NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Diabetic retinopathy is a significant life-altering complication affecting patients with diabetes. Understanding its pathogenesis, prevention, and treatment is critical to delivering effective and comprehensive care for patients with diabetes at all stages. This review discusses the risk factors, epidemiology, pathogenesis, clinical features, and treatment options for diabetic retinopathy, with an emphasis on practical information useful for endocrinologists and other non-ophthalmologists. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes and a leading cause of blindness worldwide and in the US (1-3). The individual lifetime risk of DR is estimated to be 50–60% in patients with type 2 diabetes and over 90% in patients with type 1 diabetes (4). It is the most frequent cause of blindness in adults between 20-74 years of age in developed countries (5). The same pathologic mechanisms that damage the kidneys and other organs affect the microcirculation of the eye (6). With the global epidemic of diabetes, one expects that diabetes will be the leading global cause of vision loss in many countries (1,2). While DR is specific for diabetes, other eye disorders, such as glaucoma and cataracts, occur earlier and more frequently in people with diabetes (5).
Often, by the time patients seek ophthalmologic examination and treatment, there are significant alterations of the retinal microvasculature. Therefore, it is important for non-ophthalmologists to recognize the importance of eye disease in patients with diabetes so that appropriate referral to eye-care specialists can be a part of their diabetes management program.
EPIDEMIOLOGY
In the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), the prevalence of DR in patients with type 1 diabetes was 17% in those with less than 5 years of diabetes vs 98% in those with 15 or more years of diabetes (6). Proliferative diabetic retinopathy (PDR) was absent in patients with type 1 diabetes of short duration but present in 48% of those with 15 or more years of diabetes. In patients with type 1 diabetes, the 25-year rate of progression of DR was 83%, with progression to PDR occurring in 42% of patients (7). Improvement of DR was observed in 18% of patients with type 1 diabetes. In the WESDR, 3.6% of patients with type 1 diabetes were legally blind, and 86% of the blindness was attributable to DR (8). The risk of blindness increases with the duration of diabetes.
In the WESDR, patients with type 2 diabetes of less than 5 years had a prevalence of DR of 28%, while in patients with greater than 15 years of diabetes, the prevalence was 78% (6). A considerable number of patients with type 2 diabetes (12-19%) have DR at the time of the diagnosis of diabetes (1). The prevalence of PDR was relatively low in patients with type 2 diabetes (2%) in patients with less than 5 years duration vs 16% in patients with greater than 15 years duration of diabetes (6). The prevalence of DR and PDR was greater in the patients with type 2 diabetes using insulin. In the patients with type 2 diabetes, 1.6% were legally blind, and one-third of cases of legal blindness were due to DR (8).
Of note, the WESDR cohort is 99% white, and data suggest a higher prevalence of DR in Mexican-Americans and African-Americans with type 2 diabetes (6,9,10). Asians appear to have the same or lower prevalence of DR (1,10). DR occurs in both males and females with diabetes, but males appear to be at a slightly higher risk (9). Diabetic macular edema (DME) occurs more commonly in patients with type 2 diabetes, and with the marked increase in the prevalence of type 2 diabetes, DME is becoming more common (2). DME is over two times more prevalent than PDR (9).
In a pooled analysis of 35 studies between 1980 and 2008, among 22,896 individuals with diabetes, the overall prevalence of DR was 34.6%, PDR 6.96%, DME 6.81%, and vision-threatening DR 10.2% (11). The longer the duration of diabetes, the greater the prevalence of all of these diabetic eye manifestations (11). Moreover, the prevalence of DR, PDR, and DME was greater in patients with type 1 diabetes (77%, 32%, and 14%) compared to patients with type 2 diabetes (32%, 3, and 6%) (1,11).
In developed countries the incidence and the risk of progression of DR have greatly declined in patients with type 1 and type 2 diabetes (1,2,12). The WESDR showed that from 1980 to 2007, the estimated annual incidence of PDR decreased by 77%, and vision impairment decreased by 57% in patients with type 1 diabetes (12). In an analysis of 28 studies with 27,120 patients, the rates of DR and PDR were lower among participants in 1986-2008 than in 1975-1985 (13). Thus, patients with recently diagnosed type 1 or type 2 diabetes in developed countries have a much lower risk of PDR, DME, and visual impairment as compared with patients who developed diabetes in the past (1,12). This marked decrease in the prevalence and incidence of DR and vision impairment is likely due to improved glycemia control, early screening for eye disease, and the more aggressive treatment of blood pressure (1). However, in countries with limited medical resources, this reduced risk of DR and vision impairment is not occurring (2).
In caring for patients with diabetes, health care providers must bear in mind the substantial risks of developing visual loss that these patients face and the treatments that can reduce this risk. For affected patients, diabetes-related visual loss decreases the quality of life and interferes with the performance of daily activities.
RISK FACTORS
Hyperglycemia
The most important treatable risk factor for the development of DR is hyperglycemia. In patients with both type 1 and type 2 diabetes, elevated HbA1c levels are associated with an increased risk and progression of DR (2,7,14-16). Most importantly, randomized controlled trials comparing intensive glycemic control vs. usual care demonstrated a decrease in DR. A meta-analysis of 6 relatively small randomized trials prior to the publication of the Diabetes Control and Complications Trial (DCCT) reported that after 2 to 5 years of intensive therapy the risk of retinopathy progression was significantly reduced (OR 0.49, p = 0.011) (17). Intensive therapy significantly retarded retinopathy progression to more severe states such as PDR or changes requiring laser treatment (OR 0.44, p = 0 018) (17).
The DCCT was a randomized, controlled study of intensive glycemic control (HbA1c approximately 7%) vs. usual care (HbA1c approximately 9%) in 1,441 patients with type 1 diabetes (18). This study found that intensive glucose control reduced the risk of developing retinopathy by 76% compared to usual care (18). In patients with pre-existing retinopathy, intensive control slowed progression of the DR by 54% (18). For every 10% reduction in HbA1c (e.g., 10% to 9% or 9% to 8.1%) the risk of retinopathy progression was reduced on average by 44% (19).The DCCT participants were followed in an observational Epidemiology of Diabetes Interventions and Complications (EDIC) study. During the EDIC study, the mean HbA1c levels became very similar in the intensive and usual care group, with the HbA1c of the intensive treatment group increasing to approximately 8% and the usual care group HbA1c decreasing to approximately 8% (19). Despite the similar A1c levels in the 2 groups over 30 years there continued to be an approximately 50% risk reduction of further DR progression and the development of PDR and DME in the original intensive control group, a phenomenon termed metabolic memory (19). These results indicate the need for early intensive glucose control.
In the Kumamoto study, 110 patients with type 2 diabetes were randomly assigned to a multiple insulin injection treatment group (MIT group) or to a conventional insulin injection treatment group (CIT group) and followed for 6 years (20,21). HbA1c levels were 7.1% in the MIT group and 9.4% in the CIT group. Moreover, the development of DR after 6 years was 7.7% for the MIT group and 32.0% for the CIT group in the primary-prevention cohort (no microvascular disease at baseline) (P = 0.039), and progression of DR occurred in 19.2% of the MIT group and 44.0% of the CIT group in the secondary-intervention cohort (microvascular disease at baseline) (P = 0.049). This study demonstrated that improved glycemic control reduced DR in patients with type 2 diabetes.
In the UK Prospective Diabetes Study (UKPDS), 3,867 newly diagnosed patients with type 2 diabetes were randomized to diet therapy alone or to sulfonylureas or insulin with the goal of achieving a fasting glucose of 108 mg/dL (6mMol/L) in those treated with sulfonylureas or insulin (intensive group). Over 10 years, HbA1c levels were approximately 7.0% in the patients treated with sulfonylureas/insulin therapy compared with 7.9% in the diet group. This study found a 25% reduction in the risk of microvascular endpoints, including the need for diabetic retinal laser treatment, with intensive glucose control (22). A risk reduction of 21% per 1% decrease in HbA1c was observed in this trial. Patients were closely followed after the study ended, and HbA1c levels after one year became similar in the two groups. Similar to the results seen in the DCCT/EDIC study, the benefits on microvascular disease persisted in the intensive control group, confirming the concept of metabolic memory in patients with type 2 diabetes (23).
The ACCORD study was a randomized trial that enrolled 10,251 individuals with type 2 diabetes who were at high risk for cardiovascular disease to receive either intensive or standard treatment for glycemia (HbA1c 6.4% vs. 7.5%). A subgroup of 2,856 individuals were evaluated for the effects of intensive vs. standard care at 4 years on the progression of diabetic retinopathy by 3 or more steps on the Early Treatment Diabetic Retinopathy Study Severity Scale. After 4 years, the rates of progression of diabetic retinopathy were 7.3% in the intensive group vs.10.4% in the standard therapy group (odds ratio, 0.67; P=0.003) (24). It should be noted that in an analysis of the entire ACCORD study cohort, three-line change in visual acuity was reduced in the intensive control group (HR 0.94, CI 0.89-1.00; p=0.05) but no differences in photocoagulation, vitrectomy, or severe visual loss were observed (25). Four years after the ACCORD trial ended, DR progressed in 5.8% of the intensive treatment group vs.12.7% in the standard treatment group (odds ratio 0.42, P < 0.0001) (26), once again confirming the concept of metabolic memory.
It should be noted that two large cardiovascular outcome trials, the ADVANCE trial and the VADT, failed to demonstrate a benefit of intensive glucose control on diabetic retinopathy (27,28). However, a meta-analysis of the four large cardiovascular outcome studies in patients with type 2 diabetes (UKPDS, ACCORD, ADVANCE, and VADT) found that more intensive glucose control resulted in a decrease in HbA1c of -0.90% and a 13% reduction in the need for retinal photocoagulation therapy or vitrectomy, development of PDR, or progression of DR (29). Another meta-analysis of 7 trials with 10,793 participants reported a 20% decrease in DR with intensive glycemic control (0.80, 0.67 to 0.94; P=0.009) (30).
Taken together, these results clearly demonstrate that in patients with both type 1 and type 2 diabetes, improvements in glycemic control will reduce the risk of the development and progression of DR.
Rapid Improvement in Glycemic Control
Deterioration of DR, upon initiation of intensive diabetes treatment, was described in the 1980s in patients with type 1 diabetes who were treated intensively with continuous subcutaneous insulin infusions (31-34). In patients with poor glycemic control and DR, rapidly improving glycemic control can worsen DR and, in some instances, result in PDR or DME. This worsening can occur as soon as 3 months after initiating intensive glycemic control. In the DCCT early worsening was observed at the 6- and/or 12-month visit in 13.1% of patients in the intensive treatment group and in 7.6% of patients assigned to conventional treatment (odds ratio, 2.06; P < .001) (35). In the DCCT the most important risk factors for early worsening of DR were a higher HbA1c level and reduction of this level during the first 6 months of treatment (35). It must be recognized that in the DCCT the long-term outcomes in intensively treated patients who had early worsening were similar to or more favorable than outcomes in conventionally treated patients (35). This early worsening of DR with improved glycemic control has also been described in patients with type 2 diabetes treated with insulin or GLP-1 agonists, following bariatric surgery, in pregnant women with diabetes, and following pancreatic transplants in patients with type 1 diabetes (36). The mechanism(s) leading to early worsening of DR with improvements in glycemic control are unknown (36).
While this worsening is distressing, it must be recognized that the long-term benefits of improving glycemic control on DR greatly outweigh the risks of early worsening. Ophthalmologic evaluation should be obtained prior to initiating intensive treatment and close monitoring should occur at 3-month intervals for 6 to 12 months in patients with significant pre-existing DR.
Hypertension
In the WESDR, blood pressure (BP) was not related to incidence or progression of retinopathy in the patients with type 2 diabetes using insulin or the type 2 patients not using insulin, but in the patients with type 1 diabetes systolic BP was a significant predictor of the incidence of DR (37). In contrast, in the UKPDS and other studies high BP in patients with type 2 was associated with the development of DR (2,16,38). In one prospective study the risk of DR increased by 30% for every 10 mm Hg increase in systolic BP at baseline (39).
While observational studies can show an association, randomized controlled trials are required to demonstrate causation and the benefits of treatment. A number of studies have examined the effect of lowering BP in patients with hypertension on the development and progression of DR.
STUDIES IN PATIENTS WITH HYPERTENSION
The UKPDS examined the effect of tight vs. less tight BP control in 1,148 hypertensive patients with type 2 diabetes (40). In the tight BP control group (captopril and atenolol), BP was significantly reduced compared to the less tight group (144/82 mm Hg vs.154/87 mm Hg; (P<0.0001). After nine years the tight BP control group had a 34% reduction in the deterioration of retinopathy (P=0.0004) and a 47% reduced risk (P=0.004) of deterioration in visual acuity. Additionally, patients in the tight BP group were less likely to undergo photocoagulation (RR, 0.65; P = .03), a difference primarily due to a decrease in photocoagulation due to maculopathy (RR, 0.58; P = .02) (41). In contrast to glycemic control, the benefits of lowering BP were not sustained when therapy was discontinued and the differences in blood pressure were not maintained, indicating the absence of metabolic memory (42).
The HOPE study was a randomized study that compared ramipril vs. placebo in 3,577 participants with diabetes who had a previous cardiovascular event or at least one other cardiovascular risk factor (43). The baseline BP was approximately 142/80 mm Hg, and BP decreased by 1.92/3.3 mm Hg in the ramipril group vs a 0.55 mm Hg increase in systolic BP and 2.30 mm Hg decrease in diastolic BP in the placebo group. This study was not focused on DR but did report that the need for laser was 9.4% in the ramipril group vs. 10.5% in the placebo group (22% decrease; p=0.24).
The ADVANCE study examined the effect of BP control on DR in 1,241 patients with type 2 diabetes (44). Patients were randomized to BP-lowering agents (perindopril and indapamide) or placebo and followed for approximately 4-5 years. Baseline BP was approximately 143/79 mm Hg. In the group randomized to BP medications, a decrease in systolic BP of 6.1 ± 1.2 mmHg and diastolic BP of 2.3 ± 0.6 mmHg was observed (p < 0.001 for both). Fewer patients on BP lowering therapy experienced new or worsening DR compared with those on placebo (OR 0.78; 95% CI 0.57–1.06; p = 0.12), but the difference was not quite statistically significant. Certain secondary outcomes were significantly reduced (for example DME) in the BP lowering group, but most other eye end points were not significantly decreased compared to the placebo group.
The ACCORD eye study evaluated 2,856 patients with type 2 diabetes for the effect of intensive BP control (BP<120 mm Hg) vs standard BP control (BP<140 mm Hg) on the progression of DR after 4 years of treatment (24). Systolic BP was 117 mm Hg in the intensive-therapy group and 133 mm Hg in the standard-therapy group. The progression of DR was 10.4% with intensive blood-pressure therapy vs. 8.8% with standard therapy (adjusted odds ratio, 1.23; P=0.29).
The Appropriate Blood Pressure Control in Diabetes (ABCD2) Trial was a randomized blinded trial that compared the effects of intensive versus moderate BP control in 470 patients with type 2 diabetes and hypertension (45). The intensive group was treated with either nisoldipine or enalapril, while the usual care BP group received placebo. The mean blood pressure achieved was 132/78 mm Hg in the intensive group and 138/86 mm Hg in the moderate group. Over the 5-year follow-up period, there was no difference in the progression of DR between the intensive and moderate groups.
Thus, in patients with hypertension, randomized trials of lowering BP have not consistently shown beneficial effects on DR.
BASIS FOR VARIABILITY
There are numerous possible explanations for the differences in results between these studies. First, the severity of the hypertension may be important, with greater responses in individuals with higher BP levels. Second, the magnitude of the reduction in BP may be important, with greater benefit with greater decreases in BP. Third, the duration of the study may be an important variable, with the longer the study the greater the chances of benefits. Fourth, the presence of DR at baseline and the severity of DR at baseline may influence the response to BP lowering. Fifth, patient variables such as glycemic control, age, diabetes type, duration of diabetes, etc., may influence results. Finally, the drugs used to lower BP may be a key variable as described below.
STUDIES IN PATIENTS WITH NORMAL BP
Because of the potential benefits of angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin receptor inhibitors (ARBs) (Renin-Angiotensin System (RAS) inhibitors) on microvascular disease independent of BP effects, a number of studies have explored the effects of these drugs on DR in patients without elevated BP. Below we briefly describe the largest of these studies.
The EUCLID trial was a randomized double-blind placebo-controlled trial in 354 patients with type 1 diabetes who were not hypertensive and were normoalbuminuric (85%) or microalbuminuric (46). Study participants were randomized to lisinopril or placebo and followed for 2 years. Systolic BP was 3 mm Hg lower in the lisinopril group than in the placebo group. DR progressed in 23.4% of patients in the placebo group and 13.2% of patients in the lisinopril group (p=0.02). Notably progression to PDR was also reduced in the lisinopril treated group.
