NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Suspected Cancer: Recognition and Referral. London: National Institute for Health and Care Excellence (NICE); 2015 Jun. (NICE Guideline, No. 12.)
January 2021: NICE amended the recommendation on offering quantitative faecal immunochemical tests (recommendation 1.3.4) in the short version of the guideline to include the full list of criteria for faecal testing. Faecal testing should also be offered to people without rectal bleeding aged 50 or over with unexplained abdominal pain or weight loss, or to adults under 60 with changes in bowel habit or iron-deficiency anaemia. The tables of symptoms and findings in the short version have been updated to match these changes. September 2020: Recommendation 1.3.4 in the short version of this guideline was amended to clarify when to offer faecal testing for colorectal cancer to adults without rectal bleeding. The tables on abdominal and pelvic pain, change in bowel habit and primary care investigations were updated in line with this. The wording in some recommendations was edited to incorporate text previously in footnotes. July 2017: The recommendation on page 138 (recommendation 1.3.4 in the short version of the guideline) has been stood down (this has been greyed out in the pdf) as it has been superseded by newly-published NICE diagnostics guidance. An earlier recommendation was amended to remove a link to the recommendation on page 138. June 2016: Recommendations 1 and 2 in the section on Lower gastrointestinal tract cancers 2 have been changed to say 'adults' instead of 'people' to more accurately reflect the populations they cover in the pdf.
12.1. Prostate cancer
Over 41,000 new prostate cancers are diagnosed each year in the UK, so a full-time GP will usually diagnose one new person with prostate cancer each year. Five-year survival is approximately 80%.
Prostate cancer usually presents with lower urinary tract symptoms, including nocturia, urinary frequency, and hesitancy. Haematuria can occur, as can erectile dysfunction. Some prostate cancers present with disseminated disease, typically metastases to bone.
The lower urinary symptoms overlap with those of benign prostatic hyperplasia – and the two conditions can co-exist. Digital rectal examination can help to differentiate the two, with hardness of the prostate or individual nodules being features suggestive of cancer.
Prostate specific antigen (PSA) testing is generally available in primary care, with age-specific raised values suggestive of cancer. Definitive diagnosis requires biopsy, often guided by imaging. This is performed in secondary care.
For recommendations on patient information and support and monitoring of patients whose symptoms do not meet the criteria for referral or other investigation see Chapter 4 and Chapter 5 respectively.
Clinical questions:
- What is the risk of prostate cancer in patients presenting in primary care with symptom(s)?
- Which investigations of symptoms of suspected prostate cancer should be done with clinical responsibility retained by primary care?
Clinical evidence
Signs and symptoms
Risk of bias in the included studies
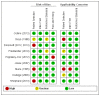
The risk of bias and applicability concerns are summarised per study in the figure below. The main issue to note is that 4/5 studies employed samples of patients that are not directly representative of an unselected symptomatic population of patients presenting to the UK-based GP and the 5th study employed a case-control design which has been shown to inflate the test accuracy characteristics. However, the statistical analyses employed by the authors may have gone some way in counteracting this influence. Three of the studies also employed reference standards that are subject to an unclear risk of bias; all of which must be born in mind when evaluating the evidence contributed by these studies.
Evidence statement
The positive predictive values for prostate cancer of single symptoms or signs presenting in a primary care setting ranged from 0.08% (for dyspepsia) to 12% (for malignant rectal exam; 5 studies, N = 7440). The studies were associated with 1-4 bias or applicability concerns (see also Table 50).
Table 50
Prostate cancer: Single symptoms.
The positive predictive values for prostate cancer of symptom pairs presenting in a primary care setting ranged from 1.8% (for haematuria + frequency/urgency) to 15% (for nocturia + malignant rectal exam; 1 study, N = 1297). This study was a case-control study (i.e, high risk of bias for patient selection; see also Table 51).
Table 51
Prostate cancer: Symptom combinations.
Investigations in primary care
Risk of bias in the included studies
The risk of bias and applicability concerns are summarised for the included study in the figure below. The main risk of bias in this study pertains to the ca 20% of missing data in this study. It is not possible to ascertain whether these data are missing in a systematic manner and whether they are likely to substantially influence the test accuracy estimates provided by this study. The only applicability concern identified for this study concerns the underspecification of the patients, that is, it is not clear from, the study whether all the patients were symptomatic patients presenting to primary care, and to the extent they are not from this patient group, the applicability to the current guideline is limited.
Evidence statement
PSA testing (1 study, N = 582) conducted in patients presenting in a primary/hospital care setting is associated with sensitivities that ranged from 77.8-88.9%, specificities that ranged from 70-90.2% and false negativity rates that ranged from 11.1-22.2% for prostate cancer. The study was associated with one bias and one applicability concern (see also Table 52).
Table 52
Prostate cancer: PSA.
Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Refer men using a suspected cancer pathway referral (for an appointment within 2 weeks) for prostate cancer if their prostate feels malignant on digital rectal examination. [new 2015] Consider a prostate-specific antigen (PSA) test and digital rectal examination to assess for prostate cancer in men with:
|
|---|---|
| Relative value placed on the outcomes considered | Signs and symptoms of prostate cancer The GDG considered the positive predictive value to be the most important outcome when identifying which signs and symptoms predict prostate cancer. Investigations in primary care for prostate cancer The GDG identified sensitivity, specificity, positive predictive values and false negative rates as relevant outcomes to this question. Although sensitivity and specificity were reported, the GDG agreed that the most informative outcomes were the positive predictive values (because these gave the risk of a person harbouring cancer), and the false negative rates (to inform whether a negative test obviated the need for further safety-netting). |
| Quality of the evidence | Signs and symptoms of prostate cancer The quality of the evidence assessed by QUADAS-II varied with only one of five studies considered to provide high quality evidence. Investigations in primary care for prostate cancer Evidence was only identified on the accuracy of PSA testing. This evidence was assessed by QUADAS-II as not being of high quality. The GDG noted some limitations of the evidence. Firstly, it was not clear whether all patients were symptomatic patients presenting to primary care. Secondly, some data are missing but it is not clear whether this was likely to substantially influence the test accuracy estimates provided. Thirdly, PSA measurement has changed since this study was published. No evidence was found pertaining to the diagnostic performance of MRI in primary care patients with suspected prostate cancer. |
| Trade-off between clinical benefits and harms | The GDG considered that a potential benefit of recommending which symptoms should prompt a suspected cancer pathway referral would be to identify those men with prostate cancer more rapidly. However, the GDG recognised the importance of recommending the “right” symptoms, in order to minimise the number of men without prostate cancer who get inappropriately referred whilst maximising the number of men with prostate cancer who get appropriately referred. In order to strike an appropriate balance between these considerations, the GDG agreed to recommend referral for those symptoms with a positive predictive value of 3% or above. The GDG were confident that at this threshold the advantages of a suspected cancer pathway referral in those with prostate cancer outweighed the disadvantages to those without. However, the GDG noted the evidence which had shown that PSA testing was a reasonably sensitive and specific test for prostate cancer and that a raised PSA level was a significant predictor of prostate cancer. Based on this evidence the GDG decided not to recommend symptoms which should prompt a suspected cancer pathway referral but instead to recommend which symptoms should prompt a PSA test and chose these symptoms based on the positive predictive values presented in the evidence. The results of this PSA test would then determine who needed a suspected cancer pathway referral. By doing this the GDG hoped to refine the group of symptomatic men being referred to those with the greatest chance of having prostate cancer. The GDG noted that Hamilton (2006) had reported loss of weight plus a benign rectal examination to have a PPV of 9.4. The GDG also noted that this PPV was based on very small numbers and no confidence intervals had been calculated for this reason. The GDG agreed that the fact that a rectal examination had been performed, strongly implied that the person also had lower urinary tract symptoms, as it would not be standard practice to perform a rectal examination for loss of weight alone. Given that recommendations had already been made on lower urinary tract symptoms were already (which would encompass people with the symptom combination cited by Hamilton (2006), the GDG agreed that a specific recommendation for this symptom combination was not required. The exception to this was those men whose prostate felt malignant on digital rectal examination. The positive predictive value of a malignant feeling prostate on digital rectal examination was so high above the 3% threshold that even after a normal PSA result, the GDG still considered that urgent referral was justified. For this reason the GDG recommended a digital rectal examination as well as PSA test for all men with relevant symptoms. The GDG noted that there was no strong primary care evidence available on which to base a recommendation for what level of PSA should prompt a suspected cancer pathway referral. They therefore agreed to accept the age-specific reference range. Due to the lack of evidence, the GDG agreed not to make any recommendations on the use of MRI in primary care patients with suspected prostate cancer. |
| Trade-off between net health benefits and resource use | The GDG noted that no relevant, published economic evaluations had been identified and no additional economic analysis had been undertaken in this area. The GDG noted that the recommendation for a suspected cancer pathway referral for a malignant prostate on digitial rectal examination is likely to be cost-neutral as it is currently standard practice. The also GDG estimated that the recommendations were likely to result in a moderate increase in PSA testing followed by a smaller increase in suspected cancer pathway referrals. The net effect of this was uncertain but the GDG agreed that any potential increase in costs would be balanced by improvements in the diagnosis of prostate cancer. |
| Other considerations | The GDG considered whether or not to specify an age range in the recommendations for which symptoms should prompt PSA testing and digital rectal examination, since prostate cancer is less common in younger men. The agreed not to do this as some risk factors, for example ethnicity, might warrant testing at a lower age. The GDG considered the situation for transgendered people, who retain any of the genital organs of their genetic sex. The recommendations for cancers generally found in a single sex, also extend to people who have the organs of that sex, whatever their gender. |
12.2. Bladder cancer
Around 10,000 new bladder cancers are diagnosed each year in the UK, meaning that a full time GP is likely to diagnose approximately 1 person with bladder cancer every 3-5 years. It is seen in both sexes, though almost three-quarters of new cases are in males. Five year survival is approximately 55%.
Several symptoms have been reported, with haematuria being the most common. Dysuria and urinary frequency are also features, especially when persistent.
Because haematuria is a symptom of several cancers, investigation strategies may need to consider more than one possible cancer site, such as kidney, prostate or endometrium. Similarly, dysuria and urinary frequency may be misattributed to urinary tract infection, especially in the elderly.
