NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014.

The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General.
Show detailsIntroduction
Tobacco use before and during pregnancy remains a major cause of reduced fertility as well as maternal, fetal, and infant morbidity and mortality. Smoking prevalence among women grew in the decades before the 1964 Surgeon General's report, Smoking and Health: Report of the Advisory Committee of the Surgeon General of the Public Health Service, and continued to increase across the 1970s as products were aggressively marketed to women (U.S. Department of Health and Human Services [USDHHS] 2001). Despite declines in recent decades, more than 400,000 live-born infants are exposed in utero to tobacco from maternal smoking annually (Hamilton et al. 2012; Tong et al. 2013). The women most likely to smoke are among the most vulnerable—those disadvantaged by low income, low education, and mental health disorders, further exacerbating the adverse health effects from smoking on mothers and their offspring (Adams et al. 2008; Holtrop et al. 2010; Maxson et al. 2012; Page et al. 2012a). Women in these groups are also less likely to quit smoking when they become pregnant and are more likely to relapse after delivery (Adams et al. 2008). Reducing the prevalence of smoking among pregnant women and women of reproductive age remains a critical component of public health efforts to improve maternal and child health.
This chapter includes the following updates to previous Surgeon General's reports:
An overview of surveillance systems of pregnant women that include data related to prenatal smoking status.
- A review of what is currently known about smoking cessation during pregnancy, including clinical- and policy-based interventions.
- A summary of the advances in our understanding of the mechanisms underlying previously established effects of tobacco on reproductive health since the 1964 report. Topics include fetal growth, preeclampsia, stillbirth and perinatal mortality, sudden infant death syndrome (SIDS), and neurocognitive development.
- Evidence reviews for outcomes not addressed or not causally related to smoking in previous Surgeon General's reports, including congenital malformations, male sexual function, neurobehavioral disorders of childhood, ectopic pregnancy, and spontaneous abortion. These topics were last reviewed in the 2004, 2006, and 2010 Surgeon General's reports.
Surveillance
Before 1989, surveillance of the prevalence of smoking during pregnancy in the United States was limited to self-reported data collected through periodic surveys, which sampled new mothers or reproductive age women, regarding their most recent pregnancy within the last 5 years (Table 9.1). The earliest data available are from the 1967 National Natality Survey, which sampled married women with live-born infants (Kleinman and Kopstein 1987). In 1989, smoking status during pregnancy was added to the U.S. Standard Certificate of Live Birth, and New York City, the District of Columbia, and all states, except California, collected this information (Tolson et al. 1991). In 2003, the U.S. Standard Certificate of Live Birth was revised to include the average number of cigarettes smoked per day during the 3 months before pregnancy and during the first, second, or third trimesters of pregnancy (Osterman et al. 2011). Because the 1989 and 2003 birth certificate smoking variables are not comparable and state uptake of the 2003 revised birth certificate has been gradual (in 2011, 38 states had implemented the 2003 revised birth certificate, and it is anticipated that all states will have made the transition by 2014), national prenatal smoking trend data after 2002 are not available. In 2002, an estimated 11.5% of singleton, live-born infants were exposed to maternal smoking in utero (Dietz et al. 2010). Of these, an estimated 5.3–7.7% of preterm deliveries, 13.1–19.0% of term low birth weight deliveries, 23.2–33.6% of SIDS, and 5.0–7.3% of preterm-related deaths were attributable to prenatal smoking.
Table 9.1
Data sources for smoking prevalence during pregnancy.
The Pregnancy Risk Assessment Monitoring System (PRAMS) is another source of state- and population-based data on smoking during pregnancy. In this survey, a questionnaire is administered 2–6 months after delivery to women with a live birth. Using 2010 data from 27 PRAMS states/sites, 23% of women who delivered live infants reported smoking in the 3 months before pregnancy; 11% in the last 3 months of pregnancy; and 16% 2–6 months after delivery (Tong et al. 2013). There was large variation by state in the prevalence of smoking during the last 3 months of pregnancy, ranging from 2.3% in New York City to 30.5% in West Virginia. Demographic groups with the highest prevalence of prenatal smoking were 20–24 year olds (17.6%), American Indian/Alaska Natives (26.0%), women with less than 12 years of education (17.4%), unmarried (18.6%), and those with an annual income of less than $15,000 per year (19.0%) (Tong et al. 2013).
Underreporting of smoking among pregnant women has been documented through biochemical confirmation of self-report in clinical trials and population-based studies. In an analysis of 1999–2006 National Health and Nutrition Examination Survey data, which compared self-reported smoking status to serum cotinine, 22.9% of pregnant smokers and 9.2% of nonpregnant smokers of reproductive age did not accurately disclose their smoking status (Dietz et al. 2010). Such nondisclosure likely contributes to underreporting of prenatal smoking status on birth certificates and in self-administered surveys. It is unknown whether and to what extent nondisclosure of smoking status has changed over time. Existing surveillance systems of pregnant women do not currently gather data on noncigarette tobacco products such as little cigars/cigarillos, hookah, snus, or electronic cigarettes.
Cessation
Smoking cessation in pregnancy has been associated with improvements in outcomes including fetal growth restriction and preterm delivery (McCowan et al. 2009; Baba et al. 2012). The first study of a smoking cessation intervention for pregnant women was published in 1976 and included brief advice from a physician to quit (Baric et al. 1976). Numerous intervention trials have been conducted since then, and results have been generally positive with regard to pregnancy outcomes (Lumley et al. 2009), although the increasing importance of including biochemical validation of cessation in research protocols has also been recognized (Kendrick et al. 1995). Behavioral counseling has been shown to have a modest effect, resulting in about an additional 1 in 20 pregnant women quitting (Lumley et al. 2009), and the current best-practice guidance for prenatal smoking cessation entails psychosocial counseling delivered in the prenatal care setting (Fiore et al. 2008; American Congress of Obstetricians and Gynecologists [ACOG] 2010). However, even with universal implementation of this best-practice approach, the prevalence of smoking among pregnant women was projected to decline by no more than approximately 1% in a model of smoking among pregnant women based on 2004 U.S. data (Kim et al. 2009). Therefore, other interventions are also needed in order to have a substantial public health impact.
In addition to behavioral interventions, a number of studies have assessed the safety and efficacy of nicotine replacement therapy (NRT) for cessation during pregnancy. A 2012 meta-analysis of six randomized controlled trials (RCTs) of NRT found no significant difference for smoking cessation in later pregnancy after using NRT as an adjunct to behavioral support as compared to control (relative risk [RR] = 1.33; 95% confidence interval [CI], 0.93–1.91, 1,745 women) (Coleman 2012). Both placebo and nonplacebo controlled studies were assessed (placebo RCTs: RR = 1.20; 95% CI, 0.93–1.56, four studies, 1,524 women; nonplacebo RCTs: RR = 7.81; 95% CI, 1.51–40.35, two studies, 221 women), suggesting clinical heterogeneity and uncontrolled biases in the nonplacebo controlled trials. There was insufficient evidence to conclude that NRT had a positive or negative effect on rates of miscarriage, stillbirth, premature birth, birthweight, low birthweight, admissions to neonatal intensive care, or neonatal death compared to the control groups. Nonadherence to NRT treatment was reported among the majority of participants in five of the six NRT trials (range of 7.2–29% of patients adhering to NRT treatment). A recent observational study of pregnant smokers utilizing national smoking cessation services in the United Kingdom found that use of a NRT patch along with a faster-acting form was associated with higher odds of quitting compared with no medication (odds ratio [OR] = 1.93; 95% CI, 1.13–3.29, p = 0.016), whereas use of a single form of NRT showed no benefit (OR = 1.06; 95% CI, 0.60–1.86, p = 0.84) (Brose et al. 2013). Research is needed to further assess the efficacy and safety of NRT as well as understanding the reasons for nonadherence to NRT treatments. Currently, ACOG (2010) recommends NRT only if behavioral therapy fails to achieve smoking cessation and it must be administered under the supervision of a physician.
In addition to the current clinical guidelines, section 4107 of the Patient Protection and Affordable Care Act, which took effect on October 1, 2010, requires state Medicaid programs to cover tobacco-cessation counseling and drug therapy for pregnant women without cost sharing. The update of Treating Tobacco Use and Dependence guidelines (Fiore et al. 2008) note that although the use of NRT exposes pregnant women to nicotine, smoking exposes them to nicotine plus numerous other chemicals that are injurious to the woman and fetus, and these concerns must be considered in the context of inconclusive evidence that cessation medications boost abstinence rates in pregnant smokers.
Studies of contingency management interventions, in which quitting is rewarded with financial incentives, show promise, including higher quit rates (34% of women in the intervention arm quit compared to 7.1% of women receiving standard care) and improvements in infant birth weight (Higgins et al. 2010, 2012). The effectiveness of contingency management across diverse populations and settings and the cost-benefit of implementing these interventions have not been evaluated.
Studies of the effects of interventions to prevent relapse after delivery have been mixed and limited by methodologic weaknesses; a 2009 Cochrane review found the evidence “insufficient to support the use of any specific behavioral intervention for helping smokers who have successfully quit for a short time to avoid relapse” (Hajek et al. 2009).
There is growing evidence that tobacco control policies may be effective in reducing the prevalence of prenatal smoking and improving birth outcomes. Studies conducted in Scotland and Belgium found that implementation of national smokefree air laws had a significant effect on reducing the prevalence of prenatal smoking and decreased the risk of preterm delivery (Mackay et al. 2012; Cox et al. 2013). In an analysis of 2000–2005 PRAMS data from 29 states linked to state tobacco control data, state tobacco control policies, taxes, and smokefree air laws were found to be effective in reducing maternal smoking (Adams et al. 2012). For example, a $1.00 increase in cigarette taxes and prices increased the quit rate among pregnant women from 44.1–48.9% and decreased the percentage who relapsed in the early postpartum period. Additionally, the same study found that implementing a full worksite smoking ban increased quits during pregnancy by an estimated 5%. Several studies of local ordinances in the United States have also documented reduced prevalence of smoking (Nguyen et al. 2013), and reductions in preterm births (Page et al. 2012b). Tobacco control policies are continually being implemented at local and state levels, and there is a need for evaluation of these policies and their effects on the prevalence of smoking and birth outcomes in pregnant women.
Advances in the Understanding of Tobacco and Reproductive Health
The 1964 Surgeon General's report stated that infants of smokers are more likely than those of nonsmokers to be born at less than 2,500 grams (g) (U.S. Department of Health, Education, and Welfare [USDHEW] 1964); since that time, the list of adverse reproductive health outcomes associated with maternal smoking has grown dramatically (see Table 4.4S). For many of these outcomes, however, the mechanisms through which tobacco acts to cause adverse effects are still not completely understood. As the landscape of commercial tobacco products changes and new nicotine-delivery devices are introduced into the market, gaining a better understanding of the underlying pathophysiologic mechanisms and the components responsible is of increasing urgency, as is identifying the most effective approaches to decrease the prevalence of prenatal and postnatal smoking.
Fetal Growth
The effects of maternal smoking during pregnancy on birth weight have been recognized since the first Surgeon General's report on smoking and health, in which it was observed that infants of smokers are more likely than those of nonsmokers to be born weighing less than 2,500 g, even after stratification by social class (USDHEW 1964).
Since the 1964 Surgeon General's report, new insights have been gained into the potential underlying mechanisms and clinical implications of reduced birth weight. In the 1960s, the terms low birth weight and preterm delivery were used interchangeably; however, recognition that they are not synonymous eventually led to the transition to the use of alternative outcomes (Wilcox 2001). Low- and normal-birth weight outcomes have largely been replaced with outcomes related specifically to gestational age and/or fetal growth. Intrauterine growth retardation (IUGR) (the lower tenth percentile for the gestational age), birth weight in units of standard deviations (z-scores), term birth weight, gestational-age adjusted birth weight, mean gestational age, and percentage of deliveries that are preterm (less than 37 completed weeks gestation) are all commonly used.
The 2004 Surgeon General's report found the evidence sufficient to infer causal relationships between smoking and fetal growth restriction and between smoking and decreased gestation/increased preterm delivery. Since then, newer studies have included consideration of the effects of active maternal smoking on both fetal growth and gestational age, and of active smoking and exposure to secondhand smoke on fetal growth. For example, in a large study of midtrimester cotinine levels and birth outcomes, women with cotinine levels indicative of exposure to secondhand smoke below the threshold for active smoking (10 nanograms/milliliter [ng/mL]), but above the limit of detection (0.05 ng/mL) were compared with women who had levels below the limit of detection. Women with cotinine levels between 0.05–10 ng/mL delivered infants with a mean overall decrease in birth weight of 109 g after adjustment for a number of variables and for gestational age. Women with cotinine levels in the active smoking range (above 10 ng/mL) delivered infants with a mean reduction in birth weight of 327g compared with women who had levels below the level of detection (Kharrazi et al. 2004). Other estimates for active smoking range from about 200–300 g (USDHHS 2001).
Studies of birth outcomes in mothers who use smokeless tobacco during pregnancy offer new insights into the mechanisms underlying reductions in birth weight among infants of smokers. It has been hypothesized that exposure to cigarette smoke results in fetal growth restriction through products of combustion (e.g., carbon monoxide [CO]) and associated hypoxia, nicotine-mediated vasoconstriction of uteroplacental vessels, or both (Lambers and Clark 1996). However, it has been questioned whether vascocontrictive effects of nicotine are sufficient to overcome placental circulatory reserve (Benowitz and Dempsey 2004). If nicotine-related mechanisms are important, negative associations between birth weight and exposure to smokeless tobacco use and to cigarette smoking would be expected. However, the associations between smokeless tobacco use and birth weight deficits found in studies that include an adjustment for gestational age are modest; estimated deficits range from 17–93 g (England et al. 2003, 2012; Gupta and Sreevidya 2004; Steyn et al. 2006; Juárez and Merlo 2013). Two of these estimates were not significant (Steyn et al. 2006; England et al. 2012). Smokeless tobacco use has also been associated with a modest increase in the risk for being small for gestational age. In a population-based study using birth registry data in Sweden, smokeless tobacco use and smoking were both associated with term small for gestational age (defined as birth weight more than two standard deviations below the mean for gestational age among term infants), but the magnitude of the association was smaller for smokeless tobacco use (adjusted odds ratio [AOR] = 1.21; 95% CI, 1.02–1.43 and AOR = 2.76; 95% CI, 2.62–2.91, respectively) (Baba et al. 2012). None of the studies of smokeless tobacco and pregnancy outcomes conducted thus far have included adjustment for exposure to secondhand smoke.
Taken together, these data provide support that nicotine makes a relatively modest contribution to the effects of tobacco use on fetal growth when compared with the larger contribution of the combination of both nicotine and products of combustion in cigarette smoke. However, it will be difficult to accurately quantify the specific contribution of nicotine until studies are done that include biomarkers of nicotine exposure (e.g., cotinine) and measures of exposure to secondhand smoke.
Studies of tobacco use and birth weight must necessarily include consideration of the concurrent effects on gestational age. Maternal smoking is associated with a 27% increase in the risk of preterm delivery compared with nonsmokers (Shah and Bracken 2000). Several studies have also found an increased risk of preterm delivery among smokeless tobacco users compared with tobacco nonusers (Gupta and Sreevidya 2004; Baba et al. 2012; England et al. 2013). In Sweden, continued snuff use and smoking during pregnancy were each associated with increased risks of preterm birth, and the magnitudes of the associations were similar to one another (adjusted estimated pooled OR = 1.29; 95 % CI, 1.17–1.43, AOR = 1.30; 95% CI, 1.25–1.36, respectively) (Baba et al. 2012). In a study of pregnant women in India, smokeless tobacco users delivered 6.2 days earlier on average than nonusers (p <0.001). In addition, smokeless tobacco use was associated with preterm delivery overall (AOR = 1.5, p = 0.05) and with preterm delivery at less than 32 and less than 28 weeks' gestation (AOR = 4.9; 95% CI, 2.1–11.8; AOR = 8.0; 95% CI, 2.6–27.2, respectively) (Gupta and Sreevidya 2004). In South Africa, snuff users delivered at a slightly reduced gestational age compared with tobacco nonusers (37.9 and 38.3 weeks, respectively, p = 0.003), but there was no significant increase in preterm delivery at less than 36 weeks gestation (Steyn et al. 2006). Together, these studies support an association between smokeless tobacco use and preterm delivery and raise important concerns about the potential effects of nicotine exposure during pregnancy.
Evidence from studies of gene-environment interactions support the hypothesis that components of tobacco other than nicotine may contribute to tobacco-related adverse pregnancy outcomes. Genes that encode enzymes associated with the metabolism of other compounds found in tobacco smoke, such as polycyclic aromatic hydrocarbons (PAHs) and nitrosamines, have been associated with adverse birth outcomes in smokers, including preterm delivery and restricted fetal growth (Wang et al. 2002; Nukui et al. 2004; Grazuleviciene et al. 2009; Aagaard-Tillery et al. 2010). For example, the effects of maternal smoking on the risk of IUGR appear to be modified by maternal CYP1A1 and GSTT1 genotypes; in one study, cigarette smoking in women with GSTT1 deletions appeared to be associated with more extreme birth weight reduction and increased risk of IUGR compared with women who used tobacco but did not have the deletion (Wang et al. 2002). In the same study, cigarette smoking in women with CYP1A1 heterozygous and homozygous variant types was also associated with more extreme reductions in birth weight and with IUGR compared with women who smoked but who had wild-type variants. Women with both the CYP1A1 variant genotype and the GSTT1 deletion had the greatest reduction in birth weight (Wang et al. 2002). In contrast, a subsequent study showed no association between gene polymorphisms of CYP1A1 and birth weight, but did show associations with GSTT1 deletions (Nukui et al. 2004). Studies of allelic variants affecting nicotine metabolism and birth weight have been inconclusive (Aagaard-Tillary et al. 2010). Additional studies of tobacco-related adverse pregnancy outcomes among women with different genotypes may help to further define pathways between different components in tobacco and adverse pregnancy outcomes.
The clinical significance of the effects of smoking on fetal growth has been a topic of debate for decades, and the relationship between smoking-related birth weight reductions and infant mortality has been studied in detail. It has long been recognized that low birth weight babies of smokers have lower mortality than low birth weight babies of nonsmokers. This phenomenon was cited early on as support that smoking improved survival, rather than causing harm (Yerushalmy 1971). An alternative explanation is that the smaller size of infants of smokers does not in itself affect survival, so smaller infants of smokers have better survival rates than other infants of the same weight. Indeed, when birth weight distributions for infants of smokers and nonsmokers and their corresponding mortality rates are examined, the infants of smokers have higher mortality at every birth weight, when each population is adjusted to its own z-scale of birth weight (Wilcox 2001). This provides strong evidence that smoking affects infant mortality and that this effect is independent of birth weight, in contrast to early explanations that smoking somehow confers an advantage to smaller babies (Yerushalmy 1971). In other words, infants of nonsmokers may be less likely to be born at a low birth weight than infants of smokers, but when they are, the underlying etiologies of low birth weight are associated with higher mortality (Wilcox 2001).
Preeclampsia
Among the most dramatic advances in our understanding of the pathophysiology of reproductive health outcomes are developments in the field of preeclampsia. Preeclampsia is a syndrome of reduced organ perfusion attributable to vasospasm and endothelial activation with an onset after 20 weeks of gestation. It is marked by proteinuria, hypertension, and dysfunction of the endothelial cells lining the uterus (Sibai et al. 2005). Smoking is inversely associated with preeclampsia; the pooled risk reduction is 32% (Conde-Agudelo et al. 1999). The 2004 Surgeon General's report found the evidence sufficient to infer a causal relationship between smoking and a reduced risk of preeclampsia (USDHHS 2004).
The discovery of an animal model in which almost all the complications of preeclampsia (hypertension, proteinuria, cerebral edema, hematologic abnormalities, and fetal growth restriction) can be initiated by administration of the anti-angiogenic protein soluble fms-like tyrosine kinase-1 (sFlt-1) to rats has led to the construction of a working model for preeclampsia (Levine and Karumanchi 2005). Pro-angiogenic factors, including VEGF, promote angiogenesis in the placenta while anti-angiogenic factors inhibit angiogenesis. Anti-angiogenic factors include sFlt-1, a splice variant of VEGF receptor 1 and a major placental inhibitor of angiogenesis. Circulating sFlt-1 binds VEGF and placental growth factor, preventing them from binding with cell-surface receptors. Production of excess sFlt-1 and other anti-angiogenic factors leads to an imbalance between pro- and anti-angiogenic factors and results in the clinical manifestation of preeclampsia including vasoconstriction and a state of generalized endothelial dysfunction. The molecular basis of placental dysregulation of angiogenic factors is currently the subject of ongoing research (Steinberg et al. 2009; Maynard and Karumanchi 2011).
A new preeclampsia model provides a plausible explanation for reduced preeclampsia risk from cigarette smoking. Smoking in pregnancy has been associated with reduced sFlt-1 levels compared with nonsmokers, in women with and without preeclampsia (Jeyabalan et al. 2008), and cigarette smoke extract decreases sFlt-1 production in placental villous explants (Maynard and Karumanchi 2011). Because a reduced risk of preeclampsia has not been observed in smokeless tobacco users (Wikström 2010; England et al. 2013), it seems likely that one or more products of combustion is responsible for the reduced risk of preeclampsia seen in smokers, and not nicotine. CO is one promising candidate for a mediator, as it has vasoprotective properties, and CO and CO-releasing molecules lower sFlt-1 and soluble endoglobin in in vitro cultures.
Stillbirth and Perinatal Mortality
In the 1969 Surgeon General's report supplement, it was stated that prenatal smoking may be associated with stillbirth (fetal death after 28 weeks gestation) and neonatal death (death within 28 days of birth) (USDHEW 1969). In the 2001 Surgeon General's report, it was noted that cigarette smoking was consistently associated with stillbirth (USDHHS 2001), with an increased risk of 40% (Cnattingius et al. 1988) to 60% (Raymond et al. 1994). Underlying factors were attributed to IUGR, placental complications, or both. Neonatal mortality was also noted to be increased in infants of smokers by 20% (Cnattingius et al. 1988; Malloy et al. 1988). Perinatal mortality was noted to be increased by 20–30% with 3.4–8.4% of perinatal deaths attributable to smoking (DiFranza and Lew 1995).
Since the 1964 Surgeon General's report, there has been significant progress in understanding the increased perinatal and infant mortality in offspring of smokers. Smoking likely increases perinatal mortality through numerous mechanisms, including abruption, placenta previa, preterm delivery, and premature and prolonged rupture of the membranes, and through physiologic responses of the fetus and newborn to stress (Meyer and Tonascia 1977). For example, an abnormal adrenal response of the fetus or neonate to hypoxia could affect cardiac function and survival (Slotkin 1998), and hypoxia from sleep apnea or airway obstruction could precipitate respiratory failure in a susceptible infant (Horne et al. 2005).
Some studies support a role for nicotine in the effects of smoking on stillbirth and perinatal mortality (see Chapter 5, “Nicotine”). Nicotinic acetylchonine receptors (nAChRs) are receptors that are ordinarily activated by endogenous acetylcholine, but that also can be stimulated by nicotine, resulting in disruption of normal cholinergic signaling (Albuquerque et al. 2009). nAChRs are expressed early in fetal development in the central, peripheral, and enteric nervous systems (reviewed by Abbott and Winzer-Serhan 2012), and transient, regional patterns of increased nAChR expression occur throughout perinatal and postnatal development. nAChRs are involved in neurogenesis, migration, differentiation, and synaptogenesis, in regulating the growth of developing neurites, guiding pathfinding of these projections, and mediating pruning of hippocampal and cortical neurons through effects on apoptosis (Dywer et al. 2008). Depending on the subunit composition, and the dose and duration of exposure, exogenous nicotine can activate or inactivate a given receptor, potentially altering fetal development. For example, animal models show that nicotine exposure in the fetus causes cell damage, and reduces cell number, and impairs synaptic activity. Receptor stimulation by nicotine leads to errors in cell development, including premature change from cell replication to differentiation and initiation of apoptosis (Slotkin et al. 1987; Slotkin 1998; Dwyer et al. 2008). Because nicotinic receptors continue to emerge after organogenesis, periods of fetal vulnerability likely extend into the second and third trimesters of pregnancy (Slotkin 1998).
Human and animal studies suggest that nAChRs in the brainstem nuclei control cardiopulmonary integration and arousal during early life (reviewed by Dwyer 2008). Gestational nicotine exposure in rat pups blunted the ventilator response to hypercapnia and hypoxia/hypercapnia in the first days of life, perhaps through effects in carotid body oxygen sensing or central processing (Huang et al. 2010). Prenatal nicotine exposure in rat pups also resulted in increased mortality in response to hypoxia (Slotkin et al. 1995), while human preterm infants of maternal smokers exhibit increased obstructive apnea and decreased arousal in response to apnea events (Sawnani et al. 2004). Additional data suggest that gestational smokeless tobacco exposure also increases the risk of apnea, of a similar magnitude to that seen with smoking (Gunnerbeck et al. 2011), further supporting a role for nicotine in underlying pathophysiologic processes.
Extensive animal research has generated plausible models to explain how nicotine could increase the risk of perinatal mortality (see Chapter 5) (Slotkin 1998). During parturition, a massive release of catecholamines from the fetal adrenal medulla protects the fetus from hypoxia and maintains blood flow to the brain and heart (Lagercrantz and Slotkin 1986). However, prenatal nicotine exposure in rat models causes immature chromaffin cells in the adrenal gland to differentiate prematurely, resulting in loss of the normal direct stimulation of the adrenal gland by hypoxia and a complete absence of catecholamine release, which in turn causes an impaired cardiac response (Slotkin 1998). This results in the loss of a critical protective response to hypoxia, which would lead to an increased risk of infant mortality (Figure 9.1) (Slotkin 1998).
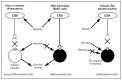
Figure 9.1
Catecholamine response to hypoxia by nicotine-exposed and unexposed rats. Source: Slotkin 1998. Reprinted with permission from American Society for Pharmacology & Experimental Therapeutics, © 1998. Note: CNS = central nervous system.
Studies of stillbirth have also been conducted among smokeless tobacco users. In a study in India, researchers reported an adjusted risk for stillbirth three times higher for mothers who used smokeless tobacco than for those who do not use any tobacco. Some evidence for a dose-response relationship was found using frequency of use (Gupta and Subramoney 2006). A previous study from India also found an increased risk of stillbirth or perinatal death with use of smokeless tobacco (primarily chewing tobacco) (Krishna 1978). In a large study of Swedish women, snuff users had an increased risk of stillbirth compared with tobacco nonusers (AOR = 1.6; 95% CI, 1.1–2.3); the risk was higher for preterm stillbirth (AOR = 2.1; 95% CI, 1.3–3.4). For women smoking 1–9 cigarettes per day and smoking more than 10 cigarettes per day, the AORs for stillbirth were 1.4 (1.2–1.7) and 2.4 (2.0–3.0), respectively. When women with preeclampsia, antenatal bleeding, or small for gestational age deliveries were excluded, the smoking-related risks of stillbirth was markedly attenuated while the elevated risk for snuff users remained at the same level. These findings suggest that the mechanisms underlying the associations between smoking and stillbirth and between smokeless tobacco use and stillbirth both involve nicotine, but other factors may also contribute to increased risk in smokers (Wikström et al. 2010).
Sudden Infant Death Syndrome
SIDS is currently defined as “…sudden death of an infant under one year of age which remains unexplained after a thorough case investigation, including performance of a complete autopsy, examination of the death scene, and review of the clinical history” (Willinger et al. 1991, p. 681). A causal association between SIDS and smoking during and after pregnancy was established in 2004 (USDHHS 2004), more than 30 years after the landmark Collaborative Perinatal Project (CPP) first described an elevated risk of SIDS in infants of smokers. Approximated 42,000 pregnant women were enrolled, making the CPP the largest U.S.-based cohort study of pregnancy and childhood to date (reviewed by Klebanoff 2009).
Major risk factors for SIDS include prone/side sleep position, soft sleep surface, maternal smoking during pregnancy, secondhand tobacco smoke, bed sharing, and overheating (American Academy of Pediatrics [AAP] 2011). Following the release in 1992 of the recommendation that infants be placed in a nonprone position for sleep, there was a dramatic drop in the number of SIDS deaths, although this decline has plateaued. As the deaths related to prone sleeping declined, the fraction of deaths attributable to smoking increased (AAP 2011). In more recent years, the fraction of SIDS deaths attributable to smoking may be stabilizing; it is estimated that 23.2–33.6% of SIDS deaths were attributable to prenatal smoking in 2002; after extrapolating, based on trends in prenatal smoking, it was estimated that 20.2–29.3% of SIDS deaths were attributable to prenatal smoking in 2009 (Dietz et al. 2010). Guidelines for death scene investigations and autopsies are available to improve standardization of data collected and, ultimately, to improve the consistency of cause-of-death determination. However, these guidelines are not universally practiced (Camperlengo et al. 2012).
A number of hypotheses regarding the underlying causes of SIDS have been proposed. These include dysfunctional and/or immature cardiorespiratory systems; dysfunctional and/or immature arousal systems, with resulting failure to respond to stressors with normal protective responses (AAP 2011); potentiation of the laryngeal chemoreflex (Thach 2008); respiratory obstruction; bacterial toxins; thermal stress; and failure of the diaphragm with inactivation of intercostal muscles (reviewed by Harper and Kinney 2010; Goldwater 2011).
Many of these hypothesized mechanisms are unified in the triple risk model of SIDS. In this model, death from SIDS occurs in the presence of three overlapping factors: (1) a vulnerable infant, (2) a critical developmental period in homeostatic control, and (3) an exogenous stressor(s). A number of different factors (including prenatal tobacco exposure) may make the infant vulnerable to sudden death during the critical period. SIDS then occurs only in the presence of exogenous stressors, such as bed sharing and overbundling (Figure 9.2) (Filiano and Kinney 1994).
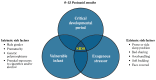
Figure 9.2
Triple-risk model for sudden infant death syndrome (SIDS). Source: Trachtenberg et al. 2012. Reprinted with permission from American Academy of Pediatrics, © 2012.
Epidemiologic data support that a high-risk scenario as described in the triple risk model could increase the risk of SIDS. In a recent meta-analysis, the authors found that the risk of SIDS associated with bed sharing was higher for infants whose mothers smoked (combined OR = 6.27; 95% CI, 3.94–9.99), than for infants whose mothers did not smoke (combined OR = 1.66; 95% CI, 0.91–3.01) (Vennemann et al. 2012). This finding suggests that the combination of the effects of tobacco exposure on infant vulnerability and stress related to the sleep environment may combine synergistically to increase the risk of death (Alsweiler et al. 2012).
Nicotine may play an important role in increasing the risk of SIDS in infants of smokers (see Chapter 5). In animal models, exogenous nicotine administered to pregnant rats alters the expression of nAChRs in the brainstem in areas involved in autonomic function, and affects fetal autonomic activity and medullary neurotransmitter receptors (Duncan et al. 2009). In studies of human infants, prenatal tobacco exposure affects recovery from hypoxia in preterm infants (Thiriez et al. 2009); infants also display impaired arousal patterns that correspond to cotinine levels (Richardson et al. 2009). These changes in autonomic function and/or arousal could increase the risk of SIDS (reviewed by AAP 2011), although a causal pathway has not been established.
Neurocognitive Development
Maternal smoking and exposure to secondhand tobacco smoke during pregnancy are hypothesized to affect physical and mental development in infancy and early childhood. Earlier Surgeon General's reports examined this topic and reported possible effects. However, at the time of the 2004 report, the evidence was considered “inadequate to infer the presence or absence of a causal relationship between maternal smoking and physical growth and neurocognitive development of children” (USDHHS 2004, p. 28), and in the 2006 Surgeon General's report's examination of the evidence related to exposure to secondhand smoke also found the evidence “inadequate” (USDHHS 2006).
Researchers have suggested that prenatal exposure to smoking impairs neurologic development and intellectual abilities through its effects on the central nervous system (reviewed by Bublitz and Stroud 2012). Although the results of studies of the effects of maternal smoking on cognitive development in infants and young children have been inconsistent, studies of the associations between maternal smoking and children's lower performance on assessments of verbal skills in general, as well as on specific language and auditory tests, have been more consistent (reviewed in USDHHS 2010). Postnatal exposure to secondhand smoke may also be important, but is difficult to separate from prenatal exposure, because the two are correlated.
Studies of the effects of prenatal smoking on infant brain structure and function in humans are limited (reviewed by Bublitz and Stroud 2012), but data from a large study of more than 5,000 pregnant women suggest that prenatal tobacco use had negative effects on fetal head growth and caused structural alterations in the cerebellum, consistent with cell loss (Roza et al. 2007). Studies of auditory brainstem responses (evoked by the brainstem and used as a measure of auditory function) in infants are consistent with the dysregulation of auditory processing associated with prenatal tobacco exposure (Peck et al. 2012), which could contribute to learning disabilities and language impairment. Studies of brain structure and function in older children with prenatal tobacco exposure are limited by the difficulty of accounting for tobacco exposure after birth and other potential confounders, such as an absence of repeated measurements of tobacco exposure from pregnancy through adolescence and the lack of prospective monitoring of developmental and behavioral outcomes (Bublitz and Stroud 2012).
