (Kraft, Schwab, Salazar, et al., 1993; Schwab, Grafman, Salazar, et al., 1993). A registry study, the Traumatic Coma Data Bank, found that two-thirds of survivors who were employed or in school before their injury returned to work within 1 year of injury (Ruff, Marshall, Crouch, et al., 1993). A few small U.S. studies have used acute hospital discharge abstracts to identify patients with TBI. In one of these, one-third of patients were cognitively impaired and 60 percent were unemployed 6 months after discharge. Only 8 of the 48 located at followup received any rehabilitation services (Dombovy and Olek, 1997). In a study of 31 patients identified from discharge records of acute care hospitals and surveyed after 2 years, many people with moderate-to-severe head injuries remained unable to work, support themselves financially, live independently, or participate in preinjury leisure activities (Dikmen, Machamer, and Temkin, 1993).
Rehabilitation programs designed for populations of TBI survivors with all levels of deficit can achieve about 50 percent employment (Ben-Yishay, Silver, Plasetsky, et al., 1987; Prigatano, 1986). The main trends of employment postinjury are summarized by Wehman, Sherron, Kregel, and others (1993) as follows: (a) unemployment rates soar in survivors of TBI postinjury; (b) unemployment stays at very high rates of 50 to 80 percent for long periods of time, even when vocational rehabilitation services are provided; (c) wages are greatly reduced from preinjury levels; and (d) there is high job turnover among survivors postinjury.
Most information about the long-term outcomes of people with traumatic brain injury comes from followup studies of patients who underwent inpatient rehabilitation. While they are useful in understanding what to expect from inpatient rehabilitation in the long run, these studies are of limited usefulness in estimating the long-term burden of TBI in the general population for two reasons.
First, these studies exclude the large numbers of survivors who do not undergo acute inpatient rehabilitation. As noted above, patients with mild or severe injuries who enter inpatient rehabilitation units are not necessarily representative of patients generally.
Second, because survivors of TBI who have a poor outcome are relatively difficult to follow up over time, the studies may overestimate the likelihood of a good outcome. In one study, for example, the investigators vigorously tried to contact 88 people who had TBI 1 year after they were discharged from acute rehabilitation (Corrigan, Bogner, Mysiw, et al., 1997); 34 (38.6 percent) of these individuals could not be reached. People intoxicated at time of injury and those with history of substance abuse were more likely to be lost to followup. The authors noted that, among those who were contacted, these characteristics were associated with a lower probability of return to work. They concluded that "systematic bias in longitudinal studies may result from subjects with substance abuse problems being lost to followup. Population estimates for return to work or school will be overestimated if those lost who have substance use problems resemble those followed" (Corrigan, Bogner, Mysiw, et al., 1997, p. 132).
Fourteen studies concerned long-term outcomes of unselected patients with TBI after acute inpatient rehabilitation (Asikainen, Kaste, and Sarna, 1996; Corrigan, Bogner, Mysiw, et al., 1997; Dombovy and Olek, 1997; Eames, Cotterill, Kneale, et al., 1996; Fearnside, Cook, McDougall, et al., 1993; Greenspan, Wrigley, Kresnow, et al., 1996; Hawkins, Lewis, and Medeiros, 1996; High, Hall, Rosenthal, et al., 1996; Rappaport, Herrero-Backe, Rappaport, et al., 1989; Sander, Kreutzer, Rosenthal, et al., 1996; Spatt, Zebenholzer, and Oder, 1997; Tennant, MacDermott, and Neary, 1995; Whitlock, 1992; Whitlock and Hamilton, 1995). Four studies were multicenter (Greenspan, Wrigley, Kresnow, et al., 1996; Sander, Kreutzer, Rosenthal, et al., 1996; Tennant, MacDermott, and Neary, 1995; Whitlock and Hamilton, 1995). The sample size for eight studies was under 100 and ranged between 181 and 525 for six studies. Followup measures were taken at < 2 years for seven studies and at > 2 years for six studies. In these studies, between 13 percent and 40 percent of subjects could not be reached for followup.
In general, patients show substantial improvements in physical, cognitive, and other functions between the time of admission to a rehabilitation facility and the time of discharge or at long-term followup. At the same time, continued morbidity and disability are common. Eight of the studies addressed postinjury return to productive activity. Postinjury unemployment ranged from 28 percent to 75 percent across these studies (Asikainen, Kaste, and Sarna, 1996; Dombovy and Olek, 1997; Fearnside, Cook, McDougall, et al., 1993; Greenspan, Wrigley, Kresnow, et al., 1996; Hawkins, Lewis, and Medeiros, 1996; Rappaport, Herrero-Backe, Rappaport, et al., 1989; Sander, Kreutzer, Rosenthal, et al., 1996; Spatt, Zebenholzer, and Oder, 1997). Employment in a job below preinjury level or with reduced hours and demand ranged from 7 percent to 34 percent.