A diagnosis of bladder cancer is generally made by cystoscopy with biopsy, performed in secondary care. Because bladder cancer shares some symptoms with other urological cancers, most haematuria clinics investigate with ultrasound before proceeding to cystoscopy.
For recommendations on patient information and support and monitoring of patients whose symptoms do not meet the criteria for referral or other investigation see Chapter 4 and Chapter 5 respectively.
Clinical questions:
- What is the risk of bladder cancer in patients presenting in primary care with symptom(s)?
- Which investigations of symptoms of suspected bladder cancer should be done with clinical responsibility retained by primary care?
Clinical evidence
Signs and symptoms
Risk of bias in the included studies
The risk of bias and applicability concerns are summarised per study in the figure below. The main bias and validity issues to note are that one study was conducted in a Belgian primary care population (Bruyninckx, 2003) and another in US primary care setting (Friedlander, 2014) and these studies are therefore only applicable to the extent that the populations are comparable to a UK GP population, another study (Hippisley-Cox 2012) only presented data for 967681 out of 1240722 eligible patients and it is unclear why, a third study (Jones, 2007) report the results for both 6 months and 3 years after first symptom presentation and it is unclear whether 3 years is too long an interval to be confident that the symptom is a result of underlying cancer, similarly, Friedlander (2014) only followed up the included patients for 180 days, which may be too short a time period. The final study (Shephard, 2012) employed a case-control design which has been shown to be associated with inflated test accuracy parameters compared to designs that incorporate random or consecutive patient selection.
Evidence statement
Haematuria (6 studies, N = 89345) presenting in a primary care setting is associated with overall positive predictive values ranging from 1.34%-10.27% for bladder cancer, which tended to be higher in men (5.47%-14.2%) than in women (2.48%-5.1%; 3 studies, total N = 49327) and to increase with age in men (up 22.1%; 2 studies, total N = 11517) and much less so in women (up to 8.53%; 2 studies, total N = 11517). All the studies were associated with 0-2 bias or applicability concern (see also Tables 53-55).
Table 53
Bladder cancer: Meta-analyses.
Table 54
Bladder cancer: Individual positive predictive values from the meta-analyses.
Table 55
Bladder cancer: Additional results reported by the individual papers.
Haematuria in combination with other symptoms presenting in a primary care setting was associated with positive predictive values ranging from 1.1% (non-visible with raised creatinine in patients ≥ 60 years; 1 study, total N = 26633) to 33.3% (with weight loss in men > 60 years old; 1 study, total N = 409) for bladder cancer. Both studies were associated with 1 bias or applicability concern (see also Table 3).
Other symptoms (than haematuria) presenting alone or in combination with each other (but not haematuria) in a primary care setting were all associated with positive predictive values ≤ 1.5% for bladder cancer (3 studies, total N = 1284137). All the studies were associated with 0-1 bias or applicability concern (see also Table 3).
Investigations in primary care
No primary care evidence was identified pertaining to the diagnostic accuracy of urine cytology, ultrasound, cystoscopy, blood HCG, urine marker NMP22, and urine marker MCM5 in patients with suspected bladder cancer where the clinical responsibility was retained by primary care.
Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Refer people using a suspected cancer pathway referral (for an appointment within 2 weeks) for bladder cancer if they are:
|
|---|---|
| Relative value placed on the outcomes considered | Signs and symptoms of bladder cancer The GDG considered the positive predictive value to be the most important outcome when identifying which signs and symptoms predict bladder cancer. Investigations in primary care for bladder cancer The GDG identified sensitivity, specificity, positive predictive values and false negative rates as relevant outcomes to this question. No evidence was found on any of these outcomes |
| Quality of the evidence | Signs and symptoms of bladder cancer The quality of the evidence as assessed by QUADAS-II varied for the positive predictive values for the different symptoms but was generally of high quality. It was noted that the majority of the evidence had merged all urinary tract cancers making it difficult to tease out the specifics related to bladder cancer. The GDG also noted that most of the evidence did not distinguish between visible and non-visible haematuria, but largely grouped these two symptoms together as haematuria. The GDG judged, based on their clinical experience, that most of that evidence was likely to reflect visible haematuria which left them with evidence from one paper about non-visible haematuria. Investigations in primary care for bladder cancer No evidence was found pertaining to the diagnostic performance of ultrasound, urine cytology, cystoscopy, blood HCG or urinary markers NMP22 and MCM5 in primary care patients with suspected bladder cancer. |
| Trade-off between clinical benefits and harms | The GDG considered that a potential benefit of recommending which symptoms should prompt a suspected cancer pathway referral would be to identify those people with bladder cancer more rapidly. However, the GDG recognised the importance of recommending the “right” symptoms, in order to minimise the number of people without bladder cancer who get inappropriately referred whilst maximising the number of people with bladder cancer who get appropriately referred. In order to strike an appropriate balance between these considerations, the GDG agreed to recommend referral for those symptoms with a positive predictive value of 3% or above. The GDG were confident that at this threshold the advantages of a suspected cancer pathway referral in those with bladder cancer outweighed the disadvantages to those without. The GDG noted, based on the evidence, that haematuria presenting in a primary care setting was associated with a positive predictive value of above 3% for bladder cancer. They therefore recommended