NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Cholesterol and triglycerides are insoluble in water and therefore these lipids must be transported in association with proteins. Lipoproteins are complex particles with a central core containing cholesterol esters and triglycerides surrounded by free cholesterol, phospholipids, and apolipoproteins, which facilitate lipoprotein formation and function. Plasma lipoproteins can be divided into seven classes based on size, lipid composition, and apolipoproteins (chylomicrons, chylomicron remnants, VLDL, VLDL remnants (IDL), LDL, HDL, and Lp (a)). Chylomicron remnants, VLDL, IDL, LDL, and Lp (a) are all pro-atherogenic while HDL is anti-atherogenic. Apolipoproteins have four major functions including 1) serving a structural role, 2) acting as ligands for lipoprotein receptors, 3) guiding the formation of lipoproteins, and 4) serving as activators or inhibitors of enzymes involved in the metabolism of lipoproteins. The exogenous lipoprotein pathway starts with the incorporation of dietary lipids into chylomicrons in the intestine. In the circulation, the triglycerides carried in chylomicrons are metabolized in muscle and adipose tissue by lipoprotein lipase releasing free fatty acids, which are subsequently metabolized by muscle and adipose tissue, and chylomicron remnants are formed. Chylomicron remnants are then taken up by the liver. The endogenous lipoprotein pathway begins in the liver with the formation of VLDL. The triglycerides carried in VLDL are metabolized in muscle and adipose tissue by lipoprotein lipase releasing free fatty acids and IDL are formed. The IDL are further metabolized to LDL, which are taken up by the LDL receptor in numerous tissues including the liver, the predominant site of uptake. Reverse cholesterol transport begins with the formation of nascent HDL by the liver and intestine. These small HDL particles can then acquire cholesterol and phospholipids that are effluxed from cells, a process mediated by ABCA1 resulting in the formation of mature HDL. Mature HDL can acquire addition cholesterol from cells via ABCG1, SR-B1, or passive diffusion. The HDL then transports the cholesterol to the liver either directly by interacting with hepatic SR-B1 or indirectly by transferring the cholesterol to VLDL or LDL, a process facilitated by CETP. Cholesterol efflux from macrophages to HDL plays an important role in protecting from the development of atherosclerosis. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Because lipids, such as cholesterol and triglycerides, are insoluble in water these lipids must be transported in association with proteins (lipoproteins) in the circulation. Large quantities of fatty acids from meals must be transported as triglycerides to avoid toxicity. These lipoproteins play a key role in the absorption and transport of dietary lipids by the small intestine, in the transport of lipids from the liver to peripheral tissues, and the transport of lipids from peripheral tissues to the liver and intestine (reverse cholesterol transport). A secondary function is to transport toxic foreign hydrophobic and amphipathic compounds, such as bacterial toxins, from areas of invasion and infection (1). For example, lipoproteins bind endotoxin (LPS) from gram negative bacteria and lipoteichoic acid from gram positive bacteria thereby reducing their toxic effects (1). In addition, apolipoprotein L1, associated with HDL particles, has lytic activity against the parasite Trypanosoma brucei brucei and lipoproteins can neutralize viruses (2,3). Thus, while this article will focus on the transport properties of lipoproteins the reader should recognize that lipoprotein may have other important roles.
STRUCTURE OF LIPOPROTEINS (4)
Lipoproteins are complex particles that have a central hydrophobic core of non-polar lipids, primarily cholesterol esters and triglycerides. This hydrophobic core is surrounded by a hydrophilic membrane consisting of phospholipids, free cholesterol, and apolipoproteins (Figure 1). Plasma lipoproteins are divided into seven classes based on size, lipid composition, and apolipoproteins (Table 1 and Figure 2).

Figure 1.
Lipoprotein Structure (figure modified from Biochemistry 39: 9763, 2000).
Table 1.
Lipoprotein Classes
| Lipoprotein | Density (g/ml) | Size (nm) | Major Lipids | Major Apoproteins |
|---|---|---|---|---|
| Chylomicrons | <0.930 | 75-1200 | Triglycerides | Apo B-48, Apo C, Apo E, Apo A-I, A-II, A-IV |
| Chylomicron Remnants | 0.930- 1.006 | 30-80 | Triglycerides Cholesterol | Apo B-48, Apo E |
| VLDL | 0.930- 1.006 | 30-80 | Triglycerides | Apo B-100, Apo E, Apo C |
| IDL | 1.006- 1.019 | 25-35 | Triglycerides Cholesterol | Apo B-100, Apo E, Apo C |
| LDL | 1.019- 1.063 | 18- 25 | Cholesterol | Apo B-100 |
| HDL | 1.063- 1.210 | 5- 12 | Cholesterol Phospholipids | Apo A-I, Apo A-II, Apo C, Apo E |
| Lp (a) | 1.055- 1.085 | ~30 | Cholesterol | Apo B-100, Apo (a) |
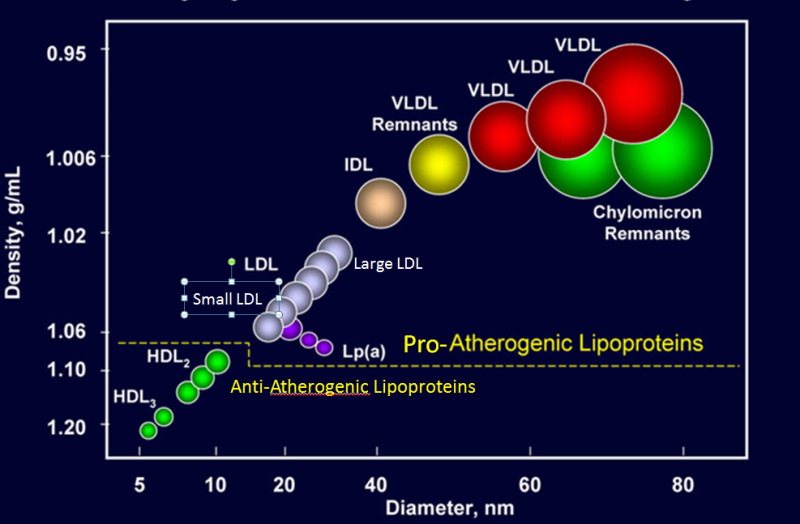
Figure 2.
Classes of Lipoproteins (figure modified from Advances Protein Chemistry 45:303, 1994).
Chylomicrons (5)
These are large triglyceride rich particles made by the intestine, which are involved in the transport of dietary triglycerides and cholesterol to peripheral tissues and liver. These particles contain apolipoproteins A-I, A-II, A-IV, A-V, B-48, C-II, C-III, and E. Apo B-48 is the core structural protein and each chylomicron particle contains one Apo B-48 molecule. The size of chylomicrons varies depending on the amount of fat ingested. A high fat meal leads to the formation of large chylomicron particles due to the increased amount of triglyceride being transported whereas in the fasting state the chylomicron particles are small carrying decreased quantities of triglyceride. The quantity of cholesterol carried by chylomicrons also can vary depending upon dietary intake.
Chylomicron Remnants (5-7)
The removal of triglyceride from chylomicrons by lipoprotein lipase in peripheral tissues results in smaller particles called chylomicron remnants. Compared to chylomicrons these particles are enriched in cholesterol and are pro-atherogenic.
Very Low-Density Lipoproteins (VLDL)
These particles are produced by the liver and are triglyceride rich. They contain apolipoprotein B-100, C-I, C-II, C-III, and E. Apo B-100 is the core structural protein and each VLDL particle contains one Apo B-100 molecule. Similar to chylomicrons the size of the VLDL particles can vary depending on the quantity of triglyceride carried in the particle. When triglyceride production in the liver is increased, the secreted VLDL particles are large. However, VLDL particles are smaller than chylomicrons.
Intermediate-Density Lipoproteins (IDL; VLDL Remnants) (6,7)
The removal of triglycerides from VLDL by muscle and adipose tissue results in the formation of IDL particles which are enriched in cholesterol. These particles contain apolipoprotein B-100 and E. These IDL particles are pro-atherogenic.
Low-Density Lipoproteins (LDL)
These particles are derived from VLDL and IDL particles and they are even further enriched in cholesterol. LDL carries the majority of the cholesterol that is in the circulation. The predominant apolipoprotein is B-100 and each LDL particle contains one Apo B-100 molecule. LDL consists of a spectrum of particles varying in size and density. An abundance of small dense LDL particles is seen in association with hypertriglyceridemia, low HDL levels, obesity, type 2 diabetes (i.e. patients with the metabolic syndrome) and infectious and inflammatory states. These small dense LDL particles are considered to be more pro-atherogenic than large LDL particles for a number of reasons (8). Small dense LDL particles have a decreased affinity for the LDL receptor resulting in a prolonged retention time in the circulation. Additionally, they more easily enter the arterial wall and bind more avidly to intra-arterial proteoglycans, which traps them in the arterial wall. Finally, small dense LDL particles are more susceptible to oxidation, which could result in an enhanced uptake by macrophages.