Studies differed widely in the methods used to measure return to work. Accounting for differences in measurement and the impact of injury severity on the probability of returning to work, it appears that more than half of survivors of TBI become unemployed as a consequence of their condition. For example, some samples combined survivors who retired with those who were unemployed or placed on disability. Also, some studies did not account for preinjury unemployment. At 1-year followup, one study reported 75 percent unemployment; preinjury unemployment for that sample was 19 percent (Hawkins, Lewis, and Medeiros, 1996). Forty-one individuals had an initial GCS of 3 to 8, suggesting a group with severe impairments, which could account for the high unemployment ratio. The study with the lowest postinjury unemployment ratio retrospectively evaluated 496 survivors up to 20 years after injury (Asikainen, Kaste, and Sarna, 1996). In that sample, 285 (58 percent) had an initial GCS of 3 to 8; postinjury unemployment was 28 percent, with an additional 14 percent working at jobs below the preinjury standard.
Seven studies used long-term followup to assess community reintegration (Eames, Cotterill, Kneale, et al., 1996; Fearnside, Cook, McDougall, et al., 1993; Hawkins, Lewis, and Medeiros, 1996; Rappaport, Herrero-Backe, Rappaport, et al., 1989; Tennant, MacDermott, and Neary, 1995; Whitlock, 1992; Whitlock and Hamilton, 1995). Type of placement at discharge from inpatient rehabilitation is often used as an indicator of community reintegration. It is difficult to compare the results of different studies because the categories for disposition at discharge vary. Also, a postinjury living status of "alone" may indicate a high level of independence and a successful recovery, or it may indicate social isolation and a decrease in quality of life. Thus, postinjury status without a measure of change from preinjury status may not be an accurate indicator of the effect of the trauma.
To estimate the impact of TBI on community integration, we categorized disposition as either discharged to home or to an institution such as a skilled nursing facility, long-term rehabilitation center, hospital, prison, etc. In six studies that measured this outcome, the proportion of people institutionalized after discharge from inpatient rehabilitation ranged from 6 percent to 52 percent (Eames, Cotterill, Kneale, et al., 1996; Fearnside, Cook, McDougall, et al., 1993; Hawkins, Lewis, and Medeiros, 1996; Rappaport, Herrero-Backe, Rappaport, et al., 1989; Whitlock, 1992; Whitlock and Hamilton, 1995).
A study performed in New South Wales, Australia, had the lowest proportion of institutionalized survivors (6 percent) (Fearnside, Cook, McDougall, et al., 1993), perhaps reflecting national cultural differences that would result in a greater number of people being discharged to home rather than to an institution. The highest proportion of institutionalized survivors in these studies was 52 percent (Whitlock, 1992). Of 23 respondents, 11 had returned home, and 12 were in skilled nursing facilities at 1-year followup. Comparing the results of these studies, it appears that institutionalization of roughly half of TBI patients persists beyond 1 year.
Injury severity and preadmission disability affect the probability that a patient will eventually go home as opposed to being institutionalized. In the studies cited above, initial injury severity and preadmission disability were measured by a wide variety of methods, including FIM scores, GCS scores, length of stay in acute care, PTA (post-traumatic amnesia) duration, coma duration, and novel measures designed by the researchers conducting the investigation; each sample contained its own mix of severity levels. Given the inconsistencies in measurement and categorization and differences due to culture and resources, the probability of a patient being in a long-term care facility cannot be estimated from these studies.
One study used the FIM to evaluate outcome at 1 year after discharge from rehabilitation (Hawkins, Lewis, and Medeiros, 1996). As measured by the FIM at 1 year after discharge, 43 of 51 survivors (84 percent) were independent in self care, 42 (82 percent) in locomotion, 27 (53 percent) in communication, and 21 (41 percent) in cognition. In another study (Whitlock, 1992), 20 of 23 patients improved on FIM scores from admission to discharge. However, for this same group, only five patients improved on the GOS between 6 months and 1 year postdischarge. Seventeen stayed the same (one patient was not included in the 1-year assignment). Other studies that used the GOS to estimate functional status present sample proportions with good outcomes ranging from 24 percent to 79 percent (Hawkins, Lewis, and Medeiros, 1996).