The Appropriate Blood Pressure Control in Diabetes (ABCD1) trial was a randomized trial in 480 normotensive type 2 diabetic subjects of more intensive vs. usual BP control (47). The intensive group was treated with either nisoldipine or enalapril, while the usual care BP group received placebo. Mean BP in the intensive group was 128/75 mm Hg vs. 137/81 mm Hg in the placebo group (P < 0.0001). After a mean follow-up of 5.4 years, the intensive BP control group demonstrated less progression of diabetic retinopathy (34% vs. 46%, P = 0.019). PDR developed in 0% of patients in the intensive therapy group vs. 3.9% in the placebo group. However, in patients who at baseline did not have DR, the number of patients developing retinopathy was similar in the two groups (39% of patients in the intensive therapy group vs. 42% in the placebo group).
The DIRECT- Prevent 1 trial was a randomized, double-blind, placebo-controlled trial in 1,421 normotensive, normoalbuminuric individuals with type 1 diabetes without retinopathy (48). Patients were randomized to candesartan or placebo and followed for 4.7 years. Mean systolic and diastolic BP was reduced by 2.6 mm Hg and 2.7 mm Hg, respectively, in the candesartan group vs. the placebo group. DR developed in 25% of the participants in the candesartan group vs. 31% in the placebo group (18% decrease).
The Direct Protect 1 was a randomized, double-blind, placebo-controlled trial in 1,905 normotensive, normoalbuminuric patients with type 1 diabetes with existing retinopathy (48). Patients were randomized to candesartan or placebo and followed for 4.7 years. Mean systolic and diastolic BP was reduced by 3.6 mm Hg and 2.5 mm Hg, respectively, in the candesartan group versus the placebo group. There was an identical 13% progression of DR in the placebo and candesartan groups, and progression to the combined secondary endpoint of PDR or clinically significant DME, or both, did not differ between the two groups.
The DIRECT-Protect 2 trial was a randomized, double-blind, placebo-controlled trial in 1,905 normoalbuminuric, normotensive, or treated hypertensive people with type 2 diabetes with mild to moderately severe retinopathy (49). Patients were randomized to candesartan or placebo and followed for 4.7 years. The decrease in systolic/diastolic blood pressure was 4.3/2.5 mm Hg greater in the candesartan group than in the placebo group in individuals who were receiving antihypertensive treatment at baseline (p<0·0001 for both), and for those not on anti-hypertensive therapy at baseline the decrease was 2.9/1.3 mm Hg (p=0.0003/p=0.0045). The risk of progression of retinopathy was non-significantly reduced by 13% in patients on candesartan compared to the placebo group (HR 0.87; p=0.20). However, regression on active treatment was increased by 34% (HR 1.34; p=0.009), and overall change towards less severe retinopathy by the end of the trial was observed in the candesartan group (odds 1.17; p=0.003).
The RASS trial was a controlled trial involving 223 normotensive patients with type 1 diabetes and normoalbuminuria and who were randomly assigned to receive losartan, enalapril, or placebo (50). The systolic and diastolic BP during the study were lower in the enalapril group (113/66 mm Hg) and the losartan group (115/66 mm Hg) than in the placebo group (117/68 mm Hg) (P<0.001 for the two systolic and P≤0.02 for the two diastolic comparisons, respectively). After 5 years progression in DR occurred in 38% of patients receiving placebo but only 25% of those receiving enalapril (P=0.02) and 21% of those receiving losartan (P=0.008).
META-ANALYSIS OF ACE INHIBITORS AND ARBS
Many of the studies described above used either an ACE inhibitor or an ARB with variable results on DR. To better understand the effect of RAS inhibitors on DR, a meta-analysis has extensively examined these studies and a number of other trials (51). In 7 studies with 3,705 participants without DR, RAS inhibitors reduced the development of DR by 27% (p= 0.00006). This decrease in the development of DR was seen in patients with both type 1 and type 2 diabetes and patients who were hypertensive or normotensive. In 16 studies with 9,580 participants with pre-existing DR, RAS inhibitors decreased the progression of DR by 13% (p=0.00006). This decrease in progression of DR was seen in patients with both type 1 and type 2 diabetes and patients who were normotensive. In hypertensive patients there was a trend (7% decrease) that was not statistically significant. It should be noted that in the hypertensive patients RAS inhibitors were compared to other hypertensive drugs, and the number of hypertensive participants was relatively small (n=839). Therefore, the absence of a decrease in progression of DR in hypertensive patients is not definitive. Six studies with 2,624 participants examined the effect of RAS inhibitors on inducing regression of DR. RAS inhibitors increased the regression of DR by 39% (p=0.00002), and this beneficial effect was seen in patients with type 1 and type 2 diabetes. ACE inhibitors were more effective in reducing the development, progression, and regression of DR than ARBs. Thus, with the data available, RAS inhibitors appear to have benefits on DR above and beyond their effects on BP control.
CONCLUSION
Observational studies have shown an association of elevated BP with a higher risk of DR. As should be obvious from the above discussion, the beneficial effects of lowering BP in hypertensive patients on DR have not produced consistent results. Several large carefully carried out studies have failed to demonstrate a beneficial effect of lowering BP on DR (ACCORD, ADVANCE, ABCD2). Potential reasons for this inconsistency were discussed above. It is unlikely that future studies will provide definitive data on this issue, as lowering BP in hypertensive patients with diabetes to prevent cardiovascular disease is essential, and therefore designing clinical trials regarding DR will be very difficult. From the clinician’s viewpoint, treating hypertension in patients with diabetes to prevent cardiovascular disease is standard therapy and may also have beneficial effects DR. Similar to the beneficial effects on renal disease, RAS inhibitors appear to decrease the development and progression of DR, and therefore when treating patients with diabetes who are hypertensive, one should be preferentially consider RAS inhibitors to lower BP in patients with or at high risk of DR. In normotensive patients the available data suggests that RAS inhibition will have beneficial effects on DR, and further studies in this population are possible and would be informative.
Hyperlipidemia
Observational studies of the association of plasma lipids with DR have been inconsistent (52) with some studies reporting an increased risk of DR with elevated lipid levels (53-57), while other studies have not observed a relationship between lipid levels and DR (10,38,58-60). Of note a Mendelian randomization study did not demonstrate a causal role of total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides on DR (61). From the clinician’s point of view the key question is whether lowering lipid levels will have a beneficial effect on DR.
FIBRATES
Small studies in the 1960’s presented evidence that treatment with clofibrate improved diabetic retinopathy (62,63). Larger randomized studies have confirmed these observations.
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was a randomized trial in patients with Type 2 diabetes. Patients were randomly assigned to receive either fenofibrate 200 mg/day (n=4895) or placebo (n=4900). Laser treatment for retinopathy was significantly lower in the fenofibrate group than in the placebo group (3.4% patients on fenofibrate vs 4.9% on placebo; p=0.0002) (64). Fenofibrate therapy reduced the need for laser therapy to a similar extent for maculopathy (31% decrease) and for proliferative retinopathy (30% decrease). In the ophthalmology sub-study (n=1012), the primary endpoint of 2-step progression of retinopathy grade did not differ significantly between the fenofibrate and control groups (9.6% patients on fenofibrate vs 12.3% on placebo; p=0.19). In patients without pre-existing retinopathy there was no difference in progression (11.4% vs 11.7%; p=0.87). However, in patients with pre-existing retinopathy, significantly fewer patients on fenofibrate had a 2-step progression than did those on placebo (3.1% patients vs 14.6%; p=0.004). A composite endpoint of 2-step progression of retinopathy grade, macular edema, or laser treatments was significantly reduced in the fenofibrate group (HR 0.66, 95% CI 0.47-0.94; p=0.022).
In the ACCORD Study a subgroup of participants was evaluated for the progression of diabetic retinopathy by 3 or more steps on the Early Treatment Diabetic Retinopathy Study Severity Scale or the development of diabetic retinopathy necessitating laser photocoagulation or vitrectomy over a four-year period (24). At 4 years, the rates of progression of diabetic retinopathy were 6.5% with fenofibrate therapy (n=806) vs. 10.2% with placebo (n=787) (adjusted odds ratio, 0.60; 95% CI, 0.42 to 0.87; P = 0.006). Of note, this reduction in the progression of diabetic retinopathy was of a similar magnitude as intensive glycemic treatment vs. standard therapy.
A double-blind, randomized, placebo-controlled study in 296 patients with type 2 diabetes mellitus and DR evaluated the effect of placebo or etofibrate on DR (65). After 12 months an improvement in ocular pathology was more frequent in the etofibrate group vs the placebo group ((46% versus 32%; p< 0.001).
The MacuFen study was a small double-blind, randomized, placebo-controlled study in 110 subjects with DME who did not require immediate photocoagulation or intraocular treatment. Patients were randomized to fenofibric acid or placebo for 1 year. Patients treated with fenofibric acid had a modest improvement in total macular volume that was not statistically significant compared to the placebo group.
Taken together these results indicate that fibrates have beneficial effects on the progression of diabetic retinopathy (66). The mechanisms by which fibrates decrease diabetic retinopathy are unknown, and whether decreases in serum triglyceride levels plays an important role is uncertain. Fibrates activate PPAR alpha, which is expressed in the retina (67). Diabetic PPARα KO mice developed more severe DR while overexpression of PPARα in the retina of diabetic rats significantly alleviated diabetes-induced retinal vascular leakage and retinal inflammation, suggesting that fibrates could have direct effects on the retina to reduce DR (67).
STATINS
Several large database studies have suggested that statin use reduces the development of DR (68-71). Unfortunately, the number of randomized clinical trials testing the hypothesis that statin therapy reduces DR development or progression is very limited.
In a study by Sen and colleagues, 50 patients with diabetes mellitus (Type 1 and 2) with good glycemic control and hypercholesterolemia and having DR were randomized to simvastatin vs. placebo (72). Visual acuity improved in four patients using simvastatin and decreased in seven patients in the placebo group and none in the simvastatin group (P = 0.009). Fundus fluorescein angiography and color fundus photography showed improvement in one patient in the simvastatin group, while seven patients showed worsening in the placebo group (P = 0.009).
In a study by Gupta and colleagues, 30 patients with type 2 diabetes with clinically significant macular edema, dyslipidemia, and grade 4 hard exudates were randomized to receive atorvastatin or no lipid lowering drugs (73). All patients received laser therapy. Ten (66.6%) of 15 patients treated with atorvastatin and two (13.3%) of 15 patients in the control group showed a reduction in hard exudates (P =.007). None of the patients treated with atorvastatin and five (33.3%) of 15 in the control group showed subfoveal lipid migration after laser photocoagulation (P =.04). Regression of macular edema was seen in nine eyes in the atorvastatin group and five in the control group (P =.27).
In a study by Narang and colleagues, 30 patients with clinically significant macular edema with a normal lipid profile were randomly treated with atorvastatin or with no lipid lowering drugs. All patients received laser therapy. After a 6-month follow-up visual acuity, macular edema and hard exudates resolution was not significantly different in the two groups.
The data on the benefit of statin therapy on DR are not very strong. Given the current recommendations to prevent cardiovascular disease, most patients with diabetes are treated with statins, and therefore it is unlikely that large randomized trials of the effect of statin therapy on DR are feasible.
OMEGA-3-FATTY ACIDS
A Study of Cardiovascular Events in Diabetes (ASCEND) was a randomized, placebo controlled, double blind, cardiovascular outcome trial of 1-gram omega-3-fatty acids (400 mg EPA and 300 mg DHA ethyl esters) vs. olive oil placebo in 15,480 patients with diabetes without a history of cardiovascular disease (primary prevention trial) (74). Total cholesterol, HDL-C, and non-HDL-C levels were not significantly altered by omega-3-fatty acid treatment (changes in TG levels were not reported). After a mean follow-up of 7.4 years the development of retinopathy and the need for laser therapy based on self-report was similar in the omega-3-fatty acid and placebo group. Thus, at this time there is no evidence that omega-3-fatty acids influence DR.
NIACIN
It has been estimated that 0.67% of patients treated with niacin develop macular edema (75).
Pregnancy
Diabetic retinopathy may progress during pregnancy and up to one year postpartum. For additional information on retinopathy during pregnancy see the chapter in Endotext on “Diabetes in Pregnancy” (76).
Genetics
Some individuals develop DR despite good glycemic control and short duration of disease, while others do not develop DR, even with poor glycemic control and longer duration of diabetes (77). Additionally, the strongest environmental factors (duration of diabetes and HbA1c) only explained about 11% of the variation in DR risk in the DCCT trial and 10% in the WESDR study (12,78). Thus, factors other than glycemic control play an important role. There is a familial relationship in the development of DR, as twin and family studies indicate a genetic basis (79,80). The differences in the prevalence of DR in different ethnic groups may be related to genetic factors (79). Unfortunately, the identification of genetic susceptibility loci for DR through candidate gene approaches, linkage studies, and GWAS has not provided conclusive results (79-81). From a clinician’s point of view, if there is a family history of DR, one should aggressively control risk factors for DR and ensure close eye follow-up.
SCREENING
The American Academy of Ophthalmology has recommended screening for diabetic retinopathy 5 years after diagnosis in patients with type 1 diabetes, and at the time of diagnosis in patients with type 2 diabetes. Patients without retinopathy should undergo dilated fundus examination annually. If mild non-proliferative diabetic retinopathy (NPDR) is present, exams should be repeated every 9 months. Patients with moderate NPDR should be examined every 6 months. In severe NPDR, exams should be conducted every 3 months. Patients with a new diagnosis of proliferative diabetic retinopathy should be examined every 2 to 3 months, until they are deemed stable, at which point examinations can be performed less frequently. During pregnancy, patients should be examined every 3 months, since retinopathy can progress rapidly in this setting (2019 AAO preferred practice pattern document for monitoring diabetic retinopathy: https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp).
The American Diabetes Association 2020 guidelines (5) recommends the following:
- Adults with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years after the onset of diabetes.
- Patients with type 2 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist at the time of the diabetes diagnosis.
- If there is no evidence of retinopathy for one or more annual eye exams and glycemia is well controlled, then screening every 1–2 years may be considered. If any level of diabetic retinopathy is present, subsequent dilated retinal examinations should be repeated at least annually by an ophthalmologist or optometrist. If retinopathy is progressing or sight-threatening, then examinations will be required more frequently.
- Programs that use retinal photography (with remote reading or use of a validated assessment tool) to improve access to diabetic retinopathy screening can be appropriate screening strategies for diabetic retinopathy. Such programs need to provide pathways for timely referral for a comprehensive eye examination when indicated.
- Women with preexisting type 1 or type 2 diabetes who are planning pregnancy or who are pregnant should be counseled on the risk of development and/or progression of diabetic retinopathy.
- Eye examinations should occur before pregnancy or in the first trimester in patients with preexisting type 1 or type 2 diabetes, and then patients should be monitored every trimester and for 1 year postpartum as indicated by the degree of retinopathy.
PATHOGENESIS
Various mechanisms account for the features of diabetic retinopathy. Histopathologic analysis shows thickening of capillary basement membranes, microaneurysm formation, loss of pericytes, capillary acellularity, and neovascularization. Microaneurysms, outpouchings of the capillary wall, serve as sites of fluid and lipid leakage, which can lead to the development of diabetic macular edema. Theories on the biochemistry of these end-organ changes include toxic effects from sorbitol accumulation, vascular damage by excessive glycosylation with crosslinking of basement membrane proteins, and activation of protein kinase C-ß2 by vascular endothelial growth factor (VEGF), leading to increased vascular permeability and endothelial cell proliferation. VEGF, produced by the retina in response to hypoxia, is believed to play a central role in the development of neovascularization (1,82).
CLINICAL FEATURES
Nonproliferative Diabetic Retinopathy (NPDR)
Studies have found that retinopathy in both insulin-dependent and non-insulin-dependent diabetes occurs 3 to 5 years or more after the onset of diabetes. In the WESDR, the prevalence of at least minimal retinopathy was almost 100% after 20 years (83). A more recent study has confirmed that at least 39% of young persons with diabetes developed retinopathy within the first 10 years (84). The earliest clinical sign of diabetic retinopathy is the microaneurysm, a red dot seen on ophthalmoscopy that varies from 15 to 60 microns in diameter (Figure 1).