Updated Evidence Reviews
Congenital Malformations
Major structural birth defects as well as other birth defects may occur because of a malformation, disruption, or deformation of one or more parts of the body, or they can result from a chromosomal abnormality. Major birth defects can have serious adverse effects on the health, development, or functional abilities of the affected child (Centers for Disease Control and Prevention [CDC] 2008). Each year, approximately 3% of newborns in the United States are born with major birth defects, and the prevalence of all major birth defects combined has remained relatively stable in the United States from the 1970s through recent years (CDC 2008).
To date, the evidence on smoking and birth defects has been most abundant and consistent for orofacial clefts. The 2004 Surgeon General's report found the evidence to be suggestive of a causal relationship between maternal smoking and orofacial clefts. The 2010 Surgeon General's report did not include conclusions related to causality. It did provide an update of studies related to smoking and orofacial clefts, which included results of a 2004 meta-analysis that found a small but positive association with maternal smoking for cleft lip with or without cleft palate (CL/P) and for cleft palate (CP) alone (Little et al. 2004a,b; USDHHS 2010). Subsequent to that meta-analysis, eight studies have shown positive associations between periconceptional maternal smoking and orofacial clefts (Little et al. 2004a; Bille et al. 2007; Romitti et al. 2007; Johansen et al. 2009; Leite and Koifman 2009; Shaw et al. 2009; Lebby et al. 2010; Zhang et al. 2011), one of which used midpregnancy cotinine levels to more accurately ascertain prenatal smoking exposure (Shaw et al. 2009); three of these (Little et al. 2004a; Honein et al. 2007 [contains data that overlap with Romitti et al. 2007]; Shaw et al. 2009) were reviewed in the 2010 Surgeon General's report. Although the magnitude of the association between periconceptional maternal smoking and orofacial clefts is relatively modest, this remains one of the most consistent findings in etiologic research on the causes of birth defects. In addition, despite some presumed misclassification of maternal smoking given that most studies rely on self-reported exposure, methodologic research suggests the finding is quite robust and corrections for likely levels of misclassification would result in somewhat higher effect estimates (MacLehose et al. 2009).
This section summarizes the evidence for associations between maternal prenatal smoking and specific birth defects. Topics include orofacial clefts, clubfoot, gastroschisis (abdominal wall defect), congenital heart defects, craniosynostosis (premature closure of cranial sutures), and anorectal atresia. This section also summarizes the 2010 Surgeon General's report review of specific genetic risk factors for congenital malformations, and their potential interactions with tobacco exposure in the etiology of birth defects.
Biologic Basis
The embryonic period is a time of rapid differentiation, and the developing organs are particularly susceptible to the effects of exogenous agents. The stage of embryonic development determines the embryo's susceptibility to environmental factors, and the embryo is most easily disturbed during the organogenesis period, from day 15 to day 60 after conception. In addition, each system or organ of an embryo has a critical period when its development may be altered. Tobacco smoke includes about 7,000 different compounds, many of which could have deleterious effects on a fetus during development and potentially cause major birth defects (Talbot 2008; Rogers 2009; USDHHS 2010). Specific constituents of concern include nicotine, CO, aromatic amines, and cadmium (Nelson 2001; Rogers 2009). The 2010 Surgeon General's report covered the biologic basis for injury to the fetus by maternal smoking at length.
Maternal smoking could interfere with normal organ development in offspring in several ways, including through fetal hypoxia, alterations in essential nutrients, teratogenic effects, and DNA damage. These effects could be related to exposure to tobacco smoke components such as CO, nicotine, cadmium, and PAHs (Chernoff 1973; Mochizuki et al. 1984; Lammer et al. 2004; Munger et al. 2004; Ziaei et al. 2005). In addition, certain populations with genetic polymorphisms may be more susceptible to damage attributable to exposure to tobacco smoke because of alterations in metabolic pathways (see the section “Smoking and Maternal and Fetal Genetic Polymorphisms” later in this chapter).
Nicotine has diverse pharmacologic and toxicologic properties, which are discussed in Chapter 5. The Office of Environmental Health Hazard Assessment of the California Environmental Protection Agency lists nicotine as a developmental toxicant. In addition, nicotine is a vasoconstrictor and is known to cross the placenta and concentrate in the fetus at levels slightly higher than those in the mother.
CO is a by-product of combustion and thus is present in tobacco smoke and is found in higher levels in smokers than nonsmokers (USDHHS 2010). CO is a potent toxin whose primary target organ is the brain, and the fetus is more susceptible to the toxic effects of CO than is the mother. Exposure to CO from which the mother will fully recover could end in permanent neurologic damage to the fetus or even death (e.g., stillbirth) (Norman and Halton 1990; Koren et al. 1991; Rogers 2009). The fetal effects of CO are well studied in animals (Koren et al. 1991; Penney 1996) and include central nervous system abnormalities in fetuses and pups of pregnant rats with long-term exposure to CO (Storm and Fechter 1985a,b; Storm et al. 1986; Fechter 1987; Carratù et al. 1993a,b; Packianathan et al. 1993). CO-induced hypoxia appears to be related to congenital anomalies including cleft lip and CP in susceptible strains of mice (Millicovsky and Johnston 1981a,b; Bronsky et al. 1986; Bailey et al. 1995). An association with cleft lip was demonstrated in a rat model in which the medication phenytoin was administered to pregnant rats to induce embryonic hypoxia (Webster et al. 2006). Subsequent human epidemiologic studies of birth defects in relation to CO levels from air pollution early in pregnancy found associations between higher CO levels and various cardiac defects, but the findings were not consistent (Ritz et al. 2002; Gilboa et al. 2005).
Thus, the evidence suggests likely impacts of nicotine and CO on fetal development. The combination of exposure to nicotine and hypoxia could decrease the supply of nutrients and oxygen to the embryonic tissues through a vasoconstrictive impact, resulting in congenital defects (Lambers and Clark 1996).
Tobacco smoke contains heavy metals, including cadmium, which is of particular concern because of its potential teratogenic effects (Chang et al. 1980; Carmichael et al. 1982). Cadmium crosses the placenta, and in animal studies has been associated with adverse effects on fetal growth (Carmichael et al. 1982; Goyer 1991) and orofacial clefts (Mulvihill et al. 1970; Ferm 1971; Chernoff 1973), limb reduction anomalies, central nervous system defects, and some other birth defects (Ferm 1971; Barr 1973; Carmichael et al. 1982; Goyer 1991).
Reductions in serum folate levels mediated by maternal smoking have also been associated with orofacial clefts (McDonald et al. 2002; Mannino et al. 2003; Ortega et al. 2004). This is further supported by two studies that found that intake of vitamins containing folic acid was associated with a decreased risk of orofacial clefts (Itikala et al. 2001; Bailey and Berry 2005). However, one large study—the National Birth Defects Prevention Study (NBDPS), a multisite population-based case-control study in 10 sites that began in 1997, did not observe an interaction between intake of folic acid and maternal smoking in the etiology of orofacial clefts (Honein et al. 2007). This is perhaps due in part because NBDPS largely enrolled cases conceived after folic acid fortification of enriched cereal grains in the United States when folic acid intake among all women was considerably higher than before fortification. However, the lack of interaction might also be due to the lack of an association between tobacco and folic acid preventable birth defects. Smoking has not been associated consistently with neural tube defects, an outcome causally associated with decreased folate and one that has been significantly reduced by folic acid fortification (Hackshaw et al. 2011).
PAHs are products of the partial combustion of carbon-containing materials and are found in tobacco smoke (International Agency for Research on Cancer 1986, 2004; U.S. Environmental Protection Agency 1992). Studies have reported direct fetotoxic and teratogenic effects associated with PAHs, as well as adverse effects on reproduction. Other effects include immunotoxicity, endocrine effects, and toxic effects on the lungs. The toxic effects and dose-response relationships described for PAHs are primarily based on experiments in animals. Lupo and colleagues (2012) reported an association between occupational PAH exposure and gastroschisis among mothers 20 years of age or older (OR = 2.53; 95% CI, 1.27–5.04), but no association among mothers younger than 20 years of age. The most commonly observed effects of PAHs in animal studies are growth retardation and fetal mortality, but a few experiments have demonstrated anatomic teratogenic effects. The number of surviving offspring is reduced in these experiments, so it appears that the dose-range over which surviving, but malformed, offspring are produced is narrow (USDHHS 2010).
Description of the Literature Review
To update the epidemiologic literature on smoking and birth defects, a comprehensive literature search was undertaken using PubMed to capture English-language publications from 1999 through July 2012. The studies included in the review presented the outcome of either all birth defects or specific types of birth defects, and their potential association with maternal smoking, paternal smoking, or maternal exposure to secondhand smoke. Search terms included the following: (1) smoking and defect, (2) smoking and cleft, (3) smoking and heart defect, (4) smoking and gastroschisis, (5) smoking and cryptorchidism, (6) smoking and atresia, (7) smoking and congenital, (8) smoking and clubfoot, (9) smoking and renal, (10) smoking and craniosynostosis, (11) smoking and hypospadias, (12) tobacco and defect, (13) tobacco and cleft, (14) tobacco and heart defect, (15) tobacco and gastroschisis, (16) tobacco and cryptorchidism, (17) tobacco and atresia, (18) tobacco and congenital, (19) tobacco and clubfoot, (20) tobacco and renal, (21) tobacco and craniosynostosis, (22) tobacco and hypospadias, (23) smoking and malformation, and (24) tobacco and malformation. Additional articles were identified by examining the reference lists of articles identified by these searches, by searching PubMed for specific investigators, and by reviewing previous Surgeon General's reports.
Methodologic Considerations
Several methodologic challenges need to be addressed in studies of maternal smoking and congenital malformations. Case definitions can be heterogeneous across studies, but most authors attempt to remove cases associated with syndromes from the analysis. Isolated defects are sometimes studied alone, and other times they are combined with multiple defects: defined as those affected by two or more major defects in different organ systems (Rasmussen and Moore 2001). In case-control studies, periconceptional smoking status is generally obtained following delivery and after women have knowledge of their child's congenital malformation, introducing possible bias due to differential disclosure of smoking status among mothers with affected versus unaffected children. When suspected, confounding needs to be addressed through adjusted, matched, or stratified analysis. In meta-analyses, it is often difficult to fully account for confounding with the variation in treatment of confounders across studies. Finally, the selection of control groups could affect the results of case-control studies. For example, the selection of control groups with conditions also potentially associated with maternal tobacco use, such as other types of malformations, could result in bias.
Epidemiologic Evidence
Tables 9.2 and 9.3S–9.8S summarize studies that examined prenatal maternal smoking as a risk factor for specific defects. Defects include orofacial clefts, clubfoot, gastroschisis, congenital heart defects, craniosynostosis, and anorectal atresia. The following sections summarize the key findings from those studies. Because Little and colleagues (2004b) completed a meta-analysis of publications through 2001, specific studies are reviewed for orofacial clefts for 2002 through July 2012 (Table 9.3S). For all other defects, the literature is reviewed from 1999 through July 2012 (Tables 9.4S–9.8S).
Table 9.2
Summary of a systematic review of maternal smoking during pregnancy and its relationship with specific congenital malformations.
Orofacial Clefts
CL/P and CP are birth defects that occur when the upper lip or the palate do not close correctly during fetal development. With a cleft lip, the tissue that makes up the lip does not join completely between the fourth and seventh week of pregnancy. With CP, the tissue that makes up the palate (roof of the mouth) does not join correctly between the sixth and ninth week of pregnancy. CL/P and CP are embryologically distinct entities, and these phenotypes have somewhat different epidemiologic characteristics. For example, CL/P is more frequent among males and CP more frequent among females; CP is less common among Hispanics than other racial/ethnic groups, but this difference is not present for CL/P (Genisca et al. 2009). Studies that assessed CL/P and CP separately for their potential association with maternal smoking found that the risk estimates for the two defect types were generally similar (Little et al. 2004a; Krapels et al. 2006; Bille et al. 2007; Honein et al. 2007).
A 2011 systematic review and meta-analysis by Hackshaw and colleagues included publications from 1959–2010. Of the 38 case-control and cohort studies, 13 showed a significant association between maternal smoking and an increased risk for CL/P, and the pooled OR was 1.28 (95% CI, 1.20–1.36). Restriction of the analysis to prospective studies did not change the findings (OR = 1.24; 95% CI not included), nor did restricting to studies with AORs (1.26; 95% CI, 1.18–1.34). The earlier meta-analysis of orofacial clefts by Little and colleagues (2004b) still contributes to this knowledge base since it included several studies that were excluded from the Hackshaw meta-analysis (Kelsey 1978; Hwang et al. 1995; Lieff 1999).
In their meta-analysis, Little and colleagues (2004b) included 24 case-control and cohort studies published between 1974–2001. The authors found a consistent association between maternal smoking and CL/P (RR = 1.34; 95% CI, 1.25–1.44) and between smoking and CP (RR = 1.22; 95% CI, 1.10–1.35). Most of the studies in this meta-analysis included consideration of confounders, such as maternal age and education; and some adjusted for partity, marital status, and race/ethnicity; and 5 addressed maternal alcohol use. Adjusted relative risks in individual studies were generally similar to crude RRs, but crude RRs were included in the meta-analysis unless only adjusted RRs were available. When the analysis was restricted to studies with isolated CL/P, or to studies that did not use controls with malformations, the findings did not change. Five of 9 studies with information about the number of cigarettes smoked per day showed evidence of a weak dose-response relationship for CL/P. Eight studies were examined for a dose-response relationship with CP; no clear evidence was observed.
Two recent studies of orofacial clefts and smoking that showed significant positive associations, and were not included in the Hackshaw meta-analysis, further strengthen the evidence for this relationship (Shaw et al. 2009; Lebby et al. 2010). One of the largest studies (Romitti et al. 2007) used NBDPS, which included data from interviews with 1,128 cases of persons with orofacial clefts. For all orofacial clefts combined, the crude OR (1.37; 95% CI, 1.20–1.57) was very similar to the ORs in both the 2004 and 2011 meta-analyses (Little et al. 2004b; Hackshaw et al. 2011), and included adjustment for folic acid, study site, prepregnancy obesity, alcohol use, gravidity, maternal age, maternal education, and maternal race/ethnicity.
As previously discussed, most studies of maternal smoking and CL/P are case-control studies, and necessarily rely on maternal self-reported smoking history obtained after delivery. This method for obtaining exposure data can result in bias if women who smoked and had adverse pregnancy outcomes are more or less likely to deny smoking than women who smoked and did not have adverse outcomes. However, in a study of the potential contributions of misclassification of maternal smoking status on studies of CL/P using Bayesian models with and without correction for reporting bias, the authors found that associations between maternal smoking and CL/P was strengthened slightly and remained significant after correction for expected levels of bias (MacLehose et al. 2009). In addition, despite other potential threats to validity, the findings on orofacial clefts and smoking have been quite consistent across study design and location.
A study in California used midpregnancy serum cotinine concentration (a metabolite of nicotine) to classify maternal smoking status (Shaw et al. 2009). Active smoking was defined as serum cotinine concentration of at least 2 ng/mL, and 11 exposed cases were identified. Maternal smoking was found to be associated with CL/P before (OR = 2.1; 95% CI, 1.0–4.4) and after adjusting for maternal age, race, and serum folate level (AOR = 2.4; 95% CI, 1.1–5.3). It is unknown to what extent the use of midpregnancy cotinine levels results in the misclassification of early pregnancy smoking; women still smoking in the middle of pregnancy were presumably also smoking in early pregnancy, but some of those smoking in early pregnancy might have stopped by the middle of pregnancy and therefore would be inappropriately included in the unexposed group, potentially attenuating the effect estimate. Although the sample size was relatively small, this key study with a biomarker for smoking exposure helps support the many consistent reports of weak associations between smoking and orofacial clefts based on self-reported smoking exposure in early pregnancy. The findings regarding a possible dose-response relationship between smoking and the risk for orofacial clefts have been mixed, with some studies finding evidence of a positive relationship (Little et al. 2004a,b; Bille et al. 2007; Honein et al. 2007) but not all (Krapels et al. 2006; Grewal et al. 2008).
The findings on exposure to secondhand smoke and orofacial clefts have been inconsistent, but there is some evidence of risk in settings where maternal smoking is relatively uncommon but exposure to secondhand smoke is high. For example, in China, the prevalence of smoking among reproductive age women is low (1.5%), while exposure to secondhand smoke is high (over 50%) (CDC 2012). Three studies conducted in China reported positive associations between maternal exposure to secondhand tobacco smoke and orofacial clefts; one included adjustment for potential confounders (occupation, flu or fever, and infant gender) (Table 9.3S) (Li et al. 2010; Jia et al. 2011; Zhang et al. 2011).
Idiopathic Talipes Equinovarus (Clubfoot)
Idiopathic talipes equinovarus or clubfoot is a serious birth defect that requires medical treatment and often surgery (Table 9.4S). There are some complexities in accurately capturing clubfoot with surveillance or research studies, and appropriately excluding those with positional foot deformities. However, despite these challenges, the findings for maternal smoking and clubfoot have been quite consistent. Six of eight studies published in 2000 or later found significant, positive associations between maternal smoking and the occurrence of congenital clubfoot (Honein et al. 2000, 2001; Skelly et al. 2002; Dickinson et al. 2008; Parker et al. 2009; Kancherla et al. 2010); three of these six studies also reported evidence of a dose-response relationship with smoking level, one did not find a dose-response relationship, and two did not assess dose.
In the study by Honein and colleagues (2000), the effect of smoking varied by self-reported family history. The OR of clubfoot associated with maternal smoking among those without a family history was 1.34 (95% CI, 1.04–1.72), but the effect estimate was much stronger, albeit imprecise (OR = 20.3; 95% CI, 7.9–52.2), for those with a positive first-degree family history. Skelly and colleagues (2002) also used self-reported smoking and found a dose-response relationship: among women who smoked 20 or more cigarettes daily, the OR for clubfoot was 3.9 (95% CI, 1.6–9.2), but among those who smoked fewer than 10 cigarettes daily it was 1.5 (95% CI, 0.9–2.5).
Dickinson and colleagues (2008) analyzed linked data from the North Carolina Birth Defects Monitoring Program and North Carolina birth certificates and health services and found a significant association between maternal smoking and clubfoot (OR = 1.40; 95% CI, 1.07–1.83), when controlling for maternal age, race/ethnicity, infant's gender, and timing of prenatal care initiation; the authors did not find a dose-response relationship. Parker and colleagues (2009) used 2001–2005 data from 10 population-based surveillance systems in the United States. Information on smoking was obtained from birth certificates and was linked to data on birth defects from surveillance systems. The ORs for clubfoot were 1.45 (95% CI, 1.32–1.60) for women who smoked 1–10 cigarettes per day and 1.88 (95% CI, 1.64–2.14) for women who smoked more than 10 cigarettes per day (Parker et al. 2009). Kancherla and colleagues (2010) analyzed population-based surveillance data from the Iowa Registry for Congenital and Inherited Disorders. The study found that maternal smoking was associated with clubfoot with an OR of 1.5 (95% CI, 1.2–1.9). It is important to note that there is a small overlap between the Parker and colleagues 2009 study (10-state analysis) and the Kancherla and colleagues 2010 study (Iowa analysis) since Iowa is 1 of 10 states included in the paper by Parker and colleagues and some of the years overlap.
Hackshaw and colleagues (2011) pooled data from 12 studies on clubfoot and smoking and found an OR of 1.28 (95% CI, 1.10–1.47) (Table 9.2). When restricted to studies that addressed potential confounders, the results were not substantially different from the pooled analysis of all studies (OR = 1.44; 95% CI, 1.20–1.71).
Gastroschisis
Gastroschisis is a congenital defect in which the abdominal contents protrude through an opening in the anterior abdominal wall. It is strongly associated with young maternal age (Rasmussen and Frias 2008). From 2000–2011, 12 studies have investigated the potential association between maternal smoking and gastroschisis and 8 reported a significant association (Table 9.5S).
A retrospective case-control study conducted from 1995–1999 in the United States and Canada examined the association of gastrochisis with maternal smoking using control infants with malformations or who were hospitalized for other reasons. The study found maternal smoking during the first 2½ months of pregnancy to be associated with gastroschisis (Werler et al. 2003), and the authors also observed evidence of a dose-response relationship. Feldkamp and colleagues (2008) used birth certificates data to assess smoking and they used birth defects surveillance data to identify cases of gastroschisis. When adjusted for maternal age and prepregnancy body mass index (BMI), the authors found a significant association between smoking during the first trimester and gastroschisis (Feldkamp et al. 2008).
Werler and colleagues (2009) compared cases and age-matched controls from NBDPS to study maternal smoking and gastroschisis. The overall AOR was 1.5 (95% CI, 1.2–1.9), and a dose-response relationship was observed. After stratification by maternal age, however, the association was present only in women who were 25 years of age or older (Werler et al. 2009). This interaction between smoking and maternal age was similar to the one described by Feldkamp and colleagues (2008). More recently, an analysis based on birth certificate data from Washington state found an association between smoking during pregnancy and gastroschisis after adjusting for birth year, maternal age, race, urban-rural residence, county of residence, paternal age, and baby's gender (Chabra et al. 2011).
Finally, Hackshaw and colleagues (2011) included 12 published studies on gastroschisis in their meta-analysis and found a significant association with maternal smoking (OR = 1.50; 95% CI, 1.28–1.76) (Table 9.2). When restricted to studies that addressed confounding, the authors found similar results (OR = 1.44; 95% CI, 1.20–1.71).
Congenital Heart Defects
Congenital heart defects are the most common type of birth defect, affecting nearly 1% of births in the United States, and including many specific types of congenital heart defects with relatively high morbidity and mortality (Reller et al. 2008). From 1999–2012, 15 published studies evaluated the association between smoking and congenital heart defects, and 9 reported significant assocations for one or more types of specific heart defects (Table 9.6S). The most consistent finding has been an associations between maternal smoking and atrial septal defects reported by 4 studies (Källén 1999a; Malik et al. 2008; Kučienė and Dulskienė 2010; Alverson et al. 2011).
Data from the Swedish Child Cardiology and Medical Birth Registries were used to assess the associations of maternal smoking with 30 categories of congenital heart defects (Källén 1999a). In this study, maternal smoking was ascertained during the first prenatal visit rather than after the pregnancy outcome was known, reducing the potential for recall bias. Significant associations were seen for transposition of the great arteries, atrial septal defects, and for patent ductus arteriosus in full-term infants. The author did not observe a dose-response relationship for the association with patent ductus arteriosus (Källén 1999a).
In the Baltimore-Washington Infant Study, the authors assessed risk factors for single ventricle defects and found ORs above unity for both maternal and paternal smoking. These findings were not significant, however, and they were not adjusted for potential confounders (Steinberger et al. 2002). A more recent analysis from the Baltimore-Washington Infant Study assessed all congenital heart defects without other birth defects and found associations between maternal smoking and secundum-type atrial septal defects, right outflow tract defects, l-transposition of the great arteries, and truncus arteriosus (Alverson et al. 2011).
Data from NBDPS was used by Malik and colleagues (2008) to study the relationship between smoking and various heart defects; the authors found that atrial septal defects were associated with smoking at all levels of exposure (1–14, 15–24, ≥25 cigarettes/day) but no dose-response relationship was observed. Other congenital heart defect phenotypes were also assessed, but did not show evidence of associations with maternal smoking. Baardman and colleagues (2012) found evidence of interactions between smoking and BMI ≥25 for all congenital heart defects (p = 0.027), septal defects (p = 0.036), conotruncal defects (p = 0.020), and for outflow tract anomalies (p = 0.024).
Hackshaw and colleagues (2011) combined all cardiovascular and congenital heart defects, but did not present results for specific phenotypes. Overall, the pooled OR from 25 published studies showed a small but significant elevation in risk for congenital heart defects (OR = 1.09; 95% CI, 1.02–1.17) (Table 9.2). When the analysis was restricted to studies that addressed confounding, the results were similar to the original analysis in both instances (OR = 1.10; 95% CI, 1.02–1.20).
Craniosynostosis
Premature fusion of one or more of the cranial suture of the skull results in craniosynostosis, a serious birth defect that usually requires surgical correction. Without timely treatment, craniosynostosis can result in serious consequences including restriction of brain growth. The critical timing of fetal exposure is unclear, but might extend beyond early pregnancy. Several publications have addressed the possible association between maternal smoking and craniosynostosis (Table 9.7S). A birth defects registry linkage study found an association between smoking and craniosynostosis among isolated cases (OR = 1.67; 95% CI, 1.27–2.19) (Källén 1999b). In addition, the author saw evidence of a dose-response relationship with smoking and differences in effects for different cranial sutures; the highest OR was observed if the sagittal suture was affected (Källén 1999b).
In the United States, maternal smoking was associated with isolated craniosynostosis in a metropolitan Atlanta, Georgia, population (OR = 1.92; 95% CI, 1.01–3.66) (Honein and Rasmussen 2000). In contrast, a case-control study using NBDPS data did not find a significant association (Carmichael et al. 2008). In the same study, for heavy smoking in the third month of pregnancy and in the second trimester, moderately increased ORs were observed (OR = 1.6; 95% CI, 0.9–2.6; and OR = 1.6; 95% CI, 0.9–2.8, respectively).
In The Netherlands, a study of infants with sagittal synostosis did not find an association with maternal smoking (Butzelaar et al. 2009). Hackshaw and colleagues (2011) analyzed five studies and found a positive association between smoking and craniosynostosis (OR = 1.33; 95% CI, 1.03–1.73) (Table 9.2). When the analysis was restricted to studies that addressed confounding, the findings did not change (OR = 1.33; 95% CI, 1.04–1.63).
Anorectal Atresia
Anorectal atresia is a defect that occurs when there is faulty separation of the rectum and urogenital system or failure of the anal membrane to rupture (Stevenson 1993). Four studies have been published since 1999 (Table 9.8S); one reported a significant association, two reported borderline/nonsignificant associations, and one found an association with paternal but not maternal smoking. Hackshaw and colleagues (2011) reviewed seven papers and reported a positive association (OR = 1.20; 95% CI, 1.06–1.36) (Table 9.2). In addition, a recent meta-analysis of risk factors for anorectal malformations reported paternal smoking as a risk factor (pooled OR = 1.53; 95% CI, 1.04–2.26) (Zwink et al. 2011).
Other Defects
There have been some studies of central nervous system defects, including neural tube defects, but case definitions have varied and most have not shown an association with maternal smoking (To and Tang 1999; Suarez et al. 2008, 2011; Van Landingham et al. 2009; Miller et al. 2010; Yin et al. 2011). Hackshaw and colleagues (2011) analyzed 17 studies and found no association between maternal smoking and anencephaly/spina bifida, but the authors found a small association with all central nervous system defects combined (pooled OR = 1.10; 95% CI, 1.01–1.19). When the analysis was restricted to studies that addressed confounding, the association was no longer signficant (OR = 1.13; 95% CI, 0.99–1.28).
Cryptorchidism or undescended testes commonly occurs with prematurity, but is typically only monitored by birth defects surveillance systems among term infants. However, it is unclear if all studies examining the potential association between cryptorchidism and smoking limited their analyses to term infants. Although a weak association between maternal smoking and cryptorchidism has been described in some studies (Akre et al. 1999; Biggs et al. 2002), other more recent studies have not reported an association (Pierik et al. 2004; Kurahashi et al. 2005a; Damgaard et al. 2008) or noted an association only among mothers who smoked heavily (Thorup et al. 2006; Jensen et al. 2007). Hackshaw and colleagues (2011) analyzed 18 studies and found a small but significant elevation in risk of cryptorchidism from maternal smoking (OR = 1.13; 95% CI, 1.02–1.25). When the analysis was restricted to studies that addressed potential confounding, the findings did not change (OR = 1.16; 95% CI, 1.08–1.25). However, the observed effect might be due at least in part to prematurity and the association between tobacco exposure and preterm birth.
Hypospadias is a birth defect in boys in which the opening of the urethra is not located at the tip of the penis, and there are different degrees of hypospadias ranging from first degree (relatively minor) to second and third degree (more severe). Most of the studies to date have not found significant associations between maternal smoking and hypospadias, and a few studies have found an inverse association (Källén 2002; Pierik et al. 2004; Carmichael et al. 2005; Brouwers et al. 2007). Hackshaw and colleagues (2011) analyzed 15 studies and found a small negative association between smoking and hypospadias (OR = 0.90; 95% CI, 0.85–0.95). When the analysis was restricted to studies that addressed confounding, the findings did not change (OR = 0.89; 95% CI, 0.83–0.96).
Smoking and Maternal and Fetal Genetic Polymorphisms
Studies of the differences in the human metabolism of toxic constituents in tobacco smoke are summarized in the 2010 Surgeon General's report (Benowitz et al. 1999; Lee et al. 2000; Yang et al. 2001; USDHHS 2010). Initial investigations of the mechanisms of maternal or fetal metabolism of tobacco smoke toxins and adverse birth outcomes were conducted in studies of birth defects, and several studies examined the potential interaction of maternal exposure to tobacco smoke and maternal and/or neonatal genotypes in association with orofacial cleft in newborns. The genetic polymorphisms that code for the expression inflammatory response and immune mediator enzymes and that were examined included TGF-α and TGF-β3, MSX1, and EPHX1, as well as gene variants of both phase I activation and phase II detoxification enzymes CYP1A1, GSTM1, GSTT1, NAT1, and NAT2. Prenatal exposure to tobacco smoke was typically measured by maternal self-reports of active smoking, exposure to secondhand smoke, and of paternal active smoking. Most of these studies examined the TGF-α genotype in neonates. In one study, genotyping was performed in both neonates and parents.
A case-control study of infants with a TGF-α *TAQ1 genotype that contained a rare allele and whose mothers had smoked during pregnancy found a significantly elevated risk for CP in offspring (Hwang et al. 1995). In a large population-based case-control study conducted by the California Birth Defects Monitoring Program registry, the risks of CP and CL with or without CP were significantly elevated among White infants with TGF-α *rare genotypes (*A2) whose mothers were heavy smokers (Shaw et al. 1996). However, three subsequent case-control studies (Christensen et al. 1999; Romitti et al. 1999; Beaty et al. 2001) that failed to replicate these findings had fewer cases and one study used a lower cutpoint for smoking than that used by Shaw and colleagues (1996). None of the five studies cited above presented regression models with terms for estimating maternal smoking levels and the TGF-α genotype interactions. Zeiger and colleagues (2005) conducted a meta-analysis of data from these 5 studies and found a marginally significant interaction between maternal smoking and infant TGF-α *allele genotypes (*A2) in relation to CP (OR = 1.95; 95% CI, 1.22–3.10). A Human Genome Epidemiology review that assessed 47 published studies on the potential association between TGF-α and orofacial clefts produced somewhat inconsistent findings (Vieira 2006), but concluded that TGF-α likely had a role in modifying the risk of orofacial clefts.
Romitti and colleagues (1999) also examined the TGF-β3 genotype and maternal smoking in relation to the risk of CP or CL/P. These researchers found a significantly elevated risk for the conditions among infants who were homozygous for the common *1 allele at the X5.1 or 5′UTR.1 site and whose mothers had smoked 10 or more cigarettes per day. There was no evidence of an interaction for infant genotypes that included the rare *2 allele.
Hartsfield and colleagues (2001) did not observe any significant interaction between maternal smoking and null GSTM1 genotypes in a case-control study of isolated cleft lip and CP. van Rooij and colleagues (2001) examined the association of maternal prenatal smoking and the maternal GSTT1 genotype and found that mothers who smoked and carried the GSTT1 null genotype had a marginally higher risk for delivering an infant with oral clefting than that of nonsmokers who carried the wild-type genotype. Although the RR was not statistically significant, it was almost five times greater when both mothers and their infants carried the GSTT1 null genotype. There was no evidence of an interaction between maternal smoking and the CYP1A1 genotype with a recessive allele in relation to oral clefting.
In a case-control study, the CYP1A1, GSTT1, and GSTM1 polymorphisms were also examined as risk factors for hypospadias (Kurahashi et al. 2005b). The study did not observe any increased risk of hypospadias among children born to mothers who smoked and had various genotypes, including CYP1A1 *MSPI variant allele genotype or the GSTT1 null genotype or GSTM1 null genotype. In a case-only, haplotypic analysis of an intronic CA repeat of the MSX1 gene in 206 infants with oral clefting, there was evidence for an interaction with maternal prenatal smoking (Fallin et al. 2003). In the Iowa study (Romitti et al. 1999), infants whose MSX1 X1.3 or MSX1 X2.4 genotype contained the *2 allele and whose mothers smoked 10 or more cigarettes per day also had a significantly elevated risk of CP. In a study of limb deficiency defects, Carmichael and colleagues (2004) did not observe any significantly elevated risk for infants with MSX1 intronic CA repeat genotype whose mothers smoked during pregnancy. In another case-control study from the California Birth Defects Monitoring Program, the NAT1 1088 genotype *A/*A and the NAT1 1095 genotype *A/*A, but not NAT2 polymorphisms, were strongly associated with isolated oral clefting in infants whose mothers had smoked during pregnancy (Lammer et al. 2004).
Evidence Synthesis
A modest but consistent association has been documented between maternal smoking during early pregnancy and orofacial clefts, and the evidence has continued to accumulate and strengthen since the 2004 Surgeon General's report. The literature is diverse and includes observations from cohort and case-control studies, and meta-analyses have produced significant pooled risk estimates even after restricting to cohort studies or studies which addressed confounding. One study with the advantage of incorporating biomarkers to objectively assess maternal smoking exposure showed a more than twofold increased risk of orofacial clefts with maternal smoking exposure. However, the results of studies examining a dose-response relationship between smoking and orofacial clefts have been mixed. Two cohort studies that both collected tobacco exposure data before delivery support the notion that a temporal relationship exists, showing an effect of maternal smoking during the time period critical for closure of the palate. Case-control studies often have significantly more power to detect risk factors for birth defects and have supported the association between maternal smoking and orofacial clefts. The plausibility of an association between maternal smoking and orofacial clefts is further supported by animal studies showing an association between cadmium and clefting and between hypoxia-inducing compounds and clefting.
