Included under terms of UK Non-commercial Government License.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Royle P, Mistry H, Auguste P, et al. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: systematic review and economic evaluation. Southampton (UK): NIHR Journals Library; 2015 Jul. (Health Technology Assessment, No. 19.51.)

Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: systematic review and economic evaluation.
Show detailsMethods
Literature searches and study selection
The search question posed in the commissioning brief was:
What is the clinical and cost-effectiveness of pan-retinal laser treatment in the management of non-proliferative (pre-proliferative) diabetic retinopathy (NPDR)?
The patient groups specified were those with early stages of NPDR (Level R2) versus the control or comparator treatment of PRP at PDR (Level R3), in any appropriate setting.
Our scoping searches gave a very low retrieval of studies that would be relevant to this search question, but did show that there were recent developments in types of laser and in the use of laser and drug combinations. Therefore, in the draft protocol we proposed a wider scope for this Technology Assessment Report than had been envisaged in the commissioning brief. This was approved by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) after being supported by the external referees. The decision problem was subsequently expanded to become:
Treatment of non-proliferative diabetic retinopathy: a review of pan-retinal photocoagulation, other forms of laser treatment, and combinations of photocoagulation and anti-VEGF drugs or inject steroids.
However, the broader searches revealed that there were no RCTs that compared patients at the NPDR level to those at later stages of PRP. Indeed, the most relevant and largest study done addressing the timing of PRP laser in the treatment of DR, the ETDRS, grouped together patients with moderate to severe NPDR and early PDR, and did not report outcomes on these groups separately.
Therefore, it seemed likely that a trial to address the original research question was needed, and, in order to inform a future study on PRP treatment of patients at the NPDR stage, we decided to further broaden the searches to capture all forms of current laser and topical drug treatment of DR at any stage, and explore if these newer treatments could be applied to patients at the NPDR stage.
The databases MEDLINE, EMBASE and The Cochrane Library were searched for previous systematic reviews or meta-analyses relevant to our search question (see Appendix 2 for search strategies). There were 94 potentially relevant records downloaded and the full text of five articles was examined by two reviewers (PR, NW). The most relevant review was one by Mohamed et al. (2007).33 Although this was a useful review, its objective was to review the best evidence for primary and secondary intervention in the management of DR, including DMO, which was a lot broader than our review, so did not address our specific research question. Also, the searches were performed in May 2007, so it was several years out of date.
We searched for RCTs for the treatment of DR. We separated the results into three categories in order to provide evidence for each of the different aspects of our decision problem (Appendix 2 shows the details of the search strategies and Figure 2 shows the flow diagram for RCTs searches).
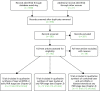
FIGURE 2
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for identifying RCTs included in Chapters 2–4.
- Trials of:
From the 102 full-text papers assessed, independently checked against the inclusion criteria by two reviewers (PR/NW), 22 references relevant to category 1 above were identified. Upon reading the full text of these references, it became evident that all were papers arising from two large RCTs, the DRS and the ETDRS, each producing many papers. Further searches were done to search specifically for publications arising from the DRS and ETDRS, and reference lists were checked, in order to obtain all the relevant papers from these two trials; this resulted in an additional 18 articles.
The excluded papers were retained and were assessed for inclusion criteria relevant to category 2 and 3 searches above, and are reviewed in Chapters 3 and 4.
Data extraction and quality assessment
The data extractions, and quality assessments (based on the Cochrane Collaboration’s risk of bias tool34), of the two trials were carried out by one reviewer (PR) and checked by a second (NW). The final number of papers reviewed was 14 from the DRS and 24 from the ETDRS.
The flow of studies is shown in Figure 2.
The Diabetic Retinopathy Study
Background
Laser photocoagulation had become widely used in the management of DR by the early 1970s in the USA. However, there was a lack of good-quality evidence supporting the risk and benefits of this procedure. Therefore, in 1971, the National Eye Institute (NEI) funded the DRS35 to evaluate photocoagulation treatment for PDR.
Study design
The DRS was a randomised, controlled clinical trial involving 15 clinical centres. A total of 1758 patients were enrolled between 1972 and 1975. Patient follow-up was completed in 1979.
The main aim of the DRS was to determine whether photocoagulation helps prevent severe visual loss (SVL) from PDR, and whether a difference exists in the efficacy and safety of argon versus xenon photocoagulation for PDR. Another objective was to obtain information on the natural history and clinical course of proliferative retinopathy.
Patients were eligible if they had best corrected visual acuity (BCVA) of 20/100 or better in each eye, and the presence of PDR in at least one eye or severe non-proliferative retinopathy in both eyes. Both eyes had to be suitable for photocoagulation. The eye to be treated was chosen randomly.
The baseline VA of the enrolled patients was equal to or better than 20/20 in approximately half of the eyes. Patients were predominantly white and had a mean age of 42.6 years; approximately 45% were classified as juvenile-onset diabetics, and there were slightly more men than women.
The principal end point was SVL, which was considered to have occurred if VA was less than 5/200 at two or more consecutively completed 4-month follow-up visits.
Quality assessment
The DRS was a high-quality trial with a low risk of bias, as shown in Table 4. The details of the design, methods and baseline results of the DRS were extensively reported in DRS report no. 6 (DRS #6).36
TABLE 4
Quality assessment and risk of bias of DRS
Treatment
One eye of each patient was randomly assigned to immediate photocoagulation and the other to follow-up without treatment, regardless of the course followed by either eye. The eye chosen for photocoagulation was randomly assigned to argon laser or to xenon arc photocoagulation. Treatment was usually completed in one or two sittings. Both treatment techniques included extensive scatter photocoagulation (PRP) and focal treatment of new vessels on the surface of the retina.
The argon treatment technique specified 800–1600 scatter burns, 500 µm in size, 0.1-second duration and direct treatment of new vessels whether on or within one disc diameter (DD) of the optic disc (NVD) or outside this area (NVE). The xenon technique was similar, but scatter burns were fewer in number, generally of longer duration, and stronger, and direct treatment was applied only to NVE on the surface of the retina. Focal treatment was also applied to microaneurysms or NVE lesions thought to be causing MO. Those treated with argon could have flat or elevated NVE treated.
Follow-up visits were planned at 4-month intervals for a minimum follow-up of 5 years, where follow-up treatment was applied as needed. BCVA was measured in both eyes by masked techniques before treatment and at 4-month intervals after treatment.