this symptom should prompt a suspected cancer pathway referral. The GDG also noted that, based on the evidence, the positive predictive value of haematuria for bladder cancer increased with age. They therefore agreed to recommend referral for those people aged 45 or over. The GDG agreed, based on their clinical experience that urinary tract infections often cause visible haematuria. They therefore recommended that if visible haematuria persists or recurs after successful treatment of urinary tract infection, a suspected cancer pathway referral should be made. The GDG acknowledged that the positive predictive values associated with urinary tract infections presenting in primary care were inconsistent for bladder cancer and that there was no evidence on recurrent (greater than two) urinary tract infections. However the GDG considered that this was a population in which cancer can be missed and therefore a non-urgent referral should be considered for people with this symptom. The GDG agreed, based on the evidence, to recommend a suspected cancer pathway referral (for an appointment within 2 weeks) for bladder cancer for people aged 60 years and over with unexplained non-visible haematuria and either dysuria or a raised white cell count on a blood test. The GDG acknowledged that no other symptoms had a high enough positive predictive value for bladder cancer to warrant making recommendations on them. The GDG noted the absence of evidence on investigations in primary care, and that the definitive test for bladder cancer is cystoscopy. However the GDG considered cystoscopy to be best performed by specialists in secondary care and therefore decided to not make any recommendations for investigations for bladder cancer in primary care. |
| Trade-off between net health benefits and resource use | The GDG noted that no relevant, published economic evaluations had been identified and no additional economic analysis had been undertaken in this area. The GDG noted that the recommendations on haematuria were likely to be cost saving as the age threshold for referral has been raised for both visible and non-visible haematuria. Investigation of persistent and recurrent urinary tract infections is a revised recommendation and this is likely to increase referrals. The recommendations on non-visible haematuria and recurrent/persistent urinary tract infection in people over 60 are likely to result in a moderate increase in costs. On this basis, the GDG estimated that overall the recommendations were likely to be either cost neutral or a small cost increase. However, they agreed that this balanced against improvements in earlier diagnosis of bladder cancer. |
| Other considerations | The GDG noted that visible haematuria is a symptom which is common to both renal and bladder cancer. It was therefore, agreed that recommendations for referral of haematuria would need to be consistent for both these cancer sites. |
12.3. Renal cancer
Over 10,000 new renal cancers are diagnosed each year in the UK. A full time GP is likely to diagnose approximately 1 person with renal cancer every 3-5 years. It is seen in both sexes, though around 60% of new diagnoses are in males. Five year survival is over 55%.
Renal cancer symptoms include haematuria, loin pain, urinary tract infections or a mass in the flank.
The symptoms overlap with other urological cancers, particularly bladder cancer.
Most renal cancers are visible on ultrasound of the kidneys – a test that is available in primary care.
Definitive diagnosis of renal cancer requires histology, performed in secondary care.
For recommendations on patient information and support and monitoring of patients whose symptoms do not meet the criteria for referral or other investigation see Chapter 4 and Chapter 5 respectively.
Clinical questions:
- What is the risk of renal cancer in patients presenting in primary care with symptom(s)?
- Which investigations of symptoms of suspected renal cancer should be done with clinical responsibility retained by primary care?
Clinical evidence
Signs and symptoms
Risk of bias in the included studies
The risk of bias and applicability concerns are summarised per study in the figure below. The main issue to note is that patient selection is associated with a number of bias or applicability concerns in most of the included studies, with some studies employing non-consecutive or non-random selection of patients and with some studies being employed in settings that are not clearly directly representative of UK-based primary care. Other areas of concern include missing data, compromised reference standards and underspecified presenting symptoms. These issues should all be born in mind when evaluating the evidence along with the fact that a large number of the included cancers were not renal cancers.
Evidence statement
Patients aged > 14 years
Haematuria (5 studies, N = 87161) presenting in a primary care setting is associated with overall positive predictive values of 0.65-6.48% for renal cancer, which tended to be higher in men (5.47-5.5%) than in women (2.48-2.6%; 2 studies, N = 48918) and to increase with age in men (up to 11.21%; 1 study, N = 11108) and less so in women (up to 8.53%; 1 study, N = 11108). The evidence was, however, compromised by a large number of the included cancers being non-renal cancers. Each of the studies was associated with 0-2 bias concern (see also Tables 56-58).
Table 56
Renal cancer: Meta-analyses.
Table 57
Renal cancer: Individual positive predictive values from the meta-analyses.
For renal cancer the positive predictive values of single symptoms (excluding haematuria; 6 studies, N = 344897) presenting in primary care ranged from 0.05% (for back pain) to 1.4% (for anaemia in men). The evidence was, however, compromised by a large number of the included cancers being non-renal cancers and ≤ 3 bias or applicability concerns associated with 4 of the 6 included studies (see also Table 58).
Table 58
Renal cancer: Patients aged > 14 years: Single symptoms.
For renal cancer the positive predictive values of symptom combinations (1 study, N = 17240) presenting in primary care ranged from 0.1% (for constipation in combination with either abdominal pain, nausea or lower urinary tract infection) to > 5% (for abdominal pain combined with microcytosis). The included study was associated with 1 bias concern (see also Table 59).
