NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
The definition of elderly is arbitrary. In this chapter we will define elderly as greater than 75 years of age because both the US and European lipids guidelines use this age to differentiate therapy recommendations. Atherosclerotic cardiovascular disease (ASCVD) is a major cause of morbidity and mortality in the elderly. Age is a key risk factor for ASCVD and with identical risk factors the 10-year risk of an ASCVD event markedly increases with age. In fact, an older individual with excellent risk factors can still have a high risk for having an ASCVD event. ASCVD begins early in life and progresses until it leads to clinical events later in life. The age that one develops clinical manifestations of ASCVD is dependent on the severity of individual risk factors, the number of risk factors, and the duration of exposure to the risk factors. Elderly individuals have a long exposure to risk factors so even when the risk factors are relatively modest the cumulative effects can be sufficient to result in clinical ASCVD events. This explains why age is such a key variable in determining the risk of developing an ASCVD event. Cardiovascular outcome studies have demonstrated that lowering LDL-C levels with statins, ezetimibe, or PCSK 9 monoclonal antibodies will reduce ASCVD events in elderly patients with pre-existing cardiovascular disease (secondary prevention). In elderly patients without cardiovascular disease (primary prevention) the available data does not definitively demonstrate a decrease in ASCVD events with statin or ezetimibe therapy but is suggestive of a benefit (note there are no primary prevention trials with PCSK9 inhibitors). Additional data is required to determine if bempedoic acid and icosapent ethyl reduce ASCVD events in patients ≥ 75 years of age. Studies are currently underway to provide definitive information on whether statin therapy is beneficial as primary prevention in the elderly. In deciding whether to treat an elderly patient with lipid lowering drugs one needs to consider the following factors; the higher the LDL-C level the greater the benefit of lowering LDL-C, the greater the decrease in LDL-C the greater the benefit, the higher the absolute risk of ASCVD the greater the benefit of lowering LDL-C, life expectancy, competing non-cardiovascular disorders, risk of drug side effects, potential for drug interactions, and patient preferences. In elderly patients without pre-existing ASCVD one should estimate the patient’s risk of developing ASCVD events and in conjunction with the general principles described above discuss with the patient a treatment plan. Determining the coronary calcium score can be helpful if there is uncertainty regarding the appropriate decision. If the decision is to treat our goal in primary prevention patients is often an LDL-C < 100mg/dL but in high-risk patients our goal may be an LDL-C < 70mg/dL. Elderly patients with ASCVD should be treated with lipid lowering drugs to reduce ASCVD unless there are contraindications. At a minimum our goal is an LDL-C < 70mg/dL but we would prefer an LDL-C < 55mg/dL if they can be achieved with a statin + ezetimibe. In very high-risk patients our goal is an LDL-C < 55mg/dL and adding a PCSK9 inhibitor may be required to achieve these levels in some patients. Age per se should not be used to withhold therapy with lipid lowering drugs that can reduce the risk of ASCVD events. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Due to a decreasing birth rate and a longer life expectancy the population is getting older. According to the US census in 2020 there were approximately 50 million people between 65 and 84 years of age (14.9% of the total population) and approximately 6 million between 85 and 99 years of age (1.89% of the total population). The number of Americans ages 65 and older is projected to increase to 82 million by 2050 (23% of the total population). World-wide there are 703 million people aged 65 or older, which is projected to reach 1.5 billion by 2050 (1 in 6 people). It is well recognized that atherosclerotic cardiovascular disease (ASCVD) increases with age and is a major cause of morbidity and mortality in the elderly. In addition to an increased risk of coronary artery disease there is more than a doubling of the prevalence of peripheral arterial disease, cerebrovascular disease, and abdominal aortic aneurism with each decade of life (1). Unfortunately, the elderly (≥ 75 years of age) have not been well represented in lipid lowering cardiovascular outcome trials.
The definition of elderly is arbitrary. In this chapter we will define elderly as greater than 75 years of age because both the US and European guidelines use this age to differentiate therapy recommendations. In both the “Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines” and the “2019 ESC/EAS Guidelines for the Management of Dyslipidemias: Lipid Modification to Reduce Cardiovascular Risk” the recommendations for those over 75 years of age differ from recommendations for younger individuals (2,3). Thus, where possible we will focus on studies in individuals greater than 75 years of age.
LIPID LEVELS IN THE ELDERLY
Lipid levels in US adults from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 are shown in Table 1 (4). Compared to 30–69-year-olds there is a slight decrease in LDL-C, non-HDL-C, and triglycerides with similar HDL-C levels in individuals 70-79 years of age. Other cross-sectional studies have reported similar results (5-11). Prospective studies with longitudinal follow-up have also observed small decreases in total cholesterol, LDL-C, and HDL-C levels in men and women as they become elderly (6,7,9,12-14). It should be noted that the changes in lipid levels reported with aging are relatively small and vary somewhat from study to study. The clinical significance of these small changes is uncertain.
Table 1.
Lipid Levels in U.S. Adults (NHANES 2003-2004)
| Age | LDL-C (mg/dL) | Non-HDL-C (mg/dL) | HDL-C (mg/dL) | Triglycerides (mg/dL) |
|---|---|---|---|---|
| 20-29 | 104 | 126 | 54 | 105 |
| 30-39 | 120 | 146 | 54 | 118 |
| 40-49 | 124 | 152 | 53 | 144 |
| 50-59 | 123 | 154 | 55 | 141 |
| 60-69 | 126 | 157 | 54 | 145 |
| 70-79 | 119 | 148 | 56 | 133 |
Studies have demonstrated that older individuals have an exaggerated postprandial lipemia compared with younger individuals (15,16). While elevated postprandial triglycerides is associated with an increased risk of ASCVD whether this plays a causal role in increasing ASCVD is uncertain.
It is well recognized that with increasing age the likelihood of other medical disorders increases and this can affect lipid levels. For example, inflammation and infections can decrease LDL-C and HDL-C levels (17). Additionally, poor nutrition due to illness or social-economic factors could decrease lipid levels in the elderly. Finally, frailty is a syndrome associated with aging and increases with age. It is usually associated with a lowering of total, LDL-C, and non-HDL cholesterol levels (18-20).
AGE IS AN IMPORTANT RISK FOR ATHEROSCLEROTIC CARDIOVASCULAR DISEASE
The clearest way to illustrate the importance of age as a key risk factor for atherosclerotic cardiovascular disease (ASCVD) is to compare the 10-year risk at different ages using an updated version of the AHA/ACC pooled cohort equation. Shown in table 2 are four examples of the effect of age on 10-year risk in different clinical situations that demonstrate the marked effect of age on ASCVD risk. Similarly, using the SCORE risk calculator for determining the 10-year risk of fatal cardiovascular disease also demonstrates the very large impact of age on risk (figure 1). It is obvious that age is a major determinant of ASCVD risk.
Table 2.
Ten Year Risk of Developing ASCVD
| Age 55 | Age 65 | Age 75 | |
|---|---|---|---|
| Male, white, BP 130, TC 200, HDL-C 45, non-smoker, no diabetes | 6.3% | 13.9% | 26.2% |
| Female, African American, BP120, TC 180, HDL-C 50, non-smoker, no diabetes | 2.8% | 6.3% | 13.2% |
| Male, African American, BP140, TC 200, HDL-C 50, smoker, no diabetes | 10.1% | 14.9% | 20.5% |
| Female, white, BP 140, TC 180, HDL-C 50, non-smoker, diabetes | 4.4% | 12.2% | 32.9% |
BP= systolic BP mm Hg, TC= total cholesterol mg/dL.
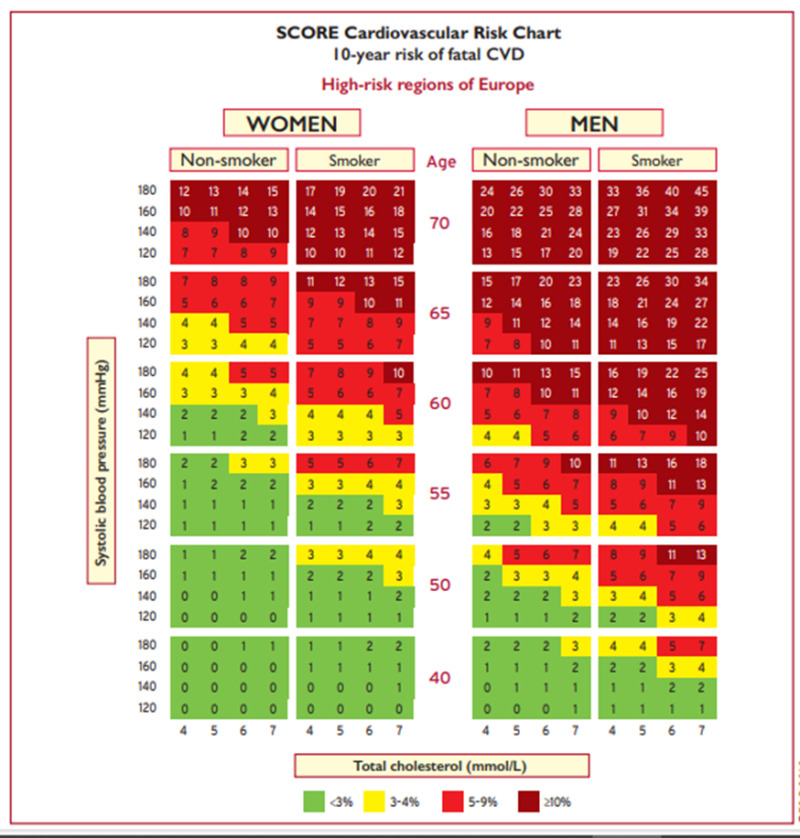
Figure 1.
Systematic Coronary Risk Estimation chart for European populations at high cardiovascular disease risk (from 2019 ESC/EAS Guidelines for the management of dyslipidaemias (3)).
In fact, an older individual with excellent risk factors can have a high risk for having an ASCVD event. For example, using the AHA/ACC pooled cohort equation a 75-year-old white male with a total cholesterol of 180mg/dL, an HDL-C of 50mg/dL, a blood pressure of 120/80 mmHg, who is not diabetic, doesn’t smoke, and is on no medications still has a 10-year risk for an ASCVD event of 21.7%. A 75-year-old white female with the same risk factors also has a relatively high 10-year risk (14.1%). Using the SCORE estimator (figure 1) for a fatal CVD event it is also apparent that many older individuals, particularly males, are at high risk even when they are non-smokers with an excellent total cholesterol and blood pressure. For example, a 70-year-old male, non-smoker with a total cholesterol of 160mg/dL and systolic BP of 120 mmHg still has a 13% 10-year risk of death from CVD.
WHY ARE OLDER INDIVIDUALS AT HIGHER RISK FOR ASCVD?
It is widely recognized that atherosclerosis begins early in life and slowly progresses ultimately resulting in clinical manifestations later in life (21). Numerous studies have demonstrated the presence of atherosclerosis in young individuals (22-27). In the Bogalusa Heart Study autopsies were performed on 204 young people 2 to 39 years of age (22,28). In the coronary arteries fatty streaks were very common (50 percent at 2 to 15 years of age and 85 percent at 21 to 39 years of age). More advanced raised fibrous-plaque lesions in the coronary arteries were present in 8 percent of individuals 2 to 15 years of age and 69 percent of individuals 26 to 39 years of age. The extent of the atherosclerotic lesions correlated positively with BMI, systolic and diastolic BP, total cholesterol, LDL-C, and triglyceride levels and negatively with HDL-C levels. The extent of the atherosclerotic lesions was greatest in individuals who had multiple risk factors. The Pathobiological Determinants of Atherosclerosis in Youth [PDAY] study examined the effect of risk factors for atherosclerosis in 1079 men and 364 women 15 through 34 years of age who died due to accidents, homicide, or suicide (23,29). Atherosclerosis of the aorta and right coronary artery was measured and increased with age, LDL-C levels, glycohemoglobin levels, BMI, and smoking while HDL-C levels were negatively associated with the extent of fatty streaks and raised lesions in the aorta and right coronary artery. Finally, in a study of US service members (mean age 25.9 years; range 18-59 years; 98.3% male) who died of combat or unintentional injuries (n= 3832) the effect of risk factors on coronary atherosclerosis was determined (27). Atherosclerosis prevalence was increased by age, dyslipidemia, hypertension, and obesity. Taken together these studies clearly demonstrate that atherosclerosis begins early in life with the prevalence increasing with age and the extent and onset of lesions is influenced by risk factors, including dyslipidemia.
Moreover, the presence of risk factors early in life is associated with an increase in atherosclerosis later in life (30-32). A meta-analysis that included 4380 participants from 4 prospective studies that collected cardiovascular risk factor data during childhood (age 3 to 18 years) and measured carotid intima-media thickness (CIMT) in adulthood (age 20 to 45 years) reported that total cholesterol, triglycerides, BP, and BMI measured in childhood were predictive of elevated CIMT in adults (33). Additionally, increased LDL-C and/or decreased HDL-C during adolescence predict an increase in CIMT later in life (34). Importantly, an increased total cholesterol or BP early in life also predicted an increased risk of developing cardiovascular disease later in life (35-38).
Genetic studies have further illustrated the key role of risk factors and duration of exposure to the risk factor as key variables determining the time when clinical manifestations of ASCVD occur. In patients with homozygous familial hypercholesterolemia (FH) LDL-C are markedly elevated and cardiovascular events can occur early in life. Greater than 50% of untreated patients with homozygous FH develop clinically significant ASCVD by the age of 30 and cardiovascular events can occur before age 10 in some patients (39). In patients with heterozygous FH LDL-C levels are elevated but not to the levels seen with homozygous FH and cardiovascular events occur later in life but still at a relatively younger age. Untreated males with heterozygous FH have a 50% risk for a fatal or non-fatal myocardial infarction by 50 years of age whereas untreated females have a 30% chance by age 60 (39). Conversely, individuals with genetic variants in PCSK9, HMG-CoA reductase, LDL receptor, NPC1L1, or ATP citrate lyase that lead to a decrease in LDL-C levels have a reduced risk of developing cardiovascular events (40,41). The relationship between genetic disorders that alter LDL-C levels and the time to develop clinical cardiovascular events is illustrated in figure 2. The figure clearly illustrates that the age when one clinically manifests ASCVD depends on the level of LDL-C. With very high LDL-C levels clinical events occur early in life and with low LDL-C levels events will occur at an older age leading to the concept of LDL years.