The DRS data were reviewed every 3 months by the Data Monitoring Committee for evidence of adverse and beneficial treatment effects.
Results (before protocol change)
In 1975 after an average of only 15 months of follow-up (range 0–38 months), the 2-year incidence of blindness was 16.3% in untreated eyes but only 6.4% in treated eyes.37 Therefore, photocoagulation had reduced the 2-year risk of blindness by about 60%. This finding was unexpected and highly statistically significant. These beneficial effects were noted to some degree in all stages of DR included in the study.
Protocol change
On the basis of these results a decision was made in 1976 (more than 3 years before the planned termination of the study) to consider photocoagulation treatment for the initially untreated eyes, which now, or in the future, would fulfil any one of the following criteria, referred to as eyes with HRCs:
- Moderate or severe new vessels on or within one DD of the optic disc.
- Mild new vessels on or within one DD of the optic disc if fresh vitreous or pre-retinal haemorrhage is present.
- Moderate or severe new vessels elsewhere (NVE), if fresh vitreous or pre-retinal haemorrhage is present, and if the area of new vessels was half the disc area or more.
Photocoagulation techniques were modified when treatment was carried out in eyes initially assigned to the untreated control groups after the 1976 protocol change. Argon treatment was preferred, and to decrease the risk of VA loss, many DRS investigators divided scatter treatment into two or more episodes, days or weeks apart.
Evidence of recovery before protocol change
Although the principal goal of photocoagulation treatment is to prevent visual loss, not to improve vision, there were eyes with some evidence of recovery, defined as VA ≥ 5/200 at any subsequent visit at 1, 2 or 3 consecutively completed follow-up visits. The percentage of eyes with some evidence of recovery at each visit were 28.6%, 12.2% and 7.7% in untreated eyes compared with 48.8%, 28.6% and 20.8% in treated eyes, respectively. Therefore, it appeared that recovery of VA was more frequent in treated than untreated eyes.
Harms
Some harmful effects of treatment were also found, including moderate losses of VA and constriction of peripheral visual field, which were greater in the xenon treated group than the argon group. The loss in sharp, central vision was temporary in some patients but persisted in others. However, DRS physicians believed that these harmful effects of photocoagulation in eyes with moderate or severe retinopathy were outweighed by the reduced risk of SVL without treatment at these stages.
Results after the protocol change
Additional follow-up after the DRS protocol change confirmed previous reports that, by 24 months, photocoagulation reduces the risk of SVL by 50% or more.
Cumulative rates of SVL for argon and xenon groups combined up to 72 months’ follow-up are shown in Table 5 (adapted from table 2, DRS #810). Although the risk of SVL in untreated eyes increases from 14% at 24 months to 36.7% at 72 months, it can be seen that over this time period the treatment effect was consistent (ranging between 56% and 59%).
TABLE 5
Cumulative event rates of SVL: cumulative event rates per 100 eyes at risk (adapted from table 2, DRS #8)
The 24-month data in Table 5 differ slightly from that presented earlier (prior to the protocol change), as 43% of the 2-year visits and all of the 4-year visits included were carried out after the 1976 protocol change. All eyes are classified in the group to which they were originally randomly assigned, ignoring treatment of control eyes.
The treatment effect was somewhat greater in the xenon group than in the argon group (data not shown), but its statistical significance was borderline, and its clinical importance was outweighed by the greater harmful treatment effects observed with the xenon technique used in the DRS.
Occurrence of severe visual loss in eyes classified according to baseline severity
As patients enrolled in DRS had a broad range of severity of DR, it was important to evaluate results for different stages. Table 6 (taken from table 2, DRS #1438) shows the cumulative 2- and 4-year rates of SVL by eyes grouped by their severity of retinopathy at baseline and treatment assignment.
TABLE 6
Cumulative 2- and 4-year rates of SVL by eyes grouped by baseline severity of retinopathy and treatment assigned (from table 2 DRS #14)
It can be seen that the treatment effect in Table 6 is substantial (except for the group without PDR at 2 years) and fairly uniform across all subgroups at both 2 and 4 years, with reductions of SVL by from 54% to 65%.
The rate of SVL for untreated eyes with proliferative retinopathy with HRCs after 24 months of follow-up is about 26% and is reduced to 11% in treated eyes. However, in eyes with proliferative retinopathy without HRCs, the untreated rate at 2 years is much lower (7.0%), and although the beneficial treatment effects are substantial (a 54% reduction in SVL), the risks without treatment are smaller, and so the harmful effects of treatment need to be given more weight than for eyes with a higher risk.
In eyes with severe NPDR the risk of SVL without photocoagulation treatment at 2 years is low (3.2%) and reduces to 2.8% (a reduction of 12.5%) only with treatment, so the risks of treatment become even more important.
Harms: argon and xenon
Decreases of VA of one or more lines and constriction of peripheral visual field due to treatment were also observed in some eyes. These changes were sometimes due to an increase in MO, and sometimes the reduction in VA was temporary. In others, the changes persisted. The changes in visual field are important because they may mean that patients can no longer meet the requirements for driving.
Visual fields were measured using the Goldman method, wherein normal fields range from 50° (superiorly) to 90° (temporally). The DRS group defined modest visual field loss as a reduction from over 30° up to 45°, and 30° or less as severe.
The UK legal requirement is VA of 6/12 (measured in metres) or better (this is equivalent to 20/40 using measurements in feet) and with regards to visual field, to have a binocular visual field of 120 ° horizontally (in the horizontal axis) and no significant defect within the central 20 °, horizontally or vertically (above or below the horizontal meridian).
These harmful effects were more frequent and more severe following the DRS xenon technique; 50% of xenon-treated eyes suffered some loss of visual field compared with 5% of the argon-treated eyes. It was also estimated that a persistent VA decrease of one line was attributable to treatment in 19% of xenon-treated eyes and a persistent decrease of two or more lines in an additional 11%. Comparable estimates for the argon group were 11% and 3%, respectively.
Xenon photocoagulation has been discontinued.
Macular oedema in the Diabetic Retinopathy Study patients (DRS #12)
The DRS39 was not designed to evaluate the effect of photocoagulation in eyes with MO. Although focal treatment was carried out in those eyes with MO assessment, its direct effect cannot be determined because it was always combined with scatter treatment.