Figure 1.
Microaneurysms and intraretinal hemorrhages in nonproliferative retinopathy. (UCSF Department of Ophthalmology)
The lesions can be difficult to distinguish from intraretinal hemorrhages on examination, but with fluorescein angiography microaneurysms can be identified easily as punctate spots of hyperfluorescence (Figure 2, 3). By contrast, hemorrhages block the background fluorescence and therefore appear dark.
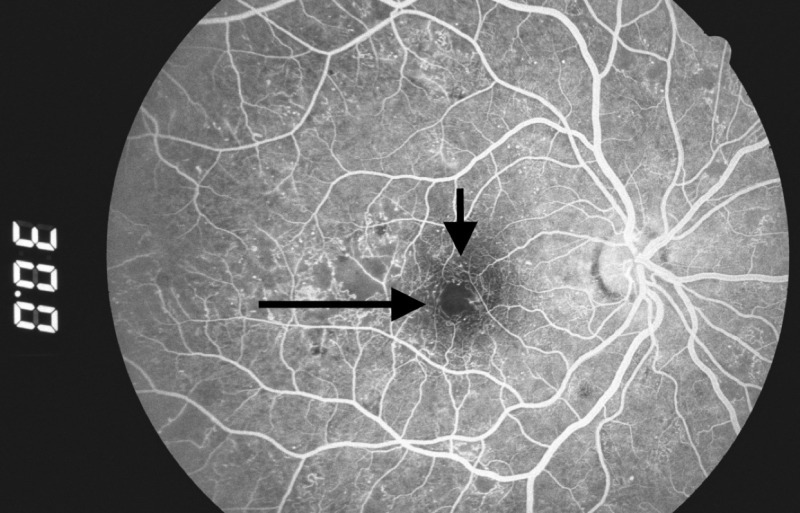
Figure 2.
Microaneurysms: hyperfluorescent dots in early phase of fluorescein angiogram (arrows). (Zuckerberg San Francisco General Hospital, Dept. of Ophthalmology)