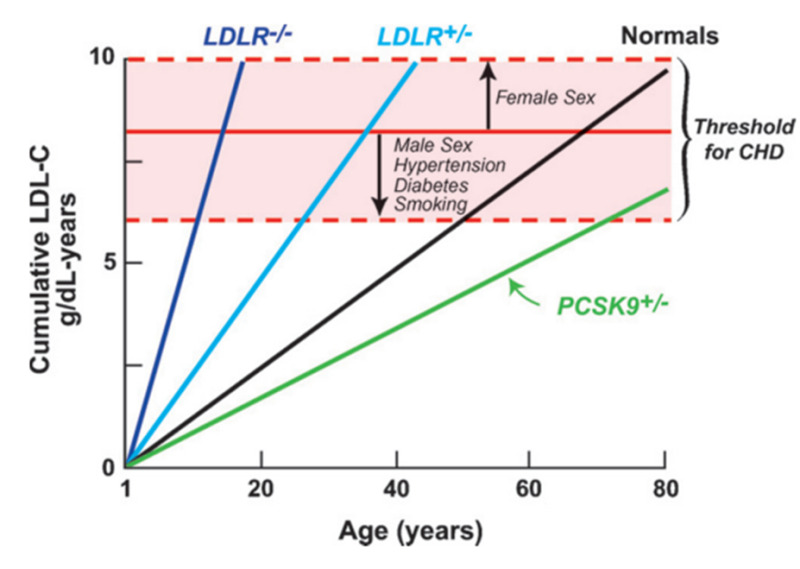
Figure 2.
Relationship between cumulative LDL-C exposure and age of developing cardiovascular disease. (from (41)).
The degree and duration of other risk factors also seems to play a role in when the clinical manifestations of ASCVD are expressed. For example, for cigarette smoking, cigarettes/day, smoking duration, and pack-years all increase the risk of cardiovascular disease (42). Interestingly smoking fewer cigarettes/day for a longer duration was more deleterious than smoking more cigarettes/day for a shorter duration (42,43). Additionally, while smoking cessation lowers the risk of ASCVD events an increased risk persists for decades after smoking cessation (44). These observations suggest that the effect of smoking is related to the number of cigarettes smoked and the duration of the smoking (i.e., pack years). Similarly, in patients with diabetes glycemic control and duration of diabetes influences the development of ASCVD complications (45-48). At any given age, a 10-year longer diabetes duration was associated with a 1.1-1.5-fold increased risk of stroke and 1.5-2.0-fold increased risk of MI (45).
Thus, ASCVD begins early in life and progresses until it leads to clinical events such as a myocardial infarction or stroke later in life. The age that one develops clinical manifestations of ASCVD is dependent on the severity of individual risk factors, the number of risk factors, and the duration of exposure to the risk factors. Elderly individuals have a long exposure to risk factors so even when the risk factors are relatively modest the cumulative effects can be sufficient to result in clinical ASCVD events. This explains why age is such a key variable in determining the risk of developing an ASCVD event.
DOES LIPID LOWERING REDUCE EVENTS IN THE ELDERLY
Below we discuss lipid lowering drug studies that report the effect on cardiovascular outcomes that are relevant to clinical decisions in elderly individuals. For additional and more detailed information on lipid lowering cardiovascular outcome studies see the Endotext chapters on “Cholesterol Lowering Drugs” and “Triglyceride Lowering Drugs” (49,50).
Statins
Few statin studies have focused on lowering LDL-C in elderly patients, which we define as individuals greater than 75 years of age. The Prosper Trial determined the effect of pravastatin 40mg/day (n= 2891) vs. placebo (n= 2913) on cardiovascular events in older subjects (70-82 years of age) with pre-existing vascular disease or who were at high risk for vascular disease (51). The average age in this trial was 75 years of age and approximately 45% had cardiovascular disease. As expected, pravastatin treatment lowered LDL-C by 34% compared to the placebo group. The primary end point was coronary death, non-fatal myocardial infarction, and fatal or non-fatal stroke, which was reduced by 15% (HR 0.85, 95% CI 0.74-0.97, p=0.014). However, in the individuals without pre-existing cardiovascular disease pravastatin did not significantly reduce ASCVD events (HR 0.94; CI 0.77–1.15). In contrast, in individuals with cardiovascular disease pravastatin therapy significantly reduced ASCVD events (HR 0.78, CI 0.66–0.93). Thus, this study demonstrated benefits of statin therapy in the elderly with cardiovascular disease but failed to demonstrate benefit in the elderly without cardiovascular disease.
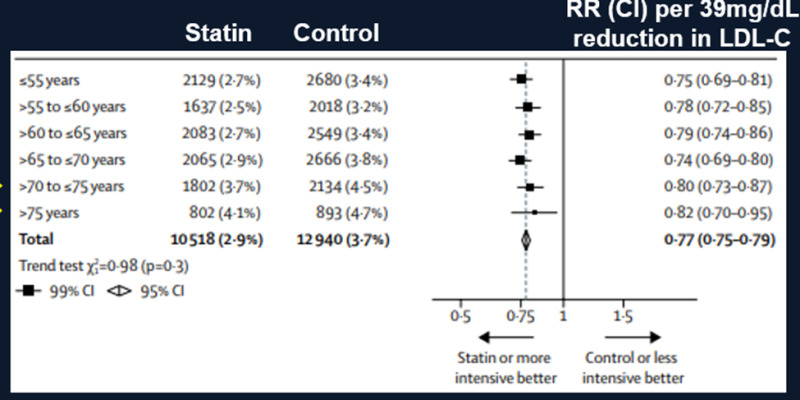
A meta-analysis by the Cholesterol Treatment Trialists of 28 trials with 14,483 of 186,854 found a reduction in LDL-C levels with statin therapy that was similar in the participants ≥75 years of age compared to younger individuals. Moreover, statin therapy resulted in a decrease in cardiovascular events in all age groups including participants ≥75 years of age (Figure 3) (52). In the participants ≥75 there was a 13% reduction in ASCVD events per 39mg/dL decrease in LDL-C (RR 0.87; 95% CI 0.77–0.99). This analysis included four trials done exclusively among people with heart failure or receiving renal dialysis, for whom statin therapy shows little or no benefit (50). A second analysis was performed after elimination of these four trials and there was an 18% reduction in ASCVD events per 39mg/dL decrease in LDL-C (RR 0.82; 95%CI 0.70-0.95). Similar to the Prosper Trial a decrease in ASCVD events was clearly demonstrated in individuals with pre-existing cardiovascular disease (secondary prevention) but in individuals without pre-existing cardiovascular disease (primary prevention) the decrease in ASCVD events was not statistically significant (Figure 4- analysis included all studies). After excluding the trials in patients with heart failure or receiving renal dialysis, statin therapy reduced major ASCVD events by 26% per 39mg/dL decrease in LDL-C (RR 0.74; 95% CI0.60 − 0.91) in patients with pre-existing cardiovascular disease but only by 8% in patients without pre-existing cardiovascular disease (RR 0.92; 95%CI 0.72 − 1.16).
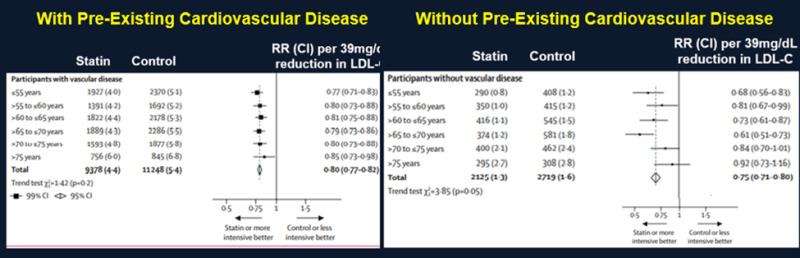
Figure 4.
Effect of Statin Treatment on Major Vascular Events in Individuals With and Without Pre-Existing Cardiovascular Disease. Modified from (44).
A statin trial not included in the Cholesterol Treatment Trialists meta-analysis was carried out in patients with an ischemic stroke or a transient ischemic attack who were treated with statins and/or ezetimibe with a target LDL-C level < than 70mg/dL (n= 1430) or an LDL-C 90mg/dL to 110mg/dL (n= 1430) (53). The primary end point was ischemic stroke, myocardial infarction, new symptoms leading to urgent coronary or carotid revascularization, or death from cardiovascular causes. The mean LDL-C level was 65mg/dL in the lower-target group and 96 mg/dL in the higher-target group. After median of 3.5 years the primary end point occurred in 8.5% of the patients in the lower-target group and 10.9% of the patients in the higher target group (HR 0.78; 95% CI 0.61 to 0.98; P=0.04). In patients < 65 years of age only a 7% decrease in the primary end point was observed (HR 0.93; 95%CI 0.63–1.36) whereas more impressive decreases in the primary endpoint were observed in patients 65-75 years of age (37% decrease; HR 0.63 95% CI 0.42–0.95) and > 75 years of age (23% decrease; HR 0.77; 95%CI 0.49–1.22). These results are consistent with the Cholesterol Treatment Trialists meta-analysis demonstrating that elderly patients with pre-existing cardiovascular disease lowering LDL-C levels reduces ASCVD events.
There are observational studies demonstrating that statin treatment for the primary prevention of ASCVD is effective in older patients (54-59). For example, in US veterans ≥75 years of age and free of ASCVD at baseline, new statin use was significantly associated with a lower risk of ASCVD events (HR 0.92; 95% CI 0.91-0.94) and cardiovascular mortality (HR 0.80; 95% CI 0.78-0.81) when compared to statin nonusers (55). Similarly, in a Danish nationwide cohort initiation of statin therapy in patients > 70 years of age without pre-existing cardiovascular disease there was a 23% lower risk of major vascular events per 39mg/dL decrease in LDL-C (HR 0.77; 95% CI 0.71-0.83), which was similar to what was observed in younger individuals (54). Finally, in nursing home residents without ASCVD statin use reduced all-cause mortality in individuals with and without dementia (59). These observational studies while suggestive of a benefit of statin therapy for primary prevention in older individuals cannot provide definitive proof as there is always the possibility of residual confounding. Nevertheless, they provide additional support that statin therapy provides benefits in elderly patients without pre-existing cardiovascular disease.
Thus, in older patients with cardiovascular disease lowering LDL-C levels with statins clearly reduces cardiovascular events but in older patients without cardiovascular disease the data demonstrating that statins reduce cardiovascular events is less robust but suggests a reduction in ASCVD events.
Ezetimibe
IMPROVE-IT TRIAL
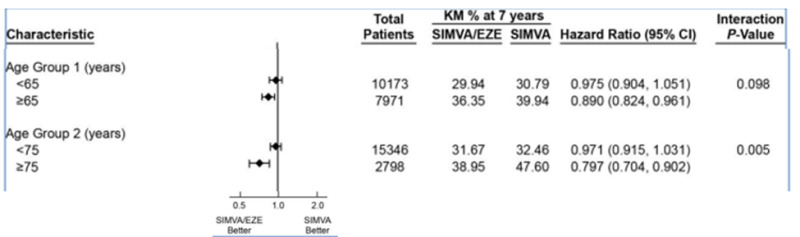
The IMPROVE-IT Trial tested whether the addition of ezetimibe to statin therapy would provide an additional beneficial effect in patients with the acute coronary syndrome (60). The IMPROVE-IT Trial was a large trial with over 18,000 patients randomized to simvastatin 40mg vs. simvastatin 40mg + ezetimibe 10mg per day. On treatment LDL-C levels were 70mg/dL in the statin alone group vs. 54mg/dL in the statin + ezetimibe group. There was a small but significant 6.4% decrease in major cardiovascular events (cardiovascular death, MI, documented unstable angina requiring rehospitalization, coronary revascularization, or stroke) in the statin + ezetimibe group (HR 0.936; 95% CI 0.887-0.988; p=0.016). Cardiovascular death, non-fatal MI, or non-fatal stroke were reduced by 10% (HR 0.90; 95% CI 0.84-0.97; p=0.003). The effect of age on the benefits of statin + ezetimibe therapy is shown in figure 5. In elderly individuals (≥ 75 years of age) the combination of ezetimibe and simvastatin reduced ASCVD events.
EWTOPIA 75 TRIAL
EWTOPIA 75 was a multicenter, randomized trial in Japan that examined the preventive efficacy of ezetimibe for patients aged ≥75 years (mean age 80.6 years), with elevated LDL-C (≥140 mg/dL) without a history of coronary artery disease (primary prevention) who were not taking lipid lowering drugs (61). Patients were randomized to ezetimibe 10mg (n=1,716) or usual care (n=1,695) and followed for 4.1 years. The primary outcome was a composite of sudden cardiac death, myocardial infarction, coronary revascularization, or stroke. In the ezetimibe group LDL-C was decreased by 25.9% and non-HDL-C by 23.1% while in the usual care group LDL-C was decreased by 18.5% and non-HDL-C by 16.5% (p<0.001 for both lipid parameters). By the end of the trial 9.6% of the patients in the usual care group and 2.1% of the ezetimibe group were taking statins. Ezetimibe reduced the incidence of the primary outcome by 34% (HR 0.66; P=0.002). Additionally, composite cardiac events were reduced by 60% (HR 0.60; P=0.039) and coronary revascularization by 62% (HR 0.38; P=0.007) in the ezetimibe group vs. the control group. There was no difference in the incidence of stroke or all-cause mortality between the groups. It should be noted that the reduction in cardiovascular events was much greater than one would expect based on the absolute difference in LDL-C levels (121mg/dL in ezetimibe group vs. 132mg/dL in usual care group). As stated by the authors “Given the open-label nature of the trial, its premature termination, and issues with follow-up, the magnitude of benefit observed should be interpreted with caution.” Nevertheless, this study suggests that lowering LDL-C in elderly individuals without cardiovascular disease can reduce ASCVD events.
RACING TRIAL
The RACING trial was a randomized, open-label trial in patients with ASCVD carried out in South Korea (62). Patients were randomly assigned to either rosuvastatin 10 mg with ezetimibe 10 mg (n= 1894) or rosuvastatin 20 mg (n= 1886). The primary endpoint was cardiovascular death, major cardiovascular events, or non-fatal stroke. The median LDL-C level during the study was 58mg/dL in the combination therapy group and 66mg/dL in the statin monotherapy group (p<0.0001). The primary endpoint occurred in 9.1% of the patients in the combination therapy group and 9.9% of the patients in the high-intensity statin monotherapy group (non-inferior). Non-inferiority was observed in patients with baseline LDL-C levels < 100mg/dL and >100mg/dL (63).
In the RACING trial 574 participants (15.2%) were aged ≥75 years and there was no difference in the primary endpoint between the combination therapy group and the high-intensity statin monotherapy group in these elderly participants (64). However, in participants ≥75 years of age moderate-intensity statin with ezetimibe combination therapy was associated with lower rates of drug related intolerance with drug discontinuation or dose reduction (2.3% vs 7.2%; P = 0.010).
This study demonstrates that moderate intensity statin plus ezetimibe was non-inferior to high-intensity statin therapy with regards to cardiovascular death, major cardiovascular events, or non-fatal stroke. The lower prevalence of discontinuation or dose reduction caused by intolerance to the study drug was seen with combination therapy indicating that using a moderate intensity dose of a statin plus ezetimibe is a useful strategy in patients that do not tolerate high intensity statin therapy or where there are concerns about statin toxicity with high doses.
