NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Benign breast disease in women is a very common finding and results in a diagnosis in approximately one million women annually in the United States (1). An understanding of the hormonal and growth factor control of breast development and function is key to the rational and systematic evaluation and treatment of patients. A firm understanding of benign breast disease is important since sequential steps are necessary to distinguish lesions which impart a high risk of subsequent breast cancer from those which do not. This chapter will review the physiology of breast function, provide histologic examples of common lesions, and detail practical approaches to evaluation and treatment. For complete coverage of all related areas of Endocrinology, please see our online FREE web-book, www.endotext.org.
BREAST PHYSIOLOGY IN WOMEN:
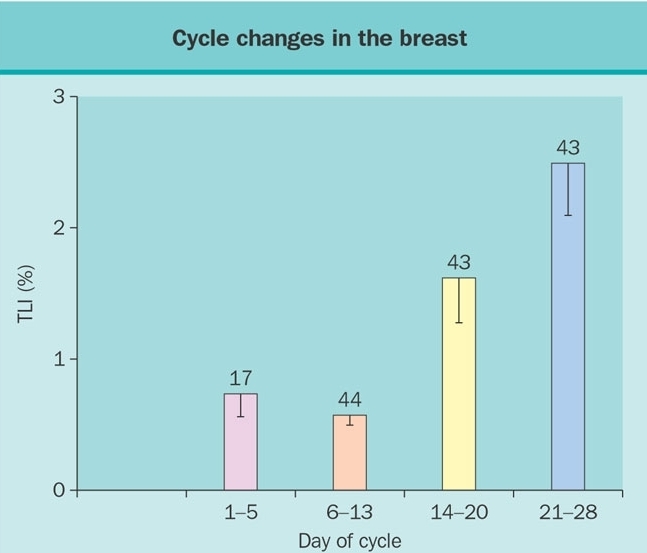
Hormones and growth factors act upon stromal and epithelial cells to regulate mammary gland development, maturation and differentiation (2). Broadly summarized, estrogen mediates development of ductal tissue; progesterone facilitates ductal branching and lobulo-alveolar development; and prolactin regulates milk protein production. At puberty, estradiol and progesterone levels increase to initiate breast development. A complex tree-like structure results and comprises 5 to 10 primary milk ducts originating at the nipple, 20 to 40 segmental ducts, and 10 to 100 sub-segmental ducts ending in glandular units called terminal duct lobular units (TDLUs) (3). During the menstrual cycle the increments in estrogen and progesterone stimulate cell proliferation during the luteal phase (Figure1). Cycle dependent apoptosis balances proliferation (4). In response to enhanced proliferation, the breast can increase by as much as 15% in size during the luteal phase.
SPECIFIC BENIGN BREAST LESIONS:
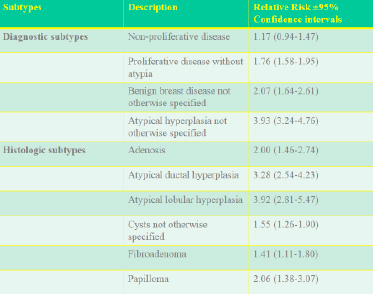
A wide variety of benign breast lesions have been described and the histologic appearance fully characterized. On a practical basis, these can be subdivided into those associated with no substantial increased risk of breast cancer ( i.e. < 1.49%), those with an increase of 1.5-2% and those with a >2% increase (Table 1) (13) .
Table 1. Relative risk of breast cancer imparted by specific benign breast lesions (Data from Reference 13)
No Increased risk:
The more common lesions Included in this category are fibrocystic changes, periductal fibrosis, hamartomas, lipomas, phylloides tumors, and neurofibromas. Another lesion is duct ectasia (Figure 5), characterized by distention of subareolar ducts and presence within them of yellowish-orange material. Penetration of the duct wall by this material may produce acute inflammatory changes in the surrounding tissues. Histologically, crystalline oval and round structures thought to be lipid in origin are present. Resolution may occur but residual periductal fibrosis and nodule formation often persist. This lesion is thought to be more frequent in cigarette smokers and represents a more chronic and extensive inflammatory process. A less common lesion is diabetic mastopathy which consists of localized or diffuse areas of fibrosis occurring in patients with diabetes (14).
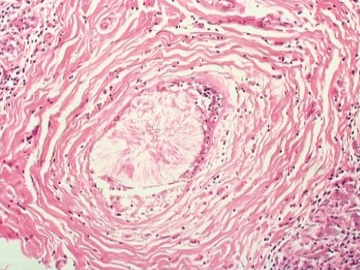
Figure 5Histological appearance of duct ectasia showing the characteristic crystalline formation of the intraluminal contents.
Abnormalities of ductal secretion may result in discharge of clear, cloudy, blue, green or black aqueous material. Stromal hyperplasia can result in nipple retraction or in palpable lesions requiring biopsy to distinguish from breast carcinoma. Histologically, the tissue may contain fibroblasts nearly exclusively or predominantly fibroblasts with admixture of glandular epithelium. Some degree of stromal hyperplasia may occur in 50-60% of normal women, particularly those in their middle and late reproductive periods. Traumatic lesions (hematomas and fat necrosis) and inflammatory conditions (granulomas and mastitis) are also not associated with an increased breast cancers (15).
1.5 To 2.0 Fold Increase In Relative Risk:
Fibroadenomas:During the early reproductive period, glandular components of the breast may respond to cyclic hormonal stimuli in an exaggerated fashion with the development of single fibroadenomas. These consist of lobular units which grow to larger than normal size and contain both epithelial and stromal elements. Fibroadenomas range in size from slightly larger than a normal single lobular unit to larger, more discrete palpable lesions. When exceeding 5 cm, these are called giant fibroadenomas which are seen primarily at puberty or during pregnancy. The incidence of fibroadenomas peaks at age 20-24 (Figure 6). The prevalence on physical examination in young women is 2% in young women but 15-23% when evaluated prospectively at autopsy (16). When complex and containing cysts >3mm in diameter, sclerosing adenosis, epithelial calcification or papillary changes, these lesions are associated with an increased risk of breast cancer (17) (Table 1).

Figure 6
Incidence of fibrocystic breast changes and fibroadenoma by age group. (Reprinted with permission of the publisher and authors from Goehring C, Morabia A. Epidemiology of Bengin Breast Disease, with special attention to histologic types Epidemiologic Reviews 19:310-327, 1997) (16). Note that the term “fibrocystic disease” is used in the legend but this term has now been changed to “fibrocystic changes”.
Other lesions:
Hyperplasia without atypia, papillomas, papillomatosis, radial scar, blunt duct adenosis, and sclerosing adenosis are also associated with an increased risk.
