NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Male androgenetic alopecia (MAA) is the most common form of hair loss in men, affecting 30-50% of men by age 50. MAA occurs in a highly reproducible pattern, preferentially affecting the temples, vertex and mid frontal scalp. Although MAA is often regarded as a relatively minor dermatological condition, hair loss impacts self-image and is a great cause of anxiety and depression in some men. MAA is increasingly identified as a risk factor for arterial stiffness and cardiovascular disease. A familial tendency to MAA and racial variation in the prevalence is well recognized, with heredity accounting for approximately 80% of predisposition. Normal levels of androgens are sufficient to cause hair loss in genetically susceptible individuals. The key pathophysiological features of MAA are alteration in hair cycle development, follicular miniaturization, and inflammation. In MAA, the anagen phase decreases with each cycle, while the length of telogen remains constant or is prolonged. Ultimately, anagen duration becomes so short that the growing hair fails to achieve sufficient length to reach the surface of the skin, leaving an empty follicular pore. Hair follicle miniaturization is the histological hallmark of androgenetic alopecia. Once the arrector pili muscle, that attaches circumferentially around the primary follicle, has detached from all secondary follicles and primary follicles have undergone miniaturization and detachment, hair loss is likely irreversible. While many men choose not to undergo treatment, topical minoxidil and oral finasteride are approved by the Food and Drug Administration (USA) for the treatment of MAA. Both medications prevent further hair loss, but only partially reverse baldness, and require continuous use to maintain the effect. Topical minoxidil is well tolerated as a 2% or 5% solution or 5% foam. There is initially accelerated hair loss for several weeks due to telogen hairs falling out. Minor adverse effects include itching of the scalp, dandruff, and erythema. Finasteride is a potent and selective antagonist of the type II 5 alpha reductase, and is not an anti-androgen. 5 alpha reductase converts testosterone into dihydrotestosterone (DHT). DHT binding to the scalp hair follicle androgen receptors produces MAA. A daily oral finasteride dose of one milligram reduces scalp dihydrotestosterone by 64% and serum dihydrotestosterone by 68%. Adverse effects, including sexual dysfunction (erectile dysfunction, low libido, anorgasmia) are uncommon, and most often resolve without discontinuing treatment. Permanent sexual adverse effects have been reported on social media and internet forums; however, the true incidence is unknown. Dutasteride inhibits type I and type II 5 alpha reductase, and it might be superior to finasteride in improving hair growth in young males. However, adverse sexual side effects are more common with dutasteride than with finasteride. Combining medications with different mechanisms of action enhances the efficacy. Topical antiandrogens, prostaglandin analogues, topical antifungals, growth factors, and laser treatment are all emerging medical treatments for MAA, yet lack the necessary research to confirm efficacy and safety. Hair transplantation involves removal of hair from the occipital scalp and re-implantation into the bald vertex and frontal scalp. With modern techniques, graft survival in excess of 90% can be reliably achieved. A combination of these therapeutic options is now available for men experiencing MAA, with favorable cosmetic outcomes possible. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Male androgenetic alopecia (MAA, male pattern baldness) is the most common cause of hair loss in men. The hair loss is progressive. Gradual conversion of terminal hairs into vellus hairs occurs in a highly reproducible pattern, denudes the scalp and leads to baldness. While some degree of androgen-dependent hair loss is universal after puberty, the prevalence of alopecia of sufficient severity to warrant a diagnosis of balding increases with advancing age. Twin studies confirm that hair loss is a genetically determined phenomenon. Observational studies in eunuchs have established the androgen-dependent nature of this condition.
The morbidity of MAA is predominately psychological, although MAA is associated with slight increased risk of melanoma and non-melanoma skin cancer of the scalp. MAA has a variable psychosocial impact on the affected individual, however premature MAA is more likely to cause emotional distress. MAA is reportedly associated with increased incidence of myocardial infarction, hypertension, and hypercholesterolemia.
Topical minoxidil and finasteride (5 alpha reductase type II inhibitor) are the only FDA approved treatments for MAA. Both agents arrest progression of hair loss and stimulate partial regrowth of hair. Dutasteride (dual 5 alpha reductase type I and II inhibitor) is more potent and has been more effective than finasteride in phase II trials but phase III trial data are limited. Hair transplantation is widely practiced in the USA and takes advantage of the relative sparing and androgen-resistant nature of donor occipital hairs.
New insights into the pathophysiology, genetic basis of MAA, and the role of androgens may help in the discovery of additional treatments for androgenetic alopecia.
EPIDEMIOLOGY
Hamilton estimated that 30% to 50% of men developed MAA by the age of 50 (1). Many Western studies have shown that there are racial as well as age-related differences in the incidence and pattern of hair loss in MAA (2).
The incidence and severity of MAA is reported to be more common in Caucasian men than other nationalities. It has been observed that advanced degrees of alopecia are more frequent and develop at an earlier age in Caucasian than in Mongolian populations (3). The onset of MAA in the Japanese occurs one decade later than in Caucasians.(4) Black, Oriental, Native American, and African-American men are more likely to have preservation of their frontal hair lines, less extensive and late onset baldness than Caucasians (1,5-7). A population study completed in Singapore supports that Chinese men are reported to have a lower incidence of MAA (8).
Age prevalence of MAA has been documented in numerous study populations. In Australia, a study of 1390 men between the ages of 40 and 69 was conducted to determine the prevalence and risk factors for MAA. The prevalence of vertex or full baldness (Figure 1) (Norwood Hamilton scale) increases with age from 31% (age 40-55) to 53% (age 65-69). A receding frontal hairline was found in 25% of men aged 40-55 and 31% aged 65-69 (9). A survey done in the USA reported a prevalence of moderate or severe MAA of 53% in the age group 40-49 (10). An increased incidence of MAA with aging has also been reported in Korean population, with type III-vertex involvement most commonly seen in the third to seventh decades (11). The prevalence of MAA in Singaporean males was reported to be 63%, increasing with age, from 32% at 17-26 years to 100% after 80 years (8).

Figure 1.
Androgenetic alopecia patterns in men.
PSYCHOSOCIAL IMPACT OF MAA
Hair is an essential part of an individual's self-image and its main significance relates to socialization. Thus, the consequences of MAA are predominantly psychological. Several studies show that the negative self-perception of balding patients appears to be consistent between Western (12, 13) and Asian cultures (14). The negative impact of MAA is often trivialized or ignored by unaffected people (15). However, there is evidence that perception by others may compound the psychological problems suffered by balding men. A Korean study of the perception of balding men by women and non-balding men found that their negative perception of men with MAA was similar to the psychosocial effects reported by the patients themselves (14). Of note, a perception of bald men looking less attractive was found in more than 90% of subjects surveyed. This view was more common in women than non-balding men. Such negative perceptions may further impair the social functioning of balding men. A recent study confirmed a high prevalence of depression and anxiety in patients experiencing androgenetic alopecia, who often utilize avoidant coping strategies (16). It is important to note however, that most affected men cope well with androgenetic alopecia, without significant impact on their psychosocial function. Thus, those who do seek help are likely to be in greater emotional distress and have been dissatisfied with any treatment they have received. The most distressed balding men are those with more extensive hair loss, those who have very early onset, and those that believe their balding is progressive (12). It is important for the physician to address the patients’ emotional responses to alopecia, including anger, anxiety, and depression, including their beliefs about the impact of their condition (16).
MAA AND DISEASE ASSOCIATIONS
Cotton et al. first suggested idea that male pattern baldness may be a risk factor for cardiovascular disease (17). This has been subsequently supported by several other studies (18-21). A recent study found that asymptomatic young men with at least Grade 3 vertex baldness have a significantly greater risk of arterial stiffness than those with normal hair status (22). However, most of these studies were conducted by non-dermatologists and no dermatologic input was included for confirmation of MAA diagnoses. These statistically-significant, though weak, associations were discovered in epidemiological, cohort, and case control studies. Severe early onset of MAA in subjects before age 30 may be associated with a higher risk for ischemic heart disease. In a retrospective study of 22,071 American subjects, men experiencing vertex balding were shown to have an increased incidence of myocardial infarction compared with frontal alopecia (23). One study showed that frontal male pattern baldness in young men was associated with increased serum cholesterol levels and higher blood pressure compared to men of similar age with no hair loss (23). Increased incidences of hypertension and elevated aldosterone levels have also been found in women with female pattern hair loss (24,25). No clear link between cardiovascular diseases and MAA has been established. High androgen levels have been postulated to cause MAA as well as atherosclerosis and thrombosis, however some data has shown no association between baldness and established coronary risk factors (26).
An increased incidence of benign prostatic hyperplasia has been associated with MAA, and MAA could be an early marker of the disease (27-29). A recent study, however, suggested that there is no relationship between androgenetic alopecia, benign prostatic hyperplasia, PSA level, and prostate volume (30). Prostate cancer has also been found to be positively associated with MAA in various studies (31,31). A large scale Australian case-control study found that vertex balding was associated with a 50% increase in risk of prostate cancer and 11 year follow up data suggests that vertex androgenetic alopecia at age of 40 years might be a marker of increased risk of early-onset prostate cancer (32,33). A meta-analysis conducted using Medline and Cochrane databases suggests that an increased risk of prostate cancer was only associated with vertex baldness, whereas other patterns appear to have no association (34). However, associations with high-grade prostate cancer were found in all patterns of MAA, being especially significant in men aged 60-69 years. An association and a pathophysiological mechanism for the link between MAA and prostate cancer also remains to be established but may involve the dual dependence of these conditions on dihydrotestosterone (35).
A meta-analysis of case-control studies (n=5) has suggested a reduced risk of testicular germ cell tumor (TGCT) in men with MAA (36). They reported that a statistically significant association was observed, suggesting that MAA might become a protective factor for the risk of TGCT. Testicular cancer is hormone-dependent as supported by its rapid increase in incidence starting at pubertal age and late onset of puberty has been inversely associated with the risk of testicular cancer. MAA and testicular cancer share some biological and epidemiologic risk factors including: aging, genetic inheritance, and androgenetic influence. Androgen metabolic pathway genetic variation studies showed that Ser312-Asn polymorphism of the luteinizing hormone receptor was linked to a decreased relative risk of TGCT(37). These conclusions have been based on a limited number of case-control studies and further research is required.
Recent studies suggest higher prevalence, comorbidity, and mortality rates of COVID-19 in males than females. A review of 59,254 individuals from 11 countries, demonstrated higher mortality of SARS-CoV-2 infection in males. A possible suggestion of MAA and worse Covid-19 outcomes has led to several studies reviewing this association (38-40). One study assessed the severity of hair loss in 1,941 admitted male symptomatic patients that tested for SARS-CoV-2 infection, consisted of 1,605 negative-tested and 336 positives. Classification of hair loss was obtained from the UK Biobank data based on the Hamilton–Norwood Scale (HNS). Severe MAA (HNS 4–7) was significantly associated with a higher rate of positive COVID-19 tests (41). In addition, severe MAA had a higher odds ratio than other important risk factors consisting of increased Body Mass Index, hypertension, dyslipidemia, and diabetes. However, mild and moderate MAA (in comparison with patients with no hair loss) did not correlate significantly with increased COVID-19 positivity. The main limitation of that study was that the MAA characterization of patients was based on self-reported data (41). Wambier et al. in a recent study, described SARS-CoV-2 infectivity through transmembrane protease serine 2 (TMPRSS2), which is associated with androgen sensitivity (42). Moreover, the prospective cohort study of Goren et al. involved 77 men admitted with COVID-19; ICU admission was significantly lower (1 out of 12 patients; 8%) in the group taking anti-androgens (dutasteride, finasteride, spironolactone, etc.) relative to the group that did not use anti-androgens (38 out of 65 patients; 58%) (43). Recent studies suggested both high and low androgen levels could lead to a severe course of COVID-19 (44). Increased tissue dihydrotestosterone (DHT) levels are seen in hyperandrogenic conditions like MAA, benign prostatic hyperplasia, and prostate cancer (45). Notably, Motopoli et al. studied androgen-deprivation therapy (ADT) in 118 prostate cancer patients and demonstrated that ADT might have a protective role against SARS-CoV-2 infection (46).
ETIOLOGY
Genetic factors and androgens both play key roles in causing “androgenic” hair loss.
Genetics and Androgenetic Alopecia
A familial tendency to MAA and racial variation in the prevalence of balding is well recognized (5,47). Twin studies identified heredity as accounting for around 80% of the predisposition to baldness (48). Genetic factors modify the magnitude of the hair follicle response to circulating androgens. Those with a strong predisposition go bald in their teenage years, while those with a weak predisposition may not go bald until they are in their 60s or 70s. Fewer than 15% of men have little or no baldness by the age of 70 (49). Osborne in 1916 suggested that the baldness gene behaved in an autosomal dominant manner in men and an autosomal recessive fashion in women (50). Happle and Küster were unable to demonstrate a bimodal distribution of phenotypes with clearly unaffected and clearly affected individuals as is usually seen in autosomal dominant disorders (51). In contrast they observed a range of phenotypes for men and women that seem to follow a normal distribution. This, together with the finding that baldness risk increases with the number of affected family members, is more consistent with polygenic inheritance. Furthermore, they noted that inherited traits due to single gene defects rarely have an incidence greater that 1:1000, while polygenic diseases are much more common, as is the case with androgenetic alopecia. The current concept of it being a polygenic inheritance is supported by an Australian study that examined the frequency of baldness in the fathers of balding men (52). Of the fifty-four father-son relationships, 81.5% of balding sons had fathers who had cosmetically significant balding. This figure greatly exceeded the proportion expected of an autosomal dominant pattern of inheritance. The same authors also described an association of male pattern baldness with a polymorphism of the androgen receptor gene on the X chromosome (52,53). The androgen receptor gene restriction fragment length polymorphism [RFLP] was found in almost all (98.1%) young bald men, older bald men (92.3%), but only in 77% of non-bald men. This polymorphism appears to be necessary for the development of MAA, but its presence in non-bald men indicates that it is necessary but not sufficient to cause the phenotype (53). In addition, several shorter triplet repeat haplotypes were found in higher frequency in bald men than in normal controls. These RFLPs appear to be associated with a functional variant of the androgen receptor (AR) gene. Of note, the androgen receptor gene is located on the X chromosome, which is passed on from mother to a male child. However, family studies have shown resemblance of hair loss between fathers and sons, which cannot be explained by AR gene mutations alone.
These data suggest that other autosomal genes may also be contributing to the phenotype. Several studies have examined the other candidate genes and chromosomal regions that can contribute to the hair loss.
Genetic association studies of 5 alpha reductase genes SRD5A1 on chromosome 5 and SRD5A2 on chromosome 2, using dimorphic intragenic restriction fragment length polymorphisms in 828 families, failed to show an association between these genes and MAA (53). However, the role of the 5 alpha reductase enzyme in MAA is evident from its role in the metabolism of testosterone to dihydrotestosterone (DHT) and the effect of 5 alpha reductase inhibitors in treating hair loss. The cytochrome p450 alpha aromatase enzyme has also been found to contribute to androgenetic alopecia. Aromatase diminishes intra-follicular testosterone by catalyzing the conversion of testosterone to estradiol. Differences exist in the expression of aromatase in balding and non-balding scalp (54). Yip et al suggest that the aromatase gene (CYP19A1) might predispose to hair loss in women (55).
Hillmer et al sought to identify new susceptibility genes in MAA (56). In a genome wide scan and fine mapping linkage study performed on 95 families, they found that there is strong evidence for an MAA susceptibility locus on chromosome 3q26 (56). This study could not confirm or exclude the relevance of chromosomes 11q22-q24, 18p11-q22, and 19p13-q13 in causing MAA. Another genome-wide association study completed by Hillmer et al found a highly significant association on chromosome 20p11 suggesting that the 20p11 locus has a role in a yet-to-be-identified androgen-independent pathway (57). A new susceptibility variant on chromosome 7p21.1 suggests HDAC9 is a 3rd candidate gene for male-pattern baldness (58).
A recent genome wide association study conducted in the United Kingdom, using 25,662 MPB cases and 17,928 controls, found 71 significantly associated loci, 30 of which were previously undescribed. These loci account for 38% of the heritability of MAA, suggesting a relatively low level of complexity in the genetic architecture (59).
GENETIC TESTING IN ANDROGENETIC ALOPECIA
A gene polymorphism-based diagnostic test that will predict the chances of future androgenetic alopecia development is now in the market (53,56). For young patients concerned about hair loss, this test may help to define the value of early treatment initiation.