Table 59
Renal cancer: Patients aged ≥ 60 years: Symptom combinations.
Patients aged < 15 years
The positive predictive values of having any childhood cancer ranged from 0.04% (for pain and musculoskeletal symptoms) to 2.19% (for hepatosplenomegaly) in all included patients, and from 0.061% (for lymphadenopathy) to 1.286% (for hepatosplenomegaly) for patients aged 0-4 years old, and from 0.049% (for bruising) to 0.154% (for ‘lump/mass/swelling’ [the PPV for hepatosplenomegaly could not be calculated as none of the controls experienced this symptom]) for patients aged 5-14 years old (all from 1 study, N = 16585). The evidence quality is somewhat compromised by the case-control design of the study (see also Tables 60-62).
Table 60
Positive predictive values for any childhood cancer: All patients.
Table 61
Positive predictive values for any childhood cancer: Patients aged 0-4 years.
Table 62
Positive predictive values for any childhood cancer: Patients aged 5-14 years.
Investigations in primary care
No primary care evidence was identified pertaining to the diagnostic accuracy of abdominal ultrasound, urine cytology, x-ray, intravenous pyelogram, or CT scan of the abdomen and pelvis in patients with suspected renal cancer where the clinical responsibility was retained by primary care.
Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Refer people using a suspected cancer pathway referral (for an appointment within 2 weeks) for renal cancer if they are aged 45 and over and have:
|
|---|---|
| Relative value placed on the outcomes considered | Signs and symptoms of renal cancer The GDG considered the positive predictive value to be the most important outcome when identifying which signs and symptoms predict renal cancer. Investigations in primary care for renal cancer The GDG identified sensitivity, specificity, positive predictive values and false negative rates as relevant outcomes to this question. No evidence was found on any of these outcomes |
| Quality of the evidence | Signs and symptoms of renal cancer The quality of the evidence as assessed by QUADAS-II varied from low to high for the positive predictive values for the different symptoms. The GDG noted some limitations of the evidence. Firstly, all the evidence with the exception of two papers had merged all urinary tract cancers making it difficult to tease out the specifics related to renal cancer. Secondly, the evidence did not distinguish between visible and non-visible haematuria, but largely grouped these two together as haematuria. The GDG judged, based on their clinical experience, that most of that evidence was likely to reflect visible haematuria. Investigations in primary care for renal cancer No evidence was found pertaining to the diagnostic accuracy of abdominal ultrasound, urine cytology, intravenous pyelogram, abdominal/pelvic CT scan or X-ray in primary care patients with suspected renal cancer. |
| Trade-off between clinical benefits and harms | The GDG considered that a potential benefit of recommending which symptoms should prompt a suspected cancer pathway referral would be to identify those people with renal cancer more rapidly. However, the GDG recognised the importance of recommending the “right” symptoms, in order to minimise the number of people without renal cancer who get inappropriately referred whilst maximising the number of people with renal cancer who get appropriately referred. In order to strike an appropriate balance between these considerations, the GDG agreed to recommend referral for those symptoms with a positive predictive value of 3% or above. The GDG were confident that at this threshold the advantages of a suspected cancer pathway referral in those with renal cancer outweighed the disadvantages to those without. The GDG noted, based on the evidence, that visible haematuria presenting in a primary care setting was associated with a positive predictive value of above 3% for renal cancer. They therefore recommended this symptom should prompt a suspected cancer pathway referral. The GDG also noted that, based on the evidence, the positive predictive value of visible haematuria for renal cancer increased with age. They therefore agreed to recommend referral for those people aged 45 or over. The GDG agreed, based on their clinical experience that urinary tract infections often cause visible haematuria. They therefore recommended that if visible haematuria persists or recurs after successful treatment of urinary tract infection, a suspected cancer pathway referral should be made. Although the symptoms of abdominal pain and microcytosis had positive predictive values above 3%, the GDG noted that referral for colorectal cancer would normally be the first direction of investigation for these symptoms. They therefore agreed not to make any recommendations for these symptoms related to renal cancer. The GDG noted the absence of evidence for investigations for renal cancer in primary care. Based on their clinical experience they considered that whilst ultrasound is an investigation commonly used to diagnose renal cancer in secondary care, it could have value as an investigation in primary care. The GDG considered that the clinical benefits of renal ultrasound performed in primary care would be to expedite renal cancer diagnosis in people whose symptoms may otherwise not be investigated. However, the GDG recognised that it was difficult to define exactly which symptoms should prompt an ultrasound and consequently some people without renal cancer may also be investigated unnecessarily. The GDG therefore felt unable to make any recommendations on primary care-based investigations for renal cancer. |
| Trade-off between net health benefits and resource use | The GDG noted that no relevant, published economic evaluations had been identified and no additional economic analysis had been undertaken in this area. The GDG noted that the recommendation for a suspected cancer pathway referral for visible haematuria is likely to result in a cost decrease because of the introduction of an age limit. However, the recommendation to refer if there is persistent/recurrent urinary tract infection is likely to represent a small to moderate increase in costs. Overall the GDG agreed these were likely to balance each other. |
| Other considerations | The GDG noted that visible haematuria is a symptom which is common to cancers of the urinary tract. It was therefore, agreed that recommendations for referral of haematuria would need to be consistent for these cancer sites. |
12.4. Testicular cancer
Over 2,000 new testicular cancers are diagnosed each year in the UK, so a full-time GP will usually diagnose one new person with testicular cancer during their career. It is atypical in terms of the age-groups affected. The peak age of onset is 30-34 years, although it can occur in older males. It is the commonest cancer in males between 16 and 24 years. Five-year survival is almost 100%.