High-Density Lipoproteins (HDL) (9-11)
These particles play an important role in reverse cholesterol transport from peripheral tissues to the liver, which is one potential mechanism by which HDL may be anti-atherogenic. In addition, HDL particles have anti-oxidant, anti-inflammatory, anti-thrombotic, and anti-apoptotic properties, which may also contribute to their ability to inhibit atherosclerosis. HDL particles are enriched in cholesterol and phospholipids. Apolipoproteins A-I, A-II, A-IV, C-I, C-II, C-III, and E are associated with these particles. Apo A-I is the core structural protein and each HDL particle may contain multiple Apo A-I molecules. In addition, using mass spectrometry proteins involved in proteinase inhibition, complement activation, and the acute-phase response have been found associated with HDL particles (12). HDL particles are very heterogeneous and can be classified based on density, size, charge, or apolipoprotein composition (Table 2).
Table 2.
Classification of HDL
| Method of classification | Types of HDL |
|---|---|
| Density gradient ultracentrifugation | HDL2, HDL3, very high-density HDL |
| Nuclear magnetic resonance | large, medium, and small |
| Gradient gel electrophoresis | HDL 2a, 2b, 3a, 3b, 3c |
| 2-dimensional gel electrophoresis | pre-beta 1 and 2, alpha 1, 2, 3, 4 |
| Apolipoprotein composition | A-I particles, A-I: A-II particles, A-I: E particles |
Lipoprotein (a) (Lp (a)) (13-16)
Lp (a) is an LDL particle that has apolipoprotein (a) attached to Apo B-100 via a disulfide bond. Lp (a) contain Apo (a) and Apo B-100 in a 1:1 molar ratio. The size of Lp(a) particles can vary greatly based on the size of apolipoprotein (a). This particle is pro-atherogenic.
APOLIPOPROTEINS (17,18)
Apolipoproteins have four major functions including 1) serving a structural role, 2) acting as ligands for lipoprotein receptors, 3) guiding the formation of lipoproteins, and 4) serving as activators or inhibitors of enzymes involved in the metabolism of lipoproteins (Table 3). Apolipoproteins thus play a crucial role in lipoprotein metabolism.
Apolipoprotein A-I (19)
Apo A-I is synthesized in the liver and intestine and is the major structural protein of HDL accounting for approximately 70% of HDL protein. It also plays a role in the interaction of HDL with ATP-binding cassette protein A1 (ABCA1), ABCG1, and class B, type I scavenger receptor (SR-B1). Apo A-I is an activator of lecithin: cholesterol acyltransferase (LCAT), an enzyme that converts free cholesterol into cholesteryl ester. High levels of Apo A-I are associated with a decreased risk of atherosclerosis.
Apolipoprotein A-II (20)
Apo A-II is synthesized in the liver and is the second most abundant protein on HDL accounting for approximately 20% of HDL protein. The role of Apo A-II in lipid metabolism is unclear. Apo A-II is a strong predictor of risk for CVD.
Apolipoprotein A-IV (21)
Apo A-IV is synthesized in the intestine during fat absorption. Apo A-IV is associated with chylomicrons and high-density lipoproteins, but is also found in the lipoprotein-free fraction. Its precise role in lipoprotein metabolism remains to be determined but studies have suggested a role for Apo A-IV in regulating food intake.
Apolipoprotein A-V (22,23)
Apo A-V is synthesized in the liver and associates with triglyceride rich lipoproteins. It is an activator of LPL mediated lipolysis and thereby plays an important role in the metabolism of triglyceride rich lipoproteins.
Apolipoprotein B-48 (24)
Apo B-48 is synthesized in the intestine and is the major structural protein of chylomicrons and chylomicron remnants. There is a single molecule of apo B-48 per chylomicron particle. There is a single apolipoprotein B gene that is expressed in both the liver and intestine. The intestine expresses a protein that is approximately ½ the size of the liver due to mRNA editing. The apobec-1 editing complex is expressed in the intestine and edits a specific cytidine to an uracil in the apo B mRNA in the intestine creating a stop codon that results in the cessation of protein translation and a shorter Apo B (Apo B-48). The portion of Apo-B that is recognized by the LDL receptor is not contained in Apo-B48 and therefore Apo B-48 is not recognized by the LDL receptor.
Apolipoprotein B-100
Apo B-100 is synthesized in the liver and is the major structural component of VLDL, IDL, and LDL. There is a single molecule of Apo B-100 per VLDL, IDL, LDL and Lp(a) particle. Apo B-100 is a ligand for the LDL receptor and therefore plays an important role in the clearance of lipoprotein particles. Certain mutations in Apo B-100 result in decreased binding to the LDL receptor and familial hypercholesterolemia (25). High levels of Apo B-100 are associated with an increased risk of atherosclerosis.
Apolipoprotein C (26-29)
The C apolipoproteins are synthesized primarily in the liver and freely exchange between lipoprotein particles and therefore are found in association with chylomicrons, VLDL, and HDL.
Apo C-II is a co-factor for lipoprotein lipase (LPL) and thus stimulates triglyceride hydrolysis and the clearance of triglyceride rich lipoproteins (26,29). Loss of function mutations in Apo C-II result in marked hypertriglyceridemia due to a failure to metabolize triglyceride rich lipoproteins (30).
Apo C-III is an inhibitor of LPL (31). Additionally, Apo C-III inhibits the interaction of triglyceride rich lipoproteins with their receptors (31). Recent studies have shown that loss of function mutations in Apo C-III lead to decreases in serum triglyceride levels and a reduced risk of cardiovascular disease. Interestingly, inhibition of Apo C-III expression results in a decrease in serum triglyceride levels even in patients deficient in lipoprotein lipase indicating that the ability of Apo C-III to modulate serum triglyceride levels is not dependent solely on regulating lipoprotein lipase activity (32).
Apolipoprotein E (33)
Apolipoprotein E is synthesized in many tissues but the liver and intestine are the primary source of circulating Apo E. Apo E exchanges between lipoprotein particles and is associated with chylomicrons, chylomicron remnants, VLDL, IDL, and a subgroup of HDL particles. There are three common genetic variants of Apo E (Apo E2, E3, and E4). ApoE2 differs from the most common isoform, Apo E3, by a single amino acid substitution where cysteine substitutes for arginine at residue 158. Apo E4 differs from Apo E3 at residue 112, where arginine substitutes for cysteine. Apo E3 and E4 are ligands for the LDL receptor while Apo E2 is poorly recognized by the LDL receptor. Patients who are homozygous for Apo E2 can develop familial dysbetalipoproteinemia (30). Apo E4 is associated with an increased risk of Alzheimer’s disease and an increased risk of atherosclerosis.
Apolipoprotein (a) (14,16)
Apo (a) is synthesized in the liver. This protein is a homolog of plasminogen and its molecular weight varies from 300,000 to 800,000. It is attached to Apo B-100 via a disulfide bond. High levels of Apo (a) are associated with an increased risk of atherosclerosis. Apo (a) is an inhibitor of fibrinolysis and can also enhance the uptake of lipoproteins by macrophages, both of which could increase the risk of atherosclerosis. The physiologic function of Apo (a) is unknown. Interestingly this apolipoprotein is found in primates but not in other species.
Table 3.
Apolipoproteins
| Apolipoprotein | MW | Primary Source | Lipoprotein Association | Function |
|---|---|---|---|---|
| Apo A-I | 28,000 | Liver, Intestine | HDL, chylomicrons | Structural protein for HDL, Activates LCAT |
| Apo A-II | 17,000 | Liver | HDL, chylomicrons | Structural protein for HDL, Activates hepatic lipase |
| Apo A-IV | 45,000 | Intestine | HDL, chylomicrons | Unknown |
| Apo A-V | 39,000 | Liver | VLDL, chylomicrons, HDL | Promotes LPL mediated TG lipolysis |
| Apo B-48 | 241,000 | Intestine | Chylomicrons | Structural protein for chylomicrons |
| Apo B-100 | 512,000 | Liver | VLDL, IDL, LDL, Lp (a) | Structural protein, Ligand for LDL receptor |
| Apo C-I | 6,600 | Liver | Chylomicrons, VLDL, HDL | Activates LCAT |
| Apo C-II | 8,800 | Liver | Chylomicrons, VLDL, HDL | Co-factor for LPL |
| Apo C-III | 8,800 | Liver | Chylomicrons, VLDL, HDL | Inhibits LPL and uptake of lipoproteins |
| Apo E | 34,000 | Liver | Chylomicron remnants, IDL, HDL | Ligand for LDL receptor |
| Apo (a) | 250,000- 800,00 | Liver | Lp (a) | Inhibits plasminogen activation |
LIPOPROTEIN RECEPTORS AND LIPID TRANSPORTERS
There are several receptors and transporters that play a crucial role in lipoprotein metabolism.