In males, the gene test can predict the chances for MAA by reporting the presence or absence of a specific variation in the androgen receptor gene found on the X chromosome. The variant androgen receptor gene causes changes in the hair follicle’s response to dihydrotestosterone, resulting in alterations in the hair growth cycle. A positive test result indicates a 70% chance of developing MAA, whereas a negative test result indicates a 70% chance of not developing MAA. The test is of value as a screening test in predicting the future chances of developing MAA rather than a confirmatory test.
Recently, a gene test has been developed that is designed to evaluate an individual’s response to finasteride therapy. The test is based on significant association of specific variations in the androgen receptor gene and the likelihood that a man will respond to finasteride therapy (60). The test provides the patient’s CAG repeat length score in the androgen receptor gene. A shorter CAG repeat length (<22) is associated with a greater likelihood that the patient will experience a significant benefit by using finasteride for the treatment of MAA. The genetic test for finasteride response helps determine if the patient will have a slight, moderate, or great response to finasteride treatment. These tests are not routinely performed in clinical practice currently.
Hormones and Androgenetic Alopecia
The role of androgen in male pattern hair loss is well established. American anatomist James Hamilton observed that castrated males did not develop MAA unless they were supplemented with testosterone (61).
Measurements of serum androgens, testosterone, dehydroepiandrosterone sulphate (DHEA), and free testosterone levels have failed to demonstrate a reproducible difference between cases and controls (62). A study that assessed different hormonal levels in MAA and age-matched controls measured elevated levels of cortisol and androstenedione in those experiencing MAA (63). This study further suggests a broad range of hormones may influence androgenetic alopecia. Even though scalp hair loss and hirsutism are essential features of hyperandrogenism in women, several investigations failed to demonstrate raised androgen levels in women (64). Therefore, it is suggested that normal levels of androgens are sufficient to cause hair loss in genetically susceptible individuals.
The observation that eunuchoidal patients with androgen-insensitivity syndrome and 5 alpha-reductase deficiency do not go bald suggests that MAA is induced by activation of follicular androgen receptors by DHT (65-67). Patients affected by Kennedy's disease, with a functional abnormality of the androgen receptor gene, have a reduced risk of MAA (68). Increased levels of DHT have been found in balding scalp compared to non-balding scalp (69).
Intrafollicular androgen over-activity may also be the result of local factors including an increased number of androgen receptors, functional polymorphisms of the androgen receptor, increased local production of DHT, and reduced local degradation of DHT (70).
Similar to the classical steroidogenic organs, such as gonads and adrenal glands, the skin and its appendages, including hair follicles, sebaceous glands, and eccrine/apocrine glands, are armed with all the necessary enzymes required for androgen synthesis and metabolism. The 5 alpha reductase enzyme plays a central role through the intrafollicular conversion of testosterone to the more active metabolite DHT (71). DHT binds the androgen receptor with 5 times the avidity of testosterone and is more potent in its ability to cause downstream activation (72). Two 5 alpha reductase isoenzymes have been characterized, based on their different pH optima and tissue expression patterns (73). Type 1 5 alpha reductase is found immunohistochemically in sebaceous glands, epidermis, eccrine sweat glands, apocrine sweat glands, and hair follicles. In the skin, the activity of the type 1 5 alpha reductase is concentrated in sebaceous glands and is significantly higher in sebaceous glands from the face and scalp compared with non-acne-prone areas. Northern blot studies reveal an abundance of type 1 mRNA in neonatal foreskin keratinocytes, followed by adult facial sebocytes, and stronger expression in dermal papilla (DP) from occipital hair cells than from beard (74). It is also found in the liver, adrenals and kidneys. Despite the wide expression pattern of type 1 enzyme, its physiological function is uncertain. The type 2 enzyme has been found by immunohistochemistry to be in the dermal papilla, the inner layer of the outer root sheath, the sebaceous ducts, and proximal inner root sheath of scalp hair follicles (75). It is also found in the prostate, testes, and liver. Type 2 5 alpha reductase accounts for about 80% of circulating DHT.
Recent studies by Hoffmann et al demonstrate that there are number of other enzymes involved in the pathogenesis of androgenetic hair loss. 17-beta- and 3-beta-hydroxysteroid dehydrogenases (HSD), with type 2 5 alpha reductase within the dermal papilla, play a central role in the intrafollicular conversion of testosterone to DHT (76). Fritsch et al suggest that small levels of some isoenzymes found in normal states may have important implications in disease states (77). Steroid sulfatase, 3-beta-HSD1, 17-beta-HSD3, and type 1 5 alpha reductase are the major steroidogenic enzymes responsible for the formation of potent androgens, whereas 17-beta-HSD2, 3-alpha-HSD, and aromatase seem to inactivate the excess androgens locally in order to achieve androgen homeostasis in the hair follicles (77).
Human hair follicles, distributed in specific sites of the body, appear to have an inherited susceptibility for androgen-dependent growth starting during puberty. Depending on the body sites, androgens have paradoxically different effects on human hair follicles. Androgens stimulate hair growth in some sites such as the beard, axillary, and pubic areas and suppress the growth of frontal scalp hair of genetically disposed individuals. Itami et al proposed that the second messenger system determines whether androgen sensitive follicles will respond to androgens by either miniaturization or enhancement (78). Androgen stimulation of cultured beard dermal papilla cells lead to increased transcription of insulin-like growth factor 1 (IGF-1) and enhanced growth of co-cultured keratinocytes. Androgen stimulation of dermal papilla cells derived from balding scalp lead to suppression of growth of co-cultured keratinocytes. This growth suppression of keratinocytes was mediated by transforming growth factor-beta1 (TGF-beta1) derived from dermal papilla cells from men with MAA, suggesting that TGF-beta1 is a paracrine mediator for MAA (79). Beard dermal papilla cells are known to secrete growth-inducing autocrine growth factors in response to testosterone, leading to an increase in dermal papilla size and enlargement of the hair follicle and hair cortex. IGF-1 has been identified as a major component of secreted cytokines (80).
Hair loss on the scalp progresses in an orderly and reproducible pattern, and is a function of factors intrinsic to each hair follicle. In vitro experiments have shown that the hair follicles are able to self-regulate their response to androgens by regulating the expression of 5 alpha reductase and androgen receptors (81-83). This self-regulation is postulated to produce the quantifiable difference in androgen receptor numbers and 5 alpha reductase activity that is observed between balding and non-balding areas of the scalp (54, 81, 82, 84). This intrinsic regulation is best demonstrated in hair transplantation experiments: occipital hairs maintain their resistance to MAA when transplanted to the vertex, and scalp hairs from the vertex transplanted to the forearm miniaturize at the same pace as hairs neighboring the donor site (85).
PATHOPHYSIOLOGY
Large terminal follicles are shed and replaced by small vellus hairs in androgenetic alopecia. Three areas of the scalp are affected preferentially: the temples, vertex scalp, and mid frontal scalp (Figure 2). Within these areas the process is strictly patterned. Bitemporal hair loss starts at the anterior hairline and moves posteriorly over the scalp. Hair loss over the vertex scalp begins centrally and radiates outwards circumferentially. Over the mid frontal scalp, hair follicle miniaturization leads to a pattern of hair loss reminiscent of a Christmas tree (86). These three zones are not affected equally leading to clinical variations in the pattern of hair loss, with some men balding more to the front while other bald more over the crown.
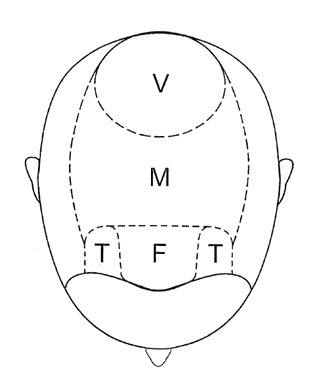
Figure 2.
Areas of the scalp. F-Frontal / M-Mid frontal / T-Temple / V-Vertex.
The 3 key features of MAA are alteration of hair cycle dynamics, follicular miniaturization, and inflammation.
Hair Cycle Dynamics and Androgenetic Alopecia
Hair is lost and replaced cyclically. Follicles undergo corresponding cyclic phases of growth, involution, quiescence, and regeneration (Figure 3). The growth phase (anagen) lasts for 3-5 years (87). As hair elongation is relatively constant at 1 cm per month, the duration of the growth phase is the primary determinant of the final hair length. At the end of anagen the involutional phase (catagen) lasts for a few weeks. The period of hair follicle quiescence (telogen) that follows lasts approximately 3 months (88). Hair follicle regeneration occurs in approximately the first week of anagen and once regenerated, the anagen phase continues until the hair reaches its final (possibly predetermined) length.
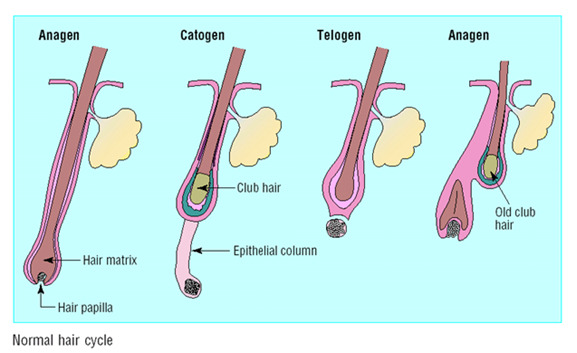
Figure 3.
Normal hair cycle - Each telogen hair is replaced by a new anagen hair.
Hair cycle in mammals has an intrinsic rhythmic behavior and this is modified by systemic and local factors. Humans have an asynchronous hair cycle and the duration of anagen and the final length of hair differ between regions of the body. A number of molecular signals including growth factors, nuclear receptors, cytokines, and intracellular signaling pathways are involved in controlling the hair cycle. Growth factors such as IGF-1, hepatocyte growth factor, keratinocyte growth factor, and vascular endothelial growth factor (VEGF) promote the anagen phase of the hair cycle. Similarly, transforming growth factor-beta (TGF beta), interleukin 1-alpha, and tumor necrosis factor-alpha promote onset of catagen (89).
In androgenetic alopecia, the duration of anagen decreases with each cycle, while the length of telogen remains constant or is prolonged; this results in a reduction of the anagen to telogen ratio (47). Balding patients often describe periods of excessive hair shedding, most noticeable while combing or washing. This is due to the relative increase in numbers of follicles in telogen. As the hair growth rate remains relatively constant, the duration of anagen growth determines hair length. Thus, with each successively foreshortened hair cycle, the length of each hair shaft is reduced. Ultimately, anagen duration becomes so short that the growing hair fails to achieve sufficient length to reach the surface of the skin, leaving an empty follicular pore. Prolongation of the kenogen phase, the lag phase or the delayed replacement of telogen hair, seems to last longer in MAA leaving a higher percentage of empty hair follicles contributing to balding (90,91). Further, the kenogen (latent phase) is prolonged in MAA, reducing hair numbers and contributing to the balding process (90).
In MAA tiny, pale hairs gradually replace large, pigmented ones. Androgens appear to reduce alopecia hair color by inhibiting dermal papilla stem cell factor (SCF) production, which is important in embryonic melanocyte migration and bulbar melanocyte pigmentation (92).
Hair Follicle Miniaturization
Hair follicle miniaturization is the histological hallmark of androgenetic alopecia (93). Hair follicles consist of mesenchymal and ectodermal components. The ectodermal part consists of an invagination of epidermis into the dermis and subcutaneous fat. The hair bulb contains the hair matrix which produces the hair shaft. The mesenchymal component is the dermal papilla, a small collection of specialized fibroblasts that is totally surrounded by the hair bulb.
In association with the changes in hair cycle dynamics, there is progressive, stepwise miniaturization of the entire follicular apparatus in MAA (Figure 4). The mesenchyme-derived dermal papilla, located in the middle of the hair bulb at the follicle base, regulates many aspects of the epithelial follicle and determines the type of hair produced (94,95). As the dermal papilla is central in the maintenance and control of hair growth, it is likely to be the target of androgen-mediated events leading to miniaturization and hair cycle changes (96-98). The constant geometric relationship between the dermal papilla size and the size of the hair matrix suggests that the size of the dermal papilla determines the size of the hair bulb and ultimately the hair shaft produced (99, 100).
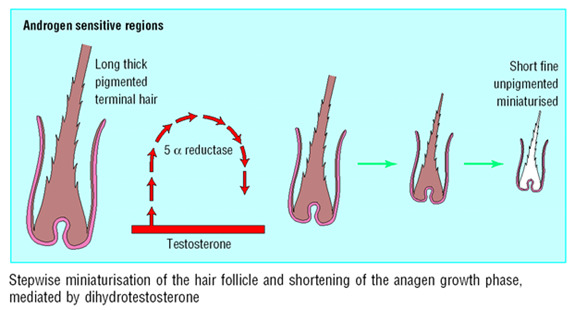
Figure 4.
Progressive miniaturization of hair in each cycle.
A greater than tenfold reduction in overall cell numbers is likely to account for the decrease in hair follicular size (101). The mechanism by which this decrease occurs is unexplained, and may be the result of either apoptotic cell death, decreased proliferation of keratinocytes (92), cell displacement with loss of cellular adhesion leading to dermal papilla fibroblasts dropping off into the dermis, or migration of dermal papilla cells into the dermal sheath associated with the outer root sheath of the hair follicle (100, 102). In vitro studies demonstrate that human balding dermal papilla cells secrete inhibitory factors, which affect the growth of both human and rodent dermal papilla cells, and factors that delay the onset of anagen in mice in vivo. These inhibitory factor(s) probably cause the formation of smaller dermal papillae and smaller hairs in MAA (103). Insulin-like growth factor binding protein 3 (IGFBP3) has a demonstrated antagonistic effect on keratinocyte proliferation in the hair follicle in transgenic mice studies (104).
Smaller follicles result in finer hairs. The caliber of hair shafts reduces from 0.08mm to less than 0.06mm. On the balding scalp, transitional indeterminate hairs represent the bridge between full-sized and miniaturized terminal hairs (105). Traditional models of MAA show follicular miniaturization occurring in a stepwise fashion. This has recently been contested, and it is now believed that the transition from terminal to vellus hair occurs as an abrupt, large step process. Either way, the cross-sectional area of individual hair shafts remains constant throughout fully developed anagen, indicating that the hair follicle, and its dermal papilla, remains the same size (105). Therefore, follicular miniaturization occurs between anagen cycles rather than within the anagen phase. This short window of androgen effect may also explain the lengthy delay experienced between clinical response and the commencement of therapy, as any pharmacological intervention will only have effect at the point of miniaturization (105).
Follicular miniaturization leaves behind stelae as dermal remnants of the full-sized follicle. These stelae, also known as fibrous tracts or streamers, extend from the subcutaneous tissue up the old follicular tract to the miniaturized hair and mark the formal position of the original terminal follicle (106, 107). Arao-Perkins bodies may be seen with elastic stains within the follicular stelae. An Arao-Perkins body begins as a small cluster of elastic fibers in the neck of the dermal papilla. These clump during catagen and remain situated at the lowest point of origin of the follicular stelae. With the progressive shortening of anagen hair seen in androgenetic alopecia, multiple elastic clumps may be found in a stela, like the rungs of a ladder (108).
In addition to the hair follicle miniaturization that leads to thin fibers in androgenetic alopecia, a reduction in anagen duration leads to shorter hair length, while an increase in telogen duration delays regeneration. This results in hairs so short and fine that they fail to achieve sufficient length to reach the surface of the scalp.
While miniaturized hairs are also seen in alopecia areata, that autoimmune condition is potentially fully reversible with treatment (e.g., corticosteroid). In contrast, MAA is only partially reversible at its best. The mechanism for the difference may be related to the attachment of arrector pili muscle and the hair follicle, which will be discussed later in this chapter.
Pattern of Hair Loss
There are 2 concurrent patterns in the hair loss; a macroscopic pattern and a microscopic pattern. The macroscopic pattern of hair loss is highly reproducible with certain zones of the scalp being affected preferentially. This is best seen over the vertex scalp where the baldness begins at a central focus and hair loss progresses radially in all directions. There are no-skip lesions. Hair transplantation studies have demonstrated that this pattern is not due to a local signal or a diffusible chemical but rather genetically imprinted in the follicle. The orderly and systematic progression of hair loss is retained even when follicles are relocated to distant sites.