Testicular cancer usually presents as a change in the shape or texture of the testis. This may be painful. It can present as disseminated disease, particularly with lymph node spread.
Testicular cancer can be seen on ultrasound of the testis, a test available in primary care.
For recommendations on patient information and support and monitoring of patients whose symptoms do not meet the criteria for referral or other investigation see Chapter 4 and Chapter 5 respectively.
Clinical questions:
- What is the risk of testicular cancer in patients presenting in primary care with symptom(s)?
- Which investigations of symptoms of suspected testicular cancer should be done with clinical responsibility retained by primary care?
Clinical evidence
Signs and symptoms
No primary care evidence was identified pertaining to the risk of testicular cancer in patients presenting with symptoms in primary care.
Investigations in primary care
No primary care evidence was identified pertaining to the diagnostic accuracy of ultrasound in patients with suspected testicular cancer where the clinical responsibility was retained by primary care.
Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Consider a suspected cancer pathway referral (for an appointment within 2 weeks) for testicular cancer in men if they have a non-painful enlargement or change in shape or texture of the testis. [new 2015] Consider a direct access ultrasound scan for testicular cancer in men with unexplained or persistent testicular symptoms. [new 2015] |
|---|---|
| Relative value placed on the outcomes considered | Signs and symptoms of testicular cancer The GDG considered the positive predictive value to be the most important outcome when identifying which signs and symptoms predict testicular cancer. No evidence was found on this outcome. Investigations in primary care for testicular cancer The GDG identified sensitivity, specificity, positive predictive values and false negative rates as relevant outcomes to this question. No evidence was found on any of these outcomes |
| Quality of the evidence | Signs and symptoms of testicular cancer No evidence was found pertaining to the positive predictive values of different symptoms of testicular cancer in primary care. Investigations in primary care for testicular cancer No evidence was found pertaining to the diagnostic accuracy of ultrasound in primary care patients with suspected testicular cancer. |
| Trade-off between clinical benefits and harms | The GDG considered that a potential benefit of recommending which symptoms should prompt a suspected cancer pathway referral would be to identify those men with testicular cancer more rapidly. However, the GDG recognised the importance of recommending the “right” symptoms, in order to minimise the number of men without testicular cancer who get inappropriately referred whilst maximising the number of men with testicular cancer who get appropriately referred. In order to strike an appropriate balance between these considerations, the GDG agreed to recommend referral for those symptoms with a positive predictive value of 3% or above. The GDG were confident that at this threshold the advantages of a suspected cancer pathway referral in those with testicular cancer outweighed the disadvantages to those without. However, in this instance, the GDG acknowledged that no evidence had been found on the positive predictive values of symptoms for testicular cancer. Despite the lack of evidence, the GDG considered that it was still important to provide guidance on which symptoms should prompt referral for suspected testicular cancer as it is a very treatable disease and diagnosis at an early stage improves outcome. However, the GDG were aware that most men presenting with scrotal symptoms do not have testicular cancer. They therefore needed to use caution when specifying which symptoms should prompt referral so that excessive referral was avoided. The GDG agreed, based on their clinical experience, that non-painful enlargement or change in shape or texture of the testis were likely to be the typical symptoms of testicular cancer and should prompt a suspected cancer pathway referral. The GDG noted, that although pain can be indicative of cancer, pain in the testes does not often result from testicular cancer. They therefore did not include this symptom in the recommendation as they agreed it would be likely to result in over-referral. The GDG acknowledged that there may be a small number of men with atypical presentations of testicular cancer, who would be missed by this recommendation. However, they agreed that if the symptoms resulted from testicular cancer, they were likely to worsen/persist rather than resolve. The GDG noted the lack of evidence on the diagnostic accuracy of ultrasound. However, based on their clinical experience, they noted that ultrasound was an accessible, non-invasive test that could be used to discriminate between malignant and non-malignant disorders of the testes. They therefore agreed to recommend that ultrasound be considered for those men with unexplained or persistent testicular symptoms in order to pick up those men with atypical presentations of testicular cancer. |
| Trade-off between net health benefits and resource use | The GDG noted that no relevant, published economic evaluations had been identified and no additional economic analysis had been undertaken in this area. The GDG noted that referral for men with a non-painful enlargement or change in shape or texture of the testis is already current practice. In addition, ultrasound is a relatively inexpensive test and given the small numbers of men likely to be scanned, this was unlikely to represent a significant additional cost. |
| Other considerations | The GDG considered the situation for transgendered people, who retain any of the genital organs of their genetic sex. The recommendations for cancers generally found in a single sex, also extend to people who have the organs of that sex, whatever their gender. |
12.5. Penile cancer
Penile cancer is rare, with around 500 cases diagnosed each year in the UK. A full time GP is likely to diagnose only one – if any – person with penile cancer during their career. Nearly all are squamous cell cancers.
