NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical Agents and Related Occupations. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100F.)
4-Aminobiphenyl was considered by previous IARC Working Groups in 1971, 1987, and 2008 (IARC, 1972, 1987, 2010). Since that time new data have become available, which have been incorporated in this Monograph, and taken into consideration in the present evaluation.
1. Exposure Data
1.1. Identification of the agent
- Chem. Abstr. Serv. Reg. No.: 92-67-1
- Chem. Abstr. Serv. Name: [1,1'-Biphenyl]-4-amine
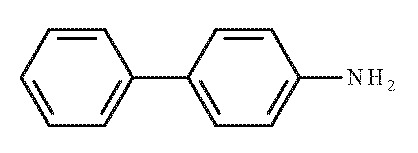
- C12H11N
- Relative molecular mass: 169.22
- Description: Colourless, crystalline solid that turns purple when exposed to air
- Solubility: Slightly soluble in cold water; soluble in acetone, chloroform, ethanol, diethyl ether, and hot water
From O’Neil (2006), Lide (2008), and IARC (2010)
1.2. Uses
4-Aminobiphenyl has been used in the past as a rubber antioxidant, as a dye intermediate, and in the detection of sulfates. It is reportedly used as a model carcinogen in mutagenicity studies and in cancer research (NTP, 2005; O’Neil, 2006; HSDB, 2009).
1.3. Human exposure
1.3.1. Occupational exposure
Historically, occupational exposure to 4-aminobiphenyl mainly occurred during its production and its use as a rubber antioxidant and dye intermediate. No exposure measurements are available for these occupational exposure situations (IARC, 2010).
Occupational exposure can also occur when workers are exposed to products contaminated with 4-aminobiphenyl, or in the case of exposure to benzidine and benzidine-based dyes, from which 4-aminobiphenyl can be metabolically released (IARC, 2010). In a study from India on workers exposed to benzidine or benzidine-based dyes and a non-exposed control group, urine samples were analysed for 4-aminobiphenyl and acetylated 4-aminobiphenyl (Ac4ABP). 4-Aminobiphenyl was found in 30 of 33 urine samples from exposed workers and in one sample from the 13 control workers. The workers exposed to benzidine had significantly higher median 4-aminobiphenyl concentrations (57 pmol/mL) than those exposed to benzidine-based dyes (29.3 pmol/mL). Ac4ABP was only detected (79.5 pmol/mL) in the urine sample that was provided by the person who had the highest 4-aminobiphenyl concentration (Beyerbach et al., 2006).
1.3.2. Non-occupational exposure
The main sources of exposure to 4-aminobiphenyl for the general population are cigarette smoking and second-hand tobacco smoke, as 4-aminobiphenyl is formed during tobacco combustion. The following amounts of 4-aminobiphenyl have been reported in unfiltered mainstream, filtered mainstream and side-stream cigarette smoke, respectively: 2.4 to 4.6 ng/cigarette; 0.2 to 23 ng/cigarette; and up to 140 ng/cigarette (Patrianakos & Hoffmann, 1979; Hoffmann et al., 1997).
Other potential sources include hair dyes and food colourant. 4-Aminobiphenyl can occur as a contaminant in 2-aminobiphenyl, which is used in the manufacture of dyes. 4-Aminobiphenyl has been detected in aniline, in the drug and cosmetic colour additive D&C Yellow No. 1, in the food dye FD&C Yellow No. 6, and in hair dyes (Richfield-Fratz et al., 1985; Chiang et al., 1999; Turesky et al., 2003; Akyüz, 2007; Bafana et al., 2007). 4-Aminobiphenyl has also been found as a contaminant in diphenylamine, a fungicide that has been used on apples.
4-Aminobiphenyl has been detected in fume from cooking oils. In a study from Taiwan, China, concentrations of 4-aminobiphenyl were 35.7 μg/m3 in fumes from cooking with sunflower oil, 26.4 μg/m3 in vegetable oil fumes and 23.3 μg/m3 in oil fumes from refined lard (Chiang et al., 1999).
Living near benzidine-contaminated sites may result in exposure to 4-aminobiphenyl, as benzidine in the environment can be degraded to 4-aminobiphenyl by certain bacteria (Bafana et al., 2007).