The microscopic pattern of hair loss refers to the pattern of hair loss within scalp follicular units (109). In contrast to beard hairs, scalp hairs exist as compound follicles with between 2 and 5 hairs emerging from a single pore. Miniaturization within these follicular units is also ordered and leads to a reduction in the number of terminal hairs per follicular unit, which can be demonstrated via dermoscopy (Figure 5). This is perceived by the affected individual as a loss of hair volume. When all the hairs within a follicular unit have miniaturized, additional denuded scalp is visible and perceived by affected individuals as baldness.
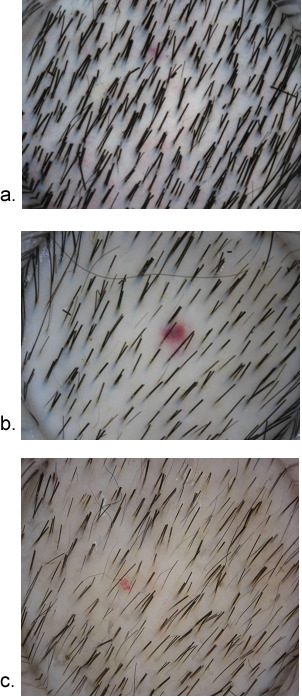
Figure 5.
Dermoscopic images of scalp in different stages of alopecia. A) Normal scalp with 2-4 hairs in most follicular units. B) Early androgenetic alopecia with mixture of multiple and single hair in follicular units. C) Advanced androgenetic alopecia with thin and single hair in most follicular units.
Inflammation
Studies suggest that inflammation is a feature in MAA even though its significance in the pathogenesis of the disease is controversial. Activated T-cells infiltrating the lower portions of follicular infundibula have been demonstrated in scalp biopsies (110). A moderate perifollicular, lymphohistiocytic infiltrate, perhaps with concentric layers of perifollicular collagen deposition, is present in some 40% of cases of androgenetic alopecia, but only 10% of normal controls (107). Occasional eosinophils and mast cells can be seen. The cellular inflammatory changes also occur around lower follicles in some cases and occasionally involve follicular stelae. A considerable difference in the inflammatory infiltrate has been observed between balding and non-balding scalp (111).
A modest degree of chronic inflammation around the upper part of hair follicles has been well described by many investigators (108, 111, 112).
Scarring
The possibility of a slow inflammatory scarring process has been suggested by the irreversibility of the hair loss, the histological evidence of fibrous tracts, and the histological similarity seen between MAA and lichen planopilaris (113).
HISTOPATHOLOGY
Histological diagnosis is rarely necessary for MAA. In patients where the diagnosis is equivocal, 4mm punch vertex scalp biopsies are the ideal specimen. Horizontal scalp biopsies have more diagnostic information than vertical biopsies (107). Triple horizontal biopsies have showed 98% diagnostic accuracy compared with 79% in a single biopsy in female androgenetic alopecia (114).
The prime feature found in scalp biopsies is the reduction in the terminal anagen hair count. The apparent reduction in the number of terminal hairs is due to progressive replacement of terminal hairs with secondary pseudo-vellus hairs, with residual angiofibrotic tracts (112). There is a change in the ratio of terminal to vellus hairs from greater than 6:1 to less than 4:1. Also, the anagen to telogen hair ratio reduces from 12:1 to 5:1 (107).
Messenger et al reported that there is an increase in vellus follicle numbers with increasing severity of hair loss in women with female pattern hair loss, suggesting that terminal follicles do indeed miniaturize (115).
Considering that hair follicle miniaturization is the key point during androgenic alopecia onset and development, diversity in hair diameter represents an important feature histologically, reflecting the different stages of miniaturization, and this accurately correlates with the clinical hair diameter diversity (116).
Arrector Pili Muscle and Androgenetic Alopecia
Hair exists as follicular units consisting of 3-5 terminal hairs per follicular unit nourished by a single arborizing arrector pili muscle (APM), which attaches circumferentially around the primary follicle with variable attachment to other follicles (103, 109). A study by Yazdabadi et al demonstrated that in MAA and female pattern hair loss, where follicle miniaturization is either irreversible or only partially reversible, there was a consistent loss of attachment of the arrector pilli muscle to vellus hair follicles (Figure 6). This was in contrast to potentially reversible alopecia areata, in which the arrector pilli muscle maintained contact with the miniaturized secondary vellus follicles (Figure 7). The study suggests that the persisting contact between the APM and follicular unit predicts reversibility of miniaturization (117).
A recent proposition for the pathogenesis and mechanism of androgenetic alopecia suggests that in the early stages of MAA, the arrector pili muscle remains attached to the primary follicle, yet loses attachment to a number of the regressing secondary follicles in some follicular units (93). As further miniaturization and detachment occurs, patients may first notice a change in their hair density and complain of thinning. Once the arrector pili muscle has detached from all secondary follicles and primary follicles have undergone miniaturization and detachment, hair loss is likely irreversible (93). This proposed mechanism establishes the importance of early treatment to halt balding prior to the loss of primary hair follicles.
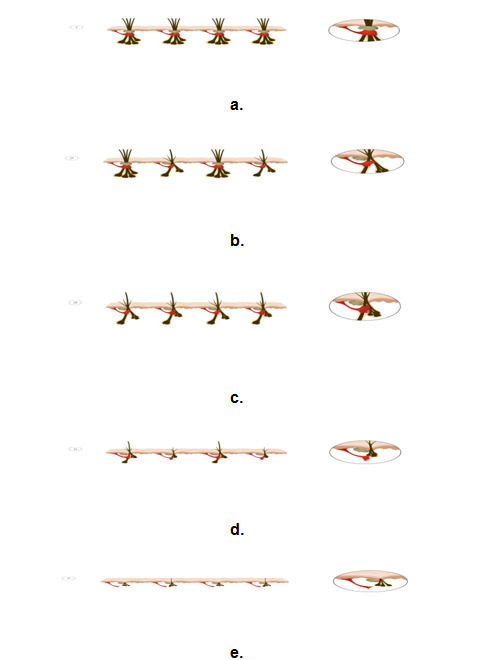
Figure 6.
Illustrations showing the progressive miniaturization within the follicular units and the detachment with the arrector pili muscle.
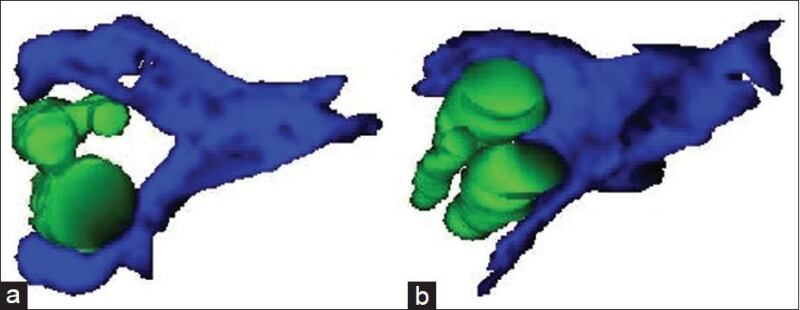
Figure 7.
Three-dimensional reconstructions of alopecia areata (a) and MAAs (b) demonstrating the loss of contact of the arrector pili muscle with the outer root sheath of the vellus hair follicle in MAA that is largely irreversible compared with maintenance of this contact of the arrector pili muscle with outer root sheath which is potentially completely reversible in alopecia area.
CLINICAL SYNDROME
The clinical appearance of MAA is universally and instantly recognizable in most cases. The progression of the hair loss occurs in an orderly manner and has been well documented by Hamilton (1) and Norwood (118) (Figure 8). These authors use a modified grading scale for male pattern hair loss (Figure 9). Affected hairs are miniaturized and there is decreased hair density. Progressive replacement of terminal hairs by vellus hairs leads to an overall decrease in hair density in affected zones as a precursor to total baldness.
Androgenetic alopecia in men typically presents with bitemporal recession and vertex balding (male pattern hair loss). However, 3.9% of Australian men (119) and 11.1% of Korean men (11) with androgenetic alopecia present with “female pattern hair loss (FPHL),” characterized by diffuse rarefaction of hair on the mid-frontal aspect of the scalp and crown, with preservation of the anterior hairline. Expert dermatologists experienced in the treatment of hair disorders participated in the development of a modified Sinclair scale for men by modeling it on the original Sinclair scale, a 5-point visual analog scale for the assessment of FPHL in women. The modified Sinclair scale similarly comprises 5 clinical photographs of men's scalps and is made up of 2 separate composite images for use in men with shorter and longer hairstyles (Figure 10) (120).
The scalp is generally normal, and periods of increased hair shedding may be accompanied by a positive “hair pull” on examination. A positive hair pull test is when hair is easily plucked from the scalp. A family history of MAA on either side of the family is seen in around 80% while in 20% of cases, there is no family history.
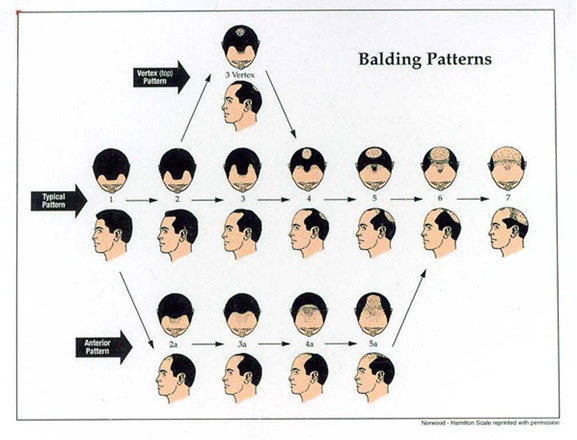
Figure 8.
The Hamilton-Norwood classification of male androgenetic alopecia.
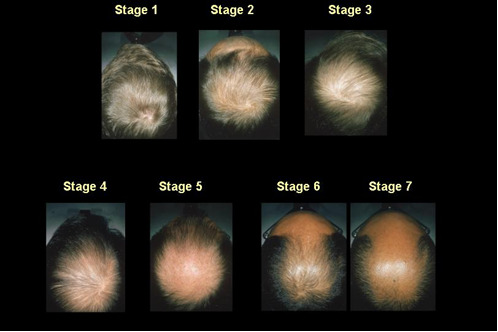
Figure 9.
Modified male pattern hair loss grading scale.
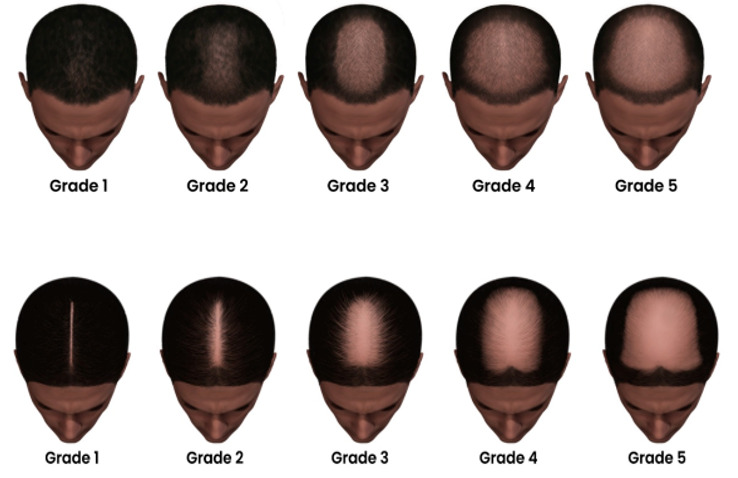
Figure 10.
Modified Sinclair Scale for female pattern hair loss (a less common type of MAA) in men with short hair and long hair.
MANAGEMENT
A number of options are available to balding men. Firstly, as the condition is not life threatening and the morbidity are variable, a reasonable option is to have no treatment and allow the balding to progress naturally. In fact, this is what the vast majority of men elect to do. Regardless of whether patients pursue treatment, an adequate explanation of the pathogenesis of the disease, how common it is in the community, and the various treatment options available form an important part of the support and counselling that should occur with each patient. It is very important to ascertain whether patients' expectations regarding treatment outcome are achievable before embarking on medical therapy. Patients should also be educated about the advantage of early treatment and the necessity of prolonged therapy.
Camouflage and Wigs
Camouflage is the simplest and easiest way to deal with mild MAA. Changing the hair styling to cover the balding scalp, adding small fibers held in place electrostatically and dyeing the scalp the same color as hair are important and cheap measures that can achieve cosmetically satisfactory results. Modern wigs can be styled, washed, and give a natural look.
Medical Management
Topical minoxidil and oral finasteride are the only two treatments currently approved by the Food and Drug Administration (USA) for androgenetic alopecia in men. Both of these medications prevent further hair loss but are only able to partially reverse the baldness. Both require continuous use to maintain the effect. As clinical response may take 6-12 months to become apparent, these agents should be used for at least one year before deciding whether to continue treatment. HairMax LaserComb, also known as low level laser therapy is also FDA-cleared in the context of androgenetic alopecia (121). These are the only treatments recognized by the FDA for treatment of androgenetic alopecia.
MINOXIDIL
Oral Minoxidil has been used to treat hypertension since the 1960s (122). Hypertrichosis as a consequence of minoxidil treatment was observed shortly thereafter and has been said to occur in 100% of the users (123, 124). These observations led to the development of topical minoxidil as a treatment for hair loss (125). It was approved by the FDA for the treatment of male androgenetic alopecia in 1984.
A number of investigators have advanced hypotheses as to the mechanism of action of minoxidil. One important hypothesis is that it has vasodilatory properties. Cutaneous blood flow was observed to increase after 10 to 15 minutes of applying topical minoxidil (126). Up-regulation of vascular endothelial growth factor (VEGF), which helps in maintaining dermal papilla vasculature and hair growth, is another important action of minoxidil (127). Li et al proposed a possible mechanism for minoxidil stimulation of VEGF from experiments on dermal papilla cells (128). They suggest that binding of minoxidil to adenosine receptors A1 and A2, as well as the sulphonylurea receptor SUR2B, activates adenosine-signaling pathways and increase the release of VEGF. Overexpression of VEGF increases perifollicular vascularization and accelerate hair growth.
The prevailing view is that minoxidil promotes hair regrowth through its action to open potassium channels (129, 130). It is postulated that minoxidil sulfate, the active metabolite, opens the adenosine triphosphate (ATP) sensitive potassium channels (KATP channel), thereby having a relaxant effect on vascular smooth muscle and rendering the intracellular potential more negative. This negative gradient promotes depletion of intracellular calcium. In the presence of calcium, epidermal growth factor has been shown to inhibit hair follicular growth in vitro. The conversion of minoxidil to minoxidil sulfate is higher in hair follicles than in the surrounding skin and may suppress EGF-induced inhibition of growth, prolonging the anagen growth phase of hair follicles (128). The effect on the cell cycle is to initiate the onset of anagen (and thereby shorten telogen duration) and to prolong the duration of anagen by delaying initiation of catagen.
Several studies have shown the effect of topical minoxidil in promoting hair growth (131, 132). A five-year follow up with topical minoxidil has shown the sustained effect of minoxidil with long term use (133). Minoxidil works as a non-specific promoter of hair growth, but the slow miniaturization of hair follicles induced by androgens continues in spite of treatment. Evidence for this is seen in a 120-week double-blind study comparing the clipped hair weight of men treated with 5% minoxidil, 2% minoxidil, and placebo and a group with no treatment (132). As expected, the minoxidil groups experienced a surge in hair weights at the induction of therapy. The 5% group was superior to the 2% group in terms of the initial peak in hair weights. Both were superior to placebo and no treatment groups. However, all groups (minoxidil, placebo, and no treatment) showed a progressive 6% per annum decrease in hair weights during the treatment period. This would mean that patients using minoxidil as mono-therapy for MAA continue to bald despite of treatment. If treatment is ceased, any positive effect on hair growth is lost in 4-6 months (134).
Minoxidil topical preparations are available in 2% and 5% solutions. Both strengths are currently in use for treatment in males, yet the 5% minoxidil solution used twice daily has shown higher efficacy than the 2% solution (135). A recent advancement in the use of minoxidil as a hair loss treatment is the development of a 5% topical foam. The traditional topical solution consists of a liquid vehicle with a tendency to spread beyond the intended site of treatment, and that takes time to dry. It also contains a high concentration of propylene glycol, a potential irritant. The newly developed topical hydroalcoholic foam is propylene glycol-free, and it is more easily applied specifically to target areas and rubbed directly on to the scalp skin. Placebo controlled double-blind trials have demonstrated that the hydroalcoholic foam is efficacious, safe and well accepted cosmetically by patients (136).
