NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal Habits and Indoor Combustions. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100E.)
Second-hand tobacco smoke was considered by a previous IARC Working Group in 2002 as “involuntary smoking” (IARC, 2004). Since that time, new data have become available, these have been incorporated into the Monograph, and taken into consideration in the present evaluation.
1. Exposure Data
Second-hand tobacco smoke comprises the smoke released from the burning tip of a cigarette (or other burned tobacco product) between puffs (called sidestream smoke (SM)) and the smoke exhaled by the smoker (exhaled mainstream smoke (MS)). Small additional amounts are contributed from the tip of the cigarette and through the cigarette paper during a puff, and through the paper and from the mouth end of the cigarette between puffs (Jenkins et al., 2000).
Second-hand tobacco smoke is also referred as ‘environmental tobacco smoke’, ‘passive smoking’ or ‘involuntary smoking’ (IARC, 2004). The terms ‘passive smoking’ or ‘involuntary smoking’ suggest that while involuntary or passive smoking is not acceptable, voluntary or active smoking is acceptable. In this document, we use the term second-hand tobacco smoke (WHO, 2010).
1.1. Chemical composition
Many studies have examined the concentrations of cigarette smoke constituents in mainstream and sidestream smoke. The composition of mainstream and sidestream smoke is qualitatively similar but quantitatively different. The ratios of sidestream to mainstream smoke vary greatly depending on the constituent. Some representative SS:MS ratios are: nicotine, 7.1; carbon monoxide, 4.8; ammonia, 455; formaldehyde, 36.5; acrolein, 18.6; benzo[a]pyrene, 16.0; N′-nitrosonornicotine (NNN), 0.43; (methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), 0.40 (Jenkins et al., 2000; IARC, 2004).
The physicochemical properties of second-hand tobacco smoke are different from those of mainstream smoke and sidestream smoke because of its rapid dilution and dispersion into the indoor environment (IARC, 2004). Concentrations of individual constituents in second-hand tobacco smoke can vary with time and environmental conditions. Field studies of these constituents and representative data have been extensively summarized (Jenkins et al., 2000; IARC, 2004). Some representative data are presented in Table 1.1 (Jenkins et al., 2000; IARC, 2004; US Department of Health and Human Services, 2006).
Table 1.1
Concentration of selected constituents in second-hand tobacco smoke.
1.2. Sources of exposure
Second-hand tobacco smoke is present in virtually all places where smoking takes place (Navas-Acien et al., 2004): at home, in the workplace, in bars, restaurants, public buildings, hospitals, public transport and educational institutions. The setting that represents the most important source of exposure differs depending on the population. For example in children, the home environment may constitute a significant source of exposure, while other sources that may contribute are schools and public transportation. Likewise, for most women, the home environment is the primary source of second-hand tobacco smoke, which may be enhanced by exposure at the workplace.
Biomarker studies have evaluated carcinogen uptake in non-smokers exposed to second-hand tobacco smoke. The NNK metabolites NNAL and its glucuronides (total NNAL) are consistently elevated in non-smokers exposed to second-hand tobacco smoke, in studies conducted in various living and occupational environments, and from infancy through adulthood (Hecht et al., 2006; Hecht, 2008). Levels of the biomarker of PAHs, urinary 1-hydroxypyrene, were significantly elevated in a large study of non-smokers exposed to second-hand tobacco smoke (Suwan-ampai et al., 2009).
1.3. Measures of exposure
A conceptual framework for considering exposure to second-hand tobacco smoke is the “microenvironmental model,” which takes the weighted sum of the concentrations of second-hand tobacco smoke in the microenvironments where time is spent, with the weights the time spent in each, as a measure of personal exposure (Jaakkola & Jaakkola, 1997). Direct measures of exposure use concentrations of second-hand tobacco smoke components in the air in the home, workplace, or other environments, combined with information on the time spent in the microenvironments where exposure took place. Measurements of tobacco smoke biomarker(s) in biological specimens also represent a direct measure of exposure to second-hand smoke (Samet & Yang, 2001; Table 1.2). Indirect measures are generally obtained by survey questionnaires. These include self-reported exposure and descriptions of the source of second-hand tobacco smoke in relevant microenvironments, most often the home and workplace (Samet & Yang, 2001).
Table 1.2
Types of indicators measuring exposure to second-hand tobacco smoke.
One useful surrogate measure, and the only available in many countries, is the prevalence of smoking among men and women. It provides a measure of the likelihood of exposure. In most countries in Asia and the Middle East, for example, the very high prevalence of smoking among men combined with the low prevalence among women would imply that most women are exposed to second-hand tobacco smoke at home (Samet & Yang, 2001).
To measure exposure to second-hand tobacco smoke in children, self-reported smoking habits of their parents are used as a surrogate (US Department of Health and Human Services, 2006). More recently, other surrogate measures such as nicotine concentrations in house dust have been considered less biased than parental smoking as they reflect cumulative smoking habits and long-term exposure rather than current patterns of smoking (Whitehead et al., 2009).
1.4. Prevalence of exposure
1.4.1. Exposure among children
(a) Overview
The most extensive population-based data on exposure to second-hand tobacco smoke among children are available through the Global Youth Tobacco Survey (GYTS) (CDC/WHO, 2009). GYTS is part of the Global Tobacco Surveillance System (GTSS), developed by the WHO and the United States’ Centers for Disease Control and Prevention (CDC) in 1998. The GYTS is a school-based survey designed to measure tobacco use and some key tobacco control measures among youth (13–15 years) using a common methodology and core questionnaire. While most GYTS are national surveys, in some countries they are limited to subnational locations. Further, countries conduct the GYTS in different years, rendering comparison across countries for the same year difficult. The GYTS questionnaire asks about children's exposure to second-hand tobacco smoke in their home or in other places in the last 7 days preceding the survey. Since its inception in 1999, over 2 million students in 160 countries representing all six WHO regions have participated in the GYTS (WHO, 2008, 2009a).
Country-level estimates on second-hand tobacco smoke exposure at home and in public places among youth are available in the WHO Reports on the global tobacco epidemic (WHO, 2008, 2009a, 2011).
(b) Exposure at home
Nearly half of youth aged 13–15 years are exposed to second-hand tobacco smoke in their homes (Fig. 1.1; CDC, 2008). Among the six WHO regions, exposure to second-hand tobacco smoke at home was highest in the European Region (77.8%) and lowest in the African region (27.6%). In the other four regions, exposure to second-hand tobacco smoke at home ranged from 50.6% in the Western Pacific Region to 34.3% in the South East Asian Region.
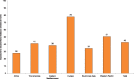
Fig. 1.1
Average prevalence (in%) of 13–15 year old children living in a home where others smoke, by WHO region, 2007
Fig. 1.2 shows the range of exposure to second-hand tobacco smoke at home by WHO region for boys and girls and for both sexes combined. The largest variations are observed in the Eastern Mediterranean Region and the European Region irrespective of sex. These variations are predominantly due to differences in parental smoking prevalence between countries, as well as the impact of the smoke-free places campaigns in place in various countries.
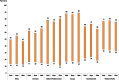
Fig. 1.2
Range of prevalence (in%) of exposure of 13–15 year old children to second-hand tobacco smoke at home, by WHO region, 2009
Country-level estimates from the Global Youth Tobacco Survey (1999–2009) are presented in Table 1.3.
Table 1.3
Prevalence of exposure to second-hand tobacco smoke at home and outside home among 13–15 year olds, by country and sex, from the Global Youth Tobacco Survey (participating countries only) — 1999–2009.
Öberg and colleagues have estimated the worldwide exposure to second-hand tobacco smoke among children by using parent's current smoking status as an indicator of exposure among children (WHO, 2010). Four out of ten children (approximately 700 million children globally) have at least one parent who currently smokes, predisposing them to exposure to second-hand tobacco smoke at home (Table 1.4). Children in the Western Pacific Region had the highest level of potential exposure (68%) while Africa had the lowest, with about 13% of children having at least one parent who smoked. In the 2010 WHO Report on global estimate of the burden of disease from second-hand smoke (WHO, 2010), country-level estimates were collected or modelled from various sources. [Data partially overlap with those of the Global Youth Tobacco Survey].
Table 1.4
Proportion of children under 15 years with one or more parent who smokes, by WHO subregion (based on survey data and modeling).
(c) Exposure outside home
Similar to second-hand tobacco smoke exposure at home, almost half of the youth are exposed to second-hand tobacco smoke in public places, according to estimates from the Global Youth Tobacco Survey (Fig. 1.3; CDC, 2008). Exposure was highest in Europe (86.1%); for the other five regions, exposure to second-hand tobacco smoke in public places ranged from 64.1% in the Western Pacific to 43.7% in Africa.
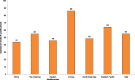
Fig. 1.3
Average prevalence (in%) of exposure of 13–15 year old children to second-hand tobacco smoke in public places, by WHO region, 2007
Fig. 1.4 presents the range of exposure to second-hand tobacco smoke outside home by WHO region for boys and girls and for both sexes combined. There are wide variations in second-hand tobacco smoke exposure outside home within each region. The largest variations are observed in the African region and the Western Pacific region irrespective of sex. This is largely influenced by the presence of smoke-free legislation for public paces in the countries, as well as levels of enforcement and public's compliance with these laws.
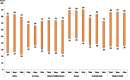
Fig. 1.4
Range of prevalence (in%) of exposure of 13–15 year old children to second-hand tobacco smoke outside their home, by WHO region, 2009
1.4.2. Exposure among adults
(a) Overview
While the GYTS offers a valuable global source for estimating exposure to second-hand tobacco smoke among children, there is no such extensive source of data for adults. Estimates of second-hand tobacco smoke exposure among adults have used the definitions of exposure based on having a spouse who smokes or exposure to tobacco smoke at work. For the countries lacking such data, exposure was estimated using a model based on smoking prevalence among men from the WHO Global InfoBase.
About one third of adults worldwide are regularly exposed to second-hand tobacco smoke (Table 1.5). The highest exposure was estimated in European Region C with 66% of the population being regularly exposed to second-hand tobacco smoke. The lowest regional exposure was estimated in the African region (4%). Differences between men and women were generally small, except in Eastern Mediterranean Region D and South East Asia Region B.
Table 1.5
Proportion of non-smoking adults exposed regularly to second-hand tobacco smoke, by WHO region (based on survey data and modeling).
(b) Exposure at home
The Global Tobacco Surveillance System, through its adult household survey “Global Adult Tobacco Survey” (GATS), collects information on key tobacco control indicators including information on second-hand tobacco smoke exposure at home, at work and several public places (WHO, 2009b). GATS is a nationally representative survey conducted among persons aged ≥ 15 years using a standardized questionnaire, sample design, data collection method, and analysis protocol. GATS results are available from 14 countries with a high tobacco burden. Additionally since 2008, The WHO STEPwise approach to surveillance (STEPS) surveys have started to collect information on exposure to second-hand tobacco smoke at home and at work, now available for 7 countries (WHO, 2009c).
In the 21 countries that have reported data on exposure to second-hand tobacco smoke, large numbers of people are exposed at home (Fig. 1.5). Exposure was highest in Sierra Leone (74%) and lowest in the British Virgin Islands (3%). Overall, differences between men and women were relatively small in most countries; in China, Cambodia and Mongolia, more women reported being exposed to second-hand tobacco smoke in their homes then men. This lack of difference implies that even when prevalence of smoking among women is low, they are exposed to second-hand tobacco smoke at home as much as men.

Fig. 1.5
Prevalence of adults exposed to second-hand tobacco smoke in their homes, in the countries that completed the Global Adult Tobacco Survey (GATS) and WHO STEPwise approach to surveillance (STEPS) surveys, 2008–2009
(c) Exposure at the workplace
The same magnitude of second-hand tobacco smoke exposure at the workplace was reported as at home (Fig. 1.6). Exposure to second-hand tobacco smoke at the workplace was highest in Sierra Leone (74%) and lowest in the British Virgin Islands (3%). However, more men reported being exposed to others’ smoke at their workplace as compared to women in all countries. This difference was most significant in Libyan Arab Jamahirya and Bangladesh. These differences could be explained by the fact that women either tend to work in places where smoking is banned, such as education or health facilities, or work predominantly with other women.

Fig. 1.6
Prevalence of adults exposed to second-hand tobacco smoke in their workplaces, in the countries that completed the Global Adult Tobacco Survey and WHO STEPwise approach to surveillance (STEPS) surveys, 2008–2009
1.5. Regulations
The World Health Organization's Framework Convention on Tobacco Control (WHO FCTC) is a multilateral treaty with legally binding obligations for its 174 Parties (as of November 2011) (WHO, 2003). This comprehensive treaty contains supply and demand reduction measures available to countries to counter the tobacco epidemic. Article 8 of the Treaty specifically addresses the need for protection from second-hand tobacco smoke, and articulates the “adoption and implementation of effective legislative, executive, administrative and /or other measures” by Parties to the Convention to this effect. Guidelines to Article 8 specify key elements needed in legislation to help countries meet the highest standards of protection from second-hand tobacco smoke and provide a clear timeline for Parties to adopt appropriate measures (within five years after entry into Force of the WHO FCTC) (WHO, 2007).
All countries, regardless of their FCTC ratification status, are taking steps to reduce second-hand tobacco smoke in public places, through either planning the steps to or implementing national smoke-free laws for public places or workplaces. In 2008, approximately 5% of the world's population (354 million) had national smoke-free laws. Fig. 1.7 provides details on the number of public places with national smoke-free legislation for all WHO Member States.
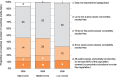
Fig. 1.7
Number and percentage of countries with number of public places covered by smoke free legislations, by income status (as of 31 December 2008)
As of December 2008, fifteen countries across the globe have legislation that provide the highest level of protection against second-hand tobacco smoke exposure. These include: Albania, Australia, Bhutan, Canada, Colombia, Guatemala, Islamic Republic of Iran, Ireland, Marshall Islands, New Zealand, Panama, Turkey, Turkmenistan, United Kingdom of Great Britain and Northern Ireland and Uruguay.
2. Cancer in Humans
2.1. Cancer of the lung
More than 50 epidemiological studies since 1981 have examined the association between second-hand tobacco smoke and lung cancer resulting in the conclusion that exposure of non-smokers to second-hand tobacco smoke is causally associated with lung cancer risk (IARC, 2004). Many studies previously available assessed the lung cancer risk among the nonsmoking spouses of smokers since it is one of the sources of adult exposure to second-hand tobacco smoke that is less likely to be subject to exposure misclassification or other bias. Several important new, cohort, case–control studies and meta-analyses have been published since 2004 that provide additional evidence confirming the causal association (Table 2.1 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.1.pdf, Table 2.2 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.2.pdf, and Table 2.3 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.3.pdf). These new studies also expand our assessment of the effect of second-hand tobacco smoke in the workplace allowing for more refined estimates of lung cancer risk. Preliminary data also suggest significant interactions between several genetic polymorphisms, second-hand tobacco smoke and lung cancer risk.
In a meta-analysis of 55 studies, including 7 cohort, 25 population based case–control studies and 23 hospital based case–control studies the pooled relative risk (RR) for lung cancer for never smoking women exposed to second-hand tobacco smoke from spouses was 1.27 (95%CI: 1.17–1.37). The relative risk for studies in North America was 1.15 (95%CI: 1.03–1.28), in Asia 1.31 (95%CI: 1.16–1.48) and Europe 1.31 (1.24–1.52) (Taylor et al., 2007).
In a meta-analysis of 22 studies that assessed the effect of second-hand tobacco smoke exposures at work, the relative risk for lung cancer among exposed non-smokers was 1.24 (95%CI: 1.18–1.29) and among those workers classified as highly exposed to second-hand tobacco smoke at work 2.01 (95%CI: 1.33–2.60) compared to those with no exposure at work (Stayner et al., 2007).