The loss of VA associated with scatter photocoagulation observed soon after treatment was especially prominent in eyes with pre-existing MO. It was also associated with the intensity of treatment. It was suggested that reducing MO by focal photocoagulation before initiating scatter treatment and dividing scatter treatment into multiple sessions with less-intense burns may decrease the risk of the visual loss associated with photocoagulation.39
Summary
Results of the DRS showed that photocoagulation reduced the 2-year incidence of SVL by more than half in eyes with PDR, both with and without HRCs. However, in eyes with NPDR, where the 2-year risk of SVL in the untreated control group was low at 3.2%, photocoagulation only reduced the risk to 2.8%. Therefore, in patients with NPDR the harmful effects of photocoagulation assume more importance. Some of the harmful effects of treatment for some patients included a moderate loss of VA and a narrowing of the visual field.
Implications of Diabetic Retinopathy Study findings for treatment of early proliferative or severe non-proliferative retinopathy
The DRS concluded that in the eyes with PDR and HRCs the risk of SVL without treatment substantially outweighs the risks of photocoagulation, and prompt treatment is usually advisable. However, as the DRS findings result from a comparison between prompt treatment versus no treatment, they did not provide evidence on the relative value of prompt treatment versus deferral of treatment in the earlier stages of DR. They recommended careful follow-up for changes with DR and when non-proliferative changes are present, the follow-up visits should be at frequent intervals.37
Finally, their conclusions stated:
Demonstration that prompt treatment of eyes with early proliferative or severe nonproliferative retinopathy is better than no treatment does not mean that prompt treatment is superior to deferral of treatment until progression occurs.37
They called for a randomised trial to examine when best to apply PRP.
Early Treatment Diabetic Retinopathy Study
Background
The ETDRS was a multicentre, randomised clinical trial designed to evaluate argon laser photocoagulation in the management of patients with non-proliferative or early PDR. It was supported by the NEI and arose from results of the DRS, which had shown that laser photocoagulation was effective in reducing the rate of SVL from an advanced stage of DR.9,40
Purpose and aims
The three principal clinical questions of ETDRS were:
- When in the course of DR is it most effective to initiate photocoagulation therapy?
- Is photocoagulation effective in the treatment of MO?
- Is aspirin effective in altering the course of DR?
This summary will focus on the first of these questions. Our main interest is between early scatter treatment of eyes with moderate to severe NPDR or PDR without HRCs and deferral of scatter treatment unless PDR with HRCs develops.
Initially, patients were also assigned randomly to aspirin (650 mg per day) or placebo. However, aspirin was not found to have an effect on retinopathy progression, so patients assigned to aspirin were pooled with those assigned to placebo.
Quality assessment
The ETDRS was a high-quality trial with a low risk of bias as shown in Table 7.
TABLE 7
Quality assessment and risk of bias of ETDRS
Patient recruitment
Recruitment of eligible patients began in December 1979 and was completed in July 1985. The 3711 patients accepted for the study, from 22 clinical centres in the USA, were followed through to 1989. Recruitment ended with 98% of the goal of 4000 patients enrolled. By study end, 706 patients had died, and, of the 2971 patients known to be alive, 164 did not have a final eye examination but all but 11 had some sort of final check.
Patient eligibility
To be eligible for the ETDRS, patients had to be aged between 18 and 70 years and to have DR in both eyes. Each eye had to meet either of the following eligibility criteria:
- No MO, VA of 20/40 or better and moderate or severe non-proliferative or early proliferative retinopathy, or
- MO, VA of 20/200 or better and mild, moderate or severe non-proliferative retinopathy or early proliferative retinopathy.
Methods for assessing outcome variables
Best corrected visual acuity was measured with logarithmic VA charts at baseline and each subsequent follow-up visit, scheduled at 4-month intervals. A standardised protocol for the collection of VA measurements was used in all clinical centres.
Stereoscopic 30° colour photographs were taken of seven standard fields at baseline, 4 months, 1 year after entry and yearly thereafter. All fundus photographs were graded according to a standardised procedure by the Fundus Photograph Reading Center staff, who had no knowledge of treatment assignments and clinical data.
Definitions of diabetic retinopathy
The ETDRS adopted the DRS definitions of severe NPDR and HR-PDR and defined moderate NPDR (see table in Appendix 1). Subsequently, the ETDRS developed a more detailed scale, which provided further subdivisions within both the NPDR and the PDR categories.6
Assessment of severity of retinopathy and macular oedema
Fundus Photograph Reading Center staff, without knowledge of treatment assignments and clinical data, followed a standardised procedure to grade fundus photographs and fluorescein angiographs for individual lesions and DR.
Randomisation procedure
To obtain information on the appropriate timing of scatter photocoagulation, one eye of each patient in the ETDRS was assigned randomly to early photocoagulation (either mild or full scatter) and the other to deferral of photocoagulation, with follow-up scheduled every 4 months and photocoagulation to be performed promptly if HR-PDR developed.
All eyes chosen for early photocoagulation were further randomised to one of two scatter photocoagulation techniques (full or mild). Full scatter involved 1200–1600 burns in two sessions, mild scatter 400–650 burns in one session. Eyes also with MO were assigned randomly to one of two timing strategies for focal photocoagulation (immediate or delayed), so that for these eyes there were four strategies of early photocoagulation.
Three categories were defined on the basis of retinopathy severity and the presence or absence of MO at baseline, and the type of photocoagulation differed for each category.
Less severe retinopathy was defined as eyes with mild to moderate non-proliferative retinopathy, and more severe retinopathy as eyes with severe non-proliferative or early PDR.
- Category 1: eyes without MO Eyes in this category had moderate to severe non-proliferative or early proliferative retinopathy.
Eyes randomised to immediate photocoagulation were further randomised to full or mild scatter.
In the deferred arm, eyes were followed up at 4-monthly intervals and received photocoagulation if PDR with HRC-PDR developed.
In both arms, delayed focal photocoagulation was initiated during follow-up if clinically significant macular oedema (CSMO) developed (i.e. MO that involved or threatened the centre of the macula).
Ideally, the trial would have separated NPDR from PDR, but this was not done.
- Category 2: eyes with MO and less severe retinopathy Eyes in this category had MO and mild to moderate NPDR.
