NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
The glucagonoma syndrome is caused by a glucagon-secreting pancreatic neuroendocrine neoplasm (panNEN)(glucagonoma). The syndrome includes: necrolytic migratory erythema, painful glossitis, cheilitis & stomatitis, weight loss, anemia, new-onset or worsening diabetes mellitus, hypoaminoacidemia, low zinc levels, deep vein thrombosis and depression. At diagnosis, a glucagonoma is usually larger than 4-5 cm in diameter and locoregional lymph node and distant metastases, particularly to the liver or bones are present. The incidence of glucagonoma syndrome is 1-2% of all panNENs. Approximately 10% of glucagonomas are associated with multiple endocrine neoplasia type 1 (MEN1). Glucagonomas highly express somatostatin receptor subtypes and, therefore, somatostatin receptor positron emission tomography (PET) CT/MRI with 68Ga- labelled somatostatin analogs (DOTATATE, DOTANOC, and DOTATOC) can be used in the localization of glucagonomas. The somatostatin receptor subtypes can also be utilized for peptide receptor radionuclide therapy with radiolabeled somatostatin analogs of metastatic glucagonomas. Other treatment options include supportive measures like amino acids, surgery, somatostatin analogs, sunitinib, everolimus, systemic cytotoxic chemotherapy, and liver-directed therapies. Glucagon cell hyperplasia and neoplasia of the endocrine pancreas is an autosomal recessive syndrome associated with hyperglucagonemia and is a genetically determined receptor disease, affecting the glucagon receptor. Patients with this disorder can develop multiple glucagonomas. However, the clinical presentation is NOT with the glucagonoma syndrome. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Glucagon is a 29-amino acid polypeptide hormone with a molecular mass of 3485 daltons, which is produced by alpha cells of the pancreas. Glucagon regulates not only glucose, but also amino acid and lipid metabolism. There is substantial evidence in humans that glucagon can also be produced outside the pancreas (1). A glucagonoma is a neuroendocrine neoplasm (NEN) secreting glucagon and pre-pro-glucagon-derived peptides.
HISTORY
In 1923, a hyperglycemic factor named “glucagon” was isolated from beef pancreas in Rochester, NY, USA by the biochemistry student Charles P. Kimball (1897-19..) and his mentor John R. Murlin (1874-1960) (2-5). In 1942, the US dermatologist S. William Becker (1894-1964) and colleagues were the first to describe the typical glucagonoma skin eruption in a patient with a pancreatic tumor (6). In 1963, Roger Unger and colleagues succeeded in recovering glucagon from extracts of pancreatic endocrine tumors found at autopsy (7). In 1966, the US pathologist Malcolm H. McGavran (1923-1999) and his associates published the first report on a patient with a glucagonoma (5,8). This 42-year-old woman presented with diabetes mellitus, anemia, a peculiar skin eruption and a metastatic pancreatic tumor. Later in the course of the disease, elevated plasma glucagon levels were found (8). The tumor was biopsied and operated by the US surgeon Hiram C. Polk Jr.(8). The characteristic cutaneous lesion which occurs in association with the glucagonoma syndrome was first described by the British dermatologist Ronald E. Church (1922-2007) and his colleague, the pathologist Walter (Bill) A.J. Crane (1925-1982) (9). The British dermatologist Darrell S. Wilkinson (1919-2009) named this lesion “necrolytic migratory erythema” (NME) in 1971 (5,10). In 1974, the British gastroenterologist Christopher N. Mallinson in collaboration with Stephen R. Bloom and colleagues described four glucagonoma patients with the glucagonoma syndrome and NME with increased plasma glucagon levels and very low plasma amino acid concentrations (11). One patient fully recovered after resection of the glucagonoma (5,11). In the same year, a similar complete response of the glucagonoma syndrome and NME after surgical resection was described by the British dermatologist R. Douglas Sweet (1917-2001)(5,12). The first well-documented cases of glucagon cell hyperplasia and neoplasia (GCHN) of the endocrine pancreas (Mahvash syndrome) were published in 2006 by the German pathologist Martin Anlauf and colleagues (13). The first clinically well-characterized case of GCHN was described by the group of the US-Chinese endocrinologist Run Yu in 2008 (14).