LDL Receptor (34)
The LDL receptor is present in the liver and most other tissues. It recognizes Apo B-100 and Apo E and hence mediates the uptake of LDL, chylomicron remnants, and IDL, which occurs via endocytosis (Figure 3). After internalization, the lipoprotein particle is degraded in lysosomes and the cholesterol is released. The delivery of cholesterol to the cell decreases the activity of HMGCoA reductase and other enzymes required for the biosynthesis of cholesterol, and the expression of LDL receptors. LDL receptors in the liver play a major role in determining plasma LDL levels (a low number of receptors is associated with high plasma LDL levels while a high number of hepatic LDL receptors is associated with low plasma LDL levels). The number of LDL receptors is regulated by the cholesterol content of the cell (35). When cellular cholesterol levels are decreased the transcription factor SREBP is transported from the endoplasmic reticulum to the Golgi where proteases cleave and activate SREBP, which then migrates to the nucleus and stimulates the expression of LDL receptors (Figure 4). Conversely, when cellular cholesterol levels are high SREBP remains in the endoplasmic reticulum in an inactive form and the expression of LDL receptors is low. As discussed later PCSK9 regulates the rate of degradation of LDL receptors.

Figure 3.
LDL Receptor Pathway (figure modified from Annual Review of Biochemistry 46: 897, 1977).

Figure 4.
SREBP Pathway (figure modified from Journal of Lipid Research 50: Supp S15, 2009).
LDL Receptor Related Protein 1 (LRP-1) (36)
LRP-1 is a member of the LDL receptor family. It is expressed in multiple tissues including the liver. LRP-1 recognizes Apo E and mediates the uptake of chylomicron remnants and IDL (VLDL remnants).
VLDL Receptor (37)
The VLDL receptor is a member of the LDL receptor family. The VLDLR is expressed in the heart, skeletal muscle, adipose tissue, endothelium, brain, macrophages, and other tissues. Interestingly it is not usually expressed in the liver but hepatic expression can be induced by endoplasmic reticulum stress and PPAR alpha activation. Apo E but not Apo B bind to the VLDL receptor thereby allowing for the uptake of triglyceride rich lipoprotein particles (VLDL and chylomicrons).
Class B Scavenger Receptor B1 (SR-B1) (38)
SR-B1 is expressed in the liver, adrenal glands, ovaries, testes, macrophages, and other cells. In the liver and steroid producing cells, it mediates the selective uptake of cholesterol esters from HDL particles. In macrophages and other cells, it facilitates the efflux of cholesterol from the cell to HDL particles.
ATP-Binding Cassette Transporter A1 (ABCA1) (39)
ABCA1 is expressed in many cells including hepatocytes, enterocytes, and macrophages. It mediates the transport of cholesterol and phospholipids from the cell to lipid poor HDL particles (pre-beta-HDL).
ATP-Binding Cassette Transporter G1 (ABCG1) (40)
ABCG1 is expressed in many different cell types and mediates the efflux of cholesterol from the cell to HDL particles.
ATP-Binding Cassette Transporter G5 and G8 (ABCG5/ABCG8) (41,42)
ABCG5 and ABCG8 are expressed in the liver and intestine and form a heterodimer. In the intestine, these transporters mediate the movement of plant sterols and cholesterol from inside the enterocyte into the intestinal lumen thereby decreasing the absorption of cholesterol and limiting the uptake of dietary plant sterols. In the liver, these transporters play a role in the movement of cholesterol and plant sterols into the bile facilitating the excretion of sterols.
Niemann-Pick C1-Like 1 (NPC1L1) (41)
NPC1L1 is expressed in the intestine and mediates the uptake of cholesterol and plant sterols from the intestinal lumen into the enterocyte. NPC1L1 is also expressed in the liver where it mediates the movement of cholesterol from hepatocytes into the bile.
ENZYMES AND TRANSFER PROTEINS INVOLVED IN LIPOPROTEIN METABOLISM
There are several enzymes and transfer proteins that play a key role in lipoprotein metabolism.
Lipoprotein Lipase (LPL) (43)
LPL is synthesized in muscle, heart, and adipose tissue, then secreted and attached to the endothelium of the adjacent blood capillaries. This enzyme hydrolyzes the triglycerides carried in chylomicrons and VLDL to fatty acids, which can be taken up by cells. The catabolism of triglycerides results in the conversion of chylomicrons into chylomicron remnants and VLDL into IDL (VLDL remnants). This enzyme requires Apo C-II as a cofactor. Apo A-V also plays a key role in the activation of this enzyme. In contrast Apo C-III and Apo A-II inhibit the activity of LPL. Insulin stimulates LPL expression and LPL activity is reduced in patients with poorly controlled diabetes, which can impair the metabolism of triglyceride rich lipoproteins leading to hypertriglyceridemia (44).
Hepatic Lipase (45)
Hepatic lipase is localized to the sinusoidal surface of liver cells. It mediates the hydrolysis of triglycerides and phospholipids in IDL and LDL leading to smaller particles (IDL is converted to LDL; LDL is converted from large LDL to small LDL). It also mediates the hydrolysis of triglycerides and phospholipids in HDL resulting in smaller HDL particles.
Endothelial Lipase (46)
Endothelial lipase plays a major role in hydrolyzing the phospholipids in HDL.
Lecithin: Cholesterol Acyltransferase (LCAT) (47)
LCAT is made in the liver. In the plasma, it catalyzes the synthesis of cholesterol esters in HDL by facilitating the transfer of a fatty acid from position 2 of lecithin to cholesterol. This allows for the transfer of the cholesterol from the surface of the HDL particle (free cholesterol) to the core of the HDL particle (cholesterol ester), which facilitates the continued uptake of free cholesterol by HDL particles by reducing the concentration of cholesterol on the surface of HDL.
Cholesteryl Ester Transfer Protein (CETP) (48,49)
This protein is synthesized in the liver and in the plasma mediates the transfer of cholesterol esters from HDL to VLDL, chylomicrons, and LDL and the transfer of triglycerides from VLDL and chylomicrons to HDL. Inhibition of CETP activity leads to an increase in HDL cholesterol and a decrease in LDL cholesterol.
Microsomal Triglyceride Transfer Protein (MTTP) (50)
MTTP is expressed primarily in the liver and small intestine and plays a crucial role in the synthesis of lipoproteins in these tissues. MTTP mediates the transfer of triglycerides to apolipoprotein B-100 in the liver to form VLDL and to apolipoprotein B-48 in the intestine to form chylomicrons.
EXOGENOUS LIPOPROTEIN PATHWAY (CHYLOMICRONS)

Figure 5.
Exogenous Lipoprotein Pathway.
Fat Absorption (51-54)
The exogenous lipoprotein pathway starts in the intestine. Dietary triglycerides (approximately 100 grams per day) are hydrolyzed to free fatty acids and monoacylglycerol by intestinal lipases and emulsified with bile acids, cholesterol, plant sterols, and fat-soluble vitamins to form micelles. While the fatty acids in the intestine are overwhelmingly accounted for by dietary intake the cholesterol in the intestinal lumen is primarily derived from bile (approximately 800-1200mg of cholesterol from bile vs. 200-500mg from diet). Plant sterols account for approximately 25% of dietary sterol intake (approximately 100-150mg/day). The cholesterol, plant sterols, fatty acids, monoacylglycerol, and fat-soluble vitamins contained in the micelles are then transported into the intestinal cells. The uptake of cholesterol and plant sterols from the intestinal lumen into intestinal cells is facilitated by a sterol transporter, Niemann-Pick C1- like 1 protein (NPC1L1) (Figure 6). Ezetimibe, a drug which inhibits intestinal cholesterol and plant sterol uptake, binds to NPC1L1 and inhibits its activity. Once in the intestinal cell the cholesterol and plant sterols may be transported back into the intestinal lumen, a process mediated by ABCG5 and ABCG8, or converted to sterol esters by acyl-CoA cholesterol acyl transferase (ACAT), which attaches a fatty acid to the sterol. Compared to cholesterol, plant sterols are poor substrates for ACAT and therefore the formation of plant sterol esters does not occur as efficiently as the formation of cholesterol esters. In humans, <5% of dietary plant sterols are absorbed and the vast majority are transported out of the intestine cell, a process mediated by ABCG5 and ABCG8, which are very efficient at effluxing plant sterols from the intestinal cell into the intestinal lumen. Patients with sitosterolemia have mutations in either ABCG5 or ABCG8 and net absorption of dietary plant sterols is increased (20-30% absorbed vs. < 5% in normal subjects) (55). Thus, ABCG5 and ABCG8 along with ACAT serve as gate keepers and block the uptake of plant sterols and likely also play an important role in determining the efficiency of cholesterol absorption (humans typically absorb only approximately 50% of dietary cholesterol with a range of 25-75%).

