NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington (DC): National Academies Press (US); 2015 Feb 10.

Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness.
Show detailsMyalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS) are debilitating conditions that affect somewhere between 836,000 and 2.5 million Americans (Jason et al., 1999, 2006b). Despite having different definitions, ME and CFS often are used interchangeably to refer to an illness characterized by profound fatigue and autonomic and neurocognitive symptoms. Throughout this report, the umbrella term “ME/CFS” is used to refer to ME and CFS. However, the literature analysis conducted in support of this study took into consideration the variability among the definitions used in the studies reviewed.
The cause of ME/CFS remains unknown, although in many cases, symptoms may be triggered by an infection or other prodromal events such as “immunization, anesthetics, physical trauma, exposure to environmental pollutants, chemicals and heavy metals, and rarely blood transfusions” (Carruthers and van de Sande, 2005, p. 1). An estimated 84 to 91 percent of patients affected by the condition are not yet diagnosed (Jason et al., 2006b; Solomon and Reeves, 2004), and people with ME/CFS often struggle with their illness for years before receiving a diagnosis. In multiple surveys, 67 to 77 percent of patients reported that it took longer than 1 year to receive a diagnosis, and about 29 percent reported that it took longer than 5 years (CFIDS Association of America, 2014; ProHealth, 2008).
Seeking and receiving a diagnosis can be a frustrating process for patients with ME/CFS for several reasons, including a lack of understanding of diagnosis and treatment of the condition among health care providers and skepticism about whether it is in fact a true medical condition. Less than one-third of medical schools include ME/CFS-specific information in their curriculum (Peterson et al., 2013), and only 40 percent of medical textbooks include information on the condition (Jason et al., 2010). Some studies on awareness of ME/CFS have found high awareness among health care providers, but many providers believe it is a psychiatric/psychological illness or at least has a psychiatric/psychological component (Brimmer et al., 2010; Jason and Richman, 2008; Unger, 2011). ME/CFS often is seen as a diagnosis of exclusion, which also can lead to delays in diagnosis or misdiagnosis of a psychological problem (Bayliss et al., 2014; Fossey et al., 2004). Once diagnosed, moreover, many people with ME/CFS report being subject to hostile attitudes from their health care providers (Anderson and Ferrans, 1997; David et al., 1991), as well as to treatment strategies that exacerbate their symptoms (Twemlow et al., 1997).
ME/CFS can cause significant impairment and disability that have negative economic consequences at the individual and societal levels. At least one-quarter of ME/CFS patients are house- or bedbound at some point in their lives (Marshall et al., 2011; NIH, 2011; Shepherd and Chaudhuri, 2001). The direct and indirect economic costs of ME/CFS to society are estimated to be between $17 and $24 billion annually (Jason et al., 2008), $9.1 billion of which can be attributed to lost household and labor force productivity (Reynolds et al., 2004). Together, high medical costs and reduced earning capacity often have devastating effects on patients' financial situations (Reynolds et al., 2004).
Literature on mortality associated with ME/CFS is sparse. One study found that cancer, heart disease, and suicide are the most common causes of death among those diagnosed with ME/CFS, and people with ME/CFS die from these causes at younger ages than others in the general population. However, the authors note that these results cannot be generalized to the overall population of ME/CFS patients because of the methodological limitations of the study (Jason et al., 2006a).
This report, based on a study conducted by a committee convened by the Institute of Medicine (IOM), documents the evidence base for ME/CFS. The committee also proposes clear and concise diagnostic criteria designed to improve the ability of health care providers to diagnose this disorder.
CHARGE TO THE COMMITTEE
The Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome was charged by the Department of Health and Human Services (HHS) sponsors with evaluating the current criteria for diagnosis of ME/CFS and recommending clinical diagnostic criteria that would address the needs of health care providers, patients, and their caregivers. Specifically, the committee was asked to
- conduct a study to identify the evidence for various clinical diagnostic criteria for ME/CFS using a process with input from stakeholders, including practicing clinicians and patients;
- develop evidence-based clinical diagnostic criteria for ME/CFS for use by clinicians, using a consensus-building methodology;
- recommend whether new terminology for ME/CFS should be adopted; and
- develop an outreach strategy for disseminating the new criteria nationwide to health professionals.