CLINICAL PRESENTATION
The majority of patients with a glucagonoma presents with new onset or worsening of diabetes mellitus (70%) accompanied by significant weight loss (60%), because glucagon hypersecretion has a catabolic effect in combination with diarrhea (15). Other symptoms include painful glossitis (Figure 1), cheilitis & angular stomatitis (41%), onychodystrophy (in females), deep vein thrombosis & pulmonary embolism (16-18), normochromic normocytic anemia (50%), hypoaminoacidemia and low plasma zinc levels (15,19,20). In rare cases, glucagonomas are associated with dilated cardiomyopathy that can be reversible after tumor control (21,22). However, the most distinct symptom in glucagonoma patients concerns skin lesions named necrolytic migratory erythema (NME) which occurs in 80% of patients (23-27). The NME has a characteristic distribution. It is usually widespread with major sites of involvement at the perioral and perigenital regions along with the fingers, legs, and feet (28). The rash starts as an erythematous lesion, progresses to form a bullous which ulcerates forming a depressed lesion that is surrounded by brown pigment (Figure 2). Patients can suffer from itchy or painful lesions. The basic process in the skin seems to be one of superficial epidermal necrosis, fragile blister formation, crusting, and healing with hyperpigmentation (17). Different stages of the cutaneous lesions may be present simultaneously (17). A painful glossitis manifested by an erythematous, mildly atrophic tongue has been associated with the cutaneous lesions (Figure 1).
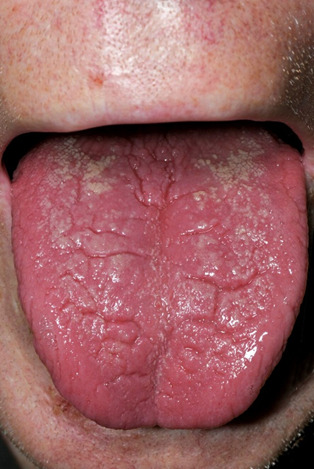
Figure 1.
Glossitisin a glucagonoma patient. Picture included with the informed consent of the patient.
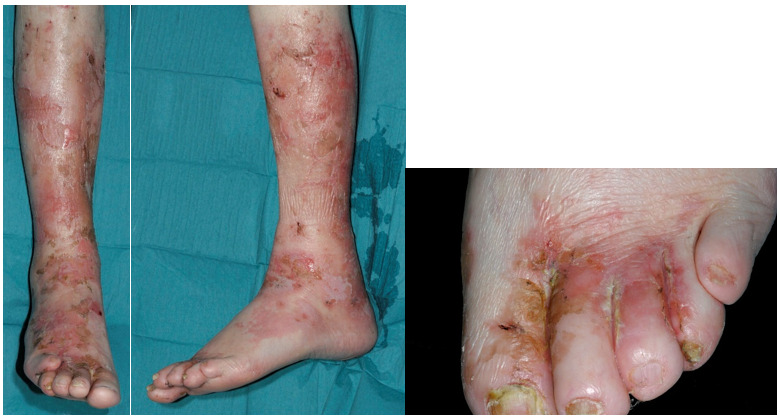
Figure 2.
Necrolytic migratory erythema in a glucagonoma patient. Pictures included with the informed consent of the patient.
The typical clinical presentation of glucagonoma is with a tumor size larger than 4-5 cm in diameter and metastatic dissemination has already occurred, particularly in the locoregional lymph nodes, liver, and bones (19,29). Secondary, or metachronous glucagon secretion in panNENs which previously were non-secreting, or secreted other peptide hormones, can also occur and is usually associated with a poor prognosis (30,31).
The clinical incidence of glucagonomas is estimated at 1-2% of panNENs and about 1-2 cases per million population (32,33). The average age of patient at diagnosis is 52.5 years, with a slight higher prevalence in females (15). The 10-year survival of a localized (and subsequently surgically resected) glucagonoma is nearly 100%, but decreases to 50% in the presence of metastatic disease (15,28,34,35). The median survival time for glucagonomas is 7.7 years (20,36,37). About 10% of glucagonomas are diagnosed in patients with multiple endocrine neoplasia type 1 (MEN1)(38,39).
GLUCAGON CELL HYPERPLASIA AND NEOPLASIA
A second, however rare, autosomal recessive syndrome associated with hyperglucagonemia is glucagon cell hyperplasia and neoplasia (GCHN) of the endocrine pancreas (Mahvash syndrome) (40,41). GCNH is a genetically determined receptor disease, affecting the glucagon receptor (GCGR). GCGR inactivation interrupts glucagon signaling in the liver, leading to disturbed metabolism of glycogen, fatty acids and amino acids. Altered function of the liver cells subsequently results in hyperplastic changes of the glucagon cells of the pancreatic islets, followed by hyperglucagonemia and the development of multiple glucagon producing NENs (41,42). In one GCHN case, a lymph node micro-metastasis was found (42) Patients are middle-aged adults who present with non-specific symptoms such as fatigue, abdominal pain, diabetes, or acute pancreatitis. Despite very high serum glucagon levels, none of the GCHN patients with GCGR mutations showed a glucagonoma syndrome, owing to the interrupted signaling of the GCGR. The only reported GCHN patient with a glucagonoma syndrome and NME harbored a wild type GCGR (42)). The pancreatic NEN (panNEN) can be visualized by somatostatin receptor imaging, since the glucagon cells express somatostatin receptor subtypes (43). GCHN follows a benign clinical course for most patients. However, follow-up of the patients is suggested, as the tumors have a metastatic potential. Glucagon also increases hepatic amino acid turnover, and thus lowers postprandial serum amino acid levels. This disturbed amino acid metabolism probably plays the most important role in the development of GCHN. The impaired glucagon signaling in the liver of GCHN patients results in chronically elevated serum amino acid levels which stimulate the secretion and proliferation of glucagon cells, leading to hyperglucagonemia, glucagon cell hyperplasia, and finally to NENs.