The relatively weak associations described in observational studies and the lack of evidence for a dose-response relationship could reflect exposure misclassification in which some women who smoke do not disclose their smoking status, attenuating the magnitude of the effect estimate. Alternatively, a weak association could result if only certain subgroups of the population, such as those with specific genetic risk factors, are at increased risk of orofacial clefts from exposure to maternal smoke. Given the strong association with a positive family history of orofacial clefts, there are likely some genetic factors that have a major impact on risk of clefting and potentially on the association between smoking and orofacial clefts.
Although the evidence base regarding other major birth defects is growing, associations with maternal smoking are less clear. The evidence has been relatively consistent for clubfoot and gastroschisis (Tables 9.4S and 9.5S), and has been somewhat less consistent for congenital heart defects, craniosynostosis, and anorectal atresia (Tables 9.6S–9.8S). The studies have been most consistent for clubfoot, but the diagnosis of this defect and ascertainment for studies can be problematic as some positional foot deformities might be erroneously included as “clubfoot”; and there could be some selection bias in who is identified as having clubfoot rather than a less serious positional foot deformity, such as if those with better access to high-quality care are more likely to have an accurate diagnosis.
Many studies reported significant associations between maternal smoking and congenital heart defects, but the findings are not consistent across the specific phenotypes. The most consistent finding to date is for an association between atrial septal defects and maternal smoking. However, the relationships between maternal smoking and these adverse outcomes deserve further study to better understand which of these outcomes might potentially be prevented.
The published data on the role of specific genetic risk factors and their interactions with maternal smoking in the etiology of birth defects has expanded over the past decade, but there has been little consistency across studies. The literature is most extensive for orofacial clefts, but it remains unclear which genes might be most important in the causal pathways associated with smoking. At this point, there are no specific genes for which the evidence is strong enough to conclude that they clearly modify the relationship between smoking and orofacial clefts. The data on gene-environment interactions for other major birth defects is much more limited than that for orofacial clefts.
Conclusions
- The evidence is sufficient to infer a causal relationship between maternal smoking in early pregnancy and orofacial clefts.
- The evidence is suggestive but not sufficient to infer a causal relationship between maternal smoking in early pregnancy and clubfoot, gastroschisis, and atrial septal heart defects.
Implications
Mothers who smoke early in pregnancy increase their risk for having an infant with an orofacial cleft. Although the attributable fraction for this exposure might be quite low, this is a completely preventable cause of a major birth defect. This risk might be greater in women with specific genetic risk factors, but research to date has not identified consistent genetic factors modifying this relationship. Efforts to reduce smoking before conception and during early pregnancy should include the provision of information on the risk of orofacial clefts.
Neurobehavorial Disorders of Childhood
This section reviews the evidence for associations between prenatal smoking and a set of neurobehavioral disorders of childhood. Previous Surgeon General's reports have considered exposure to secondhand smoke during childhood and maternal smoking during pregnancy and their effects on neurodevelopmental outcomes of children (USDHEW 1979; USDHHS 1980, 2004, 2006, 2010). However, this review goes a step further by examining prenatal exposure to tobacco smoke and these specific disorders—attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder, anxiety disorders, depression, Tourette syndrome, schizophrenia, and intellectual disability.
Biologic Basis
There are multiple biologic mechanisms through which prenatal smoke exposure could affect risk for neurobehavioral disorders in the offspring. Most research on biologic mechanisms focuses on the impact of smoking-related compounds on placental development and on nicotine's effects on the fetal brain. The effects of prenatal smoking by the mother on the placenta are described in the 2010 Surgeon General's report and include cellular and molecular abnormalities of the villous system that could lead to impaired exchange between the mother and fetus of metabolic products, oxygen, and nutrients (USDHHS 2010). A substantial body of animal research demonstrates the effects of maternal nicotine exposure on offspring neurodevelopment, including promotion of neural cell replication, initiation of a switch from cell replication to differentiation, enhancement or retardation of axonogenesis or synaptogenesis, and disruption of regulation of apoptosis (reviewed by Pauly and Slotkin 2008) (see Chapter 5). In addition, animal studies have shown associations between prenatal nicotine exposure and behavioral abnormalities also seen in children of smokers, including hyperactivity, cognitive impairment, increased anxiety, somatosensory deficits, changes in sensitivity to nicotine and other psychostimulants, and alterations in nicotine self-administration (Herrmann et al. 2008; reviewed by Pauly and Slotkin 2008). Animal studies have generally shown positive associations between prenatal exposure to nicotine and anxiogenic behavior, as well as some evidence of neurodevelopmental changes, supporting a biologic basis for this association. A 2008 review concluded that the association between prenatal nicotine exposure and anxiogenic behavior is strong in rats, but that additional research is needed to establish a link in humans (Winzer-Serhan 2008). Causal relationships between smoking and long-term cognitive and behavioral outcomes in humans are difficult to establish due to numerous potential confounding factors (Goriounova and Mansvelder 2012).
Description of the Literature Review
A systematic literature review was conducted to identify potentially relevant published research evaluating the relationship between prenatal smoking exposure and the selected neurobehavioral disorders of interest. References and abstracts were extracted from PubMed using key words for the disorders and associated MeSH terms (Table 9.9) as well as the smoking-related key words “maternal smoking.” The time period of study, 2000–2012, was chosen to cover the period following that of the previous Surgeon General's reviews on neurocognitive development, which included reference to neurobehavioral disorders (USDHHS 2004).
Table 9.9
Key words used in the systematic literature review of prenatal smoking and neurobehavioral disorders among offspring.
Epidemiologic Evidence
Disruptive Behavioral Disorders
A large number of studies have evaluated the association between prenatal tobacco smoke exposure and disruptive behavioral disorders in children, specifically ADHD, ODD, and conduct disorder (Table 9.10S). In total, 82 studies addressing the relationship between childhood disruptive behavioral disorders, or disruptive symptoms, and prenatal tobacco smoke exposure were identified and included in the review.
In addition to the 82 studies, several systematic reviews and meta-analyses have been conducted that synthesize the large number of studies that assess the association between prenatal smoking exposure and ADHD. Langley and colleagues conducted a meta-analysis of studies published before June 2005 and ultimately included 5 case-control studies that covered a total sample of 1,265 participants; the researchers reported a pooled OR of 2.40 (95% CI, 1.61–3.52) for an ADHD diagnosis among the children of mothers who smoked during pregnancy (Langley et al. 2005). Langley and colleagues further concluded that there is a dose-response relationship between the number of cigarettes smoked and ADHD symptoms. In a later study, the authors studied offspring conceived with assisted reproductive technologies and compared those who were genetically related and unrelated to the woman who underwent the pregnancy (Thapar et al. 2009). They anticipated that the association between maternal smoking and ADHD would persist regardless of whether mother and offspring were related. They found that the magnitude of the association between prenatal smoking and parent-reported ADHD symptoms was significantly higher in the related pairs than in the unrelated pairs, suggesting that the previously observed association between maternal smoking in pregnancy and ADHD could be due to unrecognized confounding related to heritability.
Two systematic reviews concluded that there may be an association between prenatal smoking exposure and childhood ADHD (Linnet et al. 2003; Latimer et al. 2012). In 2003, Linnet and colleagues reviewed the findings of 6 case-control studies and 18 cohort studies. They concluded that there may be an association between exposure to tobacco smoke in utero and ADHD and ADHD symptoms, but that a more definite conclusion could not be made with the evidence available at the time due to methodologic issues of the reviewed studies. These issues pertained to the retrospective report of exposure information, dichotomization of exposure, selective attrition, low statistical power, poor definition of the outcome of interest (ADHD), and a failure to control for potentially relevant confounders. More recently, Latimer and colleagues (2012) reviewed literature published between 1966–2009 that evaluated the relationship between disruptive behavioral disorders and environmental risk factors, including maternal smoking. The authors noted there was a large volume of literature on ADHD, and that despite methodologic limitations (including exposure measures that are highly susceptible to recall bias and the lack of adjustment for relevant sociodemographic confounders), the literature provides some evidence of a link between prenatal smoking and the presence of disruptive behavioral disorders in children. Hence, the authors of two systematic reviews concurred that there is some evidence for an association between prenatal smoking and disruptive behaviors in offspring, such as those associated with ADHD.
Seventy studies document an association between prenatal smoking exposure and disruptive behavioral symptoms or disorders, including hyperactivity (Table 9.10S). Most controlled for potential confounding variables, but many studies suffered from the methodologic limitations listed by Linnet and colleagues (2003) and Latimer and colleagues (2012), above. Retrospective reporting of prenatal smoking exposure and failure to rigorously assess child outcomes were among the methodologic limitations of these studies. A notable exception is a nested case-control study of 3,965 Danish children that were matched on age, gender, and date of birth, which used diagnoses from medical records and assessed prenatal smoking during pregnancy (Linnet et al. 2005). Linnet and colleagues concluded that, controlling for other risk factors, prenatal smoking increased the risk for hyperkinetic disorder (the ICD equivalent of ADHD: RR = 1.9; 95% CI, 1.3–2.8).
A dose-response relationship was also reported by Koshy and colleagues (2011) in a study of 1,074 school-aged children. This study found an association between parents' retrospective reports of the number of cigarettes smoked during pregnancy and current parent-reported ADHD diagnosis among their children, although the CIs were wide (smokers: OR = 3.19; 95% CI, 1.08–9.49; heavy smoker: OR = 10.03; 95% CI, 1.62–61.99).
A number of studies have directly considered genetic factors and suggest an independent contribution of genetic factors to the association between maternal smoking and ADHD (Maugham et al. 2004; Knopik et al. 2006; D'Onofrio et al. 2008; Thapar et al. 2009; Lindblad and Hjern 2010; Obel et al. 2011; Langley et al. 2012). As reviewed earlier, Thapar and colleagues (2009) used a sample of children conceived through assisted reproductive technologies and demonstrated that the association between smoking during pregnancy and ADHD symptoms was stronger and statistically significant only among biologically related mother-child dyads. Langley and colleagues (2012) documented independent associations between ADHD in offspring and both maternal and paternal smoking, suggesting that ADHD was due to genetic or household-level confounding and not only intrauterine effects. However, researchers suggest that there is a strong likelihood of genetic and socioeconomic confounding, because parents with ADHD are more likely to fall in lower socioeconomic strata with this association strongly confounded by socioeconomic factors (Lindblad and Hjern 2010).
The evidence for maternal smoking exposure and ADHD overlaps substantially with that of the other disruptive behavioral disorders (ODD and conduct disorder) and much of the research is relevant for all three behavioral disorders, due in part to phenotypic overlap. Because the majority of studies assess the association between prenatal smoking exposure and disruptive behavioral disorders or symptoms collectively, the current epidemiologic evidence may not fully characterize the independent risks for ADHD, ODD, conduct disorder, and associated symptoms. There are several notable additions to the ADHD literature that focus on ODD and conduct disorder independent of ADHD (Wakschlag et al. 2002, 2006a,b, 2010; Nigg and Breslau 2007; Becker et al. 2008; Boden et al. 2010). For example, Boden and colleagues (2010) documented a strong association between prenatal smoking exposure and ODD and conduct disorder in a birth cohort of 926 children, evaluated for ODD and conduct disorder at 14–16 years of age and smoking exposure assessed at birth (p <0.01 for both outcomes). In a longitudinal study of 823 school-aged children, Nigg and Breslau (2007) noted that after controlling for other risks, retrospective report of prenatal smoking doubled the risk of ODD and conduct disorder; the adjusted association with ADHD was not statistically significant. In summary, the evidence shows associations with increased rates of behavior problems, including ADHD, ODD, and conduct disorders. However, concerns about unresolved confounding persist, limiting the ability to draw firm conclusions.
Anxiety and Depression
Internalizing disorders are characterized by depressed mood, anxiety, somatic, and cognitive symptoms (as opposed to externalizing disorders which are characterized by antisocial behaviors, conduct problems, and impulse-control disorders). Anxiety and depression are both considered to be internalizing conditions (American Psychiatric Association 2013). Thirteen articles were included in the review of epidemiologic evidence for an association between prenatal smoking and anxiety, depression, or internalizing symptoms in general (Table 9.11S). Of the 12 articles, 10 focused on depression or anxiety, alone or in combination with each other, or internalizing behaviors in general. One reported on anxiety and ADHD but not depression, and 1 reported on depression without anxiety. The study on anxiety and ADHD found no association between maternal smoking and anxiety among a clinic-recruited group of 275 children 5–17 years of age with ADHD (Freitag et al. 2012). The study that assessed depression without anxiety was a prospective birth cohort study and no association between prenatal smoking and depressive symptoms was observed after controlling for exposure to secondhand smoke (Maughan et al. 2001).
In 7 of the 10 studies examining depression and anxiety, the authors did not find an association between prenatal smoking and depression and/or anxiety (disorders or symptoms) among the offspring at various ages (Hill et al. 2000; Kardia et al. 2003; Whitaker et al. 2006; Gatzke-Kopp and Beauchaine 2007; Biederman et al. 2009; Lavigne et al. 2011; Liu et al. 2011). In a study of 678 preschool children (4-year-olds), a retrospective report of smoking during pregnancy was not associated with meeting diagnostic criteria for depression or anxiety on the Diagnostic Interview Schedule for Children (Lavigne et al. 2011). No association was found between prenatal exposure and symptoms of anxiety or depression in 611 offspring when they were adults in a cohort study with prospective reporting of maternal smoking; however, this study did find an association of prenatal exposure and anger temperament (Liu et al. 2011).
Finally, two studies (Whitaker et al. 2006; Gatzke-Kopp and Beauchaine 2007) that used the child behavior checklist (CBCL) to measure internalizing symptoms, including depression and anxiety, found no associations between maternal smoking and offspring outcomes. In a study of 171 children 7–15 years of age with clinical levels of psychopathology, the types of symptoms were contrasted across three levels of smoking exposure: exposure to prenatal smoking, secondhand exposure to smoking among mothers during pregnancy, and no exposure (Gatzke-Kopp and Beauchaine 2007). The researchers found no association between prenatal smoking exposure (vs. no exposure or secondhand exposure of mother during pregnancy) and symptoms of depression, dysthymia, or anxiety on the CBCL; however, there was an association with externalizing symptoms. There was also no association observed in a cohort study between maternal report of smoking at birth and internalizing symptoms at 3 years of age (Whitaker et al. 2006).
In three studies the authors did report positive associations between prenatal smoking and internalizing symptoms in children as measured by the CBCL. Two of these studies were prospective (Indredavik et al. 2007; Robinson et al. 2008). Indredavik and colleagues (2007) enrolled women by 20 weeks gestation, collected information on smoking during pregnancy at enrollment, and completed a CBCL on 84 children when they were 14 years of age. Several potential confounders were examined in the adjusted analysis, including income, parental antisocial tendencies, and birth weight. The authors estimated that 19% of the variance in externalizing behaviors was accounted for by maternal prenatal smoking, and 8.9% of internalizing behaviors. Robinson and colleagues (2008) enrolled women during pregnancy (18 weeks gestation), and assessed 1,707 offspring using the CBCL at 2 and at 5 years of age. This study reported a significant association between internalizing behaviors and maternal smoking at 2 years of age (OR = 1.26; 95% CI, 1.02–1.55); but no difference was observed at 5 years of age (Robinson et al. 2008). In a retrospective study of maternal smoking and behavior disorders in children, the authors found a significant association with internalizing behaviors (OR = 1.28; 95% CI, 1.1–1.6), but not externalizing behaviors (Teramoto et al. 2005).
In summary, three studies reported associations between exposure to prenatal smoking and internalizing symptoms, both in preschool-age children and adolescents; however, four studies examining symptoms did not find an association. Of the three studies that measured smoking prospectively, two found a positive association with internalizing symptoms (Indredavik et al. 2007; Robinson et al. 2008), and one found an association with anger temperament in adulthood, but not with symptoms of depression or anxiety (Liu et al. 2011). These findings may point to a possible nonspecific association between exposure to prenatal smoking and neurobehavioral disorders or symptoms of these disorders.
Tourette Syndrome
Two articles were identified that address maternal prenatal smoking and Tourette syndrome in offspring; neither study found a significant association (Table 9.12S). The limited evidence available for review was insufficient to permit meaningful synthesis.
Schizophrenia
There have been very few articles published after 1999 that address maternal smoking and schizophrenia in offspring. These studies are limited by small sample size, the use of surrogate markers for schizophrenia rather than the disease itself, the use of inappropriate control groups, and reliance on recall of maternal smoking status obtained many years after the pregnancy (Zammit et al. 2009; Baguelin-Pinaud et al. 2010; Hunter et al. 2011). In a meta-analysis of obstetric complications and schizophrenia published in 2002, there was no significant association observed between maternal smoking and offspring schizophrenia; however, even when the samples from only two studies were included in the analysis, the pooled sample size of cases was small (Cannon et al. 2002). Therefore, the limited evidence available for review was insufficient to permit meaningful synthesis.
Intellectual Disability
For the purposes of this review, intellectual disability was defined as having intelligence quotient (IQ) scores in health or educational records that fell at or below 70 or test scores within the intellectual disability range, as indicated on psychometric tests, such as the Wechsler Intelligence Scale for Children-Revised.
Twelve articles were identified that assessed the relationship between prenatal exposure to maternal tobacco smoke and the risk of intellectual disability in their children (Table 9.13S). Of the 12 articles, 10 demonstrated a significant association between prenatal exposure to maternal tobacco smoke and intellectual disability and/or low intellectual performance in unadjusted analyses. However, the observed associations in all 10 studies were attenuated or disappeared after adjusting for maternal education and/or maternal IQ (Fried and Watkinson 2000; Cornelius et al. 2001; Fried et al. 2003; Breslau et al. 2005; Mortensen et al. 2005; Batty et al. 2006; Huijbregts et al. 2006; Alati et al. 2008; Braun et al. 2009; Lundberg et al. 2010).
Braun and colleagues (2009) examined the association between prenatal exposure to tobacco smoke and intellectual disability in early childhood using data from a cohort of children born during 1994–1996. This study defined intellectual disability as having an IQ score below 70 points and found the risk of intellectual disability was mildly elevated among 8-year-old children whose mothers smoked during pregnancy (RR = 1.52; 95% CI, 1.27–1.83), but was no longer significant (RR = 1.12; 95% CI, 0.92–1.36) after adjustment for maternal education, maternal race, maternal age, marital status, and gender of child (Braun et al. 2009).
The association between maternal smoking during pregnancy and intellectual disability was also examined in adolescents and young adults. Kafouri and colleagues (2009) assessed the relationship between cognitive functioning in adolescent offspring 12–18 years of age and maternal cigarette smoking during pregnancy. This study used an extensive 6-hour battery of tests in which cognitive abilities were evaluated based on 33 tasks measuring verbal and visual memory, visuospatial skills, verbal abilities, processing speed, motor dexterity, and resistance to interference and found no difference between the cognitive abilities in adolescent offspring that were exposed to maternal cigarette smoking during pregnancy compared to those unexposed after adjustment for maternal education.
Lundberg and colleagues (2010) examined the association between maternal smoking during pregnancy and the risk of intellectual impairment among young adult male offspring at 18 years of age. This study found an increased risk of intellectual impairment (OR = 1.91; 95% CI, 1.81–2.00), but the effect was attenuated (OR = 1.22; 95% CI, 1.14–1.31) after adjustment for other parental factors.
In a cohort study by MacArthur and colleagues (2001), there were differences in IQ as a function of the mother's pregnancy smoking behavior, but smoking did not remain an independent predictor after accounting for confounding factors. Further, the early hazards of smoking during pregnancy seemed to resolve by later childhood, with no evidence of direct long-term effects on cognitive functioning. The authors concluded that effects observed in early childhood, which arise from smoking during pregnancy, are significantly attenuated or disappear by later childhood, with no evidence of long-term effects on cognitive functioning.
Evidence Synthesis
Although specific mechanisms linking prenatal exposure to smoking with specific behavioral conditions have not been determined, there is some evidence from human and animal studies that supports a biological basis for the association between exposure to prenatal smoking and some neurobehavioral conditions. The 2004 Surgeon General's report concluded that the evidence was inadequate to infer the presence or absence of a causal relationship between maternal smoking and either physical growth or the collective neurocognitive development of children.
Although there is consistent evidence supporting an association between prenatal smoking exposure and disruptive behavioral symptoms among children, and ADHD in particular, the magnitude of the estimated associations diminishes when family, social, and psychosocial factors are included in multivariate models. Reliance on retrospective reporting of smoking history, which is subject to recall bias, limits the ability to draw conclusions about the temporal nature of the relationship; however, select studies that did collect exposure during pregnancy corroborate the direction of findings from the retrospective studies. Much of the published literature failed to control for many potential confounders and failed to use standard criteria for the assessment of outcomes.
The literature is limited and conflicting on the relationship between prenatal smoking exposure and anxiety and depression symptoms and disorders in children. The studies reviewed here showed no consistent association between exposure to prenatal smoking and a later diagnosis of depression or anxiety.
The available evidence is quite limited and mixed on the association between prenatal smoking and both Tourette syndrome and schizophrenia among exposed children. The research on these conditions is subject to significant methodologic limitations, including small sample size, lack of an appropriate control group, low response rate, and retrospective report of smoking by mothers. Given the limited information available for these conditions, conclusions cannot be made regarding the consistency, strength, specificity, or temporal nature of the potential relationship.
Although there is evidence of an association between prenatal exposure to maternal smoke and intellectual disability, several studies suggest that this finding is substantially attenuated or eliminated when controlling for maternal education, IQ, and other sociodemographic covariates. This is similar to the pattern documented among studies of maternal smoking and disruptive behavioral disorders.
Conclusions
- The evidence is suggestive, but not sufficient, to infer a causal relationship between maternal prenatal smoking and disruptive behavioral disorders, and attention deficit hyperactivity disorder in particular, among children.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and anxiety and depression in children.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and Tourette syndrome.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and schizophrenia in her offspring.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and intellectual disability.
Implications
There are high rates of neurobehavioral disorders among children, particularly disruptive behavioral disorders, depression, and anxiety (CDC 2010; Merikangas et al. 2010; Ghandour et al. 2012; Substance Abuse and Mental Health Services Administration 2012); these disorders have an impact on social and academic functioning, employment, and health throughout the lifespan. Additional research is needed to better understand the potential impact of exposure to prenatal smoking on neurodevelopment in general as well as on specific neurobehavioral conditions.
Ectopic Pregnancy and Spontaneous Abortion
The 2010 Surgeon General's report included a chapter that comprehensively covered the topic of the adverse reproductive effects of active smoking and exposure to secondhand smoke. Since the reviews carried out for the 2010 report, which included papers published up to 2009, additional significant findings have been reported for several outcomes, including spontaneous abortion and ectopic pregnancy. Building upon the reviews in the 2004 and 2010 reports, this section reassesses the state-of-the-evidence for these two outcomes, giving consideration to the more recent publications.
Ectopic Pregnancy
Ectopic pregnancy (EP) is a condition affecting 1–2% of pregnancies (CDC 1995; Van Den Eeden et al. 2005) in which implantation of a fertilized ovum takes place outside of the uterus, most often in the fallopian tubes. The etiology of EP is not fully understood, but appears to involve the motility and patency of the fallopian tubes. Risk factors associated with EP include advanced maternal age, history of prior spontaneous abortion, number of sexual partners, history of surgical procedures affecting the fallopian tubes, use of an intrauterine device (IUD) for birth control, history of sexually transmitted infections (STIs), and vaginal douching (Kendrick et al. 1997, Pisarska et al. 1998; Bouyer et al. 2003). Affected women are at increased risk of infertility and recurrent ectopic pregnancy in subsequent pregnancies, as would be expected in women with tubal damage.
The 2004 Surgeon General's report found the evidence suggestive, but not sufficient, to infer a causal relationship between smoking and ectopic pregnancy. The 2010 Surgeon General's report provided an update on studies published after the 2004 report, but did not formally evaluate the evidence for causality. This section reviews the epidemiologic and biological evidence for an association between prenatal smoking and increased risk of EP.
Biologic Basis
The human fallopian tube promotes embryonic development and transports the embryo to the uterus for implantation and has three anatomical regions: (1) the infundibulum, which picks up the oocyte cumulus complex after it is ovulated from the ovary; (2) the ampulla, where fertilization occurs; and (3) the isthmus, which conducts sperm to the ampulla and provides a site for preimplantation development (Shaw et al. 2010a). All regions have ciliated and secretory epithelial cells and smooth muscle cells, and proper functioning of each region is necessary for normal reproduction. Ciliated cells move the gametes and embryo along the tube, and can accomplish this function with no decrease in transit time, even when smooth muscle activity is blocked by a β-adrenergic agonist (Halbert et al. 1976a,b). Secretory cells secrete substances that facilitate maturation and transport of gametes; local vasculature also contributes to the secretory process and the formation of fallopian tube fluid (Shao et al. 2012).
EP is thought to be the result of the retention of the embryo within the fallopian tube due to structural damage or functional impairment of the tube, allowing implantation to occur (Shaw et al. 2010a; Shao et al. 2012). A number of studies have examined potential mechanisms through which EP may occur. For example, a reduction in the number of ciliated cells was observed in fallopian tubes containing an EP and in the biopsies of women, who were undergoing tubal surgery and later developed a tubal pregnancy (Vasquez et al. 1983). However, in a study of human fallopian tube sections from women undergoing sterilization procedures, there were no differences between smokers and nonsmokers in the density of ciliation or in the expression of ciliogenic transcription factors (Pier and Kazanjian 2013). Ciliary beat frequency was not examined in this study.
The oviduct appears to be an in vivo target of cigarette smoke and its components; and fallopian tube damage or dysfunction is believed to be involved. Contraction of both the human oviduct (Neri and Eckerling 1969) and the rabbit oviduct (Ruckebusch 1975) is altered by exposure to tobacco smoke. Inhalation of mainstream or sidestream smoke caused blebbing of the oviductal epithelium and decreased the ratio of ciliated to secretory cells in hamsters (Magers et al. 1995). In a study of hamsters, in which the oviduct was directly observed before, during, and after inhalation of tobacco smoke at doses equivalent to those received by humans, both mainstream and sidestream smoke decreased ampullary smooth muscle contractions and slowed embryo transport through the oviduct (DiCarlantonio and Talbot 1999). Nicotine altered the motility of the oviducts of rhesus monkeys (Neri and Marcus 1972), decreased oviductal blood flow (Mitchell and Hammer 1985), decreased sodium and potassium levels in oviductal epithelial cells of mice (Jin et al. 1998), and increased lactate dehydrogenase levels in the oviduct epithelium of rats (Rice and Yoshinaga 1980). These effects could alter the oocyte transport rate in the fallopian tube (Talbot and Riveles 2005). Other individual components found in tobacco smoke have also been examined. For example, cadmium decreases oocyte transport and delays intrauterine implantation in mice and rabbits (Saksena 1982; Shao et al. 2012).
Since the publication of the 2010 Surgeon General's report, several studies on mechanisms through which smoking increases the risk of EP have been published. As previously discussed, a reduction in the number of ciliated cells was observed in fallopian tubes containing an EP and in the biopsies of women undergoing tubal surgery who later developed a tubal pregnancy (Vasquez et al. 1983). However, in a study of human fallopian tube sections from women undergoing sterilization procedures, there were no differences between smokers and nonsmokers in the density of ciliation or in the expression of ciliogenic transcription factors (Pier et al. 2013).
PROKR1 is an angiogenic molecule that regulates smooth muscle contraction and is involved in intrauterine implantation. Investigators studied whether tubal receptor expression of PROKR1 was altered in women who smoked by collecting sera and fallopian tube samples from women undergoing hysterectomy (Shaw et al. 2010b). PROKR1 transcription was higher in the fallopian tubes from smokers. Cotinine treatment of fallopian tube explants and oviductal epithelial cells increased PROKR1 expression, and this effect was negated by treatment with nicotinic acetylcholine receptor α-7- antagonist. This suggests that smoking could predispose the fallopian tube to the implantation of the embryo by increases in tubal PROKR1.
Description of the Literature Review
This section explores available epidemiologic studies of the association between cigarette smoking (and other forms of tobacco use) and EP. A literature search was conducted for studies published from 2000 through November 2012 to cover the period following that of the 2004 and 2010 Surgeon General's reports using the PubMed system of the National Library of Medicine. The search terms included “smok*” and “tobacco” and “ectopic” and “preg.”
Epidemiologic Evidence
A large number of epidemiologic studies have addressed smoking and EP (Table 9.14S). Methodologic challenges to studies of EP include adequate consideration of potential confounders, such as history of previous STIs and pelvic inflammatory disease (PID) and the selection of appropriate control groups (Weiss et al. 1985).
Since the 2004 Surgeon General's report, two studies (both discussed in the 2010 Surgeon General's report) addressing previous methodologic limitations related to the selection of control groups and adjustment for confounders have been published; both found significant associations between smoking and EP (Bouyer et al. 2003; Karaer et al. 2006). These studies also found evidence of a dose-response relationship between the number of cigarettes smoked per day and EP, as have many previous studies (Handler et al. 1989; Coste et al. 1991; Saraiya et al. 1998; Bouyer et al. 2003; Karaer et al. 2006), but not all (Chow et al. 1988; Stergachis et al. 1991). Since the 2010 Surgeon General's report, an additional study has been published in which hospital discharge records from over 4 million pregnancies were examined using the National Inpatient Sample. Roeland and colleagues (2009) found an elevated risk for EP among smokers; however, smoking status was obtained from ICD-9 codes (a method with low sensitivity), and there was no adjustment for potentially important confounders.
An earlier meta-analysis of data from nine studies (Levin et al. 1982; World Health Organization [WHO] 1985; Chow et al. 1988; Handler et al. 1989; Coste et al. 1991; Kalandini et al. 1991; Stergachis et al. 1991; Parazzini et al. 1992; Phillips et al. 1992), all of which included adjustment for potential confounders, yielded an OR from the pooled data on EP from smoking of 1.77 (95% CI, 1.31–2.22) (Castles et al. 1999). In a subanalysis of three studies that adjusted for history of PID, IUD use, sterilization, and EP (Levin et al. 1982; Chow et al. 1988; Parazzini et al. 1992), the pooled OR was 1.91 (95% CI, 1.29–2.56). No subsequent meta-analyses have been conducted.
Studies that included examination of former smokers (either those who quit before conception compared with those who never smoked), or more detailed analysis of age at initiation, years since quitting, duration and/or intensity of smoking, have generally not found significant associations with past smoking (Kalandidi et al. 1991; Stergachis et al. 1991; Parazzini et al. 1992; Phillips et al. 1992; Saraiya et al. 1998; Karaer et al. 2006). However, two studies did show evidence of an increased risk in past smokers (Chow et al. 1988; Bouyer et al. 2003).
All studies reviewed included confounder-adjusted analyses except one (Roelands et al. 2009). Of these analyses, all but one (Parrazzini et al. 1992) yielded OR or RR point estimates greater than one, and in most, the association was statistically significant (WHO 1985; Chow et al. 1988; Handler et al. 1989; Kalandidi et al. 1991; Phillips et al. 1992; Saraiya et al. 1998; Bouyer et al. 2003; Karaer et al. 2006). The specific confounders addressed through adjustment or exclusions varied across studies, but most analyses included maternal demographics and a combination of factors related to past obstetrical outcomes (prior EP, spontaneous abortion), gynecological and surgical history (past PID, STIs, contraceptive use including IUDs, abdominal surgery), and lifestyle factors (number of sexual partners, douching, and age at first intercourse).
Methodologic challenges related to case-control studies of EP include overcoming bias introduced by selection of control groups, which exclude women with induced abortions. For example, a control group of women with term deliveries that excludes pregnancies ending in induced abortion would likely result in bias favoring characteristics associated with induced abortion (Weiss et al. 1985), such as smoking. Several approaches to address this issue have been suggested, such as excluding from case and control groups women most likely to seek an abortion, such as those using contraceptives and those who were unmarried at the time of conception. In four studies in this review (Chow et al. 1988; Saraiya et al. 1998; Bouyer et al. 2003; Karaer et al. 2006), cases and controls were selected to address this methodologic issue. In all four studies, adjusted models yielded significant associations, and two of these showed evidence of a dose-response relationship (Saraiya et al. 1998; Bouyer et al. 2003).
Evidence Synthesis
Although the precise mechanisms through which smoking could increase risk of EP remain unclear, in vitro and in vivo studies demonstrate that exposure to tobacco smoke adversely affects oviductal functioning and that nicotine can impair oviductal physiology. Animal studies suggest fallopian tubes exposed to cigarette smoke have decreased ciliary beat frequency, cilia-dependent oocyte retrieval rate, adhesion of the oocyte cumulus complex to the fallopian tube, and smooth muscle activity, providing evidence of biologic plausibility for a causal relationship. A number of epidemiologic studies provide consistent evidence of an independent association between maternal smoking and EP. This consistency is greater when restricted to studies which includes adjustment for important potential confounders and careful selection of control groups (Chow et al. 1988; Stergachis et al. 1991; Saraiya et al. 1998; Bouyer et al. 2003; Karaer et al. 2006). Of these studies, all but one identified smoking status as smoking at the time of conception, although this was done retrospectively. Evidence of a dose-response relationship within this group of studies was less consistent. Full adjustment for potential confounders is a methodologic limitation in studies of smoking and EP. Some studies showed minimal or no attenuation after adjustment for confounders (Coste et al. 1991; Bouyer et al. 2003), while others showed some attenuation (Parazzini et al. 1992; Phillips et al. 1992; Saraiya et al. 1998). Among studies in which crude and adjusted risks were reported, only one demonstrated a change from a significant to a nonsignificant association after adjustment (Parazinni et al. 1992).