PCSK9 Inhibitors
FOURIER TRIAL
The FOURIER trial was a randomized, double-blind, placebo-controlled trial of evolocumab vs. placebo in 27,564 patients with ASCVD and an LDL-C level of 70 mg/dL or higher who were on statin therapy (65). The primary end point was cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization and the key secondary end point was cardiovascular death, myocardial infarction, or stroke. The median duration of follow-up was 2.2 years. Baseline LDL-C levels were 92mg/dL and evolocumab resulted in a 59% decrease in LDL levels (LDL-C level on treatment approximately 30mg/dL). In this trial 6233 of the participants were > 69 years of age and the decrease in LDL was similar in participants > 69 years of age and younger individuals (66). A 14% reduction in the primary endpoint (HR 0.86; 95% CI 0.74–0.99) and a 18% reduction in the secondary endpoint (HR 0.82; 95% CI 0.69–0.98) was observed in the participants > 69 years of age, which was similar to the decreases seen in younger individuals (66). The effect of treatment with evolocumab on the primary and secondary endpoint in specific age groups is shown in table 3 (66). These results demonstrate that lowering LDL-C with a PCSK9 inhibitor decreases ASCVD events in elderly patients.
Table 3.
Effect of Evolocumab Treatment on Cardiovascular Outcomes in Different Age Groups
| < 65 | 65-75 | >75 | |
|---|---|---|---|
| Primary Endpoint | HR 0.86; 95%CI 0.78–0.94 | HR 0.86; 95%CI 0.76–0.97 | HR 0.78; 95%CI 0.60–1.02 |
| Secondary Endpoint | HR 0.79; 95%CI 0.69–0.90 | HR 0.82; 95%CI 0.70–0.95 | HR 0.78 95%CI 0.58–1.04 |
For the primary endpoint the P interaction for the three age groups = 0.84.
For the secondary endpoint the P interaction for the three age groups = 0.94.
ODYSSEY Trial
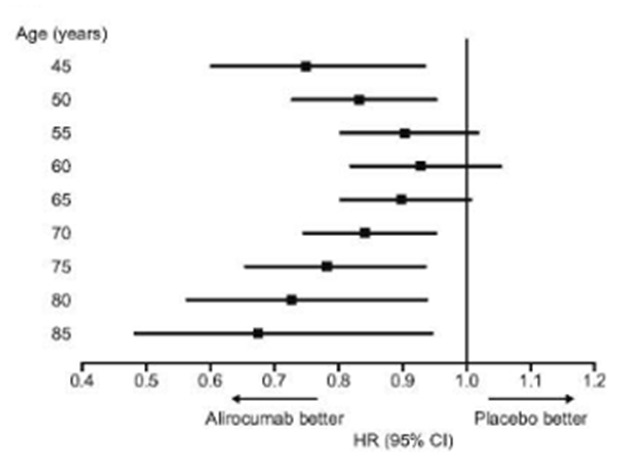
The ODYSSEY trial was a multicenter, randomized, double-blind, placebo-controlled trial involving 18,924 patients who had an acute coronary syndrome 1 to 12 months earlier, an LDL-C level of at least 70 mg/dL, a non-HDL-C level of at least 100 mg/dL, or an apolipoprotein B level of at least 80 mg/dL while on high intensity statin therapy or the maximum tolerated statin dose (67). Patients were randomly assigned to receive alirocumab 75 mg every 2 weeks or matching placebo. The dose of alirocumab was adjusted to target an LDL-C level of 25 to 50 mg/dL. The primary end point was a composite of death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. In this trial 5084 (26.9%) individuals were ≥ 65 years of age, 1007 (5.3%) ≥ 75 years of age, and 42 (0.2%) ≥ 85 years of age (68). The baseline and decrease in LDL-C levels were similar in participants ≥65 years of age and those <65 years of age (LDL-C at baseline approximately 94mg/dL and after 4 months of treatment approximately 40mg/dL) (67,68). In the individuals ≥ 65 years of age there was a 22% decrease in the primary endpoint (HR 0.78; 95% CI 0.68–0.91) and in those < 65 years of age a 11% decrease (HR 0.89; 95% CI 0.80–1.00) (68). The secondary endpoint of all-cause death, myocardial infarction, or ischemic stroke was also reduced in the ≥ 65 participants (HR 0.78; 95% CI 0.68–0.90) and < 65 participants (HR 0.91; 0.82–1.02) (68). In participants ≥ 75 years of age the primary endpoint was reduced by 15% (HR 0.85; 95% CI 0.64–1.13) (68). When plotted as a continuous variable the relative benefit of alirocumab over placebo on the primary endpoint was consistent across the entire age range (figure 6).
These two studies demonstrate that lowering LDL-C with a PCSK9 inhibitor decreases ASCVD events in elderly patients with pre-existing cardiovascular disease.
Bempedoic Acid
The CLEAR Outcome trial was a double-blind, randomized, placebo-controlled trial involving patients with cardiovascular disease or at high risk of cardiovascular disease who were unable or unwilling to take statins ("statin-intolerant" patients) (69). The patients were randomized to bempedoic acid 180 mg (n= 6992) or placebo (n= 6978) and the median duration of follow-up was 40.6 months. In this trial 44% of the participants were between ≥65 to < 75 years of age and 15% were ≥ 75 years of age. As expected, LDL-C levels were decreased by 21% in the bempedoic group compared to placebo (29mg/dL difference). The primary endpoint, death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization, was reduced by 13% in the bempedoic acid group (HR 0.87; 95% CI 0.79- 0.96; P = 0.004). The effect of age on the primary endpoint is shown in table 4.
Table 4.
Effect of Bempedoic on Cardiovascular Outcomes in Different Age Groups
| < 65 | HR 0.87; 95% CI 0.74-1.02 |
| ≥65-<75 | HR 0.83, 95% CI 0.72-0.96 |
| ≥ 75 | HR 0.95, 95% CI 0.77-1.16 |
Interaction P value = 0.60.
Niacin and Fibrates
Because of the robust effect of statins in lowering LDL-C levels and cardiovascular events recent trials of both niacin and fibrates have focused on the addition of these lipid lowering drugs to statin therapy. The AIM-HIGH trial was designed to determine if the addition of Niaspan, an extended-release form of niacin, to aggressive statin therapy would result in a further reduction in ASCVD events in patients with pre-existing cardiovascular disease (70) while the HPS 2 Thrive trial determined the effect of adding extended-release niacin (2000mg/day) combined with laropiprant, a prostaglandin D2 receptor antagonist, to statin therapy on ASCVD events in patients with pre-existing vascular disease (71). Unfortunately, both of these trials failed to demonstrate a decrease in ASCVD events with the addition of niacin to statin therapy. The absence of benefit and increased side effects from niacin therapy has markedly reduced enthusiasm for treating patients with niacin to reduce ASCVD event. For additional details on these two studies and other niacin cardiovascular outcome studies see the Endotext chapter “Triglyceride Lowering Drugs” (49).
The ACCORD-LIPID Trial was designed to determine if the addition of fenofibrate to aggressive statin therapy in patients with pre-existing cardiovascular disease or at high risk for developing cardiovascular disease would result in a further reduction in cardiovascular disease in patients with Type 2 diabetes (72). The PROMINENT trial determined whether pemafibrate, a new selective PPAR-alpha activator, in patients on statin therapy with diabetes and pre-existing cardiovascular disease or at high risk for developing cardiovascular disease would reduce cardiovascular events (73). Disappointingly, neither trial demonstrated benefits from adding a fibrate to statin therapy. For additional details on these two studies and other fibrate ASCVD outcome studies see the Endotext chapter “Triglyceride Lowering Drugs” (49).
Thus, there is currently little enthusiasm for adding either niacin or a fibrate to statin therapy to reduce ASCVD events. One should recognize that like all studies these trials have limitations, that are discussed in detail in reference (49), and it is possible that future trials could resurrect the use of niacin and/or fibrates for decreasing ASCVD.
Omega-3-Fatty Acids (Fish Oil)
Numerous studies have determined the effect of low dose fish oil (< 1 gram per day) on ASCVD and found that they do not consistently reduce the risk of cardiovascular disease (49). Described below are ASCVD outcome studies that have used higher doses.
JAPAN EPA LIPID INTERVENTION STUDY (JELIS)
JELIS was an open label study without a placebo in patients with total cholesterol levels > 254mg/dL with cardiovascular disease (n= 3,664) or without cardiovascular disease (n=14,981) who were randomly assigned to be treated with 1800 mg of EPA (Vascepa) + statin (n=9,326) or statin alone (n= 9,319) with a 5-year follow-up (74). The primary endpoint was any major coronary event, including sudden cardiac death, fatal and non-fatal myocardial infarction, and other non-fatal events including unstable angina pectoris, angioplasty, stenting, or coronary artery bypass grafting. Total cholesterol, LDL-C, and HDL-C levels were similar in the two groups but plasma TGs were modestly decreased in the EPA treated group (5% decrease in EPA group compared to controls; p = 0.0001). In the EPA plus statin group the primary endpoint occurred in 2.8% of the patients vs. 3.5% of the patients in the statin alone group (19% decrease; p = 0.011). In participants < 61 years of age the primary endpoint was reduced by 24% (HR 0.76; 95%CI 0.57–1.00) while in individuals ≥ 61 years of age the primary endpoint was reduced by 16% (HR 0.84; 95% CI 0.68–1.02; p interaction 0.57). Unstable angina and non-fatal coronary events were significantly reduced in the EPA plus statin group but in this study sudden cardiac death and coronary death did not differ between groups. Unstable angina was the main component contributing to the primary endpoint and this is a more subjective endpoint than other endpoints such as a myocardial infarction, stroke, or cardiovascular death. A subjective endpoint has the potential to be an unreliable endpoint in an open label study and is a limitation of the JELIS Study. Unfortunately, we do not have information on elderly patients (≥ 75 years).
REDUCE-IT
REDUCE-IT was a randomized, double-blind trial of 2 grams twice per day of EPA ethyl ester (icosapent ethyl) (Vascepa) vs. mineral oil placebo in 8,179 patients with hypertriglyceridemia (135mg/dL to 499mg/dL) and established cardiovascular disease or high cardiovascular disease risk (diabetes plus one risk factor) who were on stable statin therapy (75). The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina. At baseline, the median LDL-C level was 75.0 mg/dL, HDL-C level was 40.0 mg/dL, and TG level was 216.0 mg/dL. The median change in TG level from baseline to 1 year was a decrease of 18.3% (−39.0 mg/dL) in the EPA group and an increase of 2.2% (4.5 mg/dL) in the placebo group. After a median of 4.9 years the primary end-point occurred in 17.2% of the patients in the EPA group vs. 22.0% of the patients in the placebo group (HR 0.75; P<0.001), indicating a 25% decrease in events. In participants <65 years of age the primary end point was reduced by 35% (HR 0.65; 95% CI 0.54–0.78) while in participants ≥ 65 years of age the primary end point was reduced by 18% (HR 0.82; 95%CI 0.70–0.97; P Value for Interaction 0.06). The cardiovascular benefits of EPA were similar across baseline levels of TGs (<150, ≥150 to <200, and ≥200 mg per deciliter). Moreover, the cardiovascular benefits of EPA appeared to occur irrespective of the attained TG level at 1 year (≥150 or <150 mg/dL), suggesting that the ASCVD risk reduction was not associated with attainment of a normal TG level. Unfortunately, information on an elderly subgroup (≥ 75 years) is not available.
It should be noted that in this trial mineral oil was used as the placebo. In the placebo group the LDL-C, non-HDL-C, and hsCRP levels were increased compared to the EPA group during the trial (LDL-C 96mg/dL vs 85mg/dL; non-HDL-C 130mg/dL vs. 113mg/dL; hsCRP 2.8mg/L vs. 1.8mg/L). The impact of these adverse changes on clinical outcomes is uncertain and whether they contributed to the apparent beneficial effects observed in the individuals treated with EPA is unknown.
STRENGTH TRIAL
The STRENGTH Trial was a double-blind, randomized, trial comparing 4 grams per day of a carboxylic acid formulation of omega-3 fatty acids (EPA and DHA; Epanova) (n = 6,539)) vs. corn oil placebo (n = 6539) in statin-treated participants with high cardiovascular risk, hypertriglyceridemia, and low levels of HDL-C (76). Approximately 55% of patients had established cardiovascular disease and approximately 70% had diabetes. Median LDL-C level was 75.0 mg/dL, median TG level was 240 mg/dL, and median HDL-C level was 36 mg/dL. There were minimal differences in the change in LDL-C and HDL-C levels between the treated and placebo groups after treatment for 12 months but as expected there was a greater reduction in TG levels in the group treated with omega-3-fatty acids (−19.0% vs −0.9%). The primary endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina requiring hospitalization which occurred in 12.0% of individuals treated with omega-3 CA vs. 12.2% treated with corn oil (HR 0.99; P = .84). There were no significant differences between the treatment groups with regard to the risk of the individual components of the primary end point over the 3-4 years of the study. Thus, in contrast to the JELIS and REDUCE-IT trials the STRENGTH trial did not demonstrate a benefit of treatment with a mixture of omega-3-fatty acids (EPA + DHA).
OMEMI TRIAL
The OMEMI trial was a randomized trial of 1.8 grams per day of omega-3-fatty acids (930 mg EPA and 660 mg DHA) (n= 505) vs. corn oil placebo (n= 509) in patients aged 70 to 82 years with a recent myocardial infarction (2-8 weeks) (77). Baseline LDL-C was approximately 76mg/dL, HDL-C was 49mg/dL, and TGs 110mg/dL. The primary endpoint was a composite of nonfatal myocardial infarction, unscheduled revascularization, stroke, all-cause death, and heart failure hospitalization after 2 years of follow-up. The primary endpoint occurred in 21.4% of patients on omega-3-fatty acids vs. 20.0% on placebo (HR 1.08; P=0.60). TGs levels decreased 8.1% in the omega-3-fatty acid group and increased 5.1% in the placebo group (between group difference 13.2%; P<0.001) while changes in LDL-C were minimal in both groups. Thus, similar to the STRENGTH trial no benefits on cardiovascular disease were observed with EPA + DHA treatment.
SUMMARY
1) High dose EPA (JELIS and REDUCE-IT) reduced ASCVD outcomes while high dose EPA+DHA (STENGTH and OMEMI) did not decrease ASCVD outcomes.
2) The decrease in TG levels is not a major contributor to the beneficial effect of high dose EPA as the combination of high dose EPA+DHA lowers TG levels to the same degree as EPA alone without benefit. Additionally, the JELIS trial only lowered TG levels by 5% but nevertheless reduced ASCVD events. It is likely that the beneficial effects of EPA seen in the JELIS and REDUCE-IT trials are multifactorial with TG lowering making only a small contribution to the decrease in cardiovascular disease. Other actions of EPA, such as decreasing platelet function, anti-inflammation, decreasing lipid oxidation, stabilizing membranes, etc. could account for or contribute to the reduction in ASCVD events (78).