GLUCAGONOMA DIAGNOSIS
The diagnosis of the glucagonoma syndrome is based on the combination of elevated plasma glucagon levels and glucagonoma symptoms as described above. Mild elevated glucagon levels may be associated with several other diseases like cirrhosis, chronic renal failure, sepsis, acute or chronic pancreatitis, chronic hepatic failure, Cushing's syndrome, acute trauma, diabetes mellitus, diabetic ketoacidosis, stress, burns, portocaval shunting, other NENs and familial hyperglucagonemia (44). However, a fasting plasma glucagon >500 pg/ml (reference range, 70–160 pg/ml) is diagnostic for glucagonoma (45).
Anatomic and functional imaging modalities are important in the localization of a glucagonoma. As in other NENs, 3-phase CT or MRI scans must be performed for the precise localization of these tumors in the pancreas. Since glucagonomas express high numbers of different somatostatin, somatostatin receptor imaging has been used to detect distant metastases and scan-positivity has been reported in up to 97% of glucagonoma patients, (35,46). Currently, positron emission tomography (PET)-CT with 68Ga-labelled somatostatin analogs (SSAs) (DOTATATE, DOTANOC, DOTATOC) has the highest sensitivity for detecting metastases of G1-2 and some G3 pancreatic neuroendocrine tumors (panNETs) (47). In line with the work-up for all NENs, a biopsy is advised to confirm the diagnosis and for grading (Ki67 index), as the grade can influence treatment selection (48). An overview of the current staging and grading systems is provided in the chapter “Insulinoma” (49). Tumor cells are positive for general neuroendocrine markers, glucagon, and somatostatin receptor subtype 2a. (28). Skin biopsies of NME usually show psoriasiform hyperplasia of the epidermis, pallor of keratinocytes, vacuolated or dyskeratotic keratinocytes, necrosis of the upper epidermis and perivascular inflammation (17).
The diagnosis of glucagonoma relies on the presence of the glucagonoma syndrome and not on glucagon immunoreactivity in tumor cells alone. PanNENs composed of glucagon-immunoreactive cells but lacking symptoms of the glucagonoma syndrome are defined as “non-functioning glucagon-producing PanNENs”. They are frequently small and indolent tumors associated with an excellent prognosis, while glucagonomas are larger and more frequently metastatic neoplasms (50).
TREATMENT OF GLUCAGONOMA
Supportive Measures
Supportive therapy (also initiated before surgery) includes amino acid infusions (51), essential fatty acids, topic or oral zinc therapies, vitamins, minerals, and glucose control (52). Also, anticoagulant therapy has been recommended because of the increased risk of thrombosis (16,17).
Necrotic Migratory Erythema Therapy Response
Almost invariably, the NME resolves after successful removal of a glucagon-producing tumor, even if the rash has been present for several years (25,53). The NME also improves in patients who do not undergo curative resection but are treated with SSAs (54,55), everolimus, or peptide receptor radionuclide therapy (PRRT) with radiolabeled SSAs (56-58). Impressive improvement of the NME with amino acid repletion has also been described (29,51,59).
Surgery
As for all panNENs, surgery is the only curative treatment. In the occasional patient in whom a glucagonoma is discovered while the tumor is locoregionally confined, pancreatic surgery (enucleation, distal pancreatectomy with or without splenectomy, central pancreatectomy, pancreaticoduodenectomy and total pancreatectomy) should be performed to remove the glucagonoma (60,61). Post-surgery, symptoms of the glucagonoma syndrome will resolve within weeks (25). In selected patients with limited liver metastases an extended surgical resection can also be considered (62). Preoperative preparation is required including correction of malnutrition and hyperglycemia. Somatostatin analogs (SSAs) should be started to reverse the catabolic state and improve the skin rash (63). Prophylactic measures to prevent venous thrombosis, including the use of low-molecular weight heparin, should be applied to all patients during the perioperative period (29).