2. Cancer in Humans
2.1. Descriptive studies
Melick et al. (1955) reported a series of 19 cases of cancer of the urinary bladder in 171 male workers (11.1%) engaged in the production of 4-aminobiphenyl. The exposure took place in a chemical plant in the United States of America (USA) between 1935 and 1955. In a follow-up study it was reported that among 315 male workers exposed to 4-aminobiphenyl, 53 had developed bladder tumours (Melick et al., 1971).
2.2. Cohort studies
Following the cessation of industrial production of 4-aminobiphenyl in 1955, a surveillance programme in exposed workers revealed 31 of 285 men with significantly abnormal epithelial cells in urinary sediments, of whom ten were diagnosed with histologically confirmed bladder carcinoma (Melamed et al., 1960). Subsequently, 11 additional cases were found among 18 of the men reported in 1960 to have abnormal cells (Koss et al., 1965). Expanded surveillance programmes identified 35 workers with cancer of the urinary bladder among 503 workers (Koss et al., 1969) and 43 men with confirmed bladder carcinoma among 86 men with suspicious or positive histology (Melamed, 1972).
Cancer mortality was studied among 884 male workers at a chemical plant in West Virginia (USA) that produced a variety of chemicals. A ten-fold increase in mortality from bladder cancer was reported, with all nine cases having started work before 4-aminobiphenyl production ceased in the plant in 1952 (Zack & Gaffey, 1983). An analysis of mortality through 1987 showed 11 deaths from cancer of the urinary bladder among workers in jobs with possible exposure to 4-aminobiphenyl, compared to 0.54 expected (Collins et al., 1993). Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was also considered, based on reports of chloracne related to an industrial accident with TCDD in 1949, but ten of the 11 workers who died of cancer of the urinary bladder did not have chloracne. Collins et al. (1999) conducted another cohort study in the same plant and evaluated the risk for cancer of the urinary bladder associated with exposure to 4-aminobiphenyl and another bladder carcinogen, 2-mercaptobenzothiazole (MBT). Eight workers in jobs with exposure to 4-aminobiphenyl and MBT died of cancer of the urinary bladder, 0.3 deaths were expected (SMR 27.1; 95%CI: 11.7–53.4), while five workers exposed to MBT in jobs associated with little or no exposure to 4-aminobiphenyl died of cancer of the urinary bladder, compared with 1.2 expected.
2.3. Synthesis
Case reports and cohort-surveillance studies indicate a high occurrence of cancer of the urinary bladder in workers occupationally exposed to 4-aminobiphenyl, supported by evaluations of mortality in a chemical plant in the USA. Bladder cancer is strongly associated with occupational exposure to 4-aminobiphenyl.
3. Cancer in Experimental Animals
Studies on the carcinogenicity of 4-aminobiphenyl in the mouse, rat, dog, and rabbit after oral administration or after subcutaneous or intraperitoneal injection have been reviewed in previous IARC Monographs (IARC, 1972, 1987, 2010). The results of adequately conducted carcinogenicity studies are summarized in Table 3.1. There have been no additional carcinogenicity studies in animals reported since the most recent evaluation (IARC, 2010).
Table 3.1
Carcinogenicity studies of 4-aminobiphenyl in experimental animals.
4-Aminobiphenyl was tested for carcinogenicity by oral administration in three studies in mice, six studies in dogs and one study in rabbits, by subcutaneous injection in one study in mice and one study in rats and by intraperitoneal injection in four studies in mice.
Oral administration of 4-aminobiphenyl caused increased incidences of angiosarcoma (all sites) in male and female mice, bladder carcinoma in male mice (Schieferstein et al., 1985), hepatocellular carcinoma in female mice (Clayson et al., 1967; Schieferstein et al., 1985), and bladder carcinoma in male and female dogs (Walpole et al., 1954; Deichmann et al., 1958, 1965; Block et al., 1978) and in rabbits (sex not specified) (Bonser, 1962). [The Working Group noted that there were limitations in the design and reporting of these studies.] The incidence of hepatocellular adenoma and/or carcinoma was increased in male mice after subcutaneous (Gorrod et al., 1968) or intraperitoneal injection (Dooley et al., 1992; Parsons et al., 2005). [Most of these studies were designed to study tumour formation in the liver and the histopathology is limited to examination of the liver only.]