Figure 6.
Intestinal Cell and Sterol Metabolism. C= cholesterol, CE= cholesterol ester.
The pathway of absorption of free fatty acids is not well understood but it is likely that both passive diffusion and specific transporters play a role. The fatty acid transporter CD36 is strongly expressed in the proximal third of the intestine and is localized to the villi. While this transporter likely plays a role in fatty acid uptake by intestinal cells, this transporter is not essential as humans and mice deficient in this protein do not have fat malabsorption. However, in mice deficient in CD36 there is a shift in the absorption of lipid to the distal intestine, suggesting pathways that can compensate for the absence of CD36. Fatty acid transport protein 4 (FATP4) is also highly expressed in the intestine. However, mice deficient in FATP4 do not have abnormalities in fat absorption. It is likely that there are multiple pathways for the absorption of fatty acids into intestinal cells. The pathways by which monoacylglycerols are absorbed by intestinal cells remain to be defined.
Formation of Chylomicrons (51,54)
The absorbed fatty acids and monoacylglycerols are utilized to synthesize triglycerides. The key enzymes required for triglyceride synthesis are monoacylglycerol acyltransferase (MGAT) and diacylglycerol transferase (DGAT). MGAT catalyzes the addition of a fatty acid to monoacylglycerol while DGAT catalyzes the addition of a fatty acid to diacylglycerol resulting in triglyceride formation. As noted above, the majority of the cholesterol absorbed by the intestine is esterified to cholesterol esters by ACAT. The triglycerides and cholesterol esters are packaged into chylomicrons in the endoplasmic reticulum. The size and composition of the chylomicrons formed in the intestine are dependent on the amount of fat ingested and absorbed by the intestine and the type of fat absorbed. Increased fat absorption results in larger chylomicrons. The formation of chylomicrons in the endoplasmic reticulum requires the synthesis of Apo B-48 by the intestinal cell (Figure 6). Microsomal triglyceride transfer protein (MTTP) is required for the movement of lipid from the endoplasmic reticulum to the Apo B-48. The absence of MTTP results in the inability to form chylomicrons (Abetalipoproteinemia) (56). Lomitapide inhibits MTTP function and is used to treat patients with homozygous Familial Hypercholesterolemia (57).
Chylomicron Metabolism (26,31,43,58-62)
Chylomicrons are secreted into the lymph and delivered via the thoracic duct to the circulation. It should be noted that this results in the newly formed chylomicrons being delivered to the systemic circulation and not delivered directly to the liver via the portal circulation. This facilitates the delivery of the nutrients contained in the chylomicrons to muscle and adipose tissue. In muscle and adipose tissue lipoprotein lipase (LPL) is expressed at high levels. LPL is synthesized in muscle and adipocytes and transported to the luminal surface of capillaries. Lipase maturation factor 1 plays a key role in the stabilization and movement of LPL from muscle cells and adipocytes to the capillary endothelial cell surface. Glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 (GPIHBP1) binds LPL and transports it to the capillary lumen and anchors LPL to the capillary endothelium. Activation of LPL by Apo C-II, carried on the chylomicrons, leads to the hydrolysis of the triglycerides that are carried in the chylomicrons resulting in the formation of free fatty acids, which can be taken up by the adjacent muscle cells and adipocytes for either energy production or storage. Fatty acid transport proteins (FATPs) and CD36 facilitate the uptake of fatty acids into adipocytes and muscle cells. Some of the free fatty acids released from chylomicrons bind to albumin and can be transported to other tissues. Apo A-V also plays an important role in activating LPL activity. Loss of function mutations in LPL, Apo C-II, GPIHPB1, lipase maturation factor 1, and Apo A-V can result in marked hypertriglyceridemia (familial chylomicronemia syndrome) (30). In addition, there are proteins that inhibit LPL activity. Apo C-III inhibits LPL activity and loss of function mutations in this gene are associated with increases in LPL activity and decreases in plasma triglyceride levels. Similarly, angiopoietin like protein 3 and 4, which target LPL for inactivation, regulate LPL activity. Loss of function mutations in these proteins also are associated with decreases in plasma triglyceride levels. Finally, the expression of LPL by muscle cells and adipocytes is regulated by hormones (particularly insulin), nutritional status, and inflammation.
The metabolism of the triglycerides carried in the chylomicrons results in a marked decrease in the size of these particles leading to the formation of chylomicron remnants, which are enriched in cholesterol esters and acquire Apo E. As these particles decrease in size phospholipids and apolipoproteins (Apo A and C) on the surface of the chylomicrons are transferred to other lipoproteins, mainly HDL. The transfer of Apo C-II from chylomicrons to HDL decreases the ability of LPL to further breakdown triglycerides. These chylomicron remnants are cleared from the circulation by the liver. The Apo E on the chylomicron remnants binds to the LDL receptor and other hepatic receptors such as LRP and syndecan-4 and the entire particle is taken up by the hepatocytes. Apo E is crucial for this process and mutations in Apo E (for example homozygosity for the Apo E2 isoform) can result in decreased chylomicron clearance and elevations in plasma cholesterol and triglyceride levels (familial dysbetalipoproteinemia) (30).
The exogenous lipoprotein pathway results in the efficient transfer of dietary fatty acids to muscle and adipose tissue for energy utilization and storage. The cholesterol is delivered to the liver where it can be utilized for the formation of VLDL, bile acids, or secreted back to the intestine via secretion into the bile. In normal individuals, this pathway can handle large amounts of fat (100 grams or more per day) without resulting in marked increases in plasma triglyceride levels. In fact, in a normal individual, a meal containing 75 grams of fat results in only a very modest increase in postprandial triglyceride levels.
ENDOGENOUS LIPOPROTEIN PATHWAY (VLDL AND LDL)

Figure 7.
Endogenous Lipoprotein Pathway.
Formation of VLDL (50,63,64)
In the liver triglycerides and cholesterol esters are transferred in the endoplasmic reticulum to newly synthesized Apo B-100. Similar to the intestine this transfer is mediated by MTTP. The availability of triglycerides is the primary determinant of the rate of VLDL synthesis. If the supply of triglyceride is limited the newly synthesized Apo B is rapidly degraded. Thus, in contrast to many proteins the rate of synthesis of the Apo B-100 is not the major determinant of the rate of secretion. Rather the amount of lipid available determines whether Apo B-100 is degraded or secreted. MTTP is required for the early addition of lipid to Apo B-100 particles but additional lipid is added via pathways that do not require MTTP. Additionally, the size of the VLDL particles is determined by the availability of triglycerides. When triglycerides are abundant the VLDL particles are large.
The quantity of fatty acids available for the synthesis of triglycerides is the main determinant of triglyceride synthesis in the liver. The major sources of fatty acids are a) de novo fatty acid synthesis, b) the hepatic uptake of triglyceride rich lipoproteins, and c) the flux of fatty acids from adipose tissue to the liver. Diabetes, obesity, and the metabolic syndrome are common causes of an increase in hepatic triglyceride levels and the increased secretion of VLDL (44,65).
Loss of function mutations in either Apo B-100 or MTTP result in the failure to produce VLDL and marked decreases in plasma triglyceride and cholesterol levels (familial hypobetalipoproteinemia or abetalipoproteinemia) (56). The precise pathway by which the newly synthesized VLDL particles are secreted from the hepatocyte into the circulation is not resolved.