In case of unresectable metastases, treatment is focused on tumor stabilization and symptom reduction by decreasing the secretion of glucagon. In general, anti-tumor therapy is similar to non-functioning panNENs as specific data for glucagonoma is often lacking. The guidelines by ENETS, NANETS and ESMO describe the selection and sequencing of SSAs, targeted therapy, PRRT with radiolabeled SSAs and cytotoxic chemotherapy (29,64,65).
Somatostatin Analogs
SSAs are the first-line palliative treatments to control glucagon secretion and tumor growth (46). In a randomized controlled trial (CLARINET), including G1-2 panNENs, treatment with lanreotide autogel 120 mg every 4 weeks deep sc was associated with significantly prolonged median progression-free survival (PFS) of 38 months versus 18 months for placebo (66). Moreover, SSAs have been reported to decrease the NME (29,56).
Peptide Receptor Radionuclide Therapy
Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE results in a response rate of 55% for panNENs, with a median PFS of 30 months and median overall survival (OS) of 71 months (67). PRRT with 177Lu-DOTATATE for the treatment of metastatic glucagonoma has been described in small case series (58,68). The radiological response rate of glucagonomas seems to be comparable to that observed in patients with clinically non-functioning G1-2 panNETs. Of additional value is the high symptomatic response rate (71%) and the increase in quality of life after treatment with 177Lu-DOTATATE (29,58).
Everolimus
Everolimus is registered for the second-line treatment of G1-2 panNENs based on the result of the RADIANT-3 trial. In this study, 24% of patients had a functioning (= hormone-secreting) panNEN and everolimus treatment was associated with a (statistically not significant) overall survival benefit of 6.3 months (69,70). Everolimus was found to decrease plasma glucagon levels in patients with elevated plasma glucagon levels (71). However, median plasma glucagon levels in these patients were only 1.5 times the upper limit of normal suggesting that they did not suffer from the classical glucagonoma syndrome. Since everolimus can also worsen diabetes mellitus by reducing insulin secretion from the pancreas and inducing insulin resistance, its contribution to the treatment of glucagonoma patients is still unclear (29).
Sunitinib
In a randomized controlled trial in patients with G1-2 panNENs, second-line sunitinib treatment (37.5 mg/day) resulted in an increased progression-free survival by 5.9 months compared to placebo (72,73). In this trial, 5 glucagonoma patients (3%) were enrolled (72). However, the radiological and symptomatic response for this subgroup of glucagonoma patients was not separately reported (29,72,73).
Chemotherapy
Treatment of 18 glucagonoma patients with streptozotocin (STZ) and 5-fluorouracil (5-FU) resulted in an objective response in 50% of patients (35). Chemotherapy with capecitabine and temozolomide is also effective for the treatment of panNENs, but no specific data for glucagonoma are available (29,74,75).
Liver-Directed Therapy
As severity of the glucagonoma syndrome is associated with tumor burden, reducing liver tumor burden could potentially reduce symptoms of glucagonoma as well. In patients with liver-dominant disease, liver metastases can be resected or treated by bland embolization, radioembolization (SIRT), radiofrequency ablation (RFA), microwave and cryoablation, high-intensity focused ultrasound (HIFU), laser, brachytherapy and irreversible electroporation (IRE) depending on local availability (76).
REFERENCES
- 1.
- Holst JJ. Glucagon 100 years. Important, but still enigmatic. Peptides. 2023;161:170942. [PubMed: 36626940]
- 2.
- Kimball CP, Murlin JR. Aqueous extracts of pancreas. III. Some precipitation reactions of insulin. J Biol Chem. 1923;58(1):337–346.
- 3.
- Unger RH. Glucagon physiology and pathophysiology. N Engl J Med. 1971;285(8):443–449. [PubMed: 4997492]
- 4.
- Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The New Biology and Pharmacology of Glucagon. Physiol Rev. 2017;97(2):721–766. [PubMed: 28275047]
- 5.
- de Herder WW, Rehfeld JF, Kidd M, Modlin IM. A short history of neuroendocrine tumours and their peptide hormones. Best Pract Res Clin Endocrinol Metab. 2016;30(1):3–17. [PubMed: 26971840]
- 6.
- Becker SW, Kahn D, Rothman S. Cutaneous manifestations of internal malignant tumours. Arch Dermatol Syphilol 1. 1942;45:1069-1080.
- 7.
- Unger RH, Eisentraut AM, Lochner JV. Glucagon-producing tumors of the islets of Langerhans. J Clin Invest. 1963;42:987–988.
- 8.
- McGavran MH, Unger RH, Recant L, Polk HC, Kilo C, Levin ME. A glucagon-secreting alpha-cell carcinoma of the pancreas. N Engl J Med. 1966;274(25):1408–1413. [PubMed: 4286757]
- 9.