Predictors of Outcome
A large number of studies have examined the predictive ability of patient characteristics known at the time of admission to inpatient rehabilitation units (Cowen, Meythaler, DeVivo, et al., 1995; Lehmann, Steinbeck, Gobiet, et al., 1996; Malec, Smigielski, De Pompolo, et al., 1993; Saneda and Corrigan, 1992; Spatt, Zebenholzer, and Oder, 1997; Torkelson, Jellinek, Malec, et al., 1983; Vilkki, Ahola, Holst, et al., 1994; Zafonte, Hammond, Mann, et al., 1996). Variables that have been associated with long-term outcomes include:
- Preinjury characteristics such as diseases, psychological conditions, and social and economic issues.
- Age and sex.
- Severity of brain injury (site, severity, mechanism of injury, secondary insults such as hypotension or hypoxia, etc.).
- Severity and influence of extracranial injuries and complications of acute-care hospital care.
- Length of time between the initial injury and the initiation of rehabilitative treatment.
The Glascow Coma Scale (GCS) score is the instrument most intensively studied (Teasdale and Jennett, 1974). This scale, ranging from 3 to 15 points, reliably and repetitively describes the level of consciousness of the patient with TBI. When it is carefully scored at the completion of resuscitation, the GCS is highly predictive of outcome measured by the Glasgow Outcome Scale (GOS) (Jennett and Bond, 1975) at 3, 6, and 12 months after injury (Braakman, Gelpke, Habbema, et al., 1980; Choi, Narayan, Anderson, et al., 1988; Levin, Gary, Eisenberg, et al., 1990; Marshall, Gautille, Klauber, et al., 1991). However, emergency departments vary in who performs the assessment (neurosurgeon versus emergency department staff) and when it is performed (before or after blood pressure and hypoxia are stabilized) (Marion and Carlier, 1994); these variations can affect the ability of the GCS to predict outcome (Bullock, Chesnut, Clifton, et al., 1998). Attention to these details has been lacking in the literature to date, even in quasiexperimental studies that use the GCS as a covariate.
In addition to the GCS, four other indicators are useful. A recent evidence-based literature analysis done for the World Health Organization as part of the Guidelines for the Management of Severe Head Injury has outlined the operating definitions of these variables and the optimal methods for their collection and has suggested that they be controlled via multivariate analysis in all subsequent TBI outcome prediction studies (Bullock, Chesnut, Clifton, et al., 1998).
Pupils
The status of the pupils (an indicator of intracranial pressure or herniation) helps predict outcome (Marshall, Gautille, Klauber, et al., 1991).
Age
Age is usually (Jennett, Teasdale, Galbraith, et al., 1979; Vollmer, Torner, Jane, et al., 1991; Waxman, Sundine, and Young, 1991; Braakman, Gelpke, Habbema, et al., 1980; Choi, Narayan, Anderson, et al., 1988) but not always (Reeder, Rosenthal, Lichtenberg, et al., 1996) found to predict GOS and function (FIM) after rehabilitation. Age appears to be a primary predictor independent of age-related factors such as systemic illnesses (Vollmer, Torner, Jane, et al., 1991).
Systemic Hypotension
The presence of severe systemic injuries is also correlated with worse outcome (Bowers and Marshall, 1980; Klauber, Marshall, Luerssen, et al., 1989; Mayer, Walker, Shasha, et al., 1981). However, when systemic hypotension occurring during the period between injury and the end of resuscitation is controlled, the influence of systemic trauma drops out (Chesnut, Marshall, Klauber, et al., 1993). This suggests that the influence of injuries to extracranial organ systems on outcomes from TBI is primarily mediated by the associated hypotension.
Intracranial Computer Tomographic (CT) Diagnosis
It would seem logical that the location, extent, and severity of damage to the brain would be predictive of outcome from TBI. Although prediction studies have correlated outcome with various parameters consistent with severity of brain injury such as skull fracture, intracranial hematoma, or presence of surgical mass lesions (Bergman, Rockswold, Haines, et al., 1987; Braakman, Gelpke, Habbema, et al., 1980; Jennett, Teasdale, Galbraith, et al., 1979; Waxman, Sundine, and Young, 1991), no one has yet demonstrated the expected degree of anatomic specificity in predicting recovery or residual deficits. This may be largely a question of defining the extent of brain injury based on the rather limited technology of computed tomography and magnetic resonance imaging. To date, the Traumatic Coma Data Bank classification of the CT imaging of the brain during the acute-care course is the most promising method of incorporating the anatomical nature of the brain injury into a predictive model (Marshall, Bowers-Marshall, Klauber, et al., 1991).