The committee was also asked to distinguish among disease subgroups, develop a plan for updating the new criteria, and make recommendations for the plan's implementation. The statement of task requested that the committee's recommendations consider unique diagnostic issues facing people with ME/CFS, related specifically to gender and particular subgroups with substantial disability and extending across the life span. The committee was not asked to investigate the etiology, pathophysiology, pathogenesis, or treatment of ME/CFS. The complete statement of task is provided in Box 1-1.
The committee comprised 15 members with expertise in clinical care for ME/CFS, pediatrics, infectious disease, epidemiology, immunology, rheumatology, behavioral health, pain, sleep, primary care, genetics, exercise physiology, neurology/neuropathology, and clinical case definitions. In addition to their scientific expertise, two committee members are or have been patients, and one is a family member/caregiver of a patient with ME/CFS.
CONTEXT FOR THIS STUDY
This study was sponsored by the Office on Women's Health within HHS, the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration, the Agency for Healthcare Research and Quality, and the Social Security Administration. The study was commissioned in response to a recommendation from HHS's Chronic Fatigue Syndrome Advisory Committee (CFSAC), which comprises 11 voting members, including the chair, who provide advice and recommendations to the Secretary of HHS on issues related to ME/CFS. Of the 11 members, 7 are required to be scientists with demonstrated expertise in ME/CFS biomedical research, and 4 should have expertise in health care delivery, private health care services or insurers, or voluntary organizations working with ME/CFS patients.1 In 2012, the CFSAC recommended that HHS “promptly convene … at least one stakeholders' (ME/CFS experts, patients, advocates) workshop in consultation with the CFSAC members to reach a consensus for a case definition useful for research, diagnosis and treatment of ME/CFS beginning with the 2003 Canadian Consensus Definition for discussion purposes.”2 Given the well-established and well-regarded consensus process used by the IOM, HHS contracted with the IOM in September 2013 to conduct this study.
In the weeks that followed, many advocates were greatly disappointed that HHS did not follow the CFSAC recommendation as it was intended. Patients, advocates, researchers, and clinicians expressed strong opposition to the study, arguing that the IOM lacks the expertise to develop clinical case definitions and that the inclusion of non-ME/CFS experts in this process would move the science backward. An open letter was sent to the Secretary of HHS, signed by 38 U.S.-based biomedical researchers and clinicians, declaring that consensus had been reached on the use of the Canadian clinical case definition (often called the Canadian Consensus Criteria [CCC]) for diagnosis of ME/CFS, and requesting that the IOM study be canceled and the funds used to support further ME/CFS research instead (see Chapter 3 for a discussion of current diagnostic criteria) (An Open Letter, 2013).
THE COMMITTEE'S APPROACH
The committee held five meetings and two public sessions during the course of its work (see Appendix A for the agendas for the public sessions). Throughout the study, many people with ME/CFS and their family members and friends, as well as the study sponsors and other organizations and individuals, provided valuable input to the committee about their concerns, burdens, hopes, and challenges. Some quotes throughout the report highlight perspectives shared during the public sessions and in emails to the committee. In addition to its meetings and public sessions, the committee sought information from relevant concurrent research efforts and undertook a comprehensive review of the scientific literature and other available evidence on ME/CFS. The committee consulted with a health communications specialist and a statistician to obtain additional expertise in addressing its statement of task. The committee's approach to this study is described in more detail below.
Public Sessions and Public Comments
In the two public sessions, the committee heard testimony from patients, their family members, advocates, and researchers in the field. The committee also reviewed and carefully considered hundreds of comments received from the public.3
Input from Other Groups
As requested in its statement of task, the committee sought data from the ongoing CDC Multi-Site Clinical Study of CFS. The CDC study has collected standardized data on major illness domains of CFS from patients in seven practices around the country (Unger, 2013). Dr. Elizabeth Unger, principal investigator of the CDC study, presented the study's preliminary results at the committee's first public session. Throughout the study, the committee communicated questions and data requests to CDC through the IOM staff. Dr. Unger and her team provided analyses requested by the committee.4 The committee recognizes that these findings are preliminary and have not been independently verified, and thus should be interpreted with caution. These data are appropriately cited throughout this report.
The study's statement of task also directed the committee to seek input from NIH's Evidence-based Methodology Workshop for ME/CFS, a process now referred to as Pathways to Prevention (P2P). The NIH P2P workshop was originally intended to complement the present study by developing a research case definition for ME/CFS (CFSAC, 2012). However, in remarks on behalf of the P2P workshop process at the committee's first public session, Susan Maier, Deputy Director for NIH's Office of Research on Women's Health, stated that the goal of the P2P workshop was not to develop a research case definition but to suggest a research agenda for ME/CFS based on an unbiased review of the evidence. She also expressed a desire to work with this committee throughout the P2P process. However, the planning group for the P2P workshop declined to share any data with the committee.