Epidemiologic studies combined with in vitro and in vivo studies, document the consistency of findings and biologic plausibility that maternal smoking adversely affects the oviduct in ways that increase the risk of EP.
Conclusion
The evidence is sufficient to infer a causal relationship between maternal active smoking and ectopic pregnancy.
Implications
Data from animal and epidemiologic studies support smoking as a causal risk factor for EP. The evidence of an association between smoking and EP is sufficient to warrant intensified efforts to promote smoking cessation among women of reproductive age and during preconception care. More research is needed to better characterize potential mechanisms, through which smoking affects the success of implantation and placentation.
Spontaneous Abortion
Spontaneous abortion (SAB) is typically defined as the involuntary termination of an intrauterine pregnancy before 20 weeks of gestation, although some studies define SAB as occurring before 28 weeks. Studies have reported recognized SABs in approximately 12% of pregnancies, and, most occur before 12 weeks gestation (Regan et al. 1989). However, very early pregnancy loss may go unrecognized and/or unreported. An estimated 31% of all conceptions end in pregnancy loss, and 22% of conceptions end before the pregnancy is recognized (Wilcox et al. 1988). Studies of embryonic tissue from SABs suggest that 22–61% of losses have an abnormal karyotype (Kline et al. 1989). In addition to fetal abnormalities, other factors that likely contribute to SAB include anatomical abnormalities of the mother's uterus, immunologic disturbances, thrombotic disorders, and endocrine abnormalities (Christianson 1979; Cramer and Wise 2000; Regan and Rai 2000). Infections may also play a role, but data are limited and inconsistent (Cramer and Wise 2000; McDonald and Chambers 2000; Matovina et al. 2004; Rai and Regan 2006).
The 2004 Surgeon General's report found the evidence suggestive, but not sufficient, to infer a causal relationship between smoking and SAB. The 2010 Surgeon General's report provided an update on studies published after the 2004 report, but did not formally evaluate the evidence for causality. This section reviews the epidemiologic and biological evidence for associations between prenatal smoking and increased risk of SAB.
Biologic Basis
The mechanisms through which smoking may increase the risk of SAB are unclear. Mechanistic pathways that have been evaluated through in vitro studies include the effects of tobacco exposure on uterine microvasculature, cytotrophoblast invasion, mitotic activity, differentiation, and attachment during placental development, and on embryonic development (Talbot 2008). In vivo studies also suggest an effect of tobacco and/or nicotine on oocyte quality and embryo development (reviewed by Soares and Melo 2008). Other proposed pathways include fetal hypoxia from exposure to CO, and vasoconstrictive and antimetabolic effects resulting in placental insufficiency and the subsequent death of the embryo or fetus (Salafia and Schiverick 1999; Practice Committee of the American Society for Reproductive Medicine 2012). Finally, cadmium is absorbed from cigarette smoke and has been associated with numerous adverse effects on reproductive function, including retardation of trophoblast development, placental necrosis, abnormal embryonic development, and interference with cell adhesion in the postimplantation embryo (Thompson and Bannigan 2008).
Description of the Literature Review
This section explores available epidemiologic studies of the association between cigarette smoking (and other forms of tobacco use) and SAB. A literature search was conducted for studies published from 2000 through November 2012 to cover the period following that of the 2004 and 2010 Surgeon General's reports using the PubMed system of the National Library of Medicine. The search terms included “smok*,” “tobacco,” “abortion,” “miscarriage,” and “preg.”
Epidemiologic Evidence
A large number of epidemiologic studies have addressed smoking and SAB (Table 9.15S). However, methodologic challenges have made the study of potential associations between maternal smoking and SAB difficult. Many early pregnancy losses are not recognized or reported, making it difficult to study losses across the full gestational age span, unless women are enrolled in a study before conception. The etiology of SAB is multifactorial and the mechanisms are not well understood; thus, the ability to clinically categorize cases of SAB is limited, especially in large epidemiologic studies. Because it is unlikely that tobacco exposure would have similar effects on SAB risk in women across etiologic subgroups, combining cases of SAB could bias potential associations with tobacco toward the null. For example, many early pregnancy losses are associated with karyotypically abnormal embryos. Although it would be optimal to study SAB cases with normal and abnormal embryo karyotype separately, the embryo karyotype is unknown in many studies. Full adjustment for potential confounding from other exposures, such as alcohol use, substance abuse, and STIs, is difficult, especially in large studies. In case-control studies, the selection of an appropriate control group is important in order to avoid bias. For example, women with term births (a commonly selected control group) may differ in their prevalence of smoking from women who have elective terminations and preterm births (often omitted from control groups). Finally, exposure misclassification due to maternal nondisclosure of smoking status, or other factors, could result in bias toward the null.
Most studies reviewed at the time of the 2004 Surgeon General's report indicated an increased risk of SAB in active smokers. In a meta-analysis of data from 13 studies, the pooled crude ORs for SAB in smokers were slightly elevated at 1.24 (95% CI, 1.19–1.30) for cohort studies and 1.32 (1.18–1.48) for case-control studies (DiFranza and Lew 1995). Finally, in the largest study to date of karyotyped miscarriages (n = 2,376), Kline and colleagues (1995) observed an association between active smoking and SAB that was confined to losses of chromosomally normal conceptions (AOR = 1.3; 95% CI, 1.1–1.7). This finding supports the association that smoking increases risk of SAB through toxic effects that occur during gestation. However, George and colleagues (2006) also found that smoking was significantly associated with SAB with unknown and abnormal fetal karyotype, but not with SAB with normal karyotype. The number of SAB cases with a normal karyotype was small (n = 75) and a large percentage of SAB cases were of unknown karyotype (George et al. 2006). Further, the collection of samples for cotinine measurement in both studies by George and colleagues (2006) and by Ness and colleagues (1999) occurred at the time of the miscarriage, and so cotinine levels did not necessarily reflect tobacco exposure at or before conception. There have been no new studies since the 2010 Surgeon General's report that have examined the risk of SAB from maternal smoking by karyotype.
Eight new studies from a number of different countries were evaluated in the 2010 report and included case-control (Chatenoud et al. 1998; Ness et al. 1999; Rasch 2003; Wisborg et al. 2003; George et al. 2006; Nielsen et al. 2006), cohort (Windham et al. 1999), and cross-sectional (Mishra et al. 2000) study designs. Analyses included adjustment for various potential confounders, such as use of oral contraceptives, IUDs (Nielsen et al. 2006), alcohol and caffeine (Chatenoud et al. 1998; Ness et al. 1999; Windham et al. 1999; Rasch 2003; Wisborg et al. 2003; George et al. 2006), illicit substances (Ness et al. 1999), history of STIs (Ness et al. 1999), and folate levels (George et al. 2006). Five of eight studies found significant positive associations between smoking and SAB in adjusted models (Chatenoud et al. 1998; Ness et al. 1999; Mishra et al. 2000; George et al. 2006; Nielsen et al. 2006); in one study the association was not significant for smoking overall, but was significant when the number of cigarettes smoked per day among smokers was examined (AOR = 1.20; 95% CI, 1.04–1.39 per 5 cigarettes/day) (Nielsen et al. 2006).
Two studies used cotinine to verify exposure to tobacco smoke and found relatively higher risks of SAB compared with other studies (AORs = 1.8; 95% CI, 1.3–2.6; and 2.1; 95% CI, 1.4–3.3) (Ness et al. 1999; George et al. 2006). In contrast, two studies of Danish women (one large cohort study of 24,608 pregnant women and one case-control study of women with SAB or a live fetus at 6–16 weeks gestation) found no association between smoking and SAB after adjustment for multiple potential confounders (Rasch 2003; Wisborg et al. 2003). In a cohort study of pregnant women at 12 weeks gestation or less and enrolled in a prepaid health plan, Windham and colleagues (1999) did not find a significant association between smoking and SAB (AOR = 1.3; 95% CI, 0.9–1.9). In the latter study, however, the association was marginally significant when the analysis was restricted to loss after 10 weeks gestation (AOR = 1.6; 95% CI, 1.0–2.4) (SAB at a later gestational age is more likely to have a normal karyotype than an SAB at an earlier gestational age).
Since the publication of the 2010 Surgeon General's report, several studies have examined the effects of maternal active smoking on SAB risk, with mixed results (Table 9.15S). In a case-control study of Japanese women with early SAB and using women with term births as a control group, AORs for smoking 1–19 and 20 or more cigarettes per day were 1.30 (95% CI, 0.84–2.02) and 2.39 (95% CI, 1.26–4.53), respectively (p for trend = 0.02) (Baba et al. 2011). ORs were adjusted for numerous factors, including BMI and alcohol intake.
In a cross-sectional survey of cosmetologists, realtors, teachers, nurses, and retail clerks, Gallicchio and colleagues (2009) found a significant association between smoking and SAB after adjusting for age, race, education, and alcohol use (AOR = 1.53; 95% CI, 1.09–2.16).
Maconochie and colleagues (2007) conducted a nested case-control study and found a significant association between smoking and SAB for women smoking 11–20 cigarettes per day (AOR = 1.68; 95% CI, 1.16–2.42), but not for smoking 1–10 or more than 20 cigarettes per day and Bhattacharya and colleagues (2010) also found a modest but significant association (AOR = 1.13; 95% CI, 1.05–1.22), but results were adjusted only for age and year of event, and smoking status was obtained from medical coding and was missing for a large proportion of women. Only one of the studies reviewed included evidence of a dose-response relationship (Baba et al. 2011). The remaining studies found no evidence (Maconochie et al. 2007) or did not report associations by cigarettes smoked per day (Gallicchio et al. 2009; Bhattacharya et al. 2010). Other studies found no association between smoking and SAB (Blohm et al. 2008; Zhang et al. 2010; Campbell et al. 2011).
Several studies have examined the role of maternal smoking and SAB among women undergoing assisted reproductive technology (methods to achieve pregnancy by artificial or partially artificial means) procedures. Because women receiving these services undergo intense follow-up, the timing of conception and tobacco exposure status at conception are often known, allowing researchers to overcome methodologic limitations often present in other populations. In a meta-analysis of studies addressing the effects of tobacco use on outcomes among assisted reproductive technology patients, the pooled OR for SAB was 2.65 (95% CI, 1.33–5.30) (Waylen et al. 2009). However, studies included in this meta-analysis had several methodologic limitations, including the inability to control for confounders (Harrison et al. 1990; Pattinson et al. 1991; Hughes et al. 1992; Maximovich et al. 1995; Gustafson et al. 1996; Soares et al. 2007), use of repeated measures without documentation that the appropriate statistical analysis was used (Hughes et al. 1992; Winter et al. 2002), and poorly defined smoking status (Maximovich et al. 1995; Winter et al. 2002). In a subanalysis of three studies which were unlikely to be affected by confounding due to maternal age, the association between smoking and SAB was no longer significant (OR = 1.88; 95% CI, 0.55–6.27) (Waylen et al. 2009).
Evidence Synthesis
In summary, there are multiple potential mechanisms through which smoking during pregnancy could increase risk for SAB. Several studies published since the 2004 Surgeon General's report address previous methodologic limitations. These studies have included consideration of a number of potentially important confounders, such as alcohol and illicit substance use, and history of STIs. Studies using biochemical validation of tobacco exposure had positive and significant associations (Ness et al. 1999; George et al. 2006). Evidence of a dose-response relationship was found in some recent studies (Nielsen et al. 2006; Baba et al. 2011), but not in all (Mishra et al. 2000; Maconochie et al. 2007). Overall, results of epidemiologic studies remain mixed, and many studies have important methodologic limitations, including reliance on women with term births as controls, lack of data on many relevant confounders, unknown embryonic/fetal karyotype, and uncertainty regarding level of exposure to tobacco during periods critical to the outcome.
Conclusion
The evidence is suggestive, but not sufficient, to infer a causal relationship between maternal active smoking and spontaneous abortion.
Implications
SAB is multifactorial and the mechanisms are not yet well understood; however, the evidence of an association between smoking and SAB is sufficient to warrant intensified efforts to promote smoking cessation before conception and during early prenatal care. More research is needed to better characterize potential mechanisms through which smoking might affect the success of implantation and placentation.
Male Sexual Function
Erectile dysfunction (ED) is defined as the persistent inability of a man to attain and maintain an erection that is adequate for satisfactory sexual performance (NIH Consensus Development Panel on Impotence 1993). According to the National Health and Social Life Survey, 18% of U.S. men, 50–59 years of age, had ED in 1992 (Laumann et al. 1999). This prevalence rate relied on a probability sample that included 1,410 men, 18–59 years of age. Later, the National Health and Nutrition Examination Survey of 2001–2002 estimated that 18.4% of U.S. men, 20 years of age and older, had ED and that the condition affected 18 million men nationwide (Saigal et al. 2006; Selvin et al. 2007). Using data from the Massachusetts Male Aging Study (Feldman et al. 1994), estimates of the prevalence of complete ED among men 40–70 years of age exceeded 10% during 1987–1989; estimates of at least mild ED exceeded 50%. According to an estimate derived from longitudinal results of the Massachusetts Male Aging Study in the late 1990s, 25.9 cases of new-onset (incidence) ED occurred per 1,000 men annually (Johannes et al. 2000).
Hormonal derangement, psychogenic factors, neurologic disorders, and vascular insufficiency have been implicated in the etiology of ED, as have several other factors. Objectively demonstrable ED has been found in patients who have had a myocardial infarction, undergone coronary artery bypass surgery, suffered a cerebrovascular accident, or have peripheral vascular disease or hypertension (Melman and Gingell 1999). In addition, reports of patients with vasculogenic ED have suggested predisposing vasculopathic risk factors, such as cigarette smoking, high-fat diets, higher risk serum lipid levels, hypertension, physical inactivity, and obesity (Goldstein and Hatzichristou 1994; Kendirci et al. 2007; Miner and Billups 2008). Several large epidemiologic studies have explored the extent to which these factors impair erectile function (Feldman et al. 1994, 2000; Derby et al. 2000a,b; Johannes et al. 2000). The results of these studies imply that modifying risk factors may reduce the occurrence of ED. For example, Esposito and colleagues (2004, 2009) assessed the effects of increased physical activity and weight loss on erectile function in overweight men and found that both could improve penile function.
A growing body of literature shows that tobacco smoke adversely affects sexual health and erectile function in particular (Bornman and du Plessis 1986; Juenemann et al. 1987; Mannino et al. 1994; Polsky et al. 2005; Shiri et al. 2006; He et al. 2007; Kupelian et al. 2007, 2010; Harte and Meston 2008; Tostes et al. 2008). Cigarette smoking may affect erectile function through its atherogenic effects on penile vasculature in a manner that is analogous to the effects of heart disease on coronary circulation. This chapter summarizes and evaluates current observational, clinical, and experimental data that link cigarette smoking with ED, including the relevant pathophysiologic concepts.
Conclusions from Previous Surgeon General's Reports
The 2004 report indicated that on the basis of case series and population-based studies as well as experimental evidence from human and animal studies, cigarette smoking is a risk factor for erectile dysfunction (USDHHS 2004). However, the evidence was considered not sufficient to infer a causal relationship.
Biologic Basis
One possible mechanism for ED is smoking-induced endothelial dysfunction of the penile vasculature. Both the endothelium of the blood vessels supplying the penis and the lining of the lacunar spaces within that organ release vasoactive substances that contribute to the control of the relaxation of smooth muscle that is required for erection (Lue and Tanagho 1987; Lue 2000).
Saenz de Tejada and colleagues (1989), as part of an investigation of the consequences of diabetes mellitus on endothelial function in the penis of men with ED, examined the effect of smoking on penile vasculature. Using isolated strips of human corpora cavernosa of the penis taken at surgery, researchers compared isometric tension results from impotent men with and without diabetes who were smokers (i.e., with at least a 5 pack-year1 history of smoking) or nonsmokers. They found that a history of smoking was not associated with greater impairment of endothelium-mediated relaxation responses.
In a study of rats, Xie and colleagues (1997) examined the long-term effects of smoking on the endothelial synthesis of nitric oxide (NO) in the penis; NO is the principal vasoactive mediator of penile erection (Burnett 1997). In the study, rats were passively exposed to cigarette smoke for 60 minutes at a time once per day, 5 days per week, for 8 weeks. Immunoblot analyses of the protein expression of eNOS in penile tissue from exposed rats did not reveal any diminution of eNOS expression in a comparison with control rats. Overall, however, the study confirmed that NOS enzymatic activity (which combines neuronal and endothelial sources) and specifically the protein expression of the neuronal form of NOS in the penis were markedly reduced in rats that were passively exposed to cigarette smoke compared with unexposed rats. These findings suggest that smoking selectively impairs neuronal mechanisms, particularly the neuronally based NO signal transduction pathway associated with penile erection. The rat model, however, may not be relevant for humans.
Several studies in humans have demonstrated reduced endothelium-derived NO production as a result of acute and chronic smoking (Celermajer et al. 1993; Shen et al. 1996; Adams et al. 1997; Puranik and Celermajer 2003; Brunner et al. 2005; Tostes et al. 2008). In addition, the adverse effect of chronic smoking on vascular medial elastic fibers has been cited as a possible contributor to smoking-induced ED (Ambrose and Barua 2004; Guo et al. 2006). The critical effects of smoking-induced oxidative stress on ED, mediated through the formation of superoxide radicals, have been evaluated. Support for a role of oxidative injury includes the generation of superoxides by cavernosal smooth muscle cells following noxious stimuli, inhibition of cavernosal smooth muscle relaxation by inhibition of copper/zinc superoxide dismutase, improvement of diabetes-related erectile function in animal models following therapy using antioxidants or free oxygen radical scavengers, and the elevated production of cavernosal reduced nicotinamide adenine dinucleotide phosphate oxidase-derived superoxides that has been observed in vasculogenic ED (Mok et al. 1998; DeYoung et al. 2004; Koupparis et al. 2005; Shukla et al. 2005; Kovanecz et al. 2006; Ozkara et al. 2006; Hotston et al. 2007; Tostes et al. 2008).
Saenz de Tejada and colleagues (1989), looked at whether smoking affects the neurogenic mechanisms that are responsible for erection. The researchers found that the impairment of neurogenically mediated relaxation of penile smooth muscle obtained from smokers (in an analysis that combined results from men with and without diabetes) did not differ from the impairment observed in nonsmokers (men with and without diabetes). Adaikan and Ratnam (1988) found that the actions of nicotine are both contractile and relaxant. If ED results from exogenously administered nicotine during smoking, it may be because of the acute vasoactive modulatory effects of this agent on the penile vasculature.
In a randomized, double-blind, placebo-controlled trial, Harte and Meston (2008) investigated the acute effects of an intermediate dose of nicotine on physiological and subjective sexual arousal in nonsmoking men. The study measured objective (through assessments of penile circumference via plethysmography) and subjective (through self-reports) differences in response to sexual stimuli with and without acute nicotine exposure in 28 men and found a 23% reduction in physiological sexual arousal with exposure to nicotine. The study's authors attributed these findings to the sympathomimetic effects of nicotine-causing vasoconstriction (i.e., anti-erectogenic) through the release of epinephrine and norepinephrine (Lue and Tanagho 1987; Harte and Meston 2008). Based on self-reports from men in this study, sexual arousal did not decrease after the administration of nicotine. Thus, the authors postulated that the effects of nicotine were more likely physiological than cognitive.
Clinical Evidence
Studies on Penile Tumescence
The monitoring of nocturnal penile tumescence (NPT) is a noninvasive diagnostic technique that can quantify the physiology of erection during the naturally occurring cycle of sleep-related erections. These spontaneous episodes of tumescence normally accompany rapid eye movement sleep and are diminished in men with ED that is presumed to be organic (vasculogenic, neurogenic, anatomic, or endocrinologic) (Karacan et al. 1978; Allen and Brendler 1992). Several early investigations of the objective basis for vasculogenic ED applied NPT monitoring. Elist and colleagues (1984) confirmed NPT-monitored abnormalities in 20 smokers with ED, of whom 7 (35%) displayed normal NPT-monitored results after 6 weeks of not smoking. Virag and colleagues (1985) found that smokers constituted 72% of patients with abnormal NPT results but only 32% of patients with normal NPT results. Karacan and colleagues (1978), in a study of 168 heavy smokers (one or more packs of cigarettes smoked per day) and 632 light smokers (less than one pack smoked per day), found that during a sleep-related erection the penis was significantly less rigid at each decade of life after 30 years of age in heavy smokers than in light smokers. The study also found that the duration of maximal tumescence was significantly lower among heavy smokers 30 years of age and younger and those 51–60 years of age than in age-equivalent light smokers.
In an investigation of 314 smokers with ED, Hirshkowitz and colleagues (1992) found a significant inverse correlation between penile rigidity during a sleep-related erection and number of cigarettes smoked per day (r = -0.12; p = 0.04). These investigators also showed that the duration of maximal tumescence was significantly shorter at the penile base (p ≤0.05), and the duration of detumescence (i.e., the decline from full erection to flaccidity) was also shorter (p = 0.06), among men who smoked 40 or more cigarettes per day than among men who smoked 1–19 or 20–39 cigarettes per day (p = 0.14).
Vascular Hemodynamics of the Penis
Impaired blood flow to the penis has been assessed using various measurement techniques. A widely used early method was the Doppler ultrasound of arterial pulsations in the flaccid, unstimulated penis. Although this method is no longer used, findings from studies that used this method remain relevant with respect to the pathogenesis of smoking-related vascular disease of the penis. The penile-brachial index (PBI)—the ratio of penile to brachial systolic blood pressures—can be calculated from values obtained through Doppler ultrasound. Reduced PBI values have been associated with impairment of the erectile process (Kempczinski 1979). Using Doppler ultrasound, Wabrek and colleagues (1983) did not find a significant association between cigarette smoking and abnormal PBI values, and Virag and colleagues (1985) also did not find an independent effect of smoking on PBI. The latter study, however, revealed a synergistic effect of smoking on PBI in combination with such other arterial risk factors as diabetes, hyperlipidemia, and hypertension.
Smokers in a study by Condra and colleagues (1986), however, had significantly lower PBI values than did nonsmokers. In another study, DePalma and colleagues (1987) found that cigarette smoking carried a significantly higher probability of abnormal (49%) than normal (28%) vascular laboratory findings, including those for PBI—an effect that was not observed for age, hypertension, diabetes, or prior myocardial infarction. A study by Hirshkowitz and colleagues (1992) found consistent reductions in PBI among 314 cigarette smokers with ED. The investigators found significant correlations between the number of cigarettes smoked per day and the magnitude of these reductions in PBI for the left dorsal artery (r = -0.14; p = 0.01) and right cavernosal artery (r = -0.13; p <0.03).
More recent investigations have used a pharmacologic stimulus in combination with duplex ultrasonography to characterize the vascular competence of penile arteries. This technique has been used since the discovery that a pharmacologic stimulus to induce an artificial erection provides a better assessment of the physiologic responsiveness of these arteries than that provided during the resting state (Abber et al. 1986). Using this technique and applying a combined set of ultrasonographic parameters to establish normal vascular findings, Shabsigh and colleagues (1991) found a consistent, marginally significant difference in vascular impairment between smokers and nonsmokers. Kadioğlu and colleagues (1995) also observed that penile vascular parameters were abnormal to a greater extent among smokers than among nonsmokers, although the differences were not significant. Overall, PBI testing suggests deleterious effects of smoking on the resting-state circulation of the penis, and sonographic evaluation of the penis following pharmacostimulation additionally suggests deleterious effects of smoking on changes in dynamic blood flow in that organ.
To better understand the hemodynamic mechanisms involved in the development of ED among smokers, Elhanbly and colleagues (2004) studied 109 patients with ED (71 current smokers and 38 nonsmokers). Evaluation included the monitoring of nocturnal penile tumescence and rigidity (NPTR) with a device called a RigiScan, followed by pharmacopenile duplex ultrasonography and redosing pharmacocavernosometry. NPTR results were abnormal for 86% of smokers and 55% of nonsmokers (p = 0.02), but the difference in peak systolic velocity of the cavernosal artery between smokers and nonsmokers (26.8 and 31.2 centimeters/second, respectively) was not significant. The latter finding suggests that vascular pathology in ED is more likely related to veno-occlusive dysfunction than to pure arterial insufficiency. Further vascular testing in the study by Elhanbly and colleagues (2004) with redosing pharmacocavernosometry revealed abnormal maintenance flow (>5 mL/minute) in 89% of smokers but only 47% of nonsmokers (p <0.01). Based on these findings, including the higher incidence of abnormal maintenance flow in the smoker group, the authors concluded that veno-occlusive dysfunction plays a substantial role in the development of ED in smokers.
Vascular Morphology
Clinicians and researchers have frequently used arteriographic studies to characterize the vascular anatomy of the penis in patients with ED. For example, investigators have used arteriography to confirm the presence and location of arteriographic lesions in smokers with ED. In one study, Virag and colleagues (1985) found a 67.8% prevalence of arteriographic abnormalities in the four main blood vessels of the penis among patients in whom organic ED had been established by NPT monitoring, of whom 86% were smokers. Similarly, Bahren and colleagues (1988) found that 82% of their patient groups with arteriographically proven peripheral arteriosclerotic lesions were heavy smokers. In a study by Forsberg and colleagues (1989), men with ED underwent screening studies of penile blood flow to identify abnormalities. Using pharmacostimulation and angiography in 17 men, the study found significant distal lesions of penile vessels in all but 1 of the 17 men; 14 (82%) of the men were identified as smokers. Later, Rosen and colleagues (1991) conducted a comprehensive evaluation of penile circulation in cigarette smokers with ED. According to the study, smoking represents a significant independent risk factor in the development of atherosclerotic lesions in the internal pudendal and common penile arteries. This study also determined that the number of pack-years smoked was independently associated with hemodynamically significant atherosclerotic disease in the hypogastric-cavernous arterial bed supplying the penis: for each 10 pack-years of smoking, the RR of this disease was 1.31 (95% CI, 1.05–1.64) compared with 1.03 (95% CI, 1.01–1.05) for 1 pack-year of smoking.
Histopathology
Mersdorf and colleagues (1991), who investigated the effects of cigarette smoking on erectile tissue, found degenerative tissue changes (including decreases in smooth muscle content, sinusoidal endothelium, nerve fibers, and capillaries and an increase in collagen density) in the erectile tissue of smokers. These alterations are consistent with the alterations of tissue observed in other vascular diseases.
Experimental Evidence
This section reviews experiments carried out in humans and animals to test the effects of cigarette smoking on erectile function (Table 9.16S). Experimental approaches can control for exposure to cigarette smoking and provide the possibility of a rigorous evaluation of the consequences of smoking for ability to achieve an erection.
Human Studies
Gilbert and colleagues (1986) may have been the first to report on an experimental evaluation of the hypothesized association between cigarette smoking and ED. The study made polygraphic recordings of the erections in smokers as they viewed erotic videos. The study population consisted of 42 males who self-reported to be heterosexual cigarette smokers, 18–44 years of age, in good health. Unknown to the experimenter, participants were assigned to and randomly selected from three groups: one group smoked high-nicotine cigarettes during the experiment (0.9 milligrams [mg] nicotine/cigarette), a second group smoked low-nicotine cigarettes (0.002 mg nicotine/cigarette), and a third, the control group, sucked on a hard mint candy. Before the experiment, smokers were required to abstain from smoking for 2 hours. At baseline, measures of cardiovascular responses were obtained as participants watched erotic videos. The study found that smoking two, but not one, high-nicotine cigarettes significantly decreased the rate at which the diameter of the penis increased in a comparison with the other two conditions (low-nicotine cigarettes, control) during the erectile stimulus (p <0.001). The study also determined that high-nicotine cigarettes caused significantly more vasoconstriction and a higher heart rate than did low-nicotine cigarettes. Glina and colleagues (1988) monitored intracavernous pressures to try to determine whether cigarette smoking interfered with vasoactive, drug-induced erectile responses. Twelve chronic smokers, 22–65 years of age, were not permitted to smoke on test days, except if directed. Each participant underwent pharmacostimulation at baseline and 1 week later immediately after exposure to nicotine (smoking two cigarettes, each with 1.3 mg of nicotine). Investigators obtained measurements of intracavernous pressure 20 minutes after pharmacostimulation. The study found that all 12 men obtained an erection (by clinical judgment) at baseline, compared with only 4 (33%) men after smoking two cigarettes, corresponding to a significant decrease in mean intracavernous pressures from 85.83 millimeters of mercury (Hg) at baseline to 53.50 mm Hg after smoking. In a visual depiction of the effects of cigarette smoking on arterial flow to the penis, Levine and Gerber (1990) described a pelvic arteriographic study of a man, 38 years of age, who had a 25 pack-year smoking history when he presented for evaluation of ED. A complete baseline evaluation, including pelvic arteriographic studies, showed no abnormalities. However, repeat pelvic arteriography immediately after the patient smoked two cigarettes revealed a decrease in the caliber of the entire pudendal artery and nonvisualization of the deep penile artery. The investigators suggested that acute vasospasm was responsible for the observed effects.
A study of smoking cessation by Guay and colleagues (1998) enrolled 10 men, 32–62 years of age, who had at least a 30 pack-year smoking history and were currently smoking one pack of cigarettes or more per day. Participants used the RigiScan technique at home to monitor NPTR. The study required the monitoring of sleep-related penile erections on two successive nights—the first night following a usual day of smoking and the second night following discontinuation of smoking for a 24-hour interval. An additional component of the study involved repeat monitoring for 1 month in four men who did not smoke, although these men were administered transdermal nicotine patches (21 mg) during that time. The study found that erectile parameters improved to a statistically significant degree in the men who had stopped smoking for 24 hours. Erectile parameters improved even more in the men who did not smoke but wore a nicotine patch for 1 month. The study investigators concluded that eliminating cigarette smoking improves erectile function and that chemicals contained in cigarette smoke other than nicotine are primarily responsible for the damaging effects.
Sighinolfi and colleagues (2007) also evaluated the acute effects of smoking cessation on penile hemodynamics. These investigators assessed 20 active smokers, 31–48 years of age, who had ED, per the five-item International Index of Erectile Function (IIEF) questionnaire. These smokers had consumed 20–40 cigarettes per day for a mean of 7 years (range: 5–8 years). Participants underwent penile color Doppler ultrasonography following pharmacostimulation at baseline and underwent Doppler ultrasonography again at 24–36 hours after they withdrew from smoking. At baseline, 10 (50%) of the 20 participants had abnormal peak systolic velocity values and 15 (75%) had abnormal end diastolic velocity values. But after they withdrew from smoking, none of the 20 had an abnormal peak systolic velocity and only 3 (15%) had abnormal end diastolic velocity values. The study suggests that chronic cigarette smoking adversely affects erection, with a predominant effect on the veno-occlusive function of the penis.
Animal Studies
Animal models provide another useful approach to investigating the association between cigarette smoking and ED. Juenemann and colleagues (1987) used an in vivo canine model to monitor arterial inflow, intracavernous pressure, and venous outflow of the penis during stimulation of the cavernous nerve to produce an erection without perfusion of the penis, as well as with regulated penile perfusion before and after acute inhalation of cigarette smoke (1.4 mg nicotine per cigarette). After exposure to smoking (one to six cigarettes), and compared with nonsmoking conditions at baseline, peak arterial inflow was significantly diminished, peak intracavernous pressure was significantly diminished and could not be maintained, and venous outflow was not significantly restricted. Measurable serum nicotine and cotinine levels obtained in the dogs following exposure to smoking were consistent with concentrations found in human smokers, but no changes in arterial blood gases or systemic blood pressure were observed throughout the investigation. The study concluded that smoking exerts a localized deleterious effect on the neurovascular mechanisms required for penile erection, with a particular impairment of the veno-occlusive mechanism that is associated with maintaining an erection.
Xie and colleagues (1997) used a rat model to evaluate the long-term effects of cigarette smoking on erection. Investigators monitored neurostimulated erections in vivo after exposing rats to a constant influx of cigarette smoke in an enclosed cage for a 60-minute session once per day, 5 days per week, for 8 weeks. Compared with controls, smoke-exposed rats exhibited increased intracavernous pressure, but they also developed systemic hypertension. After standardizing intracavernous pressures to systemic blood pressures in the rats exposed to cigarette smoke, intracavernous pressures were not different between exposed rats and controls.
Description of the Literature Review
This section explores available observational data on the association between cigarette smoking (and other forms of tobacco use) and ED. A literature search conducted through May 2010, using the PubMed system of the National Library of Medicine, was supplemented with professional knowledge of other resources. The search terms included “erectile dysfunction and smoking” and “erectile dysfunction and tobacco.”
Epidemiologic Evidence
Unlike quantitative data on tobacco smoking and erectile performance, observational data rely on self-reporting and other subjective instruments (e.g., logs, questionnaires, and inventories of sexual function). A single-item assessment (e.g., “Do you experience difficulty getting and/or maintaining an erection that is rigid enough for satisfactory sexual intercourse?”) has been widely used, particularly for population-based epidemiologic studies (Derby et al. 2000a).