3) Whether EPA has special properties that resulted in the reduction in ASCVD events in the REDUCE-IT trial or there were flaws in the trial design (i.e., the use of mineral oil as the placebo) is uncertain and debated. It should be noted that in the REDUCE-IT trial LDL-C and non-HDL-C levels were increased by approximately 10% in the mineral oil placebo group (75). Additionally, Apo B levels were increased by 7% (6mg/dL) by mineral oil (75). Finally, an increase in hsCRP (20-30%) and other biomarkers of atherosclerosis (oxidized LDL-C, IL-6, IL-1 beta, and lipoprotein-associated phospholipase A2) were noted in the mineral oil group (75,79). In the STRENGTH trial there were no differences in LDL-C, Non-HDL-C, HDL-C, Apo B, or hsCRP levels between the treated vs. placebo groups (76). Whether EPA has special properties compared to DHA leading to a reduction in cardiovascular events or the mineral oil placebo resulted in adverse changes increasing ASCVD in the placebo resulting in an artifactual decrease in the EPA group is debated (80,81). Ideally, another large randomized ASCVD trial with EPA ethyl ester (icosapent ethyl) (Vascepa) using a placebo other than mineral oil would help resolve this controversy. In the meantime, clinicians will need to use their clinical judgement on whether to treat patients with modest elevations in TG levels with EPA (icosapent ethyl; Vascepa) balancing the potential benefits of treatment vs. the potential side effects.
Summary of Lipid Lowering Drug Studies
The above results clearly demonstrate that lowering LDL-C levels with statins, ezetimibe, or PCSK 9 monoclonal antibodies will reduce ASCVD events in elderly patients (≥ 75 years of age) with pre-existing cardiovascular disease. In elderly patients without cardiovascular disease (primary prevention) the available data does not definitively demonstrate a decrease in ASCVD events with statin or ezetimibe therapy but is suggestive of a benefit (note there are no primary prevention trials with PCSK9 inhibitors). Additional data is required to determine if bempedoic acid and icosapent ethyl reduce ASCVD events in patients ≥ 75 years of age.
Studies in Progress
Studies are currently underway to provide definitive information on whether statin therapy is beneficial as primary prevention in the elderly. STAREE (NCT02099123) is a multicenter randomized trial in Australia of atorvastatin 40mg vs. placebo in adults ≥ 70 years of age without cardiovascular disease and PREVENTABLE (NCT04262206) is a multicenter randomized trial in the USA of atorvastatin vs. placebo in adults ≥ 75 years of age without cardiovascular disease (82,83). Other trials in the elderly that are in progress include SCOPE (NCT03770312) which is a multicenter randomized trial in Korea of low intensity vs. high intensity statin therapy in adults 76-85 years of age without CVD and SITE (Statins In The Elderly) (NCT02547883) which is a trial in France of patients ≥ 75 years of age on statin therapy who will be randomized to continue statin therapy or stop statin therapy.
SIDE EFFECTS OF LIPID LOWERING DRUGS
In this section we will describe the potential side effects of lipid lowering drugs with an emphasis on side effects likely to be seen in the elderly. Elderly patients may be more susceptible to side effects due to decreased renal function, decreased drug metabolism by the liver, polypharmacy leading to drug interactions, and co-morbidities. For a detailed discussion of the side effects of lipid lowering drugs see the Endotext chapters entitled “Cholesterol Lowering Drugs” and “Triglyceride Lowering Drugs” (49,50).
Statins
An umbrella review of meta-analyses of observational studies and randomized controlled trials examined 278 unique non-CVD outcomes from 112 meta-analyses of observational studies and 144 meta-analyses of RCTs and found that the only adverse effects associated with statin therapy were the development of diabetes and muscle disorders (84). For a detailed discussion of the side effects of statin therapy a scientific statement from the American Heart Association provides a comprehensive review (85).
DIABETES
After many years of statin use it was recognized that statins increase the risk of developing diabetes. In a meta-analysis of 13 trials with over 90,000 subjects, there was a 9% increase in the incidence of diabetes during follow-up among subjects receiving statin therapy (86). All statins appear to increase the risk of developing diabetes. In comparisons of intensive vs. moderate statin therapy, Preiss et al observed that patients treated with intensive statin therapy had a 12% greater risk of developing diabetes compared to subjects treated with moderate dose statin therapy (87). Older subjects, obese subjects, and subjects with high glucose levels were at a higher risk of developing diabetes while on statin therapy. In the Prosper trial in elderly subjects (70-82 years of age; average age 75), diabetes developed in 6.6% of patients treated with pravastatin vs. 5.1% of patients in the placebo group (51). Thus, statins may be unmasking and accelerating the development of diabetes that would have occurred naturally in these subjects at some point in time. In patients without risk factors for developing diabetes, treatment with statins does not appear to greatly increase the risk of developing diabetes.
In balancing the benefits and risks of statin therapy it is important to recognize that an increase in plasma glucose levels is a surrogate marker for an increased risk of developing micro and macrovascular disease (i.e., an increase in plasma glucose per se is not an event but rather increases the risk of future events). In contrast, statin therapy is preventing actual clinical events that cause morbidity and mortality. Furthermore, it may take many years for an elevated blood glucose to induce diabetic complications while the reduction in cardiovascular events with statin therapy occurs relatively quickly (after one-year benefits are seen). Finally, the number of patients needed to treat with statins to avoid one cardiovascular event is much lower (10-20 depending on the type of patient) than the number of patients needed to treat to cause one patient to develop diabetes (100–200 for one extra case of diabetes) (88). Patients on statin therapy, particularly those with risk factors for the development of diabetes, should be periodically screened for the development of diabetes with measurement of fasting glucose and/or HbA1c levels.
COGNITIVE DYSFUNCTION
Several randomized clinical trials have examined the effect of statin therapy on cognitive function and have not indicated any increased risk (for review see (89-92)). The Prosper Trial was designed to determine whether statin therapy will reduce cardiovascular disease in older subjects (age 70-82) (51). In this trial cognitive function was assessed repeatedly and no difference in cognitive decline was found in subjects treated with pravastatin compared to placebo (51,93). In the Heart Protection Study over 20,000 patients were randomized to simvastatin 40mg or placebo and again no significant differences in cognitive function was observed between the statin vs. placebo group (94). Additionally, a Cochrane review examined the effect of statin therapy in patients with established dementia and identified 4 studies with 1154 participants (95). In this analysis no benefit or harm of statin therapy on cognitive function could be demonstrated in this high-risk group of patients. Thus, randomized clinical trials do not indicate a significant association between statins and cognitive function.
MUSCLE
The most common side effect of statin therapy is muscle symptoms and many patients will discontinue the use of statins due to muscle symptoms. These can range from life threatening rhabdomyolysis, which is very rare, to myalgias, which are a common complaint (96). The risk of serious muscle disorders due to statin therapy is very small, particularly if one is aware of the potential drug interactions that increase the risk. In a case-control study with a cohort of 252,460 new users of lipid-lowering medications in U.S. health plans 21 cases of rhabdomyolysis were compared to 200 controls without rhabdomyolysis (97). Statin users >65 years of age had four times the risk of hospitalization for rhabdomyolysis than those under age 65.
The Cholesterol Treatment Trialists analyzed individual participant data on the development of muscle symptoms from 19 double-blind trials of statin versus placebo with 123,940 participants and four double-blind trials of a more intensive vs. a less intensive statin regimen with 30,724 participants (98). After a median follow-up of 4.3 years 27.1% of the individuals taking a statin vs. 26.6% on placebo reported muscle pain or weakness representing a 3% increase greater than placebo (risk ratio- 1.03; 95% CI 1.01-1.06) (Table 5). The specific muscle symptoms caused by statin therapy, myalgia, muscle cramps or spasm, limb pain, other musculoskeletal pain, or muscle fatigue or weakness were similar to those caused by placebo. The slight increase in muscle symptoms in the statin treated individuals was manifest in the first year of therapy but in the later years muscle symptoms were similar in the statin treated and placebo groups. The relative risk of statin induced muscle symptoms was greater in women than men. Intensive statin treatment with 40-80 mg atorvastatin or 20-40 mg rosuvastatin resulted in a higher risk of muscle symptoms than less intensive or moderate-intensity regimens but different statins at equivalent LDL-C lowering doses had similar effects on muscle symptoms. As shown in figure 7 muscle pain or weakness was slightly increased in patients > 65 years of age and similar in patients > 65 and ≤ 75 and those > 75 years of age. It should be noted that in individuals > 75 years of age the occurrence of muscle pain or weakness occurred in 39.6% of the individuals on the placebo, demonstrating the very high occurrence of muscle symptoms in this age group.
This study demonstrates that there is a small increase in muscle symptoms that primarily manifests in the first year of therapy. Statin therapy caused approximately 11 additional complaints of muscle pain or weakness per 1000 patients during the first year, but little excess in later years. Of particularly note is that 26.6% of patients taking a placebo had muscle symptoms demonstrating a very high frequency of this clinical complaint (even higher in patients > 75). Given the high prevalence of muscle complaints and the small increase attributed to statins it is very difficult to determine if a muscle complaint is actually due to the statin, which presents great clinical difficulties in patient management.
Table 5.
Effect of Statin vs. Placebo on Muscle Symptoms
| Symptom | Statin Events | Placebo Events | RR (95% CI) |
|---|---|---|---|
| Myalgia | 12.0% | 11.7% | 1·03 (0·99–1·08) |
| Other musculoskeletal pain | 13.3 | 13.0 | 1·03 (0·99–1·08) |
| Any muscle pain | 26.9% | 26.3% | 1·03 (1·01–1·06) |
| Any muscle pain or weakness | 27.1% | 26.6% | 1·03 (1·01–1·06) |
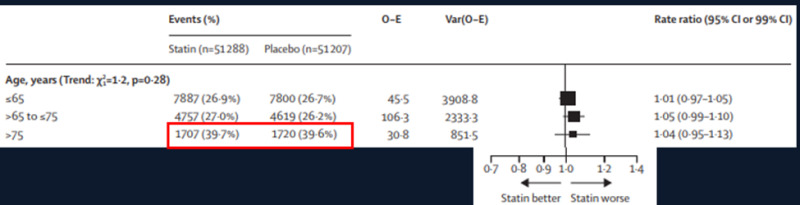
Figure 7.
Occurrence of muscle pain or weakness in different age groups in the Cholesterol Treatment Trialists meta-analysis.
While the results of the randomized trials suggest that muscle symptoms are not frequently induced by statin therapy, in typical clinical settings a significant percentage of patients are unable to tolerate statins due to muscle symptoms (in many studies as high as 5-25% of patients) (99-101). Additionally, when patients know that they are taking a statin they are more likely to have muscle symptoms (i.e. the nocebo effect) (102). Clinically differentiating statin induced myalgias from placebo induced myalgias is difficult, as there are no specific symptoms, signs, or biomarkers that clearly distinguish between the two. Thus, while statin induced myalgias are a real entity careful studies have shown that in the majority of patients with “statin induced muscle symptoms” the symptoms are not actually due to statin therapy. In the clinic it is difficult to be certain whether the muscle symptoms are actually due to true statin intolerance or to other factors.
A detailed discussion of statin induced muscle symptoms and a clinical approach to this problem is presented in the Endotext chapter entitled “Cholesterol Lowering Drugs” (50). In the section “Patients with Statin Intolerance” in this chapter we discuss the clinical approach to treating these patients.
Ezetimibe
Ezetimibe has not demonstrated significant side effects.
PCSK9 Monoclonal Antibodies
In a subgroup of patients from the FOURIER trial a prospective study of cognitive function (EBBINGHAUS Study) was carried out and no significant differences in cognitive function was observed over a median of 19 months in the PCSK9 treated vs. placebo group (103).
An issue of concern is whether lowering LDL-C to very low levels has the potential to cause toxicity. In a number of the PCSK9 studies a significant number of patients had LDL-C levels < 25mg/dL. For example, in the Odyssey long term study 37% of patients on alirocumab had two consecutive LDL-C levels below 25mg/dL and in the Osler long term study in patients treated with evolocumab 13% had values below 25mg/dL (104,105). In these short term PCSK9 studies, toxicity from very low LDL-C levels has not been observed. Additionally, in patients with Familial Hypobetalipoproteinemia LDL levels can be very low and these patients do not have any major disorders other than hepatic steatosis, which is not mechanistically due to low LDL-C levels (106). Similarly, there are rare individuals who are homozygous for loss of function mutations in the PCSK9 gene and they also do not appear to have major medical issues (107). Finally, in a number of statin trials and the IMPROVE-IT trial (statin + ezetimibe) there have been patients with very low LDL-C levels and an increased risk of side effects has not been consistently observed in those patients (108-111). Thus, with the limited data available there does not appear to be a major risk from markedly lowering LDL-C levels.
Bempedoic Acid
In clinical trials, 26% of bempedoic acid-treated patients with normal baseline uric acid values experienced hyperuricemia one or more times versus 9.5% in the placebo group (package insert). In the CLEAR Outcomes trial elevated uric acid levels occurred in 10.9% of the patients on bempedoic acid compared to 5.6% taking the placebo (69). Elevations in blood uric acid levels may lead to the development of gout. Gout was reported in 1.5% of patients treated with bempedoic acid vs. 0.4% of patients treated with placebo. The risk for gout attacks were higher in patients with a prior history of gout (11.2% for bempedoic acid treatment vs. 1.7% in the placebo group) (package insert). In patients with no prior history of gout only 1% of patients treated with bempedoic acid and 0.3% of the placebo group had a gouty attack (package insert). In the CLEAR Outcomes trial gout was increased in the bempedoic acid group (3.1% vs. 2.1%) (69).
In clinical trials tendon rupture occurred in 0.5% of patients treated with bempedoic acid vs. 0% of placebo treated patients and involved the rotator cuff (the shoulder), biceps tendon, or Achilles tendon (package insert). Tendon rupture occurred within weeks to months of starting bempedoic acid and occurred more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders. In the CLEAR Outcomes trial tendon rupture was similar in the bempedoic acid and placebo group (bempedoic acid 1.2% and placebo 0.9%) (69).
Bempedoic acid treatment resulted in a mean increase in serum creatinine of 0.05 mg/dL compared to baseline. Approximately 3.8% of patients treated with bempedoic acid had BUN levels that doubled vs. 1.5% in the placebo group and about 2.2% of patients treated with bempedoic acid had creatinine values that increased by 0.5 mg/dL vs. 1.1% in the placebo group (package insert). Renal function returned to baseline when bempedoic acid was discontinued. In the CLEAR Outcomes trial renal impairment was increased in the bempedoic acid group (11.5% vs.8.6%) as was the change from baseline creatinine (0.05±0.2 mg/dL vs. 0.01±0.2 mg/dL) (69).