4. Other Relevant Data
4.1. Aromatic amines: metabolism, genotoxicity, and cancer susceptibility
Biotransformation pathways and genotoxic effects of aromatic amines are described in detail in IARC (2010); highlights are summarized below.
Exposures to aromatic amines, such as 2-naphthylamine, 4-aminobiphenyl and benzidine in the textile dye and rubber tyre industries have long been known to cause cancer of the urinary bladder in humans. These substances also induce neoplasms at multiple organ sites in laboratory animals. Tobacco smoke and hair dyes are major non-occupational sources of exposure to arylamines, as is demonstrated by the detection of aminobiphenyl-haemoglobin adducts (Bryant et al., 1988; Ward et al., 1996; Beyerbach et al., 2006), which can be 3- to 10-fold more abundant in tobacco smokers than in nonsmokers (Yu et al., 2002). Other environmental sources of exposure are likely to exist as well, because biomarkers derived from aromatic amines, such as haemoglobin adducts or urinary metabolites have been identified also in non-smokers who are not occupationally exposed to these chemicals.
Multiple metabolic pathways are involved in the activation of aromatic amines to DNA-reactive intermediates. Metabolism is initiated in the liver with either N-oxidation (by cytochrome P-450-associated enzymes) or N-acetylation (by N-acetyltransferase 2, NAT2). N-oxidation to N-hydroxyarylamine is mainly mediated by CYP1A2, but also CYP1A1 and CYP4B1 iso-enzymes may play a role (Landi et al., 1996; Ketelslegers et al., 2009). NAT2-catalysed N-acetylation can provide a detoxification pathway for aromatic amines since it reduces the amount of parent compound that may undergo CYP-mediated N-hydroxylation. The N-hydroxy metabolite is highly electrophilic: N-hydroxyaminobiphenyl, the oxidation product of 4-aminobiphenyl (4-ABP) forms adducts with hepatic DNA at the C8 position of deoxyguanosine and deoxyadenosine in rats (Jones & Sabbioni, 2003). N-hydroxyarylamines may be transported in free form to the blood or be conjugated with glucuronide. The acid-labile glucuronidated intermediate is excreted via the kidney and hydrolysed in the bladder lumen where it eventually forms the N-hydroxy metabolite again. The acidic pH of urine enhances the hydrolysis reaction and thus represents an additional risk factor for aromatic amine-related bladder cancer. NAT1-mediated O-acetylation may represent the final activation step of N-hydroxyarylamines; it takes place in the bladder epithelium and forms N-acetoxyarylamine. Breakdown of this unstable aromatic acetoxy ester produces the highly reactive aryl nitrenium ion that may serve as electrophilic intermediate leading to DNA adducts and tumour initiation. The highly active NAT1*10 isoform was correlated with higher levels of arylamine-DNA adducts in the human bladder (Kadlubar & Badawi, 1995).
Other activation pathways of aromatic amines to DNA-reactive intermediates include the sulfotransferase-mediated activation of N-hydroxyarylamine to an N-sulfate ester (Chou et al., 1995), the myeloperoxidase- (Lakshmi et al., 2000) and lactoperoxidase-mediated pathways that catalyse activation in the mammary gland (Gorlewska-Roberts et al., 2004), the peroxidative activation by prostaglandin H synthase (Flammang et al., 1989) – likely predominant in extra-hepatic tissues with low levels of cytochrome P450 isoenzymes –, and non-enzymatic protonation of the N-hydroxylamine nitrogen (Beland et al., 1983). Genotoxic aromatic amines may induce tumour formation at different sites depending on substrate specificity and different bio-activation pathways. Inter-individual variability in prostaglandin H synthases in the urinary bladder and in myeloperoxidases in the lung may account for differences in target-site susceptibility to aromatic amines in cigarette smokers (Flammang et al., 1989).
The genotoxic effects of aromatic amines are well established on the basis of mutagenicity and clastogenicity observed in numerous in vitro and in vivo assays that show the capability of these compounds to form DNA adducts after metabolic activation to electrophilic intermediates. The predominant site for covalent binding of aromatic amines to DNA is the C8 position of guanine, but adducts at other sites, including C8 of adenine and N2 of guanine, have also been identified (Beland et al., 1983; Kaderlik et al., 1993; Lin et al., 1994; Rothman et al., 1996a). As DNA adducts may lead to somatic point mutations, it is reasonable to assume that activated aromatic amines may lead to bladder-tumour development by inducing mutations in key genes such as the TP53 tumour suppressor gene (Sørlie et al., 1998; Feng et al., 2002) and the H-RAS gene (Boulalas et al., 2009), both involved in bladder carcinogenesis.