In a large cohort study conducted in 10 European countries (European Prospective Investigation into Cancer and Nutrition, EPIC), it was estimated that the hazard ratio (HR) for lung cancer risk from second-hand tobacco smoke exposure at home and/or at work for never smokers and ex-smokers (at least 10 years) was 1.34 (0.85−2.13) (Vineis et al., 2007a). The main component of this risk was attributable to exposure at the workplace, resulting in a hazard ratio of 1.65 (1.04–2.63). The overall hazard ratio between childhood exposure and the risk of lung cancer in adulthood was 2.00 (0.94–4.28); among children with daily exposure for many hours each day the hazard ratio was 3.63 (1.19–11.12). In a separate analysis of workplace exposure to second-hand tobacco smoke in this cohort women were observed to have a lung cancer hazard ratio of 2.13 (1.6–3.4) (Veglia et al., 2007).
In a large population-based cohort study conducted in Japan, findings confirmed that exposure to second-hand tobacco smoke is a risk factor for lung cancer among Japanese women (Kurahashi et al., 2008). Compared with women married to never smokers, the hazard ratio for all lung cancer incidence was 1.34 (95%CI:0.81–2.21) and for adenocarcinoma 2.03 (95%CI:1.07–3.86). For adenocarcinoma dose–response relationships were seen for both intensity (P for trend = 0.02) and total amount (P for trend = 0.03) of the husband’s smoking. Exposure to second-hand tobacco smoke at the workplace also increased the risk of lung cancer (HR, 1.32; 95%CI: 0.85–2.04).
Data from a cohort study of women from Shanghai, China also found that exposure to second-hand tobacco smoke is associated with lung cancer mortality. Exposure to second-hand tobacco smoke at work was associated with a significantly increased mortality to lung cancer (HR 1.79, 95%CI: 1.09–2.93) but the risk was not significant for exposure to husband’s second-hand tobacco smoke (HR 1.09, 95%CI: 0.74–1.61) (Wen et al., 2006). In a case–control study of lung cancer among lifetime non-smoking Chinese men living in Hong Kong Special Administrative Region a non-significant association between all lung cancer and ever being exposed to household and/or workplace second-hand tobacco smoke was observed (OR, 1.11, 95%CI: 0.74–1.67) but a significant increase was observed for adenocarcinoma (OR, 1.68, 95%CI: 1.00–2.38) (Tse et al., 2009).
In a long-term case–control study of lung cancer cases at the Massachusetts General Hospital, study participants exposed to second-hand tobacco smoke at work and at leisure were at a significantly greater risk (OR, 1.30, 95%CI: 1.08–1.57) if the exposure occurred between birth and 25 years of age. If the exposures occurred after the age of 25 years the risk was not elevated (OR, 0.66, 95%CI: 0.21–1.57) but the confidence limits are wide for this subgroup analysis (Asomaning et al., 2008).
In two other cohort studies, one conducted in California (Enstrom & Kabat, 2003) and another in New Zealand (Hill et al., 2007) no excess risk was observed among lifelong non-smokers exposed to second-hand tobacco smoke. In the California study the relative risk was 0.99 (95%CI: 0.72–1.37) based on 126 lung cancer cases. [The confidence intervals in this study are relatively wide and they include the current IARC estimate of lung cancer risk from second-hand tobacco smoke exposure. In addition the opportunity for substantial misclassification of second-hand tobacco smoke exposure is great because exposures outside the home were not assessed and the second-hand tobacco smoke exposures were not re-evaluated after enrollment into the study.] Hill et al. (2007) observed no association between second-hand tobacco smoke exposure in a census enumeration of current second-hand tobacco smoke exposure at home and linkage to cancer registries three years later. The authors suggest that this may be a result of either the misclassification of total second-hand tobacco smoke exposure since exposures outside the home were not assessed and/or the fact that a 3-year follow-up after exposure ascertainment may have been too short to capture important exposures before the diagnosis of lung cancer.
One case–control study (Wenzlaff et al., 2005) and one case-only study (Bonner et al., 2006) assessed lung cancer risk associated with second-hand tobacco smoke exposure and several polymorphisms. In the case–control study, individuals were stratified by household second-hand tobacco smoke exposure (yes/no), those with CYP1B1 Leu432Val genotype alone or in combination with Phase II enzyme polymorphisms were more strongly associated with lung cancer risk if they also were exposed to at least some household second-hand tobacco smoke exposure compared to those that had no exposure. In the case-only study a significant interaction was observed between lung cancer risk, second-hand tobacco smoke and a GSTM1 (null) genotype (OR, 2.28, 95%CI:1.15–4.51).
2.2. Cancer of the breast
2.2.1. Overview of studies
The relationship between exposure to second-hand tobacco smoke and breast cancer has been comprehensively reviewed in the peer reviewed literature (Johnson, 2005; Miller et al., 2007) and in reports from national and international committees (IARC, 2004, 2009; California Environmental Protection Agency, 2005; US. Department of Health and Human Services, 2006; Collishaw et al., 2009). These reviews have drawn different conclusions. IARC (2004) characterized the evidence as “inconsistent,” based on studies published or in press by June, 2002. A US Surgeon General Report (2006) concluded that the evidence was “suggestive but not sufficient” to infer a causal relationship between second-hand tobacco smoke and breast cancer, whereas reviews by the California Environmental Protection Agency (CalEPA) in 2005 and by a panel of researchers in this area convened in Canada (Collishaw et al., 2009) designated the evidence for second-hand tobacco smoke as “consistent with a causal association in younger primarily premenopausal women.”
A total of 16 new studies have been published since the previous IARC Monograph (IARC, 2004). These include three cohort studies (Reynolds et al., 2004; Hanaoka et al., 2005; Pirie et al., 2008) (Table 2.4 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.4.pdf), and 13 new case–control studies (Lash & Aschengrau, 2002; Alberg et al., 2004; Gammon et al., 2004; Shrubsole et al., 2004; Bonner et al., 2005; Sillanpää et al., 2005; Lissowska et al., 2006; Mechanic et al., 2006; Roddam et al., 2007; Rollison et al., 2008; Slattery et al., 2008; Ahern et al., 2009; Young et al., 2009) (Table 2.5 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.5.pdf). Table 2.5 also presents two case–control studies not discussed previously (Zhao et al., 1999; Liu et al., 2000). Several meta-analyses have also been published as new data became available (California Environmental Protection Agency, 2005; Johnson, 2005; US. Department of Health and Human Services, 2006; Pirie et al., 2008; IARC, 2009).
The largest of the cohort studies, identified 2518 incident breast cancers among 224917 never smokers followed for an average of 3.5 years in the British Million Women Study (Pirie et al., 2008). The cohort was drawn from women, age 50–64 years, participating in mammography screening programmes. Nearly all cases were post-menopausal and the overall analyses pertain to postmenopausal breast cancer. No relationship was observed between breast cancer risk and smoking by a parent at the time of birth and/or age 10 years (HR, 0.98; 95%CI: 0.88–1.08); the results were also null for smoking by a current partner (HR, 1.02; 95%CI: 0.89–1.16) or exposure to the combination of parental and spousal smoking (HR, 1.03; 95%CI: 0.90–1.19). Pirie et al. (2008) also present a meta-analysis of studies of second-hand smoke and breast cancer risk, separating studies by cohort or case–control design. No overall association was observed in the cohort studies. These largely represent postmenopausal breast cancer, so the analysis was not stratified by menopausal status. An overall association was seen in the case–control studies, similar to the findings of other meta-analyses (California Environmental Protection Agency, 2005; US. Department of Health and Human Services, 2006; IARC, 2009). [Pirie et al. (2008) focus on the discrepancy between the cohort and case–control results and propose that the associations observed in early case–control studies can likely be explained by recall bias. The study has been criticized for the lack of information on occupational exposures to second-hand smoke (Collishaw et al., 2009).]
A second large cohort study (Reynolds et al., 2004) identified 1998 women diagnosed with breast cancer during five years of follow-up of the California Teachers Study. Analyses were based on 433 women with pre/peri-menopausal breast cancer and 1361 women with postmenopausal cancer. No association was observed between post-menopausal breast cancer and residential exposure to second-hand tobacco smoke in childhood or adulthood. No association was initially reported with pre/peri-menopausal breast cancer in analyses based on menopausal status at enrollment (RR 0.93, 95%CI: 0.71–1.22). When menopausal status was defined by age at diagnosis rather than by age at enrollment, the hazard ratio for premenopausal breast cancer among women exposed in both childhood and adulthood increased to 1.27 (95%CI: 0.84–1.92) (Reynolds et al., 2006).
Hanaoka et al. (2005) identified 162 incident breast cancer cases during a nine-year follow-up of 20169 Japanese women, age 40–59 years, who reported no history of active smoking when enrolled in the Japan Public Health Center (JPHC) study in 1990. Nearly three quarters (72%) of the women reported exposure to second-hand tobacco smoke. About half of the women were premenopausal when enrolled in the study, although there were only nine unexposed cases among the pre-menopausal women. The multivariate-adjusted relative risk for breast cancer among all exposed women irrespective of menopausal status was 1.1 (95%CI: 0.8–1.6) compared to those classified as unexposed. The corresponding relative risks for women who were pre- or postmenopausal at baseline were 2.6 (95%CI: 1.3–5.2) and 0.7 (95%CI: 0.4–1.0), respectively.
Six of the 13 new population-based case–control studies included more than 1000 cases each (Shrubsole et al., 2004; Bonner et al., 2005; Lissowska et al., 2006; Mechanic et al., 2006; Slattery et al., 2008; Young et al., 2009) (Table 2.5 on-line). None of these 13 studies showed an overall increase in breast cancer risk associated with second-hand tobacco smoke exposure in Caucasians. The incidence of premenopausal breast cancer was associated with one or more indices of second-hand tobacco smoke exposure in all four studies that stratified the results by menopausal status (Gammon et al., 2004; Shrubsole et al., 2004; Bonner et al., 2005; Slattery et al., 2008) although the association was not always statistically significant (Gammon et al., 2004; Bonner et al., 2005; Fig. 2.1). Associations were also reported between second-hand tobacco smoke exposure and overall breast cancer risk in African Americans (Mechanic et al., 2006) and with premenopausal breast cancer in Hispanics/American Indians (Slattery et al., 2008). The associations observed in these case–control studies are generally weaker than those reported in earlier case–control studies. Whereas the relative risk estimates reported in the earlier studies often equalled or exceeded 2.0 (Sandler et al., 1985a; Lash & Aschengrau, 1999; Zhao et al., 1999; Johnson & Repace, 2000; Liu et al., 2000) or 3.0 (Smith et al., 1984; Morabia et al., 1996; Liu et al., 2000; Morabia et al., 2000), the estimates in the later studies were mostly under 1.5, even in studies that reported positive associations.
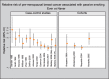
Fig. 2.1
Relative risk of pre-menopausal breast cancer associated with second-hand tobacco smoke. Ever vs Never
2.2.2. Issues affecting the interpretation of studies
One important consideration in evaluating these data has been the lack of a strong and convincing relationship between active smoking and breast cancer. Several theories have been advanced to explain why second-hand tobacco smoke might have a stronger effect on breast cancer than active smoking (California Environmental Protection Agency, 2005; Johnson, 2005; Collishaw et al., 2009). Central to these is the hypothesis that active smoking may have counterbalancing protective and detrimental effects on breast cancer risk that, in combination, produce little or no overall association, whereas second-hand tobacco smoke may have only an adverse effect on risk. The weakness of this theory is that there is little direct evidence (see Section 4) identifying the mechanism by which active smoking may cause the proposed [protective] antiestrogenic effects. Without knowing the mechanism, it has been impossible to prove that active smoking has this effect but exposure to second-hand tobacco smoke does not. A second hypothesis that has been advanced is that second-hand tobacco smoke may have a greater effect on pre- than on postmenopausal breast cancer. This theory was proposed by CalEPA in 2005 (Johnson & Glantz, 2008) based on analyses of studies available at the time, and was subsequently questioned by some (US. Department of Health and Human Services, 2006) but not all (Collishaw et al., 2009) subsequent reviews. [Because this arose as an a posteriori observation rather than as an a priori hypothesis, it must be confirmed by independent studies.] The strongest support for the hypothesis comes from a cohort study in Japan (Hanaoka et al., 2005), which reported significantly increased risk (RR 2.6, 95%CI: 1.3–5.2) of premenopausal breast cancer in women who previously reported having ever lived with a regular smoker or ever being exposed to second-hand tobacco smoke for at least one hour per day in settings outside the home. However, the referent group in this analysis included only nine unexposed cases. No associations were observed with post-menopausal breast cancer. A weak association between second-hand tobacco smoke exposure and premenopausal breast cancer was reported in the California Teachers cohort, when menopausal status was defined by age at diagnosis rather than age at entry into the study (Reynolds et al., 2006). In case–control studies published since the CalEPA review (California Environmental Protection Agency, 2005) that reported results stratified by menopausal status, Bonner et al. (2005) and Slattery et al. (2008) reported stronger associations with pre- than with post-menopausal breast cancer, although the only statistically significant association with premenopausal breast cancer was in Hispanic or American Indian women who had second-hand tobacco smoke exposure of more than ten hours per week (OR, 2.3, 95%CI:1.2–4.5) (Slattery et al., 2008). In a case–control study of breast cancer in women age 36–45 years Roddam et al. (2007) observed no increased risk in premenopausal women who, since age 16, were married to or lived with a boyfriend who smoked for at least one year.
Two other explanations for inconsistencies in the evidence relate to the fundamental design differences between cohort and case–control studies. A critical advantage of cohort studies is that they collect information on exposures before the disease of interest is diagnosed, thus preventing knowledge of disease status influencing how participants recall and/or report their exposures. Recall bias is especially challenging in case–control studies of exposures that are difficult to measure, when recollection of the frequency and intensity of exposure is necessarily subjective. In counterpart, an important advantage of case–control studies is that they can collect more detailed information on the exposure of interest than is usually possible in cohort studies. Together, these factors create what has been described as “a tension” between the potential for recall or selection bias in case–control studies, and the reduced possibility of collecting full “lifetime exposure histories” in cohort studies (Collishaw et al., 2009). The discrepancy in the results from case–control and cohort studies is seen especially in the earlier case–control studies, which found much stronger associations than those observed in most recent studies. Five studies in particular (Smith et al., 1984; Morabia et al., 1996; Zhao et al., 1999; Johnson & Repace, 2000; Kropp & Chang-Claude, 2002) were considered by Collishaw et al. (2009) as having the most complete information on lifetime exposure to second-hand tobacco smoke from all sources. At the same time, these studies are among the most susceptible to recall bias for two reasons. The first is a general problem of case–control studies, in that cases are more likely to remember and report potentially hazardous exposures than controls. Second, recall bias is potentially more problematic when subjective considerations can influence reporting. It is easier to report smoking by a parent or spouse than it is to remember exposures from other sources that possibly occurred many years ago in daily life. Exposure to second-hand tobacco smoke was highly prevalent in the decades following World War II in Europe and North America. It would be unusual for someone not to be exposed. The studies that the California Environmental Protection Agency (2005) considered to have the best information on exposure to second-hand tobacco smoke are also those which rely more heavily on recall of past exposures outside the home. Moreover, inclusion in the referent group in these studies is also vulnerable to recall bias. Previous reviews by IARC (2004) and the US Surgeon General (US. Department of Health and Human Services, 2006) have expressed concern about potential biases that may be introduced by relying on a small and unusual subgroup (the unexposed to active smoking and second-hand tobacco smoke) as the referent category in these studies. Recall bias remains a plausible explanation for why the association with second-hand tobacco smoke is stronger in studies that collect “lifetime exposure histories” than in those that rely on parental or spousal smoking. In addition, publication bias cannot be ruled out because the reporting of association limited by subgroup (pre-menopausal) could have been selective.