VLDL Metabolism (6,58)
VLDL particles are transported to peripheral tissues where the triglycerides are hydrolyzed by LPL and fatty acids are released. This process is very similar to that described above for chylomicrons and there is competition between the metabolism of chylomicrons and VLDL. High levels of chylomicrons can inhibit the clearance of VLDL. The removal of triglycerides from VLDL results in the formation of VLDL remnants (Intermediate density lipoproteins (IDL)). These IDL particles are relatively enriched in cholesterol esters and acquire Apo E from HDL particles. In a pathway analogous to the removal of chylomicron remnants these IDL particles can be removed from the circulation by the liver via binding of Apo E to LDL and LRP receptors. However, while the vast majority of chylomicron remnants are rapidly cleared from the circulation by the liver, only a fraction of IDL particles are cleared (approximately 50% but varies). The remaining triglycerides in the IDL particles are hydrolyzed by hepatic lipase leading to a further decrease in triglyceride content and the exchangeable apolipoproteins are transferred from the IDL particles to other lipoproteins leading to the formation of LDL. These LDL particles predominantly contain cholesterol esters and Apo B-100. Thus, LDL is a product of VLDL metabolism.
LDL Metabolism (34,66-69)
The levels of plasma LDL are determined by the rate of LDL production and the rate of LDL clearance, both of which are regulated by the number of LDL receptors in the liver. The production rate of LDL from VLDL is partially determined by hepatic LDL receptor activity with a high LDL receptor activity resulting in a decrease in LDL production due to an increase in IDL uptake. Conversely, low LDL receptor activity results in an increase in LDL production formation due to a decrease in IDL uptake. With regards to LDL clearance, approximately 70% of circulating LDL is cleared via hepatocyte LDL receptor mediated endocytosis with the remainder taken up by extrahepatic tissues. An increase in the number of hepatic LDL receptors therefore increases LDL clearance leading to a decrease in plasma LDL levels. Conversely, a decrease in hepatic LDL receptors slows LDL clearance leading to an increase in plasma LDL levels. Thus, the level of hepatic LDL receptors plays a key role in regulating plasma LDL levels. Many of the drugs used to lower plasma LDL levels, such as the statins, ezetimibe, PCSK9 inhibitors, bile acid sequestrants and bempedoic acid lower plasma LDL levels by increasing the number of hepatic LDL receptors (57).
The levels of LDL receptors in the liver are mainly regulated by the cholesterol content of the hepatocyte. As cholesterol levels in the cell decrease, inactive sterol regulatory element binding proteins (SREBPs), which are transcription factors that mediate the expression of LDL receptors and key genes involved in cholesterol and fatty acid metabolism, are transported from the endoplasmic reticulum to the Golgi where proteases cleave the SREBPs into active transcription factors (Figure 4). These active SREBPs move to the nucleus where they stimulate the transcription of the LDL receptor and enzymes required for cholesterol synthesis, including HMG-CoA reductase, the rate limiting enzyme in cholesterol synthesis. If cholesterol levels in the cell are high, then the SREBPs remain in the endoplasmic reticulum in an inactive form and do not stimulate LDL receptor synthesis. In addition, cholesterol in the cell is oxidized and oxidized sterols activate LXR, a nuclear hormone receptor that is a transcription factor, which stimulates the transcription of E3 ubiquitin ligase that mediates the ubiquitination and degradation of the low-density lipoprotein receptor (Inducible degrader of the low-density lipoprotein receptor (IDOL)). Thus, the cell can sense the availability of cholesterol and regulate LDL receptor activity. If the cholesterol content of the cell is decreased LDL receptor activity is increased to allow for the increased uptake of cholesterol. Conversely, if the cholesterol content of the cell is increased LDL receptor activity is decreased and the uptake of LDL by the cell is diminished. Statins, ezetimibe, bile acid sequestrants, and bempedoic acid decrease hepatic cholesterol levels thereby increasing LDL receptor levels and decreasing plasma LDL levels (57). Finally, the LDL receptor is targeted for degradation by PCSK9, a secreted protein that binds to the LDL receptor and enhances LDL receptor degradation in the lysosomes. Loss of function mutations in PCSK9 and drugs that inhibit PCSK9 result in increased LDL receptor activity and decreased LDL levels while gain of function mutations in PCSK9 lead to decreased LDL receptor activity and elevations in LDL levels.
Thus, the endogenous lipoprotein pathway facilitates the movement of triglycerides synthesized in the liver to muscle and adipose tissue. Additionally, it also provides a pathway for the transport of cholesterol from the liver to peripheral tissues.
HDL METABOLISM AND REVERSE CHOLESTEROL TRANSPORT (38-40,47,48,70,71)

Figure 8.
HDL Metabolism.
HDL Formation
Several steps are required to generate mature HDL particles. The first step involves the synthesis of the main structural protein contained in HDL, Apo A-I. Apo A-I is synthesized predominantly by the liver and intestine. After Apo A-I is secreted, it acquires cholesterol and phospholipids that are effluxed from hepatocytes and enterocytes. The efflux of cholesterol and phospholipids to the newly synthesized lipid poor Apo A-I (pre-beta HDL) is facilitated by ABCA1. Patients with loss of function mutations in ABCA1 (Tangiers disease) fail to lipidate the newly secreted Apo A-I leading to the rapid catabolism of Apo A-I and very low HDL levels (72). Using mice with targeted knock-out of ABCA1 it has been shown that HDL cholesterol levels are reduced by 80% in mice lacking ABCA1 in the liver and 30% in mice lacking ABCA1 in the intestine. While initially cholesterol and phospholipids are obtained from the liver and intestine, HDL also acquires lipid from other tissues and from other lipoproteins. Muscle cells, adipocytes, and other tissues express ABCA1 and ABCG1 and are able to transfer cholesterol and phospholipids to Apo A-I particles. Additionally, as noted above, newly formed HDL can also obtain cholesterol and phospholipids from chylomicrons and VLDL during their lipolysis by LPL. This accounts for the observation that patients with high plasma triglyceride levels due to decreased clearance frequently have low HDL cholesterol levels. Additionally, phospholipid transfer protein (PLTP) facilitates the movement of phospholipids between lipoproteins; mice lacking PLTP have a marked reduction in HDL cholesterol and Apo A-I levels. Finally, the lipolysis of triglyceride rich lipoproteins also results in the transfer of apolipoproteins from these particles to HDL.
HDL Cholesterol Esterification
As noted earlier the cholesterol in the core of HDL is esterified (cholesterol esters). The cholesterol that is effluxed from cells to HDL is free cholesterol and is localized on the surface of HDL particles. In order to form mature large spherical HDL particles with a core of cholesterol esters the free cholesterol transferred from cells to the surface of HDL particles must be esterified. LCAT, an HDL associated enzyme catalyzes the transfer of a fatty acid from phospholipids to free cholesterol resulting in the formation of cholesterol esters. The cholesterol ester formed is then able to move from the surface of the HDL particle to the core allowing additional free cholesterol to be transferred from cells to HDL particles. Apo A-I is an activator of LCAT and facilitates this esterification process. LCAT activity is required for the formation of large HDL particles. LCAT deficiency in humans results in decreased HDL cholesterol and Apo A-I levels and a higher percentage of small HDL particles (72).
HDL Metabolism
Lipases and transfer proteins play an important role in determining the size and composition of HDL particles. The cholesterol ester carried in the core of HDL particles may be transferred to Apo B containing particles in exchange for triglyceride. This transfer is mediated by CETP and results in HDL enriched in triglycerides which may then be metabolized by lipases. Humans deficient in CETP activity have very high HDL cholesterol levels and large HDL particles (72). CETP also impacts LDL cholesterol levels and the absence of CETP results in a decrease in LDL cholesterol. Mice do not have CETP and have relatively high HDL cholesterol levels and low LDL cholesterol levels. Hepatic lipase hydrolyzes both triglycerides and phospholipids in HDL. The triglycerides that are transferred to HDL by CETP activity are catabolized by hepatic lipase resulting in the formation of small HDL particles and Apo A-I more easily disassociates from small HDL resulting in the release of Apo A-I and increased Apo A-I degradation. Genetic deficiency of hepatic lipase results in a modest elevation in HDL cholesterol levels and larger HDL particles (72). Hepatic lipase activity is increased in insulin resistant states and this is associated with reduced HDL cholesterol levels. Endothelial cell lipase is a phospholipase that hydrolyzes the phospholipids carried in HDL particles. In mice increased endothelial lipase activity results in decreased HDL cholesterol levels while decreased endothelial lipase activity increases HDL cholesterol levels.