Greater Than A Two-Fold Risk:
Atypical hyperplasia
This lesion can be subdivided into atypical ductal hyperplasia (figure 7) and atypical lobular hyperplasia but both impart a similar increase in relative risk of breast cancer of 3 to 4 fold (18) (Table 1). In the first ten years after diagnosis of AH, the breast tumors occur predominantly in the same breast but subsequently, occur with equal frequency in the contralateral as in the ipsilateral breast (19). These observations suggest the AH is both a precursor lesion for breast cancer and a marker of an underlying predisposing condition termed a “field defect” or alternatively a “mutator phenotype (15) When the amount of hyperplastic tissue increases according to specific criteria (20) the lesions are no longer called benign but are classified as ductal carcinoma in situ. Recent emphasis has been directed toward identification of molecular genetic markers which could predict which patients with AH are at increased risk of developing breast cancer. Over-expression of HER-2/neu is one such marker (21). The presence of aberrant p53, p21, interleukin 6, and TNF alpha and aneuploidly on flow cytometery have also been reported as markers of increased risk (22).
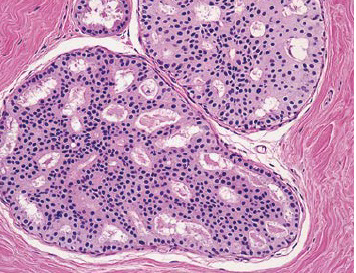
Figure 7Photomicrograph of atypical ductal hyperplasia histology reprinted from Hartmann LC et al (23).
Recent data from the Mayo Clinic report the absolute rates of breast cancer during prolonged 20+ year follow-up and the important role of multifocality (23;24). As shown in Figure 8, the risk approximates 24% with one lesion, 36% with two and 47% with 3 or more lesions. The increased risk with multifocality has been confirmed in an independent cohort follow at Vanderbilt (18;19). Although previous studies indicated that family history added to this risk (18), the updated data suggest that neither family history or other risk factors independently contribute to breast cancer risk (23). It is important to emphasize the AH appears to impart a risk of breast cancer which is lower but may approach that from BrCa1 and 2 mutations. As noted above, the presence of atypical hyperplasia imparts a risk over time of both ipsilateral and contralateral breast cancer (Figure 9).
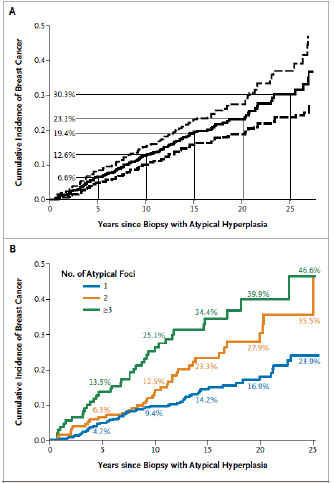
Figure 8
Incidence of breast cancer in cohort of patients with a histologic diagnosis of atypical hyperplasia. Top panel: mean (solid line) and 95% confidence limits (dashed lines). Bottom panel: Incidence in patients with 1, 2, or 3 or more independent lesions. Reprinted with the permission of the authors and publisher from Hartmann LC et al (23).
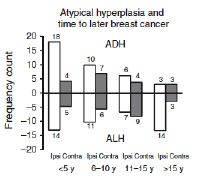
Figure 9
Ipsilateral versus contralateral distribution of breast cancers diagnosed over time in patients initially diagnosed with atypical hyperplasia. Reproduced with the permission of the authors and publishers from Hartmann LC et al (24).
Lobular Carcinoma in situ:
Even though called lobular carcinoma in situ, this lesion is not generally considered to have reached the neoplastic stage but is analogous to atypical hyperplasia. This lesion imparts approximately a 10 fold increase in relative risk of breast cancer developing over time. The subsequent breast cancer can occur in the same or contralateral breast; accordingly, this lesion is thought to represent a filed defect or mutator phenotype which increases breast cancer risk but the lesion itself is not a precursor of breast cancer.
Mammographic Density And Breast Cancer Risk:
The percent breast density on mammography correlates with breast cancer risk as shown on Figure 10. When comparing the lowest density category with highest, the relative risk is increased by 5.3 fold. Recent studies examined the histologic composition of dense and non-dense breast tissue. When dense lesions are biopsied and compared to areas of low density, they are found to contain a higher proportion of stroma and glandular tissue and lesser amount of fat (26). Notably, dense lesions contain more of the enzyme aromatase, when quantitated by an immunologic histologic score after staining with an aromatase monoclonal antibody (27). These findings are likely associated with a higher local production of estradiol and could explain the higher incidence of breast cancer with both of these lesions. Taking these observations together, determination of breast density assessed by Bi-RADS criteria or quantitated with a computer assisted method of determining breast density, provides the most powerful means of predicting risk of breast cancer over time (Figure 10).
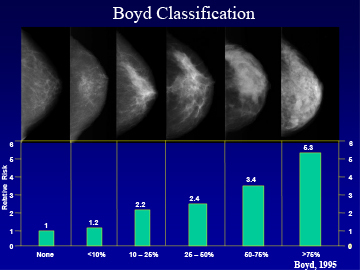
Figure 10
The percentage of breast tissue which is dense on a mammogram is determined by computer assisted analysis and classified as none, <10%, 10-25%, 25-50%, 50-75%, >75%.The relative risk of breast cancer increases with each step of increased breast density. Adapted from that data of Boyd et al (25).
ETIOLOGY OF BENIGN BREAST DISORDERS
Clinical observations in women receiving estrogens and anti-estrogens suggest that hormonal events play a role in the etiology of benign breast lesions. In post-menopausal women receiving estrogens ± progestins for >8 years, the prevalence of benign breast lesions increased by 1.7 fold (95 percent CI 1.06-2.72) (28). In the Women’s Health Initiative study (WHI), the use of estrogen plus progestin was associated with a 74% increase in the risk of benign proliferative breast disease [hazard ratio, 1.74; 95% confidence interval (CI), 1.35-2.25] (29). The anti-estrogen, tamoxifen, when used for breast cancer prevention, was associated with a 28 percent (RR 0.72, 95 percent Confidence Intervals 0.65-0.79) reduction in prevalence of benign breast lesions, including adenosis, cysts, duct ectasia, and hyperplasia (30).
Underlying and acquired genetic changes are also associated with benign breast lesions. Loss of heterozygosity (LOH), a finding caused by deletions of small segments of DNA (31;32) is commonly found in benign breast lesions. Women frequently have multi-focal lesions, each of which exhibit loss of heterozygosity (LOH) of differing regions of DNA. Women with BRCA1/2 mutations are found to have a high frequency of multiple benign or malignant breast lesions when bilateral mastectomy specimens are meticulously examined (33). These findings support the current theory of an underlying predisposition to mutations in some patients as the cause of multiple breast lesions (34). In the past, this phenomenon was termed a “field effect” and more recently, a “mutator phenotype” (34).