[The Working Group noted that adjustment for potential confounders using the questionnaire data on other established risk factors for breast cancer did not eliminate the association with second-hand tobacco smoke in these studies. However, this does not resolve concerns about the possibility of recall or publication bias.]
Several meta-analyses have been published, largely showing similar results but leading to substantially different interpretations of the evidence (California Environmental Protection Agency, 2005; US Department of Health and Human Services, 2006; Johnson, 2007; IARC, 2009). The California Environmental Protection Agency (2005) calculated a pooled estimate for second-hand tobacco smoke and breast cancer risk of 1.11 (95%CI: 1.04–1.19) in all women and 1.38 (95%CI: 1.21–1.56) in premenopausal women, based on 19 studies and a fixed effects model. These estimates increased to 1.89 (95%CI: 1.57–2.27) for all women and 2.18 (95%CI: 1.70–2.79) in premenopausal women when the analysis was restricted to the subset of studies considered to have the best exposure data.
Based on these analyses, the California Environmental Protection Agency (2005) and Collishaw et al. (2009) emphasized the positive association with premenopausal breast cancer in their conclusion that the evidence is “consistent with a causal relationship” whereas the US Surgeon General (US Department of Health and Human Services, 2006) was more cautious in characterizing the evidence as “suggestive but not sufficient.”
[The Working Group noted that the criterion used by IARC specifies “sufficient evidence of carcinogenicity in which chance, bias and confounding could be ruled out with reasonable confidence.” This is a more stringent definition than “consistent with a causal relationship.”]
2.3. Cancers of the upper aerodigestive tract
2.3.1. Upper areodigestive tract combined
Cancers of the upper aerodigestive tract traditionally comprise cancers of the oral cavity, pharynx, larynx and oesophagus. However, some epidemiological studies have examined only head and neck cancers restricted to tumours of the oral cavity, pharynx and larynx. Four case–control studies (Tan et al., 1997; Zhang et al., 2000; Lee et al., 2008; Ramroth et al., 2008) assessed the effects of second-hand tobacco smoke on head and neck cancers combined and separately for oral cavity, oropharynx or larynx cancers (Table 2.6 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.6.pdf).
In a hospital-based case–control study in the USA, including only non smoking cases and controls, Tan et al. (1997) detected high risk of head and neck cancer among those ever exposed to second-hand tobacco smoke at home or at work. Women presented higher risk at home (OR, 7.3; P < 0.001) than men (OR, 1.1; P < 0.79). On the other hand, men showed higher risk at work (OR, 11.6; P < 0.001) than women (OR, 8.9; P < 0.002). [The authors did not provide the percentages of the telephone interviews done with the spouses of cases and controls. Probably, this is the main weakness of this study and differential misclassification of exposure to second-hand tobacco smoke could not be excluded. The analysis was performed without adjustment for potential confounding variables.] In a study in the USA, Zhang et al. (2000) observed an increased risk (OR, 2.4; 95%CI: 0.9–6.8) with lifetime second-hand tobacco smoke exposure (ever/never) for head and neck cancers, adjusted for age, sex, ethnicity, education, alcohol drinking, pack-years of cigarette smoking, and marijuana consumption.
Lee et al. (2008) pooled the data from several studies including cases of head and neck cancers and controls (population and hospital) from central Europe, Latin America and United States. Two groups were examined separately, never tobacco users and never tobacco and alcohol users. Among never tobacco users, no association was observed between ever exposure to second-hand tobacco smoke at home or at work and the risk for head and neck cancers. Among never tobacco and alcohol users, a non-statistically significant risk (or 1.30; 95%ci: 0.94–1.81) was observed. When considering specific anatomical sites, only laryngeal cancer risk was increased when ever exposed to second-hand tobacco smoke in a lifetime, detected among never tobacco users (OR, 1.71; 95%CI: 0.98–3.00) and among never tobacco and alcohol users (OR, 2.90; 95%CI: 1.09–7.73).
In Germany, in a population-based case–control study on laryngeal cancer, Ramroth et al. (2008) observed a non-statistically significant risk (OR, 2.0; 95%CI: 0.39–10.7) for exposure to second-hand tobacco smoke (ever/never) at home and in workplaces among nonsmokers.
(a) Evidence of a dose–response
Zhang et al. (2000) observed a dose–response relationship with the intensity of exposure to second-hand tobacco smoke (never, moderate and heavy) on head and neck cancers (P = 0.025); those at heavy level of exposure at home or at work showed highest risk for head and neck cancer (OR, 3.6; 95%CI: 1.1–11.5). However, the classification of exposure to second-hand tobacco smoke at work as never, occasionally or regularly did not show any dose–response effect; and the risk for the groups of occasionally or regularly exposed at home were similar and non statistically significant.
Lee et al. (2008) explored the intensity and duration of sexposure to second-hand tobacco smoke. For intensity the number of hours of exposure per day was considered at home (0–3 hours, > 3 hours) or at the workplace (never, 1–3 hours and > 3 hours). Among both groups of never tobacco users and never tobacco and alcohol users non-statistically significant risks of head and neck cancers were observed for those exposed for > 3 hours per day at home or at work. For duration the number of years of exposure at home and at work was considered (never, 1–15 years, and > 15 years). Among never tobacco users, there was a trend of increase in risk for head and neck cancers with greater number of years of exposure at home, but not at work. Among never tobacco and alcohol users, the duration of exposure showed a trend for exposure both at work or at home.
Considering specific anatomical sites, for cancer of the oral cavity no dose–response effect was observed with increasing number of years of exposure to second-hand tobacco smoke at home or at work. For cancer of the pharynx, a dose–response effect was observed with increasing number of years of exposure to second-hand tobacco smoke with only at home, in both never tobacco users and never tobacco and alcohol users. For cancer of the larynx, a dose–response effect was noted with increasing number of years of exposure to second-hand tobacco smoke at home among never tobacco users and at work among never tobacco and alcohol users. Among never tobacco and alcohol users, all the odd ratios (OR) were statistically significantly elevated for > 15 years of exposure at home or at work for head and neck cancers overall and separately for cancer of the pharynx, and only at work for cancer of the larynx.
2.3.2. Cancers of the nasopharynx, and nasal cavity and accessory sinuses
The relationship between exposure to second-hand tobacco smoke and risk for these rare cancers of the upper respiratory tract has been examined in one cohort study (Hirayama, 1984; Table 2.7 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.7.pdf) and five case–controls studies (Fukuda & Shibata, 1990; Yu et al., 1990; Zheng et al., 1993; Cheng et al., 1999; Yuan et al., 2000; Table 2.6 on-line). A positive association was found in most of these studies.
Hirayama (1984) found an increased risk of sinonasal cancer in women (histology not noted) associated with increasing numbers of cigarettes smoked by husbands of nonsmoking women. When compared with nonsmoking women married to nonsmokers, wives whose husbands smoked had a relative risk of 1.7 (95%CI: 0.7–4.2) for 1–14 cigarettes per day, 2.0 (95%CI: 0.6–6.3) for 15–19 cigarettes per day and 2.55 (95%CI: 1.0–6.3) for ≥ 20 cigarettes per day (P for trend = 0.03).
Fukuda & Shibata (1990) reported the results of a Japanese case–control study based on 169 cases of squamous-cell carcinoma of the maxillary sinus and 338 controls matched on sex, age and residence in Hokkaido, Japan. Among nonsmoking women, a relative risk of 5.4 (P < 0.05) was associated with exposure in the household to second-hand tobacco smoke from one or more smokers. Active smoking was associated with an increased risk for squamous-cell carcinoma of the maxillary sinus in men in the same study.
Zheng et al. (1993) used data from the 1986 US National Mortality Followback Survey to assess risk for cancer of the nasal cavity and sinuses in relation to exposure to second-hand tobacco smoke in white men. A total of 147 deaths from cancer of the nasal cavity and sinuses was compared to 449 controls who had died from other causes (excluding any causes strongly linked to alcohol and/or tobacco use). Data were obtained from postal questionnaires completed by next-of-kins. Among nonsmokers, patients with nasal cancer were more likely to have a spouse who smoked cigarettes (RR, 3.0; 95%CI: 1.0–8.9) after adjustment for age and alcohol use. When the analysis of cases was restricted to those with cancer of the maxillary sinus, the risk was somewhat higher (RR 4.8; 95%CI: 0.9–24.7). The risks reported for active smoking and exposure to second-hand tobacco smoke were of similar magnitude in this study.
Neither second-hand tobacco smoke exposure during childhood nor exposure during adulthood were positively associated with an increased risk for nasopharyngeal cancer in a study in Taiwan, China (Cheng et al., 1999). Although histological type was not specified, all cases were histologically confirmed. Among never-smokers, the risk estimates for cumulative exposure to second-hand tobacco smoke (pack-person-years) in childhood declined as exposure increased (P for trend = 0.05); a similar but non-significant inverse relationship was found for exposure during adulthood. Significant elevations in risk for nasopharyngeal cancer were observed for active smokers in this study. [The Working Group noted that the exposure assessment was relatively detailed and that the estimates of relative risk were adjusted for age, sex, education and family history of nasopharyngeal cancer.]
A large population-based case–control study conducted in Shanghai, China, included 935 cases of nasopharyngeal carcinoma and 1032 population controls randomly selected from a population-registry and frequency-matched by sex and 5-year age group (Yuan et al., 2000). All cases were histologically confirmed, but the cell type was not specified. The study subjects were interviewed face to face, and the response rates were 84% for cases and 99% for controls. In female never-smokers, a consistent increase in risk related to exposure to second-hand tobacco smoke during childhood was noted. The relative risk was 3.4 (95%CI: 1.4–8.1) if the mother smoked; 3.0 (95%CI: 1.4–6.2) if the father smoked; 2.7 (95%CI: 1.1–6.9) if another household member smoked and 3.0 (95%CI: 1.4–6.2) if any household member smoked. Risks associated with exposure to second-hand tobacco smoke during adulthood in women were also statistically significantly increased. For male never-smokers, the associations were weaker and were not statistically significantly elevated for exposure during childhood and adulthood. [The Working Group noted that this was a large, well conducted study that included a detailed exposure assessment and adjustment for numerous potential confounders.]
2.4. Leukaemia and lymphomas
Kasim et al. (2005) analysed the risk of leukaemia in adults after exposure to second-hand tobacco smoke (Table 2.8 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.8.pdf). This case–control study was based on postal questionnaires. There was a slightly increased risk (P for trend = 0.001) with increasing duration of exposure to second-hand tobacco smoke. The association was limited to chronic lymphocytic leukaemia and was stronger for occupational exposures to second-hand tobacco smoke.
2.5. Other cancers in adults
2.5.1. All cancer combined
Hirayama (1984), Sandler et al. (1985b), and Miller (1990) observed a significant association between exposure to second-hand tobacco smoke and overall cancer incidence or mortality. Nishino et al. (2001) also studied all cancers combined and reported a relative risk of 1.1 (95%CI: 0.92–1.4) associated with husband’s smoking.
2.5.2. Cancers of the gastrointestinal tract
In addition to the studies reviewed previously (Sandler et al. 1988; Gerhardsson de Verdier et al., 1992; Mao et.al., 2002), ten new studies have been identified: two cohort (Nishino et al., 2001; Hooker et al., 2008; Table 2.13 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.13.pdf); seven case–control (Sandler et al., 1985a, b; Slattery et al., 2003; Lilla et al., 2006; Wang et al., 2006; Duan et al., 2009; Verla-Tebit et al., 2009; Table 2.14 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.14.pdf) and one case-only study (Peppone et al., 2008; Table 2.15 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.15.pdf). Two studies (Sandler et al., 1985a; Wang et al., 2006) did not provide risk estimates of gastrointestinal cancers for never smokers and are not discussed further. [No data for these studies are included in the tables.]
Sandler et al. (1985b) observed a relative risk of 0.7 and 1.3 for cancer of the digestive system from exposure to maternal and paternal passive smoke, respectively. [No CIs were provided and the numbers of never smokers exposed were small.] Verla-Tebit et al. (2009) found no evidence of an increased risk for colorectal cancer associated with exposure to second-hand tobacco smoke overall.
(a) Cancer of the colorectum
Nishino et al. (2001) observed no association with husband’s smoking for cancer of the colon (RR 1.3; CI: 0.65–2.4) or of the rectum (RR 1.8; 0.85–3.9).
Four studies investigated risk for cancer or the colon and/or rectum by sex. Sandler et al. (1988) reported an increased risk for colorectal cancer in men (RR 3.0; 95%CI: 1.8–5.0) but a protective effect in women (RR 0.7; 95%CI: 0.6–1.0). Slattery et al. (2003) noted that rectal cancer was significantly associated with exposure to second-hand tobacco smoke in men (OR, 1.5; 95%CI: 1.1–2.2 for never smokers) but not in women. Hooker et al. (2008) reported an effect among men only, with a significantly increased risk for rectal cancer in the 1963 cohort (RR 5.8, 95%CI: 1.8–18.4) but not the 1975 cohort. Gerhardsson de Verdier et al. (1992) found an increased risk for rectal cancer in men (RR 1.9; 95%CI: 1.0–3) and for colon cancer in women (RR 1.8; 95%CI: 1.2–2.8). [The Working Group noted that it is unclear whether the analysis was restricted to never-smokers.]
When analysing different sources of exposure to second-hand tobacco smoke, Verla-Tebit et al. (2009) found no evidence of an increased risk for cancer of the colorectum associated with exposure to second-hand tobacco smoke specifically during childhood or at work, but observed a significant increase in risk associated with spousal exposure.
Peppone et al. (2008) noted that considerable exposure to second-hand tobacco smoke, especially during childhood, was more likely to lead to an earlier-age diagnosis of cancer of the colorectum.
In exploring the association of cancer of the colorectum with exposure to second-hand tobacco smoke and NAT1 and NAT2 status, Lilla et al. (2006) noted that risk may only be relevant among genetically susceptible (NAT1 and NAT2 status) individuals.
(b) Cancer of the stomach
Nishino et al. (2001) observed no association with husband’s smoking for cancer of the stomach (RR, 0.95; 95%CI: 0.58–1.6).
The two studies on the association of exposure to second-hand tobacco smoke with stomach cancer by subsite (cardia versus distal) gave contradictory results. In one study (Mao et al., 2002) a positive trend (P = 0.03) in risk for cancer of the gastric cardia was associated with lifetime exposure to second-hand tobacco smoke (residential plus occupational) in never smoking men, with a relative risk of 5.8 (95%CI: 1.2–27.5) at the highest level of exposure (≥ 43 years); no increased risks or trends were observed for distal gastric cancer. In the other study, Duan et al. (2009) an increased risk for distal gastric cancer was found, but not for gastric cardia [Data were not analysed by sex due to small sample size].
2.5.3. Cancer of the pancreas
Six studies have been identified on the association of exposure to second-hand tobacco smoke with cancer of the pancreas: three cohort (Nishino et al., 2001; Gallicchio et al., 2006; Bao et al., 2009; the latter two are summarized in Table 2.17 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.17.pdf) and three case–control (Villeneuve et al., 2004; Hassan et al., 2007; Lo et al., 2007; the former two studies are summarized in Table 2.18 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.18.pdf).
(a) Exposure in adulthood
Data from the majority of the studies (Nishino et al., 2001; Villeneuve et al., 2004; Gallicchio et al., 2006; Hassan et al., 2007; Bao et al., 2009) suggested lack of an association of cancer of the pancreas with never smokers exposed to second-hand tobacco smoke in adulthood at home or at work. (RR 1.2 (95%CI: 0.45–3.1) and 1.21 (95%CI: 0.60–2.44) respectively).