Figure 3.
Two minutes later, fluorescein leakage from the microaneurysms gives them a hazy appearance. (Zuckerberg San Francisco General Hospital, Dept. of Ophthalmology)
The severity of NPDR can be graded as mild, moderate, severe, or very severe. In mild disease, microaneurysms are present with hemorrhage or hard exudates (lipid transudates). In moderate NPDR, these findings are associated with cotton-wool spots (focal infarcts of the retinal nerve fiber layer or areas of axoplasmic stasis) or intraretinal microvascular abnormalities (vessels that may be either abnormally dilated and tortuous retinal vessels, or intraretinal neovascularization). The “4-2-1 rule” is used to diagnose severe NPDR: criteria are met if hemorrhages and microaneurysms are present in 4 quadrants, or venous beading (Figure 4) is present in 2 quadrants, or moderate intraretinal microvascular abnormalities are present in 1 quadrant. In very severe NPDR, two of these features are present.
The correct evaluation and staging of NPDR is important as a means of assessing the risk of progression. In the ETDRS, eyes with very severe NPDR had a 60-fold increased risk of developing high-risk proliferative retinopathy after 1 year compared with eyes with mild NPDR (85). For eyes with mild or moderate NPDR, early treatment with laser was not warranted, as the benefits in preventing vision loss did not outweigh the side effects (1). By contrast, in very severe NPDR, early laser treatment was often helpful.