The cholesterol carried on HDL is primarily delivered to the liver. The uptake of HDL cholesterol by the liver is mediated by SR-BI, which promotes the selective uptake of HDL cholesterol. The HDL particle binds to SR-BI and the cholesterol in HDL is transported into the liver without internalization of the HDL particle. A smaller cholesterol depleted HDL particle is formed, which is then released back into the circulation. In SR-BI deficient mice there is a marked increase in HDL cholesterol levels. Interestingly the risk of atherosclerosis is increased in these SR-BI deficient mice despite an increase in HDL cholesterol levels. Notably, while HDL cholesterol levels are increased in SR-B1 deficient mice the reverse cholesterol transport pathway is actually reduced. While in mice the physiological importance of the hepatic SR-BI pathway is clear, the role in humans is uncertain. In mice, the movement of cholesterol from peripheral tissues to the liver is dependent solely on SR-BI while in humans CETP can facilitate the transport of cholesterol from HDL to Apo B containing lipoproteins, which serves as an alternative pathway for the transport cholesterol to the liver.
Apo A-I is metabolized independently of HDL cholesterol. Most of the Apo A-I is catabolized by the kidneys with the remainder catabolized by the liver. Lipid free or lipid poor Apo A-I is filtered by the kidneys and then taken up by the renal tubules. The size of the Apo A-I particle determines whether it can be filtered by the kidneys and hence the degree of lipidation of Apo A-I determines the rate of catabolism. Conditions or disease states (for example Tangiers disease, which is due to a mutation in ABCA1, or LCAT deficiency) that result in lipid poor HDL led to the accelerated catabolism of Apo A-I by the kidney. Apo A-I binds to cubilin, which in conjunction with megalin, a member of the LDL receptor gene family, leads to the uptake and degradation of filtered Apo A-I by renal tubular cells. While the liver is also involved in the catabolism of Apo A-I, the mechanisms are poorly understood. HDL particles may contain Apo E and it is therefore possible that Apo E containing HDL particles are taken up via the LDL receptor and other Apo E receptors in the liver and degraded.
Reverse Cholesterol Transport (73-78)
Peripheral cells accumulate cholesterol through the uptake of circulating lipoproteins and de novo cholesterol synthesis. Most cells do not have a mechanism for catabolizing cholesterol. Cells that synthesize steroid hormones can convert cholesterol to glucocorticoids, estrogen, testosterone, etc. Intestinal cells, sebocytes, and keratinocytes can secrete cholesterol into the intestinal lumen or onto the skin surface thereby eliminating cholesterol. However, in order for most cells to decrease their cholesterol content reverse cholesterol transport is required. From a clinical point of view, the ability of macrophages in the arterial wall to efficiently efflux cholesterol into the reverse cholesterol transport pathway may play an important role in the prevention of atherosclerosis.
As noted earlier ABCA1 plays an important role in the efflux of cholesterol to lipid poor pre-beta Apo A-I particles (Figure 9). ABCG1 plays an important role in the efflux of cholesterol from cells to mature HDL particles. In some studies, SR-B1 also plays a role in the efflux of cholesterol to mature HDL particles. Additionally, passive diffusion of cholesterol from the plasma membrane to HDL may also contribute to cholesterol efflux. The levels of both ABCA1 and ABCG1 are increased by LXR activation. LXR is a nuclear hormone transcription factor that is activated by oxysterols. As the cholesterol levels in a cell increase the formation of oxysterols increases leading to the activation of LXR resulting in an increase in ABCA1 and ABCG1 expression, which will result in the enhanced efflux of cholesterol from the cell to HDL. Additionally, ABCA1 and ABCG1 mRNAs are targeted for degradation by miR-33, a microRNA that is embedded within the SREBP2 gene. An increase in cellular cholesterol decreases the expression of SREBP2 leading to a decrease in miR-33 resulting in enhanced LXR expression. Thus, the decrease in SREBP2 transcription will lead to a decrease in LDL receptor activity and a reduction in cholesterol uptake, while simultaneously, a decrease in miR-33 will lead to an increase in LXR activity stimulating the expression of ABCA1 and ABCG1 resulting in increased cholesterol efflux. Conversely a decrease in cellular cholesterol levels will increase SREBP2 expression resulting in an increase in LDL receptor activity and an increase in miR-33, which will result in a decrease in LXR activity, decreased expression of ABCA1 and ABCG1, and a reduction in cholesterol efflux. Together changes in cholesterol uptake mediated by the LDL receptor and cholesterol efflux mediated by ABCA1 and ABCG1 will maintain cellular cholesterol homeostasis.

Figure 9.
Cholesterol Efflux from Macrophages (modified from J. Clinical Investigation 116: 3090, 2006).
Once cholesterol is transferred from cells to HDL there are two pathways for the cholesterol to be transported and taken up by the liver. As discussed earlier, HDL can interact with hepatic SR-BI receptors resulting in the selective uptake of cholesterol from HDL particles. Alternatively, CETP can transfer cholesterol from HDL particles to Apo B containing particles with the subsequent uptake of Apo B containing lipoproteins by the liver. After the delivery of cholesterol to the liver there are several pathways by which the cholesterol can be eliminated. Cholesterol can be converted to bile acids and secreted in the bile. Alternatively, cholesterol can be directly secreted into the bile. ABCG5 and ABCG8 promote the transport of cholesterol into the bile and the expression of these genes is enhanced by LXR activation. Thus, an increase in hepatic cholesterol levels leading to increased oxysterol production will activate LXR resulting in the increased expression of ABCG5 and ABCG8 facilitating the secretion of cholesterol in the bile.
Evidence suggests that reverse cholesterol transport plays an important role in protecting from the development of atherosclerosis. It should be noted that HDL cholesterol levels may not be indicative of the rate of reverse cholesterol transport. As described above reverse cholesterol transport involves several steps and the level of HDL cholesterol may not accurately reflect these steps. For example, studies have shown that the ability of HDL to promote cholesterol efflux from macrophages can vary. Thus, the same level of HDL cholesterol may not have equivalent abilities to mediate the initial step of reverse cholesterol transport.
LIPOPROTEIN (a) (14-16,79)

Figure 10.
Lp (a).
Lp (a) consists of an LDL molecule and a unique apolipoprotein (a), which is attached to the Apo B-100 of the LDL via a single disulfide bound. Lp (a) contain Apo (a) and Apo B-100 in a 1:1 molar ratio. Like Apo B-100, apo (a) is also made by hepatocytes. Apo (a) contains multiple kringle motifs that are similar to the kringle repeats in plasminogen. The number of kringle repeats can vary and thus the molecular weight of apo (a) can range from 250,000 to 800,000. The levels of Lp (a) in plasma can vary more than a 1000-fold ranging from undetectable to greater than 100mg/dl. Lp (a) levels largely reflect Lp (a) production rates, which are primarily genetically regulated and not greatly affected by environmental factors. Individuals with high molecular weight Apo (a) proteins tend to have lower levels of Lp (a) while individuals with low molecular weight Apo (a) tend to have higher levels. It is hypothesized that the liver is less efficient in secreting high molecular weight Apo (a). The mechanism of Lp (a) clearance is uncertain but does not appear to primarily involve LDL receptors. Therapies that accelerate LDL clearance and lower LDL levels do not lower Lp (a) levels (for example statin therapy). The kidney appears to play an important role in Lp (a) clearance as kidney disease is associated with delayed clearance and elevations in Lp (a) levels.
Elevated plasma Lp(a) levels are associated with an increased risk of atherosclerosis. Apo (a) is an inhibitor of fibrinolysis and enhances the uptake of lipoproteins by macrophages, both of which could account for the increased the risk of atherosclerosis in individuals with elevated Apo (a) levels. Additionally, Lp (a) is the major lipoprotein carrier of oxidized phospholipids, which are inflammatory and could also increase the risk of atherosclerosis. The physiologic function of Apo (a) is unknown. Apo (a) is found in primates but not in other species.
ACKNOWLEDGEMENTS
This work was supported by grants from the Northern California Institute for Research and Education.
REFERENCES
- 1.
- Feingold KR, Grunfeld C. Lipids: a key player in the battle between the host and microorganisms. J Lipid Res 2012; 53:2487-2489 [PMC free article: PMC3494250] [PubMed: 23075464]
- 2.
- Nielsen LB, Nielsen MJ, Moestrup SK. Lipid metabolism: an apolipoprotein-derived weapon combating trypanosoma infection. Curr Opin Lipidol 2006; 17:699-701 [PubMed: 17095915]
- 3.
- Feingold KR. The bidirectional link between HDL and COVID-19 infections. J Lipid Res 2021; 62:100067 [PMC free article: PMC7963524] [PubMed: 33741421]
- 4.
- Smith LC, Pownall HJ, Gotto AM, Jr. The plasma lipoproteins: structure and metabolism. Annu Rev Biochem 1978; 47:751-757 [PubMed: 209732]
- 5.