Lo et al. (2007) reported an odd ratio of 6.0 (95%CI: 2.4 −14.8) for never smokers (both sexes combined) exposed to second-hand tobacco smoke in Egypt. [The Working Group noted the small numbers of cases, the use of hospital controls and the small proportion of the cases (35%) with histopathological confirmation. Data are not included in Table 2.18].
(b) Exposure during childhood
In the Nurses’ Health Study, Bao et al. (2009) noted an increased risk for cancer of the pancreas (RR 1.42; 95%CI: 1.07–1.89) for maternal but not for paternal smoking (RR 0.97; 95%CI: 0.77–1.21) during childhood.
2.5.4. Cancer of the kidney (renal cell carcinoma)
Two case–control studies have been published on the association of exposure to second-hand tobacco smoke with cancer of the kidney (specifically renal cell carcinoma) since IARC (2004) (Hu et al., 2005; Theis et al., 2008; Table 2.19 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.19.pdf). In both studies a significantly increased risk associated with exposure to second-hand tobacco smoke among never smokers was reported.
2.5.5. Cancer of the urinary bladder
A total of seven studies and one meta-analysis have considered the association between exposure to second-hand tobacco smoke and cancer of the urinary bladder: three cohort studies (Zeegers et al., 2002; Bjerregaard et al., 2006; Alberg et al., 2007; Table 2.9, available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.9.pdf), four case–control studies (Burch et al., 1989; Chen et al., 2005a; Samanic et al., 2006; Jiang et al., 2007; Table 2.10 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.10.pdf), and one meta-analysis (Van Hemelrijck et al., 2009).
(a) Population-based exposure-response relationship
Burch et al. (1989) and Zeegers et al. (2002) reported no increased risk for cancer of the urinary bladder [Data are not included in the Tables]. Van Hemelrijck et al. (2009) reported a meta-relative risk of 0.99 (95%CI: 0.86–1.14) for never smokers exposed to second-hand tobacco smoke. [Data not included in Table. The Working Group noted the marked variation in risk in the analyses by sex and by timing of exposure to second-hand tobacco smoke during adulthood or childhood].
In the European Prospective Investigation into Cancer and Nutrition (EPIC) study, Bjerregaard et al. (2006) compared ever versus never exposed to second-hand tobacco smoke as an adult or a child: the risk for cancer of the urinary bladder increased for exposures during childhood (OR, 1.38; 95%CI: 1.00–1.90), and was stronger for never-smokers (OR, 2.02; 95%CI: 0.94–4.35).
Alberg et al. (2007) analysed data from two cohorts of non-smoking women in the USA exposed to second-hand smoke at home. An association with exposure to second-hand tobacco smoke was found in the 1963 cohort (RR, 2.3; 95%CI: 1.0–5.4) but not in the 1975 cohort (RR, 0.9; 95%CI: 0.4–2.3). [The Working Group noted the small number of cases available for some of the risk estimates.]
In a study assessing occupational exposure to second-hand tobacco smoke (Samanic et al., 2006), the risk for cancer of the urinary bladder was increased in the highest exposure category among women (RR, 3.3; 95%CI: 1.1–9.5) but not among men (RR, 0.6; 95%CI: 0.2–1.4).
(b) Molecular-based exposure-response relationship
4-aminobiphenyl (4-ABP) can form DNA adducts and induce mutations, and cigarette smoke is the most prominent source of exposure to 4-aminobiphenyl in humans (see Section 4). Jiang et al. (2007) used variation in 4-ABP-haemoglobin adducts levels to assess exposure to second-hand tobacco smoke and reported a significantly increased risk with increasing lifetime exposure among never-smoking women exposed in adulthood or childhood.
Chen et al. (2005a) hypothesized that the ability to detoxify arsenic (a risk factor urinary bladder cancer) through methylation may modify risk related to second-hand tobacco smoke exposure. Results of the adjusted analyses show that a high primary methylation index associates with lower risk of cancer of the urinary bladder (OR, 0.37; 95%CI: 0.14–0.96, p interaction = 0.11) in second-hand tobacco smoke exposed subjects compared to unexposed. In endemic area the ability to methylate arsenic may play a role in reducing the risk of cancer of the urinary bladder associated with second-hand tobacco smoke exposure. [The Working Group noted that the small number of cases and the use of hospital controls limit the validity of inferences from this study].
Using case–control data for never and former smokers nested within the EPIC study Vineis et al. (2007b) examined susceptibility in genes involved in oxidative stress (such as NQO1, MPO, COMT, MnSOD), in phase I (such as CYP1A1 and CYP1B1) and phase II (such as GSTM1, and GSTT1) metabolizing genes, and in methylenetetrahydrofolate (MTHFR). GSTM1 deletion was strongly associated with risk for urinary bladder cancer in never smokers (OR, 1.75; 95%CI: 0.89–3.43), and a similar association was noted for former smokers and for men.
2.5.6. Cancer of the cervix
The cohort studies evaluated previously (Hirayama, 1984; Jee et al., 1999; Nishino et al., 2001) consistently indicated the lack of association between exposure to second-hand tobacco smoke and cancer of the uterine cervix, while the informative case–control studies (Sandler et al., 1985b; Slattery et al., 1989; Scholes et al., 1999) suggested a non-statistically significant increase in risk.
A total of 10 new studies have been identified: one cohort study (Table 2.11 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.11.pdf) and nine case–control studies (Buckley et al., 1981; Brown et al., 1982; Hellberg et al., 1986; Hirose et al., 1996; Coker et al., 2002; Wu et al., 2003; Tay & Tay, 2004; Sobti et al., 2006; Tsai et al., 2007; Table 2.12 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.12.pdf). Three early case–control studies (Buckley et al., 1981; Brown et al., 1982; Hellberg et al., 1986) did not look at risk of exposure to second-hand tobacco smoke in never smoking women, and are not further discussed.
(a) Squamous cell carcinoma of the cervix
A significant increase risk for invasive cancer of the uterine cervix associated with exposure to second-hand tobacco smoke during adulthood was found in three case–control studies (Hirose et al., 1996; Wu et al., 2003; Tay & Tay, 2004) and one cohort study (Trimble et al., 2005).
(b) Cervical intraepithelial lesions and neoplasia
An earlier case–control study (Coker et al., 1992) found no statistically significant association between exposure to second-hand tobacco smoke and CIN II/III in non-smokers, after adjustment for age, race, education, number of partners, contraceptive use, history of sexually transmitted disease and history of Pap smear. A later study (Coker et al., 2002) looked at risk of low grade and high grade cervical squamous intraepithelial lesions (LSIL and HSIL, respectively) in HPV positive never-smokers and reported a significant association with exposure to second-hand tobacco smoke. In a community-based case–control study, Tsai et al. (2007) observed a markedly increased risk for both CIN1 and CIN2 in both HPV-positive and HPV-negative women exposed to second-hand tobacco smoke. Only Coker et al. (2002) and Tsai et al. (2007) controlled for HPV status in women.
Sobti et al. (2006) reported that cervical cancer risk is increased in individuals exposed to second-hand tobacco smoke with GSTM1 (null), GSTT1 (null) and GSTP1 (Ile105Val) genotypes, with odd ratios ranging from 6.4 to 10.2.
2.5.7. Cancer of the ovary
One cohort study (Nishino et al., 2001) and two case–control studies (Goodman & Tung, 2003; Baker et al., 2006; Table 2.16 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.16.pdf) have been published on the association of exposure to second-hand tobacco smoke with cancer of the ovary. In all three studies a null or inverse association of cancer of the ovary for never smokers exposed to second-hand tobacco smoke was found. Nishino et al. (2001) observed no association with husband’s smoking (RR 1.7; 95%CI: 0.6- 5.2). Goodman & Tung (2003) reported no association of exposure to second-hand tobacco smoke during childhood with risk of cancer of the ovary. Baker et al. (2006) reported a decreased risk of cancer of the ovary for never smokers exposed to second-hand tobacco smoke (OR, 0.68; 95%CI: 0.46–0.99), with similar findings for former and current smokers.
2.5.8. Tumours of the brain and CNS
A total of three case–control studies (Ryan et al., 1992; Hurley et al., 1996; Phillips et al., 2005) have considered the association of second-hand tobacco smoke and cancers of the brain and central nervous system. Ryan et al. (1992) reported an increased risk of meningioma associated with spousal exposure, particularly among women (RR 2.7; 95%CI: 1.2–6.1). In a case–control study of gliomas in Australia no association was found for exposure to second-hand tobacco smoke in never smokers (RR 0.97, 95%CI: 0.61–1.53) (both sexes combined) (Hurley et al., 1996). However Phillips et al. (2005) found that spousal smoking was associated with an increased risk for intracranial meningioma in both sexes combined (OR, 2.0; 95%CI: 1.1–3.5), the risk increased with increasing duration of exposure (P for trend = 0.02).
2.5.9. Other cancers
One case–control study on hepatocellular cancer (Hassan et al., 2008) and one on cancer of the testis (McGlynn et al., 2006) were published since IARC (2004). Hassan et al. (2008) did not find an association with exposure to second-hand tobacco smoke and hepatocellular cancer, while that of McGlynn et al. (2006) did not support the hypothesis that maternal smoking is related to the development of cancer of the testis (Table 2.20 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.20.pdf). However, these studies provide limited information on the association of exposure to second-hand tobacco smoke with the risk of these cancers.
2.6. Parental tobacco smoking and childhood cancers
2.6.1. Overview
A large number of studies have evaluated the association of cancer risk in childhood with exposure to parental smoking. However, childhood cancers are extremely heterogeneous, both between major cancer sites and within subtypes. In addition, given the rarity of childhood cancers, studies of specific cancer sites and subtypes that have adequate sample sizes and detailed exposure assessments are difficult to achieve.
(a) Smoking exposure assessment
Parental smoking before and during pregnancy exposes germ cells (spermatozoa and ova) and/or the fetus to the same chemical mixture and levels of tobacco smoke as during active smoking, while post-natal exposure to parental tobacco smoking exposes the offspring to second-hand tobacco smoke. Some studies distinguish whether exposure to parental smoking was preconceptional, in utero or postnatal. Even when a study reports only on one time period, exposure may have occurred at all three periods. Exposures to tobacco smoking during each of these periods tend to correlate, in particular, paternal smoking is less likely to change during and after pregnancy. In addition, paternal and maternal smoking habits are highly correlated (Boffetta et al., 2000).
Most studies assessed the number of cigarettes smoked per day (e.g. 0–10, 11–20, 20+) and, when data were available, some assessed continuous consumption of cigarettes per day. One study reported exposure in pack-years (Lee et al., 2009). The SEARC international case–control study assessed polycyclic aromatic hydrocarbons (PAHs) as the main exposure of interest and obtained information on both tobacco smoke and occupational exposures (Cordier et al., 2004).
(b) Bias and confounding
Whitehead et al. (2009) evaluated the adequacy of self-reported smoking histories on 469 homes of leukaemia cases and controls and found that nicotine concentrations derived from interview responses to a structured questionnaire strongly correlated to measured levels in dust samples.
The major confounders for the relationship between parental smoking and childhood cancers were markers of socioeconomic status, race or ethnicity, birth weight or gestational age, parental age, sex and age of the case child. In most studies matching or adjusting for these confounders was performed as appropriate. In some studies matching was performed for birth order and centre of diagnosis.
2.6.2. All childhood cancers combined
In addition to the four cohort and 10 case–control studies reviewed by IARC (2004), three case–control studies have examined the role of second-hand tobacco smoke in relation to risk for all childhood cancers combined (Sorahan et al., 2001; Pang et al., 2003; Sorahan & Lancashire, 2004; Table 2.21 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.21.pdf).
(a) Intensity and timing of parental smoking
In a follow-up of the Inter-Regional Epidemiological Study of Childhood Cancer (IRESCC) by McKinney et al. (1987), a statistically significant positive trend with daily paternal smoking before pregnancy was observed when cases were compared with controls selected from General Practitioners’ (GPs’) lists, but not from hospitals; an inverse trend was noted for maternal smoking before pregnancy when cases were compared with hospital, but not with General Practitioners, controls (Sorahan et al., 2001).
In the United Kingdom Childhood Cancer Study (UKCCS), Pang et al. (2003) observed a similar pattern of increasing risk with increasing intensity of paternal preconception smoking, and of decreasing risk for increasing maternal smoking before and during pregnancy for all diagnoses combined, and for most individual diagnostic groups.
In the most recent report from the Oxford Survey of Childhood Cancers (OSCC), the risk of death from all childhood cancers combined was not associated with maternal smoking, but was consistently associated with paternal smoking alone or in combination with maternal smoking, in both adjusted and unadjusted analyses [Ex-smokers of more than 2 years before birth of the survey child were assimilated to non-smokers] (Sorahan & Lancashire, 2004).
(b) Bias and confounding
The significant trends observed by Sorahan et al. (2001) and Pang & Birch (2003) did not diminish when adjusted for potential confounding covariates or with simultaneous analysis of parental smoking habits. The relationship between maternal smoking and birth weight reported by Sorahan et al. (2001) suggested that self-reported maternal smoking was equally reliable for cases and for controls. However, comparisons of smoking patterns with national data suggested that control parents in this study were heavier smokers.
2.6.3. Leukaemias and lymphomas
Since IARC (2004), one cohort study (Mucci et al., 2004) (Table 2.22 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.22.pdf), eleven case–control studies (Table 2.23 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.23.pdf), and one meta-analysis (Lee et al., 2009) (Table 2.24 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.24.pdf) have evaluated the association of parental tobacco smoking with the risk for lymphatic and haematopoietic cancers.
(a) Duration and intensity of exposure
From a meta-analysis of 30 studies published before 1999 Boffetta et al. (2000) reported no statistically significant association for all lymphatic and haematopoietic neoplasms and noted evidence of publication bias for the available data.
Lee et al. (2009) performed a meta-analysis of twelve studies on paternal smoking and risk of childhood leukaemia. Paternal smoking before conception of the index child was significantly associated with the risk for acute leukaemia (AL) and acute lymphoblastic leukaemia (ALL) (Fig. 2.2).

Fig. 2.2
Meta-analysis of the association between paternal smoking and childhood leukaemia
In a cohort study, maternal smoking was associated with a lower risk of acute lymphoblastic leukaemia, a higher risk of acute myeloid leukaemia (AML) particularly among heavy smokers, and a slight excess risk for non-Hodgkin lymphoma (NHL) (Mucci et al., 2004).
Because of the diversity of types of exposure (paternal, maternal, parental), of timing of exposure (preconception, in utero, post-natally) and of the outcome, the case–control studies are briefly summarized individually.