CLINICAL MANIFESTATIONS:
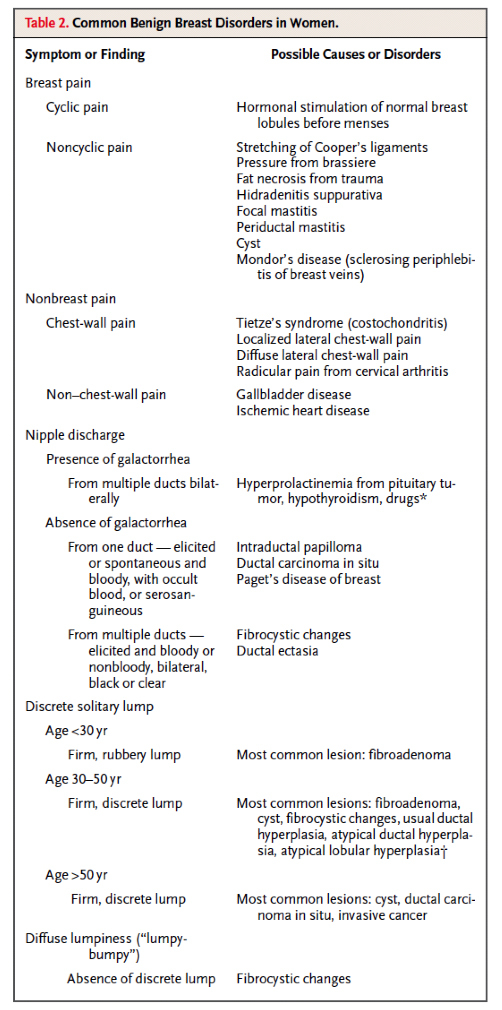
Clinical presentations of benign breast disease are divided into those with pain, lumps, or breast discharge (Table 2).
Breast Pain:
Cyclic breast pain
Usually occurs during the late luteal phase of the menstrual cycle, in association with the premenstrual syndrome or independently (35-40), and resolves at the onset of menses (35;36;38). A recent study in 1171 healthy American women indicated that 11% experience moderate to severe cyclic breast pain and 58%, mild discomfort (39). Breast pain interfered with usual sexual activity in 48% and with physical (37%), social (12%), and school (8%) activity in others. A role for caffeine, iodine deficiency, alterations in fatty acid levels in the breast, fat intake in the diet, and psychological factors in the etiology of breast pain remain unproven. Non-cyclic breast pain is unrelated to the menstrual cycle. Detection of focal tenderness is helpful diagnostically and suggests a tender cyst, rupture through the wall of an ectatic duct, or a particularly tender area of breast nodularity. Acute enlargement of cysts and periductal mastitis may cause severe, localized pain of sudden onset.
Non-breast pain:
When arising from the chest wall, pain may be mistakenly attributed to the breast. Pain localized to a limited area and characterized as burning or knife like in nature suggests this possibility. Several distinct subtypes can be distinguished including localized or diffuse lateral chest wall pain, radicular pain from cervical arthritis and Tietze’s syndrome or costochondritis. The method to distinguish between breast and non-breast pain by careful physical examination is illustrated on Figure 11, panels A-D.
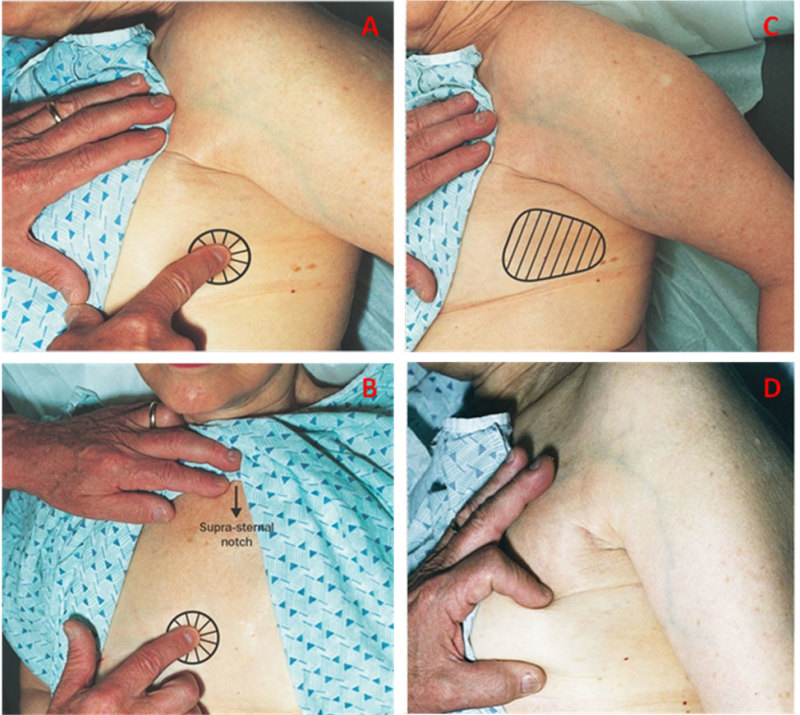
Figure 11. A
Focal chest wall pain—lateral. The patient is turned 90º on her side so that breast tissue is no longer under the area of palpation. The index finger elicits a focal area of pain. B. Focal chest wall pain over costochondral junctions anteriorly. C. Diffuse lateral chest wall pain. With the patient turned over 90 degrees on her side, pain is elicited over a wider area of the chest wall. D. Verification that squeezing breast tissue does not elicit pain ensures that the pain is not related to the breast but represents chest wall pain.
Breast Nodule:
Proliferation of ductal or lobular tissue causes histologic changes that are manifested by the presence of palpable lumps or nodules. Patients often present with the finding of a new breast nodule on self-exam or are found to have a lump by their health care provider. Ninety percent of these new nodules in premenopausal women are benign and usually represent fibroadenomas in the early reproductive period (16). In the middle reproductive period, focal areas of fibrosis, hyperplasia, or cyst formation are more likely. In the later reproductive period, hyperplasia, cysts, and carcinoma in situ are more common. Some lesions present with symptoms suggesting the cause. With duct ectasia, penetration of the duct wall by lipid material may be associated with a nodule exhibiting acute redness, and causing pain, and fever; after resolution, a subareolar nodule persists.
Other specific lesions present as lumps. These include multiple papillomas, sclerosing adenosis, and radial scars. Multiple papillomas may present as breast lumps, nodules on ultrasound, or may be the cause of bloody nipple discharge and can be seen on ductography. Sclerosing adenosis is a lobular lesion with increased fibrous tissue and interspersed glandular cells. It can present as a mass or a suspicious finding on mammograms. Radial scars are a pathologic diagnosis, usually diagnosed following mammography or palpation and then biopsy. Radial scars are characterized microscopically by a fibroelastic core with radiating ducts and lobules and impart a minimally increased risk of breast cancer similar to that of proliferative changes without atypia (41).
Nipple discharge:
6.8 percent of referrals to physicians for breast concerns result from this symptom. Although particularly distressing to the patient, only 5 percent are found to have serious underlying pathology. Age is an important factor with respect to risk of malignancy (42). In one series, 3 percent of women younger than age 40, 10 percent between 40 and 60 and 32 percent >60 with nipple discharge as their only symptom were found to have a malignancy.