Organ specificity and inter- and intra-species differences in cancer susceptibility to aromatic amines are likely related to polymorphisms in genes that regulate DNA-repair, since deficient DNA-repair capacity is associated with increased bladder cancer risk (Lin et al., 2005), and to polymorphisms in genes that encode enzymes involved in activation or detoxification pathways. The NAT2 slow-acetylator genotype accounts for a greater risk for cancer of the urinary bladder in individuals exposed to 2-naphthylamine or 4-aminobiphenyl (Yu et al., 2002) and for a lower risk in workers exposed to benzidine (Carreón et al., 2006). Conflicting findings between studies may be a consequence of the interdependence of pathways of arylamine metabolism and of the capability of N-acetyltransferases both to detoxify the parent compound and to activate metabolites at different rates in different tissues.
4.2. 4-Aminobiphenyl
N-hydroxylation of 4-aminobiphenyl (4-ABP) in human and rat-liver microsomes is primarily catalysed by CYP1A2 (Kimura et al., 1999), an enzyme with a large inter-individual variability (Butler et al., 1989), and by extra-hepatic cytochrome P450s including CYP1A1, CYP1B1, and CYP2A13 (Shimada et al., 1996; Nakajima et al., 2006). In extra-hepatic tissues, the binding of 4-ABP to DNA may be catalysed by peroxidase enzymes, such as prostaglandin H synthase (Flammang et al., 1989).
The major 4-ABP-DNA adduct identified in human bladder and lung is N-(deoxyguanosin-8-yl)-4-ABP (Lin et al., 1994): other adducts include N-(deoxyadenosin-8-yl)-4-ABP and N-(deoxyguanosin-N2-yl)-4-ABP (Beland et al., 1983). N-(deoxyguanosin-8-yl)-4-ABP has also been detected in female breast tissue of both smokers and non-smokers (Lin et al., 1994; Faraglia et al., 2003) indicating that 4-ABP-reactive intermediates are distributed systemically and/or that multiple organs are capable of activating 4-ABP or its metabolites. Experiments in animals show that 4-ABP induces bladder tumours in mice, rabbits, and dogs, liver tumours, mammary gland tumours and angiosarcomas in mice, and intestinal tumours in rats. Increased levels of 4-ABP-haemoglobin adducts are associated with cigarette smoking (Bryant et al., 1988), and occupational exposure to 4-ABP is associated with an increased risk for cancer of the urinary bladder (Beyerbach et al., 2006).
To explore the role of various metabolic intermediates in the mutageniticy of aromatic amines, the DNA-damaging potential of 4-ABP was studied in different bacterial strains. In S. typhimurium in the presence of S-9-mediated metabolic activation, 4-ABP was found to induce mutations such as frameshifts and base substitutions in TA98 and TA100 strains, respectively (Chung et al., 2000), and oxidant-induced mutations in TA102, suggesting an oxidative mechanism (Makena & Chung, 2007). 4-ABP-induced DNA damage was mainly due to activation by NAT1 (Oda, 2004) and was increased with higher O-acetyltransferase activity (Dang & McQueen, 1999), thus demonstrating the potentially important role of N-acetoxy-4-ABP in the mutagenicity of this aromatic amine. In E. coli, 4-ABP induced base-pair substitutions predominantly at G sites, including G→T, G→C transversions, and G→A transitions (Verghis et al., 1997). In addition, G→C transversion mutations were triggered by incorporating an oligonucleotide containing the N-(deoxyadenosin-8-yl)-4-ABP adduct into the single-stranded DNA of the cloning vector, demonstrating the role of this adduct in 4-ABP-induced mutagenesis.
4-ABP induced mutations at the HPRT locus and chromosomal instability in human bladder epithelial cells. In 4-ABP-induced liver tumours in B6C3F1 and CD-1 mice, primarily C→A and A→T mutations, respectively, were detected at codon 61 of the H-Ras gene. 4-ABP also increased the mutation frequency in the bladder, liver, and bone marrow of mice. In human bladder cells treated with N-hydroxy-4-ABP, preferential sites of adduct formation in TP53 were at codons 175, 248, 280, and 285, which are mutational hotspots for cancer of the urinary bladder (Feng et al., 2002).