Early photocoagulation for these eyes consisted of (1) immediate focal photocoagulation to treat the MO, which was seen as a greater threat to vision than the retinopathy, with scatter photocoagulation (with further randomisation to mild or full) added if severe non-proliferative or early proliferative retinopathy developed during follow-up and (2) immediate scatter photocoagulation (with further randomisation to mild or full), with focal photocoagulation delayed for at least 4 months.
Eyes assigned to delayed focal photocoagulation received treatment at the 4-month visit if the oedema had not improved clinically and the VA score had not increased by five or more letters by that time. Focal photocoagulation was initiated at the 8-month visit if the oedema was not substantially improved, as demonstrated by either a return of an initially thickened macular centre to normal thickness or improvement in VA score by 10 or more letters. At and after the 12-month visit, initiation of focal photocoagulation was required for all eyes assigned to early PRP if they had CSMO and had not yet received focal photocoagulation. So focal was not given if the MO improved.
In the deferred arm, eyes were followed up at 4-monthly intervals and received scatter photocoagulation if HRC-PDR developed. They could receive focal photocoagulation if CSMO developed. Note that this group could only receive scatter PRP if HRC-PDR developed, whereas the early treatment arm could have PRP if they progressed to severe NPDR, early PDR or HRC-PDR.
- Category 3: eyes with MO and more severe retinopathy Eyes in this category had MO and severe non-proliferative or early PDR.
Early photocoagulation for these eyes consisted of (1) immediate focal and scatter photocoagulation (with random allocation to mild or full) or (2) immediate scatter photocoagulation (randomisation to mild or full), with focal photocoagulation delayed for at least 4 months. The same procedure as described above for initiating focal photocoagulation at or after 4 months was used.
In the deferred arm, eyes were followed up at 4-monthly intervals and received photocoagulation if HRC-PDR developed.
Thus, in each of the three categories there are four different randomly allocated strategies for the timing and extent of early photocoagulation. All eyes received scatter (mild or full) originally, and if the retinopathy progressed to HRC-PDR, the mild scatter group received full scatter. Eyes that had MO, or developed it, received full focal photocoagulation treatment. (Approximately 85% of eyes with MO at baseline eventually received focal photocoagulation compared with only 40% of eyes without MO at baseline.)
In the deferred arms, the initial protocol specified that full scatter be given if HRC-PDR developed. The protocol was modified in 1985 to allow focal photocoagulation if CSMO was present. This was because the data had by then shown that focal photocoagulation reduced visual loss in eyes with CSMO.
Early Treatment Diabetic Retinopathy Study photocoagulation technique
Argon laser was chosen for photocoagulation in the ETDRS. The photocoagulation treatment techniques used were based on those used in the DRS and on the clinical experience of the ETDRS investigators.
Major features of the scatter and focal photocoagulation techniques used in the ETDRS are shown in the table in Appendix 3.
Full scatter Full scatter treatment consisted of a spot size of 500 µm and exposure time of 0.1 second, used with power adjusted to obtain moderately intense white burns that do not spread to become appreciably larger than 500 µm. It was estimated that a total of 1200–1600 burns were required to complete the full scatter treatment. The protocol specified that division of scatter treatment be applied in two or more episodes, in the hope of reducing the incidence of adverse treatment effects. If applied in two episodes, these were to be no less than 2 weeks apart; if in three or more episodes, these must be at least 4 days apart. No more than 900 scatter burns were to be applied in a single episode, and the initial treatment session was to be completed within 5 weeks.
Mild scatter Mild scatter treatment involved a spot size, exposure time and intensity the same as for full scatter treatment, in order to produce burns of the same strength. Burns were placed at least one burn diameter apart and scattered uniformly across the same zone of retina as specified or full scatter, using 400–650 burns, usually applied at a single episode.
Focal photocoagulation Focal photocoagulation for MO consisted of the application of argon laser burns to focal lesions (such as leaking microaneurysms as determined by FA or areas of retinal ischaemia) located between 500 and 3000 µm from the centre of the macula.
Definition of terms used in the Early Treatment Diabetic Retinopathy Study
A definition of the terms as used in the ETDRS studies is given in Table 8.
TABLE 8
Early Treatment Diabetic Retinopathy Study: definition of terms
End points
The primary end point for assessment of early photocoagulation was the development of SVL. This was defined as VA < 5/200 at two consecutive follow-up visits (scheduled at 4-month intervals). BCVA was measured at 6 weeks and 4 months after randomisation. The procedure was repeated every 4 months thereafter.
Other end points evaluated included either severe visual loss or vitrectomy (SVLV), and change between baseline and follow-up visits in visual field, colour vision or retinopathy. Visual fields were assessed by Goldman perimetry and identification of scotomas.
Study power
Power calculations for the primary end point of SVL assumed that 10% of eyes assigned to deferral would develop SVL within 5 years. With 2000 eyes assigned to the deferral group and their 2000 fellow eyes assigned to early photocoagulation, a 40% reduction in the rate of SVL could be detected with 98% power.
Statistical methods
Comparisons of end points expressed as proportions of events were made with two-sample tests of equality of proportions. Comparisons of continuous variables were based on the two-sample z-test of equality of means.
Because multiple end points in the different groups were compared several times for the Data Monitoring Committee, a 0.01 level of probability was used for the primary end points rather than 0.05. Observed z-values of ± 2.58 or more extreme (corresponding to a 0.01 level for a single test of significance) were considered statistically significant.9
Baseline characteristics
The baseline characteristics of the ETDRS patients, by assignment of scatter photocoagulation are shown in Table 9.
TABLE 9
Baseline characteristics of the ETDRS patients (from table 6, ETDRS #7)
Of the 3711 patients randomised, 56% were male, 52% were between 50 and 70 years of age, 57% had a duration of diabetes between 10 and 19 years, and 30% were classified as having type 1 diabetes.
By today’s standards, control of blood glucose, BP and cholesterol would not be considered satisfactory; 19% had systolic blood pressure (SBP) of 160 mmHg or more, and 42% had HbA1c of 10% or more; 36% had total cholesterol level over 6.2 mmol/l. The mean HbA1c was over 12%.
Groups were well balanced for all characteristics, except that a significantly greater proportion in the full scatter group had higher diastolic BP.
In 75% of ETDRS patients both eyes belonged to the same baseline category. Within each baseline category there were no large differences in mean VA scores between groups of eyes assigned to various strategies for early photocoagulation and eyes assigned to deferral of photocoagulation. Randomised treatment groups were comparable. Adherence to the assigned strategy for photocoagulation at the initial treatment session was reviewed and found to be over 98% for application of the assigned scatter and/or focal photocoagulation.