Omega-3-Fatty Acids
At very high doses, omega-3-fatty acids can inhibit platelets and prolong bleeding time. However, at the recommended doses this has not been a major clinical problem but nevertheless when patients are on anti-platelet drugs one should be alert for the possibility of bleeding problems (Package Inserts for Lovaza, Vascepa, and Epanova). In the REDUCE-IT trial serious bleeding events occurred in 2.7% of the patients in the icosapent ethyl group and in 2.1% in the placebo group (P=0.06) (75). There were no fatal bleeding events in either group and the rates of hemorrhagic stroke, serious central nervous system bleeding, and serious gastrointestinal bleeding were not significantly higher in the EPA group than in the placebo group. In the STRENGHT trial any bleeding events and major bleeding events were similar in the omega-3 fatty acid group and placebo group (76). A recent review found no evidence for discontinuing the use of omega-3 fatty acid treatment before invasive procedures or when given in combination with other agents that affect bleeding (112).
An increase in new-onset atrial fibrillation was observed in the REDUCE-IT trial in the patients treated with icosapent ethyl 4 grams/day (5.3% vs. 3.9%) and in the STRENGTH trial in the patients treated with omega-3-fatty acids (2.2% vs 1.3%)
CURRENT GUIDELINES AND LDL-C GOALS
This section discusses guidelines as they pertain to elderly patients.
2018 AHA/ACC/Multi-Society Report
The following summarizes the 2018 AHA/ACC guidelines (2).
PRIMARY PREVENTION
- For individuals >75 years of age, randomized controlled trials of statin therapy do not provide strong evidence for benefit, so clinical assessment of risk status in a clinician–patient risk discussion is needed for deciding whether to continue or initiate statin treatment.
- In individuals ≥ 75 years of age with an LDL-C level of 70 to 189mg/dL, initiating a moderate-intensity statin may be reasonable. Goal is to reduce LDL-C by 30-49% (note these guidelines recommend percent reduction rather than absolute LDL-C goals).
- In individuals ≥ 75 years of age it may be reasonable to stop statin therapy when functional decline (physical or cognitive), multimorbidity, frailty, or reduced life-expectancy limits the potential benefits of statin therapy.
- A shared decision-making process between clinicians and patients that individualizes decisions is indicated, with regular periodic reassessment.
- Determining coronary artery calcium (CAC) score will help in determining which patients will benefit the most. For older adults with CAC scores of zero, the likelihood of benefits from statin therapy does not outweigh the risks. Limiting statin therapy to those with CAC scores greater than zero, combined with clinical judgment and patient preference, could provide a valuable awareness with which to inform shared decision-making.
SECONDARY PREVENTION
- In patients ≥75 years of age with clinical ASCVD, it is reasonable to initiate moderate- or high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug–drug interactions, as well as patient frailty and patient preferences. The goal of moderate statin therapy is to reduce LDL-C by 30-49% and the goal of high-intensity statin therapy is to reduce LDL-C by ≥ 50%. In very high-risk patients, a goal of an LDL-C < 70mg/dL and non-HDL-C < 100mg/dL is reasonable.
- In patients ≥75 years of age who are tolerating high-intensity statin therapy, it is reasonable to continue high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug-drug interactions, as well as patient frailty and patient preferences.
PATIENTS WITH DIABETES
- In patients ≥ 75 years of age with diabetes mellitus and who are already on statin therapy, it is reasonable to continue statin therapy.
- In patients ≥ 75 years of age with diabetes mellitus without cardiovascular disease it may be reasonable to start moderate statin therapy after a clinician-patient discussion of the potential benefits and risks of therapy. The goal is to decrease LDL-C by 30-49%.
2019 ESC/EAS Guidelines
The following summarizes the 2019 ESC/EAS guidelines (3).
- Treatment with statins is recommended for older people with ASCVD in the same way as for younger patients.
- Treatment with statins is recommended for primary prevention, according to the level of risk, in older people aged ≤ 75 years.
- Initiation of statin treatment for primary prevention in older people aged >75 years may be considered, if at high-risk or above.
- It is recommended that the statin is started at a low dose if there is significant renal impairment and/or the potential for drug interactions, and then titrated upwards to achieve LDL-C treatment goals.
- In patients at very-high risk in primary or secondary prevention the goal is a 50% reduction in LDL-C and an LDL-C < 55mg/dL.
- In patients at high risk in primary or secondary prevention the goal is a 50% reduction in LDL-C and an LDL-C < 70mg/dL.
The ESC/EAS criteria for very high risk and high risk are shown in table 6.
Table 6.
ESC/EAS Criteria for Very-High Risk and High Risk for ASCVD Events
| Very High Risk Documented ASCVD or unequivocal on imaging DM with target organ damage or at least three major risk factors, or early onset of T1DM of long duration (>20 years) Severe CKD (eGFR <30 mL/min/1.73 m2) A calculated SCORE ≥ 10% for 10-year risk of fatal CVD Familial Hypercholesterolemia with ASCVD or with another major risk factor |
| High Risk Markedly elevated single risk factors, in particular LDL-C >190 mg/dL or BP ≥ 180/110 mmHg Patients with Familial Hypercholesterolemia without other major risk factors Patients with DM without target organ damage, with DM duration ≥ 10 years, or another additional risk factor Moderate CKD (eGFR 30-59 mL/min/1.73 m2) A calculated SCORE ≥ 5% and <10% for 10-year risk of fatal CVD. |
Our Approach
Our approach is based on concepts taken from both the ACC/AHA and ESC/EAS guidelines (i.e., we try to utilize the best ideas from each guideline). There are several general principles regarding lipid lowering therapy that should be considered in deciding who to treat (113).
- The higher the LDL-C level the greater the benefit of lowering LDL-C.
- The greater the decrease in LDL-C the greater the benefit.
- The higher the absolute risk of ASCVD the greater the benefit of lowering LDL-C.
Additional factors that need to be considered, particularly in elderly patients, include
- Life expectancy.
- Competing non-cardiovascular disorders.
- Risk of drug side effects.
- Potential for drug interactions.
- Patient preferences.
PRIMARY PREVENTION
Given the absence of definitive outcome trials demonstrating the benefit of decreasing LDL-C levels in patients ≥ 75 years of age without cardiovascular disease one must use clinical judgement in deciding who to treat. It should be recognized, as discussed in detail earlier, that the available evidence suggests that decreasing LDL-C will reduce ASCVD events in the elderly. Our approach is to determine ASCVD risk and then balance the risk with competing factors such as life expectancy, non-cardiovascular disorders, potential for drug interactions, and patient preferences. We use the approach described below to determine risk.
Step 1- Calculate the 10-year risk of an ASCVD event using the AHA/ACC pooled cohort equation. In Europe one can use the SCORE OP risk prediction algorithms (114). This will provide an estimate of the risk of the patient having an ASCVD event/death.
Step 2- To gain further insight on the risk of ASCVD one can determine if patient has any risk enhancing factors (tables 7 and 8). This can help further stratify the patient’s risk.
Step 3- If after discussion with the patient, you and/or the patient is uncertain on the level of risk and the appropriate treatment plan obtaining a coronary calcium score (CAC) can be helpful. A CAC score of zero indicates low risk for ASCVD and allows one to not start statin therapy (2). Note that a CAC score of zero in cigarette smokers, patients with diabetes mellitus, those with a strong family history of ASCVD, and possibly chronic inflammatory conditions such as HIV, may still be associated with a substantial 10-year risk (2).
Following these steps, we can estimate the risk for ASCVD events and in conjunction with the general principles described above discuss with the patient a treatment plan. If the decision is to treat our goal is often an LDL-C < 100mg/dL and non-HDL-C < 130mg/dL but in high-risk patients our goal may be an LDL-C < 70mg/dL and non-HDL-C < 100mg/dL.
Table 7.
Risk-Enhancing Factors
| Family history of premature ASCVD (males, age <55 y; females, age <65 y) |
| Primary hypercholesterolemia (LDL-C ≥160mg/dL; non-HDL-C ≥190mg/dL |
| Metabolic syndrome (increased waist circumference, elevated triglycerides [>175 mg/dL], elevated blood pressure, elevated glucose, and low HDL-C [<40 mg/dL in men; <50 in women mg/dL] are factors; tally of 3 makes the diagnosis) |
| Chronic kidney disease (eGFR 15–59 mL/min/1.73 m2 with or without albuminuria; not treated with dialysis or kidney transplantation) |
| Chronic inflammatory conditions such as psoriasis, RA, or HIV/AIDS |
| History of premature menopause (before age 40 y) and history of pregnancy-associated conditions that increase later ASCVD risk such as preeclampsia |
| High-risk race/ethnicities (e.g., South Asian ancestry) |
| Lipid/biomarkers: Associated with increased ASCVD risk |
| Persistently* elevated, primary hypertriglyceridemia (≥175 mg/dL); |
| If measured: |
| 1. Elevated high-sensitivity C-reactive protein (≥2.0 mg/L) |
| 2. Elevated Lp(a) ≥50 mg/dL or ≥125 nmol/L |
| 3. Elevated apoB ≥130 mg/dL |
| 4. ABI <0.9 |
ABI= ankle-brachial index, RA= rheumatoid arthritis.
Modified from reference (2).
Table 8.
Factors Modifying Systematic Coronary Risk Estimation Risks
| Social deprivation: the origin of many of the causes of CVD. |
| Obesity and central obesity as measured by the body mass index and waist circumference, respectively. |
| Physical inactivity. |
| Psychosocial stress |
| Family history of premature CVD (men: <55 years and women: <60 years). |
| Chronic immune-mediated inflammatory disorder. |
| Major psychiatric disorders. |
| Treatment for HIV infection. |
| Atrial fibrillation. |
| Left ventricular hypertrophy. |
| Chronic kidney disease. |
| Obstructive sleep apnea syndrome. |
| Metabolic associated fatty liver disease. |
Modified from reference (3).
PATIENTS WITH DIABETES
In patients ≥ 75 with diabetes without pre-existing cardiovascular our approach is very similar to that described for primary prevention. In addition to the risk enhancers listed in tables 7 and 8 there are specific diabetes risk enhancers that clinicians should factor in their decisions (table 9). Also, as noted above, in the presence of diabetes a zero CAC score is not as strong an indicator of low risk for ASCVD as in non-diabetics.
In patients with diabetes because they usually have multiple risk factors and are at high risk for ASCVD events our typical LDL-C goal is < 70mg/dL and non-HDL-C < 100mg/dL. In the rare situation where there are minimal other risk factors an LDL-C goal < 100mg/dL and non-HDL-C < 130mg/dL is reasonable.
Table 9.
Diabetes-Specific Risk Enhancers That Are Independent of Other Risk Factors
| Long duration (≥10 years for type 2 diabetes or ≥20 years for type 1 diabetes) |
| Albuminuria ≥30 mcg of albumin/mg creatinine |
| eGFR <60 mL/min/1.73 m |
| Retinopathy |
| Neuropathy |
| ABI <0.9 |
ABI= ankle-brachial index.
Modified from reference (2).
SECONDARY PREVENTION
Studies have shown that lowering LDL-C levels with statins, ezetimibe, and PCSK9 monoclonal antibodies reduces ASCVD events in older adults with ASCVD. Thus, unless there are contraindications older patients with ASCVD should be treated with lipid lowering drugs to reduce ASCVD events. In elderly patients we will often employ a modest statin dose (for example atorvastatin 10-20mg or rosuvastatin 5-10mg) in combination with ezetimibe 10mg and then increase the statin dose, if necessary, based on lipid levels and the patient tolerating the treatment regimen. At a minimum our goal is an LDL-C < 70mg/dL and a non-HDL-C level < 100mg/dL but we would prefer lower values (ideally LDL-C < 55mg/dL and non-HDL-C < 85mg/dL) if they can be achieved with a statin + ezetimibe. In very high-risk patients (table 10) our goal is an LDL-C < 55mg/dL and non-HDL-C < 85mg/dL and adding a PCSK9 inhibitor may be required to achieve these levels in some patients.
Table 10.
Criteria for Very High Risk
| Very high-risk includes a history of multiple major ASCVD events or one major ASCVD event and multiple high-risk conditions. |
| Major ASCVD Events |
| Recent ACS (within the past 12 months) |
| History of MI (other than recent ACS event) |
| History of ischemic stroke |
| Symptomatic peripheral arterial disease (history of claudication with ABI <0.85, or previous revascularization or amputation) |
| High-Risk Conditions |
| Age ≥65 y |
| Heterozygous familial hypercholesterolemia |
| History of prior coronary artery bypass surgery or percutaneous coronary intervention outside of the major ASCVD event(s) |
| Diabetes mellitus |
| Hypertension |
| CKD (eGFR 15-59 mL/min/1.73 m2) |
| Current smoking |
| Persistently elevated LDL-C (LDL-C ≥100 mg/dL [≥2.6 mmol/L]) despite maximally tolerated statin therapy and ezetimibe |
| History of congestive Heart Failure |
ABI= ankle-brachial index; ACS= acute coronary syndrome.
Based on reference (2).
TREATMENT
Lifestyle
The lifestyle changes described below are recommended for all adults and are not specific for elderly individuals or for individuals with cardiovascular disease. The lifestyle changes recommended will lower lipid levels and are likely to reduce the risk of ASCVD.
EXERCISE
There is little debate that exercise is beneficial and that all individuals should be physically active. It is recommended that individuals participate in at least 150 minutes of moderate-intensity aerobic physical activity (for example 30 minutes 5 times per week) or 75 minutes per week of vigorous-intensity physical activity (115,116). Additionally, it is recommended that individuals participate in 2 days per week of muscle-strengthening activity (116). Because of the loss of muscle mass with aging it is very important to incorporate resistance training into the exercise program of elderly individuals.
A meta-analysis of exercise in the older individuals (>60 years of age) found that aerobic exercise decreased triglyceride and LDL-C levels and increased HDL-C levels while resistance exercise decreased LDL-C levels (117). Exercise also increases fitness and helps with weight loss. It should be noted that many elderly individuals may have substantial medical and social barriers to participating in exercise programs. Comorbidities, such as osteoarthritis, may limit exercise tolerance and make exercise challenging. Older individuals should be encouraged to be as active as possible.
DIET
For a detailed discussion of the effect of diet on lipids, lipoproteins and ASCVD see the Endotext chapter entitled “The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels (118). There is general agreement on what constitutes a healthy diet and a brief summary of the Guidelines for Americans 2020-2025 is shown in table 11 and the guidelines from the American College of Cardiology/American Heart Association are shown in table 12.
Table 11.