- Church RE, Crane WA. A cutaneous syndrome associated with islet-cell carcinoma of the pancreas. Br J Dermatol. 1967;79(5):284–286. [PubMed: 4290599]
- 10.
- Wilkinson DS. Necrolytic migratory erythema with pancreatic carcinoma. Proc R Soc Med. 1971;64(12):1197–1198. [PMC free article: PMC1813174] [PubMed: 5131260]
- 11.
- Mallinson CN, Bloom SR, Warin AP, Salmon PR, Cox B. A glucagonoma syndrome. Lancet. 1974;2(7871):1–5. [PubMed: 4134714]
- 12.
- Sweet RD. A dermatosis specifically associated with a tumour of pancreatic alpha cells. Br J Dermatol. 1974;90(3):301–308. [PubMed: 4207228]
- 13.
- Anlauf M, Schlenger R, Perren A, Bauersfeld J, Koch CA, Dralle H, Raffel A, Knoefel WT, Weihe E, Ruszniewski P, Couvelard A, Komminoth P, Heitz PU, Klöppel G. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30(5):560–574. [PubMed: 16699310]
- 14.
- Yu R, Nissen NN, Dhall D, Heaney AP. Nesidioblastosis and hyperplasia of alpha cells, microglucagonoma, and nonfunctioning islet cell tumor of the pancreas: review of the literature. Pancreas. 2008;36(4):428–431. [PubMed: 18437091]
- 15.
- Soga J, Yakuwa Y. Glucagonomas/diabetico-dermatogenic syndrome (DDS): a statistical evaluation of 407 reported cases. J Hepatobiliary Pancreat Surg. 1998;5(3):312–319. [PubMed: 9880781]
- 16.
- Georgiou GK, Gizas I, Katopodis KP, Katsios CS. Non-secreting benign glucagonoma diagnosed incidentally in a patient with refractory thrombocytopenic thrombotic purpura: report of a case. Surg Today. 2015;45(10):1317–1320. [PubMed: 25373364]
- 17.
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-Associated Necrolytic Migratory Erythema: The Broad Spectrum of the Clinical and Histopathological Findings and Clues to the Diagnosis. Am J Dermatopathol. 2019;41(3):e29–e32. [PubMed: 30124507]
- 18.
- Delli Colli M, Alamri BN, Palma L, Rivera J. A Glucagonoma Presenting as Cerebral Vein Thrombosis and Diabetes. Case Rep Endocrinol. 2022;2022:7659341. [PMC free article: PMC9054441] [PubMed: 35498123]
- 19.
- Hofland J, Kaltsas G, de Herder WW. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr Rev. 2020;41(2):371–403. [PMC free article: PMC7080342] [PubMed: 31555796]
- 20.
- Song X, Zheng S, Yang G, Xiong G, Cao Z, Feng M, Zhang T, Zhao Y. Glucagonoma and the glucagonoma syndrome. Oncol Lett. 2018;15(3):2749–2755. [PMC free article: PMC5778850] [PubMed: 29435000]
- 21.
- Chang-Chretien K, Chew JT, Judge DP. Reversible dilated cardiomyopathy associated with glucagonoma. Heart. 2004;90(7):e44. [PMC free article: PMC1768315] [PubMed: 15201270]
- 22.
- Demir OM, Paschou SA, Ellis HC, Fitzpatrick M, Kalogeropoulos AS, Davies A, Thompson J, Davies SW, Grapsa J. Reversal of dilated cardiomyopathy after glucagonoma excision. Hormones (Athens). 2015;14(1):172–173. [PubMed: 25553769]
- 23.
- Li W, Yang X, Deng Y, Jiang Y, Xu G, Li E, Wu Y, Ren J, Ma Z, Dong S, Han L, Ma Q, Wu Z, Wang Z. Necrolytic migratory erythema is an important visual cutaneous clue of glucagonoma. Sci Rep. 2022;12(1):9053. [PMC free article: PMC9156669] [PubMed: 35641533]
- 24.
- Feldmann R, Wahl S, Steiner A. Normoglycemic glucagonoma syndrome associated with necrolytic migratory erythema. J Eur Acad Dermatol Venereol. 2018;32(8):e306–e307. [PubMed: 29419911]
- 25.
- Banerjee A, Shah SR. Necrolytic Migratory Erythema Associated with a Glucagonoma. N Engl J Med. 2020;383(6):e39. [PubMed: 32757527]
- 26.
- Halvorson SA, Gilbert E, Hopkins RS, Liu H, Lopez C, Chu M, Martin M, Sheppard B. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28(11):1525–1529. [PMC free article: PMC3797362] [PubMed: 23681843]
- 27.