Literature Review
Throughout the study, the IOM staff maintained an EndNote library to organize the committee's research. The foundation for this library was a broad search of eight databases for all articles related to ME, CFS, ME/CFS, and other terms used to describe this disorder (such as post-viral fatigue syndrome) published since 1950. This search was run regularly to identify peer-reviewed articles published through May 30, 2014. Additional citations and grey literature (i.e., non-commercially published) were identified by the IOM staff, committee members, and the public and from references in pertinent articles. See Figure 1-1 for additional information on the initial search results.
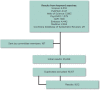
FIGURE 1-1
Initial results (as of January 2014) of the committee's broad literature search. NOTE: Through May 2014 the committee received regular updates on this search strategy. The committee also received additional literature from members of the public and identified (more...)
The committee was charged with reviewing the evidence for various diagnostic clinical criteria for ME/CFS. Existing diagnostic criteria refer to a minimum of nine distinct symptoms, so the evidence for clinical criteria spans a wide range of disciplines. After a preliminary review of the literature, the committee directed the IOM staff to divide the articles into topics most central to its work: eight symptoms or symptom categories (for children/adolescents and adults) and three additional topics. For some of these topics, the committee reviewed all of the relevant literature, while for others, the committee conducted targeted literature searches. All of the topic areas are listed below; those in italics were identified for targeted searches.
- Symptoms or symptom categories
- – Autonomic manifestations
- – Fatigue
- – Immune manifestations
- – Neurocognitive manifestations
- – Neuroendocrine manifestations
- – Pain
- – PEM (post-exertional malaise)
- – Sleep
- Additional topics
- – Disability and impairment
- – Infections and ME/CFS
- – Symptom constructs and clusters
For the topics identified for targeted searches, the committee and the IOM staff worked together to identify priority research questions and to develop targeted search strategies for gathering literature relevant to answering these questions. The targeted searches were run in the same eight databases as the general search and also included articles published from 1950 through May 30, 2014. The IOM staff evaluated the results of each targeted search according to predefined inclusion and exclusion criteria, and they provided a list of abstracts to research groups of two to five committee members assigned to each topic. The research groups reviewed the abstracts and identified articles appropriate for full-text review. Articles addressing diagnosis (e.g., a particular biomarker) and prognosis (e.g., the relationship between a feature and an outcome) and those defining manifestations of ME/CFS subgroups (cluster of symptoms and signs) were selected. The overall intent was to identify information on symptoms and objectively measurable signs (such as laboratory and imaging abnormalities) that are associated with ME/CFS and could be useful in defining ME/CFS or discriminating it—or subgroups—from other conditions. The results of the targeted searches are presented in Tables 1-1 and 1-2.
TABLE 1-1
Targeted Search Results: Adults.
TABLE 1-2
Targeted Search Results: Pediatrics.
The research groups read the full-text articles and extracted information into spreadsheets, including information about study populations, sample sizes, methods, findings, and conclusions. The data extraction spreadsheets also included items adapted from Quality Assessment of Diagnostic Accuracy Studies (QUADAS) criteria (Whiting et al., 2003) and Hoy and colleagues (2012) to help assess study quality. The research groups presented summaries of the literature and assessments of its quality to the entire committee.
Committee's Deliberation and Consensus Process
The committee carefully considered the testimony and public comments received and the results of the literature review as it deliberated on its recommendations concerning (1) symptoms and abnormalities that must be present to make the diagnosis of ME/CFS, (2) symptoms and abnormalities that can support the diagnosis but are not required in all cases, and (3) exclusionary and comorbid conditions. The committee adapted a “GRADE grid” to record individual judgments as to whether there is sufficient evidence that certain symptoms and abnormalities define either ME/CFS or a particular subtype of ME/CFS (see Appendix B for the grid template) (Guyatt et al., 2008; Jaeschke et al., 2008). The collated judgments were used to facilitate further discussion, which led to consensus among the committee members on final recommendations regarding diagnostic criteria. The committee then considered the recommended criteria and revisited the public comments to inform its decision making on whether a different name or set of names might be appropriate for ME/CFS.