Other Variables
Other variables that have been suggested as predictive of outcome, as measured by GOS, include mechanism of injury (Waxman, Sundine, and Young, 1991), brainstem reflexes (Born, Albert, Hans, et al., 1985), evoked potentials (Anderson, Bundlie, and Rockswold, 1984), cerebrospinal fluid (CSF) catecholamines (Woolf, Hamill, Lee, et al., 1987), degree and severity of intracranial hypertension (Alberico, Ward, Choi, et al., 1987; Jones, Andrews, Midgley, et al., 1994; Marmarou, Anderson, Ward, et al., 1991), jugular venous desaturation (Gopinath, Robertson, Contant, et al., 1994; Jones, Andrews, Midgley, et al., 1994; Robertson, Contant, Gokaslan, et al., 1992), cerebral perfusion pressure (Gopinath, Robertson, Contant, et al., 1994; Jones, Andrews, Midgley, et al., 1994; Robertson, Contant, Gokaslan, et al., 1992), fever (Jones, Andrews, Midgley, et al., 1994), and in-hospital hypotension (Chesnut, Marshall, Piek, et al., 1993; Jones, Andrews, Midgley, et al., 1994). The statistical independence of these various factors remains to be clearly delineated. It cannot be suggested at this time that they be included as potential injury severity confounding variables in rehabilitation studies. When planning such investigations, however, the present state of the literature must be assessed since some of these indexes, or variations thereof, may develop as mandated covariables.
Two other indexes, duration of PTA and coma, are frequently used in quasiexperimental studies to adjust for severity of injury. Both of these are determined at some time following the injury.
The use of PTA originated with Russell and colleagues in the 1930s (Russell, 1932; Russell, 1935; Russell, 1971; Russell and Nathan, 1946; Russell and Smith, 1961). Russell classified injuries with PTA < 5 minutes as very mild; 5 to 60 minutes as mild; 1 to 14 hours as moderate; > 24 hours as severe; > 1 week as very severe; and > 4 weeks as extremely severe. In a study of 1,766 patients, Russell and Smith (1961) found the duration of PTA to be the single best predictor of neurological outcome.
In many studies, PTA is measured retrospectively by reviewing patient charts. Unfortunately, retrospective PTA is unreliable (Gronwall and Wrightson, 1980). PTA is best determined prospectively using as an index the attainment of a criterion score (e.g., 85 percent) on the Galveston Orientation and Amnesia Test (GOAT) (Levin, O'Donnell, and Grossman, 1979).
It is difficult to reconcile PTA with the more commonly used GCS score as an index of TBI severity. Using a PTA of > 24 hours as their criterion for the diagnosis of severe TBI, Bishara and colleagues found that 81 percent of such patients attained a good outcome (GOS 4-5) at 1 year (Bishara, Partridge, Godfrey, et al., 1992). This contrasts with only 43 percent of patients achieving such an outcome in the Traumatic Coma Data Bank (TCDB), where severe head injury was defined as a postresuscitation GCS = 8 (Marshall, Gautille, Klauber, et al., 1991). Such a discrepancy suggests that these two indexes cannot be used interchangeably as they will be predictive of markedly different courses of recovery.
Duration of coma has been used to quantify the severity of brain injury and to predict outcome. Patients in coma for < 20 days frequently regain independence in functional activities, whereas those who remain in coma > 20 days are usually profoundly disabled (Jones, 1981; Pazzaglia, Frank, Frank, et al., 1975). Like the PTA, duration of coma is unreliable when determined retrospectively, and it is not interchangeable with the GCS score. In addition, its determination can be confounded by the use of medications which are commonly administered during treatment of TBI patients.
Average LOS in acute care after TBI has been used as a gross indicator of the "sickness" of the patient during the immediate, posttraumatic period. More recent studies have reported acute care stays ranging from 20 to 60 days (Lehmkulh, Hall, Mann, et al., 1993; Mackay, Bernstein, Chapman, et al., 1992; Sakata, Ostby, and Leung, 1991; Sparadeo and Gill, 1989). Unfortunately, this variable is sensitive to socioeconomic issues, which may be difficult to control when using it as an indicator of trauma severity.
The above considerations reveal that the prediction of outcome based on physiologic indicators of TBI remains in a state of active development. At present, a credible attempt to control for (or match on) severity of illness should include the five best physiologic indicators described above. Older studies frequently do not use these indicators, the importance of which was not clearly established until recently.
Even today, retrospective analyses are hampered by the lack of reliability and absence of the necessary data in patient charts. Properly approaching this problem in the future will require a coordinated effort in data collection beginning at admission and continuing through rehabilitation wherein common definitions are used throughout.
Footnotes
- 1
Use of language in reference to people with TBI is based on a survey of current usage. "Survivor" isused through the course of a person's life. "Patient" is used when the person is an inpatient. "Client" is used in general outside the patient setting. Otherwise,"a person (or people) with TBI" is the preferred term.
- Introduction and Background - Rehabilitation for Traumatic Brain InjuryIntroduction and Background - Rehabilitation for Traumatic Brain Injury
Your browsing activity is empty.
Activity recording is turned off.
See more...