- Gaiser CA, Dhawan N. Paradoxical presentation of glucagonoma with delayed onset of necrolytic migratory erythema. Am J Med. 2015;128(2):e1–2.
- 28.
- Wermers RA, Fatourechi V, Wynne AG, Kvols LK, Lloyd RV. The glucagonoma syndrome. Clinical and pathologic features in 21 patients. Medicine (Baltimore). 1996;75(2):53–63. [PubMed: 8606627]
- 29.
- Alexandraki KI, Kaltsas G, Grozinsky-Glasberg S. Emerging therapies for malignant insulinomas and glucagonomas. Endocr Relat Cancer. 2023
- 30.
- Crona J, Norlén O, Antonodimitrakis P, Welin S, Stålberg P, Eriksson B. Multiple and Secondary Hormone Secretion in Patients With Metastatic Pancreatic Neuroendocrine Tumours. J Clin Endocrinol Metab. 2016;101(2):445–452. [PubMed: 26672633]
- 31.
- de Mestier L, Hentic O, Cros J, Walter T, Roquin G, Brixi H, Lombard-Bohas C, Hammel P, Diebold MD, Couvelard A, Ruszniewski P, Cadiot G. Metachronous hormonal syndromes in patients with pancreatic neuroendocrine tumors: a case-series study. Ann Intern Med. 2015;162(10):682–689. [PubMed: 25984844]
- 32.
- Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol. 2012;26(6):737–753. [PMC free article: PMC3627221] [PubMed: 23582916]
- 33.
- Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, Hassan M, Evans DB. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14(12):3492–3500. [PMC free article: PMC2077912] [PubMed: 17896148]
- 34.
- Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ. Glucagonoma and the glucagonoma syndrome - cumulative experience with an elusive endocrine tumour. Clin Endocrinol (Oxf). 2011;74(5):593–598. [PubMed: 21470282]
- 35.
- Kindmark H, Sundin A, Granberg D, Dunder K, Skogseid B, Janson ET, Welin S, Oberg K, Eriksson B. Endocrine pancreatic tumors with glucagon hypersecretion: a retrospective study of 23 cases during 20 years. Med Oncol. 2007;24(3):330–337. [PubMed: 17873310]
- 36.
- Keutgen XM, Nilubol N, Kebebew E. Malignant-functioning neuroendocrine tumors of the pancreas: A survival analysis. Surgery. 2016;159(5):1382–1389. [PubMed: 26704781]
- 37.
- Song L, Zhai X, Yu S, Ma Y, Wang F, Yu X, Tao S, Lian Y, Yang M, Tao W, Fan Q. Clinical analysis of 547 patients with neuroendocrine tumors in a Chinese population: A single-center study. Cancer Med. 2019;8(8):3729–3737. [PMC free article: PMC6639184] [PubMed: 31127690]
- 38.
- Lévy-Bohbot N, Merle C, Goudet P, Delemer B, Calender A, Jolly D, Thiéfin G, Cadiot G. Prevalence, characteristics and prognosis of MEN 1-associated glucagonomas, VIPomas, and somatostatinomas: study from the GTE (Groupe des Tumeurs Endocrines) registry. Gastroenterol Clin Biol. 2004;28(11):1075–1081. [PubMed: 15657529]
- 39.
- Pieterman CRC, van Leeuwaarde RS, van den Broek MFM, van Nesselrooij BPM, Valk GD. Multiple Endocrine Neoplasia Type 1. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000.
- 40.
- Yu R. Mahvash Disease: 10 Years After Discovery. Pancreas. 2018;47(5):511–515. [PubMed: 29702528]
- 41.
- Sipos B, Klöppel G. Glucagon cell hyperplasia and neoplasia: a recently recognized endocrine receptor disease. Endocr Relat Cancer. 2023
- 42.
- Sipos B, Sperveslage J, Anlauf M, Hoffmeister M, Henopp T, Buch S, Hampe J, Weber A, Hammel P, Couvelard A, Höbling W, Lieb W, Boehm BO, Klöppel G. Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J Clin Endocrinol Metab. 2015;100(5):E783–788. [PubMed: 25695890]
- 43.
- Henopp T, Anlauf M, Schmitt A, Schlenger R, Zalatnai A, Couvelard A, Ruszniewski P, Schaps KP, Jonkers YM, Speel EJ, Pellegata NS, Heitz PU, Komminoth P, Perren A, Klöppel G. Glucagon cell adenomatosis: a newly recognized disease of the endocrine pancreas. J Clin Endocrinol Metab. 2009;94(1):213–217. [PubMed: 18957496]
- 44.