5. Evaluation
There is sufficient evidence in humans for the carcinogenicity of 4-aminobiphenyl. 4-Aminobiphenyl causes cancer of the urinary bladder.
There is sufficient evidence in experimental animals for the carcinogenicity of 4-aminobiphenyl.
There is strong mechanistic evidence indicating that the carcinogenicity of 4-aminobiphenyl in humans operates by a genotoxic mechanism of action that involves metabolic activation, formation of DNA adducts, and induction of mutagenic and clastogenic effects. Metabolic activation to DNA-reactive intermediates occurs by multiple pathways including N-oxidation in the liver, O-acetylation in the bladder, and peroxidative activation in the mammary gland and other organs.
4-Aminobiphenyl is carcinogenic to humans (Group 1).
References
- Akyüz M. Simultaneous determination of aliphatic and aromatic amines in indoor and outdoor air samples by gas chromatography-mass spectrometry. Talanta. 2007;71:486–492. [PubMed: 19071331] [CrossRef]
- Bafana A, Devi SS, Krishnamurthi K, Chakrabarti T. Kinetics of decolourisation and biotransformation of direct black 38 by C. hominis and P. stutzeri. Appl Microbiol and Biotechnol. 2007;74:1145–1152. [PubMed: 17318544] [CrossRef]
- Beland FA, Beranek DT, Dooley KL, et al. Arylamine-DNA adducts in vitro and in vivo: their role in bacterial mutagenesis and urinary bladder carcinogenesis. Environmental Health Perspectives. 1983;49:125–134. [PMC free article: PMC1569140] [PubMed: 6339219] [CrossRef]
- Beyerbach A, Rothman N, Bhatnagar VK, et al. Hemoglobin adducts in workers exposed to benzidine and azo dyes. Carcinogenesis. 2006;27:1600–1606. [PubMed: 16497705] [CrossRef]
- Block NL, Sigel MM, Lynne CM, et al. The initiation, progress, and diagnosis of dog bladder cancer induced by 4-aminobiphenyl. Invest Urol. 1978;16:50–54. [PubMed: 689839]
- Bonser GM (1962) Precancerous changes in the urinary bladder. Perugia: 435–439.
- Boulalas I, Zaravinos A, Karyotis I, et al. Activation of RAS family genes in urothelial carcinoma. The Journal of Urology. 2009;181:2312–2319. [PubMed: 19303097] [CrossRef]
- Bryant MS, Vineis P, Skipper PL, Tannenbaum SR. Hemoglobin adducts of aromatic amines: associations with smoking status and type of tobacco. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:9788–9791. [PMC free article: PMC282866] [PubMed: 3200858] [CrossRef]
- Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:7696–7700. [PMC free article: PMC298137] [PubMed: 2813353] [CrossRef]
- Carreón T, Ruder AM, Schulte PA, et al. NAT2 slow acetylation and bladder cancer in workers exposed to benzidine. International Journal of Cancer. 2006;118:161–168. [PubMed: 16003747] [CrossRef]
- Chiang T-A, Pei-Fen W, Ying LS, et al. Mutagenicity and aromatic amine content of fumes from heated cooking oils produced in Taiwan. Food and Chemical Toxicology. 1999;37:125–134. [PubMed: 10227736] [CrossRef]
- Chou HC, Lang NP, Kadlubar FF. Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s). Cancer Res. 1995;55:525–529. [PubMed: 7834621]
- Chung KT, Chen SC, Wong TY, et al. Mutagenicity studies of benzidine and its analogs: structure-activity relationships. Toxicological Sciences. 2000;56:351–356. [PubMed: 10910993] [CrossRef]