On commencing treatment, minoxidil may cause a surge in the growth of miniaturized hairs and induction of anagen from resting hair follicles. This may produce a rapid hair shedding of previous telogen hairs 2-8 weeks after treatment initiation. This temporary shedding may be interpreted as a clinical indication that the minoxidil is having a beneficial effect and the hair shedding usually resolves after a few weeks.
Hypertrichosis (fine hairs on non-androgen-sensitive skin) on the face and hands is a common side effect observed following topical and oral minoxidil. Itching of the scalp, increased dandruff, and erythema are commonly reported with topical minoxidil preparations. Contact allergic dermatitis to minoxidil can occur and could either be due to minoxidil itself or more commonly to propylene glycol in the vehicle (137). Patch testing is worthwhile in differentiating the cause of contact dermatitis and the new minoxidil form which does not contain propylene glycol is an alternative in these patients.
Oral Minoxidil is not licensed for the use in androgenetic alopecia; however, it is increasingly being utilized for a variety of hair disorders including MAA (138). An open-label, prospective, single-arm study of thirty men aged 24–59 years with AGA types III vertex to V were treated with oral minoxidil 5 mg once daily for 24 weeks (139). They found a significant increase in total hair counts from baseline at weeks 12 and 24 (both p = 0.007). Photographic assessment of the vertex area by an expert panel revealed 100% improvement, with 43% of patients showing excellent improvement. The frontal area also showed a significant response but less than that of the vertex area. Common side effects were hypertrichosis (93%) and pedal edema (10%). No serious cardiovascular adverse events and abnormal laboratory findings were observed. The reassuring safety profile of oral minoxidil has also been reflected in a large multicenter study evaluating the safety of oral minoxidil in a total of 1404 patients (943 women [67.2%] and 461 men [32.8%]) with a mean age of 43 years (range 8-86). The most frequent adverse effect was hypertrichosis (15.1%). Systemic adverse effects included lightheadedness (1.7%), fluid retention (1.3%), tachycardia (0.9%), headache (0.4%), periorbital edema (0.3%), and insomnia (0.2%), leading to drug discontinuation in 29 patients (1.2%). No life-threatening adverse effects were observed (140).
FINASTERIDE
Finasteride is a synthetic azo-steroid that is a potent and highly selective antagonist of type II 5 alpha reductase. It is not an anti-androgen. It binds irreversibly to the enzyme and inhibits the conversion of testosterone to dihydrotestosterone. Thus, while the pharmacokinetic half-life is approximately eight hours, the biological effect persists for much longer. Finasteride was initially FDA approved for use in benign prostate hyperplasia (BPH) and was approved to treat MAA in 1997. The underlying principle for its use in MAA is the reduction of DHT production in order to limit its action on scalp hair follicles.
Various studies have demonstrated the beneficial effects of finasteride in MAA, with the most benefits seen in patients with primarily type III vertex or type IV Hamilton/Norwood hair loss (141-147). Finasteride has been reported to slow the progression of MAA and to produce partial regrowth in about two thirds of men (141). A study measuring hair counts using macrophotographs found that both total and anagen hair counts increase with treatment of finasteride (142). A significant increase in the anagen to telogen ratio was also achieved. This demonstrates the ability of finasteride to stimulate conversion of hair follicles into the anagen phase, possibly through reversion of the decrease in anagen phase and the increase in lag phase. A study looking at scalp biopsies shows that finasteride stimulates an increase in terminal hair counts and a decrease in vellus hair counts (143). Other studies have used hair count and hair weight as objective measures of outcome and demonstrated that both increase, with hair weight increasing to a larger extent (144, 148). Factors that affect hair weight include the number of hairs, hair growth rate and hair thickness. These findings show the ability of finasteride to reverse the miniaturization process, producing hair of greater length and thickness, and possibly with a greater growth rate.
A daily oral finasteride dose of one milligram reduces scalp DHT by 64% and serum DHT by 68% (145). Finasteride was originally given for benign prostatic hyperplasia at 5mg daily. For the treatment of androgenetic alopecia, dose ranging studies have found no significant difference in clinical benefit between five and one milligram daily regimens, nor is there any significant further reduction of scalp or serum DHT levels (135). In practice, finasteride can be administered at either a dose of one milligram per day, or at longer intervals. A 5-year multinational study looking at the effect of finasteride on treatment of MAA found it to be superior to placebo (146). The placebo group suffered a progressive decline in hair count, losing approximately 26% of terminal hairs compared to baseline counts at the end of the 5-year study. In contrast, patients on finasteride experienced a 10% increase in hair count at the end of the first year. Hair count declined somewhat thereafter but remained above baseline, remaining at 5% above baseline hair count after 5 years of treatment. The rate of decline in hair count in the finasteride group is significantly less than that of the placebo group. Taken together, there is a progressive increase in the difference between treatment and placebo group over time. This demonstrates the effects of finasteride in stimulating a substantial amount of hair regrowth, reaching its peak efficacy after one year of treatment, and slowing the progression of hair loss thereafter. At the end of the first year, some in the placebo group were swapped onto receiving finasteride for the remaining four years. These patients demonstrated a decrease in hair count during the first year with placebo, followed by an improvement in the subsequent four years with finasteride. The improvement is similar to that of the group who received finasteride for five years throughout the study. However, mean hair count level is less than that of the patients who have taken finasteride "a year earlier" at all comparable time points, with the difference being similar to the amount of hair loss sustained during the year of placebo treatment. This shows the relative benefits of early commencement of treatment with finasteride. Some of the finasteride patients were also crossed-over to receive placebo after a year of finasteride treatment. A decrease in hair count was observed twelve months later, demonstrating the reversal of the beneficial effects of treatment obtained during the first year.
Further evidence of the efficacy of finasteride in the treatment of MAA is seen in a randomized, double-blind, placebo-controlled twin study (147). At month 12, all subjects in the finasteride group demonstrated an increase in hair count, while a decrease was found in 44% of the placebo group. Serum DHT levels were significantly decreased in the finasteride group, with no significant change observed in the placebo group. Global photography assessment shows significant improvement on hair growth in vertex and superior-frontal scalp in the finasteride group, with no significant differences between treatment groups observed in the temporal or anterior hairline views. This finding shows the relative effectiveness of finasteride for protecting hair loss over the vertex and superior-frontal regions of the scalp, in comparison to the minimal response over the temporal and the anterior hairline regions. An open randomized comparative study with 5% topical minoxidil and oral finasteride 1 milligram a day showed significantly more hair growth in the finasteride group (149).
One Japanese study shows that the hair growth with finasteride continues to increase with continuing treatment without significant side effects (150). A recent 10-year study of 118 men treated with 1 mg/day finasteride for androgenic alopecia found that 86% of men continued to benefit from treatment over the entire course of 10 years — showing increased or stable rates of hair growth and only 14% experiencing any further hair loss (151). Sexual side effects are a main concern when treating patients for male pattern baldness with finasteride. The evidence available to date about the safety of the drug is controversial and needs to be further evaluated. In view of this, it is very important to properly counsel patients before the treatment.
Long-term studies have reported few adverse effects when using finasteride. In the finasteride group loss of libido was reported in 1.9% and erectile dysfunction (ED) in 1.4% in the first year. The placebo groups reported these same events with frequencies of 1.3% and 0.6% respectively. These events appeared to resolve on cessation of the drug and, in some ceased with continued treatment. It has been suggested that even these figures overstate the true incidence of sexual dysfunction (106, 148, 152). A recent review of the efficacy and safety of 5-alpha reductase inhibitors for the treatment of androgenetic alopecia concluded that sexual side effects are uncommon and most often resolve spontaneously even without discontinuing treatment (153).
However, a recent study by Irwig et al conducted standardized interviews with 71 otherwise healthy men aged 21-46 years taking finasteride (154). The subjects reported the onset of sexual side effects associated with the temporary use of finasteride, with symptoms persisting for at least three months despite stopping the drug. The study revealed that the subjects reported new-onset persistent sexual dysfunction (low libido, ED, and problems with orgasm) associated with the use of finasteride. Total sexual dysfunction score increased for both before and after finasteride use (P< 0.0001 for both). The small number of patients, selection bias, recall bias prior to finasteride use, and the absence of serum hormone analyses were the limiting factors of the study. The study recommended that physicians treating MAA should discuss the potential risk levels with patients while prescribing the drug. Furthermore, a meta-analysis in 2015 describes available toxicity information for finasteride as limited, of poor quality, and systematically biased (155). Further pharmaco-epidemiological studies, with clear evaluation of adverse events and their duration, may be needed.
In view of the conflicting and continuing data and importance of the subject, the International Society of Hair Restoration Surgery (ISHRS) established a Task Force on Finasteride Adverse Event Controversies to evaluate published data and make recommendations. ISHRS recommend that finasteride use for MAA is entirely at the discretion of the patient given that the male pattern hair loss is largely a cosmetic condition. However, the treating physician should provide full information about the drug to enable the patient to make an informed decision.
Of note, older men on finasteride experienced a 50% reduction in serum prostate specific antigen [PSA] levels, which could result in an underestimation of prostatic cancer risk. Previous recommendations in the urology literature state that PSA levels remain valid whilst patients are on finasteride, but the value should be doubled to correct for the finasteride effect (156, 157). Men between 18 to 41 years old are thought to have a negligible decrease in measured PSA levels. More recent studies now suggest that finasteride treatment at the 5mg/day dose affects the serum PSA concentration in a time-dependent manner (158). In the Prostate Cancer Prevention Trial, the adjustment factor needed to be increased from 2 at 24 months to 2.5 at 7 years after the initiation of finasteride (159). In a study conducted with men aged 40-60 years taking 1mg finasteride /day for 48 weeks, results suggest that existing recommendations for the adjustment of serum PSA concentration in prostate-cancer screening in men taking 5 mg/day finasteride should also apply to men taking the 1 mg/day preparation for MAA (160). Limited data suggest that reduction in PSA of malignant origin appears to be no greater than the percentage reduction in PSA of benign origin (161). The free/total PSA ratio, currently used to help differentiate benign from malignant processes in the prostate, remains valid during treatment with finasteride, as finasteride does not affect the free/total PSA ratio in men with benign prostatic hyperplasia (but might decrease the ratio in men with prostate cancer) (156).
The effect of finasteride on the incidence and severity of prostate cancer has been extensively investigated and conflicting evidence for the risk of increased incidence of high-grade prostate cancer associated with finasteride prevents its use as a chemoprevention agent. In a trial involving 18,882 men older than 54 years with a normal digital rectal examination and a serum PSA equal to or less than 3ng/ml, men were randomized to finasteride 5mg daily or placebo groups. There was a 25% reduction in prostate cancer prevalence in those taking finasteride (162). However, 6.4% of the men taking finasteride developed histologically high-grade cancer (Gleason score 7-10), compared with 5.1% of those in the placebo group. Recent data suggest that several confounding factors could have contributed to the above results. It is suggested that morphological and histological alterations, the degree of sampling error induced by the reduction of prostate volume, differential sensitivity of the biopsy between the groups, and increased sensitivity of PSA in detecting prostate cancer with finasteride may have contributed to an apparent increase of higher grade cancers (163-167). Alternatively, finasteride use has also been suggested to significantly improve prostate cancer detection with digital rectal examination (168).
Topical finasteride has been investigated as potential variation in drug delivery. While a 0.05% of finasteride solution applied to the scalp was well absorbed and produced a 40% reduction in serum DHT, it had shown no effect on hair regrowth. One explanation for this observation is that inhibition of prostatic DHT production is an important factor in preventing hair loss with finasteride, i.e. a significant reduction in circulating DHT is required in addition to the local blockade of 5 alpha reductase at the hair follicle (169). However, a double blind, randomized clinical study between oral finasteride and topical finasteride showed similar efficacy after 18 months in one study (170). Further studies are needed to assess the efficacy of topical finasteride.
Medical treatment should be continued indefinitely, as the benefit will not be maintained upon ceasing therapy. Up to one year of treatment may be required before any clinical response is noticeable.
Baseline and follow up photographs are helpful in monitoring the response to treatment, but unlikely to detect changes of less than 20% in hair density. The authors make use of a camera mounted on a stereotactic device; a system that is identical to the set-up used in the phase III finasteride trials (169). Photographs are taken of the vertex and frontal hairline at six-monthly to yearly intervals and hair densities at these time points can be readily compared. This set-up is proving to be useful in the long-term monitoring of treatment response (Figure 10 & 11). Patients are able to observe their regrowth during treatment, while the photographs serve as a motivating factor, improving long-term patient compliance to medical treatment. Similar set-ups using digital photographs also appear useful (171).
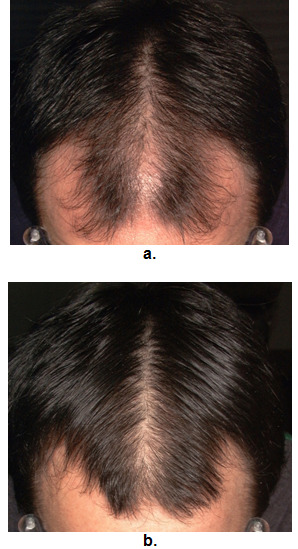
Figure 11.
Photographic evaluation of treatment response to finasteride. a - pretreatment. b - post treatment.

Figure 11.
Hair photography using the stereotactic device.
Finasteride is a teratogen, pregnancy category X drug and is therefore contraindicated in pregnancy. Male rats exposed to finasteride in utero develop hypospadias with cleft prepuce, decreased anogenital distance, reduced prostate weight, and altered nipple formation (160). As the drug is secreted in the semen and can be absorbed through the vagina during intercourse, it was originally advocated that men taking finasteride should avoid unprotected intercourse with pregnant women. In practice, the concentration of finasteride in the semen is well below the minimum effect dosage, and no recommendations regarding the use of condoms are made in the product information leaflet. To date there are no reports of adverse pregnancy outcomes among women whose partners take finasteride.
Finasteride has demonstrable small effect on semen parameters in normal men including decreased total sperm count, semen volume, sperm concentration, and sperm motility but no apparent effect on sperm morphology (172). With regards to long-term safety, finasteride has now been in use for over 10 years. Many recipients are elderly men taking 5mg per day. Very few side-effects have been observed. There is no effect of long-term use on bone mineral density (173-174). Reversible painful gynecomastia has been reported and the incidence is thought to be around 0.001% (175, 176). Depression measured by increased Beck Depression Inventory (BDI) and Hospital Anxiety and Depression Scale (HADS) before and after treatment has been reported (177).
Finasteride related reports of suicidality and psychological adverse events have led to coining of the term post finasteride syndrome and the creation of organizations such as the Post-Finasteride Syndrome Foundation (178). In 2011, a post-marketing report was sent to the US Food and Drug Administration suggesting that finasteride might be linked to depression, self-harm, and suicide (178). In the last 5 years, health authorities in Canada, Korea, New Zealand, and the United Kingdom have also acknowledged these potential adverse effects and issued warnings for finasteride (178). There is a potential biological basis linking finasteride with depression and anxiety. Some reports suggest that men with depression have lower levels of the neurosteroid allopregnanolone, which is produced by the 5α-reductase enzyme and has antidepressant and anxiolytic effects (179).
Topical finasteride application for the treatment of MAA was first explored by Mazarella et al. in a study involving 52 patients (28 men). They reported at 6 months of treatment, a progressive and significant decrease in the rate of hair loss was observed in the topical finasteride versus placebo group, with no significant changes in plasma levels of total testosterone, free testosterone, and DHT between treatment groups (180). Pirraccin et al. reported a phase III randomized control trial reviewing safety and efficacy of topical finasteride spray solution (181). A total of 458 patients were randomized, 323 completed the study and 446 were evaluated for safety. Their primary endpoint was defined as change from baseline in target area hair count at week 24. They found this was significantly greater with topical finasteride than placebo (adjusted mean change 20.2 vs. 6.7 hairs; P<0.001), and numerically similar between topical and oral finasteride. No serious adverse events were reported. As maximum plasma finasteride concentrations were >100 times lower, and reduction from baseline in mean serum DHT concentration was less (34.5 vs. 55.6%), with topical versus oral finasteride, there is less likelihood of systemic sexual adverse events related to a decrease in DHT with topical finasteride (181).