A multi-item questionnaire to distinguish between erectile and ejaculatory dysfunction was developed by the Krimpen Study in The Netherlands (Blanker et al. 2001). This type of questionnaire has been useful as a single, direct, practical tool to ascertain the presence of ED. However, this methodology, as with any self-report, introduces the possibility of information bias, probably in this case with a tendency toward underreporting ED. Differential underreporting of this condition by smoking status would bias estimates of the effects of smoking.
Case Series
Cigarette smoking has been linked to ED in several clinical reports, most of which would qualify as observational case series. As such, they are limited by not having true comparison groups, but they are reviewed here because they are cited often in the literature, and data from more formal studies are limited.
Wabrek and colleagues (1983) studied men who were referred to a hospital-based medical sexology program for evaluation and management of ED. Of 120 men, 50% were smokers, including users of cigarettes, cigars, or pipes. Elsewhere, in a study of 440 men who were referred for clinical evaluation of ED, 64% were smokers, defined as smoking more than 15 cigarettes per day for at least 15 years (Virag et al. 1985). Bornman and du Plessis (1986) observed similar results among 300 men who were screened for impotence at an andrology clinic. Of those who were diagnosed with either psychogenic or vasculogenic impotence, 62% were smokers and had smoked approximately 25 cigarettes per day for more than 20 years.
Condra and colleagues (1986) attempted to provide comparative information using a study of 178 men who were referred for clinical evaluation for ED. In all, 51.4% of the men were current cigarette smokers and 81% were either current or former cigarette smokers. These estimates exceeded the 38.6% and 58.3% estimates, respectively, that were ascertained from the general population (Canada) using concurrent survey data.
Finally, Tengs and Osgood (2001) identified 19 clinical studies of ED involving 3,819 men that had been published in the previous 20 years. Pooling the prevalence of current smoking across the series, they found that 40% of those with ED were current smokers.
Cross-Sectional Studies
Cross-sectional, random surveys of sample populations offer more population-based appraisals of the association of cigarette smoking and ED (Table 9.17S).
The Vietnam Experience Study of 1985–1986 surveyed 4,462 U.S. Army Vietnam-era veterans, 31–49 years of age (Mannino et al. 1994). The study found prevalence rates of ED of 2.2% among never smokers, 2.0% among former smokers, and 3.7% among current smokers (p = 0.005). The association was significant for current smokers (OR = 1.5; 95% CI, 1.0–2.2) even after adjusting for such factors as vascular disease, psychiatric problems, hormonal factors, substance abuse, marital status, race, and age.
In Italy, a cross-sectional study by Parazzini and colleagues (2000) assessed the prevalence of ED in 2,010 men, 18 years of age and older, in 1996–1997. After controlling for multiple variables—including age, marital status, socioeconomic status, and chronic diseases—the authors found an increased risk of ED for current smokers (OR = 1.7; 95% CI, 1.2–2.4; p <0.05) and former smokers (OR = 1.6; 95% CI, 1.1–2.3; p <0.05) compared with lifetime nonsmokers.
The Krimpen Study described previously was a community-based investigation conducted in Rotterdam, The Netherlands, between 1995–1998 that surveyed 1,688 men, 50–78 years of age (Blanker et al. 2001). In this study, smokers were more likely than nonsmokers to report ED (AOR = 1.6; 95% CI, 1.1–2.3; p <0.05). In Spain, Martin-Morales and colleagues (2001) conducted a cross-sectional study of the prevalence of ED in 1998–1999. Among 2,476 men, 25–70 years of age, the authors found that cigarette smoking was significantly associated with ED (AOR = 2.5; 95% CI, 1.64–3.80; p <0.05).
To investigate relationships between smoking and both risk of ED and the prognosis for the condition, Shiri and colleagues (2005) performed a population-based study of 1,442 men, 50–75 years of age, in Finland who had responded to a series of baseline and follow-up questionnaires. The risk for ED from smoking was relatively small (OR = 1.4; 95% CI, 0.9–2.3), and the authors also found that smokers had reduced odds of recovering from ED compared with never smokers (OR = 0.6; 95% CI, 0.2–1.4). In Australia, the association between cigarette smoking and ED was examined as part of the 2001 Australian Study of Health and Relationships. This major national survey of sexual and reproductive health had a large, representative sample of 8,367 Australian men, 16–59 years of age, who were interviewed between mid-2001 and mid-2002 (Millett et al. 2006). The study found that smokers were more likely than nonsmokers to have ED. This association was stronger for heavier smokers: 20 cigarettes or fewer smoked per day (AOR = 1.24; 95% CI, 1.0–1.52; p <0.05), more than 20 cigarettes smoked per day (AOR = 1.39; 95% CI, 1.05–1.83; p <0.05).
In the Global Study of Sexual Attitudes and Behaviors, Moreira and colleagues (2006) investigated the prevalence of sexual problems in Korea. Here, the evaluation of sexual dysfunction relied entirely on self-reporting through a nonvalidated questionnaire. Among the 600 men, 40–80 years of age, who completed the survey, both current and former smoking was associated with erectile and ejaculatory dysfunction.
In Hong Kong, Lam and colleagues (2006b) conducted a cross-sectional survey of 819 Chinese men, 31–60 years of age, to evaluate the association between smoking and ED, which was defined as self-reported dissatisfaction with and/or erection difficulty during sexual intercourse. The authors also used a questionnaire that had not been validated. The authors found that smoking 20 or more cigarettes per day was associated with a 47% increased risk of ED (OR = 1.47; 95% CI, 1.00–2.16; p <0.05) when never smoking was the referent. This study also found that the risk of dissatisfaction with sexual intercourse was significantly lower for former smokers than smokers who were consuming 20 or more cigarettes per day.
In another Asian study, He and colleagues (2007) reported on the association between cigarette smoking and ED among 7,864 Chinese men, 35–74 years of age, who did not have clinical vascular disease. The evaluation examined serum concentrations of cholesterol and triglycerides, the assessment of clinical vascular disease was based on self-reports (Gades et al. 2008). The authors reported a significant dose-response relationship between the risk of ED and cigarette smoking: OR = 1.41 (95% CI, 1.09–1.81). In a comparison with never smokers, the study also found a significant dose-response relationship between the number of cigarettes smoked per day and risk of ED: smoking 1–10 cigarettes per day (age-adjusted OR = 1.22; 95% CI, 0.88–1.68); 11–20 cigarettes per day (age-adjusted OR = 1.39; 95% CI, 1.05–1.85); more than 20 cigarettes per day (age-adjusted OR = 1.70; 95% CI, 1.13–2.56). The authors suggested that cigarette smoking may contribute to approximately 11.8 million cases of ED in China.
Cohort Studies
The Health Professionals Follow-up Study began in 1986 as a prospective cohort study of heart disease and cancer among 51,529 male health professionals in the United States. In a cross-sectional analysis of 34,282 of these men, 53–90 years of age, that controlled for age, marital status, and other variables, Bacon and colleagues (2003) found an increased probability of ED among current smokers versus nonsmokers (OR = 1.3; 95% CI, 1.1–1.4; p <0.05). In another study, Bacon and colleagues (2006) examined prospectively the impact of obesity, physical activity, alcohol use, and smoking on the development of ED among 22,086 men, 40–75 years of age, in the same cohort. The RR of developing ED during the 14-year follow-up among smokers was 1.5 (95% CI, 1.3–1.7). According to the authors, obesity and smoking were positively associated and physical activity was inversely associated with ED.
Findings from the baseline phase of the Massachusetts Male Aging Study—a community-based survey conducted from 1987–1989 of 1,290 men, 40–70 years of age, living in the Boston, Massachusetts, area—did not support an independent association between cigarette smoking and ED (Feldman et al. 1994). Here, the probabilities of complete ED were 11.0% for smokers and 9.3% for nonsmokers, which included former smokers and never smokers (p >0.20). However, the prospective phase of the Massachusetts Male Aging Study, which extended over a median of 9 years, found the comorbidity-adjusted rate of incident ED to be significantly higher among cigarette smokers (24%) than nonsmokers (14%) (OR = 1.97; 95% CI, 1.07–3.63; p = 0.03) (Feldman et al. 2000). The classification of ED on this study was based on an algorithm derived by a discriminant analysis of 13 questions.
When performing cross-sectional analyses of predictors of ED using the baseline data from the Massachusetts Male Aging Study, Kleinman and colleagues (2000) used two new methods for classifying ED. Their field study method, which corresponded to the approach used by Feldman and colleagues (2000), relied on responses to an original questionnaire from men who were attending a urology clinic and answers to a single question to self-rate ED. Their second method was based on responses to an expanded follow-up questionnaire given to a sample of men in the clinic. The field study method found an association between smoking and ED (OR = 1.39; 95% CI, 1.07–1.80), but the expanded questionnaire did not (OR = 0.95; 95% CI, 0.72–1.22).
Using data from the Boston Area Community Health (BACH) survey, Kupelian and colleagues (2007) assessed associations between active and passive smoking and ED. The study used the IIEF questionnaire to assess ED among a random sample of 2,301 racially and ethnically diverse men (approximately one-third each Black, White, and Hispanic), 30–79 years of age (mean age: 47.6 years). After controlling for age and various comorbidities, the study found an association between increased pack-years and greater severity of ED. The dose-response relationship was most prominent among those with 20 or more pack-years of exposure. The BACH survey did not collect information on time since quitting smoking, and so the impact of cessation on erectile function over time could not be assessed.
Finally, Kupelian and colleagues (2010) investigated the relative contributions of modifiable risk factors to ED in a follow-up study of the BACH survey and obtained results consistent with previously published data. The authors found that increased duration and intensity of smoking were associated with greater risk of ED.
Dose-Response Relationships
Several epidemiologic analyses have explored relationships between the amount of exposure to tobacco and the extent of ED. Among currently smoking veterans, the Vietnam Experience Study of 1985–1986 did not show any relationship between ED and the number of cigarettes smoked daily or the number of years of smoking (Mannino et al. 1994). In contrast, in an Italian cross-sectional study, Parazzini and colleagues (2000) found that duration of smoking was associated with an increased risk of ED: for men who smoked less than 20 years, the OR was 1.2 (95% CI, 1.0–7.4); and for men who smoked 20 or more years, the OR was 1.6 (95% CI, 1.1–2.3).
In a case-control study of Canadian men, 50–80 years of age, Polsky and colleagues (2005) investigated the associations between an array of lifestyle and medical factors, including smoking and taking drugs for cardiovascular disease, and ED. The study compared 101 men who had clinically diagnosed ED with 234 controls who had various benign urological conditions. Based on questionnaires completed by participants, the estimated OR was 2.2 (95% CI, 1.17–3.94; p value not reported) for ED in former smokers compared with nonsmokers. The OR for current smokers was not elevated, however, raising the possibility of reverse causation. The study found that those with at least 10 pack-years of smoking had twice the risk of ED as never smokers. The fact that current smoking was not a risk factor for ED was attributed by the authors to the possible bias introduced by the potentially higher likelihood of smokers with symptoms of ED to be encouraged and motivated to quit smoking and thus not be included as smokers in this study.
Interactions with Other Risk Factors, Medications
Several studies have analyzed the combined effects of cigarette smoking and other risk factors in the development of ED. Goldstein and colleagues (1984) examined the clinical characteristics of 19 potent patients who underwent pelvic irradiation for prostate cancer. Fourteen of the 15 patients who displayed diminished erectile capacity after radiation were cigarette smokers, but only 1 of the 4 who preserved their previous erectile capacity was a cigarette smoker. The strong association of cigarette smoking with erectile impairment after radiation in this study led investigators to propose a synergistic role of smoking, and conceivably other vasculopathic risk factors, in radiation-associated ED.
In the baseline phase of the Massachusetts Male Aging Study, Feldman and colleagues (1994) found that cigarette smoking was not an independent risk factor for ED. In that same study, however, the associations of several risk factors with ED were greatly increased in current cigarette smokers. This synergy was demonstrated for persons who had ED and were being treated for heart disease (from 21% for current nonsmokers to 56% for current smokers), treated for treated hypertension (from 8.5% to 20%), and not treated for arthritis (from 9.4% to 20%) and for persons who were receiving various medications, including cardiac drugs (from 14% to 41%), antihypertensives (from 7.5% to 21%), and vasodilators (from 21% to 52%). Similarly, in an Italian cross-sectional study, smoking increased the AORs for ED associated with diabetes by 13% and with hypertension by 39% (Parazzini et al. 2000).
Shiri and colleagues (2006) investigated the role of vascular disease in causing smoking-related ED in men—50, 60, or 70 years of age—in Finland. This questionnaire-based study assessed responses to a series of three surveys that were mailed to the study cohort (3,143 men in 1994; 2,837 men in 1999; and 2,510 men in 2004). Compared with never smokers who did not develop vascular disease (defined as hypertension, heart disease, or cerebrovascular disease), the study found that the risk of developing ED was approximately three times as high (adjusted incidence density ratio = 3.1; 95% CI, 1.3–7.5) among men who smoked in 2004 and developed vascular disease during the study period. An increased risk of ED was not demonstrated for smokers who did not develop vascular disease (adjusted incidence density ratio = 1.0; 95% CI, 0.5–1.8). The study also found that former smokers who had ED at the start of the study in 1994 had a significantly increased risk of developing vascular disease during the remainder of the study period. The authors concluded that smoking may cause ED because it can cause vascular disease, and they further noted the possible utility of this diagnosis (ED) as a marker of silent vascular disease in former smokers.
Gades and colleagues (2005) conducted a questionnaire-based study to evaluate the association between smoking and ED in a randomly selected cohort of 1,329 men, 40–79 years of age, from Minnesota. The authors found that among smokers, the OR for ED decreased with increasing age. In comparisons with never smokers and former smokers in the same age groups, current smokers in their forties had the highest odds of ED (OR = 2.74; 95% CI, 0.44–16.89), followed by smokers in their sixties (OR = 1.70; 95% CI, 0.82–3.51), fifties (OR = 1.38; 95% CI, 0.51–3.74), and seventies (OR = 0.77; 95% CI, 0.27–2.21). The declining effect with increasing age may reflect the increasing prevalence of risk factors for ED other than smoking at older ages.
Effects of Smoking Cessation
Forsberg and colleagues (1989) presented case reports of two cigarette smokers, 20 and 27 years of age, whose ED returned in concordance with improved results for penile vascular tests following cessation. In a study by Elist and colleagues (1984), 8 of 20 men with ED who had smoked one to two packs of cigarettes per day for at least 5 years recovered the ability to achieve functional erections after abstaining from cigarette smoking for 6 weeks. In this study, objective testing criteria confirmed that 7 responders recovered normal erectile activity from levels that were abnormal at baseline.
Population-based reports offer additional perspectives on the premise that modifying smoking behavior affects the occurrence of ED. For example, the Vietnam Experience Study of 1985–1986 determined that the prevalence of ED was comparable between former smokers and nonsmokers and that the prevalence estimates for those groups were significantly lower than those for current smokers (Mannino et al. 1994). Similarly, the longitudinal phase of the Massachusetts Male Aging Study determined that incident ED was no more likely among former smokers than among nonsmokers, but it was more common in current smokers than in former smokers and nonsmokers (Feldman et al. 2000).
These results from population-based studies could suggest that smoking cessation leads to a recovery of functional erection status. However, this conclusion is challenged by results from the prospective evaluation of men who discontinued smoking during the almost 9-year follow-up period of the Massachusetts Male Aging Study (Feldman et al. 2000). According to that analysis, the covariate-adjusted incidence of ED was not significantly reduced after smoking cessation (p = 0.28). The nature of the population in the Massachusetts study merits emphasis, however, because the men who quit smoking had started at an early age (mean age: 16.6 years) and had accumulated substantial lifetime exposure to tobacco smoke before quitting (mean pack-years: 39.4). Data from other studies help to refine our understanding of the effects of cessation on ED. For example, Derby and colleagues (2000b) found that cessation during middle age—after a significant lifetime exposure to cigarette smoke—may fail to modify the occurrence of ED because long-term vascular effects of smoking may persist after cessation.
Travison and colleagues (2007) analyzed the data from the Massachusetts Male Aging Study and found that smoking and self-assessed health status were associated with progression only. Specifically, the odds of a progression of ED doubled with smoking status. The study did not reveal a corresponding decrease in likelihood of remission (i.e., by stopping smoking). The authors concluded that abstaining from smoking may help to protect against the progression of ED, but smoking has little effect on the likelihood of remission once ED begins.
Evidence Synthesis
Mounting evidence indicates that cigarette smoking constitutes a risk factor for ED. The consistency of such a relationship is supported by case series, cross-sectional, and prospective population-based studies that have evaluated rates of ED among smokers. The population-based studies afford a more accurate observational basis for this assessment than do uncontrolled case series, but the number of such studies is limited. Prospective cohort studies are particularly critical in providing evidence not subject to the various limitations of cross-sectional studies. Their results confirm the temporality of the association (i.e., smoking appropriately antedates ED). Several studies demonstrate an increased risk with greater exposure to cigarette smoke. Observational findings demonstrate that cessation of cigarette smoking may lead to a recovery of erectile function only if the discontinuation occurs after a limited extent of lifetime smoking.
The coherence of the relationship between smoking and ED is supported by studies that indicate plausible mechanisms for such a connection. The acute deleterious effects of smoking on erectile function result at least in part from nicotine in cigarette smoke. Nicotine pharmacologically induces vasospasm of penile arteries, thus altering the dynamics of the local blood flow required for erection. The chronic deleterious effects of smoking on erectile function result from impaired vascular physiology of the erectile tissue, as evidenced by degenerative morphologic changes in the tissue of smokers. Studies in animals point to damaging effects of smoking on tissue-dependent erection regulatory factors. In sum, several lines of evidence support the inference of a causal relationship between cigarette smoking and ED.
Conclusion
The evidence is sufficient to infer a causal relationship between smoking and erectile dysfunction.
Implications
The clinical studies and basic scientific research summarized in this section support a causal relationship between cigarette smoking and ED. The current knowledge about the condition affirms recommendations for quitting smoking to limit the risk of ED. Promoting abstinence from smoking to prevent ED is clinically appropriate.
Evidence Summary
This chapter summarizes the consequences of smoking across a wide array of adverse reproductive health effects both immediate and longer-term. The evidence reviewed shows that smoking affects the likelihood of pregnancy, the outcome of pregnancy, and the future health of the child.
This report returns to the topic of smoking during pregnancy and congenital malformations. The 2004 Surgeon General's report found the evidence to be suggestive to infer a causal relationship between maternal smoking during pregnancy and orofacial clefts. Substantial additional evidence supports the strengthening of this conclusion to sufficient. For other congenital abnormalities, the evidence was not sufficient to infer causality and was quite limited in extent for some.
Evidence reviews were also conducted on a number of neurobehavioral disorders, including disorders not included in previous reports: ADHD, ODD, conduct disorder, anxiety, depression, Tourette syndrome, schizophrenia, and intellectual disability. Data show consistent support for an association between maternal prenatal smoking and childhood disruptive behavioral disorders, and ADHD in particular; but the results are attenuated when adjusted for sociodemographic and psychosocial factors. The evidence was determined to be suggestive but not sufficient to infer causality. Additional studies are needed that prospectively collect data on smoking exposure during pregnancy and control for relevant confounders.
Studies of maternal prenatal smoking and anxiety and/or depression did not show significant associations in either children or adult offspring, although a small number of studies found associations with internalizing symptoms in children at ages ranging from 2–14 years of age; positive findings in children at 2 years of age were no longer present by 5 years of age. Additional prospective, longitudinal studies are needed to understand the association of maternal prenatal smoking and both symptoms and diagnoses of anxiety and depression throughout childhood and into adolescence. The evidence was determined to be inadequate to infer a causal relationship.
Data on prenatal smoking and Tourette syndrome and schizophrenia were very limited and did not consistently show significant associations. The evidence for these two outcomes was determined to be inadequate to infer a causal relationship.
Studies of smoking and intellectual disability in child and young adult offspring have not shown significant associations after adjustment for maternal education, IQ, and/or other sociodemographic variables. Evidence was determined to be inadequate to infer a causal relationship; however, additional prospective studies that collect and control for potential confounding variables could benefit the field.
New evidence on two other reproductive health outcomes, EP and SAB, has strengthened support for a causal association for EP and is suggestive of an effect on SAB.
Finally, this report finds the evidence sufficient to infer that smoking adversely affects male reproductive functioning. The 2004 Surgeon General's report found the evidence to be suggestive, but additional experimental and observational studies have been carried out and there are several documented pathways by which smoking impairs male sexual functioning. The 2004 report found that smoking reduced fertility. Thus, for couples who smoke and want to have children, their smoking decreases the likelihood of a successful pregnancy.
Chapter Conclusions
Congenital Malformations
The evidence is sufficient to infer a causal relationship between maternal smoking in early pregnancy and orofacial clefts.
- The evidence is suggestive but not sufficient to infer a causal relationship between maternal smoking in early pregnancy and clubfoot, gastroschisis, and atrial septal heart defects.
Neurobehavioral Disorders of Childhood
- The evidence is suggestive but not sufficient to infer a causal relationship between maternal prenatal smoking and disruptive behavioral disorders, and attention deficit hyperactivity disorder in particular, among children.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and anxiety and depression in children.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and Tourette syndrome.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and schizophrenia in her offspring.
- The evidence is insufficient to infer the presence or absence of a causal relationship between maternal prenatal smoking and intellectual disability.
Ectopic Pregnancy
- The evidence is sufficient to infer a causal relationship between maternal active smoking and ectopic pregnancy.
Spontaneous Abortion
- The evidence is suggestive but not sufficient to infer a causal relationship between maternal active smoking and spontaneous abortion.
Male Sexual Function
- The evidence is sufficient to infer a causal relationship between smoking and erectile dysfunction.
References
- Aagaard-Tillery K, Spong CY, Thom E, Sibai B, Wendel G Jr., Wenstrom K, Samuels P, Simhan H, Sorokin Y, Miodovnik M, et al. Pharmacogenomics of maternal tobacco use: metabolic gene polymorphisms and risk of adverse pregnancy outcomes. Obstetrics and Gynecology. 2010;115(3):568–77. [PMC free article: PMC3263385] [PubMed: 20177288]
- Abber JC, Lue TF, Orvis BR, McClure RD, Williams RD. Diagnostic tests for impotence: a comparison of papaverine injection with the penile-brachial index and nocturnal penile tumescence monitoring. Journal of Urology. 1986;135(5):923–5. [PubMed: 3959242]
- Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Critical Reviews in Toxicology. 2012;42(4):279–303. [PubMed: 22394313]
- Adaikan PG, Ratnam SS. Pharmacology of penile erection in humans. Cardiovascular and Interventional Radiology. 1988;11(4):191–4. [PubMed: 2852054]
- Adams EK, Markowitz S, Kannan V, Dietz PM, Tong VT, Malarcher AM. Reducing prenatal smoking: the role of state policies. American Journal of Preventive Medicine. 2012;43(1):34–40. [PubMed: 22704743]
- Adams KE, Melvin CL, Raskind-Hood CL. Sociodemographic, insurance, and risk profiles of maternal smokers post the 1990s: how can we reach them? Nicotine & Tobacco Research. 2008;10(7):1121–9. [PubMed: 18629721]
- Adams MR, Jessup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: reversibility with oral L-arginine but not vitamin C. Journal of the American College of Cardiology. 1997;29(3):491–7. [PubMed: 9060883]
- Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, Madden PA, Haber JR, Jacob T, Bucholz KK, et al. The effects of maternal smoking during pregnancy on offspring outcomes. Preventive Medicine. 2010;50(1-2):13–8. [PMC free article: PMC2813884] [PubMed: 20026103]
- Akre O, Lipworth L, Cnattingius S, Sparen P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999;10(4):364–9. [PubMed: 10401869]
- Alati R, Macleod J, Hickman M, Sayal K, May M, Smith GD, Lawlor DA. Intrauterine exposure to alcohol and tobacco use and childhood IQ: findings from a parental-offspring comparison within the Avon Longitudinal Study of Parents and Children. Pediatric Research. 2008;64(6):659–66. [PubMed: 18670372]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiological Reviews. 2009;89(1):73–120. [PMC free article: PMC2713585] [PubMed: 19126755]
- Allen R, Brendler C. Nocturnal penile tumescence with polysomnography (NPT-PSG) remains the only objectively validated procedure for differential diagnosis of organic versus psychogenic erectile impotence. In: Lue TF, editor. World Book of Impotence. London: Smith-Gordon; 1992. pp. 75–80.
- Alsweiler JM, Harding JE, Bloomfield FH. Tight glycemic control with insulin in hyperglycemic preterm babies: a randomized controlled trial. Pediatrics. 2012;129(4):639–47. [PubMed: 22430450]
- Altink ME, Arias-Vasquez A, Franke B, Slaats-Willemse DI, Buschgens CJ, Rommelse NN, Fliers EA, Anney R, Brookes KJ, Chen W, et al. The dopamine receptor D4 7-repeat allele and prenatal smoking in ADHD-affected children and their unaffected siblings: no gene-environment interaction. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49(10):1053–60. [PMC free article: PMC2870715] [PubMed: 19017022]
- Altink ME, Slaats-Willemse DI, Rommelse NN, Buschgens CJ, Fliers EA, Arias-Vasquez A, Xu X, Franke B, Sergeant JA, Faraone SV, et al. Effects of maternal and paternal smoking on attentional control in children with and without ADHD. European Child and Adolescent Psychiatry. 2009;18(8):465–75. [PMC free article: PMC2718195] [PubMed: 19288168]
- Alverson CJ, Strickland MJ, Gilboa SM, Correa A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics. 2011;127(3):e647–53. [PubMed: 21357347]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American College of Cardiology. 2004;43(10):1731–7. [PubMed: 15145091]
- American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128(5):1030–9. [PubMed: 22007004]
- American Congress of Obstetricians and Gynecologists. Committee opinion no. 471: smoking cessation during pregnancy. Obstetrics and Gynecology. 2010;116(5):1241–4. [PubMed: 20966731]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013.
- Anselmi L, Menezes AM, Barros FC, Hallal PC, Araujo CL, Domingues MR, Rohde LA. Early determinants of attention and hyperactivity problems in adolescents: the 11-year follow-up of the 1993 Pelotas (Brazil) birth cohort study. Cadernos de Saúde Publica. 2010;26(10):1954–62. [PubMed: 20963293]
- Baardman ME, Kerstjens-Frederikse WS, Corpeleijn E, de Walle HE, Hofstra RM, Berger RM, Bakker MK. Combined adverse effects of maternal smoking and high body mass index on heart development in offspring: evidence for interaction? Heart. 2012;98(6):474–9. [PubMed: 22294555]
- Baba S, Noda H, Nakayama M, Waguri M, Mitsuda N, Iso H. Risk factors of early spontaneous abortions among Japanese: a matched case-control study. Human Reproduction. 2011;26(2):466–72. [PubMed: 21156724]
- Baba S, Wikström AK, Stephansson O, Cnattingius S. Influence of smoking and snuff cessation on risk of preterm birth. European Journal of Epidemiology. 2012;27(4):297–304. [PubMed: 22430122]
- Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Annals of Internal Medicine. 2003;139(3):161–8. [PubMed: 12899583]
- Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. A prospective study of risk factors for erectile dysfunction. Journal of Urology. 2006;176(1):217–21. [PubMed: 16753404]
- Baguelin-Pinaud A, Robert S, Menard JF, Thibaut F. Prenatal exposure to tobacco and risk for schizophrenia: a retrospective epidemiological study. Comprehensive Psychiatry. 2010;51(2):106–9. [PubMed: 20152288]
- Bahren W, Gall H, Scherb W, Stief C, Thon W. Arterial anatomy and arteriographic diagnosis of arteriogenic impotence. Cardiovascular and Interventional Radiology. 1988;11(4):195–210. [PubMed: 3147134]
- Bailey LB, Berry RJ. Folic acid supplementation and the occurrence of congenital heart defects, orofacial clefts, multiple births, and miscarriage. American Journal of Clinical Nutrition. 2005;81(5):1213S–7S. [PubMed: 15883454]
- Bailey LJ, Johnston MC, Billet J. Effects of carbon monoxide and hypoxia on cleft lip in A/J mice. Cleft Palate-Craniofacial Journal. 1995;32(1):14–9. [PubMed: 7727482]
- Ball SW, Gilman SE, Mick E, Fitzmaurice G, Ganz ML, Seidman LJ, Buka SL. Revisiting the association between maternal smoking during pregnancy and ADHD. Journal of Psychiatric Research. 2010;44(15):1058–62. [PubMed: 20413131]
- Bandiera FC, Richardson AK, Lee DJ, He JP, Merikangas KR. Secondhand smoke exposure and mental health among children and adolescents. Archives of Pediatric and Adolescent Medicine. 2011;165(4):332–8. [PMC free article: PMC3075798] [PubMed: 21464381]
- Baric L, MacArthur C, Sherwood M. A study of health education aspects of smoking in pregnancy. International Journal of Health Education. 1976;19(Suppl):1–17.