- Clayson DB, Lawson TA, Pringle JA. The carcinogenic action of 2-aminodiphenylene oxide and 4-aminodiphenyl on the bladder and liver of the C57 X IF mouse. Br J Cancer. 1967;21:755–762. [PMC free article: PMC2008181] [PubMed: 6074696] [CrossRef]
- Collins JJ, Strauss ME, Levinskas GJ, Conner PR. The mortality experience of workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a trichlorophenol process accident. Epidemiology. 1993;4:7–13. [PubMed: 8420584]
- Collins JJ, Strauss ME, Riordan SG. Mortalities of workers at the Nitro plant with exposure to 2-mercaptobenzothialzole. Occup Environ Med. 1999;56:667–671. [PMC free article: PMC1757670] [PubMed: 10658544]
- Dang LN, McQueen CA. Mutagenicity of 4-aminobiphenyl and 4-acetylaminobiphenyl in Salmonella typhimurium strains expressing different levels of N-acetyltransferase. Toxicology and Applied Pharmacology. 1999;159:77–82. [PubMed: 10495770] [CrossRef]
- Deichmann WB, Radomski J, Glass E, et al. Synergism among oral carcinogens. 3. simultaneous feeding of four bladder carcinogens to dogs. Ind Med Surg. 1965;34:640–649. [PubMed: 14334179]
- Deichmann WB, Radomski JL, Anderson WA, et al. The carcinogenic action of p-aminobiphenyl in the dog; final report. Ind Med Surg. 1958;27:25–26. [PubMed: 13491120]
- Dooley KL, Von Tungeln LS, Bucci T, et al. Comparative carcinogenicity of 4-aminobiphenyl and the food pyrolysates, Glu-P-1, IQ, PhIP, and MeIQx in the neonatal B6C3F1 male mouse. Cancer Letters. 1992;62:205–209. [PubMed: 1596864] [CrossRef]
- Faraglia B, Chen SY, Gammon MD, et al. Evaluation of 4-aminobiphenyl-DNA adducts in human breast cancer: the influence of tobacco smoke. Carcinogenesis. 2003;24:719–725. [PubMed: 12727801] [CrossRef]
- Feng Z, Hu W, Rom WN, et al. 4-aminobiphenyl is a major etiological agent of human bladder cancer: evidence from its DNA binding spectrum in human p53 gene. Carcinogenesis. 2002;23:1721–1727. [PubMed: 12376482] [CrossRef]
- Flammang TJ, Yamazoe Y, Benson RW, et al. Arachidonic acid-dependent peroxidative activation of carcinogenic arylamines by extrahepatic human tissue microsomes. Cancer Res. 1989;49:1977–1982. [PubMed: 2495173]
- Gorlewska-Roberts KM, Teitel CH, Lay JO Jr, et al. Lactoperoxidase-catalysed activation of carcinogenic aromatic and heterocyclic amines. Chemical Research in Toxicology. 2004;17:1659–1666. [PubMed: 15606142] [CrossRef]
- Gorrod JW, Carter RL, Roe FJ. Induction of hepatomas by 4-aminobiphenyl and three of its hydroxylated derivatives administered to newborn mice. J Natl Cancer Inst. 1968;41:403–410. [PubMed: 4299538]
- Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Preventive Medicine. 1997;26:427–434. [PubMed: 9245661] [CrossRef]
- HSDB (2009) Hazardous Substances Data Bank: 4-Aminobiphenyl. National Library of Medicine. Last updated: 1/15/04 [http://toxnet
.nlm.nih .gov/cgi-bin/sis/htmlgen?HSDB] - IARC. Some Inorganic Substances, Chlorinated Hydrocarbons, Aromatic Amines, N-Nitroso Compounds, and Natural Products. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. 1972;1:1–184.