- Boden G, Owen OE. Familial hyperglucagonemia--an autosomal dominant disorder. N Engl J Med. 1977;296(10):534–538. [PubMed: 189188]
- 45.
- Stacpoole PW. The glucagonoma syndrome: clinical features, diagnosis, and treatment. Endocr Rev. 1981;2(3):347–361. [PubMed: 6268399]
- 46.
- Corrias G, Horvat N, Monti S, Basturk O, Lin O, Saba L, Bodei L, Reidy DL, Mannelli L. Malignant transformation of glucagonoma with SPECT/CT In-111 OctreoScan features: A case report. Medicine (Baltimore). 2017;96(50):e9252. [PMC free article: PMC5815774] [PubMed: 29390362]
- 47.
- Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, Brandau W, Bockisch A, Boy C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52(12):1864–1870. [PubMed: 22072704]
- 48.
- Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense RT, Kulke M, Oeberg K, Rindi G, Sorbye H, Welin S. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology. 2017;105(3):196–200. [PubMed: 28190015]
- 49.
- de Herder WW, Zandee WT, Hofland J. Insulinoma. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000.
- 50.
- Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33(1):115–154. [PubMed: 35294740]
- 51.
- Thomaidou E, Nahmias A, Gilead L, Zlotogorski A, Ramot Y. Rapid Clearance of Necrolytic Migratory Erythema Following Intravenous Administration of Amino Acids. JAMA Dermatol. 2016;152(3):345–346. [PubMed: 26581030]
- 52.
- Jin XF, Spampatti MP, Spitzweg C, Auernhammer CJ. Supportive therapy in gastroenteropancreatic neuroendocrine tumors: Often forgotten but important. Rev Endocr Metab Disord. 2018;19(2):145–158. [PubMed: 29464446]
- 53.
- Doherty GM. Rare endocrine tumours of the GI tract. Best Pract Res Clin Gastroenterol. 2005;19(5):807–817. [PubMed: 16253902]
- 54.
- Saavedra C, Lamarca A, Hubner RA. Resolution of necrolytic migratory erythema with somatostatin analogue in a patient diagnosed with pancreatic glucagonoma. BMJ Case Rep. 2019;12(8)
- 55.
- Lo CH, Ho CL, Shih YL. Glucagonoma with necrolytic migratory erythema exhibiting responsiveness to subcutaneous octreotide injections. Qjm. 2014;107(2):157–158. [PubMed: 23389434]
- 56.
- Grozinsky-Glasberg S, Shimon I, Korbonits M, Grossman AB. Somatostatin analogues in the control of neuroendocrine tumours: efficacy and mechanisms. Endocr Relat Cancer. 2008;15(3):701–720. [PubMed: 18524947]
- 57.
- Mavi ME, Tuncel M. Treatment of Glucagonoma-Related Necrolytic Migratory Erythema With Peptide Receptor Radionuclide Therapy. Clin Nucl Med. 2021;46(12):1002–1003. [PubMed: 34034327]
- 58.
- Zandee WT, Brabander T, Blazevic A, Kam BLR, Teunissen JJM, Feelders RA, Hofland J, de Herder WW. Symptomatic and Radiological Response to 177Lu-DOTATATE for the Treatment of Functioning Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab. 2019;104(4):1336–1344. [PubMed: 30566620]
- 59.
- Norton JA, Kahn CR, Schiebinger R, Gorschboth C, Brennan MF. Amino acid deficiency and the skin rash associated with glucagonoma. Ann Intern Med. 1979;91(2):213–215. [PubMed: 111595]
- 60.
- Cao X, Wang X, Lu Y, Zhao B, Shi J, Guan Q, Zhang X. Spleen-preserving distal pancreatectomy and lymphadenectomy for glucagonoma syndrome: A case report. Medicine (Baltimore). 2019;98(38):e17037. [PMC free article: PMC6756711] [PubMed: 31567941]
- 61.
- Wei J, Song X, Liu X, Ji Z, Ranasinha N, Wu J, Miao Y. Glucagonoma and Glucagonoma Syndrome: One Center's Experience of Six Cases. J Pancreat Cancer. 2018;4(1):11–16. [PMC free article: PMC5999015] [PubMed: 30631852]
- 62.
- Gaujoux S, Gonen M, Tang L, Klimstra D, Brennan MF, D'Angelica M, Dematteo R, Allen PJ, Jarnagin W, Fong Y. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol. 2012;19(13):4270–4277. [PubMed: 22752376]
- 63.
- Kimbara S, Fujiwara Y, Toyoda M, Chayahara N, Imamura Y, Kiyota N, Mukohara T, Fukunaga A, Oka M, Nishigori C, Minami H. Rapid improvement of glucagonoma-related necrolytic migratory erythema with octreotide. Clin J Gastroenterol. 2014;7(3):255–259. [PubMed: 26183746]
- 64.
- Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK. OʼDorisio TM, Halperin DM, Fishbein L, Eads J, Hope TA, Singh S, Salem R, Metz DC, Naraev BG, Reidy-Lagunes DL, Howe JR, Pommier RF, Menda Y, Chan JA. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(7):863–881. [PubMed: 32675783]
- 65.
- Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–860. [PubMed: 32272208]
- 66.
- Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. [PubMed: 25014687]
- 67.
- Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res. 2017;23(16):4617–4624. [PubMed: 28428192]
- 68.
- Makis W, McCann K, Riauka TA, McEwan AJ. Glucagonoma Pancreatic Neuroendocrine Tumor Treated With 177Lu DOTATATE Induction and Maintenance Peptide Receptor Radionuclide Therapy. Clin Nucl Med. 2015;40(11):877–879. [PubMed: 26204206]
- 69.
- Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. 2016;34(32):3906–3913. [PMC free article: PMC5791842] [PubMed: 27621394]
- 70.
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [PMC free article: PMC4208619] [PubMed: 21306238]
- 71.
- Pavel ME, Chen D, He W, Cushman S, Voi M, de Vries EGE, Baudin E, Yao JC. Everolimus Effect on Gastrin and Glucagon in Pancreatic Neuroendocrine Tumors. Pancreas. 2017;46(6):751–757. [PubMed: 28609362]
- 72.
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. [PubMed: 21306237]
- 73.
- Fazio N, Kulke M, Rosbrook B, Fernandez K, Raymond E. Updated Efficacy and Safety Outcomes for Patients with Well-Differentiated Pancreatic Neuroendocrine Tumors Treated with Sunitinib. Target Oncol. 2021;16(1):27–35. [PMC free article: PMC7810649] [PubMed: 33411058]
- 74.
- Garcia-Carbonero R, Rinke A, Valle JW, Fazio N, Caplin M, Gorbounova V. J OC, Eriksson B, Sorbye H, Kulke M, Chen J, Falkerby J, Costa F, de Herder W, Lombard-Bohas C, Pavel M, Antibes Consensus Conference p. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2: Chemotherapy. Neuroendocrinology. 2017;105(3):281–294. [PubMed: 28380493]
- 75.
- Garcia-Carbonero R, Anton-Pascual B, Modrego A, Riesco-Martinez MDC, Lens-Pardo A, Carretero-Puche C, Rubio-Cuesta B, Soldevilla B. Advances in the Treatment of Gastroenteropancreatic Neuroendocrine Carcinomas: are we moving forward? Endocr Rev. 2023
- 76.
- Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, Davidson B, Siegler M, Caplin M, Solcia E, Schilsky R. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15(1):e8–21. [PubMed: 24384494]
- Characteristics and treatment options of glucagonomas: a national study from the French Group of Endocrine Tumors and ENDOCAN-RENATEN network.[Eur J Endocrinol. 2023]Characteristics and treatment options of glucagonomas: a national study from the French Group of Endocrine Tumors and ENDOCAN-RENATEN network.Perrier M, Brugel M, Gérard L, Goichot B, Lièvre A, Lepage C, Hautefeuille V, Do Cao C, Smith D, Thuillier P, et al. Eur J Endocrinol. 2023 Dec 6; 189(6):575-583.
- Endocrine pancreatic tumors with glucagon hypersecretion: a retrospective study of 23 cases during 20 years.[Med Oncol. 2007]Endocrine pancreatic tumors with glucagon hypersecretion: a retrospective study of 23 cases during 20 years.Kindmark H, Sundin A, Granberg D, Dunder K, Skogseid B, Janson ET, Welin S, Oberg K, Eriksson B. Med Oncol. 2007; 24(3):330-7.
- Dermatitis, glossitis, stomatitis, cheilitis, anemia and weight loss: a classic presentation of pancreatic glucagonoma.[W V Med J. 2002]Dermatitis, glossitis, stomatitis, cheilitis, anemia and weight loss: a classic presentation of pancreatic glucagonoma.Povoski SP, Zaman SA, Ducatman BS, McFadden DW. W V Med J. 2002 Jan-Feb; 98(1):12-4.
- Review Glucagonoma syndrome.[Am J Med. 1987]Review Glucagonoma syndrome.Bloom SR, Polak JM. Am J Med. 1987 May 29; 82(5B):25-36.
- Review Insulinoma.[Endotext. 2000]Review Insulinoma.de Herder WW, Hofland J. Endotext. 2000
- Glucagon & Glucagonoma Syndrome - EndotextGlucagon & Glucagonoma Syndrome - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