Figure 4.
Venous beading (arrows) in a case of proliferative diabetic retinopathy. (UCSF Department of Ophthalmology)
Capillary closure can also result in macular ischemia, another cause of vision loss in NPDR. This can be identified clinically as an enlargement of the normal foveal avascular zone on fluorescein angiography (Figure 5).

Figure 5.
Capillary dropout around the fovea (white arrow) and in the temporal macula (black arrow). (Zuckerberg San Francisco General Hospital, Dept. of Ophthalmology)
Diabetic Macular Edema (DME)
Macular edema may be present at all the stages of diabetic retinopathy and is the most common cause of vision loss in nonproliferative diabetic retinopathy. Because of the increased vascular permeability and breakdown of the blood-retinal barrier, fluid and lipids leak into the retina and cause it to swell. This causes photoreceptor dysfunction, leading to vision loss when the center of the macula, the fovea, is affected. In the ETDRS, diabetic macular edema (DME) was characterized as "clinically significant" if any of the following were noted (Figure 6): retinal thickening within 500 microns of the fovea, hard exudates within 500 microns of the fovea if associated with adjacent retinal thickening, or an area of retinal thickening 1 disc diameter or larger if any part of it is located within 1 disc diameter of the fovea (86).

Figure 6.
Clinically significant macular edema with hard exudates in the fovea. Cotton-wool spots are present near the major vessels. (UCSF Department of Ophthalmology)
Although the cause of the microvascular changes in diabetes is not fully understood, the deficient oxygenation of the retina may induce an overexpression of vascular endothelial growth factor (VEGF), with a consequent increase in vascular leakage and retinal edema (87). Besides ischemia, inflammation may also play a role in the development of macular edema in diabetic retinopathy. In fact, elevated levels of extracellular carbonic anhydrase have been discovered in the vitreous of patients with diabetic retinopathy (88). Carbonic anhydrase may originate from retinal hemorrhages and erythrocyte lysis and may activate the kallikrein-mediated inflammatory cascade, contributing to the development of DME.
Optical Coherence Tomography (OCT) is a widely used imaging technique that provides high-resolution imaging of the retina (Figure 7) (89). Working as an “optical ultrasound,” OCT projects a light beam and then acquires the light reflected from the retina to provide a cross-sectional image. Most patients with DME have diffuse retinal thickening or cystoid macular edema (presence of intraretinal cystoid-like spaces). In some patients, DME may be associated with posterior hyaloidal traction, serous retinal detachment or traction retinal detachment (90). Cystoid macular edema and posterior hyaloid traction are significantly associated with worse visual acuity (90).
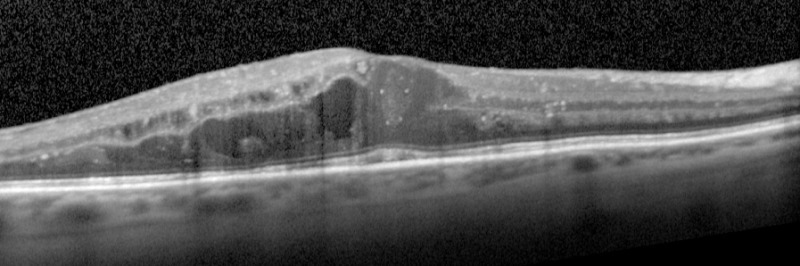
Figure 7.
OCT image showing diabetic macular edema (UCSF Department of Ophthalmology).
Proliferative Diabetic Retinopathy (PDR)
In proliferative diabetic retinopathy, many of the changes seen in NPDR are present in addition to neovascularization that extends along the surface of the retina or into the vitreous cavity (Figure 8). These vessels are in loops that may form a network of radiating spokes or may appear disorganized. In many cases the vessels are first noted on the surface of the optic disc, although they can be easily missed due to their fine caliber. Close inspection often reveals that these new vessels cross over both the normal arteries and the normal veins of the retina, a sign of their unregulated growth.