- Julve J, Martin-Campos JM, Escola-Gil JC, Blanco-Vaca F. Chylomicrons: Advances in biology, pathology, laboratory testing, and therapeutics. Clin Chim Acta 2016; 455:134-148 [PubMed: 26868089]
- 6.
- Chait A, Ginsberg HN, Vaisar T, Heinecke JW, Goldberg IJ, Bornfeldt KE. Remnants of the Triglyceride-Rich Lipoproteins, Diabetes, and Cardiovascular Disease. Diabetes 2020; 69:508-516 [PMC free article: PMC7085249] [PubMed: 32198194]
- 7.
- Krauss RM, King SM. Remnant lipoprotein particles and cardiovascular disease risk. Best Pract Res Clin Endocrinol Metab 2023; 37:101682 [PubMed: 35718703]
- 8.
- Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002; 43:1363-1379 [PubMed: 12235168]
- 9.
- Asztalos BF, Niisuke K, Horvath KV. High-density lipoprotein: our elusive friend. Curr Opin Lipidol 2019; 30:314-319 [PubMed: 31145119]
- 10.
- Thakkar H, Vincent V, Sen A, Singh A, Roy A. Changing Perspectives on HDL: From Simple Quantity Measurements to Functional Quality Assessment. J Lipids 2021; 2021:5585521 [PMC free article: PMC8096543] [PubMed: 33996157]
- 11.
- Thomas SR, Zhang Y, Rye KA. The pleiotropic effects of high-density lipoproteins and apolipoprotein A-I. Best Pract Res Clin Endocrinol Metab 2023; 37:101689 [PubMed: 36008277]
- 12.
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007; 117:746-756 [PMC free article: PMC1804352] [PubMed: 17332893]
- 13.
- Kostner KM, Kostner GM. Lipoprotein (a): a historical appraisal. J Lipid Res 2017; 58:1-14 [PMC free article: PMC5234731] [PubMed: 27821413]
- 14.
- Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 2016; 57:1953-1975 [PMC free article: PMC5087876] [PubMed: 27677946]
- 15.
- Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res 2016; 57:1339-1359 [PMC free article: PMC4959873] [PubMed: 27074913]
- 16.
- Khovidhunkit W. Lipoprotein(a). In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2023.
- 17.
- Mahley RW, Innerarity TL, Rall SC, Jr., Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res 1984; 25:1277-1294 [PubMed: 6099394]
- 18.
- Breslow JL. Human apolipoprotein molecular biology and genetic variation. Annu Rev Biochem 1985; 54:699-727 [PubMed: 3896129]
- 19.
- Frank PG, Marcel YL. Apolipoprotein A-I: structure-function relationships. J Lipid Res 2000; 41:853-872 [PubMed: 10828078]
- 20.
- Chan DC, Ng TW, Watts GF. Apolipoprotein A-II: evaluating its significance in dyslipidaemia, insulin resistance, and atherosclerosis. Ann Med 2012; 44:313-324 [PubMed: 21501035]
- 21.
- Wang F, Kohan AB, Lo CM, Liu M, Howles P, Tso P. Apolipoprotein A-IV: a protein intimately involved in metabolism. J Lipid Res 2015; 56:1403-1418 [PMC free article: PMC4513983] [PubMed: 25640749]
- 22.
- Hubacek JA. Apolipoprotein A5 fifteen years anniversary: Lessons from genetic epidemiology. Gene 2016; 592:193-199 [PMC free article: PMC7108956] [PubMed: 27496343]
- 23.
- Sharma V, Forte TM, Ryan RO. Influence of apolipoprotein A-V on the metabolic fate of triacylglycerol. Curr Opin Lipidol 2013; 24:153-159 [PMC free article: PMC3645348] [PubMed: 23241513]
- 24.
- Anant S, Davidson NO. Molecular mechanisms of apolipoprotein B mRNA editing. Curr Opin Lipidol 2001; 12:159-165 [PubMed: 11264987]
- 25.
- Levenson AE, de Ferranti SD. Familial Hypercholesterolemia. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2023.
- 26.
- Wolska A, Dunbar RL, Freeman LA, Ueda M, Amar MJ, Sviridov DO, Remaley AT. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017; 267:49-60 [PMC free article: PMC5705268] [PubMed: 29100061]
- 27.
- Ramms B, Gordts P. Apolipoprotein C-III in triglyceride-rich lipoprotein metabolism. Curr Opin Lipidol 2018; 29:171-179 [PubMed: 29547399]
- 28.
- D'Erasmo L, Di Costanzo A, Gallo A, Bruckert E, Arca M. ApoCIII: A multifaceted protein in cardiometabolic disease. Metabolism 2020; 113:154395 [PubMed: 33058850]
- 29.
- Wolska A, Reimund M, Remaley AT. Apolipoprotein C-II: the re-emergence of a forgotten factor. Curr Opin Lipidol 2020; 31:147-153 [PubMed: 32332429]
- 30.
- Patni N, Ahmad Z, Wilson DP. Genetics and Dyslipidemia. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2023.
- 31.
- Taskinen MR, Boren J. Why Is Apolipoprotein CIII Emerging as a Novel Therapeutic Target to Reduce the Burden of Cardiovascular Disease? Curr Atheroscler Rep 2016; 18:59 [PMC free article: PMC5018018] [PubMed: 27613744]
- 32.
- Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, Yang Q, Hughes SG, Geary RS, Arca M, Stroes ESG, Bergeron J, Soran H, Civeira F, Hemphill L, Tsimikas S, Blom DJ, O'Dea L, Bruckert E. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N Engl J Med 2019; 381:531-542 [PubMed: 31390500]
- 33.
- Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl) 2016; 94:739-746 [PMC free article: PMC4921111] [PubMed: 27277824]
- 34.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431-438 [PMC free article: PMC2740366] [PubMed: 19299327]
- 35.
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell 2006; 124:35-46 [PubMed: 16413480]
- 36.
- van de Sluis B, Wijers M, Herz J. News on the molecular regulation and function of hepatic low-density lipoprotein receptor and LDLR-related protein 1. Curr Opin Lipidol 2017; 28:241-247 [PMC free article: PMC5482905] [PubMed: 28301372]
- 37.
- Krauss RM, Lu JT, Higgins JJ, Clary CM, Tabibiazar R. VLDL receptor gene therapy for reducing atherogenic lipoproteins. Mol Metab 2023; 69:101685 [PMC free article: PMC9950951] [PubMed: 36739970]
- 38.
- Trigatti BL. SR-B1 and PDZK1: partners in HDL regulation. Curr Opin Lipidol 2017; 28:201-208 [PubMed: 28134663]
- 39.
- Wang S, Smith JD. ABCA1 and nascent HDL biogenesis. Biofactors 2014; 40:547-554 [PMC free article: PMC4294467] [PubMed: 25359426]
- 40.
- Baldan A, Tarr P, Lee R, Edwards PA. ATP-binding cassette transporter G1 and lipid homeostasis. Curr Opin Lipidol 2006; 17:227-232 [PubMed: 16680026]
- 41.
- Kidambi S, Patel SB. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: a review. Xenobiotica 2008; 38:1119-1139 [PubMed: 18668442]
- 42.
- Patel SB, Graf GA, Temel RE. ABCG5 and ABCG8: more than a defense against xenosterols. J Lipid Res 2018; 59:1103-1113 [PMC free article: PMC6027916] [PubMed: 29728459]
- 43.
- Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol 2016; 27:233-241 [PubMed: 27031275]
- 44.
- Feingold KR. Dyslipidemia in Patients with Diabetes. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2023.
- 45.
- Kobayashi J, Miyashita K, Nakajima K, Mabuchi H. Hepatic Lipase: a Comprehensive View of its Role on Plasma Lipid and Lipoprotein Metabolism. J Atheroscler Thromb 2015; 22:1001-1011 [PubMed: 26194979]
- 46.
- Yasuda T, Ishida T, Rader DJ. Update on the role of endothelial lipase in high-density lipoprotein metabolism, reverse cholesterol transport, and atherosclerosis. Circ J 2010; 74:2263-2270 [PubMed: 20962428]
- 47.
- Ossoli A, Simonelli S, Vitali C, Franceschini G, Calabresi L. Role of LCAT in Atherosclerosis. J Atheroscler Thromb 2016; 23:119-127 [PubMed: 26607351]
- 48.
- Mabuchi H, Nohara A, Inazu A. Cholesteryl ester transfer protein (CETP) deficiency and CETP inhibitors. Mol Cells 2014; 37:777-784 [PMC free article: PMC4255097] [PubMed: 25410905]
- 49.