Schüz et al. (1999) showed that the risk for acute childhood leukaemias was inversely related to maternal smoking during pregnancy. Paternal smoking before pregnancy showed no association with leukaemia risk for any smoking category. Sorahan et al. (2001) reported a non-significant positive association between risk for acute lymphoblastic leukaemia and daily cigarette smoking by fathers before pregnancy, and a non-significant inverse association between risk for acute lymphoblastic leukaemia and daily smoking by mothers before pregnancy. Down Syndrome children are highly susceptible to the development of acute leukaemia. In a case–control study of 27 children with acute leukaemia and Down Syndrome compared with 58 Down Syndrome children without acute leukaemia Mejía-Aranguré et al. (2003) found that paternal smoking of more than 10 cigarettes/day, both preconception and after birth of the index child was associated with acute leukaemia. In the UKCC case–control study (Pang et al., 2003), paternal but not maternal preconception tobacco smoking of 1–19 cigarettes/day was associated with an increased risk of leukaemia, and a similar pattern was reported for lymphoma. Menegaux et al. (2005) reported no increased risk of acute lymphoblastic leukaemia or acute nonlymphocytic leukaemia (ANLL) associated with any category of post-natal exposure to tobacco smoking (i.e. maternal smoking during breastfeeding or after, paternal smoking after birth, other smokers at home), except for an increased risk of acute nonlymphocytic leukaemia with paternal smoking. In a later study, (Menegaux et al., 2007) reported no association between acute and parental smoking, by subtype (acute myeloid leukaemia or acute lymphoblastic leukaemia) or by time of exposure, with the exception of an increased risk of acute lymphoblastic leukaemia associated with maternal smoking during pregnancy. Chang et al. (2006) reported no risk for acute leukaemia, acute lymphoblastic leukaemia or acute myeloid leukaemia associated with maternal smoking either by period of smoking (preconception, during pregnancy, post-natally) or by amount smoked. Paternal preconception smoking was strongly associated with risk for acute myeloid leukaemia both by period and intensity of smoking. When both paternal preconception smoking and maternal postnatal smoking were considered, the risk for acute lymphoblastic leukaemia was stronger. Rudant et al. (2008) reported a significant positive association between paternal smoking and acute lymphoblastic leukaemia, acute myeloid leukaemia, Burkitt lymphoma, and anaplastic large cell non-Hodgkin lymphoma, with increasing relative risks (RR) with increasing number of cigarettes smoked. No associations with Hodgkin lymphoma or other types of non-Hodgkin lymphoma were observed. Non-significantly elevated risks were observed for maternal smoking during pregnancy for acute lymphoblastic leukaemia and non-Hodgkin lymphoma, but not in the highest category of 10 or more cigarettes/day. MacArthur et al. (2008) reported non-significantly elevated risk estimates for acute lymphoblastic leukaemia and acute myeloid leukaemia with maternal smoking, but not with paternal smoking, before and during pregnancy. Lee et al. (2009) in Seoul, Republic of Korea, reported that paternal smoking was associated with a significantly increased risk of acute leukaemia and acute lymphoblastic leukaemia in a dose–response manner. The proportion of mothers who smoked was too low (6.1% in controls) to analyse risk in association with maternal smoking.
(b) Potential confounders
In the study of Down Syndrome children (Mejía-Aranguré et al., 2003), the adjustment models did not show any interaction between paternal alcoholism and smoking. Menegaux et al. (2005) examined the association of parental smoking and maternal alcohol and coffee intake during pregnancy with the risk for childhood leukaemia. They found no association of acute lymphoblastic leukaemia or acute nonlymphocytic leukaemia with maternal smoking during pregnancy but an association with maternal alcohol and coffee consumption.
(c) Effect modification
Cigarette smoke is a known germ-cell mutagen in mice (Yauk et al., 2007), a likely germ-cell mutagen in humans (see Section 4.1.3a) and alters gene expression (see Section 4.1.4). Infante-Rivard et al. (2000) first assessed the role of parental smoking and CYP1A1 genetic polymorphisms with leukaemia and reported no statistically significant association with leukaemia overall. However, a case-only subanalysis suggested that the effect of parental smoking may be modified by variant alleles in the CYP1A1 gene: CYP1A1*2B tended to decrease risks and CYP1A1*2A and CYP1A1*4 increased the risks associated with smoking in the second and third trimesters of pregnancy. Clavel et al. (2005) examined the role of metabolic polymorphisms in the CYP1A1, GSTM1, GSTP1, GSTT1 and NQO1 genes. The slow EPHX1 allele (exon 3 homozygous genotype) was negatively associated with leukaemia, in particular acute lymphoblastic leukaemia, whereas the fast EPHX1 allele (exon 4 homozygous genotype) was positively associated with leukaemia overall. A non-significant association with acute lymphoblastic leukaemia was noted for the homozygous NQO1*2 genotype. There was a significant interaction of the CYP1A1*2A allele with smoking in the case-only analysis and a not significant interaction, but similar in magnitude, in the case–control analysis. A significant interaction was also observed with the GSTM1 deletion in the case-only analysis, but not in the case–control analysis. Lee et al. (2009) genotyped five single-nucleotide CYP1A1 polymorphisms: acute lymphoblastic leukaemia risk was significantly increased for cases without the CGACC haplotype and with paternal smoking or the presence of at least one smoker in the home.
RAS is the second most mutated gene in smoking-associated lung tumours (Section 4.1.3b). RAS mutations have been consistently correlated with myeloid leukaemias in adults and children, in particular with occupationally-associated adult myeloid leukemias (Taylor et al., 1992; Barletta et al., 2004). Wiemels et al. (2005) studied the relationship of RAS mutations, hyperdiploidy (> 50 chromosomes) and smoking in a case series of 191 acute leukaemia. Smoking was negatively associated with hyperdiploidy (possibly due to the sensitivity of the hyperdiploid clone and consequent differential survival) and hyperdiploid acute leukaemia cases had the highest rates of RAS mutations. [Paternal smoking in the three months before pregnancy was less frequent among hyperdiploids than among non-hyperdiploids.]
2.6.4. Cancers of the brain and central nervous system
Since IARC (2004), the association of exposure to parental smoking and risk for childhood brain and central nervous system (CNS) tumours has been examined in one cohort study (Brooks et al., 2004; Table 2.25 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.25.pdf), six case–control studies (Schüz et al., 1999; Sorahan et al., 2001; Filippini et al., 2002; Pang et al., 2003; Cordier et al., 2004; Plichart et al., 2008; Table 2.26 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.26.pdf), and one meta-analysis (Huncharek et al., 2002; Table 2.27 available at http://monographs.iarc.fr/ENG/Monographs/vol100E/100E-02-Table2.27.pdf).
A meta-analysis of 30 studies published before 1999 indicated no significant increase in risk for CNS tumours associated with maternal smoking during pregnancy and an increased risk for brain tumours with paternal smoking (Boffetta et al., 2000).
Huncharek et al. (2002) included one cohort and eleven case–control studies in a meta-analysis and found no clear association of maternal smoking during pregnancy with risk for childhood brain tumours, and a null risk estimate for all CNS tumours (even when the analysis was restricted to astrocytomas, the main brain tumour type). The results were comparable and consistently null for all sensitivity analyses conducted (Table 2.27 on-line).
Brooks et al. (2004) analysing the Swedish birth cohort study observed that children, in particular those aged 2–4 years, whose mother smoked during pregnancy, had an increased incidence of childhood brain tumours; the increase in risk was similar for benign and malignant brain tumours and most apparent for astrocytomas (Table 2.25 on-line).
Schüz et al. (1999) evaluated parental smoking and CNS tumour risk in children < 15 years from the German Childhood Cancer Registry (see Table 2.26 on-line). No association with risk of CNS tumours was observed for either maternal smoking during pregnancy or paternal smoking before pregnancy. Sorahan et al. (2001) found no significant association or trends of risk of CNS tumours with either paternal or maternal smoking, except for low level of maternal exposure [the latter analysis is based on only eleven exposed cases and one control, yielding a very wide confidence interval]. Filippini et al. (2002) observed no association between risk of childhood brain tumours and parental smoking before pregnancy, maternal smoking, regular maternal exposure to second-hand tobacco smoke during pregnancy, or exposure of the child to second-hand tobacco smoke during its first year of life. The results did not vary by child’s age at diagnosis, type of CNS tumour or study centre. Plichart et al. (2008) reported no association for maternal smoking during pregnancy with CNS tumours, while paternal smoking preconception showed a significant association, especially for astrocytomas. When assessing parental exposure to PAHs, Cordier et al. (2004) observed an association of paternal exposure to occupational PAHs preconception with all childhood brain tumours and with astroglial tumours, but no trend of increasing risk with increased exposure. Paternal smoking alone was associated with a risk for astroglial tumours when compared with non-smoking, non-occupationally-exposed fathers. Pang et al. (2003) found a decreased CNS risk with maternal smoking of more than 20 cigarettes/day preconception, in both unadjusted and adjusted analyses. In the analyses by histological subgroups a statistically significant decreased risk was associated with maternal smoking during pregnancy for primitive neuroectodermal tumours.
2.6.5. Hepatoblastoma
Hepatoblastoma is an embryonal tumour presumably of fetal origin and prenatal exposures are likely more important than post-natal. In some children, a diagnosis of hepatoblastoma is evident at birth or shortly thereafter, with a median age at diagnosis of 12 months. The ability of hepatoblastoma tumour cells to synthesize α-fetoprotein (AFP), a major serum protein synthesized by fetal liver cells, also suggests a fetal origin. Also, hepatoblastomas, like many other embryonal tumours, are associated with Beckwith-Wiedemann syndrome and hemihypertrophy, further suggesting a gestational oncogenic event (DeBaun & Tucker, 1998). Data were available for both maternal and paternal exposures from two studies (Pang et al., 2003; Sorahan & Lancashire, 2004) while two other studies (McLaughlin et al., 2006; Pu et al., 2009) were limited to data on maternal smoking, available from birth certificates and medical records, respectively (Table 2.28). Most of these studies had limited sample sizes given the extreme rarity of these tumours.
Table 2.28
Studies of parental tobacco smoking and childhood hepatoblastoma.
(a) Parental smoking exposure
After adjustment for relevant covariates, Pang et al. (2003) observed a statistically significant increased risk of hepatoblastoma in association with maternal preconception smoking (OR, 2.68; 95%CI: 1.16–6.21, P = 0.02) in a somewhat dose-dependent manner (P = 0.058). The association with parental smoking was strongest (relative to neither parent smoking) when both parents smoked (OR, 4.74; 95%CI: 1.68–13.35, P = 0.003). Sorahan & Lancashire (2004) found no increased risk associated with maternal or paternal smoking alone compared to non-smokers, in both adjusted and unadjusted analyses. In contrast, parental smoking (paternal and maternal smoking combined) was strongly and consistently associated with an increased risk for hepatoblastoma in both adjusted and unadjusted analyses.
In a record-based case–cohort study only maternal smoking was examined (McLaughlin et al., 2006). Extremely low birth weight (< 1000 g) was strongly associated with hepatoblastoma. After adjustement for birth weight, a statistically significant elevated risk for hepatoblastoma was found with maternal smoking (RR 2.1; 95%CI: 1.0–4.2). The increased risk was stronger for children diagnosed at the age of two years or older (RR 6.0 versus 1.4). Also, the relarive risk for maternal smoking and hepatoblastoma was stronger for children with normal birth weight [> 2500 g] than for low birth weight children. For cases of hepatoblastoma diagnosed after the age of two years, the relative risk for maternal smoking among children with normal birth weight was also stronger than that among children with low birth weight.
Another study on maternal smoking only was conducted in Chonquing, China (Pu et al., 2009). After adjustment for birth weight, a significantly increased risk for hepatoblastoma was found for maternal smoking (RR 2.9; 95%CI: 1.1–4.2). Adjustments for maternal age, maternal body mass index and sex of the baby did not change the odd ratios. When analyses were stratified by birth weight, the odd ratio associated with maternal smoking for children with a birth weight greater than 2500 g was increased almost fourfold. Stratification by age at diagnosis showed that the risk increased almost fivefold with diagnosis at the age of two years or over. [The Working Group noted that since information regarding mother’s smoking status for both cases and controls was obtained before diagnosis the potential for biased recall of maternal exposures during pregnancy is reduced].
(b) Bias and confounding
The known risk factors for hepatoblastoma include low and very low birth weights (< 2000 g and < 1000 g, respectively), maternal age and use of assisted reproductive technologies. All studies adjusted for maternal age, and low birth weight was addressed in three of them (Pang & Birch, 2003; McLaughlin et al., 2006; Pu et al., 2009). Assisted reproductive technologies were not considered to be an important potential confounder of these studies.
Spector & Ross (2003) argued that the association of hepatoblastoma with parental smoking observed by Pang et al. (2003) might be confounded by birth weight. In their response, Pang & Birch (2003) showed evidence supporting their initial conclusion: the comparable results for maternal smoking, smoking by both parents and maternal smoking for cases diagnosed at an older age, i.e. one year or older, before and after adjustment for birth weight, appear to rule out low birth weight as an explanation for the association.
Also, both later studies (McLaughlin et al., 2006; Pu et al., 2009) reported higher relative risks for children with normal birth weight compared to those with low birth weight, particularly in cases diagnosed after the age of two years.
2.6.6. Other childhood cancers
Several other childhood cancers have been studied in relation to parental tobacco smoke exposures, namely neuroblastoma, nephroblastoma, bone tumours, Wilms tumour, soft tissue sarcomas, other neoplasms of the reticuloendothelial system, and childhood germ cell tumours. The data are few and inconsistent (Schüz et al., 1999; Sorahan et al., 2001; Chen et al., 2005b; Table 2.28).
2.7. Synthesis
2.7.1. Lung
The totality of evidence available to date firmly establishes that exposure to second-hand tobacco smoke at home and at the workplace is causally associated with lung cancer risk in both men and women. This association has been observed in studies from North America, Europe, and Asia. Emerging evidence is also suggesting that exposure to second-hand tobacco smoke among children significantly enhances the risk of lung cancer in adulthood.
2.7.2. Breast
A large number of cohort studies, case–control studies and meta-analyses have assessed the association between exposure to second-hand tobacco smoke and breast cancer. Recent large cohort studies in Europe and North America showed no association between second-hand tobacco smoke and breast cancer. Positive associations in one or more subgroups were reported from some case–control studies; however, these associations were weaker in more recent studies compared with earlier studies.
Explorative analyses focusing on premenopausal breast cancer have suggested that second-hand tobacco smoke may preferentially cause premenopausal breast cancer. Positive associations were largely reported from case–control studies, in which both recall and publication bias cannot be ruled out. Case–control studies that collect a lifetime exposure history are particularly vulnerable to subjective and differential reporting of exposures that occurred long in the past from sources that are difficult to quantify. Overall, the results for an association with premenopausal breast cancer are also inconsistent.
2.7.3. Upper aerodigestive tract combined
Most evidence of the association between second-hand tobacco smoke and upper aerodigestive tract cancers, and the subsites of the oral cavity, pharynx and larynx, comes from a pooled analysis. Overall, the association between second-hand tobacco smoke exposure and cancers of the larynx and pharynx is less than causal.
2.7.4. Nasopharynx, and nasal cavity and accessory sinuses
There is some evidence from a cohort and case–control study that exposure to second-hand tobacco smoke increases the risk of sinonasal cancer; for cancer of the nasopharynx, the evidence is contradictory.
2.7.5. Other sites
Overall, data are conflicting and sparse for the association of exposure to second-hand tobacco smoke with all cancers combined, cancers of the gastrointestinal tract combined, and cancers of the stomach, colon, rectum, pancreas, liver (hepatocellular carcinoma), kidney (renal cell carcinoma), urinary bladder, cervix, ovary, testes, and brain and central nervous system.
2.7.6. Childhood cancers
(a) All childhood cancers combined
Four cohort studies, 13 case–control studies and one meta-analysis have assessed the association of parental tobacco smoking with childhood cancers, all sites combined, in offspring. Most of the early studies only assessed the contribution of maternal smoking, whereas recent studies generally assessed both paternal and maternal smoking, and at various time periods (preconception, during pregnancy, post-natally). Overall, the evidence for an association between parental smoking and childhood cancer (all sites combined) remains inconsistent and may be subject to bias. Nevertheless, a fairly consistent association of paternal tobacco smoking with childhood cancers is beginning to emerge, which is stronger in studies with more specific exposure assessments.
(b) Leukaemias and lymphomas
Two cohort studies, 27 case–control studies and 2 meta-analyses have examined the association of childhood haematopoietic malignancies (leukaemia and lymphoma) with exposure to parental smoking (paternal, maternal or both). All studies examined leukaemia, and a large number of these addressed non-Hodgkin lymphoma.