Results
Severe visual loss
All eyes in ETDRS had low rates of SVL, whether they received early photocoagulation (2.6%) or were in the deferral group (3.7%) at 5 years.
The relative risk (RR) of SVL for the entire period of follow-up in eyes assigned to early photocoagulation (including all strategies) compared with eyes assigned to deferral photocoagulation was 0.77 (99% CI 0.56 to 1.06), calculated using a Cox proportional hazards model with retinopathy severity and presence or absence of MO at baseline as covariates.
The RRs of SVL with photocoagulation compared with deferral for all baseline retinopathy categories when all photocoagulation strategies are compared are summarised in Table 10. It can be seen from the CIs that in none of the categories was the RR statistically significant.
TABLE 10
Relative risk of SVL for eyes assigned to early photocoagulation (combining all strategies for photocoagulation) compared with deferral
Data for the development of SVL for all baseline categories are shown in Table 11, which gives estimates of RR in each of the categories. Analyses for the 5-year follow-up period demonstrated no statistically significant differences between any of the strategies for early photocoagulation and deferral within each category.
TABLE 11
Development of SVL (taken from table 7, ETDRS #9)
The eyes assigned to full scatter showed a trend towards a greater treatment effect than eyes assigned to mild scatter in the first two categories. The RR of SVL for the entire period of follow-up for all categories combined in eyes assigned to early full scatter compared with eyes assigned to deferral was 0.69 (99% CI 0.45 to 1.05); in eyes assigned to mild scatter the RR was 0.84 (99% CI 0.57 to 1.25); so neither early or full scatter showed a significant decrease in RR, but full was slightly better than mild at preventing SVL.
Both the severity of retinopathy and the presence of MO at baseline were both significantly associated with the development of SVL. The RR (adjusting for the presence of MO) for the development of SVL for eyes with more severe retinopathy compared with eyes with less severe retinopathy was 2.41 (99% CI 1.73 to 3.37). Similarly, the RR (adjusting for severity of retinopathy) for the development of SVL for eyes with MO compared with eyes without MO was 1.73 (99% CI 1.17 to 2.57).
Causes of severe visual loss in the Early Treatment Diabetic Retinopathy Study
Severe visual loss developed in 257 eyes (219 persons); however, 17 of these 257 eyes with SVL had insufficient follow-up and were not included in the analysis. Of the 240 eyes left for analysis, 149 eyes (127 persons) did not recover to 5/200 or better at any visit (persistent SVL) and VA improved in 91 eyes.41
The most common cause of SVL was vitreous or pre-retinal haemorrhage, occurring in 125 (52.1%) of the 240 eyes included in the analysis. The second and third most common causes were MO (13.8%), and macular or retinal detachment (7.1%).
When patients with persistent SVL were compared with patients without persistent SVL, they were found to have higher mean levels of HbA1c (10.4% vs. 9.7%; p = 0.001) and higher levels of cholesterol (244.1 vs. 228.5 mg/dl; p = 0.0081) at baseline.41
The low frequency of SVL in ETDRS is probably due to the use of PRP as soon as HR-PDR developed, and to vitrectomy when required.
Severe visual loss: subgroup analysis of type 1 versus type 2 diabetes
Patients were categorised into type 1 and type 2 diabetes in order to conduct a subgroup analysis of the ETDRS data to determine whether the effects of photocoagulation on SVL in patients differed by type of diabetes.42
The benefit of early photocoagulation for SVL was statistically significantly greater in patients with type 2 diabetes than in those with type 1 diabetes. (Cox regression for SVL: interaction of early photocoagulation and type of diabetes; p = 0.0003). However, the reduction was small and the risk was low in the deferral group in which only 3.7% developed SVL. (Note that the definition used was truly severe – very low levels of vision). Also, because of the high correlation between age and type of diabetes, a subgroup analysis by age showed similar results. The results varied amongst the categories, and according to outcome. In patients with mild to moderate NPDR at baseline, a small benefit of laser in reducing SVLV was seen in both types of diabetes with no interaction between laser treatment and type of diabetes. In patients with more severe retinopathy (severe NPDR or early PDR) there was no difference in SVLV in type 1 diabetes between early and deferred laser, but a large difference in type 2, partly because they had much poorer outcomes than those with type 1.42
If we use progression to HRC-PDR as the outcome, statistically significant benefit is seen in both types of diabetes. If we use reduction in VA, there is a large difference between early and deferred laser in patients with type 2 diabetes and clinically significant MO who had severe NPDR or early PDR at baseline but little in patients with type 1. If we look only at those who did not have CSMO at baseline, there is no difference in type 2 between early and deferred groups.
If we use legal blindness (defined in ETDRS as VA worse than 20/100), patients with type 2 diabetes again show a significant difference between early and deferred groups, whereas no difference is seen in type 1, but the frequency of this outcome was much higher in type 2.
The difference between the types of diabetes may be due to chance. As the ETDRS authors stated, many analyses were done and chance could lead to ‘statistically significant’ results. They show this quite neatly by doing a subgroup analysis on date of birth, which showed a statistically significant interaction.42
Vitrectomy
The initial ETDRS protocol said that vitrectomy should be done after SVL had occurred, but this was changed after the results of the Diabetic Retinopathy Vitrectomy Study appeared in 1985, and earlier vitrectomy was performed, either 1 month after detection or as soon as progressive retinal detachment occurred.43 This meant that vitrectomy was performed in many ETDRS patients who had not developed SVL.
Vitrectomy was performed at least once in 208 (243 eyes) of the 3711 patients (the overall vitrectomy numbers suggest that about 18% of eyes had more than one vitrectomy.) At baseline, eyes undergoing vitrectomy were more likely to have severe non-proliferative or worse retinopathy. Also, there were no differences in the mean VA scores or percentages with clinically significant MO. It appears that all patients who had vitrectomy, did so after developing HRC-PDR, on average 21 months before vitrectomy. About 20% had SVL before vitrectomy.44
The majority of patients undergoing vitrectomy had type 1 diabetes. The indications for vitrectomy were either vitreous haemorrhage (53.9%) or retinal detachment with or without vitreous haemorrhage (46.1%).
The cumulative rates of vitrectomy were 3.9% and 2.2% in the deferred and early groups, respectively, so this outcome was about as common as SVL.