A careful history characterizes breast discharge as either spontaneous or expressible. On examination, one can detect by careful inspection whether the discharge emanates from a single or multiple ducts. Nipple discharge can be divided into physiologic and pathologic types. Characteristics of physiologic discharge include non-spontaneous, multiple duct, bilateral, and non-bloody. Pathologic discharge is characterized as spontaneous, serous or bloody, usually unilateral and usually single duct. Reassuring characteristics are that it must be expressed; is green yellow, brown or milky; that it is bilateral and involves multiple ducts. Spontaneous discharge, whether serous or bloody, requires careful evaluation. A hemoccult card or urine dipstick can be used to test for occult blood if the discharge is spontaneous, unilateral, and from one duct. Cytologic examination is not recommended. Milky discharge (galactorrhea) should be evaluated with measurement of a serum prolactin level. If the discharge is physiologic and the patient is under 35, only reassurance is necessary. Screening mammogram is recommended for patients over 35 with physiologic discharge. Pathologic discharge requires diagnostic mammogram, galactography (Figure 12) (43), and referral to a surgeon.
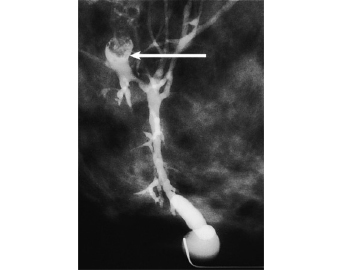
Figure 12
Galactogram illustrating space occupying lesion. A catheter is inserted into the duct from which the bloody discharge emerges. Contrast material is then injected through the catheter. The various branches of the duct are outlined.
The presence of crusting, scaling, and flaking of the nipple could be a manifestation of Paget’s disease of breast and underlying cancer or of dermatologic problems. The approach is to obtain a history of other dermatologic problems, or a history of change in soap or clothing. If absent, a diagnostic mammogram should be obtained if the patient is over 35. In a large recent series of 1251 patients with nipple discharge, 433 had unilateral discharge and 194 had no breast lump in association with this symptom. Of these, the lesions found included solitary papilloma (n=49), minimal breast cancer (n=20), fibrocystic disease(n=11), papillomatosis (n=7), lobular cancer (n=5) and duct ectasia (n=2)(42) (30A). For women with bloody discharge from a single duct, galactography is warranted. Filling defects can be due to intraductal papilloma, intraductal carcinoma, papillomatosis, debris, or air bubbles.
APPROACH TO THE PRACTICAL MANAGEMENT OF BENIGN BREAST DISEASE
A detailed history and physical exam systematically evaluates the entire breast and chest wall and focuses on areas involving the patient’s symptoms (Table 3). Diagnostic studies may then be ordered. For lumps, “The Triple Test” is recommended which includes palpation, imaging and percutaneous biopsy (either core or fine needle aspirate <FNA>). Mammography, often in conjunction with ultrasound examination (44-47) is required for evaluation of discrete palpable lesions in women over 35 whereas ultrasound provides an optional substitute in younger women (43). Round dense lesions on mammography often represent cysts which require only ultrasonography to distinguish them from solid lesions. Complex cysts containing both fluid and solid matter require biopsy. For solid lesions, radiographically or ultrasonically directed core biopsy provides highly discriminative information regarding the presence or absence of malignancy. Core biopsy utilizes a large cutting needle deployed with a spring loaded, automated biopsy instrument and obtains tissue suitable for histologic analysis familiar to most pathologists. FNA frequently yields sufficient cellular material to allow adequate cytologic evaluation but requires an experienced cytopathologist. However, the amount of material obtained is insufficient to render a diagnosis in 35-47% of non-palpable lesions (48) and core biopsy is recommended. The exact role of MRI in evaluating breast lesions is currently being determined (49). Galactography (ductography) is useful for detection of focal lesions in a single duct. Cytology of nipple discharge is of limited value with the sensitivity of detecting malignancy only 35 to 47 percent (50).
Diagnostic Procedures
Ideally a team including a radiologist experienced in mammography, ultrasound, MRI and core needle biopsy as well as an internist, gynecologist, or surgeon with expertise in breast diseases should be involved in the evaluation of patients with breast disorders. MRI mammography, ductography, or ultrasound may be utilized (Figures 12 and 13). Information to be obtained by a focused history and physical examination are outlined on Table 3. The method of documenting whether breast pain is chest wall related is illustrated on Figure 11 A-D. Imaging has become an integral part of the management of benign breast disorders.
Imaging studies:
Mammography is useful for evaluation of palpable lesions, particularly in those over 35. Digital mammography is preferred because of its ability to penetrate through dense breast tissue which is commonly found in younger women. Ultrasound is often used as initial evaluation of a palpable mass in women under age 35. If a simple cyst is present, no further evaluation is necessary (Figure 13). If not, mammography may also be necessary to fully evaluate the lump. If the mass has findings suggestive of a fibroadenoma by ultrasound and mammography, short term follow-up and re-imaging can be considered (usually performed in 6 months). Experts are divided as to the necessity to biopsy all fibroadenomas. MRI is more sensitive than digital mammography but false positives are more common (49). Routine yearly MRI is now recommended for women whose lifetime risk of breast cancer is > 20%. As an illustration of its sensitivity, 3% of the contralateral breasts in women with diagnosed breast cancer are found to have a second lesion in the opposite breast when examined by MRI (49).
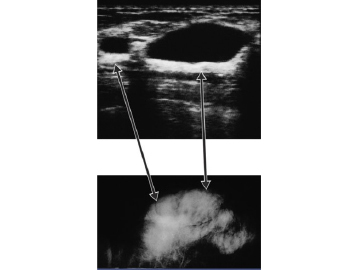
Figure 13
Upper panel illustrates by ultrasound a non-dense black area representing cyst fluid. The lower panel is the corresponding area on mammogram showing a dense area. With the combination of mammogram and ultrasound, the lesion can be shown to be a cyst.
Findings On Imaging Studies
Fibrocystic change typically presents on mammogram as round or oval, well defined masses that can be subsequently shown to represent cysts on ultrasound (Figure 13). Diffusely scattered dystrophic calcifications may also be found on the mammogram. Consequently, the goal of mammographic evaluation is to provide reassurance to the patient and physician that the risk of neoplasm is low. Aspiration of cysts is usually necessary only in a those cases where the mass does not fulfill all criteria for a simple cyst or if the cyst is painful. Biopsy may be necessary to confirm the benign nature of calcifications, particularly if clustered, linear or variously shaped.