The body of evidence suggests a consistent association of leukaemia (and lymphoma) with paternal smoking preconception and with combined parental smoking, with risk ratios ranging from 1.5 to 4.0. Maternal tobacco smoking during pregnancy generally showed modest increases in risk, or null or inverse relationships. The combined effects of preconception and post-conception exposures to tobacco smoke were highly significant.
Several studies on lymphoma risk associated with parental smoking showed significantly elevated risks associated with paternal tobacco smoking preconception. The analyses had small samples sizes, and biases due to participation, recall and response, especially related to exposure, cannot be ruled out.
(c) Brain and central nervous system
The association of childhood tumours of the brain and central nervous system with parental smoking was assessed in two cohort studies, 21 case–control studies and 2 meta-analyses. Overall these studies do not show an association with either paternal smoking, largely preconception, or maternal smoking prior, during or after pregnancy, or by CNS types, gliomas and primitive neuroectodermal tumours. The strongly positive associations noted in some studies for paternal tobacco smoking with astrocytomas are offset by the lack of association with childhood brain tumours reported by the large UK Childhood Cancer Study.
(d) Hepatoblastoma
Four informative case–control studies provided data on the association between parental smoking and hepatoblastomas. Two studies reported on both maternal and paternal smoking, while the two others assessed only maternal smoking. In one study where a large number of categories of childhood cancers (n = 25) were assessed, the only childhood cancer that showed an association with parental smoking was hepatoblastoma. This original observation was confirmed in three later studies, with relative risks ranging from 2.0 to 5.5. Chance, bias and confounding were adequately addressed in the data from the studies available. The evidence for the association of parental smoking with hepatoblastoma is convincing, with an emphasis on prenatal exposures.
(e) Other childhood cancers
Most of the associations reported for the other childhood cancers, notably soft tissue sarcomas, rhabdomyosarcomas, Ewing’s sarcoma, neuroblastoma, Wilms tumour, reticuloendothelial sarcomas and germ cell tumours were null, with a few isolated and inconsistent positive observations.
3. Cancer in Experimental Animals
3.1. Simulated second-hand tobacco smoke
Simulated second-hand tobacco smoke, frequently a mixture of 89% sidestream and 11% mainstream smoke, generated from cigarettes by smoking machines (Teague et al., 1994) has been tested for carcinogenicity in adult mice of strains that are genetically susceptible to induction of lung tumours (Malkinson, 1992). Mice were exposed in inhalation chambers. Several studies reported no increase in lung tumour incidence or multiplicity in mice exposed to simulated second-hand tobacco smoke for 5–9 months and killed immediately thereafter (Witschi et al., 1995, 1997a; Finch et al., 1996). It was suggested that the lack of tumour response in simulated second-hand tobacco smoke-exposed mice might be due to treatment-induced stress (as determined by the increased plasma corticosterone level) that has been shown to attenuate lung tumorigenesis (Stinn et al., 2005a).
In subsequent studies from several laboratories (Table 3.1), an increased multiplicity and often increased incidence of lung tumours was reported in male and female A/J mice exposed for five months and kept in filtered air for another four months (Witschi et al., 1997a, b, 1998, 1999; D’Agostini et al., 2001) or longer (Witschi et al., 2006) before the mice were killed. Similar results were obtained with Swiss albino mice (Witschi et al., 2002). In these studies, no nasal tumours were observed in smoke-exposed mice.
Table 3.1
Carcinogenicity studies of inhalation exposure to simulated second-hand tobacco smokea in A/J mice, transgenic mice with a dominant negative p53 mutation, and Wistar rats as a function of length of the post-exposure recovery period.
In one study, male and female transgenic mice with a dominant negative p53 mutation on an A/J background were exposed to simulated second-hand tobacco smoke for 9.5 continuous months or for 5 months followed by recovery in air for 4.5 months. Transgenic mice exposed by either regimen developed significantly higher incidence and multiplicity of lung tumours than sham-exposed control transgenic mice (DeFlora et al., 2003). Neither lung tumour incidence nor multiplicity was increased in smoke-exposed wild-type control mice in this study.
In one study, male and female rats exposed to room-aged sidestream cigarette smoke by nose-only inhalation for 24 months and then killed had no increased incidence of lung or other tumours in comparison with fresh-air controls. Lung tumours were not significantly increased in rats exposed for 24 months and kept until 30 months of age (Stinn et al., 2005b).
3.2. Sidestream smoke condensate
In one study, sidestream cigarette smoke condensate applied to the shaved skin of female NMRI mice lower back, at total weekly doses of 5, 10 and 15 mg, for 3 months caused benign and malignant skin tumours and mammary carcinomas in mice observed for their lifespan and was more potently carcinogenic in this assay than mainstream smoke condensate. No cutaneous or subcutaneous tumours developed in any of three control groups (P < 0.001) (Mohtashamipur et al., 1990). In one study, fractionated sidestream cigarette smoke condensates were implanted into the lungs of female rats. The fraction containing PAHs with four and more rings (dose, 1.06 mg/rat) induced 5 lung carcinomas in 35 treated rats; fractions containing no PAHs or PAHs with two or three rings (16 mg/rat) had little or no carcinogenic effect (Grimmer et al., 1988).
3.3. Observational studies of companion animals
In one study, sinonasal cancers occurred more frequently in pet dogs of long-nosed breeds which lived in homes with at least one smoker (Reif et al., 1998), but no such excess risk was seen in a second study (Bukowski et al., 1998). A marginal excess risk of lung cancer was observed in dogs aged 10 years or less and exposed to household tobacco smoke in one study (Reif et al., 1992). Risk of bladder cancer in dogs was not related to exposure to household cigarette smoke in another study (Glickman et al., 1989).
Risk of malignant lymphoma was increased in pet cats exposed to household tobacco smoke in one study (Bertone et al., 2002), but the conclusion that this association was causal has been questioned (Denson, 2003). In another study by the same group (Bertone et al., 2003), exposure of pet cats to household tobacco smoke was also associated with a non-significant 2-fold increase in risk of oral squamous cell carcinoma.
3.4. Synthesis
Several studies showed consistent increases in lung tumour multiplicity and often lung tumour incidence in inbred strain A/J mice and in transgenic mice with a dominant negative p53 tumour suppressor gene exposed by inhalation. In addition, in one report, skin and mammary tumours were induced in NMRI mice exposed to sidestream cigarette smoke condensate applied topically to the skin.
4. Other Relevant Data
See Section 4 of the Monograph on Tobacco Smoking in this volume.
5. Evaluation
There is sufficient evidence in humans for the carcinogenicity of second-hand tobacco smoke. Second-hand tobacco smoke causes cancer of the lung. Also, a positive association has been observed between exposure to second-hand tobacco smoke and cancers of the larynx and the pharynx.
There is sufficient evidence in experimental animals for the carcinogenicity of mixtures of mainstream and sidestream tobacco smoke.
There is sufficient evidence in experimental animals for the carcinogenicity of sidestream tobacco smoke condensates.
Second-hand tobacco smoke is carcinogenic to humans (Group 1).
References
- Ahern TP, Lash TL, Egan KM, Baron JA. Lifetime tobacco smoke exposure and breast cancer incidence. Cancer Causes Control. 2009;20:1837–1844. [PubMed: 19533391] [CrossRef]
- Alberg AJ, Daudt A, Huang HY, et al. N-acetyltransferase 2 (NAT2) genotypes, cigarette smoking, and the risk of breast cancer. Cancer Detect Prev. 2004;28:187–193. [PubMed: 15225898] [CrossRef]
- Alberg AJ, Kouzis A, Genkinger JM, et al. A prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smoke. Am J Epidemiol. 2007;165:660–666. [PubMed: 17204516] [CrossRef]
- Asomaning K, Miller DP, Liu G, et al. Second hand smoke, age of exposure and lung cancer risk. Lung Cancer. 2008;61:13–20. [PMC free article: PMC2515267] [PubMed: 18191495] [CrossRef]
- Baker JA, Odunuga OO, Rodabaugh KJ, et al. Active and passive smoking and risk of ovarian cancer. Int J Gynecol Cancer. 2006;16 Suppl 1:211–218. [PubMed: 16515593] [CrossRef]
- Bao Y, Giovannucci E, Fuchs CS, Michaud DS. Passive smoking and pancreatic cancer in women: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2292–2296. [PMC free article: PMC2927115] [PubMed: 19602702] [CrossRef]
- Barletta E, Gorini G, Vineis P, et al. Ras gene mutations in patients with acute myeloid leukaemia and exposure to chemical agents. Carcinogenesis. 2004;25:749–755. [PubMed: 14688017] [CrossRef]
- Bertone ER, Snyder LA, Moore AS. Environmental tobacco smoke and risk of malignant lymphoma in pet cats. Am J Epidemiol. 2002;156:268–273. [PubMed: 12142262] [CrossRef]
- Bertone ER, Snyder LA, Moore AS. Environmental and lifestyle risk factors for oral squamous cell carcinoma in domestic cats. J Vet Intern Med. 2003;17:557–562. [PubMed: 12892308] [CrossRef]
- Bjerregaard BK, Raaschou-Nielsen O, Sørensen M, et al. Tobacco smoke and bladder cancer–in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2006;119:2412–2416. [PubMed: 16894557] [CrossRef]
- Boffetta P, Trédaniel J, Greco A. Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: A meta-analysis. Environ Health Perspect. 2000;108:73–82. [PMC free article: PMC1637845] [PubMed: 10620527] [CrossRef]
- Bonner MR, Bennett WP, Xiong W, et al. Radon, secondhand smoke, glutathione-S-transferase M1 and lung cancer among women. Int J Cancer. 2006;119:1462–1467. [PubMed: 16642467] [CrossRef]
- Bonner MR, Nie J, Han D, et al. Secondhand smoke exposure in early life and the risk of breast cancer among never smokers (United States). Cancer Causes Control. 2005;16:683–689. [PubMed: 16049807] [CrossRef]
- Brooks DR, Mucci LA, Hatch EE, Cnattingius S. Maternal smoking during pregnancy and risk of brain tumors in the offspring. A prospective study of 1.4 million Swedish births. Cancer Causes Control. 2004;15:997–1005. [PubMed: 15801484] [CrossRef]
- Brown DC, Pereira L, Garner JB. Cancer of the cervix and the smoking husband. Can Fam Physician. 1982;28:499–502. [PMC free article: PMC2306385] [PubMed: 21286079]
- Buckley JD, Harris RW, Doll R, et al. Case-control study of the husbands of women with dysplasia or carcinoma of the cervix uteri. Lancet. 1981;2:1010–1015. [PubMed: 6118477] [CrossRef]
- Bukowski JA, Wartenberg D, Goldschmidt M., John A. Bukowski Daniel Wartenberg. Environmental causes for sinonasal cancers in pet dogs, and their usefulness as sentinels of indoor cancer risk. J Toxicol Environ Health A. 1998;54:579–591. [PubMed: 9726781] [CrossRef]
- Burch JD, Rohan TE, Howe GR, et al. Risk of bladder cancer by source and type of tobacco exposure: a case-control study. Int J Cancer. 1989;44:622–628. [PubMed: 2793235] [CrossRef]
- California Environmental Protection Agency (2005). Health Effects Assessment for ETS: Final. California Environmental Protection Agency, Office of Environmental Health Hazard Assesment: Sacramento, CA.
- CDC. Centers for Disease Control and Prevention. Global Youth Tobacco Surveillance, 2000–2007. Morbidity and Mortality Weekly Report. 2008;57:1–21. [PubMed: 18219269]
- CDC/WHO (2009). Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO). Global Youth Tobacco Survey (GYTS). Atlanta GA. (http://www
.cdc.gov/tobacco /global/gyts/index.htm, (accessed 27 November 2011). - Chang JS, Selvin S, Metayer C, et al. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006;163:1091–1100. [PubMed: 16597704] [CrossRef]
- Chen YC, Su HJ, Guo Y-LL, et al. Interaction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancer. Cancer Causes Control. 2005;16:75–81. a. [PubMed: 15868449] [CrossRef]
- Chen Z, Robison L, Giller R, et al. Risk of childhood germ cell tumors in association with parental smoking and drinking. Cancer. 2005;103:1064–1071. b. [PubMed: 15685619] [CrossRef]
- Cheng YJ, Hildesheim A, Hsu MM, et al. Cigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in Taiwan. Cancer Causes Control. 1999;10:201–207. [PubMed: 10454065] [CrossRef]
- Clavel J, Bellec S, Rebouissou S, et al. Childhood leukaemia, polymorphisms of metabolism enzyme genes, and interactions with maternal tobacco, coffee and alcohol consumption during pregnancy. Eur J Cancer Prev. 2005;14:531–540. [PubMed: 16284498] [CrossRef]
- Coker AL, Bond SM, Williams A, et al. Active and passive smoking, high-risk human papillomaviruses and cervical neoplasia. Cancer Detect Prev. 2002;26:121–128. [PubMed: 12102146] [CrossRef]
- Coker AL, Rosenberg AJ, McCann MF, Hulka BS. Active and passive cigarette smoke exposure and cervical intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev. 1992;1:349–356. [PubMed: 1305466]
- Collishaw NE et al. (2009). Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk, in OTRU Special Report Series. Ontario Tobacco Research Unit: Toronto.