Consultants
To fulfill its statement of task with respect to developing an outreach strategy for disseminating the diagnostic criteria for ME/CFS, the committee consulted with a communication specialist with expertise in dissemination for health care professionals. The committee worked with the consultant to explain the needs and priorities for and the audiences to be reached with this strategy. After an initial meeting, the consultant worked with a group of three committee members to develop the strategy, which was discussed during the last committee meeting. The final outreach strategy presented in this report incorporates the committee's review and feedback.
The committee also consulted with a statistician who reviewed and summarized 18 papers that use statistical methods to analyze symptom data from ME/CFS patients. The committee discussed the information provided by this consultant to determine whether the data presented in these papers could be used to identify subgroups of patients with ME/CFS.
OVERVIEW OF THE REPORT
The remainder of this report is organized as follows:
- Chapter 2 provides additional background information on ME/CFS, including its history, its terminology, and its burden and impact.
- Chapter 3 presents a comparison of five current sets of case definitions and diagnostic criteria, a discussion that supports the committee's proposal for new terminology for ME/CFS. This chapter also presents the committee's assessment of the literature on ME/CFS symptom constructs and clusters.
- Chapter 4 reviews the evidence on the major symptoms of ME/CFS (fatigue, post-exertional malaise [PEM], sleep abnormalities, neurocognitive manifestations, and orthostatic intolerance and autonomic dysfunction).
- Chapter 5 reviews the evidence on other symptoms and manifestations commonly presented in ME/CFS patients, such as pain, immune abnormalities, neuroendocrine abnormalities, and an association with infection.
- Chapter 6 reviews the evidence on pediatric ME/CFS.
- Chapter 7 presents the committee's recommendations for new diagnostic criteria and new terminology for ME/CFS and for updating of the new diagnostic criteria.
- Chapter 8 presents the committee's plan for dissemination of the new criteria to health professionals nationwide.
REFERENCES
- An Open Letter. An open letter to the honorable Kathleen Sebelius, U.S. Secretary of Health and Human Services. 2013. [January 12, 2015]. September 23, 2013. https://dl
.dropboxusercontent .com/u/89158245 /Case%20Definition %20Letter%20Sept%2023%202013.pdf. - Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. Journal of Nervous and Mental Disease. 1997;185(6):359–367. [PubMed: 9205421]
- Bayliss K, Goodall M, Chisholm A, Fordham B, Chew-Graham C, Riste L, Fisher L, Lovell K, Peters S, Wearden A. Overcoming the barriers to the diagnosis and management of chronic fatigue syndrome/ME in primary care: A meta synthesis of qualitative studies. BMC Family Practice. 2014;15(1):44. [PMC free article: PMC3973969] [PubMed: 24606913]
- Brimmer DJ, Fridinger F, Lin JM, Reeves WC. U.S. healthcare providers' knowledge, attitudes, beliefs, and perceptions concerning chronic fatigue syndrome. BMC Family Practice. 2010;11:28. [PMC free article: PMC2875206] [PubMed: 20406491]
- Carruthers BM, van de Sande MI. Myalgic encephalomyelitis/chronic fatigue syndrome: A clinical case definition and guidelines for medical practitioners: An overview of the Canadian consensus document. Vancouver, BC: Carruthers and van de Sande; 2005.
- CFIDS (Chronic Fatigue and Immune Dysfunction Syndrome) Association of America. ME/CFS road to diagnosis survey. Charlotte, NC: CFIDS Association of America; 2014.
- CFSAC (Chronic Fatigue Syndrome Advisory Committee). The twenty-second meeting of the Chronic Fatigue Syndrome Advisory Committee. Washington, DC: U.S. Department of Health and Human Services; Oct 3, 2012. 2012.
- David AS, Wessely S, Pelosi AJ. Chronic fatigue syndrome: Signs of a new approach. British Journal of Hospital Medicine. 1991;45(3):158–163. [PubMed: 2029596]
- Fossey M, Libman E, Bailes S, Baltzan M, Schondorf R, Amsel R, Fichten CS. Sleep quality and psychological adjustment in chronic fatigue syndrome. Journal of Behavioral Medicine. 2004;27(6):581–605. [PubMed: 15669445]
- Guyatt GH, Oxman AD, Vist GE, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal. 2008;336(7650):924–926. [PMC free article: PMC2335261] [PubMed: 18436948]
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. Journal of Clinical Epidemiology. 2012;65(9):934–939. [PubMed: 22742910]
- Jaeschke R, Guyatt GH, Dellinger P, Schünemann H, Levy MM, Kunz R, Norris S, Bion J. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. British Medical Journal. 2008;337:a744. [PubMed: 18669566]
- Jason LA, Richman JA. How science can stigmatize: The case of chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome. 2008;14(4):85–103.
- Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, McCready W, Huang CF, Plioplys S. A community-based study of chronic fatigue syndrome. Archives of Internal Medicine. 1999;159(18):2129–2137. [PubMed: 10527290]
- Jason L, Corradi K, Gress S, Williams S, Torres-Harding S. Causes of death among patients with chronic fatigue syndrome. Health Care for Women International. 2006a;27(7):615–626. [PubMed: 16844674]
- Jason L, Torres-Harding S, Njok M. CFIDS Chronicle. 2006b. [March 3, 2015]. The face of CFS in the U.S; pp. 16–21. http://www
.researchgate .net/profile/Leonard_Jason /publication /236995875_The_Face_of_CFS_in_the_U .S/links /00b7d51acf6823bccb000000.pdf . - Jason LA, Benton MC, Valentine L, Johnson A, Torres-Harding S. The economic impact of ME/CFS: Individual and societal costs. Dynamic Medicine. 2008;7(6) [PMC free article: PMC2324078] [PubMed: 18397528]
- Jason LA, Paavola E, Porter N, Morello ML. Frequency and content analysis of chronic fatigue syndrome in medical text books. Australian Journal of Primary Health. 2010;16(2):174–178. [PMC free article: PMC3691015] [PubMed: 21128580]
- Marshall R, Paul L, Wood L. The search for pain relief in people with chronic fatigue syndrome: A descriptive study. Physiotherapy Theory & Practice. 2011;27(5):373–383. [PubMed: 21039301]
- NIH (National Institutes of Health). State of the knowledge workshop. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) research: Workshop report. Bethesda, MD: Office of Research on Women's Health, NIH, U.S. Department of Health and Human Services; 2011.
- Peterson TM, Peterson TW, Emerson S, Regalbuto E, Evans MA, Jason LA. Coverage of CFS within U.S. medical schools. Universal Journal of Public Health. 2013;1(4):177–179.
- ProHealth. A profile ME/CFS patients: How many years and how many doctors. 2008. [August 13, 2014]. http://www
.prohealth .com/library/showarticle.cfm?libid=13672 . - Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome. Cost Effectiveness and Resource Allocation. 2004;2(4) [PMC free article: PMC449736] [PubMed: 15210053]
- Shepherd C, Chaudhuri A. ME/CFS/PVFS: An exploration of the key clinical issues. Buckingham, UK: The ME Association; 2001.
- Solomon L, Reeves WC. Factors influencing the diagnosis of chronic fatigue syndrome. Archives of Internal Medicine. 2004;164(20):2241–2245. [PubMed: 15534161]
- Twemlow SW, Bradshaw SL Jr, Coyne L, Lerma BH. Patterns of utilization of medical care and perceptions of the relationship between doctor and patient with chronic illness including chronic fatigue syndrome. Psychological Reports. 1997;80(2):643–658. [PubMed: 9129381]
- Unger A. CFS knowledge and illness management behavior among U.S. healthcare providers and the public. Paper read at IACFS/ME Biennial International Conference Ottawa; Ontario, Canada: 2011.
- Unger E. Measures of CFS in a multi-site clinical study. Paper read at FDA Scientific Drug Development Workshop; Washington, DC: Apr 26, 2013. 2013.
- Whiting P, Rutjes AW, Reitsma JB, Bossuy PM, Kleijnen J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology. 2003;3(25):1–13. [PMC free article: PMC305345] [PubMed: 14606960]
Footnotes
- 1
The HHS website can be accessed at http://www
.hhs.gov/advcomcfs /charter/index.html (accessed January 13, 2015). - 2
CFSAC recommendations can be accessed at http://www
.hhs.gov/advcomcfs /recommendations/10032012.html (accessed January 13, 2015). - 3
Public testimony and other materials submitted to the committee are available upon request from the National Academies' Public Access Records Office.
- 4
Ibid.
- Introduction - Beyond Myalgic Encephalomyelitis/Chronic Fatigue SyndromeIntroduction - Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Your browsing activity is empty.
Activity recording is turned off.
See more...