Penile cancer is usually seen as a raised lesion. Because of its rarity, few studies have reported its clinical features. It can be difficult to differentiate penile cancer from the commoner lesions seen with some sexually transmitted diseases.
It is often possible to diagnose a typical penile cancer visually, but confirmation of the diagnosis is generally made by excision biopsy in secondary care.
For recommendations on patient information and support and monitoring of patients whose symptoms do not meet the criteria for referral or other investigation see Chapter 4 and Chapter 5 respectively.
Clinical questions:
- What is the risk of penile cancer in patients presenting in primary care with symptom(s)?
- Which investigations of symptoms of suspected penile cancer should be done with clinical responsibility retained by primary care?
Clinical evidence
Signs and symptoms
No primary care evidence was identified pertaining to the risk of testicular cancer in patients presenting with symptoms in primary care.
Investigations in primary care
No primary care evidence was identified pertaining to the diagnostic accuracy of tests used in patients with suspected penile cancer where the clinical responsibility was retained by primary care.Cost-effectiveness evidence
Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendation | Consider a suspected cancer pathway referral (for an appointment within 2 weeks) for penile cancer in men if they have either:
|
|---|---|
| Relative value placed on the outcomes considered | Signs and symptoms of penile cancer The GDG considered the positive predictive value to be the most important outcome when identifying which signs and symptoms predict penile cancer. No evidence was found on this outcome. Investigations in primary care for penile cancer The GDG identified sensitivity, specificity, positive predictive values and false negative rates as relevant outcomes to this question. No evidence was found on any of these outcomes. |
| Quality of the evidence | Signs and symptoms of penile cancer No evidence was found pertaining to the positive predictive values of different symptoms of penile cancer in primary care. Investigations in primary care for penile cancer No evidence was found pertaining to the diagnostic accuracy of tests used in primary care patients with suspected penile cancer. |
| Trade-off between clinical benefits and harms | The GDG considered that a potential benefit of recommending which symptoms should prompt a suspected cancer pathway referral would be to identify those men with penile cancer more rapidly. However, the GDG recognised the importance of recommending the “right” symptoms, in order to minimise the number of men without penile cancer who get inappropriately referred whilst maximising the number of men with penile cancer who get appropriately referred. In order to strike an appropriate balance between these considerations, the GDG agreed to recommend referral for those symptoms with a positive predictive value of 3% or above. The GDG were confident that at this threshold the advantages of a suspected cancer pathway referral in those with penile cancer outweighed the disadvantages to those without. However, in this instance, the GDG acknowledged that no evidence had been found on the positive predictive values of symptoms for penile cancer. Despite the lack of evidence, the GDG considered that it was still important to provide guidance on which symptoms should prompt referral for suspected penile cancer. The GDG noted that, based on their clinical experience, penile lesions can be a symptom of penile cancer. However they acknowledged that most penile lesions are caused by sexually transmitted infections rather than cancer. They therefore agreed that a suspected cancer pathway referral should only be recommended after sexually transmitted infections had been excluded as the cause of a penile lesion, in order to reduce inappropriate urological referrals. The GDG also agreed that referral should be considered for those men with other unexplained or persistent symptoms of foreskin and/or glans. The GDG discussed whether an age threshold should be included in the recommendations, as penile cancer is rare in men under 60. However it was noted that the demographics of penile cancer may be changing to include younger men. The GDG therefore agreed not to include an age threshold in the recommendations. Due to the lack of evidence, the GDG were not able to recommend a particular test for the primary care investigation of penile cancer. Equally, the GDG were not able to recommend that no tests be done in primary care. Therefore they agreed not to make any recommendations on this issue. |
| Trade-off between net health benefits and resource use | The GDG noted that no relevant, published economic evaluations had been identified and no additional economic analysis had been undertaken in this area. The GDG considered that the recommendations made were similar to current clinical practice and therefore would not require additional funding. In addition, they noted that penile cancer is very rare and does not affect many men. They therefore agreed the recommendations were likely to be cost-neutral. |
| Other considerations | The GDG noted that the previous guidance had made specific recommendations about men with Peyronie's disease. It was agreed that this group of men would be covered by the recommendation made and did not require specific mention. The GDG considered the situation for transgendered people, who retain any of the genital organs of their genetic sex. The recommendations for cancers generally found in a single sex, also extend to people who have the organs of that sex, whatever their gender. |