- Cordier S, Monfort C, Filippini G, et al. Parental exposure to polycyclic aromatic hydrocarbons and the risk of childhood brain tumors: The SEARCH International Childhood Brain Tumor Study. Am J Epidemiol. 2004;159:1109–1116. [PubMed: 15191928] [CrossRef]
- D’Agostini F, Balansky RM, Bennicelli C, et al. Pilot studies evaluating the lung tumor yield in cigarette smoke-exposed mice. Int J Oncol. 2001;18:607–615. [PubMed: 11179494]
- De Flora S, Balansky RM, D’Agostini F, et al. Molecular alterations and lung tumors in p53 mutant mice exposed to cigarette smoke. Cancer Res. 2003;63:793–800. [PubMed: 12591728]
- DeBaun MR, Tucker MA. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr. 1998;132:398–400. [PubMed: 9544889] [CrossRef]
- Denson KW. Re: Environmental tobacco smoke and risk of malignant lymphoma in pet cats. Am J Epidemiol. 2003;158:1227–1228, author reply 1227–1228. [PubMed: 14692044]
- Duan L, Wu AH, Sullivan-Halley J, Bernstein L. Passive smoking and risk of oesophageal and gastric adenocarcinomas. Br J Cancer. 2009;100:1483–1485. [PMC free article: PMC2694436] [PubMed: 19352383] [CrossRef]
- Enstrom JE, Kabat GC. Environmental tobacco smoke and tobacco related mortality in a prospective study of Californians, 1960–98. BMJ. 2003;326:1057. [PMC free article: PMC155687] [PubMed: 12750205] [CrossRef]
- Filippini G, Maisonneuve P, McCredie M, et al. Relation of childhood brain tumors to exposure of parents and children to tobacco smoke: the SEARCH international case-control study. Surveillance of Environmental Aspects Related to Cancer in Humans. Int J Cancer. 2002;100:206–213. [PubMed: 12115571] [CrossRef]
- Finch GL, Nikula KJ, Belinsky SA, et al. Failure of cigarette smoke to induce or promote lung cancer in the A/J mouse. Cancer Lett. 1996;99:161–167. [PubMed: 8616820] [CrossRef]
- Fukuda K, Shibata A. Exposure-response relationships between woodworking, smoking or passive smoking, and squamous cell neoplasms of the maxillary sinus. Cancer Causes Control. 1990;1:165–168. [PubMed: 2102286] [CrossRef]
- Gallicchio L, Kouzis A, Genkinger JM, et al. Active cigarette smoking, household passive smoke exposure, and the risk of developing pancreatic cancer. Prev Med. 2006;42:200–205. [PubMed: 16458957] [CrossRef]
- Gammon MD, Eng SM, Teitelbaum SL, et al. Environmental tobacco smoke and breast cancer incidence. Environ Res. 2004;96:176–185. [PubMed: 15325878] [CrossRef]
- Gerhardsson de Verdier M, Plato N, Steineck G, Peters JM. Occupational exposures and cancer of the colon and rectum. Am J Ind Med. 1992;22:291–303. [PubMed: 1519614] [CrossRef]
- Glickman LT, Schofer FS, McKee LJ, et al. Epidemiologic study of insecticide exposures, obesity, and risk of bladder cancer in household dogs. J Toxicol Environ Health. 1989;28:407–414. [PubMed: 2593174] [CrossRef]
- Goodman MT, Tung KH. Active and passive tobacco smoking and the risk of borderline and invasive ovarian cancer (United States). Cancer Causes Control. 2003;14:569–577. [PubMed: 12948288] [CrossRef]
- Grimmer G, Brune H, Dettbarn G, et al. Contribution of polycyclic aromatic compounds to the carcinogenicity of sidestream smoke of cigarettes evaluated by implantation into the lungs of rats. Cancer Lett. 1988;43:173–177. [PubMed: 3203336] [CrossRef]
- Hanaoka T, Yamamoto S, Sobue T, et al. Japan Public Health Center-Based Prospective Study on Cancer and Cardiovascular Disease Study Group. Active and passive smoking and breast cancer risk in middle-aged Japanese women. Int J Cancer. 2005;114:317–322. [PubMed: 15540214] [CrossRef]
- Hassan MM, Abbruzzese JL, Bondy ML, et al. Passive smoking and the use of noncigarette tobacco products in association with risk for pancreatic cancer: a case-control study. Cancer. 2007;109:2547–2556. [PMC free article: PMC2215306] [PubMed: 17492688] [CrossRef]
- Hassan MM, Spitz MR, Thomas MB, et al. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: case-control study. Int J Cancer. 2008;123:1883–1891. [PMC free article: PMC2673571] [PubMed: 18688864] [CrossRef]
- Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–171. [PMC free article: PMC2556958] [PubMed: 18052103] [CrossRef]
- Hecht SS, Carmella SG, Le KA, et al. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol Biomarkers Prev. 2006;15:988–992. [PubMed: 16702381] [CrossRef]
- Hellberg D, Valentin J, Nilsson S. Smoking and cervical intraepithelial neoplasia. An association independent of sexual and other risk factors? Acta Obstet Gynecol Scand. 1986;65:625–631. [PubMed: 3799159] [CrossRef]
- Hill SE, Blakely T, Kawachi I, Woodward A. Mortality among lifelong nonsmokers exposed to secondhand smoke at home: cohort data and sensitivity analyses. Am J Epidemiol. 2007;165:530–540. [PubMed: 17172631] [CrossRef]
- Hirayama T. Cancer mortality in nonsmoking women with smoking husbands based on a large-scale cohort study in Japan. Prev Med. 1984;13:680–690. [PubMed: 6536942] [CrossRef]
- Hirose K, Tajima K, Hamajima N, et al. Subsite (cervix/endometrium)-specific risk and protective factors in uterus cancer. Jpn J Cancer Res. 1996;87:1001–1009. [PMC free article: PMC5921205] [PubMed: 8878465] [CrossRef]
- Hooker CM, Gallicchio L, Genkinger JM, et al. A prospective cohort study of rectal cancer risk in relation to active cigarette smoking and passive smoke exposure. Ann Epidemiol. 2008;18:28–35. [PubMed: 17900927] [CrossRef]
- Hu J, Ugnat AM., Canadian Cancer Registries Epidemiology Research Group. Active and passive smoking and risk of renal cell carcinoma in Canada. Eur J Cancer. 2005;41:770–778. [PubMed: 15763654] [CrossRef]
- Huncharek M, Kupelnick B, Klassen H. Maternal smoking during pregnancy and the risk of childhood brain tumors: a meta-analysis of 6566 subjects from twelve epidemiological studies. J Neurooncol. 2002;57:51–57. [PubMed: 12125967] [CrossRef]
- Hurley SF, McNeil JJ, Donnan GA, et al. Tobacco smoking and alcohol consumption as risk factors for glioma: a case-control study in Melbourne, Australia. J Epidemiol Community Health. 1996;50:442–446. [PMC free article: PMC1060316] [PubMed: 8882229] [CrossRef]
- IARC (2009). Evaluating the effectiveness of smoke-free policies. IARC Handbook of Cancer Prevention. Vol. 13. Lyon: International Agency for Research on Cancer. http://www
.iarc.fr/en /publications/pdfs-online/prev/index1 .php. - IARC. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article: PMC4781536] [PubMed: 15285078]
- Infante-Rivard C, Krajinovic M, Labuda D, Sinnett D. Parental smoking, CYP1A1 genetic polymorphisms and childhood leukemia (Québec, Canada). Cancer Causes Control. 2000;11:547–553. [PubMed: 10880037] [CrossRef]
- Jaakkola MS, Jaakkola JJK. Assessment of exposure to environmental tobacco smoke. Eur Respir J. 1997;10:2384–2397. [PubMed: 9387970] [CrossRef]
- Jee SH, Ohrr H, Kim IS. Effects of husbands’ smoking on the incidence of lung cancer in Korean women. Int J Epidemiol. 1999;28:824–828. [PubMed: 10597977] [CrossRef]
- Jenkins RA, Guerin MR, Tomkins BA (2000) The Chemistry of Environmental Tobacco Smoke: Composition and Measurement, Second Edition., Jenkins RA, Guerin MR, Tomkins BA, editors. London: Lewis Boca Raton.
- Jiang X, Yuan JM, Skipper PL, et al. Environmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles County. Cancer Res. 2007;67:7540–7545. [PubMed: 17671226] [CrossRef]
- Johnson KC. Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer. 2005;117:619–628. [PubMed: 15929073] [CrossRef]
- Johnson KC. Re: more data regarding the effects of passive smoking on breast cancer risk among younger women. Int J Cancer. 2007;120:2519–2520. [PubMed: 17304510] [CrossRef]
- Johnson KC, Glantz SA. Evidence secondhand smoke causes breast cancer in 2005 stronger than for lung cancer in 1986. Prev Med. 2008;46:492–496. [PMC free article: PMC2737483] [PubMed: 18182169] [CrossRef]
- Johnson KC, Repace J. Lung cancer and passive smoking. Turning over the wrong stone. BMJ. 2000;321:1221–, author reply 1222–1223. [PMC free article: PMC1118969] [PubMed: 11073521] [CrossRef]
- Kasim K, Levallois P, Abdous B, et al. Canadian Cancer Registries Epidemiology Research Group. Environmental tobacco smoke and risk of adult leukemia. Epidemiology. 2005;16:672–680. [PubMed: 16135944] [CrossRef]
- Kropp S, Chang-Claude J. Active and passive smoking and risk of breast cancer by age 50 years among German women. Am J Epidemiol. 2002;156:616–626. [PubMed: 12244030] [CrossRef]
- Kurahashi N, Inoue M, Liu Y, et al. JPHC Study Group. Passive smoking and lung cancer in Japanese non-smoking women: a prospective study. Int J Cancer. 2008;122:653–657. [PubMed: 17935128] [CrossRef]
- Lash TL, Aschengrau A. Active and passive cigarette smoking and the occurrence of breast cancer. Am J Epidemiol. 1999;149:5–12. [PubMed: 9883788]
- Lash TL, Aschengrau A. A null association between active or passive cigarette smoking and breast cancer risk. Breast Cancer Res Treat. 2002;75:181–184. [PubMed: 12243511] [CrossRef]
- Lee KM, Ward MH, Han S, et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res. 2009;33:250–258. [PMC free article: PMC2787091] [PubMed: 18691756] [CrossRef]
- Lee YC, Boffetta P, Sturgis EM, et al. Involuntary smoking and head and neck cancer risk: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17:1974–1981. [PMC free article: PMC2561190] [PubMed: 18708387] [CrossRef]
- Lilla C, Verla-Tebit E, Risch A, et al. Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol Biomarkers Prev. 2006;15:99–107. [PubMed: 16434594] [CrossRef]
- Lissowska J, Brinton LA, Zatonski W, et al. Tobacco smoking, NAT2 acetylation genotype and breast cancer risk. Int J Cancer. 2006;119:1961–1969. [PubMed: 16721725] [CrossRef]
- Liu L, Wu K, Lin X, et al. Passive Smoking and Other Factors at Different Periods of Life and Breast Cancer Risk in Chinese Women who have Never Smoked - A Case-control Study in Chongqing, People’s Republic of China. Asian Pac J Cancer Prev. 2000;1:131–137. [PubMed: 12718680]
- Lo AC, Soliman AS, El-Ghawalby N, et al. Lifestyle, occupational, and reproductive factors in relation to pancreatic cancer risk. Pancreas. 2007;35:120–129. [PubMed: 17632317] [CrossRef]
- MacArthur AC, McBride ML, Spinelli JJ, et al. Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: the cross-Canada childhood leukemia study. Cancer Causes Control. 2008;19:283–295. [PubMed: 18283545] [CrossRef]
- Malkinson AM. Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992;52 Suppl:2670s–2676s. [PubMed: 1562998]
- Mao Y, Hu J, Semenciw R, White K., Canadian Cancer Registries Epidemiology Research Group. Active and passive smoking and the risk of stomach cancer, by subsite, in Canada. Eur J Cancer Prev. 2002;11:27–38. [PubMed: 11917206] [CrossRef]
- McGlynn KA, Zhang Y, Sakoda LC, et al. Maternal smoking and testicular germ cell tumors. Cancer Epidemiol Biomarkers Prev. 2006;15:1820–1824. [PubMed: 17035387] [CrossRef]
- McKinney PA, Cartwright RA, Saiu JM, et al. The inter-regional epidemiological study of childhood cancer (IRESCC): a case control study of aetiological factors in leukaemia and lymphoma. Arch Dis Child. 1987;62:279–287. [PMC free article: PMC1778298] [PubMed: 3646026] [CrossRef]
- McLaughlin CC, Baptiste MS, Schymura MJ, et al. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163:818–828. [PubMed: 16510543] [CrossRef]
- Mechanic LE, Millikan RC, Player J, et al. Polymorphisms in nucleotide excision repair genes, smoking and breast cancer in African Americans and whites: a population-based case-control study. Carcinogenesis. 2006;27:1377–1385. [PubMed: 16399771] [CrossRef]
- Mejía-Aranguré JM, Fajardo-Gutiérrez A, Flores-Aguilar H, et al. Environmental factors contributing to the development of childhood leukemia in children with Down’s syndrome. Leukemia. 2003;17:1905–1907. [PubMed: 12970794] [CrossRef]
- Menegaux F, Ripert M, Hémon D, Clavel J. Maternal alcohol and coffee drinking, parental smoking and childhood leukaemia: a French population-based case-control study. Paediatr Perinat Epidemiol. 2007;21:293–299. [PubMed: 17564585] [CrossRef]
- Menegaux F, Steffen C, Bellec S, et al. Maternal coffee and alcohol consumption during pregnancy, parental smoking and risk of childhood acute leukaemia. Cancer Detect Prev. 2005;29:487–493. [PubMed: 16289502] [CrossRef]
- Miller GH. The impact of passive smoking: cancer deaths among nonsmoking women. Cancer Detect Prev. 1990;14:497–503. [PubMed: 2224913]
- Miller MD, Marty MA, Broadwin R, et al. California Environmental Protection Agency. The association between exposure to environmental tobacco smoke and breast cancer: a review by the California Environmental Protection Agency. Prev Med. 2007;44:93–106. [PubMed: 17027075] [CrossRef]
- Mohtashamipur E, Mohtashamipur A, Germann PG, et al. Comparative carcinogenicity of cigarette mainstream and sidestream smoke condensates on the mouse skin. J Cancer Res Clin Oncol. 1990;116:604–608. [PubMed: 2254379] [CrossRef]
- Morabia A, Bernstein M, Héritier S, Khatchatrian N. Relation of breast cancer with passive and active exposure to tobacco smoke. Am J Epidemiol. 1996;143:918–928. [PubMed: 8610705]
- Morabia A, Bernstein MS, Bouchardy I, et al. Breast cancer and active and passive smoking: the role of the N-acetyltransferase 2 genotype. Am J Epidemiol. 2000;152:226–232. [PubMed: 10933269] [CrossRef]
- Mucci LA, Granath F, Cnattingius S. Maternal smoking and childhood leukemia and lymphoma risk among 1,440,542 Swedish children. Cancer Epidemiol Biomarkers Prev. 2004;13:1528–1533. [PubMed: 15342456]
- Navas-Acien A, Peruga A, Breysse P, et al. Secondhand tobacco smoke in public places in Latin America, 2002–2003. JAMA. 2004;291:2741–2745. [PubMed: 15187056] [CrossRef]
- Nishino Y, Tsubono Y, Tsuji I, et al. Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control. 2001;12:797–802. [PubMed: 11714107] [CrossRef]
- Pang D, Birch JM. Reply: smoking and hepatoblastoma:confounding by birth weight? Br J Cancer. 2003;89:603. [PMC free article: PMC2394388] [PubMed: 12888836] [CrossRef]
- Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer. 2003;88:373–381. [PMC free article: PMC2747546] [PubMed: 12569379] [CrossRef]
- Peppone LJ, Mahoney MC, Cummings KM, et al. Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. J Cancer Res Clin Oncol. 2008;134:743–751. [PMC free article: PMC3038611] [PubMed: 18264728] [CrossRef]
- Phillips LE, Longstreth WT Jr, Koepsell T, et al. Active and passive cigarette smoking and risk of intracranial meningioma. Neuroepidemiology. 2005;24:117–122. [PubMed: 15637448] [CrossRef]
- Pirie K, Beral V, Peto R, et al. Million Women Study Collaborators. Passive smoking and breast cancer in never smokers: prospective study and meta-analysis. Int J Epidemiol. 2008;37:1069–1079. [PubMed: 18544575] [CrossRef]
- Plichart M, Menegaux F, Lacour B, et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy and childhood malignant central nervous system tumours: the ESCALE study (SFCE). Eur J Cancer Prev. 2008;17:376–383. [PMC free article: PMC2746823] [PubMed: 18562965] [CrossRef]
- Pu CL, Guo CB, Jin XQ, et al. [Retrospective analysis of maternal and infant birth features of hepatoblastoma patients] Zhonghua Gan Zang Bing Za Zhi. 2009;17:459–461. [PubMed: 19567028]
- Ramroth H, Dietz A, Becher H. Environmental tobacco smoke and laryngeal cancer: results from a population-based case-control study. Eur Arch Otorhinolaryngol. 2008;265:1367–1371. [PubMed: 18379814] [CrossRef]
- Reif JS, Bruns C, Lower KS. Cancer of the nasal cavity and paranasal sinuses and exposure to environmental tobacco smoke in pet dogs. Am J Epidemiol. 1998;147:488–492. [PubMed: 9525536]
- Reif JS, Dunn K, Ogilvie GK, Harris CK. Passive smoking and canine lung cancer risk. Am J Epidemiol. 1992;135:234–239. [PubMed: 1546698]
- Reynolds P, Hurley S, Goldberg D., California Teachers Study Steering Committee. Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer. 2006;119:239–1, author reply 240–241. [PubMed: 16432834] [CrossRef]
- Reynolds P, Hurley S, Goldberg DE, et al. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J Natl Cancer Inst. 2004;96:29–37. [PubMed: 14709736] [CrossRef]
- Roddam AW, Pirie K, Pike MC, et al. Active and passive smoking and the risk of breast cancer in women aged 36–45 years: a population based case-control study in the UK. Br J Cancer. 2007;97:434–439. [PMC free article: PMC2360334] [PubMed: 17579618] [CrossRef]
- Rollison DE, Brownson RC, Hathcock HL, Newschaffer CJ. Case-control study of tobacco smoke exposure and breast cancer risk in Delaware. BMC Cancer. 2008;8:157. [PMC free article: PMC2424067] [PubMed: 18518960] [CrossRef]
- Rudant J, Menegaux F, Leverger G, et al. Childhood hematopoietic malignancies and parental use of tobacco and alcohol: the ESCALE study (SFCE). Cancer Causes Control. 2008;19:1277–1290. [PubMed: 18618277] [CrossRef]
- Ryan P, Lee MW, North B, McMichael AJ. Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51:20–27. [PubMed: 1563840] [CrossRef]
- Samanic C, Kogevinas M, Dosemeci M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev. 2006;15:1348–1354. [PubMed: 16835335] [CrossRef]
- Samet J, Yang G (2001) Passive smoking, women and children. In: Women and the Tobacco Epidemic, Challenges for the 21st Century, Samet, J. and Yoon, S.-Y., editors. (WHO/NMH/TF1/ 01.1), Geneva: World Health Organization.