The 5-year vitrectomy rates for eyes grouped by their initial photocoagulation assignment were 2.1% of eyes assigned to early full scatter photocoagulation group, 2.5% of eyes assigned to the early mild scatter group, and 4.0% of eyes assigned to the deferral group (based on ETDRS #1744 – ETDRS #99 gives a figure of 3.9% for the deferred group).
Comparison of eyes assigned to deferral of photocoagulation with eyes assigned to early photocoagulation showed no statistically significant difference in post-vitrectomy VA results; however, it should be noted that because they all developed HR-PDR before vitrectomy, most (88%) had had PRP, most with full scatter. After vitrectomy, results in immediate and deferred groups were similar – the outcome of surgery was not affected by delaying PRP. Also, there was no statistically significant difference between eyes that received either less than full scatter or no photocoagulation compared with eyes that received full scatter photocoagulation.44
Severe visual loss or vitrectomy
The ETDRS #740 (the design paper) does not mention vitrectomy as an outcome. However, the final analysis used as one outcome, the combination of SVL and vitrectomy (SVLV), based on the reasoning that vitrectomy had saved an unknown number of eyes from SVL, and because vitrectomy could be considered an indicator of vitreous haemorrhage that had failed to clear.
The RR of SVLV at end of follow-up for eyes assigned to early photocoagulation compared with eyes assigned to deferred photocoagulation was statistically significant at 0.67 (99% CI 0.52 to 0.87).9
The RRs of SVLV by baseline categories were:
- no MO = 0.78 (99% CI 0.47 to 1.29)
- MO and less severe retinopathy = 0.55 (99% CI 0.33 to 0.94)
- MO and more severe retinopathy = 0.68 (99% CI 0.47 to 0.99).
So, once again, eyes in category 1 had a lower reduction in RR than eyes in the MO groups.
The VA immediately before vitrectomy was 5/200 or worse in 67%, but afterwards only about 28% were left with such poor vision. About 20% had VA better than 20/40 at 3 years, so vitrectomy was highly beneficial in most.
Development of high-risk characteristics proliferative diabetic retinopathy
Results for the development of HRC-PDR by baseline retinopathy category and photocoagulation strategy are shown in Table 12.
TABLE 12
Development of HRC-PDR by photocoagulation strategy (taken from table 5, ETDRS #9)
Compared with deferral of photocoagulation, early photocoagulation reduced the rate of progression to HR-PDR in each baseline category (Mantel–Cox test: p < 0.001 for each strategy of early photocoagulation compared with deferral, except for immediate focal and mild scatter photocoagulation in eyes with MO and less severe retinopathy; p = 0.09). The reduction was greater in eyes with full scatter than mild scatter, essentially similar for all categories.
The RRs are adjusted for retinopathy severity and the presence or absence of MO.
The deferral arms afforded the possibility to determine the natural history of retinopathy by examining the 5-year rate of progression to the HR-PDR stage. The risks of progression in the deferral arms were 38.5% in the eyes with no MO and more severe retinopathy, 26.7% in eyes with MO and less severe retinopathy and 61.3% in eyes with MO and more severe retinopathy.
Table 13 shows the development of HRC-PDR in eyes assigned to deferral by baseline retinopathy severity level. It can been seen that the risk of progression increases steadily with severity of retinopathy at baseline, with 5-year rates increasing from 15.5% in eyes with mild NPDR, to 56% in eyes with severe NPDR, up to 74.5% in eyes with moderate proliferative retinopathy.
TABLE 13
Development of HRC-PDR in all eyes assigned to deferral by baseline retinopathy severity level (based on table 6, ETDRS #9)
In all categories, the 5-year risk of HRC-PDR was lowest in eyes that had full scatter PRP and highest in the deferred group. Full scatter reduced HRC-PDR by 50% and mild scatter by 25% compared with the deferred group.
Results after lens extraction
Lens surgery was performed on 205 patients (270 eyes) of the 3711 patients in the ETDRS, during follow-up that ranged from 4 to 9 years. Those having surgery were more likely to be white, older and have type 1 diabetes. Most of the lens surgery was done because of cataract; however, some may have been performed because of lens opacity that developed during or after vitrectomy.45
Eyes assigned to early photocoagulation were more likely than eyes assigned to deferral of photocoagulation to have received scatter and/or focal photocoagulation before lens surgery. However, 64.8% of eyes assigned to deferral of photocoagulation also had scatter and/or focal photocoagulation before lens surgery.
A large proportion of all operated-on eyes had improved VA postoperatively. Eyes assigned to early photocoagulation had a trend towards a better VA outcome after lens surgery than eyes assigned to deferral, but this was not statistically significant (p = 0.04).
Moderate visual loss
Percentages of eyes in which moderate visual loss occurred are shown for each baseline category in Table 14 for up to 5 years of follow-up. Moderate visual loss in the deferred groups was commoner at 5 years in the two MO groups (prevalence 30.2% and 32.1%) than in category 1 with no MO (17.6%).
TABLE 14
Occurrence of moderate visual loss (taken from table 9, ETDRS #9)
It can been seen that for all baseline categories full scatter photocoagulation appeared to have an adverse effect on moderate visual loss at both the 6-week and 4-month follow-up visits. This effect was also seen to a lesser extent with mild scatter. For eyes without MO there was a statistically significant effect of higher moderate visual loss in eyes for full scatter up to 2 years. At 3 and 5 years there was a no significant difference in eyes with photocoagulation compared with deferral.
In eyes with MO and less severe retinopathy the increase in moderate visual loss was statistically significant at 4 months, and 1 year for eyes with full scatter, but at 2, 3 and 5 years there were no significant differences. At the 5-year follow-up there was a statistically significant decrease in moderate visual loss in eyes with mild scatter and a non-significant decrease in eyes with full scatter. Eyes with immediate focal photocoagulation appeared to show a statistically significant beneficial effect of early photocoagulation for all follow-up points, beginning with the first year.
In eyes with MO and more severe retinopathy, there was a significant increase in moderate visual loss at 6 weeks for all strategies of photocoagulation. At 4 months this was seen only for eyes with immediate focal and full scatter. The only other significant difference was a lower rate at 2 years for eyes with immediate focal and mild scatter.
The summary of ETDRS #99 notes that scatter photocoagulation was not effective in reducing moderate visual loss in patients with MO.