- Shrestha S, Wu BJ, Guiney L, Barter PJ, Rye KA. Cholesteryl ester transfer protein and its inhibitors. J Lipid Res 2018; 59:772-783 [PMC free article: PMC5928430] [PubMed: 29487091]
- 50.
- Hooper AJ, Burnett JR, Watts GF. Contemporary aspects of the biology and therapeutic regulation of the microsomal triglyceride transfer protein. Circ Res 2015; 116:193-205 [PubMed: 25552696]
- 51.
- Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev 2012; 92:1061-1085 [PMC free article: PMC3589762] [PubMed: 22811425]
- 52.
- D'Aquila T, Hung YH, Carreiro A, Buhman KK. Recent discoveries on absorption of dietary fat: Presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta 2016; 1861:730-747 [PMC free article: PMC5503208] [PubMed: 27108063]
- 53.
- Hussain MM. Intestinal lipid absorption and lipoprotein formation. Curr Opin Lipidol 2014; 25:200-206 [PMC free article: PMC4265799] [PubMed: 24751933]
- 54.
- Kindel T, Lee DM, Tso P. The mechanism of the formation and secretion of chylomicrons. Atheroscler Suppl 2010; 11:11-16 [PubMed: 20493784]
- 55.
- Liebeskind A, Peterson AL, Wilson D. Sitosterolemia. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2023.
- 56.
- Shapiro MD, Feingold KR. Monogenic Disorders Causing Hypobetalipoproteinemia. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2021.
- 57.
- Feingold KR. Cholesterol Lowering Drugs. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2021.
- 58.
- Dallinga-Thie GM, Franssen R, Mooij HL, Visser ME, Hassing HC, Peelman F, Kastelein JJ, Peterfy M, Nieuwdorp M. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis 2010; 211:1-8 [PMC free article: PMC3924774] [PubMed: 20117784]
- 59.
- Dijk W, Kersten S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr Opin Lipidol 2016; 27:249-256 [PubMed: 27023631]
- 60.
- Fong LG, Young SG, Beigneux AP, Bensadoun A, Oberer M, Jiang H, Ploug M. GPIHBP1 and Plasma Triglyceride Metabolism. Trends Endocrinol Metab 2016; 27:455-469 [PMC free article: PMC4927088] [PubMed: 27185325]
- 61.
- Peterfy M. Lipase maturation factor 1: a lipase chaperone involved in lipid metabolism. Biochim Biophys Acta 2012; 1821:790-794 [PMC free article: PMC3288453] [PubMed: 22063272]
- 62.
- Young SG, Fong LG, Beigneux AP, Allan CM, He C, Jiang H, Nakajima K, Meiyappan M, Birrane G, Ploug M. GPIHBP1 and Lipoprotein Lipase, Partners in Plasma Triglyceride Metabolism. Cell Metab 2019; 30:51-65 [PMC free article: PMC6662658] [PubMed: 31269429]
- 63.
- Tiwari S, Siddiqi SA. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol 2012; 32:1079-1086 [PMC free article: PMC3334296] [PubMed: 22517366]
- 64.
- Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 2011; 22:353-363 [PMC free article: PMC3163828] [PubMed: 21616678]
- 65.
- Feingold KR. Obesity and Dyslipidemia. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2023.
- 66.
- Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 2015; 161:161-172 [PMC free article: PMC4525717] [PubMed: 25815993]
- 67.
- Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 2007; 32:71-77 [PMC free article: PMC2711871] [PubMed: 17215125]
- 68.
- Zhang L, Reue K, Fong LG, Young SG, Tontonoz P. Feedback regulation of cholesterol uptake by the LXR-IDOL-LDLR axis. Arterioscler Thromb Vasc Biol 2012; 32:2541-2546 [PMC free article: PMC4280256] [PubMed: 22936343]
- 69.
- Brown MS, Radhakrishnan A, Goldstein JL. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annual review of biochemistry 2017; [PMC free article: PMC5828883] [PubMed: 28841344]
- 70.
- Rosenson RS, Brewer HB, Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905-1919 [PMC free article: PMC4159082] [PubMed: 22508840]
- 71.
- Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res 2014; 55:168-179 [PMC free article: PMC3886656] [PubMed: 23812558]
- 72.
- Shapiro MD, Feingold KR. Monogenic Disorders Altering HDL Levels. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2021.
- 73.
- Zhao Y, Van Berkel TJ, Van Eck M. Relative roles of various efflux pathways in net cholesterol efflux from macrophage foam cells in atherosclerotic lesions. Curr Opin Lipidol 2010; 21:441-453 [PubMed: 20683325]
- 74.
- Lee-Rueckert M, Escola-Gil JC, Kovanen PT. HDL functionality in reverse cholesterol transport--Challenges in translating data emerging from mouse models to human disease. Biochim Biophys Acta 2016; 1861:566-583 [PubMed: 26968096]
- 75.
- Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med 2008; 263:256-273 [PubMed: 18271871]
- 76.
- Siddiqi HK, Kiss D, Rader D. HDL-cholesterol and cardiovascular disease: rethinking our approach. Curr Opin Cardiol 2015; 30:536-542 [PubMed: 26192490]
- 77.
- Moore KJ, Rayner KJ, Suarez Y, Fernandez-Hernando C. The role of microRNAs in cholesterol efflux and hepatic lipid metabolism. Annu Rev Nutr 2011; 31:49-63 [PMC free article: PMC3612434] [PubMed: 21548778]
- 78.
- Ouimet M, Barrett TJ, Fisher EA. HDL and Reverse Cholesterol Transport. Circ Res 2019; 124:1505-1518 [PMC free article: PMC6813799] [PubMed: 31071007]
- 79.
- Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism 2013; 62:479-491 [PMC free article: PMC3547132] [PubMed: 23040268]
- ABSTRACT
- INTRODUCTION
- STRUCTURE OF LIPOPROTEINS ()
- APOLIPOPROTEINS ()
- LIPOPROTEIN RECEPTORS AND LIPID TRANSPORTERS
- ENZYMES AND TRANSFER PROTEINS INVOLVED IN LIPOPROTEIN METABOLISM
- EXOGENOUS LIPOPROTEIN PATHWAY (CHYLOMICRONS)
- ENDOGENOUS LIPOPROTEIN PATHWAY (VLDL AND LDL)
- HDL METABOLISM AND REVERSE CHOLESTEROL TRANSPORT ()
- LIPOPROTEIN (a) ()
- ACKNOWLEDGEMENTS
- REFERENCES
- Review Lipid and Lipoprotein Metabolism.[Endocrinol Metab Clin North Am...]Review Lipid and Lipoprotein Metabolism.Feingold KR. Endocrinol Metab Clin North Am. 2022 Sep; 51(3):437-458. Epub 2022 Jul 4.
- Review [Physiology and pathophysiology of the metabolism of lipoproteins].[Wien Med Wochenschr. 1994]Review [Physiology and pathophysiology of the metabolism of lipoproteins].Sandhofer F. Wien Med Wochenschr. 1994; 144(12-13):286-90.
- Review Interrelationship of triglycerides with lipoproteins and high-density lipoproteins.[Am J Cardiol. 1990]Review Interrelationship of triglycerides with lipoproteins and high-density lipoproteins.Gotto AM Jr. Am J Cardiol. 1990 Sep 4; 66(6):20A-23A.
- Cholesteryl ester transfer activity in liver disease and cholestasis, and its relation with fatty acid composition of lipoprotein lipids.[Clin Chim Acta. 1996]Cholesteryl ester transfer activity in liver disease and cholestasis, and its relation with fatty acid composition of lipoprotein lipids.Iglesias A, Arranz M, Alvarez JJ, Perales J, Villar J, Herrera E, Lasunción MA. Clin Chim Acta. 1996 Apr 30; 248(2):157-74.
- Dissociation of high density lipoprotein precursors from apolipoprotein B-containing lipoproteins in the presence of unesterified fatty acids and a source of apolipoprotein A-I.[J Lipid Res. 1991]Dissociation of high density lipoprotein precursors from apolipoprotein B-containing lipoproteins in the presence of unesterified fatty acids and a source of apolipoprotein A-I.Musliner TA, Long MD, Forte TM, Nichols AV, Gong EL, Blanche PJ, Krauss RM. J Lipid Res. 1991 Jun; 32(6):917-33.
- Introduction to Lipids and Lipoproteins - EndotextIntroduction to Lipids and Lipoproteins - Endotext
- Dnpep aspartyl aminopeptidase [Rattus norvegicus]Dnpep aspartyl aminopeptidase [Rattus norvegicus]Gene ID:301529Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