- Barr M Jr. The teratogenicity of cadmium chloride in two stocks of Wistar rats. Teratology. 1973;7(3):237–42. [PubMed: 4268328]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Human Development. 2003;75(1-2):21–33. [PubMed: 14652157]
- Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring's cognitive ability: empirical evidence for complete confounding in the U.S. national longitudinal survey of youth. Pediatrics. 2006;118(3):943–50. [PubMed: 16950984]
- Beaty TH, Wang H, Hetmanski JB, Fan YT, Zeiger JS, Liang KY, Chiu YF, Vanderkolk CA, Seifert KC, Wulfsberg EA, et al. A case-control study of nonsyndromic oral clefts in Maryland. Annals of Epidemiology. 2001;11(6):434–42. [PubMed: 11454503]
- Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of dopamine transporter genotype with prenatal smoke exposure on ADHD symptoms. Journal of Pediatrics. 2008;152(2):263–9. [PubMed: 18206700]
- Benowitz N, Dempsey D. Pharmacotherapy for smoking cessation during pregnancy. Nicotine & Tobacco Research. 2004;6(Suppl 2):S189–S202. [PubMed: 15203821]
- Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P 3rd. Ethnic differences in N-glucuronidation of nicotine and cotinine. Journal of Pharmacology and Experimental Therapeutics. 1999;291(3):1196–203. [PubMed: 10565842]
- Bhattacharya S, Townend J, Bhattacharya S. Recurrent miscarriage: are three miscarriages one too many? Analysis of a Scottish population-based database of 151,021 pregnancies. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2010;150(1):24–7. [PubMed: 20207064]
- Biederman J, Monuteaux MC, Faraone SV, Mick E. Parsing the associations between prenatal exposure to nicotine and offspring psychopathology in a nonreferred sample. Journal of Adolescent Health. 2009;45(2):142–8. [PMC free article: PMC2759300] [PubMed: 19628140]
- Biederman J, Petty CR, Bhide PG, Woodworth KY, Faraone S. Does exposure to maternal smoking during pregnancy affect the clinical features of ADHD? Results from a controlled study. World Journal of Biological Psychiatry. 2012;13(1):60–4. [PMC free article: PMC3732048] [PubMed: 21545244]
- Biggs ML, Baer A, Critchlow CW. Maternal, delivery, and perinatal characteristics associated with cryptorchidism: a population-based case-control study among births in Washington State. Epidemiology. 2002;13(2):197–204. [PubMed: 11880761]
- Bille C, Olsen J, Vach W, Knudsen VK, Olsen SF, Rasmussen K, Murray JC, Andersen AM, Christensen K. Oral clefts and life style factors—a case-cohort study based on prospective Danish data. European Journal of Epidemiology. 2007;22(3):173–81. [PubMed: 17295096]
- Bird MT, Robbins JM, Druschel C, Cleves MA, Yang S, Hobbs CA. Demographic and environmental risk factors for gastroschisis and omphalocele in the National Birth Defects Prevention Study. Journal of Pediatric Surgery. 2009;44(8):1546–51. [PubMed: 19635303]
- Blanker MH, Bohnen AM, Groeneveld FP, Bernsen RM, Prins A, Thomas S, Bosch JL. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community-based study. Journal of the American Geriatrics Society. 2001;49(4):436–42. [PubMed: 11347788]
- Blohm F, Friden B, Milsom I. A prospective longitudinal population-based study of clinical miscarriage in an urban Swedish population. BJOG: An International Journal of Obstetrics and Gynaecology. 2008;115(2):176–82. discussion 83. [PubMed: 18081599]
- Boden JM, Fergusson DM, Horwood LJ. Risk factors for conduct disorder and oppositional/defiant disorder: evidence from a New Zealand birth cohort. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(11):1125–33. [PubMed: 20970700]
- Bornman MS, du Plessis DJ. Smoking and vascular impotence: a reason for concern. South African Medical Journal. 1986;70(6):329–30. [PubMed: 3750136]
- Bos-Veneman NG, Kuin A, Minderaa RB, Hoekstra PJ. Role of perinatal adversities on tic severity and symptoms of attention deficit/hyperactivity disorder in children and adolescents with a tic disorder. Journal of Developmental and Behavioral Pediatrics. 2010;31(2):100–6. [PubMed: 20110829]
- Botto LD, Lynberg MC, Erickson JD. Congenital heart defects, maternal febrile illness, and multivitamin use: a population-based study. Epidemiology. 2001;12(5):485–90. [PubMed: 11505164]
- Bouyer J, Coste J, Shojaei T, Pouly JL, Fernandez H, Gerbaud L, Job-Spira N. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. American Journal of Epidemiology. 2003;157(3):185–94. [PubMed: 12543617]
- Braun JM, Daniels JL, Kalkbrenner A, Zimmerman J, Nicholas JS. The effect of maternal smoking during pregnancy on intellectual disabilities among 8-year-old children. Paediatric and Perinatal Epidemiology. 2009;23(5):482–91. [PubMed: 19689499]
- Brennan PA, Hammen C, Sylvers P, Bor W, Najman J, Lind P, Montgomery G, Smith AK. Interactions between the COMT Val108/158Met polymorphism and maternal prenatal smoking predict aggressive behavior outcomes. Biological Psychology. 2011;I(1):99–105. [PMC free article: PMC3081881] [PubMed: 21356266]
- Breslau N, Chilcoat HD. Psychiatric sequelae of low birth weight at 11 years of age. Biological Psychiatry. 2000;47(11):1005–11. [PubMed: 10838069]
- Breslau N, Paneth N, Lucia VC, Paneth-Pollak R. Maternal smoking during pregnancy and offspring IQ. International Journal of Epidemiology. 2005;34(5):1047–53. [PubMed: 16085682]
- Bronsky PT, Johnston MC, Sulik KK. Morphogenesis of hypoxia-induced cleft lip in CL/Fr mice. Journal of Craniofacial Genetics and Developmental Biology. Supplement. 1986;2:113–28. [PubMed: 3491106]
- Brose LS, McEwen A, West R. Association between nicotine replacement therapy use in pregnancy and smoking cessation. Drug and Alcohol Dependence. 2013;132(3):660–4. [PubMed: 23680076]
- Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Risk factors for hypospadias. European Journal of Pediatrics. 2007;166(7):671–8. [PubMed: 17103190]
- Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Journal of Hypertension. 2005;23(2):233–46. [PubMed: 15662207]
- Bublitz MH, Stroud LR. Maternal smoking during pregnancy and offspring brain structure and function: review and agenda for future research. Nicotine & Tobacco Research. 2012;14(4):388–97. [PMC free article: PMC3313781] [PubMed: 22180574]
- Burke JD, Loeber R, Mutchka JS, Lahey BB. A question for DSM-V: which better predicts persistent conduct disorder—delinquent acts or conduct symptoms? Criminal Behavior and Mental Health. 2002;12(1):37–52. [PubMed: 12357256]
- Burnett AL. Nitric oxide in the penis: physiology and pathology. Journal of Urology. 1997;157(1):320–4. [PubMed: 8976289]
- Buschgens CJ, Swinkels SH, van Aken MA, Ormel J, Verhulst FC, Buitelaar JK. Externalizing behaviors in preadolescents: familial risk to externalizing behaviors, prenatal and perinatal risks, and their interactions. European Child and Adolescent Psychiatry. 2009;18(2):65–74. [PubMed: 18587681]
- Button TM, Thapar A, McGuffin P. Relationship between antisocial behaviour, attention-deficit hyperactivity disorder and maternal prenatal smoking. British Journal of Psychiatry. 2005;187:155–60. [PubMed: 16055827]
- Butzelaar L, Breugem CC, Hanlo P, Mink van der Molen AB. Is isolated sagittal synostosis an isolated condition? Journal of Craniofacial Surgery. 2009;20(2):399–401. [PubMed: 19276825]
- Campbell S, Lynch J, Esterman A, McDermott R. Pre-pregnancy predictors linked to miscarriage among Aboriginal and Torres Strait Islander women in North Queensland. Australian and New Zealand Journal of Public Health. 2011;35(4):343–51. [PubMed: 21806729]
- Camperlengo LT, Shapiro-Mendoza CK, Kim SY. Sudden infant death syndrome: diagnostic practices and investigative policies, 2004. American Journal of Forensic Medicine and Pathology. 2012;33(3):197–201. [PubMed: 21030849]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. American Journal of Psychiatry. 2002;159(7):1080–92. [PubMed: 12091183]
- Cardy AH, Barker S, Chesney D, Sharp L, Maffulli N, Miedzybrodzka Z. Pedigree analysis and epidemiological features of idiopathic congenital talipes equinovarus in the United Kingdom: a case-control study. BMC Musculoskeletal Disorders. 2007;8:62. [PMC free article: PMC1939995] [PubMed: 17610748]
- Carmichael NG, Backhouse BL, Winder C, Lewis PD. Teratogenicity, toxicity and perinatal effects of cadmium. Human Toxicology. 1982;1(2):159–86. [PubMed: 6757102]
- Carmichael SL, Ma C, Rasmussen SA, Honein MA, Lammer EJ, Shaw GM. Craniosynostosis and maternal smoking. Birth Defects Research Part A: Clinical and Molecular Teratology. 2008;82(2):78–85. [PubMed: 18050313]
- Carmichael SL, Shaw GM, Laurent C, Lammer EJ, Olney RS. Hypospadias and maternal exposures to cigarette smoke. Paediatric and Perinatal Epidemiology. 2005;19(6):406–12. [PubMed: 16269066]
- Carmichael SL, Shaw GM, Yang W, Lammer EJ, Zhu H, Finnell RH. Limb deficiency defects, MSX1, and exposure to tobacco smoke. American Journal of Medical Genetics. Part A. 2004;125A(3):285–9. [PubMed: 14994238]
- Carratù MR, Cagiano R, De Salvia MA, Cuomo V. Wallerian degeneration in rat sciatic nerve: comparative evaluation between prenatal and postnatal exposure to carbon monoxide. Society for Neuroscience Abstracts. 1993;19(Part 1):834. [abstract].1993.
- Carratù MR, Renna G, Giustino A, De Salvia MA, Cuomo V. Changes in peripheral nervous system activity produced in rats by prenatal exposure to carbon monoxide. Archives of Toxicology. 1993;67(5):297–301. [PubMed: 8368938]
- Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. American Journal of Preventive Medicine. 1999;16(3):208–15. [PubMed: 10198660]
- Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–55. [PubMed: 8222109]
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990–1992. Morbidity and Mortality Weekly Report. 1995;44(3):46–8. [PubMed: 7823895]
- Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. Morbidity and Mortality Weekly Report. 2008;57(1):1–5. [PubMed: 18185492]
- Centers for Disease Control and Prevention. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. Morbidity and Mortality Weekly Report. 2010;59(44):1439–43. [PubMed: 21063274]
- Centers for Disease Control and Prevention. Current tobacco use and secondhand smoke exposure among women of reproductive age—14 countries, 2008–2010. Morbidity and Mortality Weekly Report. 2012;61(43):877–82. [PubMed: 23114255]
- Centers for Disease Control and Prevention. National Health Interview Survey. 2013. [May 23, 2013]. < http://www
.cdc.gov/nchs/nhis.htm>. - Centers for Disease Control and Prevention. National Survey of Family Growth. 2013. [May 23 2013]. < http://www
.cdc.gov/nchs/nsfg.htm>. - Chabra S, Gleason CA, Seidel K, Williams MA. Rising prevalence of gastroschisis in Washington State. Journal of Toxicology and Environmental Health Part A. 2011;74(5):336–45. [PubMed: 21240733]
- Chambers CD, Chen BH, Kalla K, Jernigan L, Jones KL. Novel risk factor in gastroschisis: change of paternity. American Journal of Medical Genetics. Part A. 2007;143(7):653–9. [PubMed: 17163540]
- Chang LW, Wade PR, Pounds JG, Reuhl KR. Prenatal and neonatal toxicology and pathology of heavy metals. Advances in Pharmacology and Chemotherapy. 1980;17:195–231. [PubMed: 7004140]
- Chatenoud L, Parazzini F, di Cintio E, Zanconato G, Benzi G, Bortolus R, La Vecchia C. Paternal and maternal smoking habits before conception and during the first trimester: relation to spontaneous abortion. Annals of Epidemiology. 1998;8(8):520–6. [PubMed: 9802597]
- Chernoff N. Teratogenic effects of cadmium in rats. Teratology. 1973;8(1):29–32. [PubMed: 4737490]
- Chevrier C, Bahuau M, Perret C, Iovannisci DM, Nelva A, Herman C, Vazquez MP, Francannet C, Robert-Gnansia E, Lammer EJ, et al. Genetic susceptibilities in the association between maternal exposure to tobacco smoke and the risk of nonsyndromic oral cleft. American Journal of Medical Genetics. Part A. 2008;146A(18):2396–406. [PubMed: 18698632]
- Chow WH, Daling JR, Weiss NS, Voigt LF. Maternal cigarette smoking and tubal pregnancy. Obstetrics and Gynecology. 1988;71(2):167–70. [PubMed: 3336551]
- Christensen K, Olsen J, Norgaard-Pedersen B, Basso O, Stovring H, Milhollin-Johnson L, Murray JC. Oral clefts, transforming growth factor alpha gene variants, and maternal smoking: a population-based case-control study in Denmark, 1991–1994. American Journal of Epidemiology. 1999;149(3):248–55. [PubMed: 9927220]
- Christianson RE. Gross differences observed in the placentas of smokers and nonsmokers. American Journal of Epidemiology. 1979;110(2):178–87. [PubMed: 463872]
- Cnattingius S, Haglund B, Meirik O. Cigarette smoking as risk factor for late fetal and early neonatal death. British Medical Journal. 1988;297(6643):258–61. [PMC free article: PMC1833960] [PubMed: 3416144]
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2012;(9) Art No.: CD010078. [PubMed: 22972148] [CrossRef]
- Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. American Journal of Obstetrics and Gynecology. 1999;181(4):1026–35. [PubMed: 10521771]
- Condra M, Morales A, Owen JA, Surridge DH, Fenemore J. Prevalence and significance of tobacco smoking in impotence. Urology. 1986;27(6):495–8. [PubMed: 3716047]
- Cornelius JR, Lynch K, Martin CS, Cornelius MD, Clark DB. Clinical correlates of heavy tobacco use among adolescents. Addictive Behaviors. 2001;26(2):273–7. [PubMed: 11316382]
- Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine & Tobacco Research. 2007;9(7):739–50. [PMC free article: PMC2593871] [PubMed: 17577803]
- Coste J, Job-Spira N, Fernandez H. Increased risk of ectopic pregnancy with maternal cigarette smoking. American Journal of Public Health. 1991;81(2):199–201. [PMC free article: PMC1404958] [PubMed: 1990859]
- Cox B, Martens E, Nemery B, Vangronsveld J, Nawrot TS. Impact of a stepwise introduction of smokefree legislation on the rate of preterm births: analysis of routinely collected birth data. British Medical Journal. 2013;346:f441. [PMC free article: PMC3573179] [PubMed: 23412829]
- Cramer DW, Wise LA. The epidemiology of recurrent pregnancy loss. Seminars in Reproductive Medicine. 2000;18(4):331–9. [PubMed: 11355791]
- Cresci M, Foffa I, Ait-Ali L, Pulignani S, Gianicolo EA, Botto N, Picano E, Andreassi MG. Maternal and paternal environmental risk factors, metabolizing GSTM1 and GSTT1 polymorphisms, and congenital heart disease. American Journal of Cardiology. 2011;108(11):1625–31. [PubMed: 21890078]
- Damgaard IN, Jensen TK, Petersen JH, Skakkebaek NE, Toppari J, Main KM. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PloS One. 2008;3(8):e3051. [PMC free article: PMC2516600] [PubMed: 18725961]
- DePalma RG, Emsellem HA, Edwards CM, Druy EM, Shultz SW, Miller HC, Bergsrud D. A screening sequence for vasculogenic impotence. Journal of Vascular Surgery. 1987;5(2):228–36. [PubMed: 3820400]
- Derby CA, Araujo AB, Johannes CB, Feldman HA, McKinlay JB. Measurement of erectile dysfunction in population-based studies: the use of a single question self-assessment in the Massachusetts Male Aging Study. International Journal of Impotence Research. 2000;12(4):197–204. [PubMed: 11079360]
- Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56(2):302–6. [PubMed: 10925098]
- DeYoung L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. Journal of Andrology. 2004;25(5):830–6. [PubMed: 15292117]
- DiCarlantonio G, Talbot P. Inhalation of mainstream and sidestream cigarette smoke retards embryo transport and slows muscle contraction in oviducts of hamsters (Mesocricetus auratus). Biology of Reproduction. 1999;61(3):651–6. [PubMed: 10456841]
- Dickinson KC, Meyer RE, Kotch J. Maternal smoking and the risk for clubfoot in infants. Birth Defects Research Part A: Clinical and Molecular Teratology. 2008;82(2):86–91. [PubMed: 18022868]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. American Journal of Preventive Medicine. 2010;39(1):45–52. [PubMed: 20547278]
- DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. Journal of Family Practice. 1995;40(4):385–94. [PubMed: 7699353]
- D'Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Development and Psychopathology. 2008;20(1):139–64. [PMC free article: PMC3737574] [PubMed: 18211732]
- Draper ES, Rankin J, Tonks AM, Abrams KR, Field DJ, Clarke M, Kurinczuk JJ. Recreational drug use: a major risk factor for gastroschisis? American Journal of Epidemiology. 2008;167(4):485–91. [PubMed: 18063593]
- Duncan WC, McDonald SE, Dickinson RE, Shaw JL, Lourenco PC, Wheelhouse N, Lee KF, Critchley HO, Horne AW. Expression of the repulsive SLIT/ROBO pathway in the human endometrium and Fallopian tube. Molecular Human Reproduction. 2010;16(12):950–9. [PMC free article: PMC2992050] [PubMed: 20651036]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Research Part C: Embryo Today. 2008;84(1):30–44. [PubMed: 18383130]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacology and Therapeutics. 2009;122(2):125–39. [PMC free article: PMC2746456] [PubMed: 19268688]
- Elhanbly S, Abdel-Gaber S, Fathy H, El-Bayoumi Y, Wald M, Niederberger CS. Erectile dysfunction in smokers: a penile dynamic and vascular study. Journal of Andrology. 2004;25(6):991–5. [PubMed: 15477374]
- Elist J, Jarman WD, Edson M. Evaluating medical treatment of impotence. Urology. 1984;23(4):374–5. [PubMed: 6710711]
- Ellis LC, Berg-Nielsen TS, Lydersen S, Wichstrom L. Smoking during pregnancy and psychiatric disorders in preschoolers. European Child and Adolescent Psychiatry. 2012;21(11):635–44. [PubMed: 22767183]
- England LJ, Kim SY, Shapiro-Mendoza CK, Wilson HG, Kendrick JS, Satten GA, Lewis CA, Tucker MJ, Callaghan WM. Effects of maternal smokeless tobacco use on selected pregnancy outcomes in Alaska Native women: a case-control study. Acta Obstetricia et Gynecologica Scandinavica. 2013;92(6):648–55. [PMC free article: PMC5881106] [PubMed: 23551054]
- England LJ, Kim SY, Shapiro-Mendoza CK, Wilson HG, Kendrick JS, Satten GA, Lewis CA, Whittern P, Tucker MJ, Callaghan WM. Maternal smokeless tobacco use in Alaska Native women and singleton infant birth size. Acta Obstetricia et Gynecologica Scandinavica. 2012;91(1):93–103. [PubMed: 21902677]
- England LJ, Levine RJ, Mills JL, Klebanoff MA, Yu KF, Cnattingius S. Adverse pregnancy outcomes in snuff users. American Journal of Obstetrics and Gynecology. 2003;189(4):939–43. [PubMed: 14586330]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(6):630–41. [PubMed: 11392340]
- Esposito K, Ciotola M, Giugliano F, Maiorino MI, Autorino R, De SM, Giugliano G, Nicoletti G, D'Andrea F, Giugliano D. Effects of intensive lifestyle changes on erectile dysfunction in men. Journal of Sexual Medicine. 2009;6(1):243–50. [PubMed: 19170853]
- Esposito K, Giugliano F, Di PC, Giugliano G, Marfella R, D'Andrea F, D'Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2004;291(24):2978–84. [PubMed: 15213209]
- Fallin MD, Hetmanski JB, Park J, Scott AF, Ingersoll R, Fuernkranz HA, McIntosh I, Beaty TH. Family-based analysis of MSX1 haplotypes for association with oral clefts. Genetic Epidemiology. 2003;25(2):168–75. [PubMed: 12916025]
- Fechter LD. Neurotoxicity of prenatal carbon monoxide exposure. Research Report of the Health Effects Institute. 1987;(12):3–22. [PubMed: 3269253]
- Feldkamp ML, Alder SC, Carey JC. A case control population-based study investigating smoking as a risk factor for gastroschisis in Utah, 1997–2005. Birth Defects Research Part A: Clinical and Molecular Teratology. 2008;82(11):768–75. [PubMed: 18985693]
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. Journal of Urology. 1994;151(1):54–61. [PubMed: 8254833]
- Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, McKinlay JB. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts Male Aging Study. Preventive Medicine. 2000;30(4):328–38. [PubMed: 10731462]
- Ferm VH. Developmental malformations induced by cadmium. A study of timed injections during embryogenesis. Biology of the Neonate. 1971;19(1):101–7. [PubMed: 5003301]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biology of the Neonate. 1994;65(3-4):194–7. [PubMed: 8038282]
- Fiore MC, Jaén C, Baker T, Bailey W, Benowitz N, Curry S, Dorfman S, Froelicher E, Goldstein M, Healton C, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service; 2008. Clinical Practice Guideline.
- Forsberg L, Hederstrom E, Olsson AM. Severe arterial insufficiency in impotence confirmed with an improved angiographic technique: the impact of smoking and some other etiologic factors. European Urology. 1989;16(5):357–60. [PubMed: 2776807]
- Freitag CM, Hanig S, Schneider A, Seitz C, Palmason H, Retz W, Meyer J. Biological and psychosocial environmental risk factors influence symptom severity and psychiatric comorbidity in children with ADHD. Journal of Neural Transmission. 2012;119(1):81–94. [PubMed: 21626412]
- Fried PA, Watkinson B. Visuoperceptual functioning differs in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology. 2000;22(1):11–20. [PubMed: 10642110]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology. 2003;25(4):427–36. [PubMed: 12798960]
- Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, Kahn RS. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124(6):e1054–63. [PMC free article: PMC2853804] [PubMed: 19933729]
- Gades NM, Nehra A, Jacobson DJ, McGree ME, Girman CJ, Rhodes T, Roberts RO, Lieber MM, Jacobsen SJ. Association between smoking and erectile dysfunction: a population-based study. American Journal of Epidemiology. 2005;161(4):346–51. [PubMed: 15692078]
- Gades NM, Nehra A, Jacobson DJ, McGree ME, Sauver JL, Jacobsen SJ. Re: cigarette smoking and erectile dysfunction among Chinese men without clinical vascular disease. American Journal of Epidemiology. 2008;167(7):882–3. [PubMed: 18296706]
- Gallicchio L, Miller S, Greene T, Zacur H, Flaws JA. Cosmetologists and reproductive outcomes. Obstetrics and Gynecology. 2009;113(5):1018–26. [PubMed: 19384116]
- Gatzke-Kopp LM, Beauchaine TP. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry and Human Development. 2007;38(4):255–69. [PMC free article: PMC2711763] [PubMed: 17520361]
- Genisca AE, Frias JL, Broussard CS, Honein MA, Lammer EJ, Moore CA, Shaw GM, Murray JC, Yang W, Rasmussen SA. Orofacial clefts in the National Birth Defects Prevention Study, 1997–2004. American Journal of Medical Genetics. Part A. 2009;149A(6):1149–58. [PMC free article: PMC3111146] [PubMed: 19441124]
- George L, Granath F, Johansson AL, Anneren G, Cnattingius S. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology. 2006;17(5):500–5. [PubMed: 16837826]
- Ghandour RM, Kogan MD, Blumberg SJ, Jones JR, Perrin JM. Mental health conditions among school-aged children: geographic and sociodemographic patterns in prevalence and treatment. Journal of Developmental and Behavioral Pediatrics. 2012;33(1):42–54. [PubMed: 22218014]
- Gilbert DG, Hagen RL, D'Agostino JA. The effects of cigarette smoking on human sexual potency. Addictive Behaviors. 1986;11(4):431–4. [PubMed: 3812052]
- Gilboa SM, Mendola P, Olshan AF, Langlois PH, Savitz DA, Loomis D, Herring AH, Fixler DE. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. American Journal of Epidemiology. 2005;162(3):238–52. [PubMed: 15987727]
- Glina S, Reichelt AC, Leao PP, Dos Reis JM. Impact of cigarette smoking on papaverine-induced erection. Journal of Urology. 1988;140(3):523–4. [PubMed: 3411666]
- Goldstein I, Feldman MI, Deckers PJ, Babayan RK, Krane RJ. Radiation-associated impotence: a clinical study of its mechanism. JAMA: the Journal of the American Medical Association. 1984;251(7):903–10. [PubMed: 6694291]
- Goldstein I, Hatzichristou D. Epidemiology of impotence. In: Bennett A, editor. Impotence: Diagnosis and Management of Erectile Dysfunction. Philadelphia: W.B. Saunders; 1994. pp. 1–17.
- Goldwater PN. A perspective on SIDS pathogenesis. the hypotheses: plausibility and evidence. BMC Medicine. 2011;9:64. [PMC free article: PMC3127778] [PubMed: 21619576]
- Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harbor Perspectives in Medicine. 2012;2(12):a012120. [PMC free article: PMC3543069] [PubMed: 22983224]
- Goyer RA. Transplacental transfer of cadmium and fetal effects. Fundamental and Applied Toxicology. 1991;16(1):22–3. [PubMed: 2019348]
- Gray RF, Indurkhya A, McCormick MC. Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics. 2004;114(3):736–43. [PubMed: 15342847]
- Grazuleviciene R, Danileviciute A, Nadisauskiene R, Vencloviene J. Maternal smoking, GSTM1 and GSTT1 polymorphism and susceptibility to adverse pregnancy outcomes. International Journal of Environmental Research and Public Health. 2009;6(3):1282–97. [PMC free article: PMC2672398] [PubMed: 19440446]
- Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Research Part A: Clinical and Molecular Teratology. 2008;82(7):519–26. [PMC free article: PMC2861577] [PubMed: 18481814]
- Gruslin A, Perkins SL, Manchanda R, Fleming N, Clinch JJ. Maternal smoking and fetal erythropoietin levels. Obstetrics and Gynecology. 2000;95(4):561–4. [PubMed: 10725490]
- Guay AT, Perez JB, Heatley GJ. Cessation of smoking rapidly decreases erectile dysfunction. Endocrine Practice. 1998;4(1):23–6. [PubMed: 15251760]
- Gunnerbeck A, Wikstrom AK, Bonamy AK, Wickstrom R, Cnattingius S. Relationship of maternal snuff use and cigarette smoking with neonatal apnea. Pediatrics. 2011;128(3):503–9. [PubMed: 21873701]
- Guo X, Oldham MJ, Kleinman MT, Phalen RF, Kassab GS. Effect of cigarette smoking on nitric oxide, structural, and mechanical properties of mouse arteries. American Journal of Physiology: Heart and Circulatory Physiology. 2006;291(5):H2354–H61. [PubMed: 16815989]
- Gupta PC, Subramoney S. Smokeless tobacco use and risk of stillbirth: a cohort study in Mumbai, India. Epidemiology. 2006;17(1):47–51. [PubMed: 16357594]
- Gupta PC, Subramoney S. Smokeless tobacco use, birth weight, and gestational age: population based, prospective cohort study of 1,217 women in Mumbai, India. British Medical Journal. 2004;328(7455):1538. [PMC free article: PMC437147] [PubMed: 15198947]
- Gustafson O, Nylund L, Carlstrom K. Does hyperandrogenism explain lower in vitro fertilization (IVF) success rates in smokers? Acta Obstetricia et Gynecologica Scandinavica. 1996;75(2):149–56. [PubMed: 8604602]
- Gustafsson P, Kallen K. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Developmental Medicine and Child Neurology. 2011;53(3):263–8. [PubMed: 20964677]
- Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update. 2011;17(5):589–604. [PMC free article: PMC3156888] [PubMed: 21747128]
- Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2009;(1) Art. No.: CD003999. [PubMed: 19160228] [CrossRef]
- Halbert SA, Tam PY, Adams RJ, Blandau RJ. An analysis of the mechanisms of egg transport in the ampulla of the rabbit oviduct. Gynecologic Investigation. 1976;7(5):306–20. [PubMed: 1001998]
- Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science. 1976;191(4231):1052–3. [PubMed: 1251215]
- Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2011. National Vital Statistics Reports. 2012;61(5):1–18. [PubMed: 24979973]
- Handler A, Davis F, Ferre C, Yeko T. The relationship of smoking and ectopic pregnancy. American Journal of Public Health. 1989;79(9):1239–42. [PMC free article: PMC1349696] [PubMed: 2764200]
- Harper RM, Kinney HC. Potential mechanisms of failure in the sudden infant death syndrome. Current Pediatric Reviews. 2010;6(1):39–47. [PMC free article: PMC3392684] [PubMed: 22792083]
- Harrison KL, Breen TM, Hennessey JF. The effect of patient smoking habit on the outcome of IVF and GIFT treatment. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1990;30(4):340–2. [PubMed: 2082890]
- Harte CB, Meston CM. Acute effects of nicotine on physiological and subjective sexual arousal in nonsmoking men: a randomized, double-blind, placebo-controlled trial. Journal of Sexual Medicine. 2008;5(1):110–21. [PMC free article: PMC2864030] [PubMed: 17971108]
- Hartsfield JK Jr, Hickman TA, Everett ET, Shaw GM, Lammer EJ, Finnell RA. Analysis of the EPHX1 113 polymorphism and GSTM1 homozygous null polymorphism and oral clefting associated with maternal smoking. American Journal of Medical Genetics. 2001;102(1):21–4. [PubMed: 11471167]
- Hay DF, Pawlby S, Waters CS, Perra O, Sharp D. Mothers' antenatal depression and their children's antisocial outcomes. Child Development. 2010;81(1):149–65. [PubMed: 20331659]
- He J, Reynolds K, Chen J, Chen CS, Wu X, Duan X, Reynolds R, Bazzano LA, Whelton PK, Gu D. Cigarette smoking and erectile dysfunction among Chinese men without clinical vascular disease. American Journal of Epidemiology. 2007;166(7):803–9. [PubMed: 17623743]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Current Opinion in Pediatrics. 2008;20(2):184–90. [PubMed: 18332716]
- Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, Higgins TM, Solomon LJ. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105(11):2023–30. [PMC free article: PMC2970671] [PubMed: 20840188]
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, Bernstein IM. Financial incentives for smoking cessation among pregnant and newly postpartum women. Preventive Medicine. 2012;55(Suppl):S33–40. [PMC free article: PMC3399924] [PubMed: 22227223]
- Hill SY, Lowers L, Locke-Wellman J, Shen SA. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. Journal of Studies on Alcohol. 2000;61(5):661–8. [PMC free article: PMC4128282] [PubMed: 11022804]
- Hirshkowitz M, Karacan I, Howell JW, Arcasoy MO, Williams RL. Nocturnal penile tumescence in cigarette smokers with erectile dysfunction. Urology. 1992;39(2):101–7. [PubMed: 1736499]
- Holtrop JS, Meghea C, Raffo JE, Biery L, Chartkoff SB, Roman L. Smoking among pregnant women with Medicaid insurance: are mental health factors related? Maternal and Child Health Journal. 2010;14(6):971–7. [PubMed: 19838777]
- Honein MA, Paulozzi LJ, Moore CA. Family history, maternal smoking, and clubfoot: an indication of a gene-environment interaction. American Journal of Epidemiology. 2000;152(7):658–65. [PubMed: 11032161]
- Honein MA, Paulozzi LJ, Watkins ML. Maternal smoking and birth defects: validity of birth certificate data for effect estimation. Public Health Reports. 2001;116(4):327–35. [PMC free article: PMC1497341] [PubMed: 12037261]
- Honein MA, Rasmussen SA. Further evidence for an association between maternal smoking and craniosynostosis. Teratology. 2000;62(3):145–6. [PubMed: 10935977]
- Honein MA, Rasmussen SA, Reefhuis J, Romitti PA, Lammer EJ, Sun L, Correa A. Maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts. Epidemiology. 2007;18(2):226–33. [PubMed: 17202867]
- Horne RS, Parslow PM, Harding R. Postnatal development of ventilatory and arousal responses to hypoxia in human infants. Respiratory Physiology & Neurobiology. 2005;149(1-3):257–71. [PubMed: 15876558]
- Hotston MR, Jeremy JY, Bloor J, Koupparis A, Persad R, Shukla N. Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-alpha in cavernosal vascular smooth muscle cells: mediation by superoxide. British Journal of Urology International. 2007;99(3):612–8. [PubMed: 17176295]
- Hougland KT, Hanna AM, Meyers R, Null D. Increasing prevalence of gastroschisis in Utah. Journal of Pediatric Surgery. 2005;40(3):535–40. [PubMed: 15793731]
- Huang YH, Brown AR, Cross SJ, Cruz J, Rice A, Jaiswal S, Fregosi RF. Influence of prenatal nicotine exposure on development of the ventilatory response to hypoxia and hypercapnia in neonatal rats. Journal of Applied Physiology. 2010;109(1):149–58. [PMC free article: PMC4631539] [PubMed: 20431025]
- Hughes EG, YoungLai EV, Ward SM. Cigarette smoking and outcomes of in-vitro fertilization and embryo transfer: a prospective cohort study. Human Reproduction. 1992;7(3):358–61. [PubMed: 1587943]
- Huijbregts SC, Seguin JR, Zoccolillo M, Boivin M, Tremblay RE. Associations of maternal prenatal smoking with early childhood physical aggression, hyperactivity-impulsivity, and their co-occurrence. Journal of Abnormal Child Psychology. 2007;35(2):203–15. [PMC free article: PMC1915590] [PubMed: 17294130]
- Huijbregts SCJ, Séguin JR, Zelazo PD, Parent S, Japel C, Tremblay RE. Interrelations between maternal smoking during pregnancy, birth weight and sociodemographic factors in the prediction of early cognitive abilities. Infant and Child Development. 2006;15(6):593–607. [PMC free article: PMC5370203] [PubMed: 28360824]
- Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophrenia Bulletin. 2011;37(6):1200–8. [PMC free article: PMC3196957] [PubMed: 20403924]
- Hutchinson J, Pickett KE, Green J, Wakschlag LS. Smoking in pregnancy and disruptive behaviour in 3-year-old boys and girls: an analysis of the UK Millennium Cohort Study. Journal of Epidemiology and Community Health. 2010;64(1):82–8. [PMC free article: PMC10088058] [PubMed: 19887578]
- Hwang SJ, Beaty TH, Panny SR, Street NA, Joseph JM, Gordon S, McIntosh I, Francomano CA. Association study of transforming growth factor alpha (TGF alpha) TaqI polymorphism and oral clefts: indication of gene-environment interaction in a population-based sample of infants with birth defects. American Journal of Epidemiology. 1995;141(7):629–36. [PubMed: 7702037]
- Indredavik MS, Brubakk AM, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatrica. 2007;96(3):377–82. [PMC free article: PMC2049061] [PubMed: 17407460]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans: Tobacco Smoking. Vol. 38. Lyon (France): International Agency for Research on Cancer; 1986. [PMC free article: PMC7681469] [PubMed: 1683674]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking. Vol. 83. Lyon (France): International Agency for Research on Cancer; 2004. [PMC free article: PMC4781536] [PubMed: 15285078]
- Itikala PR, Watkins ML, Mulinare J, Moore CA, Liu Y. Maternal multivitamin use and orofacial clefts in offspring. Teratology. 2001;63(2):79–86. [PubMed: 11241430]
- Jensen MS, Toft G, Thulstrup AM, Bonde JP, Olsen J. Cryptorchidism according to maternal gestational smoking. Epidemiology. 2007;18(2):220–5. [PubMed: 17202869]
- Jeyabalan A, Powers RW, Durica AR, Harger GF, Roberts JM, Ness RB. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. American Journal of Hypertension. 2008;21(8):943–7. [PMC free article: PMC2613772] [PubMed: 18566591]
- Jia ZL, Shi B, Chen CH, Shi JY, Wu J, Xu X. Maternal malnutrition, environmental exposure during pregnancy and the risk of non-syndromic orofacial clefts. Oral Diseases. 2011;17(6):584–9. [PubMed: 21535328]
- Jin Z, Jin M, Nilsson BO, Roomans GM. Effects of nicotine administration on elemental concentrations in mouse granulosa cells, maturing oocytes and oviduct epithelium studied by X-ray microanalysis. Journal of Submicroscopic Cytology and Pathology. 1998;30(4):517–20. [PubMed: 9851060]
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. Journal of Urology. 2000;163(2):460–3. [PubMed: 10647654]
- Johansen AM, Wilcox AJ, Lie RT, Andersen LF, Drevon CA. Maternal consumption of coffee and caffeine-containing beverages and oral clefts: a population-based case-control study in Norway. American Journal of Epidemiology. 2009;169(10):1216–22. [PMC free article: PMC2727209] [PubMed: 19342400]
- Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. American Journal of Psychiatry. 1998;155(3):355–64. [PubMed: 9501745]
- Juárez SP, Merlo J. The effect of Swedish snuff (snus) on offspring birthweight: a sibling analysis. PloS One. 2013;8(6):e65611. [PMC free article: PMC3680479] [PubMed: 23776512]
- Juenemann KP, Lue TF, Luo JA, Benowitz NL, Abozeid M, Tanagho EA. The effect of cigarette smoking on penile erection. Journal of Urology. 1987;138(2):438–41. [PubMed: 3599273]
- Kadioğlu A, Erdoğru T, Karşidağ K, Dinççağ N, Satman I, Yilmaz MT, Tellaloğlu S. Evaluation of penile arterial system with color Doppler ultrasonography in nondiabetic and diabetic males. European Urology. 1995;27(4):311–4. [PubMed: 7656908]
- Kafouri S, Leonard G, Perron M, Richer L, Seguin JR, Veillette S, Pausova Z, Paus T. Maternal cigarette smoking during pregnancy and cognitive performance in adolescence. International Journal of Epidemiology. 2009;38(1):158–72. [PubMed: 19039007]
- Kahn RS, Khoury J, Nichols WC, Lanphear BP. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. Journal of Pediatrics. 2003;143(1):104–10. [PubMed: 12915833]
- Kalandidi A, Doulgerakis M, Tzonou A, Hsieh CC, Aravandinos D, Trichopoulos D. Induced abortions, contraceptive practices, and tobacco smoking as risk factors for ectopic pregnancy in Athens, Greece. British Journal of Obstetrics and Gynaecology. 1991;98(2):207–13. [PubMed: 2004058]
- Källén K. Maternal smoking and congenital heart defects. European Journal of Epidemiology. 1999;15(8):731–7. [PubMed: 10555617]
- Källén K. Maternal smoking and craniosynostosis. Teratology. 1999;60(3):146–50. [PubMed: 10471899]
- Källén K. Multiple malformations and maternal smoking. Paediatric and Perinatal Epidemiology. 2000;14(3):227–33. [PubMed: 10949214]
- Källén K. Role of maternal smoking and maternal reproductive history in the etiology of hypospadias in the offspring. Teratology. 2002;66(4):185–91. [PubMed: 12353215]
- Kancherla V, Romitti PA, Caspers KM, Puzhankara S, Morcuende JA. Epidemiology of congenital idiopathic talipes equinovarus in Iowa, 1997–2005. American Journal of Medical Genetics. Part A. 2010;152A(7):1695–700. [PMC free article: PMC6034619] [PubMed: 20583169]
- Karacan I, Salis PJ, Williams RL. The role of the sleep laboratory in diagnosis and treatment of impotence. In: Williams RL, Karacan I, editors. Sleep Disorders: Diagnosis and Treatment. New York: John Wiley & Sons; 1978. pp. 353–82.
- Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2006;46(6):521–7. [PubMed: 17116058]
- Karatza AA, Giannakopoulos I, Dassios TG, Belavgenis G, Mantagos SP, Varvarigou AA. Periconceptional tobacco smoking and Xisolated congenital heart defects in the neonatal period. International Journal of Cardiology. 2011;148(3):295–9. [PubMed: 19951824]
- Kardia SL, Pomerleau CS, Rozek LS, Marks JL. Association of parental smoking history with nicotine dependence, smoking rate, and psychological cofactors in adult smokers. Addictive Behaviors. 2003;28(8):1447–52. [PubMed: 14512067]
- Kelsey JL, Dwyer T, Holford TR, Bracken MB. Maternal smoking and congenital malformations: an epidemiological study. Journal of Epidemiology and Community Health. 1978;32(2):102–7. [PMC free article: PMC1060926] [PubMed: 355285]
- Kempczinski RF. Role of the vascular diagnostic laboratory in the evaluation of male impotence. American Journal of Surgery. 1979;138(2):278–82. [PubMed: 464231]
- Kendirci M, Trost L, Sikka SC, Hellstrom WJ. The effect of vascular risk factors on penile vascular status in men with erectile dysfunction. Journal of Urology. 2007;178(6):2516–20. [PubMed: 17937942]
- Kendrick JS, Atrash HK, Strauss LT, Gargiullo PM, Ahn YW. Vaginal douching and the risk of ectopic pregnancy among black women. American Journal of Obstetrics and Gynecology. 1997;176(5):991–7. [PubMed: 9166157]
- Kendrick JS, Zahniser SC, Miller N, Salas N, Stine J, Gargiullo PM, Floyd RL, Spierto FW, Sexton M, Metzger RW, et al. Integrating smoking cessation into routine public prenatal care: the Smoking Cessation in Pregnancy project. American Journal of Public Health. 1995;85(2):217–22. [PMC free article: PMC1615299] [PubMed: 7856781]
- Kharrazi M, DeLorenze GN, Kaufman FL, Eskenazi B, Bernert JT Jr., Graham S, Pearl M, Pirkle J. Environmental tobacco smoke and pregnancy outcome. Epidemiology. 2004;15(6):660–70. [PubMed: 15475714]
- Kim SY, England LJ, Kendrick JS, Dietz PM, Callaghan WM. The contribution of clinic-based interventions to reduce prenatal smoking prevalence among US women. American Journal of Public Health. 2009;99(5):893–8. [PMC free article: PMC2667844] [PubMed: 19299672]
- Klebanoff MA. The Collaborative Perinatal Project: a 50-year retrospective. Paediatric and Perinatal Epidemiology. 2009;23(1):2–8. [PMC free article: PMC2646177] [PubMed: 19228308]
- Kleinman JC, Kopstein A. Smoking during pregnancy, 1967–80. American Journal of Public Health. 1987;77(7):823–5. [PMC free article: PMC1647213] [PubMed: 3592035]
- Kleinman KP, Feldman HA, Johannes CB, Derby CA, McKinlay JB. A new surrogate variable for erectile dysfunction status in the Massachusetts male aging study. Journal of Clinical Epidemiology. 2000;53(1):71–8. [PubMed: 10693906]
- Kline J, Levin B, Kinney A, Stein Z, Susser M, Warburton D. Cigarette smoking and spontaneous abortion of known karyotype. Precise data but uncertain inferences. American Journal of Epidemiology. 1995;141(5):417–27. [PubMed: 7879786]
- Kline J, Stein Z, Susser M. Conception to Birth: Epidemiology of Prenatal Development. New York: Oxford University Press; 1989.
- Knopik VS, Heath AC, Bucholz KK, Madden PA, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: a female twin study. Pharmacology, Biochemistry, and Behavior. 2009;93(3):313–21. [PMC free article: PMC2758781] [PubMed: 19341765]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Waldron M, Martin NG. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36(10):1461–71. [PubMed: 16734942]
- Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, Slutske WS, Grant JD, McLaughlin TL, Todorov A, et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychological Medicine. 2005;35(5):625–35. [PubMed: 15918339]
- Kollins SH, Garrett ME, McClernon FJ, Lachiewicz AM, Morrissey-Kane E, FitzGerald D, Collins AL, Anastopoulos AD, Ashley-Koch AE. Effects of postnatal parental smoking on parent and teacher ratings of ADHD and oppositional symptoms. Journal of Nervous and Mental Disease. 2009;197(6):442–9. [PMC free article: PMC3678953] [PubMed: 19525745]
- Koren G, Sharav T, Pastuszak A, Garrettson LK, Hill K, Samson I, Rorem M, King A, Dolgin JE. A multicenter, prospective study of fetal outcome following accidental carbon monoxide poisoning in pregnancy. Reproductive Toxicology. 1991;5(5):397–403. [PubMed: 1806148]
- Koshy G, Delpisheh A, Brabin BJ. Childhood obesity and parental smoking as risk factors for childhood ADHD in Liverpool children. Attention Deficit Hyperactive Disorder. 2011;3(1):21–8. [PubMed: 21432615]
- Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, Hartikainen AL, Jarvelin MR. Maternal smoking and hyperactivity in 8-year-old children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(7):826–33. [PubMed: 12819442]
- Koupparis AJ, Jeremy JY, Muzaffar S, Persad R, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47 NAD[P]H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. British Journal of Urology International. 2005;96(3):423–7. [PubMed: 16042742]
- Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. British Journal of Urology International. 2006;98(1):116–24. [PubMed: 16831155]
- Krapels IP, Raijmakers-Eichhorn J, Peters WH, Roelofs HM, Ras F, Steegers-Theunissen RP. The I,105V polymorphism in glutathione S-transferase P1, parental smoking and the risk for nonsyndromic cleft lip with or without cleft palate. European Journal of Human Genetics. 2008;16(3):358–66. [PubMed: 18159215]
- Krapels IP, Zielhuis GA, Vroom F, de Jong-van den Berg LT, Kuijpers-Jagtman AM, van der Molen AB, Steegers-Theunissen RP. Periconceptional health and lifestyle factors of both parents affect the risk of live-born children with orofacial clefts. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76(8):613–20. [PubMed: 16955502]
- Krishna K. Tobacco chewing in pregnancy. British Journal of Obstetrics and Gynaecology. 1978;85(10):726–8. [PubMed: 708654]
- Kučienė R, Dulskienė V. Maternal socioeconomic and lifestyle factors during pregnancy and the risk of congenital heart defects. Medicina (Kaunas, Lithuania). 2009;45(11):904–9. [PubMed: 20051723]
- Kučienė R, Dulskienė V. Parental cigarette smoking and the risk of congenital heart septal defects. Medicina (Kaunas, Lithuania). 2010;46(9):635–41. [PubMed: 21252599]
- Kukla L, Hruba D, Tyrlik M. Maternal smoking during pregnancy, behavioral problems and school performances of their school-aged children. Central European Journal of Public Health. 2008;16(2):71–6. [PubMed: 18661809]
- Kupelian V, Araujo AB, Chiu GR, Rosen RC, McKinlay JB. Relative contributions of modifiable risk factors to erectile dysfunction: results from the Boston Area Community Health (BACH) Survey. Preventive Medicine. 2010;50(1-2):19–25. [PMC free article: PMC2813912] [PubMed: 19944117]
- Kupelian V, Link CL, McKinlay JB. Association between smoking, passive smoking, and erectile dysfunction: results from the Boston Area Community Health (BACH) Survey. European Urology. 2007;52(2):416–22. [PMC free article: PMC2139983] [PubMed: 17383811]
- Kurahashi N, Kasai S, Shibata T, Kakizaki H, Nonomura K, Sata F, Kishi R. Parental and neonatal risk factors for cryptorchidism. Medical Science Monitor. 2005;11(6):CR274–CR83. [PubMed: 15917718]
- Kurahashi N, Sata F, Kasai S, Shibata T, Moriya K, Yamada H, Kakizaki H, Minakami H, Nonomura K, Kishi R. Maternal genetic polymorphisms in CYP1A1, GSTM1 and GSTT1 and the risk of hypospadias. Molecular Human Reproduction. 2005;11(2):93–8. [PubMed: 15579657]
- Lagercrantz H, Slotkin TA. The “stress” of being born. Scientific American. 1986;254(4):100–7. [PubMed: 3961465]
- Lahti J, Raikkonen K, Sovio U, Miettunen J, Hartikainen AL, Pouta A, Taanila A, Joukamaa M, Jarvelin MR, Veijola J. Early-life origins of schizotypal traits in adulthood. British Journal of Psychiatry. 2009;195(2):132–7. [PubMed: 19648543]
- Lam PK, Torfs CP. Interaction between maternal smoking and malnutrition in infant risk of gastroschisis. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76(3):182–6. [PubMed: 16498669]
- Lam TH, Abdullah AS, Ho LM, Yip AW, Fan S. Smoking and sexual dysfunction in Chinese males: findings from men's health survey. International Journal of Impotence Research. 2006;18(4):364–9. [PubMed: 16355108]
- Lambe M, Hultman C, Torrång A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17(5):524–30. [PubMed: 16878043]
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Seminars in Perinatology. 1996;20(2):115–26. [PubMed: 8857697]
- Lammer EJ, Shaw GM, Iovannisci DM, Van WJ, Finnell RH. Maternal smoking and the risk of orofacial clefts: susceptibility with NAT1 and NAT2 polymorphisms. Epidemiology. 2004;15(2):150–6. [PubMed: 15127906]
- Langley K, Heron J, Smith GD, Thapar A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. American Journal of Epidemiology. 2012;176(3):261–8. [PMC free article: PMC3406617] [PubMed: 22791738]
- Langley K, Holmans PA, van den Bree MB, Thapar A. Effects of low birth weight, maternal smoking in pregnancy and social class on the phenotypic manifestation of Attention Deficit Hyperactivity Disorder and associated antisocial behaviour: investigation in a clinical sample. BMC Psychiatry. 2007;7:26. [PMC free article: PMC1913513] [PubMed: 17584500]
- Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatrica. 2005;57(6):359–71. [PubMed: 16402008]
- Latimer K, Wilson P, Kemp J, Thompson L, Sim F, Gillberg C, Puckering C, Minnis H. Disruptive behaviour disorders: a systematic review of environmental antenatal and early years risk factors. Child: Care, Health, and Development. 2012;38(5):611–28. [PubMed: 22372737]
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA: the Journal of the American Medical Association. 1999;281(6):537–44. [PubMed: 10022110]
- Lavigne JV, Hopkins J, Gouze KR, Bryant FB, LeBailly SA, Binns HJ, Lavigne PM. Is smoking during pregnancy a risk factor for psychopathology in young children? A methodological caveat and report on preschoolers. Journal of Pediatric Psychology. 2011;36(1):10–24. [PMC free article: PMC3021806] [PubMed: 20484331]
- Lebby KD, Tan F, Brown CP. Maternal factors and disparities associated with oral clefts. Ethnicity and Disease. 2010;20(1 Suppl 1):S1–S9. [PMC free article: PMC2994716] [PubMed: 20521404]
- Lee CZ, Royce FH, Denison MS, Pinkerton KE. Effect of in utero and postnatal exposure to environmental tobacco smoke on the developmental expression of pulmonary cytochrome P450 monooxygenases. Journal of Biochemical and Molecular Toxicology. 2000;14(3):121–30. [PubMed: 10711627]
- Lehn H, Derks EM, Hudziak JJ, Heutink P, van Beijsterveldt TC, Boomsma DI. Attention problems and attention-deficit/hyperactivity disorder in discordant and concordant monozygotic twins: evidence of environmental mediators. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(1):83–91. [PubMed: 17195733]
- Leite IC, Koifman S. Oral clefts, consanguinity, parental tobacco and alcohol use: a case-control study in Rio de Janeiro, Brazil. Brazil Oral Research. 2009;23(1):31–7. [PubMed: 19488469]
- Levin AA, Schoenbaum SC, Stubblefield PG, Zimicki S, Monson RR, Ryan KJ. Ectopic pregnancy and prior induced abortion. American Journal of Public Health. 1982;72(3):253–6. [PMC free article: PMC1649809] [PubMed: 7058965]
- Levine LA, Gerber GS. Acute vasospasm of penile arteries in response to cigarette smoking. Urology. 1990;36(1):99–100. [PubMed: 2368241]
- Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clinical Obstetrics and Gynecology. 2005;48(2):372–86. [PubMed: 15805796]
- Li Z, Liu J, Ye R, Zhang L, Zheng X, Ren A. Maternal passive smoking and risk of cleft lip with or without cleft palate. Epidemiology. 2010;21(2):240–2. [PubMed: 20081540]
- Lieff S, Olshan AF, Werler M, Strauss RP, Smith J, Mitchell A. Maternal cigarette smoking during pregnancy and risk of oral clefts in newborns. American Journal of Epidemiology. 1999;150(7):683–94. [PubMed: 10512422]
- Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine & Tobacco Research. 2010;12(4):408–15. [PubMed: 20176681]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. American Journal of Psychiatry. 2003;160(6):1028–40. [PubMed: 12777257]
- Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E, Henriksen TB. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116(2):462–7. [PubMed: 16061604]
- Little J, Cardy A, Arslan MT, Gilmour M, Mossey PA. Smoking and orofacial clefts: a United Kingdom-based case-control study. Cleft Palate-Craniofacial Journal. 2004;41(4):381–6. [PubMed: 15222794]
- Little J, Cardy A, Munger RG. Tobacco smoking and oral clefts: a meta-analysis. Bulletin of the World Health Organization. 2004;82(3):213–8. [PMC free article: PMC2585921] [PubMed: 15112010]
- Liu T, Gatsonis CA, Baylin A, Kubzansky LD, Loucks EB, Buka SL. Maternal smoking during pregnancy and anger temperament among adult offspring. Journal of Psychiatric Research. 2011;45(12):1648–54. [PMC free article: PMC3210329] [PubMed: 21890149]
- Lue TF. Erectile dysfunction. New England Journal of Medicine. 2000;342(24):1802–13. [PubMed: 10853004]
- Lue TF, Tanagho EA. Physiology of erection and pharmacological management of impotence. Journal of Urology. 1987;137(5):829–36. [PubMed: 3553617]
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2009;(3) Art No.: CD001055. [PMC free article: PMC4090746] [PubMed: 19588322] [CrossRef]
- Lundberg F, Cnattingius S, D'Onofrio B, Altman D, Lambe M, Hultman C, Iliadou A. Maternal smoking during pregnancy and intellectual performance in young adult Swedish male offspring. Paediatric and Perinatal Epidemiology. 2010;24(1):79–87. [PMC free article: PMC3653250] [PubMed: 20078833]
- Lupo PJ, Langlois PH, Reefhuis J, Lawson CC, Symanski E, Desrosiers TA, Khodr ZG, Agopian AJ, Waters MA, Duwe KN, et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons: effects on gastroschisis among offspring in the National Birth Defects Prevention Study. Environmental Health Perspectives. 2012;120(6):910–5. [PMC free article: PMC3385431] [PubMed: 22330681]
- MacArthur C, Knox EG, Lancashire RJ. Effects at age nine of maternal smoking in pregnancy: experimental and observational findings. BJOG: An International Journal of Obstetrics and Gynaecology. 2001;108(1):67–73. [PubMed: 11213007]
- Mackay DF, Nelson SM, Haw SJ, Pell JP. Impact of Scotland's smokefree legislation on pregnancy complications: retrospective cohort study. PLoS Medicine. 2012;9(3):e1001175. [PMC free article: PMC3295815] [PubMed: 22412353]
- MacLehose RF, Olshan AF, Herring AH, Honein MA, Shaw GM, Romitti PA. Bayesian methods for correcting misclassification: an example from birth defects epidemiology. Epidemiology. 2009;20(1):27–35. [PMC free article: PMC5753791] [PubMed: 19234399]
- Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage—results from a UK-population-based case-control study. BJOG: An International Journal of Obstetrics and Gynaecology. 2007;114(2):170–86. [PubMed: 17305901]
- Magers T, Talbot P, DiCarlantonio G, Knoll M, Demers D, Tsai I, Hoodbhoy T. Cigarette smoke inhalation affects the reproductive system of female hamsters. Reproductive Toxicology. 1995;9(6):513–25. [PubMed: 8597648]
- Malik S, Cleves MA, Honein MA, Romitti PA, Botto LD, Yang S, Hobbs CA. Maternal smoking and congenital heart defects. Pediatrics. 2008;121(4):e810–e6. [PubMed: 18381510]
- Malloy MH, Kleinman JC, Land GH, Schramm WF. The association of maternal smoking with age and cause of infant death. American Journal of Epidemiology. 1988;128(1):46–55. [PubMed: 3381835]
- Mannino DM, Klevens RM, Flanders WD. Cigarette smoking: an independent risk factor for impotence? American Journal of Epidemiology. 1994;140(11):1003–8. [PubMed: 7985647]
- Mannino DM, Mulinare J, Ford ES, Schwartz J. Tobacco smoke exposure and decreased serum and red blood cell folate levels: data from the Third National Health and Nutrition Examination Survey. Nicotine & Tobacco Research. 2003;5(3):357–62. [PubMed: 12791531]
- Martin-Morales A, Sanchez-Cruz JJ, Saenz de Tejada I, Rodriguez-Vela L, Jimenez-Cruz JF, Burgos-Rodriguez R. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. Journal of Urology. 2001;166(2):569–74. [PubMed: 11458070]
- Mateja WA, Nelson DB, Kroelinger CD, Ruzek S, Segal J. The association between maternal alcohol use and smoking in early pregnancy and congenital cardiac defects. Journal of Women's Health (Larchmont). 2012;21(1):26–34. [PubMed: 21895513]
- Mathews CA, Bimson B, Lowe TL, Herrera LD, Budman CL, Erenberg G, Naarden A, Bruun RD, Freimer NB, Reus VI. Association between maternal smoking and increased symptom severity in Tourette's syndrome. American Journal of Psychiatry. 2006;163(6):1066–73. [PubMed: 16741208]
- Matovina M, Husnjak K, Milutin N, Ciglar S, Grce M. Possible role of bacterial and viral infections in miscarriages. Fertility and Sterility. 2004;81(3):662–9. [PubMed: 15037417]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Archives of General Psychiatry. 2004;61(8):836–43. [PubMed: 15289282]
- Maughan B, Taylor C, Taylor A, Butler N, Bynner J. Pregnancy smoking and childhood conduct problems: a causal association? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42(8):1021–8. [PubMed: 11806683]
- Maximovich A, Beyler SA. Cigarette smoking at time of in vitro fertilization cycle initiation has negative effect on in vitro fertilization-embryo transfer success rate. Journal of Assisted Reproduction and Genetics. 1995;12(2):75–7. [PubMed: 7670278]
- Maxson PJ, Edwards SE, Ingram A, Miranda ML. Psychosocial differences between smokers and non-smokers during pregnancy. Addictive Behaviors. 2012;37(2):153–9. [PubMed: 22000409]
- Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Seminars in Nephrology. 2011;31(1):33–46. [PMC free article: PMC3063446] [PubMed: 21266263]
- McCowan LM, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, Moss-Morris R, North RA. SCOPE Consortium. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. British Medical Journal. 2009;338:b1081. [PMC free article: PMC2661373] [PubMed: 19325177]
- McDonald HM, Chambers HM. Intrauterine infection and spontaneous midgestation abortion: is the spectrum of microorganisms similar to that in preterm labor? Infectious Diseases in Obstetrics and Gynecology. 2000;8(5-6):220–7. [PMC free article: PMC1784699] [PubMed: 11220481]
- McDonald SD, Perkins SL, Jodouin CA, Walker MC. Folate levels in pregnant women who smoke: an important gene/environment interaction. American Journal of Obstetrics and Gynecology. 2002;187(3):620–5. [PubMed: 12237638]
- Melman A, Gingell JC. The epidemiology and pathophysiology of erectile dysfunction. Journal of Urology. 1999;161(1):5–11. [PubMed: 10037356]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–9. [PMC free article: PMC2946114] [PubMed: 20855043]
- Mersdorf A, Goldsmith PC, Diederichs W, Padula CA, Lue TF, Fishman IJ, Tanagho EA. Ultrastructural changes in impotent penile tissue: a comparison of 65 patients. Journal of Urology. 1991;145(4):749–58. [PubMed: 2005694]
- Meyer MB, Tonascia JA. Maternal smoking, pregnancy complications, and perinatal mortality. American Journal of Obstetrics and Gynecology. 1977;128(5):494–502. [PubMed: 560121]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(4):378–85. [PubMed: 11931593]
- Miller EA, Manning SE, Rasmussen SA, Reefhuis J, Honein MA. Maternal exposure to tobacco smoke, alcohol and caffeine, and risk of anorectal atresia: National Birth Defects Prevention Study 1997–2003. Paediatric and Perinatal Epidemiology. 2009;23(1):9–17. [PubMed: 19228309]
- Miller EA, Rasmussen SA, Siega-Riz AM, Frias JL, Honein MA. Risk factors for non-syndromic holoprosencephaly in the National Birth Defects Prevention Study. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2010;154C(1):62–72. [PubMed: 20104597]
- Millett C, Wen LM, Rissel C, Smith A, Richters J, Grulich A, de Visser R. Smoking and erectile dysfunction: findings from a representative sample of Australian men. Tobacco Control. 2006;15(2):136–9. [PMC free article: PMC2563576] [PubMed: 16565463]
- Millicovsky G, Johnston MC. Hyperoxia and hypoxia in pregnancy: simple experimental manipulation alters the incidence of cleft lip and palate in CL/Fr mice. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(9):5722–23. [PMC free article: PMC348841] [PubMed: 6946511]
- Millicovsky G, Johnston MC. Maternal hyperoxia greatly reduces the incidence of phenytoin-induced cleft lip and palate in A/J mice. Science. 1981;212(4495):671–72. [PubMed: 7221553]
- Miner M, Billups KL. Erectile dysfunction and dyslipidemia: relevance and role of phosphodiesterase type-5 inhibitors and statins. Journal of Sexual Medicine. 2008;5(5):1066–78. [PubMed: 18331271]
- Mirilas P, Mentessidou A, Kontis E, Asimakidou M, Moxham BJ, Petropoulos AS, Emmanouil-Nikolousi EN. Parental exposures and risk of nonsyndromic orofacial clefts in offspring: a case-control study in Greece. International Journal of Pediatric Otorhinolaryngology. 2011;75(5):695–9. [PubMed: 21450350]
- Mishra GD, Dobson AJ, Schofield MJ. Cigarette smoking, menstrual symptoms and miscarriage among young women. Australian and New Zealand Journal of Public Health. 2000;24(4):413–20. [PubMed: 11011470]
- Mitchell LE, Murray JC, O'Brien S, Christensen K. Evaluation of two putative susceptibility loci for oral clefts in the Danish population. American Journal of Epidemiology. 2001;153(10):1007–15. [PubMed: 11384957]
- Mochizuki M, Maruo T, Masuko K, Ohtsu T. Effects of smoking on fetoplacental-maternal system during pregnancy. American Journal of Obstetrics and Gynecology. 1984;149(4):413–20. [PubMed: 6203408]
- Mok JS, Paisley K, Martin W. Inhibition of nitrergic neurotransmission in the bovine retractor penis muscle by an oxidant stress: effects of superoxide dismutase mimetics. British Journal of Pharmacology. 1998;124(1):111–8. [PMC free article: PMC1565368] [PubMed: 9630350]
- Monuteaux MC, Blacker D, Biederman J, Fitzmaurice G, Buka SL. Maternal smoking during pregnancy and offspring overt and covert conduct problems: a longitudinal study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(9):883–90. [PubMed: 16930382]
- Morales-Suarez-Varela MM, Bille C, Christensen K, Olsen J. Smoking habits, nicotine use, and congenital malformations. Obstetrics and Gynecology. 2006;107(1):51–7. [PubMed: 16394039]
- Moreira ED Jr, Kim SC, Glasser D, Gingell C. Sexual activity, prevalence of sexual problems, and associated help-seeking patterns in men and women aged 40–80 years in Korea: data from the Global Study of Sexual Attitudes and Behaviors (GSSAB). Journal of Sexual Medicine. 2006;3(2):201–11. [PubMed: 16490013]
- Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. A dose-response relationship between maternal smoking during late pregnancy and adult intelligence in male offspring. Paediatric and Perinatal Epidemiology. 2005;19(1):4–11. [PubMed: 15670102]
- Motlagh MG, Katsovich L, Thompson N, Lin H, Kim YS, Scahill L, Lombroso PJ, King RA, Peterson BS, Leckman JF. Severe psychosocial stress and heavy cigarette smoking during pregnancy: an examination of the pre- and perinatal risk factors associated with ADHD and Tourette syndrome. European Child and Adolescent Psychiatry. 2010;19(10):755–64. [PMC free article: PMC3932440] [PubMed: 20532931]
- Motlagh MG, Sukhodolsky DG, Landeros-Weisenberger A, Katsovich L, Thompson N, Scahill L, King RA, Peterson BS, Schultz RT, Leckman JF. Adverse effects of heavy prenatal maternal smoking on attentional control in children with ADHD. Journal of Attention Disorders. 2011;15(7):593–603. [PMC free article: PMC3974616] [PubMed: 20616372]
- Mulvihill JE, Gamm SH, Ferm VH. Facial formation in normal and cadmium-treated golden hamsters. Journal of Embryology and Experimental Morphology. 1970;24(2):393–403. [PubMed: 5491988]
- Munger RG, Sauberlich HE, Corcoran C, Nepomuceno B, Daack-Hirsch S, Solon FS. Maternal vitamin B-6 and folate status and risk of oral cleft birth defects in the Philippines. Birth Defects Research Part A: Clinical and Molecular Teratology. 2004;70(7):464–71. [PubMed: 15259036]
- Murin S, Rafii R, Bilello K. Smoking and smoking cessation in pregnancy. Clinics in Chest Medicine. 2011;32(1):75–91. viii. [PubMed: 21277451]
- Murray J, Irving B, Farrington DP, Colman I, Bloxsom CA. Very early predictors of conduct problems and crime: results from a national cohort study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2010;51(11):1198–207. [PubMed: 20633069]
- Nelson E. The miseries of passive smoking. Human and Experimental Toxicology. 2001;20(2):61–83. [PubMed: 11327513]
- Neri A, Eckerling B. Influence of smoking and adrenaline (epinephrine) on the uterotubal insufflation test (Rubin test). Fertility and Sterility. 1969;20(5):818–28. [PubMed: 5822864]
- Neri A, Marcus SL. Effect of nicotine on the motility of the oviducts in the rhesus monkey: a preliminary report. Journal of Reproduction and Fertility. 1972;31(1):91–7. [PubMed: 4627858]
- Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, Kline J. Cocaine and tobacco use and the risk of spontaneous abortion. New England Journal of Medicine. 1999;340(5):333–9. [PubMed: 9929522]
- Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biological Psychiatry. 2007;61(12):1320–8. [PubMed: 17157268]
- Nguyen KH, Wright RJ, Sorensen G, Subramanian SV. Association between local indoor smoking ordinances in Massachusetts and cigarette smoking during pregnancy: a multilevel analysis. Tobacco Control. 2013;22(3):184–9. [PMC free article: PMC3401240] [PubMed: 22166267]
- Nielsen A, Hannibal CG, Lindekilde BE, Tolstrup J, Frederiksen K, Munk C, Bergholt T, Buss L, Ottesen B, Gronbaek M, et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstetricia et Gynecologica Scandinavica. 2006;85(9):1057–65. [PubMed: 16929410]
- Nigg JT, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(3):362–9. [PubMed: 17314722]
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference: impotence. JAMA: the Journal of the American Medical Association. 1993;270(1):83–90. [PubMed: 8510302]
- Nomura Y, Marks DJ, Halperin JM. Prenatal exposure to maternal and paternal smoking on attention deficit hyperactivity disorders symptoms and diagnosis in offspring. Journal of Nervous and Mental Disease. 2010;198(9):672–8. [PMC free article: PMC3124822] [PubMed: 20823730]
- Norman CA, Halton DM. Is carbon monoxide a workplace teratogen? A review and evaluation of the literature. Annals of Occupational Hygiene. 1990;34(4):335–47. [PubMed: 2240988]
- Nukui T, Day RD, Sims CS, Ness RB, Romkes M. Maternal/newborn GSTT1 null genotype contributes to risk of preterm, low birthweight infants. Pharmacogenetics. 2004;14(9):569–76. [PubMed: 15475730]
- Obel C, Linnet KM, Henriksen TB, Rodriguez A, Jarvelin MR, Kotimaa A, Moilanen I, Ebeling H, Bilenberg N, Taanila A, et al. Smoking during pregnancy and hyperactivity-inattention in the offspring—comparing results from three Nordic cohorts. International Journal of Epidemiology. 2009;38(3):698–705. [PubMed: 18250076]
- Obel C, Olsen J, Henriksen TB, Rodriguez A, Jarvelin MR, Moilanen I, Parner E, Linnet KM, Taanila A, Ebeling H, et al. Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?—Findings from a sibling design. International Journal of Epidemiology. 2011;40(2):338–45. [PubMed: 21075808]
- Ortega RM, Requejo AM, López-Sobaler AM, Navia B, Mena MC, Basabe B, Andrés P. Smoking and passive smoking as conditioners of folate status in young women. Journal of the American College of Nutrition. 2004;23(4):365–71. [PubMed: 15310741]
- Osterman MJ, Martin JA, Mathews TJ, Hamilton BE. Expanded Data From the New Birth Certificate, 2008. 7. Vol. 59. Hyattsville (MD): National Center for Health Statistics; 2011. National Vital Statistics Reports. [PubMed: 21848043]
- Ozkara H, Alan C, Atukeren P, Uyaner I, Demirci C, Gumustas MK, Alici B. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manuplation in rat penis. Chinese Journal of Physiology. 2006;49(3):160–6. [PubMed: 16970248]
- Packianathan S, Cain CD, Stagg RB, Longo LD. Ornithine decarboxylase activity in fetal and newborn rat brain: responses to hypoxic and carbon monoxide hypoxia. Brain Research: Developmental Brain Research. 1993;76(1):131–40. [PubMed: 8306425]
- Page RL, Padilla YC, Hamilton ER. Psychosocial factors associated with patterns of smoking surrounding pregnancy in fragile families. Maternal and Child Health Journal. 2012;16(1):249–57. [PMC free article: PMC3252496] [PubMed: 21197563]
- Page RL 2nd, Slejko JF, Libby AM. A citywide smoking ban reduced maternal smoking and risk for preterm births: a Colorado natural experiment. Journal of Women's Health (Larchmont). 2012;21(6):621–7. [PubMed: 22401497]
- Palili A, Kolaitis G, Vassi I, Veltsista A, Bakoula C, Gika A. Inattention, hyperactivity, impulsivity—epidemiology and correlations: a nationwide greek study from birth to 18 years. Journal of Child Neurology. 2011;26(2):199–204. [PubMed: 20921568]
- Parazzini F, Menchini FF, Bortolotti A, Calabro A, Chatenoud L, Colli E, Landoni M, Lavezzari M, Turchi P, Sessa A, et al. Frequency and determinants of erectile dysfunction in Italy. European Urology. 2000;37(1):43–9. [PubMed: 10671784]
- Parazzini F, Tozzi L, Ferraroni M, Bocciolone L, La Vecchia C, Fedele L. Risk factors for ectopic pregnancy: an Italian case-control study. Obstetrics and Gynecology. 1992;80(5):821–6. [PubMed: 1407922]
- Parker SE, Mai CT, Strickland MJ, Olney RS, Rickard R, Marengo L, Wang Y, Hashmi SS, Meyer RE. Multistate study of the epidemiology of clubfoot. Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85(11):897–904. [PubMed: 19697433]
- Patient Protection and Affordable Care Act. U.S. Statutes at Large. 2010. p. 119. Public Law 111-148.