Visual field
The cumulate distribution of visual field scores obtained using the Goldman 1/4e test object at baseline, 4- and 48-month visits showed no difference in distributions of visual field between categories of assigned strategies at baseline.9 The Goldman method is less sensitive than methods used today.
By the 4-month visit, eyes assigned to deferral of photocoagulation showed no significant change in scores compared with baseline. By contrast, at 4 months all three baseline categories of eyes assigned to immediate full scatter photocoagulation had significantly greater loss of visual field than eyes assigned to deferral (p < 0.001). Eyes with mild scatter also showed a lower loss of visual field. There was a statistically significant difference (p < 0.001) between the loss of visual field between eyes assigned to immediate full and immediate mild scatter. So mild scatter may be less effective, but has fewer adverse effects.
The visual field worsened in all groups from baseline to 4 years. The scores for eyes assigned to immediate full scatter remained significantly (p < 0.001) worse than for eyes assigned to deferral. This reflects the harm done by PRP.
Colour vision
Colour vision was measured using the Farnsworth–Munsell 100 hue test at baseline, and at 8-month and 4-year follow-up visits. There was significant impairment of colour vision at baseline, with 50% of the ETDRS population having colour vision scores worse than 95% of the normal population. Colour vision is a macular function so should not be affected by PRP to the peripheral retina, but might be affected by focal or grid laser for MO.
Eyes with more severe retinopathy, both without and with MO, showed no significant difference at any visit between eyes assigned to any strategy of early photocoagulation and eyes assigned to deferral. All of the eyes with MO and more severe retinopathy assigned to early photocoagulation had scatter photocoagulation as part of their initial treatment.
However, for eyes with less severe retinopathy and MO assigned to immediate focal and delayed scatter photocoagulation, there was less loss of colour vision at the 4-year visit (p < 0.001) comparing the combination of both groups of eyes assigned to immediate focal with eyes assigned to deferral.
Summary and conclusions
Severe visual loss
The primary end point of the ETDRS was the development of SVL. The 5-year RR of SVL for eyes assigned to early photocoagulation (combining all strategies for photocoagulation) compared with deferral for all baseline categories combined was 0.77 (99% CI 0.56 to 1.06). Thus, it was shown that early photocoagulation reduces the risk of SVL by about 23%, but the 99% CI overlapped with no difference.
When analysed by baseline retinopathy category it was shown that eyes with MO and less severe retinopathy had a lower RR of 0.59 (99% CI 0.32 to 1.09) and eyes with MO and more severe retinopathy showed a RR 0.70 (99% CI 0.44 to 1.11), respectively. Eyes with no MO and more severe retinopathy had a higher RR of 1.37 (99% CI 0.67 to 2.77) but the CIs were wide.
Severe visual loss or vitrectomy
The combined end point of SVLV showed a 33% reduction with early photocoagulation compared with deferral, with a RR of 0.67 (99% CI 0.52 to 0.97). As noted above, about 20% of eyes that had vitrectomy had SVL before vitrectomy but the rest did not, and many improved thereafter.
High-risk proliferative retinopathy
Early photocoagulation resulted in a significant reduction in the rate of developing high-risk proliferative retinopathy compared with deferral of photocoagulation. Strategies for photocoagulation that included immediate full scatter reduced the rate of developing high-risk proliferative retinopathy by approximately 50%, whereas strategies that included immediate mild scatter reduced that rate by approximately 25%.
When eyes assigned to deferral were stratified according to baseline retinopathy, the rate of progression to the high-risk stage generally increased as the retinopathy increased.
Harms
There were some harmful effects associated with early scatter photocoagulation. Adverse effects of moderate visual loss were shown more frequently at 6 weeks and 4 months compared with eyes assigned to deferral, but this loss was not shown in any group by the 3-year follow-up.
There was evidence of a significant loss of visual field in all groups at 4 years and this was worse for eyes assigned to full scatter. Also, colour vision showed some reduction at 4 years in the category of eyes with less severe retinopathy and MO assigned to immediate focal and delayed scatter photocoagulation.
The Early Treatment Diabetic Retinopathy Study conclusions and recommendations
Data from the ETDRS demonstrated that early photocoagulation reduced the risk of developing SVL, and the risk of progression of retinopathy. However, the rates of SVL were low in both the early photocoagulation and deferral groups, and statistical significance using 99% CIs was obtained for SVLV but not for DVL alone.
When making the decision whether to initiate scatter photocoagulation, the side effects must be carefully considered. For most eyes that have not yet reached the high-risk proliferative stage, these side effects of scatter photocoagulation must be balanced with the possible small benefit of early photocoagulation in reducing the risk of SVL.9
The ETDRS recommended that:
Provided careful follow-up can be maintained, scatter photocoagulation is not recommended for eyes with mild or moderate non-proliferative retinopathy. When retinopathy is more severe, scatter photocoagulation should be considered and usually should not be delayed if the eye has reached the high-risk proliferative stage.
Discussion
Rates of progression to SVL in ETDRS were low. They might be even lower now, with tighter control of blood glucose, BP and lipids. Tighter control of metabolic factors especially glycaemia can also slow progression. There was a fear that if HbA1c is reduced too quickly, retinopathy may temporarily worsen, usually, but not always, temporarily – the ‘glycaemic re-entry’ phenomenon.46 This phenomenon may date from the days when patients were left poorly controlled on oral agents for years and then started on insulin, and is less common now. However, it is still seen in pregnancy if that stimulates a rapid improvement in control – a dramatic drop in HbA1c may be associated with a deterioration in retinopathy.
The diagnosis of sight-threatening retinopathy may be a powerful motivating factor.
The differences were more marked in progression to HR-PDR, so perhaps with longer follow-up the SVL differences would have increased, though not if they were carefully monitored and PRP given once HRC appeared. However, it should be borne in mind that in category 2 (MO and less severe retinopathy) the deferred group could receive PRP only once they reached HR-PDR, whereas the early photocoagulation groups could have ‘rescue’ PRP from the severe NPDR stage onwards. So there was some imbalance in application of rescue laser.