For round masses or round calcifications on a first mammogram, the risk of cancer is less than 2%, and repeat mammography in 6 months is recommended. These lesions are termed “probably benign” using the lexicon terminology required by the Mammography Quality Standards Act (in the USA). If the risk is believed to be greater, core biopsy is recommended. Stereotactically directed core biopsy is ideal for evaluation of calcifications and provides highly discriminative information regarding the presence or absence of malignancy. If this technique is not available, insertion of a wire into the lesion radiographically followed by surgical excision or mere removal of a palpable lesion is warranted.
CLINICAL GUIDELINES FOR EVALUATION OF NODULES AND DISCHARGE
Careful examination distinguishes solitary, discrete, dominant, persistent masses from vague nodularity and thickening. Practice Guidelines of the Society of Surgical Oncology (51) recommend the following evaluation: In women less than age 35, all dominant discrete palpable lesions require referral to a surgeon. If vague nodularity, thickening or asymmetrical nodularity is present, the examination is repeated at midcycle after one or two menstrual cycles. If the abnormality resolves, the patient is reassured and if not, referred to a surgeon. Breast imaging may be appropriate. In women > age 35 with a dominant mass, a diagnostic mammogram (and frequently a sonogram) (44-47) is obtained and the patient referred to a surgeon. With vague nodularity or thickening, one obtains a mammogram with repeat physical exam at mid-cyle 1 to 2 months later and refers to surgeon if the abnormality persists. Post-menopausal women are referred for surgical consultation after a mammogram. For gross cysts (i.e. >4 cm), the guidelines suggest needle aspiration with repeat imaging within six months. If the aspirated fluid does not contain blood, the fluid is discarded without further histologic analysis unless the cyst contains solid components (i.e. complex cyst). If the fluid contains blood or if the cyst is complex, the, fluid is sent for cytology and consultation from a surgeon requested. With persistent refilling of the same cyst after aspiration, surgical consultation is warranted.
Usual practice requires “the triple test” with palpation, mammography (often in conjunction with ultrasonography) and biopsy in women over age 35 with dominant masses. When mammography is negative but a dominant mass is present, biopsy is required to rule out malignancy since lobular carcinoma may not be seen on mammograms. In those younger, mammography may be omitted if ultrasound and biopsy yield definitive information. Many experts omit biopsy in younger women with lesions characteristic of fibroadenoma on ultrasound and elect to follow carefully with serial ultrasounds at six monthly intervals for two years and yearly thereafter. Since careful studies have shown that a lesion appearing benign on mammography and ultrasound is benign >99 percent of the time, clinical judgment may allow follow-up without biopsy in experienced hands (44-47). However, other experienced surgeons disagree and believe that all fibroadenomas require diagnostic core biopsy or FNA and especially in BRCA mutation carriers in whom medullary cancer may be found. Biopsy confirmation of a fibroadenoma eliminates the need for serial ultrasounds. For those with a diagnosis of ADH (Atypical Ductal Hyperplasia) on FNA or core biopsy, excisional biopsy is then required since more complete resection often changes the diagnosis to DCIS.
Breast discharge is evaluated according to the algorithm illustrated below on Figure 14. Careful attention to several factors are necessary including determination whether the discharge arises from one duct or multiple ducts, is bloody, or is milky.
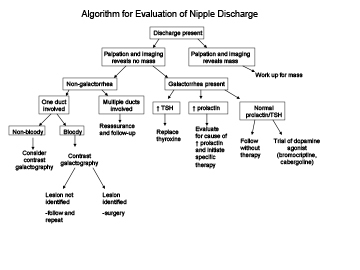
Figure 14Algorithm to assess breast discharge.
TREATMENT OF BENIGN BREAST DISEASE IN WOMEN
The initial step in evaluating pain is to distinguish true breast pain from chest wall pain (Figure 11). Several well designed, randomized, controlled, double blind, cross over trials have validated the efficacy of medical therapy for cyclic mastalgia. Based upon these studies, we categorize therapies as definitely effective, definitely ineffective, possibly effective, and insufficiently studied. For classification as definitely effective, two or more randomized trials are required. For the category, possibly effective, one randomized trial must be positive in some respect but others may be negative. For the category definitely ineffective, prospective trials must be uniformly negative. For the category, insufficiently studied, only one randomized trial, either negative or positive is available. For full details and references see Table 162-1 in Endocrinology, Fourth Edition, LJ DeGroot and JL Jameson, editors, WB Saunders Company, Philadelphia publishers; Chapter 162 Benign Breast Disorders, RJ Santen page 2194)
Danazol, bromocriptine, and tamoxifen have been proven to be effective (52-55) (Figure 15). Linoleic acid in the form of evening primrose oil has been shown effective in two randomized trials but not in the third, the largest trial. Its role in treatment therefore remains uncertain (56-58) . Vitamin E is considered definitely ineffective and iodine and vaginal progesterone possibly effective. Medroxyprogesterone acetate, caffeine avoidance, and progesterone have not been sufficiently studied. Several other therapies have not been examined in randomized controlled trials but are likely to be beneficial since they are based upon physiologic principles. For example, precise fitting of a bra to provide support for pendulous breasts has been reported to relieve pain in observational studies. GnRH agonist analogues are used to lower LH, FSH, and estradiol levels and to create a temporary post-menopausal state (59;60). Onset of menopause is known to reduce the frequency of breast pain. This therapy is reserved for patients in whom all other measures fail and the pain is considered severe. Reduction of the dosage of estrogens in post-menopausal women or addition of an androgen to estrogen replacement therapy (e.g. Estrotestâ tablets) appears to be beneficial in reducing breast pain (personal observations of author).
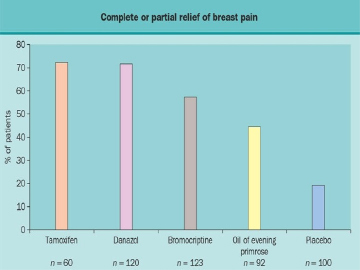
Figure 15
Relative efficacy of agents to treat breast pain. These data are from the Breast Clinic in Cardiff, Wales and represent observational studies and not randomized, controlled efficacy trails.
Relative efficacy of effective therapies:
No large randomized, controlled studies have compared the relative efficacy of danazol, bromocriptine, evening primrose oil and tamoxifen. Figure 15 rank orders them according to efficacy based upon data from individual reports from the same clinic. Minimal data are available from clinical trials which involve direct head to head comparisons. It should be noted that overall responses to danazol, bromocriptine and evening primrose oil are lower in those with non-cyclic pain than those with cyclic pain. However, not all studies have carefully excluded patients with non-breast pain and therefore conclusions regarding non-cyclic pain should be considered tentative. The approach to non-breast pain is outlined in Figure 16.