- IARC. Overall Evaluations of Carcinogenicity. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Supplement. 1987;7:1–440. [PubMed: 3482203]
- IARC. Some aromatic amines, organic dyes, and related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;99:1–678. [PMC free article: PMC5046080] [PubMed: 21528837]
- Jones CR, Sabbioni G. Identification of DNA adducts using HPLC/MS/MS following in vitro and in vivo experiments with arylamines and nitroarenes. Chemical Research in Toxicology. 2003;16:1251–1263. [PubMed: 14565767] [CrossRef]
- Kaderlik KR, Talaska G, DeBord DG, et al. 4,4'-Methylene-bis(2-chloroaniline)-DNA adduct analysis in human exfoliated urothelial cells by 32P-postlabeling. Cancer Epidemiology, Biomarkers & Prevention. 1993;2:63–69. [PubMed: 8420614]
- Kadlubar FF, Badawi AF. Genetic susceptibility and carcinogen-DNA adduct formation in human urinary bladder carcinogenesis. Toxicology Letters. 1995;82–83:627–632. [PubMed: 8597119] [CrossRef]
- Ketelslegers HB, Godschalk RW, Eskens BJ, et al. Potential role of cytochrome P450–1B1 in the metabolic activation of 4-aminobiphenyl in humans. Molecular Carcinogenesis. 2009;4:685–691. [PubMed: 19274671] [CrossRef]
- Kimura S, Kawabe M, Ward JM, et al. CYP1A2 is not the primary enzyme responsible for 4-aminobiphenyl-induced hepatocarcinogenesis in mice. Carcinogenesis. 1999;20:1825–1830. [PubMed: 10469630] [CrossRef]
- Koss LG, Melamed MR, Kelly E. Further cytologic and histologic studies of bladder lesions in workers exposed to para-aminodiphenyl: progress report. J Natl Cancer Inst. 1969;43:233–243. [PubMed: 5796385]
- Koss LG, Melamed MR, Ricci A, et al. Carcinogenesis in the human urinary bladder: observations after exposure to para-aminodiphenyl. N Engl J Med. 1965;272:767–770. [PubMed: 14263636]
- Lakshmi VM, Hsu FF, Davis BB, Zenser TV. N-Acetylbenzidine-DNA adduct formation by phorbol 12-myristate-stimulated human polymorphonuclear neutrophils. Chemical Research in Toxicology. 2000;13:785–792. [PubMed: 10956067] [CrossRef]
- Landi MT, Zocchetti C, Bernucci I, et al. Cytochrome P4501A2: enzyme induction and genetic control in determining 4-aminobiphenyl-hemoglobin adduct levels. Cancer Epidemiology, Biomarkers & Prevention. 1996;5:693–698. [PubMed: 8877060]
- Lide DR, editor (2008) CRC Handbook of Chemistry and Physics, 89th Ed., Boca Raton, FL, CRC Press, p. 3–16.
- Lin D, Lay JO Jr, Bryant MS, et al. Analysis of 4-aminobiphenyl-DNA adducts in human urinary bladder and lung by alkaline hydrolysis and negative ion gas chromatography-mass spectrometry. Environmental Health Perspectives. 1994;102 Suppl 6:11–16. [PMC free article: PMC1566844] [PubMed: 7889831] [CrossRef]
- Lin J, Kadlubar FF, Spitz MR, et al. A modified host cell reactivation assay to measure DNA repair capacity for removing 4-aminobiphenyl adducts: a pilot study of bladder cancer. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:1832–1836. [PubMed: 16030125] [CrossRef]
- Makena P, Chung KT. Evidence that 4-aminobiphenyl, benzidine, and benzidine congeners produce genotoxicity through reactive oxygen species. Environmental and Molecular Mutagenesis. 2007;48:404–413. [PubMed: 17370336] [CrossRef]
- Melamed MR. Diagnostic cytology of urinary tract carcinoma. A review of experience with spontaneous and carcinogen induced tumors in man. Eur J Cancer. 1972;8:287–292. [PubMed: 5074773]
- Melamed MR, Koss LG, Ricci A, Whitmore WF. Cytohistological observations on developing carcinoma of the urinary bladder in man. Cancer. 1960;13:67–74.
- Melick WF, Escue HM, Naryka JJ, et al. The first reported cases of human bladder tumors due to a new carcinogen-xenylamine. J Urol. 1955;74:760–766. [PubMed: 13278983]
- Melick WF, Naryka JJ, Kelly RE. Bladder cancer due to exposure to para-aminobiphenyl: a 17-year followup. J Urol. 1971;106:220–226. [PubMed: 5099312]
- Nakajima M, Itoh M, Sakai H, et al. CYP2A13 expressed in human bladder metabolically activates 4-aminobiphenyl. International Journal of Cancer. 2006;119:2520–2526. [PubMed: 16988941] [CrossRef]
- NTP. NTP 11th Report on Carcinogens: Vinyl Chloride. Rep Carcinog. 2005;(11):1–A32. [PubMed: 19826456]
- O’Neil MJ, editor (2006) The Merck Index, Whitehouse Station, NJ, Merck & Co., Inc., p. 204.