DUTASTERIDE
Dutasteride inhibits both type I and type II 5-alpha reductase. It is approximately 3 times and more than 100 times more potent than finasteride at inhibiting the type I and II 5-alpha reductase isoenzymes respectively (182). Dutasteride can decrease serum DHT by more than 90%, while finasteride decreases serum DHT by 70% (182, 183).
The serum half-life of dutasteride is 4 weeks as compared with a serum half-life of 6-8 hours for finasteride. There is persistent suppression of DHT level after dutasteride is ceased. For this reason, patients taking dutasteride should not donate blood until at least 6 months after stopping their medication, to prevent administration to a pregnant transfusion recipient (184). Dutasteride 0.5mg dose is FDA approved for the treatment of benign prostatic hyperplasia while its use in MAA is ‘off label’.
A phase II randomized placebo-controlled study of dutasteride versus finasteride showed that the effect of dutasteride was dose dependent and 2.5mg of dutasteride was superior to 5mg finasteride in improving scalp hair growth in men between the ages of 21 and 45 years (184). It was also able to produce hair growth earlier than finasteride. This was evidenced by target area hair counts and clinical assessment at 12 and 24 weeks. In addition, a recent randomized, double blind, placebo-controlled study on the efficacy of dutasteride 0.5mg/day in identical twins demonstrated that dutasteride was able to significantly reduce hair loss progression in men with MAA (185). A single case report showed improvement of hair loss with dutasteride 0.5mg in a woman who had failed to show any response to finasteride (186).
In one phase III study dutasteride 0.5mg daily showed significantly higher efficacy than placebo based on subject self-assessment and by investigator and panel photographic assessment (187). There was no major difference in adverse events between the two groups, the treatment and placebo groups. However, this study was limited to only 6 months. Another more recent phase III trial found that dutasteride 0.5mg was statistically superior to finasteride 1mg and placebo at 24 weeks (188).
Side effects, including decreased libido, impotence, and gynecomastia, are slightly more common with dutasteride than with finasteride (184, 189). Reduction in the sperm count and the volume has been reported with dutasteride (187, 190). There is no effect on bone density (189). The issue of 5alpha reductase inhibitor use and prostate cancer is considered in another Endotext chapter.
Dutasteride mesotherapy or microinjections were developed as an alternative to systemic antiandrogens in view of several men being reluctant to treat their MAA with systemic 5a-reductase enzyme inhibitors due to risk of adverse sexual side-effects. Saceda-Corralo et al. reported improvement of AGA in men treated with 1 mL of intradermal dutasteride 0.01% mesotherapy. Another study evaluated intradermal injection of dutasteride in 28 male patients with AGA over 11 weeks (191). Villarreal-Villarreal et al. reviewed dutasteride injections combined with oral minoxidil versus oral minoxidil alone and found a better response on the vertex area compared to the frontal area. They hypothesize that dutasteride might be more effective in vertex due to the greater concentration of 5 alpha reductase compared to the frontal zone. There have been no reports of sexual dysfunction in these patients and the main adverse event experienced is pain during the injections (192, 193).
Emerging Medical Therapy
TOPICAL ANTIANDROGENS
Oral anti-androgens (e.g., spironolactone, cyproterone acetate) have been widely used to treat women with androgenetic alopecia. However, these are contraindicated in men due to its feminization effects. A topical anti-androgen, fluridil has been rationally developed for use in male androgenetic alopecia. It is designed to be locally metabolized, not systemically resorbable, and degradable into inactive metabolites without systemic anti-androgenic activity (194). A double blind, placebo-controlled study showed that patients using topical fluridil had an increase in the anagen to telogen ratio, and the maximum attainable effect was achieved within the first 90 days of daily use. No side effects on libido and sexual performance have been found. Nevertheless, a long-term study is required to further investigate fluridil's long-term safety and effectiveness in male androgenetic alopecia.
Clascoterone (cortexolone 17α-proprionate) is a novel androgen receptor inhibitor, and recent trials reporting its topical use in acne have demonstrated promising results. Clascoterone antagonizes dihydrotestosterone (DHT) through competitive binding with cytoplasmic androgen receptors as it shares the same fused four-ring backbone structure (195). In August 2020, clascoterone cream 1% received its first approval in the USA for the treatment of acne vulgaris in patients 12 years of age or older (196). Clascoterone has been found to have the same efficacy as finasteride in vitro (197).
LATANOPROST
The prostaglandin analogue latanoprost stimulates hair growth supposedly by prolonging the anagen phase of the hair cycle. Lengthening of eyelashes and eyebrows has been observed when latanoprost is used topically for glaucoma (198). In a placebo controlled study, latanoprost was able to significantly increase hair density compared with baseline and placebo and may also encourage pigmentation (199).
TOPICAL ANTIBIOTICS AND ANTIFUNGALS
The role of inflammation in the pathogenesis of MAA is not clear. In particular, the significance of inflammatory cells close to the infra infundibulum of transitional hairs remains obscure. A study conducted in 20 men who used a lotion containing antimicrobials, piroctoneolamine and triclosan, regularly for 18 months showed a decrease in the density of activated T cells in the region of the follicular infra-infundibulum and isthmus over time (200). Trichograms taken at 3-month intervals suggested signs of hair regrowth with moderate increase in density of transitional hairs. Further studies are needed to confirm the effect of topical antimicrobials as a therapeutic option for MAA.
Topical ketoconazole shampoo has been shown to increased hair growth in both humans and in rodents when compared with placebo (201). Oral ketoconazole has been beneficial in treating hirsutism but the potential side effects do not warrant its use for androgenic alopecia. Ketoconazole shampoo is a good additive treatment and thought to be having anti-inflammatory and anti-androgenic properties, and it will also help associated seborrheic dermatitis if present (202).
GROWTH FACTORS
The growth and development of hair follicles is influenced by a number of different growth factors and cytokines. Use of such growth factors to promote hair growth, topically or subcutaneously, is a potential therapeutic target. Preliminary investigations using animal models have shown positive results. A phase I, double-blind clinical trial designed to evaluate the safety of a bioengineered, non-recombinant, human cell–derived formulation containing follistatin, keratinocyte growth factor (KGF), and vascular endothelial growth factor (VEGF) was performed to assess the efficacy in stimulating hair growth (203). Twenty-six subjects were entered into the study and none showed an adverse reaction to the single intradermal injection. After one year, a statistically significant increase in total hair count continued to be seen.
Platelet-rich plasma (PRP) isolated from whole blood can be used for its growth factors and stimulatory mediators. Some hair transplant surgeons use this product to encourage transplanted graft growth (204). Platelet-rich plasma is also available as a stand-alone treatment for MAA, with recent limited data suggesting positive regrowth with minimal side effects (205, 206). It is hypothesized that platelet-rich plasma stimulates hair growth by improving follicle vascularization, inhibiting apoptosis and therefore, prolonging the anagen phase, and inducing a faster transition from the telogen to the anagen phase (207).
LASER TREATMENT
Laser/light treatment for hair loss has become very popular in the last few years for a number of dermatological conditions. It has also been promoted as a preventative measure against MAA. Several different manufacturers provide lasers and light sources of varying wavelengths and with different suggested modes of use. Whilst there is evidence that laser light can stimulate hair growth at some wavelengths, the biological mechanism by which it occurs has not been defined and clinical data from large scale, placebo controlled trials is lacking (208-210). The most well-recognized modality are low-level laser/light therapies (LLLT), such as the HairMax Lasercomb®, and He-Ne laser, which are considered safe, effective, and easily accessible treatment options (211). Fractional laser is hypothesized that it induces hair growth when restricted to appropriate settings by upregulating the Wnt/βcatenin pathways and efficiently penetrating the scalp (212). Although the exact mechanisms of certain fractional lasers on hair regrowth are still uncertain, trauma stimulated wound healing likely plays a role. Hair follicle stem cells provide progeny that migrate to the epidermal defect and promote re-epithelialization (212).
Laser-assisted drug delivery is an evolving technology with potentially broad clinical applications. Lasers stimulate drug delivery by means of 3 processes; 1) tissue ablation; 2) photomechanical waves, resulting from the conversion of light into mechanical energy giving the laser a therapeutic effect; and 3) non-ablative resurfacing where thermal and physical injuries disrupt the skin barrier, promoting the delivery of medications through these laser channels (213). A prospective study comparing the efficacy and safety of fractional erbium-glass (Er:Glass) laser used in combination with topical 5% minoxidil versus 5% minoxidil alone for the treatment of male AGA. They treated 30 men with AGA who were randomized to 24 weeks of split-scalp treatment using fractional Er:Glass laser and 5% minoxidil on one side (combined therapy) or 5% minoxidil alone on the other side (monotherapy). The primary outcome was the difference in hair density and diameter, from baseline, between two treatment sides, at week 24. The secondary outcome was a global photographic assessment, evaluated by two dermatologists and the participants. Results demonstrated that combination therapy provided significantly superior results for both the primary and secondary outcomes (214). Further studies are required to formally evaluate the role of LAD in MAA treatment algorithm.
CLINICAL TRIALS
There is currently an open label study, evaluating the safety, tolerability, and efficacy in both males and females with androgenetic alopecia being treated with HMI-115 over a 24-week treatment period (215). HMI-115 is a potent monoclonal antibody, blocking the prolactin (PRL) receptor-mediated pathway in a non-competitive manner. It can be administered subcutaneously. The antibody was well tolerated in a clinical Ph I study (combined single and multiple dosing). The antibody was effective in stimulating hair growth in aged stump-tailed macaques, nearly doubling the number of terminal hairs after 6 months even in previously fully bald areas and showing a sustainable impact even after 2 years post treatment (216). The stump-tail macaque model is considered one of the rare predictive animal models for male and female pattern hair loss in humans (217).
Surgical Treatments
Hair transplantation involves removal of hair from the occipital scalp and re-implantation into the bald vertex and frontal scalp. With modern techniques, graft survival in excess of 90% can be reliably achieved. Prerequisites for the procedure are stabilization of the hair loss with medical treatment and good donor hair population on the occipital hair.
The modern hair transplant technique was started in Japan in the 1930s, where small punch grafts were used to cover damaged eyebrows or lashes.(218) Norman Orentreich reported on the use of autografts and proposed the term "donor dominance", in that the hair taken from the androgen resistant occipital scalp remain androgen resistant when implanted into the androgen sensitive bald areas of the scalp (85).
In 1995, Bernstein, Rassman et al introduced "Follicular Unit Transplantation," where hair is transplanted in naturally occurring units of 1-4 hair (219). In "Follicular Unit Transplantation", donor hair can be harvested in two different ways:
Strip Harvesting - a strip of scalp 8 - 14 mm and 20 – 30 cm is removed under local anesthesia, from the occipital scalp and the wound is then sutured back together. The donor hair is then separated into follicular units and then transplanted into the balding area. The main disadvantage of this method is that it will leave a linear scar in the donor occipital area.
∙Follicular Unit Extraction (FUE) Harvesting - individual follicles of occipital hair are removed under local anesthesia with 1mm punch biopsies. Each unit is then reinserted back into the scalp in the bald areas using a micro blade. The advantages of this technique are that there are no visible scars and takes a shorter time to heal than strip harvesting.
Both techniques can achieve good results, but follicular unit transplantations have the advantage of being able to achieve much greater hair densities. It is preferable that surgical candidates have frontal or mid-frontal hair loss as opposed to hair loss at the vertex, and their donor hair density needs to be adequate to support the surgery (i.e., > 40 follicular units/cm2). Also, thicker donor hairs are able to create better coverage compared with finer hair. Disadvantages of hair transplants are increased time and labor requirements, which translates to greater cost for the patient. Transplanted hairs seem to immediately go into a telogen resting phase after insertion. Thus, surgical results can only be adequately assessed after no less than three months after surgery. There is always a degree of graft failure. Various reasons account for dead grafts, including the skill of the surgeon, the density of graft placement, careless handling and preparation of the graft units, and desiccation of the grafts whilst awaiting insertion.
Scalp reductions result in a more unnatural look with excision scars tending to be more noticeable over time (220). In addition, the inability to predict further hair loss over time in each patient has meant that the procedures are now uncommonly performed.
Combination of Medical, Medical and Surgical Therapy
An open, randomized, parallel-group study comparing the efficacy of available medications as monotherapy or combined therapy (finasteride alone, finasteride and 2% topical minoxidil, topical minoxidil alone and finasteride and ketoconazole shampoo) showed that finasteride in combination with either topical minoxidil or ketoconazole showed significantly better hair regrowth than with finasteride as monotherapy and showed no difference in the incidence of side effects. It is inferred that the combination of medication with different mechanisms of action enhances the efficacy (221). A recent case study showed that adding dutasteride 0.5mg in a patient who had poor response to finasteride had marked improvement of hair loss (222).
Topical minoxidil and finasteride can be a useful adjunct to hair transplant surgery for MAA. Without adjuvant medical therapy to prevent progression of balding process, an unnatural appearance can evolve over time. A successful medical treatment is key to stopping hair loss progression and offering a better guarantee of a sustained long-term transplant outcome. Hair transplant surgeons therefore recommend medical therapy should first be initiated prior to reviewing the option of transplantation (223). Studies have found that topical use of minoxidil in perioperative period could prevent the usual shedding that occurs 1 to 2 weeks after transplantation and speed the time for regrowth (224). These results were confirmed by a double-blind trial, which showed that significantly less grafted hair was lost during the shedding period (225). Use of topical minoxidil as a premedication in hair transplant surgery has the advantage of stabilizing the hair loss, increasing the number of hairs in anagen phase and decreasing post-surgical telogen effluvium. Minoxidil should be stopped 2 to 3 days before surgery to minimize skin irritation and to reduce the theoretical risk of intraoperative bleeding caused by vasodilation. Therapy should be restarted in 1-2 weeks. A randomized, double-blind trial in 79 men with MAA using finasteride 1 mg daily or placebo 4 weeks before and 48 weeks after hair transplantation demonstrated that the treatment group had significant improvement from baseline, in comparison with placebo group (226).
CONCLUSION
MAA is increasingly common among men as they age. Many men find it a distressing and unwelcome event and increasing numbers are seeking treatment to prevent further hair loss and reverse the process. In addition, MAA may be a marker of increased risk of cardiovascular diseases. The hair follicle is a complex organ biologically. The changes in the hair follicles that lead to baldness have caught the interest of stem cell scientists, geneticists, developmental biologists, and immunologists and hair biology has become an increasingly fruitful field of scientific endeavor. A number of therapeutic options are now available for these men with favorable cosmetic outcomes possible in the majority of cases.
REFERENCES
- 1.
- Hamilton JB. Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 1951;53(3):708–728. [PubMed: 14819896]
- 2.
- Olsen EA. Disorders of Hair Growth: Diagnosis and Treatment. McGraw-Hill, Medical Pub. Division; 2003.
- 3.
- Montagna W, Ellis RA. The Biology of Hair Growth. Elsevier; 2013.
- 4.
- Ishino A, Uzuka M, Tsuji Y, Nakanishi J, Hanzawa N, Imamura S. Progressive decrease in hair diameter in Japanese with male pattern baldness. J. Dermatol. 1997;24(12):758–764. [PubMed: 9492438]
- 5.
- Setty LR. Hair patterns of the scalp of white and Negro males. American Journal of Physical Anthropology. 1970;33(1):49–55. [PubMed: 5431486]
- 6.
- Smith MA. Male-Type Alopecia, Alopecia Areata, and Normal Hair in Women. Archives of Dermatology. 1964;89(1):95. [PubMed: 14070844]
- 7.
- Muller SA. Alopecia: syndromes of genetic significance. J. Invest. Dermatol. 1973;60(6):475–492. [PubMed: 4581946]
- 8.
- Tang PH, Chia HP, Cheong LL, Koh D. A community study of male androgenetic alopecia in Bishan. Singapore. Singapore Med. J. 2000;41(5):202–205. [PubMed: 11063167]
- 9.
- Severi G, Sinclair R, Hopper JL, English DR, McCredie MRE, Boyle P, Giles GG. Androgenetic alopecia in men aged 40-69 years: prevalence and risk factors. Br. J. Dermatol. 2003;149(6):1207–1213. [PubMed: 14674898]
- 10.
- Rhodes T, Girman CJ, Savin RC, Kaufman KD, Guo S, Lilly FR, Siervogel RM, Chumlea WC. Prevalence of male pattern hair loss in 18-49 year old men. Dermatol. Surg. 1998;24(12):1330–1332. [PubMed: 9865198]
- 11.
- Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br. J. Dermatol. 2001;145(1):95–99. [PubMed: 11453914]
- 12.
- Cash TF. The psychological effects of androgenetic alopecia in men. J. Am. Acad. Dermatol. 1992;26(6):926–931. [PubMed: 1607410]
- 13.
- Budd D, Himmelberger D, Rhodes T, Cash TE, Girman CJ. The effects of hair loss in European men: a survey in four countries. Eur. J. Dermatol. 2000;10(2):122–127. [PubMed: 10694311]
- 14.
- Lee H-J, Ha S-J, Kim D, Kim H-O, Kim J-W. Perception of men with androgenetic alopecia by women and nonbalding men in Korea: how the nonbald regard the bald. Int. J. Dermatol. 2002;41(12):867–869. [PubMed: 12492971]
- 15.
- Passchier J. Quality of life issues in male pattern hair loss. Dermatology. 1998;197(3):217–218. [PubMed: 9812023]
- 16.
- Tabolli S, Sampogna F, di Pietro C, Mannooranparampil TJ, Ribuffo M, Abeni D. Health Status, Coping Strategies, and Alexithymia in Subjects with Androgenetic Alopecia. American Journal of Clinical Dermatology. 2013;14(2):139–145. [PubMed: 23413102]
- 17.
- Cotton SG, Nixon JM, Carpenter RG, Evans DW. Factors discriminating men with coronary heart disease from healthy controls. Br. Heart J. 1972;34(5):458–464. [PMC free article: PMC486958] [PubMed: 5031636]
- 18.
- Herrera CR, D’Agostino RB, Gerstman BB, Bosco LA, Belanger AJ. Baldness and coronary heart disease rates in men from the Framingham Study. Am. J. Epidemiol. 1995;142(8):828–833. [PubMed: 7572959]
- 19.
- Ford ES, Freedman DS, Byers T. Baldness and ischemic heart disease in a national sample of men. Am. J. Epidemiol. 1996;143(7):651–657. [PubMed: 8651226]
- 20.
- Lesko SM, Rosenberg L, Shapiro S. A case-control study of baldness in relation to myocardial infarction in men. JAMA. 1993;269(8):998–1003. [PubMed: 8429606]
- 21.
- Schnohr P, Lange P, Nyboe J, Appleyard M, Jensen G. Gray hair, baldness, and wrinkles in relation to myocardial infarction: the Copenhagen City Heart Study. Am. Heart J. 1995;130(5):1003–1010. [PubMed: 7484729]
- 22.
- Agac MT, Bektas H, Korkmaz L, Cetin M, Erkan H, Gurbak I, Hatem E, Celik S. Androgenetic alopecia is associated with increased arterial stiffness in asymptomatic young adults. Journal of the European Academy of Dermatology and Venereology. 2015;29(1):26–30. [PubMed: 24628808]
- 23.
- Lotufo PA, Chae CU, Ajani UA, Hennekens CH, Manson JE. Male pattern baldness and coronary heart disease: the Physicians’ Health Study. Arch. Intern. Med. 2000;160(2):165–171. [PubMed: 10647754]
- 24.
- Arias-Santiago S, Gutiérrez-Salmerón MT, Buendía-Eisman A, Girón-Prieto MS, Naranjo-Sintes R. Hypertension and aldosterone levels in women with early-onset androgenetic alopecia. British Journal of Dermatology. 2010;162(4):786–789. [PubMed: 19906217]
- 25.
- Ahouansou S, Le Toumelin P, Crickx B, Descamps V. Association of androgenetic alopecia and hypertension. Eur. J. Dermatol. 2007;17(3):220–222. [PubMed: 17478384]
- 26.
- Ellis JA, Stebbing M, Harrap SB. Male pattern baldness is not associated with established cardiovascular risk factors in the general population. Clin. Sci. 2001;100(4):401–404. [PubMed: 11256978]
- 27.
- Oh BR, Kim SJ, Moon JD, Kim HN, Kwon DD, Won YH, Ryu SB, Park YI. Association of benign prostatic hyperplasia with male pattern baldness. Urology. 1998;51(5):744–748. [PubMed: 9610587]
- 28.
- Chen W, Yang C-C, Chen G-Y, Wu M-C, Sheu H-M, Tzai T-S. Patients with a large prostate show a higher prevalence of androgenetic alopecia. Archives of Dermatological Research. 2004;296(6):245–249. [PubMed: 15517324]
- 29.
- Arias-Santiago S, Arrabal-Polo MA, Buenda-Eisman A, Arrabal-Martn M, Gutirrez-Salmern MT, Girn-Prieto MS, Jimenez-Pacheco A, Calonje JE, Naranjo-Sintes R, Zuluaga-Gomez A, Ortega SS. Androgenetic alopecia as an early marker of benign prostatic hyperplasia. Journal of the American Academy of Dermatology. 2012;66(3):401–408. [PubMed: 21835498]
- 30.
- Dastgheib L, Shirazi M, Moezzi I, Dehghan S, Sadati M-S. Is there a relationship between androgenic alopecia and benign prostatic hyperplasia? Acta Med. Iran. 2015;53(1):30–32. [PubMed: 25597602]
- 31.
- Hawk E, Breslow RA, Graubard BI. Male pattern baldness and clinical prostate cancer in the epidemiologic follow-up of the first National Health and Nutrition Examination Survey. Cancer Epidemiol. Biomarkers Prev. 2000;9(5):523–527. [PubMed: 10815699]
- 32.
- Giles GG, Severi G, Sinclair R, English DR, McCredie MRE, Johnson W, Boyle P, Hopper JL. Androgenetic alopecia and prostate cancer: findings from an Australian case-control study. Cancer Epidemiol. Biomarkers Prev. 2002;11(6):549–553. [PubMed: 12050096]
- 33.
- Muller DC, Giles GG, Sinclair R, Hopper JL, English DR, Severi G. Age-Dependent Associations between Androgenetic Alopecia and Prostate Cancer Risk. Cancer Epidemiology, Biomarkers & Prevention. 2013;22(2):209–215. [PubMed: 23074289]
- 34.
- Amoretti A, Laydner H, Bergfeld W. Androgenetic alopecia and risk of prostate cancer: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 2013;68(6):937–943. [PubMed: 23395589]
- 35.
- Denmark-Wahnefried W, Schildkraut JM, Thompson D, Lesko SM, McIntyre L, Schwingl P, Paulson DF, Robertson CN, Anderson EE, Walther PJ. Early onset baldness and prostate cancer risk. Cancer Epidemiol. Biomarkers Prev. 2000;9(3):325–328. [PubMed: 10750672]
- 36.
- Zhou J, Xia S, Li T, Liu R. Association between male pattern baldness and testicular germ cell tumor: a meta-analysis. BMC Cancer. 2019;19(1):53. [PMC free article: PMC6329191] [PubMed: 30634927]
- 37.
- Ingles SA, Liu SV, Pinski J. LHRH and LHR genotypes and prostate cancer incidence and survival. Int. J. Mol. Epidemiol. Genet. 2013;4(4):228–234. [PMC free article: PMC3852642] [PubMed: 24319538]
- 38.
- Moravvej H, Pourani MR, Baghani M, Abdollahimajd F. Androgenetic alopecia and COVID-19: A review of the hypothetical role of androgens. Dermatol. Ther. 2021;34(4):e15004. [PMC free article: PMC8209856] [PubMed: 34033224]
- 39.
- Ghafoor R, Ali SM, Patil A, Goldust M. Association of androgenetic alopecia and severity of coronavirus disease 2019. J. Cosmet. Dermatol. 2022;21(3):874–879. [PubMed: 34918457]
- 40.
- Wambier CG, Vaño-Galván S, McCoy J, Gomez-Zubiaur A, Herrera S, Hermosa-Gelbard Á, Moreno-Arrones OM, Jiménez-Gómez N, González-Cantero A, Fonda-Pascual P, Segurado-Miravalles G, Shapiro J, Pérez-García B, Goren A. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The “Gabrin sign.” J. Am. Acad. Dermatol. 2020;83(2):680–682. [PMC free article: PMC7242206] [PubMed: 32446821]
- 41.
- Lee J, Yousaf A, Fang W, Kolodney MS. Male balding is a major risk factor for severe COVID-19. Journal of the American Academy of Dermatology. 2020;83(5):e353–e354. [PMC free article: PMC7373684] [PubMed: 32707256]
- 42.
- Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J. Am. Acad. Dermatol. 2020;83(1):308–309. [PMC free article: PMC7151476] [PubMed: 32283245]
- 43.
- Goren A, Wambier CG, Herrera S, McCoy J, Vaño-Galván S, Gioia F, Comeche B, Ron R, Serrano-Villar S, Ramos PM, Cadegiani FA, Kovacevic M, Tosti A, Shapiro J, Sinclair R. Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men. J. Eur. Acad. Dermatol. Venereol. 2021;35(1):e13–e15. [PMC free article: PMC7536996] [PubMed: 32977363]
- 44.
- Kalra S, Bhattacharya S, Kalhan A. Testosterone in COVID-19 - Foe, Friend or Fatal Victim? Eur Endocrinol. 2020;16(2):88–91. [PMC free article: PMC7572157] [PubMed: 33117437]
- 45.
- McCoy J, Cadegiani FA, Wambier CG, Herrera S, Vaño‐Galván S, Mesinkovska NA, Ramos PM, Shapiro J, Sinclair R, Tosti A, Goren A. 5‐alpha‐reductase inhibitors are associated with reduced frequency of COVID‐19 symptoms in males with androgenetic alopecia. Journal of the European Academy of Dermatology and Venereology. 2021;35(4) [PubMed: 33135263] [CrossRef]
- 46.
- Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone GM, Cavalli A, Pagano F, Ragazzi E, Prayer-Galetti T, Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Annals of Oncology. 2020;31(8):1040–1045. [PMC free article: PMC7202813] [PubMed: 32387456]
- 47.
- Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev. Mol. Med. 2002;4(22):1–11. [PubMed: 14585162]
- 48.
- Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic basis of male pattern baldness. J. Invest. Dermatol. 2003;121(6):1561–1564. [PubMed: 14675213]
- 49.
- Ellis JA, Harrap SB. The genetics of androgenetic alopecia. Clin. Dermatol. 2001;19(2):149–154. [PubMed: 11397593]
- 50.
- Osborn. Inheritance of baldness: various patterns due to heredity and sometimes present at birth—a sex-limited character—dominant in man—women not bald …. J. Hered. [CrossRef]
- 51.
- Küster W, Happle R. The inheritance of common baldness: Two B or not two B ? Journal of the American Academy of Dermatology. 1984;11(5):921–926. [PubMed: 6512048]
- 52.
- Ellis JA, Stebbing M, Harrap SB. Genetic Analysis of Male Pattern Baldness and the 5α-Reductase Genes. J. Invest. Dermatol. 1998;110(6):849–853. [PubMed: 9620288]
- 53.
- Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J. Invest. Dermatol. 2001;116(3):452–455. [PubMed: 11231320]
- 54.
- Sawaya ME, Price VH. Different Levels of 5α-Reductase Type I and II, Aromatase, and Androgen Receptor in Hair Follicles of Women and Men with Androgenetic Alopecia. J. Invest. Dermatol. 1997;109(3):296–300. [PubMed: 9284093]
- 55.
- Yip L, Zaloumis S, Irwin D, Severi G, Hopper J, Giles G, Harrap S, Sinclair R, Ellis J. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. British Journal of Dermatology. 2009;161(2):289–294. [PubMed: 19438456]
- 56.
- Hillmer AM, Flaquer A, Hanneken S, Eigelshoven S, Kortüm A-K, Brockschmidt FF, Golla A, Metzen C, Thiele H, Kolberg S, Reinartz R, Betz RC, Ruzicka T, Hennies HC, Kruse R, Nöthen MM. Genome-wide Scan and Fine-Mapping Linkage Study of Androgenetic Alopecia Reveals a Locus on Chromosome 3q26. Am. J. Hum. Genet. 2008;82(3):737–743. [PMC free article: PMC2427264] [PubMed: 18304493]
- 57.
- Hillmer AM, Brockschmidt FF, Hanneken S, Eigelshoven S, Steffens M, Flaquer A, Herms S, Becker T, Kortüm A-K, Nyholt DR, Zhao ZZ, Montgomery GW, Martin NG, Mühleisen TW, Alblas MA, Moebus S, Jöckel K-H, Bröcker-Preuss M, Erbel R, Reinartz R, Betz RC, Cichon S, Propping P, Baur MP, Wienker TF, Kruse R, Nöthen MM. Susceptibility variants for male-pattern baldness on chromosome 20p11. Nature Genetics. 2008;40(11):1279–1281. [PubMed: 18849994]
- 58.
- Brockschmidt FF, Heilmann S, Ellis JA, Eigelshoven S, Hanneken S, Herold C, Moebus S, Alblas MA, Lippke B, Kluck N, Priebe L, Degenhardt FA, Jamra RA, Meesters C, Jöckel K-H, Erbel R, Harrap S, Schumacher J, Fröhlich H, Kruse R, Hillmer AM, Becker T, Nöthen MM. Susceptibility variants on chromosome 7p21.1 suggest HDAC9 as a new candidate gene for male-pattern baldness. British Journal of Dermatology. 2011;165(6):1293–1302. [PubMed: 22032556]
- 59.
- Pirastu N, Joshi PK, de Vries PS, Cornelis MC, McKeigue PM, Keum N, Franceschini N, Colombo M, Giovannucci EL, Spiliopoulou A, Franke L, North KE, Kraft P, Morrison AC, Esko T, Wilson JF. GWAS for male-pattern baldness identifies 71 susceptibility loci explaining 38% of the risk. Nat. Commun. 2017;8(1):1584. [PMC free article: PMC5691155] [PubMed: 29146897]
- 60.
- Yamazaki M, Sato A, Toyoshima K-E, Kojima Y, Okada T, Ishii Y, Kurata S, Yoshizato K, Tsuboi R. Polymorphic CAG repeat numbers in the androgen receptor gene of female pattern hair loss patients. J. Dermatol. 2011;38(7):680–684. [PubMed: 21352305]
- 61.
- Hamilton JB. Male hormone stimulation is prerequisite and an incitant in common baldness. Am. J. Anat. 1942;71(3):451–480.
- 62.
- Pitts RL. Serum elevation of dehydroepiandrosterone sulfate associated with male pattern baldness in young men. J. Am. Acad. Dermatol. 1987;16(3 Pt 1):571–573. [PubMed: 2950147]
- 63.
- Schmidt JB. Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacol. 1994;7(1-2):61–66. [PubMed: 8003325]
- 64.
- Schmidt JB, Lindmaier A, Trenz A, Schurz B, Spona J. Hormone studies in females with androgenic hairloss. Gynecol. Obstet. Invest. 1991;31(4):235–239. [PubMed: 1832134]
- 65.
- Hamilton JB. Effect of castration in adolescent and young adult males upon further changes in the proportions of bare and hairy scalp. J. Clin. Endocrinol. Metab. 1960;20:1309–1318. [PubMed: 13711016]
- 66.
- Wilson JD, Griffin JE, Russell DW. Steroid 5 alpha-reductase 2 deficiency. Endocr. Rev. 1993;14(5):577–593. [PubMed: 8262007]
- 67.
- Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186(4170):1213–1215. [PubMed: 4432067]
- 68.
- Sinclair R, Greenland KJ, van Egmond S, Hoedemaker C, Chapman A, Zajac JD. Men with Kennedy disease have a reduced risk of androgenetic alopecia. British Journal of Dermatology. 2007;157(2):290–294. [PubMed: 17596176]
- 69.
- Schweikert HU, Wilson JD. Regulation of Human Hair Growth by Steroid Hormones. II. Androstenedione Metabolism in Isolated Hairs. The Journal of Clinical Endocrinology & Metabolism. 1974;39(6):1012–1019. [PubMed: 4430701]
- 70.
- Randall VA, Julie Thornton M, Hamada K, Messenger AG. Androgen Action in Cultured Dermal Papilla Cells from Human Hair Follicles. Skin Pharmacology and Physiology. 1994;7(1-2):20–26. [PubMed: 8003318]
- 71.
- Kaufman KD. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol. Clin. 1996;14(4):697–711. [PubMed: 9238328]
- 72.