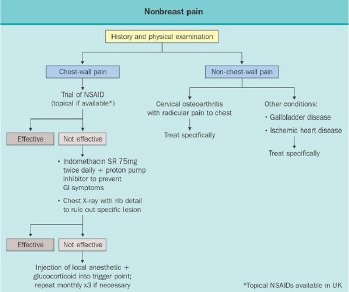
Figure 16Algorithm to treat non-breast pain
WOMEN AT HIGH RISK FOR BREAST CANCER
A major consideration for women who present with breast problems is whether they have a higher than normal risk of developing breast cancer. Certain breast lesions such as fibrocystic changes are associated with no increased risk of subsequent breast cancer (Table I). A 1.5 to 2-fold greater risk of development of breast cancer over a 20 year period of follow-up occurs with proliferative lesions including ductal hyperplasia, lobular hyperplasia without atypia, sclerosing adenosis, diffuse papillomatosis and complex fibroadenomas. A recent report also suggested that radial scars increase relative risk by 1.8, a risk similar to that found in proliferative disease without atypia. It should be noted that the Gail model for assessing breast cancer risk, which is based predominantly on reproductive factors, underestimates the long term risk of breast cancer in women with benign breast disease. On the other hand, the five year prediction is more accurate (61).
The presence of dense breast tissue on mammography has also been reported to be a predictor of increased incidence of breast cancer (Figure 10).Two components of this finding must be considered: one, the presence of high breast density makes it more difficult to read mammograms and masks the sensitivity of finding a breast cancer initially but identifies it later and two, there is an increased risk of breast cancer associated with increased breast density. With long tem follow-up studies, masking is not the explanation for the increased breast cancer risk (62).
According to classic twin studies, heritability accounts for approximately 60% of the variation in breast density (63;64). Breast cancer risk is also increased in association with high plasma estradiol and testosterone levels in postmenopausal women (62;65) and 20 kg or more weight gain (66) in the pre-menopausal years.
Another risk factor is use of hormone replacement therapy. Current data from the Women’s Health Initiative suggests a 26% increase in relative risk of breast cancer with conjugated equine estrogen (CEE) when combined with medroxyprogesterone acetate when used for an average of 7.1 years. (67). This risk is probably increased further in women starting this therapy shortly after the menopause (i.e. a short gap between onset of menopause and start of menopausal hormone therapy)(68). Starting this therapy a long time after experiencing menopause (long gap) is associated with a lesser relative risk (68). The use of estrogen alone in the WHI was associated with a trend toward reduction of risk of breast cancer at five years and a statistically significant reduction in those adhering to therapy (68). In a follow up report, after 5 years of use and 8 additional follow-up years, the reported decrease of 23% was statistically significant (69) (manson). Available data suggest that the effects of menopausal hormone therapy in the WHI is a class effect and not related to the specific type of estrogen or progestin. One study, however, suggests that use of crystalline progesterone as the progestogen is associated with a lesser risk than use of medroxyprogesterone acetate (70).
To aid in assessing breast cancer risk, a questionnaire developed by Gail, utilizes answers to 7 questions to calculate the 5 year and lifetime risk of developing breast cancer (71). This model has recognized deficiencies in that it does not consider second degree relatives with breast cancer, proliferative lesions of breast other than ADH, alcohol intake, obesity, or birth control pill and menopausal hormone therapy (MHT) use. Nonetheless, the Gail model has been prospectively validated in over 6000 women followed for an average of 4.5 years. A more recently developed model, the Tyrer–Cuzick (IBIS II) model (72) incorporates second degree relatives, obesity and use of MHT into its risk calculations and provides more robust information than the Gail model.
BREAST CANCER PREVENTION
Patients with benign breast lesions imparting an increased risk of breast cancer can be offered tamoxifen (or raloxifene) as a prevention strategy. The risk of breast cancer is determined using the Gail or Tyrer-Cuzick model and the benefits versus risks of tamoxifen evaluated. Risk factors not included in the Gail or Claus models include degree of breast density, plasma free estradiol levels, bone density, weight gain after menopause, and waist-hip ratio (25;65;66;73). Current recommendations suggest that women with a five year risk of breast cancer of over 1.67 percent and no contraindications to tamoxifen should be informed about the possibility of taking tamoxifen for five years (74-77). A recent overview has shown a 38 percent reduction of the relative risk of breast cancer with tamoxifen but benefits may be offset by increased risks of thromboembolic phenomena, endometrial cancer, and maturation of cataracts (78). The Star trial addressed whether raloxifene might be preferable to tamoxifen and (79) demonstrated relative equivalence. However, of interest was the fact that tamoxifen prevented more non-invasive breast cancers than did raloxifene (79). More intensive and frequent screening with multi-modality imaging (i.e. digital or standard mammography plus ultrasound or MRI) may be required in high risk patients. Atypical hyperplasia lesions appear to be more amenable to breast cancer prevention as reviewed from non-head-to-head studies showing prevention ranging from 62 to 75% reduction in four separate randomized studies (23) when compared to a 38% reduction in women selected to be at high risk based on reproductive factors (78).
REFERENCE LIST
(1) Figueroa JD, Pfeiffer RM, Brinton LA, Palakal MM, Degnim AC, Radisky D et al. Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: a nested case-control study. Breast Cancer Research & Treatment 2016;159(1):163-172.
(2) Russo J. Hormonal control of breast development. In: DeGroot LJ, Jameson JL, editors. Endocrinology. fourth edition ed. Philadelphia, PA: WB Saunders; 2001. p. 2181-2188.
(3) Osborne MP. Breast anatomy and development. In: Harris JR, Osborne CK, Morrow M, Lippman ME, editors. Diseases of the Breast. Philadelphia: Lippincott Williams and Wilkins; 2000. p. 1-13.
(4) Going JJ, Anderson TJ, Battersby S, MacIntyre CC. Proliferative and secretory activity in human breast during natural and artificial menstrual cycles. American Journal of Pathology 1988;130(1):193-204.
(5) Hughes LE, Mansel RE, Webster DJ. Aberrations of normal development and involution (ANDI): a new perspective on pathogenesis and nomenclature of benign breast disorders. Lancet 1987;2:1316-1319.
(6) Love SM, Gelman RS, Silen W. Sounding board. Fibrocystic "disease" of the breast--a nondisease? New England Journal of Medicine 1982;307(16):1010-1014.
(7) Pearlman MD, Griffin JL. Benign breast disease. [Review] [30 refs]. Obstetrics & Gynecology 2010;116(3):747-758.
(8) Baker SB, Burkey BA, Thornton P, LaRossa D. Juvenile gigantomastia: presentation of four cases and review of the literature. [Review] [16 refs]. Annals of Plastic Surgery 525;46(5):517-525.
(9) Huh SJ, Oh H, Peterson MA, Almendro V, Hu R, Bowden M et al. The Proliferative Activity of Mammary Epithelial Cells in Normal Tissue Predicts Breast Cancer Risk in Premenopausal Women. Cancer Research 2016;76(7):1926-1934.
(10) Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ et al. The genomic landscapes of human breast and colorectal cancers.[see comment]. Science 2007;318(5853):1108-1113.