Figure 8.
Active neovascularization in PDR. Fibrovascular proliferation overlies the optic disc (white arrow). Loops of new vessels are especially prominent superior to the disc and extending into the macula, where leakage of fluid has led to deposition of a ring of hard exudate around the neovascular net (black arrow). (UCSF Department of Ophthalmology)
New vessels can also appear on the iris, a condition known as rubeosis iridis (Figure 9). When this occurs, careful inspection of the anterior chamber angle is essential, as growth of neovascularization in this location can obstruct aqueous fluid outflow and cause neovascular glaucoma.

Figure 9.
Rubeosis iridis in a case of PDR. Abnormal new vessels are growing along the surface of the iris (arrows). (UCSF Dept. of Ophthalmology)
Neovascularization can remain relatively stable or it can grow rapidly; progression can be noted ophthalmoscopically over a period of weeks. Preretinal new vessels often develop an associated white, fibrous tissue component that can increase in size as the vessels regress. The resulting fibrovascular membrane may then develop new vessels at its edges. This cycle of growth and fibrous transformation of diabetic neovascularization is typical. The proliferation occurs on the anterior surface of the retina, and the vessels extend along the posterior surface of the vitreous body. Fibrous proliferation takes place on the posterior vitreous surface; when the vitreous detaches, the vessels can be pulled forward and the thickened posterior vitreous surface can be seen ophthalmoscopically, highlighted by areas of fibrovascular proliferation.
The severity of PDR can be classified as to the presence or absence of high-risk characteristics. As determined in the Diabetic Retinopathy Study, eyes are classified as high-risk if they have 3 of the following 4 characteristics: the presence of any neovascularization; neovascularization on or within 1-disc diameter of the optic disc; a moderate to severe amount of neovascularization (greater than 1/3 disc area neovascularization of the disc, or greater than 1/2 disc area if elsewhere), or vitreous hemorrhage.
Vision loss in proliferative diabetic retinopathy results from three main causes. First, vitreous hemorrhage occurs because the neovascular tissue is subject to vitreous traction. Coughing or vomiting may also trigger a hemorrhage. Hemorrhage may remain in the preretinal space between the retina and the posterior vitreous surface, in which case it may not cause much vision loss if located away from the macula (Figure 10). In other cases, though, hemorrhage can spread throughout the entire vitreous cavity, causing a diffuse opacification of the visual media with marked vision loss (Figure 11, 12).

Figure 10.
Preretinal hemorrhage: blood trapped between the retina and the vitreous in a case of incomplete vitreous detachment. Visual acuity is unaffected. (UCSF Department of Ophthalmology)

Figure 11.
Left: moderate vitreous hemorrhage; vision = 20/150. Right: 1 year later after spontaneous clearing of the hemorrhage; vision = 20/30. (Zuckerberg San Francisco General Hospital, Dept. of Ophthalmology)

Figure 12.
Dense vitreous hemorrhage almost completely obscuring the view of the fundus. (Zuckerberg San Francisco General Hospital, Dept. of Ophthalmology)
Another cause of severe vision loss in PDR is retinal detachment. As the fibrovascular membranes and vitreous contract, their attachments to the retina can cause focal elevations of the retina, resulting in a traction retinal detachment (Figure 13). In other cases the retinal vessels can be avulsed or retinal holes may be created by this traction, leading to a combined traction-rhegmatogenous retinal detachment (Figure 14).

Figure 13.
Marked fibrosis with traction exerted on the retina outside the central macula (arrows). The macula does not appear to be elevated centrally. (UCSF Dept. of Ophthalmology)

Figure 14.
Traction retinal detachment outside the macula. Note elevation of retinal vessel out of the plane of focus (white arrow). Scatter photocoagulation scars are seen peripherally (black arrow). (UCSF Dept. of Ophthalmology)
Finally, patients with PDR may have macular nonperfusion or coexisting diabetic macular edema that causes vision loss through photoreceptor dysfunction.
TREATMENT
Tight glucose and blood pressure control are critical systemic factors in controlling the progression of diabetic retinopathy. Ocular complications of diabetes are addressed directly through treatment with laser photocoagulation, intravitreal injections, or surgery. Laser treatment has been the primary approach to vision-threatening diabetic retinopathy for decades. Recent randomized clinical trials have demonstrated that intravitreal anti-VEGF agents are more effective than laser under certain conditions.
Laser Photocoagulation for NPDR
Diabetic macular edema is believed to result from fluid and lipid transudation from microaneurysms and telangiectatic capillaries. Focal laser photocoagulation is used to heat and close the microaneurysms, causing them to stop leaking (Figure 15). Macular edema often improves following this form of treatment. Some clinicians apply laser burns in a grid pattern overlying areas of retinal edema without directing treatment to specific microaneurysms; this method can also be effective in reducing retinal thickening. The mechanism by which grid laser treatment achieves these results is not known.
The ETDRS found that the risk of moderate visual loss in eyes with diabetic macular edema was reduced by 50% by photocoagulation (91,92). At 3 years, 24% of untreated eyes experienced a 3-line decrease in vision compared with 12% of treated eyes. Eyes meeting the criteria for clinically significant macular edema in which the edema was closest to the center were most likely to benefit from treatment. Side effects of laser treatment can include scotomata, noticeable immediately after the procedure, if treatment is performed too close to the fovea. Late enlargement of laser scars can also occur, causing delayed visual loss. Inadvertent photocoagulation of the fovea is a risk of the procedure. Since the amount of energy used is minimal, the treatment is performed under topical anesthesia.
In the ETDRS study, only a very small percentage of eyes improved with focal laser treatment, highlighting the fact that the goal of laser treatment is not to improve vision, but rather to stabilize it and prevent worsening. It is also true that inclusion criteria for that study were based on the presence of “clinically significant” macular edema threatening the macula, even if the visual acuity was not yet reduced. For this reason, it has been argued that the study enrolled patients with excellent visual acuity, making it difficult to demonstrate small improvements in vision after laser treatment.
Due to the recent evidence on the efficacy and safety of anti-VEGF therapy for diabetic macular edema, different modalities of laser therapy have been proposed. Laser may be able to stabilize macular edema and reduce the need for multiple anti-VEGF injections. Modified ETDRS laser techniques include lower intensity laser burns, and they take particular care in maintaining a greater space from the center of the fovea (93). Subthreshold laser therapy and minimalistic FA-guided treatment of microaneurysms may also induce less damage to the macula than the classic ETDRS approach (94).

Figure 15.
Focal laser scars in the macula following treatment for macular edema (arrow). Edema has resolved. (Zuckerberg San Francisco General Hospital, Dept. of Ophthalmology)
Laser Photocoagulation for PDR
Scatter laser photocoagulation, also known as panretinal photocoagulation (PRP), is an important treatment modality for PDR and severe NPDR (92). Laser spots are placed from outside the major vascular arcades to the equator of the eye, with burns spaced approximately 1/2 to 1 burn width apart (Figure 16, 17). Although the treatment destroys normal retina, the central vision is unaffected since all spots are placed outside the macula. The theory underlying this treatment is that photocoagulation of the ischemic peripheral retina decreases the elaboration of vasoproliferative factors contributing to PDR. Indeed, VEGF levels in the vitreous are increased in eyes with neovascularization, and they are lower after scatter photocoagulation (95). Other factors such as insulin-like growth factor-1 are similarly elevated in the vitreous of eyes with PDR (96).
Side effects of scatter photocoagulation can include decreased night vision and dark adaptation, and visual field loss. The procedure can be painful, so treatment may be divided into several sessions, and either topical or retrobulbar anesthesia may be used.

Figure 16.
Scatter photocoagulation scars in an eye with active PDR. Note that all scatter laser scars are located outside the macula. (UCSF Department of Ophthalmology)