Guidelines for Americans 2020-2025
| Recommend | Limit |
|---|---|
| Vegetables of all types—dark green; red and orange; beans, peas, and lentils; starchy; and other vegetables | Added sugars—Less than 10 percent of calories per day |
| Fruits, especially whole fruit | Saturated fat—Less than 10 percent of calories per day |
| Grains, at least half of which are whole grain | Sodium—Less than 2,300 milligrams per day |
| Dairy, including fat-free or low-fat milk, yogurt, and cheese, and/or lactose-free versions and fortified soy beverages and yogurt as alternatives | Alcoholic beverages—Adults can choose not to drink or to drink in moderation by limiting intake to 2 drinks or less in a day for men and 1 drink or less in a day for women |
| Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products | |
| Oils, including vegetable oils and oils in food, such as seafood and nuts |
Full guideline is available at DietaryGuidelines.gov.
Table 12.
ACC/AHA Dietary Recommendations to Reduce Risk of ASCVD (115)
| 1. A diet emphasizing intake of vegetables, fruits, legumes, nuts, whole grains, and fish is recommended |
| 2. Replacement of saturated fat with dietary monounsaturated and polyunsaturated fats can be beneficial |
| 3. A diet containing reduced amounts of cholesterol and sodium can be beneficial |
| 4. As a part of a healthy diet, it is reasonable to minimize the intake of processed meats, refined carbohydrates, and sweetened beverages |
| 5. As a part of a healthy diet, the intake of trans fats should be avoided |
A summary of the effect of individual dietary constituents on lipid and lipoprotein levels is shown in table 13 (118). This table summarizes the results of numerous randomized trials examining the effect of dietary manipulations on lipid and lipoprotein levels.
Table 13.
Summary of the Effect of Dietary Constituents on Lipid and Lipoproteins
SFA | Increase LDL-C and modest increase HDL-C |
| MUFA and PUFA | Decrease LDL-C |
| TFA | Increase LDL-C and decrease HDL-C |
| Cholesterol | Increase LDL-C |
| Carbohydrates | Increase TGs, increase greater with simple sugars particularly fructose |
| Fiber | Decrease LDL-C |
| Phytosterols | Decrease LDL-C |
SFA= saturated fatty acids, MUFA= monounsaturated fatty acids, PUFA= polyunsaturated fatty acids, TFA= trans fatty acids.
There is a huge literature describing the effect of diet on the risk of ASCVD and this literature is often conflicting and controversial (118). Several well recognized investigators have discussed the limitations of the information linking various diets and dietary constituents and the risk of disease (119,120). The major problem is that almost all of the information is based on observational studies and well conducted randomized trials measuring important ASCVD outcomes are very rare. Observational studies can demonstrate associations but do not definitively indicate that there is a cause-and-effect relationship. Unrecognized confounding variables can result in false associations.
Some of the more recent randomized dietary trials that have examined the effect of diet on ASCVD events are described below. For a discussion of other studies see reference (118). The PREDIMED trial employing a Mediterranean diet (increased monounsaturated fats) reduced the incidence of major ASCVD events (121). In this multicenter trial, carried out in Spain, over 7,000 patients at high risk for developing ASCVD were randomized to three diets (primary prevention trial). A Mediterranean diet supplemented with extra-virgin olive oil, a Mediterranean diet supplemented with mixed nuts, or a control diet. The average age of participants in this trial was 67. In the patients assigned to the Mediterranean diets there was 29% decrease in the primary end point (MI, stroke, and death from ASCVD). Subgroup analysis demonstrated that the Mediterranean diet was equally beneficial in patients < 70 and ≥ 70 years of age. The Mediterranean diet resulted in only a small but significant increase in HDL-C levels and a small decrease in both LDL-C and TG levels, suggesting that the beneficial effects were not mediated by changes in lipids (122). The CORDIOPREV study and the Lyon Diet Heart Study were randomized trials that demonstrated that a Mediterranean diet reduces ASCVD events in patients with cardiovascular disease (secondary prevention) (123,124). Unfortunately, these studies did not have a sufficient number of patients > 70 years of age for analysis of the effect of the diet in older patients with pre-existing cardiovascular disease.
The results of these three randomized trials indicate that following a Mediterranean type diet reduces ASCVD. It is likely that the beneficial effects of the Mediterranean diet on ASCVD is mediated by multiple mechanisms with alterations in lipid levels making only a minor contribution.
LIPID LOWERING DRUGS
Current guidelines and lipid lowering goals are discussed in the guidelines section above. In this section we will focus on clinical decisions regarding the use of lipid lowering drugs. To maximize benefits of lipid lowering therapy we think it is important to achieve LDL-C goals.
Elderly Patients on Lipid Lowering Therapy
In elderly patients on lipid lowering therapy, we usually continue therapy if they are tolerating the medications without side effects. We will periodically check a lipid panel to make sure that they are achieving the goals of therapy. If not, we will adjust the lipid lowering medications to achieve the desired LDL-C goals. We will make changes in therapy if circumstances change. For example, if a patient develops metastatic cancer and is transferred to palliative or Hospice care we will stop the lipid lowering therapy. Similarly, if a new drug is required the current lipid lowering drugs may need to be changed to avoid drug interactions. Thus, in most patients continuing lipid lowering therapy is appropriate.
Primary Prevention in Elderly Patients
In elderly patients we usually initiate therapy using moderate-intensity statin therapy if therapy is indicated as discussed above. We typically use either atorvastatin 10-20mg or rosuvastatin 5-10mg. Our reason for using these statins is that if one needs to lower LDL-C further we can just increase the dose of the statin and not need to start a new statin. In certain circumstances we might use another statin to avoid drug interactions (for example in a patient living with HIV we might use pitavastatin). After 6-12 weeks on statin therapy, we check a lipid panel and if the patient is having any medication side effects. If the LDL-C is not at goal we will either increase the statin dose or if we feel that the patient is at risk for statin toxicity add ezetimibe 10mg instead. In a healthy elderly patient at high risk for ASCVD (for example high LDL-C, diabetes, and hypertension) we do not hesitate to use high-intensity statin therapy (atorvastatin 40-80mg and rosuvastatin 20-40mg) plus ezetimibe 10mg to achieve the LDL-C goal.
Secondary Prevention in Elderly Patients
Unless than is a contraindication we frequently start these patients on high-intensity statin therapy. After 6-12 weeks on statin therapy, we check a lipid panel and if the patient is having any medication side effects. We will often add ezetimibe as studies have shown that the greater the lowering of LDL-C the greater the reductions in ASCVD events. Additionally, ezetimibe is generic (i.e. inexpensive) and doesn’t typically cause side effects. We will use PCSK9 inhibitors following the principle that the higher the LDL-C and the greater the risk of ASCVD events the greater the cost effectiveness of using expensive PCSK9 inhibitors.
Mixed Hyperlipidemia
In patients with mixed hyperlipidemia (elevated LDL-C and triglyceride levels) Initial drug therapy should also be a statin unless triglyceride levels are greater than 500-1000mg/dL. If triglycerides are > 500-1000mg/dL initial therapy is directed at lowering triglyceride levels (49,125). In addition to lowering LDL-C levels, statins are also effective in lowering triglyceride levels particularly when the triglycerides are elevated. If LDL-C is not lowered sufficiently ezetimibe is a reasonable next step. The approach to the patient whose LDL-C levels are at goal but the triglycerides and non-HDL-C are still elevated is not clearly defined. As discussed above studies have failed to demonstrate that adding a fibrate or niacin reduces ASCVD events. The REDUCE-IT trial has demonstrated that icosapent ethyl (Vascepa) decreases ASCVD events in this patient population but as discussed in detail above the results of this study are debated because the mineral oil placebo increased LDL-C. non-HDL-C, hsCRP, and other biomarkers associated with an increased risk of ASCVD events. It is debated by various experts whether the beneficial effect seen in this study was due to the positive effects of icosapent ethyl or to negative effects of the placebo. Clinicians will need to use their clinical judgement on whether to treat patients with elevations in TG and non-HDL-C levels with icosapent ethyl balancing the potential benefits of treatment vs. the potential side effects. In making this decision in our elderly patients it is worth noting that in participants <65 years of age the primary end point was reduced by 35% (HR 0.65; 95% CI 0.54–0.78) while in participants ≥ 65 years of age the primary end point was reduced by 18% (HR 0.82; 95%CI 0.70–0.97; P Value for Interaction 0.06). Information on patients ≥ 75 years of age is not available.
Patients with Statin Intolerance
Statin intolerance is frequently due to myalgias but on occasion can be due other issues, such as increased liver or muscle enzymes, cognitive dysfunction, or other neurological disorders. The percentage of patients who are “statin intolerant” varies greatly but in clinical practice a significant number of patients have difficulty taking statins.
It can be difficult to determine if the muscle symptoms that occur when a patient is taking a statin are actually due to the statin or are unrelated to statin use. The first step in a “statin intolerant patient” is to take a careful history of the nature and location of the muscle symptoms and the timing of onset in relation to statin use to determine whether the presentation fits the typical picture for statin induced myalgias. The characteristic findings with a statin induced myalgia are shown in table 14 and findings that are not typical for statin induced myalgia are shown in table 15. The disappearance of symptoms within a few weeks of stopping statins and the reappearance after restarting statins is very suggestive of the symptoms being due to true statin intolerance. An on-line tool (htpp://tools.acc.org/statinintolerance/#!/) and an app produced by the ACC/AHA are available. This tool characterizes patients based on 8 criteria into possible vs. unlikely to have statin induced muscle symptoms (table 16).
Table 14.
Characteristic Findings with Statin Induced Myalgia
| Symmetric |
| Proximal muscles |
| Muscle pain, tenderness, weakness, cramps |
| Symptom onset < 4 weeks after starting statin or dose increase |
| Improves within 2-4 weeks of stopping statin |
| Cramping is unilateral and involves small muscles of hands and feet |
| Same symptoms occur with re-challenge within 4 weeks |
Table 15.
Symptoms Atypical in Statin Induced Myalgia
| Unilateral |
| Asymmetric |
| Small muscles |
| Joint or tendon pain |
| Shooting pain, muscle twitching or tingling |
| Symptom onset > 12 weeks |
| No improvement after discontinuing statin |
Table 16.
Diagnosis of Statin Associated Muscle Symptoms
| Symptom timing |
| Symptom type |
| Symptom location |
| Sex |
| Age |
| Race/ethnicity |
| CK elevation > 5 times the upper limit of normal |
| Known risk factors for statin induced muscle symptoms and non-statin causes of muscle symptoms |
One should also check a CK level but this is almost always in the normal range. If the CK is not elevated and the symptoms do not suggest a statin induced myalgia one can often reassure the patient and continue statin therapy. This is often successful and studies have shown that many patients that stop taking statins due to “statin induced myalgia” can be successfully treated with a statin (50). If the CK is elevated it should be repeated after instructing the patient to avoid exercise for 48 hours. Also, the CK levels should be compared to CK levels prior to starting therapy if available. If the CK remains elevated (3x upper limit of normal) the statin should be discontinued. Similarly, if the CK is normal but the symptoms are suggestive of a statin induced myalgia the statin should also be discontinued. The next step is to determine if one can identify reversible factors that could be increasing statin toxicity (hypothyroidism, drug interactions). If none are identified the next step after the myalgias have resolved is to try a low dose of a different statin that is metabolized by a different pathway (for example instead of atorvastatin, which is metabolized by the CYP3A4 pathway, rosuvastatin, which has a different pathway of metabolism). Because statin side effects are dose related, a low dose of a statin may often be tolerated. One can also try several different statins as sometimes a patient may tolerate one statin and not others. A meta-analysis has shown that every other day administration of statins is as effective as daily administration in lowering lipid levels and therefore is a very reasonable strategy (126). In some instances, using a long-acting statin (rosuvastatin or atorvastatin) 1-3 times per week can work (we usually start with once per week and then slowly increase frequency as tolerated) (127). In these circumstances (low doses or 1-3 times per week) the reduction in LDL-C may not be sufficient but one can use combination therapy with other drugs such as ezetimibe, bempedoic acid, or PCSK9 inhibitors to achieve LDL-C target goals.
If after trying various approaches a patient still has myalgias and is unable to tolerate statin therapy one needs to utilize other approaches to lower LDL-C levels. We typically use the ezetimibe and bempedoic acid combination pill (Nexlizet), which can lower the LDL-C level by approximately 40%, which is often sufficient (128). If needed one could add a PCSK9 inhibitor to further decrease LDL-C.
CONCLUSION
ASCVD is a major cause of morbidity and mortality in elderly patients. In elderly patients with pre-existing ASCVD randomized clinical trials have shown that lipid lowering drug therapy with statins, ezetimibe, and PCSK9 inhibitors reduce ASCVD events. Thus, most elderly patients with ASCVD should be treated with lipid lowering drugs unless there are contraindications such as limited life expectancy, competing non-cardiovascular disorders, high risk of drug interactions or drug side effects. In elderly patients without ASCVD if they are already taking lipid lowering drugs and if they are tolerating the medications without side effects continuing therapy is usually reasonable as long as the clinical circumstances have not changed. In elderly patients not on lipid lowering therapy and without cardiovascular disease studies have suggested that lipid lowering therapy is beneficial but further studies are required to definitively demonstrate benefit. In these patients one needs to determine the patient’s risk for ASCVD events and then discuss the potential benefits and side effects with the patient to make a shared decision on whether to initiate therapy. Age per se should not be used to withhold therapy with lipid lowering drugs that can reduce the risk of ASCVD events.
ACKNOWLEDGEMENTS
This work was supported by grants from the Northern California Institute for Research and Education. The authors would like to thank Dan Streja, the original author of this chapter, who provided the framework for this updated chapter.
REFERENCES
- 1.
- Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, Berger JS. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 2013; 61:1736-1743 [PubMed: 23500290]
- 2.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr., Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 139:e1082-e1143 [PMC free article: PMC7403606] [PubMed: 30586774]
- 3.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41:111-188 [PubMed: 31504418]
- 4.
- Ghandehari H, Kamal-Bahl S, Wong ND. Prevalence and extent of dyslipidemia and recommended lipid levels in US adults with and without cardiovascular comorbidities: the National Health and Nutrition Examination Survey 2003-2004. Am Heart J 2008; 156:112-119 [PubMed: 18585505]
- 5.
- Laurenzi M, Mancini M. Plasma lipids in elderly men and women. Eur Heart J 1988; 9 Suppl D:69-74 [PubMed: 3378557]
- 6.
- Weijenberg MP, Feskens EJ, Kromhout D. Age-related changes in total and high-density-lipoprotein cholesterol in elderly Dutch men. Am J Public Health 1996; 86:798-803 [PMC free article: PMC1380397] [PubMed: 8659652]
- 7.
- Abbott RD, Sharp DS, Burchfiel CM, Curb JD, Rodriguez BL, Hakim AA, Yano K. Cross-sectional and longitudinal changes in total and high-density-lipoprotein cholesterol levels over a 20-year period in elderly men: the Honolulu Heart Program. Ann Epidemiol 1997; 7:417-424 [PubMed: 9279451]
- 8.