- Androgen influence on hair growth. In: Skin, Hair, and Nails. CRC Press; 2003:353–374.
- 73.
- Itami S, Kurata S, Sonoda T, Takayasu S. Characterization of 5α-Reductase in Cultured Human Dermal Papilla Cells from Beard and Occipital Scalp Hair. Journal of Investigative Dermatology. 1991;96(1):57–60. [PubMed: 1987297]
- 74.
- Chen W, Thiboutot D, Zouboulis CC. Cutaneous androgen metabolism: basic research and clinical perspectives. J. Invest. Dermatol. 2002;119(5):992–1007. [PubMed: 12445184]
- 75.
- Bayne Bayne, Flanagan Einstein, Ayala Chang, Azzolina Whiting, Mumford Thiboutot, Singer Harris. Immunohistochemical localization of types 1 and 2 5α-reductase in human scalp. British Journal of Dermatology. 1999;141(3):481–491. [PubMed: 10583052]
- 76.
- Hoffmann R, Happle R. Current understanding of androgenetic alopecia. Part I: etiopathogenesis. Eur. J. Dermatol. 2000;10(4):319–327. [PubMed: 10846263]
- 77.
- Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J. Invest. Dermatol. 2001;116(5):793–800. [PubMed: 11348472]
- 78.
- Itami S, Kurata S, Takayasu S. Androgen Induction of Follicular Epithelial Cell Growth Is Mediated via Insulin-like Growth Factor-I from Dermal Papilla Cells. Biochemical and Biophysical Research Communications. 1995;212(3):988–994. [PubMed: 7626139]
- 79.
- Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen‐inducible TGF‐β1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understanding paradoxical effects of androgen on human hair growth. The FASEB Journal. 2002;16(14):1967–1969. [PubMed: 12397096]
- 80.
- Thornton MJ, Hamada K, Messenger AG, Randall VA. Androgen-dependent beard dermal papilla cells secrete autocrine growth factor(s) in response to testosterone unlike scalp cells. J. Invest. Dermatol. 1998;111(5):727–732. [PubMed: 9804329]
- 81.
- Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of scalp. Journal of Endocrinology. 1992;133(1):141–147. [PubMed: 1517703]
- 82.
- Thornton MJ, Laing I, Hamada K, Messenger AG, Randall VA. Differences in testosterone metabolism by beard and scalp hair follicle dermal papilla cells. Clinical Endocrinology. 1993;39(6):633–639. [PubMed: 8287580]
- 83.
- Itami S, Kurata S, Takayasu S. 5α-Reductase Activity in Cultured Human Dermal Papilla Cells from Beard Compared with Reticular Dermal Fibroblasts. Journal of Investigative Dermatology. 1990;94(1):150–152. [PubMed: 2295831]
- 84.
- Boudou P. Increased scalp skin and serum 5 alpha-reductase reduced androgens in a man relevant to the acquired progressive kinky hair disorder and developing androgenetic alopecia. Archives of Dermatology. 1997;133(9):1129–1133. [PubMed: 9301590]
- 85.
- Orentreich N. Autografts in alopecias and other selected dermatological conditions. Ann. N. Y. Acad. Sci. 1959;83:463–479. [PubMed: 14429008]
- 86.
- Olsen EA. Female pattern hair loss: Clinical features and potential hormonal factors. Journal of the American Academy of Dermatology. 2001;45(3):S69. [PubMed: 11511856]
- 87.
- Kligman AM. The Human Hair Cycle11From the Department of Dermatology of the University of Pennsylvania, Philadelphia, Pa. This research was supported by U.S. Army Research Contract DA 49-007-MD-154. Journal of Investigative Dermatology. 1959;33(6):307–316. [PubMed: 14409844]
- 88.
- Burns T, Breathnach SM, Cox N, Griffiths CEM. Rook’s Textbook of Dermatology. John Wiley & Sons; 2013.
- 89.
- Messenger AG, de Berker DAR, Sinclair RD. Disorders of Hair. Rook’s Textbook of Dermatology 2010:1–100.
- 90.
- Courtois M, Loussouarn G, Hourseau C, Grollier JF. Hair Cycle and Alopecia. Skin Pharmacology and Physiology. 1994;7(1-2):84–89. [PubMed: 8003330]
- 91.
- Guarrera M, Rebora A. Anagen hairs may fail to replace telogen hairs in early androgenic female alopecia. Dermatology. 1996;192(1):28–31. [PubMed: 8832948]
- 92.
- Randall VA, Jenner TJ, Hibberts NA, De Oliveira IO, Vafaee T. Stem cell factor/c-Kit signalling in normal and androgenetic alopecia hair follicles. J. Endocrinol. 2008;197(1):11–23. [PubMed: 18372228]
- 93.
- Sinclair R, Torkamani N, Jones L. Androgenetic alopecia: new insights into the pathogenesis and mechanism of hair loss. F1000Res. 2015;4(F1000 Faculty Rev):585. [PMC free article: PMC4544386] [PubMed: 26339482]
- 94.
- Jahoda CAB, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311(5986):560–562. [PubMed: 6482967]
- 95.
- Jahoda CAB, Reynolds AJ. Dermal-epidermal interactions—follicle-derived cell populations in the study of hair-growth mechanisms. Journal of Investigative Dermatology. 1993;101(1):S33–S38. [PubMed: 8326152]
- 96.
- Randall VA, Hibberts NA, Hamada K. A comparison of the culture and growth of dermal papilla cells from hair follicles from non-balding and balding (androgenetic alopecia) scalp. British Journal of Dermatology. 1996;134(3):437–444. [PubMed: 8731666]
- 97.
- Obana N, Chang C, Uno H. Inhibition of Hair Growth by Testosterone in the Presence of Dermal Papilla Cells from the Frontal Bald Scalp of the Postpubertal Stumptailed Macaque1. Endocrinology. 1997;138(1):356–361. [PubMed: 8977424]
- 98.
- Oliver RF, Jahoda CAB. The Dermal Papilla and Maintenance of Hair Growth. The Biology of Wool and Hair 1988:51–67.
- 99.
- van Scott EJ, Ekel TM. Geometric Relationships Between the Matrix of the Hair Bulb and its Dermal Papilla in Normal and Alopecic Scalp11From the Dermatology Service, General Medical Branch, National Cancer Institute, National Institutes of Health, Public Health Service, U.S. Department of Health, Education and Welfare, Bethesda, Maryland. Journal of Investigative Dermatology. 1958;31(5):281–287. [PubMed: 13598935]
- 100.
- Jahoda CA. Cellular and developmental aspects of androgenetic alopecia. Exp. Dermatol. 1998;7(5):235–248. [PubMed: 9832312]
- 101.
- Elliott K, Messenger AG, Stephenson TJ. Differences in Hair Follicle Dermal Papilla Volume are Due to Extracellular Matrix Volume and Cell Number: Implications for the Control of Hair Follicle Size and Androgen Responses. Journal of Investigative Dermatology. 1999;113(6):873–877. [PubMed: 10594724]
- 102.
- Prieto VG. Androgenetic Alopecia: Analysis of Proliferation and Apoptosis. Archives of Dermatology. 2002;138(8):1101–1102. [PubMed: 12164759]
- 103.
- Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. British Journal of Dermatology. 2006;154(4):609–618. [PubMed: 16536801]
- 104.
- Weger N, Schlake T. Igfbp3 Modulates Cell Proliferation in the Hair Follicle. Journal of Investigative Dermatology. 2005;125(4):847–849. [PubMed: 16185287]
- 105.
- Sinclair R. Fortnightly review: Male pattern androgenetic alopecia. BMJ. 1998;317(7162):865–869. [PMC free article: PMC1113949] [PubMed: 9748188]
- 106.
- Whiting DA. Possible mechanisms of miniaturization during androgenetic alopecia or pattern hair loss. J. Am. Acad. Dermatol. 2001;45(3) Suppl:S81–6. [PubMed: 11511857]
- 107.
- Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. Journal of the American Academy of Dermatology. 1993;28(5):755–763. [PubMed: 8496421]
- 108.
- Pinkus H. Differential patterns of elastic fibers in scarring and non-scarring alopecias. J. Cutan. Pathol. 1978;5(3):93–104. [PubMed: 79579]
- 109.
- Yazdabadi A, Magee J, Harrison S, Sinclair R. The Ludwig pattern of androgenetic alopecia is due to a hierarchy of androgen sensitivity within follicular units that leads to selective miniaturization and a reduction in the number of terminal hairs per follicular unit. British Journal of Dermatology. 2008;159(6):1300–1302. [PubMed: 18795932]
- 110.
- Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: implications for pathogenesis. British Journal of Dermatology. 1992;127(3):239–246. [PubMed: 1390168]
- 111.
- Sueki H, Stoudemayer T, Kligman AM, Murphy GF. Quantitative and ultrastructural analysis of inflammatory infiltrates in male pattern alopecia. Acta Derm. Venereol. 1999;79(5):347–350. [PubMed: 10494708]
- 112.
- Kligman AM. The comparative histopathology of male-pattern baldness and senescent baldness. Clinics in Dermatology. 1988;6(4):108–118. [PubMed: 3214816]
- 113.
- Zinkernagel MS, Med C, Trüeb RM. Fibrosing Alopecia in a Pattern Distribution. Archives of Dermatology. 2000;136(2) [PubMed: 10677097] [CrossRef]
- 114.
- Sinclair R, Jolley D, Mallari R, Magee J. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. Journal of the American Academy of Dermatology. 2004;51(2):189–199. [PubMed: 15280836]
- 115.
- Messenger AG, Sinclair R. Follicular miniaturization in female pattern hair loss: clinicopathological correlations. British Journal of Dermatology. 2006;155(5):926–930. [PubMed: 17034520]
- 116.
- Deloche C, de Lacharrière O, Misciali C, Piraccini BM, Vincenzi C, Bastien P, Tardy I, Bernard BA, Tosti A. Histological features of peripilar signs associated with androgenetic alopecia. Archives of Dermatological Research. 2004;295(10):422–428. [PubMed: 14758487]
- 117.
- Yazdabadi A, Rufaut NW, Whiting D, Sinclair R. Miniaturized hairs maintain contact with the arrector pili muscle in alopecia areata but not in androgenetic alopecia: A model for reversible miniaturization and potential for hair regrowth. International Journal of Trichology. 2012;4(3):154. [PMC free article: PMC3500053] [PubMed: 23180923]
- 118.
- Norwood OT. Male Pattern Baldness: classification and Incidence. Southern Medical Journal. 1975;68(11):1359–1365. [PubMed: 1188424]
- 119.
- Kerkemeyer KL, de Carvalho LT, Jerjen R, John J, Sinclair RD, Pinczewski J, Bhoyrul B. Female pattern hair loss in men: A distinct clinical variant of androgenetic alopecia. Journal of the American Academy of Dermatology. 2021;85(1):260–262. [PubMed: 32950545]
- 120.
- Kerkemeyer KL, Poa JE, de Carvalho LT, Eisman S, Imperial IC, Sinclair RD, Bhoyrul B. Development and initial validation of a modified Sinclair scale for female pattern hair loss in men. Journal of the American Academy of Dermatology. 2022;86(6):1406–1408. [PubMed: 34111498]
- 121.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017;77(1):136–141.e5. [PubMed: 28396101]
- 122.
- Kosman ME. Evaluation of a new antihypertensive agent. Minoxidil. JAMA: The Journal of the American Medical Association. 1980;244(1):73–75. [PubMed: 6103968]
- 123.
- Devine BL, Fife R, Trust P M. Minoxidil for severe hypertension after failure of other hypotensive drugs. BMJ. 1977;2(6088):667–669. [PMC free article: PMC1631896] [PubMed: 902045]
- 124.
- Lietman PS, Pennisi AJ, Takahashi M, Bernstein BH, Singsen BH, Uittenbogaart C, Ettenger RB, Malekzadeh MH, Hanson V, Fine RN. Minoxidil therapy in children with severe hypertension. The Journal of Pediatrics. 1977;90(5):813–819. [PubMed: 323442]
- 125.
- Kreindler TG. Topical minoxidil in early androgenetic alopecia. Journal of the American Academy of Dermatology. 1987;16(3):718–724. [PubMed: 3549807]
- 126.
- Wester RC, Maibach HI, Guy RH, Novak E. Minoxidil stimulates cutaneous blood flow in human balding scalps: pharmacodynamics measured by laser Doppler velocimetry and photopulse plethysmography. J. Invest. Dermatol. 1984;82(5):515–517. [PubMed: 6239893]
- 127.
- Lachgar Lachgar, Charveron Gall. Bonafe. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. British Journal of Dermatology. 1998;138(3):407–411. [PubMed: 9580790]
- 128.
- Marubayashi A, Nakaya Y, Fukui K, Li M, Arase S. Minoxidil-Induced Hair Growth is Mediated by Adenosine in Cultured Dermal Papilla Cells: Possible Involvement of Sulfonylurea Receptor 2B as a Target of Minoxidil. Journal of Investigative Dermatology. 2001;117(6):1594–1600. [PubMed: 11886528]
- 129.
- Buhl AE, Waldon DJ, Conrad SJ, Mulholland MJ, Shull KL, Kubicek MF, Johnson GA, Brunden MN, Stefanski KJ, Stehle RG, Gadwood RC, Kamdar BV, Thomasco LM, Schostarez HJ, Schwartz TM, Diani AR. Potassium Channel Conductance: A Mechanism Affecting Hair Growth both In Vitro and In Vivo. Journal of Investigative Dermatology. 1992;98(3):315–319. [PubMed: 1545141]
- 130.
- Buhl AE, Conrad SJ, Waldon DJ, Brunden MN. Potassium Channel Conductance as a Control Mechanism in Hair Follicles. Journal of Investigative Dermatology. 1993;101(s1):148S–152S. [PubMed: 8326149]
- 131.
- Katz HI, Hien NT, Prawer SE, Goldman SJ. Long-term efficacy of topical minoxidil in male pattern baldness. Journal of the American Academy of Dermatology. 1987;16(3):711–718. [PubMed: 3549806]
- 132.
- Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J. Am. Acad. Dermatol. 1999;41(5 Pt 1):717–721. [PubMed: 10534633]
- 133.
- Olsen EA, Weiner MS, Amara IA, DeLong ER. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. Journal of the American Academy of Dermatology. 1990;22(4):643–646. [PubMed: 2180995]
- 134.
- Olsen EA, DeLong ER, Weiner MS. Long-term follow-up of men with male pattern baldness treated with topical minoxidil. Journal of the American Academy of Dermatology. 1987;16(3):688–695. [PubMed: 3549803]
- 135.
- Roberts JL, Fiedler V, Imperato-McGinley J, Whiting D, Olsen E, Shupack J, Stough D, DeVillez R, Rietschel R, Savin R, Bergfeld W, Swinehart J, Funicella T, Hordinsky M, Lowe N, Katz I, Lucky A, Drake L, Price VH, Weiss D, Whitmore E, Millikan L, Muller S, Gencheff C, Carrington P, Binkowitz B, Kotey P, He W, Bruno K, Jacobsen C, Terranella L, Gormley GJ, Kaufman KD. Clinical dose ranging studies with finasteride, a type 2 5α-reductase inhibitor, in men with male pattern hair loss. Journal of the American Academy of Dermatology. 1999;41(4):555–563. [PubMed: 10495375]
- 136.
- Olsen EA, Whiting D, Bergfeld W, Miller J, Hordinsky M, Wanser R, Zhang P, Kohut B. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2007;57(5):767–774. [PubMed: 17761356]
- 137.
- Friedman ES, Friedman PM, Cohen DE, Washenik K. Allergic contact dermatitis to topical minoxidil solution: Etiology and treatment. Journal of the American Academy of Dermatology. 2002;46(2):309–312. [PubMed: 11807448]
- 138.
- do Nascimento IJB, Harries M, Rocha VB, Thompson JY, Wong CH, Varkaneh HK, Guimarães NS, Rocha Arantes AJ, Marcolino MS. Effect of Oral Minoxidil for Alopecia: Systematic Review. Int. J. Trichology. 2020;12(4):147–155. [PMC free article: PMC7759057] [PubMed: 33376283]
- 139.
- Panchaprateep R, Lueangarun S. Efficacy and Safety of Oral Minoxidil 5 mg Once Daily in the Treatment of Male Patients with Androgenetic Alopecia: An Open-Label and Global Photographic Assessment. Dermatol. Ther. 2020;10(6):1345–1357. [PMC free article: PMC7649170] [PubMed: 32970299]
- 140.