Figure 17.
View of laser scars superior to the macula in the same eye. Spots are approximately one-half burn width apart. In the treated area, the retinal vessels are sclerotic (arrows). (UCSF Department of Ophthalmology)
The Diabetic Retinopathy Study evaluated the effects of scatter photocoagulation in over 1700 patients with PDR or severe NPDR. Patients had one eye randomized to treatment and one eye to observation. Treatment was shown to reduce severe visual loss by 50% (97). The ETDRS also found a positive risk-benefit ratio for early scatter treatment in patients with severe NPDR or early PDR. Interestingly, a subsequent study demonstrated that scatter laser performed at a single sitting was not worse than treatment divided over four sessions in terms of inducing macular edema or decreasing visual acuity (98).
Panretinal photocoagulation may induce or aggravate diabetic macular edema, reduce contrast sensitivity and affect the peripheral visual field (85). Macular edema can be approached by focal laser or intravitreal injections before or at the time of panretinal photocoagulation. However, it is not recommended to delay panretinal photocoagulation in high-risk PDR.
The DRCR.net study protocol S has shown that intravitreal anti-VEGF agents may be a substitute for panretinal laser treatment (99). This multicenter randomized clinical trial compared ranibizumab to PRP in patients with PDR. Mean visual acuity letter improvement at 2 years was +2.8 in the ranibizumab group vs +0.2 in the PRP group (P < 0.001). Mean peripheral visual field sensitivity loss was worse, vitrectomy was more frequent, and DME development was more common in the PRP group. Further studies are needed in order to evaluate the long-term implications of using anti-VEGF agents alone. Ranibizumab may be a reasonable treatment alternative to consider for patients with severe NPDR or non-high-risk PDR who can follow-up regularly.
Corticosteroids for DME
It has been demonstrated that corticosteroids stabilize the blood-retinal barrier, inhibiting leukostasis and modulating the expression of VEGF receptor (100). On this basis, periocular and intraocular injections and sustained-release steroid implants have been utilized for the treatment of diabetic macular edema. It should be remembered that any of these different methods to deliver corticosteroids to the macula carry a potential risk of increasing the intraocular pressure (glaucoma) and inducing cataract.
The use of intravitreal triamcinolone acetonide has become accepted as a treatment option for diabetic macular edema. Several formulations are available: Kenalog-40, which has a black box warning against intraocular use, and the preservative-free Triesence. Preliminary data from a randomized clinical trial showed that intravitreal corticosteroids induced a noticeable improvement of visual acuity and foveal thickness in patients with severe, refractory DME (101). However, intravitreal steroids do not appear to be more efficacious than laser treatment in giving a stable, sustained improvement in vision in the long run, as demonstrated by a recent large study (102).
A peribulbar corticosteroid injection is of particular interest for eyes with DME that have good visual acuity where the risks of an intravitreal injection may not be justified. Any intravitreal injection through the pars plana, in fact, may directly damage the crystalline lens or cause a severe, sight-threatening infection of the eye (bacterial endophthalmitis). Unfortunately, in 2007 a randomized clinical trial showed that peribulbar triamcinolone, with or without focal photocoagulation, is not effective in cases of mild DME with good visual acuity (103).
The fact that triamcinolone maintains measurable concentrations in the vitreous cavity for approximately 3 months stimulated further studies on sustained-release or biodegradable intraocular implants that can deliver steroids for a longer period of time.
A fluocinolone acetonide implant (Retisert) was investigated in a multicenter, randomized clinical trial for the treatment of diabetic macular edema. Although the efficacy of this surgically implanted material was demonstrated, it induced cataract in virtually all phakic patients and severe glaucoma needing surgery in 28% of eyes (104,105).
A biodegradable dexamethasone implant (Ozurdex), now approved for the treatment of DME, has demonstrated similar efficacy with more acceptable side effects. At day 90, a visual acuity improvement of 10 letters or more was seen in more eyes in the Ozurdex group (33.3%) than the observation group (12.3%; P = .007), but the statistical significance was lost at day 180 (106). The implant was generally well tolerated.
A smaller device releasing fluocinolone acetonide, implantable suturelessly with an office procedure thorough a 25-gauge needle, has been recently approved for DME in the USA (Iluvien). This implant has been evaluated in the FAME (Fluocinolone Acetonide in Diabetic Macular Edema) study where 956 patients were randomized worldwide (107). At month 36, the percentage of patients who gained ≥15 in letter score was 28% compared with 19% (P = 0.018) in the sham group. In patients who reported duration of DME ≥3 years at baseline; the percentage who gained ≥15 in letter score at month 36 was 34.0% compared with 13.4%. Almost all phakic patients in the insert group developed cataract, but their visual benefit after cataract surgery was similar to that in pseudophakic patients. The rate of glaucoma surgery at month 36 was 5% (108).
Anti-VEGF Drugs for DME
Vascular endothelial growth factor (VEGF) is an angiogenic factor that plays a key role in the breakdown of the blood–retina barrier and is significantly elevated in eyes with diabetic macular edema (109). Antibody fragments that bind VEGF and inhibit angiogenesis were originally developed as intraocular injection for the treatment of exudative age-related macular degeneration. These anti-VEGF drugs have been tested for the treatment of DME with interesting results.
The first agent that became available was Pegaptanib 0.3 mg (Macugen) (110). A randomized trial demonstrated after 2 years of therapy a gain of 6.1 letters in the pegaptanib arm versus 1.3 letters for sham (P<0.01) (111). Since it is targeted to the isoform VEGF-165 only, it is generally considered very safe but possibly less effective than newer anti-VEGF drugs.
Bevacizumab (Avastin), directed to all the isoforms of VEGF, has been used off-label for the treatment of DME worldwide. The first evidence came from a study on 121 patients with DME followed over 3 months in a phase II randomized clinical trial (112). Recently, the BOLT study demonstrated a mean gain of 8.6 letters for bevacizumab versus a mean loss of 0.5 letters when compared to classic macular laser. The patients received a mean of 13 injections over two years, and the treatment was well tolerated with no progression of macular ischemia (113).
Ranibizumab (Lucentis) binds all isoforms of VEGF and is FDA approved for the treatment of diabetic macular edema. In the Ranibizumab for Edema of the Macula in Diabetes (READ-2) study, ranibizumab-only was superior to laser and to combined therapy (114). The RESTORE study confirmed that ranibizumab monotherapy and combined with laser was superior to standard laser. At 1 year, no differences were detected between the ranibizumab and ranibizumab plus laser arms (115). A larger DRCR study supported ranibizumab plus prompt or deferred photocoagulation as a mainstay of current therapy for patients with DME (116). In the RESOLVE study, at month 12, mean visual acuity improved from baseline by 10.3±9.1 letters with ranibizumab and declined by 1.4±14.2 letters with sham (P<0.0001) (117). The RISE and RIDE studies confirmed the efficacy and the safety of intravitreal monthly injections of ranibizumab with similar results (118).
Aflibercept (Eylea), active against all VEGF-A isoforms, is also FDA-approved for the treatment of DME. In the DA-VINCI study, the different dose regimens of aflibercept demonstrated a mean improvement in visual acuity of 10 to 13 letters versus -1.3 letters for the laser group with a large proportion of eyes (about 40%) gaining 15 or more ETDRS letters at week 52 (119).
More recently, The Diabetic Retinopathy Clinical Research Network Protocol T compared bevacizumab, ranibizumab, and aflibercept in the treatment of center-involving CSME (120). When the initial visual-acuity loss was mild, there were no significant differences among study groups. However, at worse levels of initial visual acuity (20/50 or worse), aflibercept was more effective than bevacizumab. The differences between bevacizumab and ranibizumab and between ranibizumab and aflibercept were not statistically significant.
Currently, on the basis of the above evidence, anti-VEGF therapy is first-line therapy for center-involving macular edema, with possible deferred focal laser treatment. It should be mentioned that adverse side effects associated with intravitreal injections are uncommon but severe and include infectious endophthalmitis, cataract formation, retinal detachment, and elevated IOP.
Vitreous Surgery for PDR
Surgery may be necessary for eyes in advanced PDR with either vitreous hemorrhage or retinal detachment. In the case of vitreous hemorrhage, many cases will clear spontaneously. For this reason, clinicians often wait 3 to 6 months or more before performing vitrectomy surgery. If surgery is indicated because of persistent non-clearing hemorrhage, retinal detachment involving the macula, or vitreous hemorrhage with neovascularization of the anterior chamber angle (a precursor of neovascular glaucoma), then vitrectomy is performed via a pars plana approach. The vitreous is removed, fibrovascular membranes are dissected away from the retina, retinal detachment is repaired, and scatter laser treatment is applied at the time of surgery via direct intraocular application.
The Diabetic Retinopathy Vitrectomy Study assessed the value of early vitrectomy in patients with severe PDR. The study found that early intervention increased the likelihood of obtaining 20/40 vision or better in eyes with recent severe vitreous hemorrhage or severe PDR. Compared with 15% of control eyes, 25% of treated eyes achieved this level of vision at 2 years (109). In type 1 diabetes, the benefit of early surgery was even more pronounced, with 36% of treated eyes achieving 20/40 vision compared to 12% of control eyes. The importance of this study, performed between 1976 and 1983 when vitrectomy techniques were much less advanced than they are today, was that it showed conventional “watch and wait” management will not necessarily lead to the best visual outcomes in cases of severe PDR. In practice, clinicians evaluate the risks and benefits of each option before proceeding with scatter photocoagulation, vitrectomy, or observation in such cases.
Recently, the DRCR Protocol D evaluated the effects of pars plana vitrectomy in eyes with moderate vision loss from DME and vitreomacular traction. Although retinal thickness was generally reduced, visual acuity results were less consistent (121). Vitrectomy for refractory, chronic diabetic macular edema in the absence of vitreomacular traction should be reserved to selected cases.
Intravitreal ocriplasmin (Jetrea) is able to induce enzymatic vitreolysis and posterior vitreous detachment and could have a role, eventually associated with vitrectomy, in the treatment of vitreomacular traction and macular edema in diabetic retinopathy (122).
NOVEL THERAPIES FOR DIABETIC RETINOPATHY
Current therapies are limited in their ability to reverse vision loss in diabetic retinopathy. For example, although focal laser photocoagulation can help stabilize vision by reducing macular edema, it rarely improves vision. Corticosteroids induce cataract progression and intraocular pressure elevation. Anti-VEGF agents do not increase cataract formation rates but they generally need more frequent intravitreal injections, carrying the risk of endophthalmitis; they can temporary increase IOP; they might have systemic adverse effects. For addressing these issues, new sustained-release devices are being designed, and studies are ongoing to test new intravitreal medications.
The development of new treatment modalities is being guided by an understanding of the mechanisms of the disease. From this perspective, researchers are now focusing on the role of inflammation on DME. NSAIDs, anti-TNF agents (Etanercept and Remicade), mecamylamine (an antagonist of nACh receptors), and intravitreal erythropoietin are currently under investigation for the treatment of refractory diabetic macular edema (123).
In order to create a national taskforce to study and treat diabetic retinopathy, in 2002 the National Eye Institute instituted the Diabetic Retinopathy Clinical Research Network (www.drcr.net). DRCR is a collaborative network dedicated to design and carry out multicenter clinical trials on diabetic retinopathy and diabetic macular edema. The DRCR network currently includes over 150 participating sites with over 500 physicians throughout the United States.
The DRCR Network has an ongoing project to study genes involved in diabetic retinopathy.
CONCLUSION
Retinopathy remains a challenging complication of diabetes that can adversely affect a patient’s quality of life. Although ophthalmologists can often stabilize the condition or reduce vision loss, prevention and early detection remain the most effective ways to preserve good vision in patients with diabetes. Ensuring tight glucose and blood pressure control and referring patients for ophthalmologic examination are important ways in which internists and other clinicians can help to maximize their patients’ vision and therefore their quality of life. New treatments may offer greater hope for sustained visual improvement in patients with diabetic retinopathy.
REFERENCES
- 1.
- Amoaku WM, Ghanchi F, Bailey C, Banerjee S, Banerjee S, Downey L, Gale R, Hamilton R, Khunti K, Posner E, Quhill F, Robinson S, Setty R, Sim D, Varma D, Mehta H. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye (Lond). 2020;34:1–51. [PMC free article: PMC7337227] [PubMed: 32504038]
- 2.
- Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten Emerging Trends in the Epidemiology of Diabetic Retinopathy. Ophthalmic Epidemiol. 2016;23:209–222. [PubMed: 27355693]
- 3.
- Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–277. [PubMed: 26716602]
- 4.
- Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. [PubMed: 27159554]
- 5.
- American Diabetes A. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S135–S151. [PubMed: 31862754]
- 6.
- Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DW, Vinik AI, Casellini CM. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2017;102:4343–4410. [PMC free article: PMC5718697] [PubMed: 29126250]
- 7.
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859–1868. [PMC free article: PMC2761813] [PubMed: 19068374]
- 8.
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R. American Diabetes A. Diabetic retinopathy. Diabetes Care. 2003;26:226–229. [PubMed: 12502685]
- 9.
- Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–656. [PMC free article: PMC2945293] [PubMed: 20699456]
- 10.
- Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. [PMC free article: PMC2246042] [PubMed: 16490489]
- 11.
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY., Meta-Analysis for Eye Disease Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. [PMC free article: PMC3322721] [PubMed: 22301125]
- 12.
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. [PubMed: 22455417]
- 13.
- Wong TY, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, Ranganathan G, Wirostko B, Pleil A, Mitchell P. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. 2009;32:2307–2313. [PMC free article: PMC2782996] [PubMed: 19940227]
- 14.
- Tam VH, Lam EP, Chu BC, Tse KK, Fung LM. Incidence and progression of diabetic retinopathy in Hong Kong Chinese with type 2 diabetes mellitus. J Diabetes Complications. 2009;23:185–193. [PubMed: 18479945]
- 15.
- Chase HP, Jackson WE, Hoops SL, Cockerham RS, Archer PG, O'Brien D. Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA. 1989;261:1155–1160. [PubMed: 2915437]
- 16.
- Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–163. [PubMed: 11270671]
- 17.
- Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341:1306–1309. [PubMed: 8098449]
- 18.
- Diabetes Control Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [PubMed: 8366922]
- 19.
- Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, Lorenzi G, Zinman B., Dcct Edic Research Group. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62:3976–3986. [PMC free article: PMC3837056] [PubMed: 24264395]
- 20.
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. [PubMed: 7587918]
- 21.
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23 Suppl 2:B21–29. [PubMed: 10860187]
- 22.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed: 9742976]
- 23.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [PubMed: 18784090]
- 24.
- ACCORD Study Group, ACCORD Eye Study Group. Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC, Jr., Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. [PMC free article: PMC4026164] [PubMed: 20587587]
- 25.
- Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A, O'Connor P, Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I., ACCORD Trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. [PMC free article: PMC4123233] [PubMed: 20594588]
- 26.
- Action to Control Cardiovascular Risk in Diabetes Follow-On Eye Study Group. Action to Control Cardiovascular Risk in Diabetes Follow-On Study Group. Persistent Effects of Intensive Glycemic Control on Retinopathy in Type 2 Diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study. Diabetes Care. 2016;39:1089–1100. [PMC free article: PMC4915557] [PubMed: 27289122]
- 27.
- Advance Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [PubMed: 18539916]
- 28.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. [PubMed: 19092145]
- 29.
- Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, Hayward RA, Craven T, Coleman RL, Chalmers J., Collaborators on Trials of Lowering Glucose Group. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431–437. [PubMed: 28365411]
- 30.
- Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898. [PMC free article: PMC3223424] [PubMed: 22115901]
- 31.
- Hooymans JM, Ballegooie EV, Schweitzer NM, Doorebos H, Reitsma WD, Slutter WJ. Worsening of diabetic retinopathy with strict control of blood sugar. Lancet. 1982;2:438. [PubMed: 6124825]
- 32.
- Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Two-year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes. 1985;34 Suppl 3:74–79. [PubMed: 4018423]
- 33.
- Kroc Collaborative Study Group. Blood glucose control and the evolution of diabetic retinopathy and albuminuria. A preliminary multicenter trial. N Engl J Med. 1984;311:365–372. [PubMed: 6377076]
- 34.
- Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Effect of 1 year of near-normal blood glucose levels on retinopathy in insulin-dependent diabetics. Lancet. 1983;1:200–204. [PubMed: 6130243]
- 35.
- Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874–886. [PubMed: 9682700]
- 36.
- Bain SC, Klufas MA, Ho A, Matthews DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes Metab. 2019;21:454–466. [PMC free article: PMC6587545] [PubMed: 30226298]
- 37.
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Is blood pressure a predictor of the incidence or progression of diabetic retinopathy? Arch Intern Med. 1989;149:2427–2432. [PubMed: 2684072]
- 38.
- Tapp RJ, Shaw JE, Harper CA, de Courten MP, Balkau B, McCarty DJ, Taylor HR, Welborn TA, Zimmet PZ. AusDiab Study Group. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26:1731–1737. [PubMed: 12766102]
- 39.
- Leske MC, Wu SY, Hennis A, Hyman L, Nemesure B, Yang L, Schachat AP., Barbados Eye Study Group. Hyperglycemia, blood pressure, and the 9-year incidence of diabetic retinopathy: the Barbados Eye Studies. Ophthalmology. 2005;112:799–805. [PubMed: 15878059]
- 40.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article: PMC28659] [PubMed: 9732337]
- 41.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM., U. K. Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631–1640. [PubMed: 15534123]
- 42.
- Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–1576. [PubMed: 18784091]
- 43.
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–259. [PubMed: 10675071]
- 44.
- Beulens JW, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, Lu J, Mc GTSA, Grobbee DE, Stolk RP. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–2036. [PubMed: 19633827]
- 45.
- Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23 Suppl 2:B54–64. [PubMed: 10860192]
- 46.
- Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351:28–31. [PubMed: 9433426]
- 47.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–1097. [PubMed: 11849464]
- 48.
- Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, Bilous R, Sjolie AK., Direct Programme Study Group. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. [PubMed: 18823656]
- 49.
- Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N., Direct Programme Study Group. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. [PubMed: 18823658]
- 50.
- Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. [PMC free article: PMC2978030] [PubMed: 19571282]
- 51.
- Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL, Wang YG. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:263–274. [PubMed: 25660574]
- 52.
- Lim LS, Wong TY. Lipids and diabetic retinopathy. Expert Opin Biol Ther. 2012;12:93–105. [PubMed: 22122357]
- 53.
- Chew EY, Klein ML, Ferris FL 3rd, Remaley NA, Murphy RP, Chantry K, Hoogwerf BJ, Miller D. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–1084. [PubMed: 8790092]
- 54.
- Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians--the Chennai Urban Rural Epidemiology Study (CURES) Eye Study--2. Diabet Med. 2006;23:1029–1036. [PubMed: 16922712]
- 55.
- Salinero-Fort MA, San Andres-Rebollo FJ, de Burgos-Lunar C, Arrieta-Blanco FJ, Gomez-Campelo P. Madiabetes Group. Four-year incidence of diabetic retinopathy in a Spanish cohort: the MADIABETES study. PLoS One. 2013;8:e76417. [PMC free article: PMC3798464] [PubMed: 24146865]
- 56.
- Sinav S, Onelge MA, Onelge S, Sinav B. Plasma lipids and lipoproteins in retinopathy of type I (insulin-dependent) diabetic patients. Ann Ophthalmol. 1993;25:64–66. [PubMed: 8447652]
- 57.
- Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45:910–918. [PubMed: 14985310]
- 58.
- Zhou Y, Wang C, Shi K, Yin X. Relationship between dyslipidemia and diabetic retinopathy: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e12283. [PMC free article: PMC6133445] [PubMed: 30200172]
- 59.
- Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–1265. [PubMed: 1923364]
- 60.
- Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, Brancati FL, Hubbard LD, Couper D. Aric Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes : the atherosclerosis risk in communities study. Ophthalmology. 2002;109:1225–1234. [PubMed: 12093643]
- 61.
- Sobrin L, Chong YH, Fan Q, Gan A, Stanwyck LK, Kaidonis G, Craig JE, Kim J, Liao WL, Huang YC, Lee WJ, Hung YJ, Guo X, Hai Y, Ipp E, Pollack S, Hancock H, Price A, Penman A, Mitchell P, Liew G, Smith AV, Gudnason V, Tan G, Klein BEK, Kuo J, Li X, Christiansen MW, Psaty BM, Sandow K. Asian Genetic Epidemiology Network C, Jensen RA, Klein R, Cotch MF, Wang JJ, Jia Y, Chen CJ, Chen YI, Rotter JI, Tsai FJ, Hanis CL, Burdon KP, Wong TY, Cheng CY. Genetically Determined Plasma Lipid Levels and Risk of Diabetic Retinopathy: A Mendelian Randomization Study. Diabetes. 2017;66:3130–3141. [PMC free article: PMC5697951] [PubMed: 28951389]
- 62.
- Harrold BP, Marmion VJ, Gough KR. A double-blind controlled trial of clofibrate in the treatment of diabetic retinopathy. Diabetes. 1969;18:285–291. [PubMed: 4894161]
- 63.
- Duncan LJ, Cullen JF, Ireland JT, Nolan J, Clarke BF, Oliver MF. A three-year trial of atromid therapy in exudative diabetic retinopathy. Diabetes. 1968;17:458–467. [PubMed: 4875170]
- 64.
- Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG., FIELD Study Investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. [PubMed: 17988728]
- 65.
- Emmerich KH, Poritis N, Stelmane I, Klindzane M, Erbler H, Goldsteine J, Gortelmeyer R. Klin Monbl Augenheilkd. 2009;226:561–567. [Efficacy and safety of etofibrate in patients with non-proliferative diabetic retinopathy] [PubMed: 19644802]
- 66.
- Knickelbein JE, Abbott AB, Chew EY. Fenofibrate and Diabetic Retinopathy. Curr Diab Rep. 2016;16:90. [PMC free article: PMC8323449] [PubMed: 27525681]
- 67.
- Hu Y, Chen Y, Ding L, He X, Takahashi Y, Gao Y, Shen W, Cheng R, Chen Q, Qi X, Boulton ME, Ma JX. Pathogenic role of diabetes-induced PPAR-alpha down-regulation in microvascular dysfunction. Proc Natl Acad Sci U S A. 2013;110:15401–15406. [PMC free article: PMC3780907] [PubMed: 24003152]
- 68.
- Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2:894–900. [PubMed: 25217178]
- 69.
- Kang EY, Chen TH, Garg SJ, Sun CC, Kang JH, Wu WC, Hung MJ, Lai CC, Cherng WJ, Hwang YS. Association of Statin Therapy With Prevention of Vision-Threatening Diabetic Retinopathy. JAMA Ophthalmol. 2019;137:363–371. [PMC free article: PMC6459113] [PubMed: 30629109]
- 70.
- Vail D, Callaway NF, Ludwig CA, Saroj N, Moshfeghi DM. Lipid-Lowering Medications Are Associated with Lower Risk of Retinopathy and Ophthalmic Interventions among United States Patients with Diabetes. Am J Ophthalmol. 2019;207:378–384. [PubMed: 31194953]
- 71.
- Kawasaki R, Kitano S, Sato Y, Yamashita H, Nishimura R, Tajima N. Factors associated with non-proliferative diabetic retinopathy in patients with type 1 and type 2 diabetes: the Japan Diabetes Complication and its Prevention prospective study (JDCP study 4). Diabetol Int. 2019;10:3–11. [PMC free article: PMC6357241] [PubMed: 30800559]
- 72.
- Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56:1–11. [PubMed: 11879715]
- 73.
- Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675–682. [PubMed: 15059707]
- 74.
- Group ASC, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379:1540–1550. [PubMed: 30146932]
- 75.
- Domanico D, Verboschi F, Altimari S, Zompatori L, Vingolo EM. Ocular Effects of Niacin: A Review of the Literature. Med Hypothesis Discov Innov Ophthalmol. 2015;4:64–71. [PMC free article: PMC4458328] [PubMed: 26060832]
- 76.
- Buschur E, Stetson B, Barbour LA. Diabetes In Pregnancy. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossman A, Hershman JM, Hofland HJ, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Purnell J, Singer F, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA)2018.
- 77.
- Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care. 2011;34:968–974. [PMC free article: PMC3064059] [PubMed: 21447665]
- 78.
- Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN., Dcct Edic Research Group. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008;57:995–1001. [PubMed: 18223010]
- 79.
- Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132:96–107. [PMC free article: PMC3947937] [PubMed: 24201651]
- 80.
- Cho H, Sobrin L. Genetics of diabetic retinopathy. Curr Diab Rep. 2014;14:515. [PMC free article: PMC4125976] [PubMed: 24952107]
- 81.
- Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377–390. [PMC free article: PMC9639302] [PubMed: 32398868]
- 82.
- Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015;99:137–148. [PubMed: 26054568]
- 83.
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R., American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84–87. [PubMed: 14693935]
- 84.
- Henricsson M, Nystrom L, Blohme G, Ostman J, Kullberg C, Svensson M, Scholin A, Arnqvist HJ, Bjork E, Bolinder J, Eriksson JW, Sundkvist G. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS). Diabetes Care. 2003;26:349–354. [PubMed: 12547861]
- 85.
- Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:766–785. [PubMed: 2062512]
- 86.
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–1806. [PubMed: 2866759]
- 87.
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524. [PubMed: 16026270]
- 88.
- Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE, Aiello LP, Feener EP. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–188. [PubMed: 17259996]
- 89.
- Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, Izatt JA, Swanson EA, Fujimoto JG. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–229. [PubMed: 7862410]
- 90.
- Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142:405–412. [PubMed: 16935584]
- 91.
- Relhan N, Flynn HW Jr. The Early Treatment Diabetic Retinopathy Study historical review and relevance to today's management of diabetic macular edema. Curr Opin Ophthalmol. 2017;28:205–212. [PubMed: 28151747]
- 92.
- Neubauer AS, Ulbig MW. Laser treatment in diabetic retinopathy. Ophthalmologica. 2007;221:95–102. [PubMed: 17380063]
- 93.
- Writing Committee for the Diabetic Retinopathy Clinical Research N. Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, Danis RP, Davis MD, Feman SS, Ferris F, Friedman SM, Garcia CA, Glassman AR, Han DP, Le D, Kollman C, Lauer AK, Recchia FM, Solomon SD. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–480. [PMC free article: PMC2536574] [PubMed: 17420366]
- 94.
- Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev. 2012;8:274–284. [PMC free article: PMC3412206] [PubMed: 22587512]
- 95.
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. [PubMed: 7526212]
- 96.
- Simo R, Lecube A, Segura RM, Garcia Arumi J, Hernandez C. Free insulin growth factor-I and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2002;134:376–382. [PubMed: 12208249]
- 97.
- Preliminary report on effects of photocoagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1976;81:383–396. [PubMed: 944535]
- 98.
- Diabetic Retinopathy Clinical Research N. Brucker AJ, Qin H, Antoszyk AN, Beck RW, Bressler NM, Browning DJ, Elman MJ, Glassman AR, Gross JG, Kollman C, Wells JA, 3rd. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127:132–140. [PMC free article: PMC2754061] [PubMed: 19204228]
- 99.
- Writing Committee for the Diabetic Retinopathy Clinical Research Network. Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL, 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2015;314:2137–2146. [PMC free article: PMC5567801] [PubMed: 26565927]
- 100.
- Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: the past, the present, and the future. Surv Ophthalmol. 2008;53:139–149. [PubMed: 18348879]
- 101.
- Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–1538. [PubMed: 16828501]
- 102.
- Diabetic Retinopathy Clinical Research N. Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–251. [PMC free article: PMC2754047] [PubMed: 19273785]
- 103.
- Diabetic Retinopathy Clinical Research Network. Chew E, Strauber S, Beck R, Aiello LP, Antoszyk A, Bressler N, Browning D, Danis R, Fan J, Flaxel C, Friedman S, Glassman A, Kollman C, Lazarus H. Randomized trial of peribulbar triamcinolone acetonide with and without focal photocoagulation for mild diabetic macular edema: a pilot study. Ophthalmology. 2007;114:1190–1196. [PMC free article: PMC2465806] [PubMed: 17544778]
- 104.
- Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P, Levy B, Mann ES, Eliott D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580–1587. [PubMed: 21813090]
- 105.
- Schwartz SG, Flynn HW Jr. Fluocinolone acetonide implantable device for diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:347–351. [PubMed: 20939799]
- 106.
- Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, Whitcup SM. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289–296. [PubMed: 20212197]
- 107.
- Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J, Kapik B, Billman K, Kane FE, FAME Study Group . Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011; 118:626-635 e622. [PubMed: 21459216]
- 108.
- Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C, Reichel E, Soubrane G, Kapik B, Billman K, Kane FE, Green K., FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–2132. [PubMed: 22727177]
- 109.
- Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J, Zimmer-Galler I, Do DV, Campochiaro PA. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142:961–969. [PubMed: 17046701]
- 110.
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, Goldbaum M, Guyer DR, Katz B, Patel M, Schwartz SD. Macugen Diabetic Retinopathy Study G. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112:1747–1757. [PubMed: 16154196]
- 111.
- Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS., Macugen Study Group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011;118:1107–1118. [PubMed: 21529957]
- 112.
- Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, Stockdale CR. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–1867. [PMC free article: PMC2245885] [PubMed: 17698196]
- 113.
- Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972–979. [PubMed: 22491395]
- 114.
- Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB, Lit ES, Foster BS, Kruger E, Dugel P, Chang T, Das A, Ciulla TA, Pollack JS, Lim JI, Eliott D, Campochiaro PA., READ Study Group. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–2151. [PubMed: 20855114]
- 115.
- Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A., RESTORE Study Group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. [PubMed: 21459215]
- 116.
- Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM, Glassman AR, Scott IU, Stockdale CR, Sun JK., Diabetic Retinopathy Clinical Research Network. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–614. [PMC free article: PMC3096445] [PubMed: 21459214]
- 117.
- Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. [PMC free article: PMC2963502] [PubMed: 20980427]
- 118.
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS, Rise RIDE., RISE Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. [PubMed: 22330964]
- 119.
- Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, Berliner AJ, Gao B, Zeitz O, Ruckert R, Schmelter T, Sandbrink R, Heier JS. da Vinci Study Group. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658–1665. [PubMed: 22537617]
- 120.
- Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW., Diabetic Retinopathy Clinical Research Network. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123:1351–1359. [PMC free article: PMC4877252] [PubMed: 26935357]
- 121.
- Diabetic Retinopathy Clinical Research Network Writing Committee, Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, Browning DJ, Danis RP, Glassman AR, Googe JM, Kollman C, Lauer AK, Peters MA, Stockman ME. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology 2010; 117:1087-1093 e1083. [PMC free article: PMC2911350] [PubMed: 20299105]
- 122.
- Gandorfer A. Enzymatic vitreous disruption. Eye (Lond). 2008;22:1273–1277. [PubMed: 18292784]
- 123.
- Javey G, Schwartz SG, Flynn HW Jr. Emerging pharmacotherapies for diabetic macular edema. Exp Diabetes Res. 2012;2012:548732. [PMC free article: PMC3299388] [PubMed: 22474425]
- Review Nonalcoholic Fatty Liver Disease: The Overlooked Complication of Type 2 Diabetes.[Endotext. 2000]Review Nonalcoholic Fatty Liver Disease: The Overlooked Complication of Type 2 Diabetes.Akshintala D, Chugh R, Amer F, Cusi K. Endotext. 2000
- Review Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State.[Endotext. 2000]Review Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State.Gosmanov AR, Gosmanova EO, Kitabchi AE. Endotext. 2000
- Review Non-Diabetic Hypoglycemia.[Endotext. 2000]Review Non-Diabetic Hypoglycemia.Rayas MS, Salehi M. Endotext. 2000
- Review Etiology and Pathogenesis of Diabetes Mellitus in Children and Adolescents.[Endotext. 2000]Review Etiology and Pathogenesis of Diabetes Mellitus in Children and Adolescents.Yau M, Maclaren NK, Sperling MA. Endotext. 2000
- Review Osteoporosis: Prevention and Treatment.[Endotext. 2000]Review Osteoporosis: Prevention and Treatment.Zaheer S, LeBoff MS. Endotext. 2000
- Diabetic Retinopathy - EndotextDiabetic Retinopathy - Endotext
- fas AND (alive[prop]) (19636)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