- Oda Y. Analysis of the involvement of human N-acetyltransferase 1 in the genotoxic activation of bladder carcinogenic arylamines using a SOS/umu assay system. Mutat Res. 2004;554:399–406. [PubMed: 15450435]
- Parsons BL, Beland FA, Von Tungeln LS, et al. Levels of 4-aminobiphenyl-induced somatic H-ras mutation in mouse liver DNA correlate with potential for liver tumor development. Molecular Carcinogenesis. 2005;42:193–201. [PubMed: 15761837] [CrossRef]
- Patrianakos C, Hoffmann D. Chemical studies on tobacco smoke. LXIV. On the analysis of aromatic amines in cigarette smoke. J Anal Toxicol. 1979;3:150–154.
- Richfield-Fratz N, Bailey JE Jr, Bailey CJ. Determination of unsulfonated aromatic amines in FD&C Yellow No. 6 by the diazotization and coupling procedure followed by reversed-phase high-performance liquid chromatography. Journal of Chromatography. 1985;331:109–123. [PubMed: 4044733] [CrossRef]
- Rothman N, Bhatnagar VK, Hayes RB, et al. The impact of interindividual variation in NAT2 activity on benzidine urinary metabolites and urothelial DNA adducts in exposed workers. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5084–5089. a. [PMC free article: PMC39410] [PubMed: 8643532] [CrossRef]
- Schieferstein GJ, Littlefield NA, Gaylor DW, et al. Carcinogenesis of 4-aminobiphenyl in BALB/cStCrlfC3Hf/Nctr mice. European Journal of Cancer & Clinical Oncology. 1985;21:865–873. [PubMed: 2995043] [CrossRef]
- Shimada T, Hayes CL, Yamazaki H, et al. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed: 8674051]
- Sørlie T, Martel-Planche G, Hainaut P, et al. Analysis of p53, p16MTS, p21WAF1 and H-ras in archived bladder tumours from workers exposed to aromatic amines. Br J Cancer. 1998;77:1573–1579. [PMC free article: PMC2150070] [PubMed: 9635831]
- Turesky RJ, Freeman JP, Holland RD, et al. Identification of aminobiphenyl derivatives in commercial hair dyes. Chem Res Toxicol. 2003;16:1162–1173. [PubMed: 12971805] [CrossRef]
- Verghis SB, Essigmann JM, Kadlubar FF, et al. Specificity of mutagenesis by 4-aminobiphenyl: mutations at G residues in bacteriophage M13 DNA and G–>C transversions at a unique dG(8-ABP) lesion in single-stranded DNA. Carcinogenesis. 1997;18:2403–2414. [PubMed: 9450488] [CrossRef]
- Von Tungeln LS, Bucci TJ, Hart RW, et al. Inhibitory effect of caloric restriction on tumorigenicity induced by 4-aminobiphenyl and 2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine (PhIP) in the CD1 newborn mouse bioassay. Cancer Lett. 1996;104:133–136. [PubMed: 8665480] [CrossRef]
- Walpole AL, Williams MH, Roberts DC. The carcinogenic action of 4-aminodiphenyl and 3:2′-dimethyl-4-amino-diphenyl. Br J Ind Med. 1952;9:255–263. [PMC free article: PMC1037417] [PubMed: 12987579]
- Walpole AL, Williams MH, Roberts DC. Tumours of the urinary bladder in dogs after ingestion of 4-aminodiphenyl. Br J Ind Med. 1954;11:105–109. [PMC free article: PMC1037534] [PubMed: 13149742]
- Ward EM, Sabbioni G, DeBord DG, et al. Monitoring of aromatic amine exposures in workers at a chemical plant with a known bladder cancer excess. Journal of the National Cancer Institute. 1996;88:1046–1053. [PubMed: 8683635] [CrossRef]
- Yu MC, Skipper PL, Tannenbaum SR, et al. Arylamine exposures and bladder cancer risk. Mutat Res. 2002;506–507:21–28. [PubMed: 12351141]
- Zack JA, Gaffey WR. A mortality study of workers employed at the Monsanto Company plant in Nitro, West Virginia. Environ Sci Res. 1983;26:575–591.
- 4-AMINOBIPHENYL - Chemical Agents and Related Occupations4-AMINOBIPHENYL - Chemical Agents and Related Occupations
Your browsing activity is empty.
Activity recording is turned off.
See more...