(11) Bailey SL, Sigal BM, Plevritis SK. A simulation model investigating the impact of tumor volume doubling time and mammographic tumor detectability on screening outcomes in women aged 40-49 years. Journal of the National Cancer Institute 2010;102(16):1263-1271.
(12) Radisky DC, Visscher DW, Frank RD, Vierkant RA, Winham S, Stallings-Mann M et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Research & Treatment 2016;155(3):423-430.
(13) Dyrstad SW, Yan Y, Fowler AM, Colditz GA. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. [Review]. Breast Cancer Research & Treatment 2015;149(3):569-575.
(14) Kudva YC, Reynolds C, O'Brien T, Powell C, Oberg AL, Crotty TB. "Diabetic mastopathy," or sclerosing lymphocytic lobulitis, is strongly associated with type 1 diabetes. Diabetes Care 2002;25(1):121-126.
(15) Santen RJ, Mansel R. Benign breast disorders. [Review] [89 refs]. New England Journal of Medicine 2005;353(3):275-285.
(16) Goehring C, Morabia A. Epidemiology of benign breast disease, with special attention to histologic types. Epidemiologic Reviews 1997;19(2):310-327.
(17) Nassar A, Visscher DW, Degnim AC, Frank RD, Vierkant RA, Frost M et al. Complex fibroadenoma and breast cancer risk: a Mayo Clinic Benign Breast Disease Cohort Study. Breast Cancer Research & Treatment 2015;153(2):397-405.
(18) Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K et al. Benign breast disease and the risk of breast cancer.[see comment]. New England Journal of Medicine 2005;353(3):229-237.
(19) Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study.[see comment]. Journal of Clinical Oncology 2007;25(19):2671-2677.
(20) Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 1985;55(11):2698-2708.
(21) Stark A, Hulka BS, Joens S, Novotny D, Thor AD, Wold LE et al. HER-2/neu amplification in benign breast disease and the risk of subsequent breast cancer. Journal of Clinical Oncology 2000;18(2):267-274.
(22) Garcia-Tunon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. Role of tumor necrosis factor-alpha and its receptors in human benign breast lesions and tumors (in situ and infiltrative). Cancer Science 2006;97(10):1044-1049.
(23) Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast--risk assessment and management options. New England Journal of Medicine 2015;372(1):78-89.
(24) Hartmann LC, Radisky DC, Frost MH, Santen RJ, Vierkant RA, Benetti LL et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prevention Research 2014;7(2):211-217.
(25) Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention 1998;7(12):1133-1144.
(26) Ghosh K, Brandt KR, Reynolds C, Scott CG, Pankratz VS, Riehle DL et al. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Research & Treatment 2012;131(1):267-275.
(27) Vachon CM, Sasano H, Ghosh K, Brandt KR, Watson DA, Reynolds C et al. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Research & Treatment 2011;125(1):243-252.
(28) Rohan TE, Miller AB. Hormone replacement therapy and risk of benign proliferative epithelial disorders of the breast. European Journal of Cancer Prevention 1999;8(2):123-130.
(29) Rohan TE, Negassa A, Chlebowski RT, Lasser NL, McTiernan A, Schenken RS et al. Estrogen plus progestin and risk of benign proliferative breast disease. Cancer Epidemiology, Biomarkers & Prevention 2008;17(9):2337-2343.
(30) Tan-Chiu E, Wang J, Costantino JP, Paik S, Butch C, Wickerham DL et al. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. Journal of the National Cancer Institute 2003;95(4):302-307.
(31) Allred DC, Mohsin SK. Biological features of human pre-malignant breast disease. In: Harris JR, Osborne CK, Morrow M, Lippman ME, editors. Diseases of the Breast. 2nd Edition ed. Philadelphia: Lippincott Williams and Wilkins; 2000. p. 355-366.
(32) O'Connell P, Pekkel V, Fuqua SA, Osborne CK, Clark GM, Allred DC. Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. Journal of the National Cancer Institute 1998;90(9):697-703.
(33) Hoogerbrugge N, Bult P, Widt-Levert LM, Beex LV, Kiemeney LA, Ligtenberg MJ et al. High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. Journal of Clinical Oncology 2003;21(1):41-45.
(34) Lundin C, Mertens F. Cytogenetics of benign breast lesions. Breast Cancer Research & Treatment 1998;51(1):1-15.
(35) Khan SA, Apkarian AV. The characteristics of cyclical and non-cyclical mastalgia: a prospective study using a modified McGill Pain Questionnaire. Breast Cancer Research & Treatment 2002;75(2):147-157.
(36) Kessel B. Premenstrual syndrome. Advances in diagnosis and treatment. Obstetrics & Gynecology Clinics of North America 2000;27(3):625-639.
(37) Goodwin PJ, Neelam M, Boyd NF. Cyclical mastopathy: a critical review of therapy. British Journal of Surgery 1988;75(9):837-844.
(38) Goodwin PJ, Miller A, Del Giudice ME, Ritchie K. Breast health and associated premenstrual symptoms in women with severe cyclic mastopathy. American Journal of Obstetrics & Gynecology 1997;176(5):998-1005.
(39) Ader DN, Shriver CD. Cyclical mastalgia: prevalence and impact in an outpatient breast clinic sample. Journal of the American College of Surgeons 1997;185(5):466-470.
(40) Ader DN, South-Paul J, Adera T, Deuster PA. Cyclical mastalgia: prevalence and associated health and behavioral factors. Journal of Psychosomatic Obstetrics & Gynecology 2001;22(2):71-76.
(41) Berg JC, Visscher DW, Vierkant RA, Pankratz VS, Maloney SD, Lewis JT et al. Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Research & Treatment 2008;108(2):167-174.
(42) Seltzer MH, Perloff LJ, Kelley RI, Fitts WT, Jr. The significance of age in patients with nipple discharge. Surgery, Gynecology & Obstetrics 1970;131(3):519-522.
(43) Smith GE, Burrows P. Ultrasound diagnosis of fibroadenoma - is biopsy always necessary?[see comment]. Clinical Radiology 516;63(5):511-515.
(44) Shetty MK, Shah YP, Sharman RS. Prospective evaluation of the value of combined mammographic and sonographic assessment in patients with palpable abnormalities of the breast. Journal of Ultrasound in Medicine 2003;22(3):263-268.
(45) Moy L, Slanetz PJ, Moore R, Satija S, Yeh ED, McCarthy KA et al. Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology 2002;225(1):176-181.
(46) Soo MS, Rosen EL, Baker JA, Vo TT, Boyd BA. Negative predictive value of sonography with mammography in patients with palpable breast lesions. AJR American Journal of Roentgenology 2001;177(5):1167-1170.
(47) Flobbe K, Bosch AM, Kessels AG, Beets GL, Nelemans PJ, von Meyenfeldt MF et al. The additional diagnostic value of ultrasonography in the diagnosis of breast cancer. Archives of Internal Medicine 2003;163(10):1194-1199.