References
- Bouwman I, Van Der Heide WK, Van Der Veen WJ, Van Der Meer K. GPs and patients still think that lower urinary tract symptoms are an indication of prostate cancer. Huisarts en Wetenschap. 2007;50(7):321–325. [Dutch]
- Deyo RA, Diehl AK. Cancer as a cause of back pain: Frequency, clinical presentation, and diagnostic strategies. Journal of General Internal Medicine. 1988 Nov 1;3:230–238. [PubMed: 2967893]
- Friedlander DF, Resnick MJ, You C, Bassett J, Yarlagadda V, Penson DF, Barocas DA. Variation in the intensity of hematuria evaluation: A target for primary care quality improvement. American Journal of Medicine. 2014;127:633–640. [PMC free article: PMC4074456] [PubMed: 24486290]
- Hallissey MT, Allum WH, Jewkes AJ, Ellis AJ, Fielding JWL. Early detection of gastric cancer. British Medical Journal. 1990;301:513–515. [PMC free article: PMC1663798] [PubMed: 2207416]
- Hamilton W, Sharp DJ, Peters TJ, Round AP. Clinical features of prostate cancer before diagnosis: a population-based, case-control study. British Journal of General Practice. 2006;56(531):756–762. [PMC free article: PMC1920715] [PubMed: 17007705]
- Ramachandran S, Foster MC, Thomas DR, Roalfe AK, Hall RA. An audit of prostate-specific antigen and clinical symptoms in general practice. Postgraduate Medical Journal. 1998;74(867):28–32. [PMC free article: PMC2360786] [PubMed: 9538483]
- Bruyninckx R, Buntinx F, Aertgeerts B, Van, Casteren V. The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. British Journal of General Practice. 2003 Jan 1;53(486):31–35. [PMC free article: PMC1314489] [PubMed: 12564274]
- Collins GS, Altman DG. Identifying patients with undetected renal tract cancer in primary care: An independent and external validation of QCancer (renal) prediction model. Cancer Epidemiology. 2013;37:115–120. [PubMed: 23280341]
- Friedlander DF, Resnick MJ, You C, Bassett J, Yarlagadda V, Penson DF, Barocas DA. Variation in the intensity of hematuria evaluation: A target for primary care quality improvement. American Journal of Medicine. 2014;127:633–640. [PMC free article: PMC4074456] [PubMed: 24486290]
- Hippisley-Cox J, Coupland C. Identifying patients with suspected renal tract cancer in primary care: derivation and validation of an algorithm. British Journal of General Practice. 2012;62(597):e251–e260. [PMC free article: PMC3310031] [PubMed: 22520912]
- Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007 May 19;334(7602):1040. [PMC free article: PMC1871798] [PubMed: 17493982]
- Shephard EA, Stapley S, Neal RD, Rose P, Walter FM, Hamilton WT. Clinical features of bladder cancer in primary care. British Journal of General Practice. 2012;62(602):598–604. [PMC free article: PMC3426598] [PubMed: 22947580]
- Price SJ, Shephard EA, Stapley SA, Barraclough K, Hamilton WT. The risk of bladder cancer with non-visible haematuria: A primary care study using electronic records. British Journal of General Practice. 2014;64:e584–e589.
- Collins GS, Altman DG. Identifying patients with undetected renal tract cancer in primary care: An independent and external validation of QCancer (renal) prediction model. Cancer Epidemiology. 2013;37:115–120. [PubMed: 23280341]
- Deyo RA, Diehl AK. Cancer as a cause of back pain: Frequency, clinical presentation, and diagnostic strategies. Journal of General Internal Medicine. 1988;3:230–238. [PubMed: 2967893]
- Dommett RM, Redaniel MT, Stevens MCG, Hamilton W, Martin RM. Features of childhood cancer in primary care: A population-based nested case-control study. British Journal of Cancer. 2012 Feb 28;106(5):982–987. [PMC free article: PMC3307373] [PubMed: 22240793]
- Dommett RM, Redaniel T, Stevens MCG, Martin RM, Hamilton W. Risk of childhood cancer with symptoms in primary care: A population-based case-control study. British Journal of General Practice. 2013 [PMC free article: PMC3529289] [PubMed: 23336454] [CrossRef]
- Friedlander DF, Resnick MJ, You C, Bassett J, Yarlagadda V, Penson DF, Barocas DA. Variation in the intensity of hematuria evaluation: A target for primary care quality improvement. American Journal of Medicine. 2014;127:633–640. [PMC free article: PMC4074456] [PubMed: 24486290]
- Hippisley-Cox J, Coupland C. Identifying patients with suspected renal tract cancer in primary care: derivation and validation of an algorithm. British Journal of General Practice. 2012;62(597):e251–e260. [PMC free article: PMC3310031] [PubMed: 22520912]
- Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007 May 19;334(7602):1040. [PMC free article: PMC1871798] [PubMed: 17493982]
- Muris JW, Starmans R, Fijten GH, Crebolder HF, Schouten HJ, Knottnerus JA. Non-acute abdominal complaints in general practice: diagnostic value of signs and symptoms. British Journal of General Practice. 1995;45(395):313–316. [PMC free article: PMC1239267] [PubMed: 7619588]
- Oudega R, Moons KGM, Nieuwenhuis HK, van Nierop FL, Hoes AW. Deep vein thrombosis in primary care: Possible malignancy? British Journal of General Practice. 2006;56(530):693–696. [PMC free article: PMC1876636] [PubMed: 16954002]
- Shephard E, Neal R, Rose P, Walter F, Hamilton W. Clinical features of kidney cancer in primary care: A case-control study using primary care records. British Journal of General Practice. 2013 [PMC free article: PMC3609472] [PubMed: 23540481] [CrossRef]
Prostate cancer
Bladder cancer
Renal cancer
Testicular cancer
None
Penile cancer
None
- Urological cancers - Suspected CancerUrological cancers - Suspected Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...