- Sandler DP, Everson RB, Wilcox AJ. Passive smoking in adulthood and cancer risk. Am J Epidemiol. 1985;121:37–48. a. [PubMed: 3964991]
- Sandler DP, Everson RB, Wilcox AJ, Browder JP. Cancer risk in adulthood from early life exposure to parents’ smoking. Am J Public Health. 1985;75:487–492. b. [PMC free article: PMC1646286] [PubMed: 3985235] [CrossRef]
- Sandler RS, Sandler DP, Comstock GW, et al. Cigarette smoking and the risk of colorectal cancer in women. J Natl Cancer Inst. 1988;80:1329–1333. [PubMed: 3172257] [CrossRef]
- Scholes D, McBride C, Grothaus L, et al. The association between cigarette smoking and low-grade cervical abnormalities in reproductive-age women. Cancer Causes Control. 1999;10:339–344. [PubMed: 10530602] [CrossRef]
- Schüz J, Kaatsch P, Kaletsch U, et al. Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol. 1999;28:631–639. [PubMed: 10480689] [CrossRef]
- Shrubsole MJ, Gao YT, Dai Q, et al. Passive smoking and breast cancer risk among non-smoking Chinese women. Int J Cancer. 2004;110:605–609. [PubMed: 15122595] [CrossRef]
- Sillanpää P, Hirvonen A, Kataja V, et al. NAT2 slow acetylator genotype as an important modifier of breast cancer risk. Int J Cancer. 2005;114:579–584. [PubMed: 15609332] [CrossRef]
- Slattery ML, Curtin K, Giuliano AR, et al. Active and passive smoking, IL6, ESR1, and breast cancer risk. Breast Cancer Res Treat. 2008;109:101–111. [PMC free article: PMC2532584] [PubMed: 17594514] [CrossRef]
- Slattery ML, Edwards S, Curtin K, et al. Associations between smoking, passive smoking, GSTM-1, NAT2, and rectal cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:882–889. [PubMed: 14504199]
- Slattery ML, Robison LM, Schuman KL, et al. Cigarette smoking and exposure to passive smoke are risk factors for cervical cancer. JAMA. 1989;261:1593–1598. [PubMed: 2918652] [CrossRef]
- Smith EM, Sowers MF, Burns TL. Effects of smoking on the development of female reproductive cancers. J Natl Cancer Inst. 1984;73:371–376. [PubMed: 6589429]
- Sobti RC, Kaur S, Kaur P, et al. Interaction of passive smoking with GST (GSTM1, GSTT1, and GSTP1) genotypes in the risk of cervical cancer in India. Cancer Genet Cytogenet. 2006;166:117–123. [PubMed: 16631467] [CrossRef]
- Sorahan T, Lancashire RJ. Parental cigarette smoking and childhood risks of hepatoblastoma: OSCC data. Br J Cancer. 2004;90:1016–1018. [PMC free article: PMC2409629] [PubMed: 14997199] [CrossRef]
- Sorahan T, McKinney PA, Mann JR, et al. Childhood cancer and parental use of tobacco: findings from the inter-regional epidemiological study of childhood cancer (IRESCC). Br J Cancer. 2001;84:141–146. [PMC free article: PMC2363626] [PubMed: 11139329] [CrossRef]
- Spector LG, Ross JA. Smoking and hepatoblastoma: confounding by birth weight? Br J Cancer. 2003;89:602–603, author reply 603. [PMC free article: PMC2394388] [PubMed: 12888836] [CrossRef]
- Stayner L, Bena J, Sasco AJ, et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am J Public Health. 2007;97:545–551. [PMC free article: PMC1805004] [PubMed: 17267733] [CrossRef]
- Stinn W, Teredesai A, Anskeit E, et al. Chronic nose-only inhalation study in rats, comparing room-aged sidestream cigarette smoke and diesel engine exhaust. Inhal Toxicol. 2005;17:549–576. b. [PubMed: 16033752] [CrossRef]
- Stinn W, Teredesai A, Kuhl P, et al. Mechanisms involved in A/J mouse lung tumorigenesis induced by inhalation of an environmental tobacco smoke surrogate. Inhal Toxicol. 2005;17:263–276. a. [PubMed: 15814487] [CrossRef]
- Suwan-ampai P, Navas-Acien A, Strickland PT, Agnew J. Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol Biomarkers Prev. 2009;18:884–893. [PubMed: 19258471] [CrossRef]
- Tan EH, Adelstein DJ, Droughton ML, et al. Squamous cell head and neck cancer in nonsmokers. Am J Clin Oncol. 1997;20:146–150. [PubMed: 9124188] [CrossRef]
- Tay SK, Tay KJ. Passive cigarette smoking is a risk factor in cervical neoplasia. Gynecol Oncol. 2004;93:116–120. [PubMed: 15047223] [CrossRef]
- Taylor JA, Sandler DP, Bloomfield CD, et al. ras oncogene activation and occupational exposures in acute myeloid leukemia. J Natl Cancer Inst. 1992;84:1626–1632. [PubMed: 1433344] [CrossRef]
- Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. 2007;36:1048–1059. [PubMed: 17690135] [CrossRef]
- Teague SV, Pinkerton KE, Goldsmith M, et al. Sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhal Toxicol. 1994;6:79–93. [CrossRef]
- Theis RP, Dolwick Grieb SM, Burr D, et al. Smoking, environmental tobacco smoke, and risk of renal cell cancer: a population-based case-control study. BMC Cancer. 2008;8:387. [PMC free article: PMC2633310] [PubMed: 19108730] [CrossRef]
- Trimble CL, Genkinger JM, Burke AE, et al. Active and passive cigarette smoking and the risk of cervical neoplasia. Obstet Gynecol. 2005;105:174–181. [PMC free article: PMC3064987] [PubMed: 15625160] [CrossRef]
- Tsai HT, Tsai YM, Yang SF, et al. Lifetime cigarette smoke and second-hand smoke and cervical intraepithelial neoplasm–a community-based case-control study. Gynecol Oncol. 2007;105:181–188. [PubMed: 17204311] [CrossRef]
- Tse LA, Yu IT, Au JS, et al. Environmental tobacco smoke and lung cancer among Chinese nonsmoking males: might adenocarcinoma be the culprit? Am J Epidemiol. 2009;169:533–541. [PubMed: 19126588] [CrossRef]
- UKCCS Investigators. UK Childhood Cancer Study Investigators. The United Kingdom Childhood Cancer Study: objectives, materials and methods. Br J Cancer. 2000;82:1073–1102. [PMC free article: PMC2374433] [PubMed: 10737392] [CrossRef]
- US Department of Health and Human Services (2006). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [PubMed: 20669524]
- Van Hemelrijck MJ, Michaud DS, Connolly GN, Kabir Z. Secondhand smoking, 4-aminobiphenyl, and bladder cancer: two meta-analyses. Cancer Epidemiol Biomarkers Prev. 2009;18:1312–1320. [PubMed: 19336562] [CrossRef]
- Veglia F, Vineis P, Overvad K, et al. Occupational exposures, environmental tobacco smoke, and lung cancer. Epidemiology. 2007;18:769–775. [PubMed: 18062064] [CrossRef]
- Verla-Tebit E, Lilla C, Hoffmeister M, et al. Exposure to environmental tobacco smoke and the risk of colorectal cancer in a case-control study from Germany. Eur J Cancer Prev. 2009;18:9–12. [PubMed: 19077559] [CrossRef]
- Villeneuve PJ, Johnson KC, Mao Y, Hanley AJ., Canadian Cancer Registries Research Group. Environmental tobacco smoke and the risk of pancreatic cancer: findings from a Canadian population-based case-control study. Can J Public Health. 2004;95:32–37. [PMC free article: PMC6975857] [PubMed: 14768739]
- Vineis P, Hoek G, Krzyzanowski M, et al. Lung cancers attributible to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health. 2007;6:100–112. a. [PMC free article: PMC1803768] [PubMed: 17302981] [CrossRef]
- Vineis P, Veglia F, Garte S, et al. Genetic susceptibility according to three metabolic pathways in cancers of the lung and bladder and in myeloid leukemias in nonsmokers. Ann Oncol. 2007;18:1230–1242. b. [PubMed: 17496311] [CrossRef]
- Wang Z, Tang L, Sun G, et al. Etiological study of esophageal squamous cell carcinoma in an endemic region: a population-based case control study in Huaian, China. BMC Cancer. 2006;6:287. [PMC free article: PMC1774575] [PubMed: 17173682] [CrossRef]
- Wen W, Shu XO, Gao YT, et al. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study. BMJ. 2006;333:376. [PMC free article: PMC1550443] [PubMed: 16837487] [CrossRef]
- Wenzlaff AS, Cote ML, Bock CH, et al. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26:2207–2212. [PubMed: 16051642] [CrossRef]
- Whitehead T, Metayer C, Ward MH, et al. Is house-dust nicotine a good surrogate for household smoking? Am J Epidemiol. 2009;169:1113–1123. [PMC free article: PMC2727236] [PubMed: 19299402] [CrossRef]
- Wiemels JL, Zhang Y, Chang J, et al. RAS mutation is associated with hyperdiploidy and parental characteristics in pediatric acute lymphoblastic leukemia. Leukemia. 2005;19:415–419. [PubMed: 15674422] [CrossRef]
- Witschi H, Espiritu I, Dance ST, Miller MS. A mouse lung tumor model of tobacco smoke carcinogenesis. Toxicol Sci. 2002;68:322–330. [PubMed: 12151628] [CrossRef]
- Witschi H, Espiritu I, Maronpot RR. Lung tumors in 2 year old strain A/J mice exposed for 6 months to tobacco smoke. Cancer Lett. 2006;241:64–68. [PubMed: 16290922] [CrossRef]
- Witschi H, Espiritu I, Maronpot RR, et al. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18:2035–2042. a. [PubMed: 9395199] [CrossRef]
- Witschi H, Espiritu I, Peake JL, et al. The carcinogenicity of environmental tobacco smoke. Carcinogenesis. 1997;18:575–586. b. [PubMed: 9067559] [CrossRef]
- Witschi H, Espiritu I, Uyeminami D. Chemoprevention of tobacco smoke-induced lung tumors in A/J strain mice with dietary myo-inositol and dexamethasone. Carcinogenesis. 1999;20:1375–1378. [PubMed: 10383915] [CrossRef]
- Witschi H, Espiritu I, Yu M, Willits NH. The effects of phenethyl isothiocyanate, N-acetylcysteine and green tea on tobacco smoke-induced lung tumors in strain A/J mice. Carcinogenesis. 1998;19:1789–1794. [PubMed: 9806160] [CrossRef]
- Witschi H, Oreffo VI, Pinkerton KE. Six-month exposure of strain A/J mice to cigarette sidestream smoke: cell kinetics and lung tumor data. Fundam Appl Toxicol. 1995;26:32–40. [PubMed: 7657060] [CrossRef]
- WHO; World Health Organization (2002) The world health report 2002—reducing risks, promoting healthy life. Geneva: World Health Organization. http://www.who.int/whr/2002/en/ index.html (accessed Feb 18, 2010).
- WHO; World Health Organization (2003). WHO Framework Convention on Tobacco Control. Geneva: World Health Organization (updated 2004, 2005; http://www
.who.int/tobacco /framework/WHO_FCTC_english.pdf, accessed 01 November 2011). - WHO; World Health Organization (2007). WHO Guidelines for implementation of Article 8 of the WHO Framework Convention on Tobacco Control (Protection from exposure to tobacco smoke), Geneva. (http://www
.who.int/fctc /guidelines/article_8/en/index.html, accessed 17 August 2011). - WHO; World Health Organization (2008). WHO report on the global tobacco epidemic: the MPOWER package. Geneva: World Health Organization.
- WHO; World Health Organization (2009a). WHO report on the global tobacco epidemic: Implementing smoke-free environments. Geneva: World Health Organization.
- WHO; World Health Organization (2009b). Global Adult Tobacco Survey (GATS) reports and factsheets. Geneva: World Health Organization (http://www
.who.int/tobacco /surveillance/survey /gats/en/index.html, accessed 01 November 2011). - WHO; World Health Organization (2009c). STEPwise approach to surveillance (STEPS) – STEPS country reports. Geneva: World Health Organization (http://www
.who.int/chp /steps/reports/en/index.html, accessed 01 November 2009). - WHO; World Health Organization (2010)*. Global estimate of the burden of disease from second-hand smoke. Öberg M, Woodward A, Jaakkola MS, Peruga A, Prüss-Ustün A. http://whqlibdoc
.who .int/publications/2010 /9789241564076_eng.pdf (accessed November 21, 2011). - WHO; World Health Organization (2011)*. WHO report on the global tobacco epidemic: Warning about the dangers of tobacco. Geneva: World Health Organization.
- Wu MT, Lee LH, Ho CK, et al. Lifetime exposure to environmental tobacco smoke and cervical intraepithelial neoplasms among nonsmoking Taiwanese women. Arch Environ Health. 2003;58:353–359. [PubMed: 14992310]
- Yauk CL, Berndt ML, Williams A, et al. Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res. 2007;67:5103–5106. [PubMed: 17545587] [CrossRef]
- Young E, Leatherdale S, Sloan M, et al. Age of smoking initiation and risk of breast cancer in a sample of Ontario women. Tob Induc Dis. 2009;5:4. [PMC free article: PMC2656478] [PubMed: 19222858] [CrossRef]
- Yu MC, Garabrant DH, Huang TB, Henderson BE. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer. 1990;45:1033–1039. [PubMed: 2351484] [CrossRef]
- Yuan JM, Wang XL, Xiang YB, et al. Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer. 2000;85:364–369. [PubMed: 10652428] [CrossRef]
- Zeegers MP, Goldbohm RA, van den Brandt PA. A prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands). Cancer Causes Control. 2002;13:83–90. [PubMed: 11899922] [CrossRef]
- Zhang ZF, Morgenstern H, Spitz MR, et al. Environmental tobacco smoking, mutagen sensitivity, and head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2000;9:1043–1049. [PubMed: 11045786]
- Zhao Y, Shi Z, Liu L. [Matched case-control study for detecting risk factors of breast cancer in women living in Chengdu] Zhonghua Liu Xing Bing Xue Za Zhi. 1999;20:91–94. [PubMed: 10682541]
- Zheng W, McLaughlin JK, Chow WH, et al. Risk factors for cancers of the nasal cavity and paranasal sinuses among white men in the United States. Am J Epidemiol. 1993;138:965–972. [PubMed: 8256781]
- SECOND-HAND TOBACCO SMOKE - Personal Habits and Indoor CombustionsSECOND-HAND TOBACCO SMOKE - Personal Habits and Indoor Combustions
Your browsing activity is empty.
Activity recording is turned off.
See more...