- Pattinson HA, Taylor PJ, Pattinson MH. The effect of cigarette smoking on ovarian function and early pregnancy outcome of in vitro fertilization treatment. Fertility and Sterility. 1991;55(4):780–3. [PubMed: 2010004]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatrica. 2008;97(10):1331–7. [PubMed: 18554275]
- Peck JD, Neas B, Robledo C, Saffer E, Beebe L, Wild RA. Intrauterine tobacco exposure may alter auditory brainstem responses in newborns. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(4):592–6. [PubMed: 20367434]
- Penney DG. Effects of carbon monoxide exposure on developing animals and humans. In: Penney DG, editor. Carbon Monoxide. Boca Raton (FL): CRC Press; 1996. pp. 109–44.
- Phillips RS, Tuomala RE, Feldblum PJ, Schachter J, Rosenberg MJ, Aronson MD. The effect of cigarette smoking, Chlamydia trachomatis infection, and vaginal douching on ectopic pregnancy. Obstetrics and Gynecology. 1992;79(1):85–90. [PubMed: 1727593]
- Pier B, Kazanjian A, Gillette L, Strenge K, Burney RO. Effect of cigarette smoking on human oviductal ciliation and ciliogenesis. Fertility and Sterility. 2013;99(1):199–205. [PubMed: 23009827]
- Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environmental Health Perspectives. 2004;112(15):1570–6. [PMC free article: PMC1247623] [PubMed: 15531444]
- Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351(9109):1115–20. [PubMed: 9660597]
- Polsky JY, Aronson KJ, Heaton JP, Adams MA. Smoking and other lifestyle factors in relation to erectile dysfunction. British Journal of Urology International. 2005;96(9):1355–9. [PubMed: 16287457]
- Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertility and Sterility. 2012;98(5):1103–11. [PubMed: 22835448]
- Pringsheim T, Sandor P, Lang A, Shah P, O'Connor P. Prenatal and perinatal morbidity in children with Tourette syndrome and attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics. 2009;30(2):115–21. [PubMed: 19322105]
- Puranik R, Celermajer DS. Smoking and endothelial function. Progress in Cardiovascular Diseases. 2003;45(6):443–58. [PubMed: 12800127]
- Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–11. [PubMed: 16905025]
- Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstetricia et Gynecologica Scandinavica. 2003;82(2):182–8. [PubMed: 12648183]
- Rasmussen SA, Frias JL. Non-genetic risk factors for gastroschisis. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2008;148C(3):199–212. [PubMed: 18655102]
- Rasmussen SA, Moore CA. Effective coding in birth defects surveillance. Teratology. 2001;64(Suppl 1):S3–7. [PubMed: 11745837]
- Raymond EG, Cnattingius S, Kiely JL. Effects of maternal age, parity, and smoking on the risk of stillbirth. BJOG: An International Journal of Obstetrics and Gynaecology. 1994;101(4):301–6. [PubMed: 8199075]
- Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. British Medical Journal. 1989;299(6698):541–5. [PMC free article: PMC1837397] [PubMed: 2507063]
- Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Bailliere's Best Practice & Research: Clinical Obstetrics & Gynaecology. 2000;14(5):839–54. [PubMed: 11023804]
- Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. Journal of Pediatrics. 2008;153(6):807–13. [PMC free article: PMC2613036] [PubMed: 18657826]
- Rice C, Yoshinaga K. Effect of nicotine on oviducal lactate dehydrogenase during early pregnancy in the rat. Biology of Reproduction. 1980;23(2):445–51. [PubMed: 7417684]
- Richardson HL, Walker AM, Horne RS. Maternal smoking impairs arousal patterns in sleeping infants. Sleep. 2009;32(4):515–21. [PMC free article: PMC2663655] [PubMed: 19413145]
- Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. Ambient air pollution and risk of birth defects in Southern California. American Journal of Epidemiology. 2002;155(1):17–25. [PubMed: 11772780]
- Robinson M, Oddy WH, Li J, Kendall GE, de Klerk NH, Silburn SR, Zubrick SR, Newnham JP, Stanley FJ, Mattes E. Pre- and postnatal influences on preschool mental health: a large-scale cohort study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49(10):1118–28. [PubMed: 19017026]
- Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2005;46(3):246–54. [PubMed: 15755301]
- Rodriguez A, Olsen J, Kotimaa AJ, Kaakinen M, Moilanen I, Henriksen TB, Linnet KM, Miettunen J, Obel C, Taanila A, et al. Is prenatal alcohol exposure related to inattention and hyperactivity symptoms in children? Disentangling the effects of social adversity. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50(9):1073–83. [PubMed: 19298478]
- Roelands J, Jamison MG, Lyerly AD, James AH. Consequences of smoking during pregnancy on maternal health. Journal of Women's Health (Larchmt). 2009;18(6):867–72. [PMC free article: PMC2851129] [PubMed: 19514829]
- Rogers JM. Tobacco and pregnancy. Reproductive Toxicology. 2009;28(2):152–60. [PubMed: 19450949]
- Romano E, Tremblay RE, Farhat A, Cote S. Development and prediction of hyperactive symptoms from 2 to 7 years in a population-based sample. Pediatrics. 2006;117(6):2101–10. [PubMed: 16740853]
- Romitti PA, Lidral AC, Munger RG, Daack-Hirsch S, Burns TL, Murray JC. Candidate genes for nonsyndromic cleft lip and palate and maternal cigarette smoking and alcohol consumption: evaluation of genotype-environment interactions from a population-based case-control study of orofacial clefts. Teratology. 1999;59(1):39–50. [PubMed: 9988882]
- Romitti PA, Sun L, Honein MA, Reefhuis J, Correa A, Rasmussen SA. Maternal periconceptional alcohol consumption and risk of orofacial clefts. American Journal of Epidemiology. 2007;166(7):775–85. [PubMed: 17609516]
- Rosen MP, Greenfield AJ, Walker TG, Grant P, Dubrow J, Bettmann MA, Fried LE, Goldstein I. Cigarette smoking: an independent risk factor for atherosclerosis in the hypogastric-cavernous arterial bed of men with arteriogenic impotence. Journal of Urology. 1991;145(4):759–63. [PubMed: 2005695]
- Roza SJ, Verburg BO, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA, Witteman JC, Verhulst FC, Tiemeier H. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. European Journal of Neuroscience. 2007;25(3):611–7. [PubMed: 17298594]
- Ruckebusch Y. Relationship between the electrical activity of the oviduct and the uterus of the rabbit in vivo. Journal of Reproduction and Fertility. 1975;45(1):73–82. [PubMed: 1195258]
- Sacker A, Done DJ, Crow TJ, Golding J. Antecedents of schizophrenia and affective illness. Obstetric complications. British Journal of Psychiatry. 1995;166(6):734–41. [PubMed: 7663821]
- Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. New England Journal of Medicine. 1989;320(16):1025–30. [PubMed: 2927481]
- Sagiv SK, Epstein JN, Bellinger DC, Korrick SA. Pre- and postnatal risk factors for ADHD in a nonclinical pediatric population. Journal of Attention Disorders. 2013;17(1):47–57. [PMC free article: PMC3878902] [PubMed: 22298092]
- Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Predictors and prevalence of erectile dysfunction in a racially diverse population. Archives of Internal Medicine. 2006;166(2):207–12. [PubMed: 16432090]
- Saksena SK. Cadmium: its effects on ovulation, egg transport and pregnancy in the rabbit. Contraception. 1982;26(2):181–92. [PubMed: 7140294]
- Salafia C, Shiverick K. Cigarette smoking and pregnancy II: vascular effects. Placenta. 1999;20(4):273–9. [PubMed: 10329347]
- Salemi JL, Pierre M, Tanner JP, Kornosky JL, Hauser KW, Kirby RS, Carver JD. Maternal nativity as a risk factor for gastroschisis: a population-based study. Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85(11):890–6. [PubMed: 19645051]
- Sanderson M, Placek PJ, Keppel KG. The 1988 National Maternal and Infant Health Survey: design, content, and data availability. Birth. 1991;18(1):26–32. [PubMed: 2006958]
- Saraiya M, Berg CJ, Kendrick JS, Strauss LT, Atrash HK, Ahn YW. Cigarette smoking as a risk factor for ectopic pregnancy. American Journal of Obstetrics and Gynecology. 1998;178(3):493–8. [PubMed: 9539515]
- Sawnani H, Jackson T, Murphy T, Beckerman R, Simakajornboon N. The effect of maternal smoking on respiratory and arousal patterns in preterm infants during sleep. American Journal of Respiratory and Critical Care Medicine. 2004;169(6):733–8. [PubMed: 14684558]
- Schmitz M, Denardin D, Laufer Silva T, Pianca T, Hutz MH, Faraone S, Rohde LA. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(11):1338–45. [PubMed: 17075356]
- Sciberras E, Ukoumunne OC, Efron D. Predictors of parent-reported attention-deficit/hyperactivity disorder in children aged 6-7 years: a national longitudinal study. Journal of Abnormal Child Psychology. 2011;39(7):1025–34. [PubMed: 21468666]
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the U.S. American Journal of Medicine. 2007;120(2):151–7. [PubMed: 17275456]
- Shabsigh R, Fishman IJ, Schum C, Dunn JK. Cigarette smoking and other vascular risk factors in vasculogenic impotence. Urology. 1991;38(3):227–31. [PubMed: 1887536]
- Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. American Journal of Obstetrics and Gynecology. 2000;182(2):465–72. [PMC free article: PMC2706697] [PubMed: 10694353]
- Shao R, Zou S, Wang X, Feng Y, Brannstrom M, Stener-Victorin E, Billig H. Revealing the hidden mechanisms of smoke-induced fallopian tubal implantation. Biology of Reproduction. 2012;86(4):131. [PubMed: 22357544]
- Shaw GM, Carmichael SL, Vollset SE, Yang W, Finnell RH, Blom H, Midttun O, Ueland PM. Mid-pregnancy cotinine and risks of orofacial clefts and neural tube defects. Journal of Pediatrics. 2009;154(1):17–9. [PubMed: 18990410]
- Shaw GM, Wasserman CR, Lammer EJ, O'Malley CD, Murray JC, Basart AM, Tolarova MM. Orofacial clefts, parental cigarette smoking, and transforming growth factor-alpha gene variants. American Journal of Human Genetics. 1996;58(3):551–61. [PMC free article: PMC1914570] [PubMed: 8644715]
- Shaw JL, Dey SK, Critchley HO, Horne AW. Current knowledge of the aetiology of human tubal ectopic pregnancy. Human Reproduction Update. 2010;16(4):432–44. [PMC free article: PMC2880914] [PubMed: 20071358]
- Shaw JL, Oliver E, Lee KF, Entrican G, Jabbour HN, Critchley HO, Horne AW. Cotinine exposure increases Fallopian tube PROKR1 expression via nicotinic AChRalpha-7: a potential mechanism explaining the link between smoking and tubal ectopic pregnancy. American Journal of Pathology. 2010;177(5):2509–15. [PMC free article: PMC2966807] [PubMed: 20864676]
- Shen Y, Rattan V, Sultana C, Kalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. American Journal of Physiology. 1996;270(5 Pt 2):H1624–H33. [PubMed: 8928867]
- Shiri R, Hakama M, Hakkinen J, Tammela TL, Auvinen A, Koskimaki J. Relationship between smoking and erectile dysfunction. International Journal of Impotence Research. 2005;17(2):164–9. [PubMed: 15510179]
- Shiri R, Hakkinen J, Koskimaki J, Tammela TL, Auvinen A, Hakama M. Smoking causes erectile dysfunction through vascular disease. Urology. 2006;68(6):1318–22. [PubMed: 17141835]
- Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. European Journal of Pharmacology. 2005;517(3):224–31. [PubMed: 15963496]
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. [PubMed: 15733721]
- Sighinolfi MC, Mofferdin A, De SS, Micali S, Cicero AF, Bianchi G. Immediate improvement in penile hemodynamics after cessation of smoking: previous results. Urology. 2007;69(1):163–5. [PubMed: 17270641]
- Silberg JL, Parr T, Neale MC, Rutter M, Angold A, Eaves LJ. Maternal smoking during pregnancy and risk to boys' conduct disturbance: an examination of the causal hypothesis. Biological Psychiatry. 2003;53(2):130–5. [PubMed: 12547468]
- Skelly AC, Holt VL, Mosca VS, Alderman BW. Talipes equinovarus and maternal smoking: a population-based case-control study in Washington state. Teratology. 2002;66(2):91–100. [PubMed: 12210013]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? Journal of Pharmacology and Experimental Therapeutics. 1998;285(3):931–45. [PubMed: 9618392]
- Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ. Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for sudden infant death syndrome. Brain Research Bulletin. 1995;38(1):69–75. [PubMed: 7552377]
- Slotkin TA, Orband-Miller L, Queen KL, Whitmore WL, Seidler FJ. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. Journal of Pharmacology and Experimental Therapeutics. 1987;240(2):602–11. [PubMed: 2433431]
- Smidts DP, Oosterlaan J. How common are symptoms of ADHD in typically developing preschoolers? A study on prevalence rates and prenatal/demographic risk factors. Cortex. 2007;43(6):710–7. [PubMed: 17710823]
- Soares SR, Melo MA. Cigarette smoking and reproductive function. Current Opinion in Obstetrics and Gynecology. 2008;20(3):281–91. [PubMed: 18460944]
- Soares SR, Simon C, Remohi J, Pellicer A. Cigarette smoking affects uterine receptiveness. Human Reproduction. 2007;22(2):543–7. [PubMed: 17095517]
- St. Pourcain B, Mandy WP, Heron J, Golding J, Davey Smith G, Skuse DH. Links between co-occurring social-communication and hyperactive-inattentive trait trajectories. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(9):892–902 e5. [PMC free article: PMC3163265] [PubMed: 21871371]
- Steinberg G, Khankin EV, Karumanchi SA. Angiogenic factors and preeclampsia. Thrombosis Research. 2009;123(Suppl 2):S93–S9. [PubMed: 19217486]
- Steinberger EK, Ferencz C, Loffredo CA. Infants with single ventricle: a population-based epidemiological study. Teratology. 2002;65(3):106–15. [PubMed: 11877773]
- Stene-Larsen K, Borge AI, Vollrath ME. Maternal smoking in pregnancy and externalizing behavior in 18-month-old children: results from a population-based prospective study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(3):283–9. [PubMed: 19242291]
- Stergachis A, Scholes D, Daling JR, Weiss NS, Chu J. Maternal cigarette smoking and the risk of tubal pregnancy. American Journal of Epidemiology. 1991;133(4):332–7. [PubMed: 1822668]
- Stevenson RE. Rectum and anus. In: Stevenson RE, Hall JG, Goodman RM, editors. Human Malformations and related anomalies. New York: Oxford University Press; 1993. pp. 493–9.
- Steyn K, de Wet T, Saloojee Y, Nel H, Yach D. The influence of maternal cigarette smoking, snuff use and passive smoking on pregnancy outcomes: the Birth To Ten Study. Paediatric and Perinatal Epidemiology. 2006;20(2):90–9. [PubMed: 16466427]
- Stoll C, Alembik Y, Dott B, Roth MP. Risk factors in congenital abdominal wall defects (omphalocele and gastroschisi): a study in a series of 265,858 consecutive births. Annales de Génétique. 2001;44(4):201–8. [PubMed: 11755106]
- Storm JE, Fechter LD. Alteration in the postnatal ontogeny of cerebellar norepinephrine content following chronic prenatal carbon monoxide. Journal of Neurochemistry. 1985;45(3):965–9. [PubMed: 4031872]
- Storm JE, Fechter LD. Prenatal carbon monoxide exposure differentially affects postnatal weight and monoamine concentration of rat brain regions. Toxicology and Applied Pharmacology. 1985;81(1):139–46. [PubMed: 4049415]
- Storm JE, Valdes JJ, Fechter LD. Postnatal alterations in cerebellar GABA content, GABA uptake and morphology following exposure to carbon monoxide early in development. Developmental Neuroscience. 1986;8(4):251–61. [PubMed: 2881771]
- Suarez L, Felkner M, Brender JD, Canfield M, Hendricks K. Maternal exposures to cigarette smoke, alcohol, and street drugs and neural tube defect occurrence in offspring. Maternal and Child Health Journal. 2008;12(3):394–401. [PubMed: 17641961]
- Suarez L, Ramadhani T, Felkner M, Canfield MA, Brender JD, Romitti PA, Sun L. Maternal smoking, passive tobacco smoke, and neural tube defects. Birth Defects Research Part A: Clinical and Molecular Teratology. 2011;91(1):29–33. [PMC free article: PMC6034638] [PubMed: 21254356]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Mental Health Findings and Detailed Tables. 2012. [December 6, 2012]. < http://www
.samhsa.gov /data/NSDUH/2k11MH_FindingsandDetTables/Index.aspx>. - Talbot P. In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Research Part C: Embryo Today. 2008;84(1):61–72. [PubMed: 18383128]
- Talbot P, Riveles K. Smoking and reproduction: the oviduct as a target of cigarette smoke. Reproductive Biology and Endocrinology. 2005;3:52. [PMC free article: PMC1266059] [PubMed: 16191196]
- Tamura T, Munger RG, Corcoran C, Bacayao JY, Nepomuceno B, Solon F. Plasma zinc concentrations of mothers and the risk of nonsyndromic oral clefts in their children: a case-control study in the Philippines. Birth Defects Research Part A: Clinical and Molecular Teratology. 2005;73(9):612–6. [PubMed: 16104004]
- Tengs TO, Osgood ND. The link between smoking and impotence: two decades of evidence. Preventive Medicine. 2001;32(6):447–52. [PubMed: 11394947]
- Teramoto S, Soeda A, Hayashi Y, Saito K, Urashima M. Problematic behaviours of 3-year-old children in Japan: relationship with socioeconomic and family backgrounds. Early Human Development. 2005;81(6):563–9. [PubMed: 15935934]
- Thach BT. Some aspects of clinical relevance in the maturation of respiratory control in infants. Journal of Applied Physiology. 2008;104(6):1828–34. [PubMed: 18420716]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. American Journal of Psychiatry. 2003;160(11):1985–9. [PubMed: 14594745]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Rutter M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biological Psychiatry. 2009;66(8):722–7. [PMC free article: PMC2756407] [PubMed: 19596120]
- Thiriez G, Bouhaddi M, Mourot L, Nobili F, Fortrat JO, Menget A, Franco P, Regnard J. Heart rate variability in preterm infants and maternal smoking during pregnancy. Clinical Autonomic Research. 2009;19(3):149–56. [PubMed: 19255805]
- Thompson J, Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reproductive Toxicology. 2008;25(3):304–15. [PubMed: 18367374]
- Thorup J, Cortes D, Petersen BL. The incidence of bilateral cryptorchidism is increased and the fertility potential is reduced in sons born to mothers who have smoked during pregnancy. Journal of Urology. 2006;176(2):734–7. [PubMed: 16813933]
- To WW, Tang MH. The association between maternal smoking and fetal hydranencephaly. Journal of Obstetrics and Gynaecology Research. 1999;25(1):39–42. [PubMed: 10067012]
- Todd RD, Neuman RJ. Gene-environment interactions in the development of combined type ADHD: evidence for a synapse-based model. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B(8):971–5. [PubMed: 17955458]
- Tolson GC, Barnes JM, Gay GA, Kowaleski JL. The 1989 revision of the U.S. Standard Certificates and Reports. Vital and Health Statistics. Series 4: Documents and Committee Reports. 1991;(28):1–34. [PubMed: 1713727]
- Tong VT, Dietz PM, Morrow B, D'Angelo DV, Farr SL, Rockhill KM, England LJ. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 40 sites, 2000–2010. Morbidity and Mortality Weekly Report. 2013;62(SS-6):1–19. [PubMed: 24196750]
- Tostes RC, Carneiro FS, Lee AJ, Giachini FR, Leite R, Osawa Y, Webb RC. Cigarette smoking and erectile dysfunction: focus on NO bioavailability and ROS generation. Journal of Sexual Medicine. 2008;5(6):1284–95. [PMC free article: PMC3003263] [PubMed: 18331273]
- Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics. 2012;129(4):630–8. [PMC free article: PMC3356149] [PubMed: 22451703]
- Travison TG, Shabsigh R, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The natural progression and remission of erectile dysfunction: results from the Massachusetts Male Aging Study. Journal of Urology. 2007;177(1):241–6. [PubMed: 17162054]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking for Women. A Report of the Surgeon General. Washington: U.S. Department of Health and Human Services, Public Health Service, Office of the Assistant Secretary for Health, Office on Smoking and Health; 1980.
- U.S. Department of Health and Human Services. National Pregnancy and Health Survey Drug Use Among Women Delivering Live Births: 1992. Rockville (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Drug Abuse; 1996. NIH Publication No. 96-3819.
- U.S. Department of Health and Human Services. Women and Smoking. A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General–Executive Summary. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [PubMed: 20669524]
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease—The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed: 21452462]
- U.S. Department of Health Education, and Welfare. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1964. PHS Publication No. 1103.
- U.S. Department of Health Education, and Welfare. The Health Consequences of Smoking. 1969 Supplement to the 1967 Public Health Service Review. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service; 1969. DHEW Publication No. 1696-2.
- U.S. Department of Health, Education, and Welfare. Smoking and Health. A Report of the Surgeon General. Washington: U.S. Department of Health, Education, and Welfare, Office of the Assistant Secretary for Health, Office on Smoking and Health; 1979. DHEW Publication No. (PHS) 79-50066.
- U.S. Environmental Protection Agency. Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. Washington: U.S. Environmental Protection Agency, Office of Research and Development, Office of Health and Environmental Assessment; 1992. Publication No. EPA/600/6-90/006F.
- van Beynum IM, Kapusta L, Bakker MK, den HM, Blom HJ, de Walle HE. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. European Heart Journal. 2010;31(4):464–71. [PubMed: 19952004]
- van den Boogaard MJ, de CD, Krapels IP, Liu F, van DC, Sinke RJ, Lindhout D, Steegers-Theunissen RP. The MSX1 allele 4 homozygous child exposed to smoking at periconception is most sensitive in developing nonsyndromic orofacial clefts. Human Genetics. 2008;124(5):525–34. [PubMed: 18932005]
- Van Den Eeden SK, Shan J, Bruce C, Glasser M. Ectopic pregnancy rate and treatment utilization in a large managed care organization. Obstetrics and Gynecology. 2005;105(5 Pt 1):1052–7. [PubMed: 15863544]
- Van Landingham M, Nguyen TV, Roberts A, Parent AD, Zhang J. Risk factors of congenital hydrocephalus: a 10 year retrospective study. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(2):213–7. [PubMed: 18653551]
- van Rooij IA, Wegerif MJ, Roelofs HM, Peters WH, Kuijpers-Jagtman AM, Zielhuis GA, Merkus HM, Steegers-Theunissen RP. Smoking, genetic polymorphisms in biotransformation enzymes, and nonsyndromic oral clefting: a gene-environment interaction. Epidemiology. 2001;12(5):502–7. [PubMed: 11505167]
- van Rooij IA, Wijers CH, Rieu PN, Hendriks HS, Brouwers MM, Knoers NV, de Blaauw I, Roeleveld N. Maternal and paternal risk factors for anorectal malformations: a Dutch case-control study. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(3):152–8. [PubMed: 20073076]
- Vasquez G, Winston RM, Brosens IA. Tubal mucosa and ectopic pregnancy. British Journal of Obstetrics and Gynaecology. 1983;90(5):468–74. [PubMed: 6849848]
- Vennemann MM, Hense HW, Bajanowski T, Blair PS, Complojer C, Moon RY, Kiechl-Kohlendorfer U. Bed sharing and the risk of sudden infant death syndrome: can we resolve the debate? Journal of Pediatrics. 2012;160(1):44–8. e2. [PubMed: 21868032]
- Vieira AR. Association between the transforming growth factor alpha gene and nonsyndromic oral clefts: a HuGE review. American Journal of Epidemiology. 2006;163(9):790–810. [PubMed: 16495466]
- Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet. 1985;1(8422):181–4. [PubMed: 2857264]
- Wabrek AJ, Shelley MM, Horowitz LM, Bastarache MM, Giuca JE. Noninvasive penile arterial evaluation in 120 males with erectile dysfunction. Urology. 1983;22(3):230–4. [PubMed: 6604973]
- Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: a developmental framework. Development and Psychopathology. 2002;14(2):351–69. [PubMed: 12030696]
- Wakschlag LS, Henry DB, Blair RJ, Dukic V, Burns J, Pickett KE. Unpacking the association: Individual differences in the relation of prenatal exposure to cigarettes and disruptive behavior phenotypes. Neurotoxicology and Teratology. 2011;33(1):145–54. [PMC free article: PMC3674557] [PubMed: 21256429]
- Wakschlag LS, Keenan K. Clinical significance and correlates of disruptive behavior in environmentally at-risk preschoolers. Journal of Clinical Child Psychology. 2001;30(2):262–75. [PubMed: 11393926]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, Dukic V, Blair RJ, Leventhal BL, Cox NJ, et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Molecular Psychiatry. 2010;15(9):928–37. [PMC free article: PMC2905677] [PubMed: 19255579]
- Wakschlag LS, Leventhal BL, Pine DS, Pickett KE, Carter AS. Elucidating early mechanisms of developmental psychopathology: the case of prenatal smoking and disruptive behavior. Child Development. 2006;77(4):893–906. [PubMed: 16942496]
- Wakschlag LS, Pickett KE, Kasza KE, Loeber R. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(4):461–7. [PubMed: 16601651]
- Wang W, Guan P, Xu W, Zhou B. Risk factors for oral clefts: a population-based case-control study in Shenyang, China. Paediatric and Perinatal Epidemiology. 2009;23(4):310–20. [PubMed: 19523078]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA: the Journal of the American Medical Association. 2002;287(2):195–202. [PubMed: 11779261]
- Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Human Reproduction Update. 2009;15(1):31–44. [PubMed: 18927070]
- Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Research Part C: Embryo Today. 2007;81(3):215–28. [PubMed: 17963271]
- Webster WS, Howe AM, Abela D, Oakes DJ. The relationship between cleft lip, maxillary hypoplasia, hypoxia and phenytoin. Current Pharmaceutical Design. 2006;12(12):1431–48. [PubMed: 16611127]
- Weiss NS, Daling JR, Chow WH. Control definition in case-control studies of ectopic pregnancy. American Journal of Public Health. 1985;75(1):67–8. [PMC free article: PMC1646151] [PubMed: 3966602]
- Werler MM, Mitchell AA, Moore CA, Honein MA. Is there epidemiologic evidence to support vascular disruption as a pathogenesis of gastroschisis? American Journal of Medical Genetics Part A. 2009;149A(7):1399–406. [PMC free article: PMC2739090] [PubMed: 19533769]
- Werler MM, Sheehan JE, Mitchell AA. Association of vasoconstrictive exposures with risks of gastroschisis and small intestinal atresia. Epidemiology. 2003;14(3):349–54. [PubMed: 12859037]
- Whitaker RC, Orzol SM, Kahn RS. Maternal mental health, substance use, and domestic violence in the year after delivery and subsequent behavior problems in children at age 3 years. Archives of General Psychiatry. 2006;63(5):551–60. [PubMed: 16651512]
- Wikström AK, Cnattingius S, Stephansson O. Maternal use of Swedish snuff (snus) and risk of stillbirth. Epidemiology. 2010;21(6):772–8. [PubMed: 20805750]
- Wilcox AJ. On the importance—and the unimportance—of birthweight. International Journal of Epidemiology. 2001;30(6):1233–41. [PubMed: 11821313]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. New England Journal of Medicine. 1988;319(4):189–94. [PubMed: 3393170]
- Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatric Pathology. 1991;11(5):677–84. [PubMed: 1745639]
- Wilson P, Bradshaw P, Tipping S, Henderson M, Der G, Minnis H. What predicts persistent early conduct problems? Evidence from the Growing Up in Scotland cohort. Journal of Epidemiology and Community Health. 2013;67(1):76–80. [PMC free article: PMC3534305] [PubMed: 22844082]
- Windham GC, Von Behren J, Waller K, Fenster L. Exposure to environmental and mainstream tobacco smoke and risk of spontaneous abortion. American Journal of Epidemiology. 1999;149(3):243–7. [PubMed: 9927219]
- Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Human Reproduction. 2002;17(12):3220–3. [PubMed: 12456627]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Frontiers in Bioscience. 2008;13:636–49. [PubMed: 17981576]
- Wisborg K, Kesmodel U, Henriksen TB, Hedegaard M, Secher NJ. A prospective study of maternal smoking and spontaneous abortion. Acta Obstetricia et Gynecologica Scandinavica. 2003;82(10):936–41. [PubMed: 12956844]
- Woods SE, Raju U. Maternal smoking and the risk of congenital birth defects: a cohort study. Journal of the American Board of Family Practice. 2001;14(5):330–4. [PubMed: 11572537]
- World Health Organization. A multinational case-control study of ectopic pregnancy. Clinical Reproduction and Fertility. 1985;3(2):131–43. [PubMed: 4052920]
- Wu T, Fallin MD, Shi M, Ruczinski I, Liang KY, Hetmanski JB, Wang H, Ingersoll RG, Huang S, Ye X, et al. Evidence of gene-environment interaction for the RUNX2 gene and environmental tobacco smoke in controlling the risk of cleft lip with/without cleft palate. Birth Defects Research Part A: Clinical and Molecular Teratology. 2012;94(2):76–83. [PMC free article: PMC3608124] [PubMed: 22241686]
- Xie Y, Garban H, Ng C, Rajfer J, Gonzalez-Cadavid NF. Effect of long-term passive smoking on erectile function and penile nitric oxide synthase in the rat. Journal of Urology. 1997;157(3):1121–6. [PubMed: 9072555]
- Xu X, Cook RL, Ilacqua VA, Kan H, Talbott EO. Racial differences in the effects of postnatal environmental tobacco smoke on neurodevelopment. Pediatrics. 2010;126(4):705–11. [PubMed: 20855396]
- Yang M, Kunugita N, Kitagawa K, Kang SH, Coles B, Kadlubar FF, Katoh T, Matsuno K, Kawamoto T. Individual differences in urinary cotinine levels in Japanese smokers: relation to genetic polymorphism of drug-metabolizing enzymes. Cancer Epidemiology, Biomarkers & Prevention. 2001;10(6):589–93. [PubMed: 11401907]
- Yerushalmy J. The relationship of parents' cigarette smoking to outcome of pregnancy—implications as to the problem of inferring causation from observed associations. American Journal of Epidemiology. 1971;93(6):443–56. [PubMed: 5562717]
- Yin Z, Xu W, Xu C, Zhang S, Zheng Y, Wang W, Zhou B. A population-based case-control study of risk factors for neural tube defects in Shenyang, China. Child's Nervous System. 2011;27(1):149–54. [PubMed: 20582422]
- Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, Costa P, Druschel C, Hobbs CA, Romitti PA, et al. The National Birth Defects Prevention Study. Public Health Reports. 2001;116(Suppl 1):32–40. [PMC free article: PMC1913684] [PubMed: 11889273]
- Zamakhshary M, Yanchar NL. Complicated gastroschisis and maternal smoking: a causal association? Pediatric Surgery International. 2007;23(9):841–4. [PubMed: 17618440]
- Zammit S, Thomas K, Thompson A, Horwood J, Menezes P, Gunnell D, Hollis C, Wolke D, Lewis G, Harrison G. Maternal tobacco, cannabis and alcohol use during pregnancy and risk of adolescent psychotic symptoms in offspring. British Journal of Psychiatry. 2009;195(4):294–300. [PubMed: 19794196]
- Zandi M, Heidari A. An epidemiologic study of orofacial clefts in Hamedan city, Iran: a 15-year study. Cleft Palate-Craniofacial Journal. 2011;48(4):483–9. [PubMed: 20572780]
- Zeiger JS, Beaty TH, Liang KY. Oral clefts, maternal smoking, and TGFA: a meta-analysis of gene-environment interaction. Cleft Palate-Craniofacial Journal. 2005;42(1):58–63. [PubMed: 15643916]
- Zhang B, Jiao X, Mao L, Xue J. Maternal cigarette smoking and the associated risk of having a child with orofacial clefts in China: a case-control study. Journal of Cranio-Maxillo-Facial Surgery. 2011;39(5):313–8. [PubMed: 20832329]
- Zhang BY, Wei YS, Niu JM, Li Y, Miao ZL, Wang ZN. Risk factors for unexplained recurrent spontaneous abortion in a population from southern China. International Journal of Gynaecology and Obstetrics. 2010;108(2):135–8. [PubMed: 19897189]
- Ziaei S, Nouri K, Kazemnejad A. Effects of carbon monoxide air pollution in pregnancy on neonatal nucleated red blood cells. Paediatric and Perinatal Epidemiology. 2005;19(1):27–30. [PubMed: 15670105]
- Zwink N, Jenetzky E, Brenner H. Parental risk factors and anorectal malformations: systematic review and meta-analysis. Orphanet Journal of Rare Diseases. 2011;6:25. [PMC free article: PMC3121580] [PubMed: 21586115]
Footnotes
- 1
Pack-years = the number of years of smoking multiplied by the number of packs of cigarettes smoked per day.
- Reproductive Outcomes - The Health Consequences of Smoking—50 Years of ProgressReproductive Outcomes - The Health Consequences of Smoking—50 Years of Progress
Your browsing activity is empty.
Activity recording is turned off.
See more...