As reported in Table 10, the RR for progression to SVL in category 1 eyes (i.e. eyes without MO) was 1.37 (99% CI 0.67 to 2.77), i.e. the early PRP group did worse, though not statistically significantly, than in categories 2 and 3, which all had MO at baseline. It is likely that if this group was removed from the combined analysis, the overall RR would have been less than the 0.77 (99% CI 0.56 to 1.06) and the primary end point result would have been statistically significant. This might suggest that treating MO avoided SVL more than treating retinopathy. However, as reported above, the main cause of SVL was vitreous or pre-retinal haemorrhage (52%), with MO well behind at 14%, followed by macular or retinal detachment (7.0%).
The reason for the MO groups doing better than category 1 may simply be that they had a higher risk of visual loss and so more to gain.
The level of vision used for the primary outcome (less than 5/200) was very low – any trial nowadays would try to preserve vision at better levels, for example at 20/200. The ETDRS definition of moderate visual loss was defined in ETDRS #99 as loss of 15 letters or more between baseline and follow-up, which would apply today.
Pautler (2010)47 noted the clear recommendation from the ETDRS group against PRP in eyes with mild or moderate NPDR, but commented that the recommendations for severe NPDR and early PDR were much less clear – only that PRP should be considered. He suggests that ‘This cautious wording may have led physicians away from treating this group of eyes’.
Pautler (2010)47 suggests that PRP might be used in severe NPDR and early PDR in the following situations:
- bilateral DR approaching HR-PDR
- poor compliance with follow-up
- poor glycaemic control
- type 1 diabetes (despite the ETDRS result showing greater effect in type 2 diabetes)
- DMO (but treating the DMO first)
- previous SVL in the other eye
- pregnancy
- rubeosis (new vessels in the iris)
- large area of new vessels outside the macula.
He also suggests factors that might lead to postponement of PRP in eyes with severe NPDR or early PDR such as past laser harm in the other eye, good glycaemic control, no DMO, low-risk of visual loss in the fellow eye and patient preference.
In the ETDRS, PRP was applied to all midperipheral retina, whether ischaemic or not. Currently, using wide-angle FA, areas of retinal ischaemia can be adequately identified. Laser photocoagulation could be applied selectively to areas of retinal ischaemia, potentially reducing side effects of this treatment, such as visual field defects, as in the Japanese trial17 described in Chapter 3.
The risk of progressing to HRC-PDR was reduced more than that of SVL. The reduction in the risk of progression to HRC-PDR is not unexpected. PRP treatment ablates much of the retina. As it appears that retinal ischaemia drives the VEGF response required for the development and support of neovascularisation, following laser treatment there would be little chance for PDR to occur. In the ETDRS, patients were followed at 4-monthly intervals (unless a problem such as vitreous haemorrhage occurred). It is unknown whether similar results would still be observed if the trial would have allowed closer follow-up so that HR-PDR could have been treated more promptly.
One of the possible side effects of PRP that could have a negative impact in the QoL of patients undergoing this treatment is the development of peripheral visual field defects. Depending on their severity, peripheral visual field defects may prevent individuals from driving. Delaying PRP until it is clearly needed – for instance, until neovessels develop – may give individuals extra years of maintaining driving standards and better QoL. About 20% of people may not meet Driver and Vehicle Licensing Agency (DVLA) driving standards after bilateral PRP.48
The ETDRS established two groups, based on fundus examination, for the evaluation of treatment effects: (1) severe NPDR and early PDR and (2) HR-PDR. However, the presence or absence of neovascularisation clearly determines a different stage of disease, as visual loss occurs as a direct result of the neovascularisation process in most cases. This is illustrated by the fact that over half of people in the ETDRS who experienced SVL did so as a result of vitreous or pre-retinal haemorrhage. Thus, it might have been more appropriate to evaluate the effectiveness of treating with PRP at early PDR (less than HRCs) stage when compared with treating when HR-PDR characteristics had developed. A third group with severe NPDR could have also been included. Having severe NPDR and early PDR together made this group somewhat heterogeneous.
Decision problem revisited
The ETDRS was a very good quality and detailed study. However, it was conducted several decades ago, and one question is whether new developments since the time of the ETDRS have changed the balance of benefits and harms.
These developments include:
- Improvements in diabetes care, with better control of blood glucose, BP and lipids.
- Changes in laser treatment, arising from advances in laser technologies, different regimens and better targeting of laser therapy. There has been a trend to ‘lighter’ laser treatment with the aim of causing fewer adverse effects but retaining the same effectiveness.
- The advent of new drugs for DMO, which may also affect retinopathy, and, more importantly for our purposes, are being used in combination with laser photocoagulation in DMO, partly to reduce the adverse effects. Patients with both DMO and PDR will be expected to receive both PRP and anti-VEGFs, and the latter may affect the PDR.
- Advances in imaging, such as optical coherence tomography (OCT), which may make detection of DMO more reliable.
Given these changes, the next questions are:
- If it was decided to start PRP at the NPDR stage, based on the results of ETDRS, what sort of laser treatment would be used? Pattern lasers?
- If PRP was given earlier, should it be targeted at areas of retinal ischaemia, as detected by wide-angle FA, or given by conventional PRP that ablates the whole mid/peripheral retina, both perfused and non-perfused areas (NPAs)? (The same question could apply to PRP for PDR.)
- Should drug treatment, mainly with the anti-VEGF drugs, or perhaps with intravitreal steroids, also be used in combination with PRP?
These questions are addressed in the Chapters 3 and 4.
Another issue is whether modern techniques of measuring DR and MO might also affect staging of retinopathy, and aid selection of people for PRP. This might be done both by determining who is at most risk of progression to HR-PDR, and who is at most risk from damage by PRP. This might ensure that PRP is given to the people who will most benefit. It is known that eyes with MO before PRP are more likely to have a reduction in VA after PRP,9,49 and, as has been pointed out by Browning (2005)50/Browning et al. (2004)51 and Massin et al. (2006),52 ophthalmologists often have difficulty detecting MO. Browning et al. (2008)53 also reported that the probability of MO being detected by OCT, but not by clinical examination (stereoscopic slit-lamp examination), increased as the retinopathy became more severe. The advent of OCT with its very good sensitivity for detecting retinal thickening should lead to better detection of MO and consequent tailoring of laser treatment to the needs of the individual eye.
- The landmark trials: Diabetic Retinopathy Study and Early Treatment Diabetic Ret...The landmark trials: Diabetic Retinopathy Study and Early Treatment Diabetic Retinopathy Study - Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: systematic review and economic evaluation
Your browsing activity is empty.
Activity recording is turned off.
See more...