(48) Venta LA. Image guided biopsy of non-palpable breast lesions. Harris JR LM, Morrow M OCe, editors. Diseases of the Breast second edition. 149-164. 2000. Philadelphia, Lippincott Williams and Wilkins.
Ref Type: Generic
(49) Smith RA. The evolving role of MRI in the detection and evaluation of breast cancer.[comment]. New England Journal of Medicine 2007;356(13):1362-1364.
(50) Ambrogetti D, Berni D, Catarzi S, Ciatto S. The role of ductal galactography in the differential diagnosis of breast carcinoma. Radiologia Medica 1996;91(3):198-201.
(51) Morrow M, Bland KI, Foster R. Breast cancer surgical practice guidelines. Society of Surgical Oncology practice guidelines. Oncology (Huntington) 1997;11(6):877-881.
(52) Fentiman IS, Caleffi M, Brame K, Chaudary MA, Hayward JL. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet 1986;1(8476):287-288.
(53) Messinis IE, Lolis D. Treatment of premenstrual mastalgia with tamoxifen. Acta Obstetricia et Gynecologica Scandinavica 1988;67(4):307-309.
(54) O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. American Journal of Obstetrics & Gynecology 1999;180(1):1-23.
(55) Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstetrical & Gynecological Survey 2002;57(7):451-461.
(56) Pashby NL, Mansel RE, Hughes LE, Hanslip JI, Preece P. A clinical trial of evening primrose oil in mastalgi. British Journal of Surgery 1981;68:801.
(57) Preece PE, Hanslip JI, Gilbert L. Evening primrose oil ( Efamol) for mastalgia. In: Horrobin DF, editor. Clinical uses of essential fatty acid. Montreal: Eden; 1982. p. 147-154.
(58) Blommers J, de Lange-De Klerk ES, Kuik DJ, Bezemer PD, Meijer S. Evening primrose oil and fish oil for severe chronic astalgia: a randomized, double-blind, controlled trial. American Journal of Obstetrics & Gynecology 2002;187(5):1389-1394.
(59) Hamed H, Caleffi M, Chaudary MA, Fentiman IS. LHRH analogue for treatment of recurrent and refractory mastalgia. Annals of the Royal College of Surgeons of England 1990;72(4):221-224.
(60) Mansel RE, Goyal A, Preece P, Leinster S, Maddox PR, Gateley C et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. American Journal of Obstetrics & Gynecology 2004;191(6):1942-1949.
(61) Tarabishy Y, Hartmann LC, Frost MH, Maloney SD, Vierkant RA, Pankratz VS. Performance of the Gail model in individual women with benign breast disease. Journal of Clinical Oncology 2011;29(15_suppl):1525.
(62) Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. [Review] [95 refs]. Endocrine-Related Cancer 2007;14(2):169-187.
(63) Boyd NF, Jensen HM, Cooke G, Han HL, Lockwood GA, Miller AB. Mammographic densities and the prevalence and incidence of histological types of benign breast disease. Reference Pathologists of the Canadian National Breast Screening Study. European Journal of Cancer Prevention 2000;9(1):15-24.
(64) Byrne C, Schairer C, Brinton LA, Wolfe J, Parekh N, Salane M et al. Effects of mammographic density and benign breast disease on breast cancer risk (United States). Cancer Causes & Control 2001;12(2):103-110.
(65) Key T, Appleby P, Barnes I, Reeves G, Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. Journal of the National Cancer Institute 2002;94(8):606-616.
(66) Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas 1928;38(1):103-113.
(67) Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial.[see comment]. JAMA 2003;289(24):3243-3253.
(68) Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause.[see comment]. American Journal of Epidemiology 2009;170(1):12-23.
(69) Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013;310(13):1353-1368.
(70) Fournier A, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Estrogen-progestagen menopausal hormone therapy and breast cancer: does delay from menopause onset to treatment initiation influence risks?[see comment]. Journal of Clinical Oncology 2009;27(31):5138-5143.
(71) Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. Journal of the National Cancer Institute 1989;81(24):1879-1886.
(72) Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors.[see comment][erratum appears in Stat Med. 2005 Jan 15;24(1):156]. Statistics in Medicine 2004;23(7):1111-1130.
(73) Kuller LH, Cauley JA, Lucas L, Cummings S, Browner WS. Sex steroid hormones, bone mineral density, and risk of breast cancer. Environmental Health Perspectives 1997;105:Suppl-9.
(74) Chlebowski RT, Collyar DE, Somerfield MR, Pfister DG. American Society of Clinical Oncology technology assessment on breast cancer risk reduction strategies: tamoxifen and raloxifene. Journal of Clinical Oncology 1999;17(6):1939-1955.
(75) Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. Journal of the National Cancer Institute 1999;91(21):1829-1846.
(76) Levine M, Moutquin JM, Walton R, Feightner J, Canadian Task Force on Preventive Health Care and the Canadian Breast Cancer Initiative's Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Chemoprevention of breast cancer. A joint guideline from the Canadian Task Force on Preventive Health Care and the Canadian Breast Cancer Initiative's Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. CMAJ Canadian Medical Association Journal 2001;164(12):1681-1690.
(77) Chlebowski RT, Col N, Winer EP, Collyar DE, Cummings SR, Vogel VG, III et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. Journal of Clinical Oncology 2002;20(15):3328-3343.
(78) Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003;361(9354):296-300.
(79) Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. [Review] [35 refs]. Expert Review of Anticancer Therapy 2009;9(1):51-60.
- ABSTRACT
- BREAST PHYSIOLOGY IN WOMEN:
- AGE RELATED CHANGES IN BREAST MORPHOLOGY
- SPECIFIC BENIGN BREAST LESIONS:
- ETIOLOGY OF BENIGN BREAST DISORDERS
- CLINICAL MANIFESTATIONS:
- APPROACH TO THE PRACTICAL MANAGEMENT OF BENIGN BREAST DISEASE
- CLINICAL GUIDELINES FOR EVALUATION OF NODULES AND DISCHARGE
- TREATMENT OF BENIGN BREAST DISEASE IN WOMEN
- WOMEN AT HIGH RISK FOR BREAST CANCER
- BREAST CANCER PREVENTION
- REFERENCE LIST
- Review Testing for Endocrine Hypertension.[Endotext. 2000]Review Testing for Endocrine Hypertension.Hannah-Shmouni F, Melcescu E, Koch CA. Endotext. 2000
- Review Gynecomastia: Etiology, Diagnosis, and Treatment.[Endotext. 2000]Review Gynecomastia: Etiology, Diagnosis, and Treatment.Swerdloff RS, Ng JCM. Endotext. 2000
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Contraception.[Endotext. 2000]Review Contraception.Horvath S, Schreiber CA, Sonalkar S. Endotext. 2000
- Review Diabetes In Pregnancy.[Endotext. 2000]Review Diabetes In Pregnancy.Buschur E, Stetson B, Barbour LA. Endotext. 2000
- Benign Breast Disease in Women - EndotextBenign Breast Disease in Women - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...