- Grunenberger F, Lammi-Keefe CJ, Schlienger JL, Deslypere JP, Hautvast JG. Longitudinal changes in serum lipids of elderly Europeans. SENECA Investigators. Eur J Clin Nutr 1996; 50 Suppl 2:S25-31 [PubMed: 8841782]
- 9.
- Newschaffer CJ, Bush TL, Hale WE. Aging and total cholesterol levels: cohort, period, and survivorship effects. Am J Epidemiol 1992; 136:23-34 [PubMed: 1415129]
- 10.
- Ettinger WH, Wahl PW, Kuller LH, Bush TL, Tracy RP, Manolio TA, Borhani NO, Wong ND, O'Leary DH. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation 1992; 86:858-869 [PubMed: 1516198]
- 11.
- Curb JD, Reed DM, Yano K, Kautz JA, Albers JJ. Plasma lipids and lipoproteins in elderly Japanese-American men. J Am Geriatr Soc 1986; 34:773-780 [PubMed: 3771975]
- 12.
- Wilson PW, Anderson KM, Harris T, Kannel WB, Castelli WP. Determinants of change in total cholesterol and HDL-C with age: the Framingham Study. J Gerontol 1994; 49:M252-257 [PubMed: 7963277]
- 13.
- Garry PJ, Hunt WC, Koehler KM, VanderJagt DJ, Vellas BJ. Longitudinal study of dietary intakes and plasma lipids in healthy elderly men and women. Am J Clin Nutr 1992; 55:682-688 [PubMed: 1550044]
- 14.
- Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984-1994. Circulation 1997; 96:37-43 [PubMed: 9236414]
- 15.
- Katsanos CS. Clinical considerations and mechanistic determinants of postprandial lipemia in older adults. Adv Nutr 2014; 5:226-234 [PMC free article: PMC4013175] [PubMed: 24829469]
- 16.
- Spitler KM, Davies BSJ. Aging and plasma triglyceride metabolism. J Lipid Res 2020; 61:1161-1167 [PMC free article: PMC7397742] [PubMed: 32586846]
- 17.
- Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2022.
- 18.
- Zuliani G, Romagnoni F, Bollini C, Leoci V, Soattin L, Fellin R. Low levels of high-density lipoprotein cholesterol are a marker of disability in the elderly. Gerontology 1999; 45:317-322 [PubMed: 10559649]
- 19.
- Ranieri P, Rozzini R, Franzoni S, Barbisoni P, Trabucchi M. Serum cholesterol levels as a measure of frailty in elderly patients. Exp Aging Res 1998; 24:169-179 [PubMed: 9555569]
- 20.
- Pel-Littel RE, Schuurmans MJ, Emmelot-Vonk MH, Verhaar HJ. Frailty: defining and measuring of a concept. J Nutr Health Aging 2009; 13:390-394 [PubMed: 19300888]
- 21.
- Wilson DP. Is Atherosclerosis a Pediatric Disease? In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2020.
- 22.
- Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998; 338:1650-1656 [PubMed: 9614255]
- 23.
- McGill HC, Jr., McMahan CA, Malcom GT, Oalmann MC, Strong JP. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 1997; 17:95-106 [PubMed: 9012643]
- 24.
- Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, Young JB, Nissen SE. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation 2001; 103:2705-2710 [PubMed: 11390341]
- 25.
- Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea; preliminary report. J Am Med Assoc 1953; 152:1090-1093 [PubMed: 13052433]
- 26.
- McNamara JJ, Molot MA, Stremple JF, Cutting RT. Coronary artery disease in combat casualties in Vietnam. JAMA 1971; 216:1185-1187 [PubMed: 5108403]
- 27.
- Webber BJ, Seguin PG, Burnett DG, Clark LL, Otto JL. Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001-2011. JAMA 2012; 308:2577-2583 [PubMed: 23268516]
- 28.
- Newman WP, 3rd, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, Williamson GD, Webber LS, Berenson GS. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med 1986; 314:138-144 [PubMed: 3455748]
- 29.
- McGill HC, Jr., McMahan CA. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am J Cardiol 1998; 82:30T-36T [PubMed: 9860371]
- 30.
- Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA 2003; 290:2277-2283 [PubMed: 14600186]
- 31.
- Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA 2003; 290:2271-2276 [PubMed: 14600185]
- 32.
- Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation 2001; 104:2815-2819 [PubMed: 11733400]
- 33.
- Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, Chen W, Srinivasan SR, Daniels SR, Kahonen M, Laitinen T, Taittonen L, Berenson GS, Viikari JS, Raitakari OT. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation 2010; 122:2514-2520 [PubMed: 21126976]
- 34.
- Magnussen CG, Venn A, Thomson R, Juonala M, Srinivasan SR, Viikari JS, Berenson GS, Dwyer T, Raitakari OT. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol 2009; 53:860-869 [PMC free article: PMC2759186] [PubMed: 19264243]
- 35.
- Klag MJ, Ford DE, Mead LA, He J, Whelton PK, Liang KY, Levine DM. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med 1993; 328:313-318 [PubMed: 8419817]
- 36.
- Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study). J Am Coll Cardiol 2011; 58:2396-2403 [PMC free article: PMC3253414] [PubMed: 22115646]
- 37.
- Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins-Domingo K, Moran AE. Young Adult Exposure to Cardiovascular Risk Factors and Risk of Events Later in Life: The Framingham Offspring Study. PLoS One 2016; 11:e0154288 [PMC free article: PMC4854462] [PubMed: 27138014]
- 38.
- Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000; 284:311-318 [PubMed: 10891962]
- 39.
- Warden BA, Fazio S, Shapiro MD. Familial Hypercholesterolemia: Genes and Beyond. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2021.
- 40.
- Ference BA, Ray KK, Catapano AL, Ference TB, Burgess S, Neff DR, Oliver-Williams C, Wood AM, Butterworth AS, Di Angelantonio E, Danesh J, Kastelein JJP, Nicholls SJ. Mendelian Randomization Study of ACLY and Cardiovascular Disease. N Engl J Med 2019; 380:1033-1042 [PMC free article: PMC7612927] [PubMed: 30865797]
- 41.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50 Suppl:S172-177 [PMC free article: PMC2674748] [PubMed: 19020338]
- 42.
- Lubin JH, Couper D, Lutsey PL, Woodward M, Yatsuya H, Huxley RR. Risk of Cardiovascular Disease from Cumulative Cigarette Use and the Impact of Smoking Intensity. Epidemiology 2016; 27:395-404 [PMC free article: PMC5482174] [PubMed: 26745609]
- 43.
- Lubin JH, Albanes D, Hoppin JA, Chen H, Lerro CC, Weinstein SJ, Sandler DP, Beane Freeman LE. Greater Coronary Heart Disease Risk With Lower Intensity and Longer Duration Smoking Compared With Higher Intensity and Shorter Duration Smoking: Congruent Results Across Diverse Cohorts. Nicotine Tob Res 2017; 19:817-825 [PMC free article: PMC5896542] [PubMed: 27941116]
- 44.
- Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, Blaha MJ, Allison M, Heiss G, Selvin E, Coresh J, Matsushita K. Cigarette Smoking, Smoking Cessation, and Long-Term Risk of 3 Major Atherosclerotic Diseases. J Am Coll Cardiol 2019; 74:498-507 [PMC free article: PMC6662625] [PubMed: 31345423]
- 45.
- Morton JI, Lazzarini PA, Polkinghorne KR, Carstensen B, Magliano DJ, Shaw JE. The association of attained age, age at diagnosis, and duration of type 2 diabetes with the long-term risk for major diabetes-related complications. Diabetes Res Clin Pract 2022; 190:110022 [PubMed: 35905888]
- 46.
- de Jong M, Woodward M, Peters SAE. Duration of diabetes and the risk of major cardiovascular events in women and men: A prospective cohort study of UK Biobank participants. Diabetes Res Clin Pract 2022; 188:109899 [PubMed: 35525499]
- 47.
- Feingold KR. Role of Glucose and Lipids in the Atherosclerotic Cardiovascular Disease in Patients with Diabetes. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2023.
- 48.
- Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, Marre M, Patel A, Poulter N, Williams B, Chalmers J. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014; 57:2465-2474 [PubMed: 25226881]
- 49.
- Feingold KR. Triglyceride Lowering Drugs. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2024.
- 50.
- Feingold KR. Cholesterol Lowering Drugs. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2024.
- 51.
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623-1630 [PubMed: 12457784]
- 52.
- Cholesterol Treatment Trialists Colloboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019; 393:407-415 [PMC free article: PMC6429627] [PubMed: 30712900]
- 53.
- Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Bejot Y, Cabrejo L, Cha JK, Ducrocq G, Giroud M, Guidoux C, Hobeanu C, Kim YJ, Lapergue B, Lavallee PC, Lee BC, Lee KB, Leys D, Mahagne MH, Meseguer E, Nighoghossian N, Pico F, Samson Y, Sibon I, Steg PG, Sung SM, Touboul PJ, Touze E, Varenne O, Vicaut E, Yelles N, Bruckert E. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N Engl J Med 2020; 382:9 [PubMed: 31738483]
- 54.
- Andersson NW, Corn G, Dohlmann TL, Melbye M, Wohlfahrt J, Lund M. LDL-C Reduction With Lipid-Lowering Therapy for Primary Prevention of Major Vascular Events Among Older Individuals. J Am Coll Cardiol 2023; 82:1381-1391 [PubMed: 37758432]
- 55.
- Orkaby AR, Driver JA, Ho YL, Lu B, Costa L, Honerlaw J, Galloway A, Vassy JL, Forman DE, Gaziano JM, Gagnon DR, Wilson PWF, Cho K, Djousse L. Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA 2020; 324:68-78 [PMC free article: PMC7341181] [PubMed: 32633800]
- 56.
- Kim K, Lee CJ, Shim CY, Kim JS, Kim BK, Park S, Chang HJ, Hong GR, Ko YG, Kang SM, Choi D, Ha JW, Hong MK, Jang Y, Lee SH. Statin and clinical outcomes of primary prevention in individuals aged >75 years: The SCOPE-75 study. Atherosclerosis 2019; 284:31-36 [PubMed: 30870705]
- 57.
- Bergami M, Cenko E, Yoon J, Mendieta G, Kedev S, Zdravkovic M, Vasiljevic Z, Milicic D, Manfrini O, van der Schaar M, Gale CP, Badimon L, Bugiardini R. Statins for primary prevention among elderly men and women. Cardiovasc Res 2022; 118:3000-3009 [PMC free article: PMC9648819] [PubMed: 34864917]
- 58.
- Lavie G, Hoshen M, Leibowitz M, Benis A, Akriv A, Balicer R, Reges O. Statin Therapy for Primary Prevention in the Elderly and Its Association with New-Onset Diabetes, Cardiovascular Events, and All-Cause Mortality. Am J Med 2021; 134:643-652 [PubMed: 33217370]
- 59.
- O'Sullivan JL, Kohl R, Lech S, Romanescu L, Schuster J, Kuhlmey A, Gellert P, Yasar S. Statin Use and All-Cause Mortality in Nursing Home Residents With and Without Dementia: A Retrospective Cohort Study Using Claims Data. Neurology 2024; 102:e209189 [PubMed: 38412394]
- 60.
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015; 372:2387-2397 [PubMed: 26039521]
- 61.
- Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, Urabe T, Uchida Y, Hayashi M, Yokota N, Nishida H, Otonari T, Arai T, Sakuma I, Sakabe K, Yamamoto M, Kobayashi T, Oikawa S, Yamashita S, Rakugi H, Imai T, Tanaka S, Ohashi Y, Kuwabara M, Ito H. Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A Randomized, Controlled Trial. Circulation 2019; 140:992-1003 [PubMed: 31434507]
- 62.
- Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, Heo JH, Rha SW, Cho YH, Lee SJ, Ahn CM, Kim JS, Ko YG, Choi D, Jang Y, Hong MK. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet 2022; 400:380-390 [PubMed: 35863366]
- 63.
- Lee B, Hong SJ, Rha SW, Heo JH, Hur SH, Choi HH, Kim KJ, Kim JH, Kim HK, Kim U, Choi YJ, Lee YJ, Lee SJ, Ahn CM, Ko YG, Kim BK, Choi D, Hong MK, Jang Y, Kim JS. Moderate-intensity statin plus ezetimibe vs high-intensity statin according to baseline LDL-C in the treatment of atherosclerotic cardiovascular disease: A post-hoc analysis of the RACING randomized trial. Atherosclerosis 2023; 386:117373 [PubMed: 37995599]
- 64.
- Lee SH, Lee YJ, Heo JH, Hur SH, Choi HH, Kim KJ, Kim JH, Park KH, Lee JH, Choi YJ, Lee SJ, Hong SJ, Ahn CM, Kim BK, Ko YG, Choi D, Hong MK, Jang Y, Kim JS. Combination Moderate-Intensity Statin and Ezetimibe Therapy for Elderly Patients With Atherosclerosis. J Am Coll Cardiol 2023; 81:1339-1349 [PubMed: 37019580]
- 65.
- Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500-1509 [PubMed: 25773607]
- 66.
- Sever P, Gouni-Berthold I, Keech A, Giugliano R, Pedersen TR, Im K, Wang H, Knusel B, Sabatine MS, O'Donoghue ML. LDL-cholesterol lowering with evolocumab, and outcomes according to age and sex in patients in the FOURIER Trial. Eur J Prev Cardiol 2021; 28:805-812 [PubMed: 34298555]
- 67.
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med 2018; 379:2097-2107 [PubMed: 30403574]
- 68.
- Sinnaeve PR, Schwartz GG, Wojdyla DM, Alings M, Bhatt DL, Bittner VA, Chiang CE, Correa Flores RM, Diaz R, Dorobantu M, Goodman SG, Jukema JW, Kim YU, Pordy R, Roe MT, Sy RG, Szarek M, White HD, Zeiher AM, Steg PG. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J 2020; 41:2248-2258 [PMC free article: PMC7308542] [PubMed: 31732742]
- 69.
- Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, Thompson PD, Libby P, Cho L, Plutzky J, Bays HE, Moriarty PM, Menon V, Grobbee DE, Louie MJ, Chen CF, Li N, Bloedon L, Robinson P, Horner M, Sasiela WJ, McCluskey J, Davey D, Fajardo-Campos P, Petrovic P, Fedacko J, Zmuda W, Lukyanov Y, Nicholls SJ. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N Engl J Med 2023; 388:1353-1364 [PubMed: 36876740]
- 70.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255-2267 [PubMed: 22085343]
- 71.
- Hps Thrive Collaborative Group, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014; 371:203-212 [PubMed: 25014686]
- 72.
- ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr., Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563-1574 [PMC free article: PMC2879499] [PubMed: 20228404]
- 73.
- Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM, Campbell SE, Oshima R, Amarenco P, Blom DJ, Brinton EA, Eckel RH, Elam MB, Felicio JS, Ginsberg HN, Goudev A, Ishibashi S, Joseph J, Kodama T, Koenig W, Leiter LA, Lorenzatti AJ, Mankovsky B, Marx N, Nordestgaard BG, Pall D, Ray KK, Santos RD, Soran H, Susekov A, Tendera M, Yokote K, Paynter NP, Buring JE, Libby P, Ridker PM. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N Engl J Med 2022; 387:1923-1934 [PubMed: 36342113]
- 74.
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007; 369:1090-1098 [PubMed: 17398308]
- 75.
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019; 380:11-22 [PubMed: 30415628]
- 76.
- Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundstrom T, Agrawal R, Menon V, Wolski K, Nissen SE. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020; 324:2268-2280 [PMC free article: PMC7667577] [PubMed: 33190147]
- 77.
- Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DWT, Tveit A, Fagerland MW, Solheim S, Seljeflot I, Arnesen H. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction: A Randomized, Controlled Trial. Circulation 2021; 143:528-539 [PubMed: 33191772]
- 78.
- Mason RP, Libby P, Bhatt DL. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler Thromb Vasc Biol 2020; 40:1135-1147 [PMC free article: PMC7176343] [PubMed: 32212849]
- 79.
- Ridker PM, Rifai N, MacFadyen J, Glynn RJ, Jiao L, Steg PG, Miller M, Brinton EA, Jacobson TA, Tardif JC, Ballantyne CM, Mason RP, Bhatt DL. Effects of Randomized Treatment With Icosapent Ethyl and a Mineral Oil Comparator on Interleukin-1beta, Interleukin-6, C-Reactive Protein, Oxidized Low-Density Lipoprotein Cholesterol, Homocysteine, Lipoprotein(a), and Lipoprotein-Associated Phospholipase A2: A REDUCE-IT Biomarker Substudy. Circulation 2022; 146:372-379 [PubMed: 35762321]
- 80.
- Goff ZD, Nissen SE. N-3 polyunsaturated fatty acids for cardiovascular risk. Curr Opin Cardiol 2022; 37:356-363 [PubMed: 35275889]
- 81.
- Mason RP, Sherratt SCR, Eckel RH. Omega-3-fatty acids: Do they prevent cardiovascular disease? Best Pract Res Clin Endocrinol Metab 2023; 37:101681 [PubMed: 35739003]
- 82.
- Joseph J, Pajewski NM, Dolor RJ, Sellers MA, Perdue LH, Peeples SR, Henrie AM, Woolard N, Jones WS, Benziger CP, Orkaby AR, Mixon AS, VanWormer JJ, Shapiro MD, Kistler CE, Polonsky TS, Chatterjee R, Chamberlain AM, Forman DE, Knowlton KU, Gill TM, Newby LK, Hammill BG, Cicek MS, Williams NA, Decker JE, Ou J, Rubinstein J, Choudhary G, Gazmuri RJ, Schmader KE, Roumie CL, Vaughan CP, Effron MB, Cooper-DeHoff RM, Supiano MA, Shah RC, Whittle JC, Hernandez AF, Ambrosius WT, Williamson JD, Alexander KP. Pragmatic evaluation of events and benefits of lipid lowering in older adults (PREVENTABLE): Trial design and rationale. J Am Geriatr Soc 2023; 71:1701-1713 [PMC free article: PMC10258159] [PubMed: 37082807]
- 83.
- Zoungas S, Curtis A, Spark S, Wolfe R, McNeil JJ, Beilin L, Chong TT, Cloud G, Hopper I, Kost A, Nelson M, Nicholls SJ, Reid CM, Ryan J, Tonkin A, Ward SA, Wierzbicki A. Statins for extension of disability-free survival and primary prevention of cardiovascular events among older people: protocol for a randomised controlled trial in primary care (STAREE trial). BMJ Open 2023; 13:e069915 [PMC free article: PMC10083753] [PubMed: 37012015]
- 84.
- He Y, Li X, Gasevic D, Brunt E, McLachlan F, Millenson M, Timofeeva M, Ioannidis JPA, Campbell H, Theodoratou E. Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann Intern Med 2018; 169:543-553 [PubMed: 30304368]
- 85.
- Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL, 2nd, Goldstein LB, Chin C, Tannock LR, Miller M, Raghuveer G, Duell PB, Brinton EA, Pollak A, Braun LT, Welty FK. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol 2019; 39:e38-e81 [PubMed: 30580575]
- 86.
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375:735-742 [PubMed: 20167359]
- 87.
- Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011; 305:2556-2564 [PubMed: 21693744]
- 88.
- Sattar N. Statins and diabetes: What are the connections? Best Pract Res Clin Endocrinol Metab 2023; 37:101749 [PubMed: 36858834]
- 89.
- Rojas-Fernandez C, Hudani Z, Bittner V. Statins and Cognitive Side Effects: What Cardiologists Need to Know. Endocrinol Metab Clin North Am 2016; 45:101-116 [PubMed: 26893000]
- 90.
- Rojas-Fernandez CH, Goldstein LB, Levey AI, Taylor BA, Bittner V. An assessment by the Statin Cognitive Safety Task Force: 2014 update. J Clin Lipidol 2014; 8:S5-16 [PubMed: 24793442]
- 91.
- Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM. Statins and cognitive function: a systematic review. Ann Intern Med 2013; 159:688-697 [PubMed: 24247674]
- 92.
- Olmastroni E, Molari G, De Beni N, Colpani O, Galimberti F, Gazzotti M, Zambon A, Catapano AL, Casula M. Statin use and risk of dementia or Alzheimer's disease: a systematic review and meta-analysis of observational studies. Eur J Prev Cardiol 2022; 29:804-814 [PubMed: 34871380]
- 93.
- Trompet S, van Vliet P, de Craen AJ, Jolles J, Buckley BM, Murphy MB, Ford I, Macfarlane PW, Sattar N, Packard CJ, Stott DJ, Shepherd J, Bollen EL, Blauw GJ, Jukema JW, Westendorp RG. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol 2010; 257:85-90 [PubMed: 19653027]
- 94.
- Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004; 363:757-767 [PubMed: 15016485]
- 95.
- McGuinness B, Craig D, Bullock R, Malouf R, Passmore P. Statins for the treatment of dementia. Cochrane Database Syst Rev 2014:CD007514 [PMC free article: PMC11112650] [PubMed: 25004278]
- 96.
- Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA, The National Lipid Association's Muscle Safety Expert Panel. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol 2014; 8:S58-71 [PubMed: 24793443]
- 97.
- Schech S, Graham D, Staffa J, Andrade SE, La Grenade L, Burgess M, Blough D, Stergachis A, Chan KA, Platt R, Shatin D. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf 2007; 16:352-358 [PubMed: 16892458]
- 98.
- Cholesterol Treatment Trialists Collaboration. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet 2022; 400:832-845 [PMC free article: PMC7613583] [PubMed: 36049498]
- 99.
- Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403-414 [PubMed: 16453090]
- 100.
- Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182-1186 [PMC free article: PMC2517983] [PubMed: 18449611]
- 101.
- Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012; 6:208-215 [PubMed: 22658145]
- 102.
- Gupta A, Thompson D, Whitehouse A, Collier T, Dahlof B, Poulter N, Collins R, Sever P. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017; 389:2473-2481 [PubMed: 28476288]
- 103.
- Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR. Cognitive Function in a Randomized Trial of Evolocumab. N Engl J Med 2017; 377:633-643 [PubMed: 28813214]
- 104.
- Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, Civeira F, Somaratne R, Nelson P, Liu T, Scott R, Wasserman SM, Sabatine MS. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation 2014; 129:234-243 [PubMed: 24255061]
- 105.
- Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489-1499 [PubMed: 25773378]
- 106.
- Shapiro MD, Feingold KR. Monogenic Disorders Causing Hypobetalipoproteinemia. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2024.
- 107.
- McKenney JM. Understanding PCSK9 and anti-PCSK9 therapies. J Clin Lipidol 2015; 9:170-186 [PubMed: 25911073]
- 108.
- LaRosa JC, Grundy SM, Kastelein JJ, Kostis JB, Greten H. Safety and efficacy of Atorvastatin-induced very low-density lipoprotein cholesterol levels in Patients with coronary heart disease (a post hoc analysis of the treating to new targets [TNT] study). Am J Cardiol 2007; 100:747-752 [PubMed: 17719314]
- 109.
- Wiviott SD, Cannon CP, Morrow DA, Ray KK, Pfeffer MA, Braunwald E. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol 2005; 46:1411-1416 [PubMed: 16226163]
- 110.
- Everett BM, Mora S, Glynn RJ, MacFadyen J, Ridker PM. Safety profile of subjects treated to very low low-density lipoprotein cholesterol levels (<30 mg/dl) with rosuvastatin 20 mg daily (from JUPITER). Am J Cardiol 2014; 114:1682-1689 [PubMed: 25439449]
- 111.
- Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, White JA, Tershakovec AM, Cannon CP, Braunwald E. Long-term Safety and Efficacy of Achieving Very Low Levels of Low-Density Lipoprotein Cholesterol : A Prespecified Analysis of the IMPROVE-IT Trial. JAMA Cardiol 2017; 2:547-555 [PMC free article: PMC5814987] [PubMed: 28291866]
- 112.
- Wachira JK, Larson MK, Harris WS. n-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights. Br J Nutr 2014; 111:1652-1662 [PubMed: 24472372]
- 113.
- Feingold KR, Chait A. Approach to patients with elevated low-density lipoprotein cholesterol levels. Best Pract Res Clin Endocrinol Metab 2023; 37:101658 [PubMed: 35487874]
- 114.
- Score Op working group E. S. C. Cardiovascular risk collaboration. SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur Heart J 2021; 42:2455-2467 [PMC free article: PMC8248997] [PubMed: 34120185]
- 115.
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC, Jr., Virani SS, Williams KA, Sr., Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 140:e596-e646 [PMC free article: PMC7734661] [PubMed: 30879355]
- 116.
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical Activity Guidelines for Americans. JAMA 2018; 320:2020-2028 [PMC free article: PMC9582631] [PubMed: 30418471]
- 117.
- Yun H, Su W, Zhao H, Li H, Wang Z, Cui X, Xi C, Gao R, Sun Y, Liu C. Effects of different exercise modalities on lipid profile in the elderly population: A meta-analysis. Medicine (Baltimore) 2023; 102:e33854 [PMC free article: PMC10662825] [PubMed: 37478257]
- 118.
- Feingold KR. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA) 2021.
- 119.
- Ioannidis JPA. The Challenge of Reforming Nutritional Epidemiologic Research. JAMA 2018; 320:969-970 [PubMed: 30422271]
- 120.
- Nissen SE. U.S. Dietary Guidelines: An Evidence-Free Zone. Ann Intern Med 2016; 164:558-559 [PubMed: 26783992]
- 121.
- Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Fito M, Gea A, Hernan MA, Martinez-Gonzalez MA. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 2018; 378:e34 [PubMed: 29897866]
- 122.
- Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006; 145:1-11 [PubMed: 16818923]
- 123.
- de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994; 343:1454-1459 [PubMed: 7911176]
- 124.
- Delgado-Lista J, Alcala-Diaz JF, Torres-Pena JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, Ortiz-Morales AM, Gonzalez-Requero AI, Perez-Caballero AI, Yubero-Serrano EM, Rangel-Zuniga OA, Camargo A, Rodriguez-Cantalejo F, Lopez-Segura F, Badimon L, Ordovas JM, Perez-Jimenez F, Perez-Martinez P, Lopez-Miranda J. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet 2022; 399:1876-1885 [PubMed: 35525255]
- 125.
- Chait A, Feingold KR. Approach to patients with hypertriglyceridemia. Best Pract Res Clin Endocrinol Metab 2023; 37:101659 [PubMed: 35459627]
- 126.
- Awad K, Mikhailidis DP, Toth PP, Jones SR, Moriarty P, Lip GYH, Muntner P, Catapano AL, Pencina MJ, Rosenson RS, Rysz J, Banach M. Efficacy and Safety of Alternate-Day Versus Daily Dosing of Statins: a Systematic Review and Meta-Analysis. Cardiovasc Drugs Ther 2017; 31:419-431 [PubMed: 28741244]
- 127.
- Kennedy SP, Barnas GP, Schmidt MJ, Glisczinski MS, Paniagua AC. Efficacy and tolerability of once-weekly rosuvastatin in patients with previous statin intolerance. J Clin Lipidol 2011; 5:308-315 [PubMed: 21784377]
- 128.
- Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, Stroes ES, MacDougall D, Zhao X, Catapano AL. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2020; 27:593-603 [PMC free article: PMC7153222] [PubMed: 31357887]
- ABSTRACT
- INTRODUCTION
- LIPID LEVELS IN THE ELDERLY
- AGE IS AN IMPORTANT RISK FOR ATHEROSCLEROTIC CARDIOVASCULAR DISEASE
- WHY ARE OLDER INDIVIDUALS AT HIGHER RISK FOR ASCVD?
- DOES LIPID LOWERING REDUCE EVENTS IN THE ELDERLY
- SIDE EFFECTS OF LIPID LOWERING DRUGS
- CURRENT GUIDELINES AND LDL-C GOALS
- TREATMENT
- LIPID LOWERING DRUGS
- CONCLUSION
- ACKNOWLEDGEMENTS
- REFERENCES
- Review Guidelines for the Management of High Blood Cholesterol.[Endotext. 2000]Review Guidelines for the Management of High Blood Cholesterol.Grundy SM, Feingold KR. Endotext. 2000
- PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations.[BMJ. 2022]PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations.Hao Q, Aertgeerts B, Guyatt G, Bekkering GE, Vandvik PO, Khan SU, Rodondi N, Jackson R, Reny JL, Al Ansary L, et al. BMJ. 2022 May 4; 377:e069066. Epub 2022 May 4.
- 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways.[J Am Coll Cardiol. 2017]2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways.Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. J Am Coll Cardiol. 2017 Oct 3; 70(14):1785-1822. Epub 2017 Sep 5.
- Review Lowering Targeted Atherogenic Lipoprotein Cholesterol Goals for Patients at "Extreme" ASCVD Risk.[Curr Diab Rep. 2019]Review Lowering Targeted Atherogenic Lipoprotein Cholesterol Goals for Patients at "Extreme" ASCVD Risk.Rosenblit PD. Curr Diab Rep. 2019 Nov 21; 19(12):146. Epub 2019 Nov 21.
- Review Role of Glucose and Lipids in the Atherosclerotic Cardiovascular Disease in Patients with Diabetes.[Endotext. 2000]Review Role of Glucose and Lipids in the Atherosclerotic Cardiovascular Disease in Patients with Diabetes.Feingold KR. Endotext. 2000
- Evaluation and Treatment of Dyslipidemia in the Elderly - EndotextEvaluation and Treatment of Dyslipidemia in the Elderly - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...