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, Moreno-Arrones ÓM, Saceda-Corralo D, Rodrigues-Barata R, Jimenez-Cauhe J, Koh WL, Poa JE, Jerjen R, Trindade de Carvalho L, John JM, Salas-Callo CI, Vincenzi C, Yin L, Lo-Sicco K, Waskiel-Burnat A, Starace M, Zamorano JL, Jaén-Olasolo P, Piraccini BM, Rudnicka L, Shapiro J, Tosti A, Sinclair R, Bhoyrul B. Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. J. Am. Acad. Dermatol. 2021;84(6):1644–1651. [PubMed: 33639244]
- 141.
- Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, Price VH, Van Neste D, Roberts JL, Hordinsky M, Shapiro J, Binkowitz B, Gormley GJ. Finasteride in the treatment of men with androgenetic alopecia. Journal of the American Academy of Dermatology. 1998;39(4):578–589. [PubMed: 9777765]
- 142.
- Van Neste D, Fuh V, Sanchez-Pedreno P, Lopez-Bran E, Wolff H, Whiting D, Roberts J, Kopera D, Stene J-J, Calvieri S, Tosti A, Prens E, Guarrera M, Kanojia P, He W, Kaufman KD. Finasteride increases anagen hair in men with androgenetic alopecia. British Journal of Dermatology. 2000;143(4):804–810. [PubMed: 11069460]
- 143.
- Whiting DA, Waldstreicher J, Sanchex M, Kaufman KD. Measuring Reversal of Hair Miniaturization in Androgenetic Alopecia by Follicular Counts in Horizontal Sections of Serial Scalp Biopsies: Results of Finasteride 1mg Treatment of Men and Postmenopausal Women. Journal of Investigative Dermatology Symposium Proceedings. 1999;4(3):282–284. [PubMed: 10674382]
- 144.
- Price VH, Menefee E, Sanchez M, Kaufman KD. Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): Three- and 4-year results. Journal of the American Academy of Dermatology. 2006;55(1):71–74. [PubMed: 16781295]
- 145.
- Drake L, Hordinsky M, Fiedler V, Swinehart J, Unger WP, Cotterill PC, Thiboutot DM, Lowe N, Jacobson C, Whiting D, Stieglitz S, Kraus SJ, Griffin EI, Weiss D, Carrington P, Gencheff C, Cole GW, Pariser DM, Epstein ES, Tanaka W, Dallob A, Vandormael K, Geissler L, Waldsteicher J. The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. Journal of the American Academy of Dermatology. 1999;41(4):550–554. [PubMed: 10495374]
- 146.
- Gillespie JDN. Journal Review: Long-term (5-year) multinational experience with finasteride 1mg in the treatment of men with androgenetic alopecia. International Society of Hair Restoration Surgery. 2002;12(3):129–129.
- 147.
- Stough D. Dutasteride improves male pattern hair loss in a randomized study in identical twins. Journal of Cosmetic Dermatology. 2007;6(1):9–13. [PubMed: 17348989]
- 148.
- Price VH, Menefee E, Sanchez M, Ruane P, Kaufman KD. Changes in hair weight and hair count in men with androgenetic alopecia after treatment with finasteride, 1 mg, daily. Journal of the American Academy of Dermatology. 2002;46(4):517–523. [PubMed: 11907500]
- 149.
- Arca E, Açikgöz G, Taştan HB, Köse O, Kurumlu Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology. 2004;209(2):117–125. [PubMed: 15316165]
- 150.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia. J. Dermatol. 2012;39(1):27–32. [PubMed: 21980923]
- 151.
- Rossi A, Cantisani C. SCARNò M, Trucchia A, Fortuna MC, Calvieri S. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatologic Therapy. 2011;24(4):455–461. [PubMed: 21910805]
- 152.
- Tosti A, Piraccini BM, Soli M. Evaluation of sexual function in subjects taking finasteride for the treatment of androgenetic alopecia. Journal of the European Academy of Dermatology and Venereology. 2001;15(5):418–421. [PubMed: 11763381]
- 153.
- Yim E, Baquerizo Nole KL, Tosti A. 5α-Reductase inhibitors in androgenetic alopecia. Current Opinion in Endocrinology, Diabetes & Obesity. 2014;21(6):493–498. [PubMed: 25268732]
- 154.
- Irwig MS. Persistent Sexual Side Effects of Finasteride: Could They Be Permanent? The Journal of Sexual Medicine. 2012;9(11):2927–2932. [PubMed: 22789024]
- 155.
- Belknap SM, Aslam I, Kiguradze T, Temps WH, Yarnold PR, Cashy J, Brannigan RE, Micali G, Nardone B, West DP. Adverse Event Reporting in Clinical Trials of Finasteride for Androgenic Alopecia. JAMA Dermatology. 2015;151(6):600. [PubMed: 25830296]
- 156.
- Matzkin H, Barak M, Braf Z. Effect of finasteride on free and total serum prostate-specific antigen in men with benign prostatic hyperplasia. Br. J. Urol. 1996;78(3):405–408. [PubMed: 8881951]
- 157.
- Keetch DW, Andriole GL, Ratliff TL, Catalona WJ. Comparison of percent free prostate-specific antigen levels in men with benign prostatic hyperplasia treated with finasteride, terazosin, or watchful waiting. Urology. 1997;50(6):901–905. [PubMed: 9426721]
- 158.
- Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The Interpretation of Serum Prostate Specific Antigen in Men Receiving 5α-Reductase Inhibitors: A Review and Clinical Recommendations. Journal of Urology. 2006;176(3):868–874. [PubMed: 16890642]
- 159.
- Etzioni RD, Howlader N, Shaw PA, Ankerst DP, Penson DF, Goodman PJ, Thompson IM. LONG-TERM EFFECTS OF FINASTERIDE ON PROSTATE SPECIFIC ANTIGEN LEVELS: RESULTS FROM THE PROSTATE CANCER PREVENTION TRIAL. Journal of Urology. 2005;174(3):877–881. [PubMed: 16093979]
- 160.
- D’Amico AV, Roehrborn CG. Effect of 1 mg/day finasteride on concentrations of serum prostate-specific antigen in men with androgenic alopecia: a randomised controlled trial. The Lancet Oncology. 2007;8(1):21–25. [PubMed: 17196507]
- 161.
- Guess HA, Heyse JF, Gormley GJ. The effect of finasteride on prostate-specific antigen in men with benign prostatic hyperplasia. The Prostate. 1993;22(1):31–37. [PubMed: 7678930]
- 162.
- Thompson IM, Goodman PJ, Tangen CM, Scott Lucia M, Miller GJ, Ford LG, Lieber MM, Duane Cespedes R, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA. The Influence of Finasteride on the Development of Prostate Cancer. New England Journal of Medicine. 2003;349(3):215–224. [PubMed: 12824459]
- 163.
- Redman MW, Tangen CM, Goodman PJ, Scott Lucia M, Coltman CA, Thompson IM. Finasteride Does Not Increase the Risk of High-Grade Prostate Cancer: A Bias-Adjusted Modeling Approach. Cancer Prevention Research. 2008;1(3):174–181. [PMC free article: PMC2844801] [PubMed: 19138953]
- 164.
- Lucia MS, Epstein JI, Goodman PJ, Darke AK, Reuter VE, Civantos F, Tangen CM, Parnes HL, Lippman SM, La Rosa FG, Kattan MW, Crawford ED, Ford LG, Coltman CA, Thompson IM. Finasteride and High-Grade Prostate Cancer in the Prostate Cancer Prevention Trial. JNCI Journal of the National Cancer Institute. 2007;99(18):1375–1383. [PubMed: 17848673]
- 165.
- Cohen YC, Liu KS, Heyden NL, Carides AD, Anderson KM, Daifotis AG, Gann PH. Detection Bias Due to the Effect of Finasteride on Prostate Volume: A Modeling Approach for Analysis of the Prostate Cancer Prevention Trial. JNCI Journal of the National Cancer Institute. 2007;99(18):1366–1374. [PubMed: 17848668]
- 166.
- Kramer BS, Hagerty KL, Justman S, Somerfield MR, Albertsen PC, Blot WJ, Ballentine Carter H, Costantino JP, Epstein JI, Godley PA, Harris RP, Wilt TJ, Wittes J, Zon R, Schellhammer P. Use of 5-α-Reductase Inhibitors for Prostate Cancer Chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. Journal of Clinical Oncology. 2009;27(9):1502–1516. [PMC free article: PMC2668556] [PubMed: 19252137]
- 167.
- Elliott CS, Shinghal R, Presti JC. The Influence of Prostate Volume on Prostate-Specific Antigen Performance: Implications for the Prostate Cancer Prevention Trial Outcomes. Clinical Cancer Research. 2009;15(14):4694–4699. [PubMed: 19584168]
- 168.
- Thompson IM, Tangen CM, Goodman PJ, Scott Lucia M, Parnes HL, Lippman SM, Coltman CA. Finasteride Improves the Sensitivity of Digital Rectal Examination for Prostate Cancer Detection. Journal of Urology. 2007;177(5):1749–1752. [PubMed: 17437804]
- 169.
- Sinclair RD, Dawber RPR. Androgenetic alopecia in men and women. Clinics in Dermatology. 2001;19(2):167–178. [PubMed: 11397596]
- 170.
- Hajheydari Z, Akbari J, Saeedi M, Shokoohi L. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2009;75(1):47–51. [PubMed: 19172031]
- 171.
- Trüeb RM, Itin P. Itin und Schweizerische Arbeitsgruppe für Trichologie. Praxis. 2001;90(48):2087–2093. [Photographic documentation of the effectiveness of 1 mg. oral finasteride in treatment of androgenic alopecia in the man in routine general practice in Switzerland] [PubMed: 11770252]
- 172.
- Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, Walker SE, Haberer LJ, Clark RV. The Effect of 5α-Reductase Inhibition with Dutasteride and Finasteride on Semen Parameters and Serum Hormones in Healthy Men. The Journal of Clinical Endocrinology & Metabolism. 2007;92(5):1659–1665. [PubMed: 17299062]
- 173.
- Tollin SR, Rosen HN, Zurowski K, Saltzman B, Zeind AJ, Berg S, Greenspan SL. Finasteride therapy does not alter bone turnover in men with benign prostatic hyperplasia--a Clinical Research Center study. J. Clin. Endocrinol. Metab. 1996;81(3):1031–1034. [PubMed: 8772571]
- 174.
- Matsumoto AM, Tenover L. McCLUNG M, Mobley D, Geller J, Sullivan M, Grayhack J, Wessells H, Kadmon D, Flanagan M, Zhang GK, Schmidt J, Taylor AM, Lee M, Waldstreicher J. The Long-Term Effect Of Specific Type II 5??-Reductase Inhibition With Finasteride on Bone Mineral Density in Men: Results of a 4-Year Placebo Controlled Trial. The Journal of Urology. 2002:2105–2108. [PubMed: 11956450]
- 175.
- Wade MS, Sinclair RD. Reversible painful gynaecomastia induced by low dose finasteride (1 mg/day). Australas. J. Dermatol. 2000;41(1):55. [PubMed: 10715905]
- 176.
- Ferrando J, Grimalt R, Alsina M, Bulla F, Manasievska E. Unilateral gynecomastia induced by treatment with 1 mg of oral finasteride. Arch. Dermatol. 2002;138(4):543–544. [PubMed: 11939831]
- 177.
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin. Pharmacol. 2006;6:7. [PMC free article: PMC1622749] [PubMed: 17026771]
- 178.
- Nguyen D-D, Marchese M, Cone EB, Paciotti M, Basaria S, Bhojani N, Trinh Q-D. Investigation of Suicidality and Psychological Adverse Events in Patients Treated With Finasteride. JAMA Dermatol. 2021;157(1):35–42. [PMC free article: PMC7658800] [PubMed: 33175100]
- 179.
- Dubrovsky B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol. Biochem. Behav. 2006;84(4):644–655. [PubMed: 16962651]
- 180.
- Mazzarella GF, Loconsole GF, Cammisa GA, Mastrolonardo GM, Vena G. Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. J. Dermatolog. Treat. 1997;8(3):189–192.
- 181.
- Piraccini BM, Blume-Peytavi U, Scarci F, Jansat JM, Falqués M, Otero R, Tamarit ML, Galván J, Tebbs V, Massana E., Topical Finasteride Study Group. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. J. Eur. Acad. Dermatol. Venereol. 2022;36(2):286–294. [PMC free article: PMC9297965] [PubMed: 34634163]
- 182.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked Suppression of Dihydrotestosterone in Men with Benign Prostatic Hyperplasia by Dutasteride, a Dual 5α-Reductase Inhibitor. The Journal of Clinical Endocrinology & Metabolism. 2004;89(5):2179–2184. [PubMed: 15126539]
- 183.
- Dallob AL, Sadick NS, Unger W, Lipert S, Geissler LA, Gregoire SL, Nguyen HH, Moore EC, Tanaka WK. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J. Clin. Endocrinol. Metab. 1994;79(3):703–706. [PubMed: 8077349]
- 184.
- Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS. The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. Journal of the American Academy of Dermatology. 2006;55(6):1014–1023. [PubMed: 17110217]
- 185.
- Debruyne F, Barkin J, van Erps P, Reis M, Tammela TLJ, Roehrborn C. Efficacy and Safety of Long-Term Treatment with the Dual 5α-Reductase Inhibitor Dutasteride in Men with Symptomatic Benign Prostatic Hyperplasia. European Urology. 2004;46(4):488–495. [PubMed: 15363566]
- 186.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J. Drugs Dermatol. 2005;4(5):637–640. [PubMed: 16167423]
- 187.
- Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, Cho HK, Sim WY, Lew BL, Lee W-S, Park HY, Hong SP, Ji JH. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: A randomized, double-blind, placebo-controlled, phase III study. Journal of the American Academy of Dermatology. 2010;63(2):252–258. [PubMed: 20605255]
- 188.
- Harcha WG, Martínez JB, Tsai T-F, Katsuoka K, Kawashima M, Tsuboi R, Barnes A, Ferron-Brady G, Chetty D. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. Journal of the American Academy of Dermatology. 2014;70(3):489–498.e3. [PubMed: 24411083]
- 189.
- Andriole GL, Kirby R. Safety and Tolerability of the Dual 5α-Reductase Inhibitor Dutasteride in the Treatment of Benign Prostatic Hyperplasia. European Urology. 2003;44(1):82–88. [PubMed: 12814679]
- 190.
- Roehrborn CG. The Effect of 5α-Reductase Inhibition With Dutasteride and Finasteride on Bone Mineral Density, Serum Lipoproteins, Hemoglobin, Prostate Specific Antigen and Sexual Function in Healthy Young Men. Yearbook of Urology. 2008;2008:88–89. [PMC free article: PMC2684818] [PubMed: 18423697]
- 191.
- Saceda-Corralo D, Rodrigues-Barata A, Vano-Galvan S, Jaen-Olasolo P. Mesotherapy with dutasteride in the treatment of androgenetic alopecia. International Journal of Trichology. 2017;9(3):143. [PMC free article: PMC5596657] [PubMed: 28932074]
- 192.
- Villarreal-Villarreal CD, Boland-Rodriguez E, Rodríguez-León S, Le Voti F, Vano-Galvan S, Sinclair RD. Dutasteride intralesional microinjections in combination with oral minoxidil vs. oral minoxidil monotherapy in men with androgenetic alopecia: a retrospective analysis of 105 patients. J. Eur. Acad. Dermatol. Venereol. 2022;36(7):e570–e572. [PubMed: 35279887]
- 193.
- Saceda-Corralo D, Moustafa F, Moreno-Arrones Ó, Jaén-Olasolo P, Vañó-Galván S, Camacho F. Mesotherapy With Dutasteride for Androgenetic Alopecia: A Retrospective Study in Real Clinical Practice. J. Drugs Dermatol. 2022;21(7):742–747. [PubMed: 35816059]
- 194.
- Sovak M, Seligson AL, Kucerova R, Bienova M, Hajduch M, Bucek M. Fluridil, a Rationally Designed Topical Agent for Androgenetic Alopecia: First Clinical Experience. Dermatologic Surgery. 2002;28(8):678–685. [PubMed: 12174057]
- 195.
- Hebert A, Thiboutot D, Stein Gold L, Cartwright M, Gerloni M, Fragasso E, Mazzetti A. Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients With Facial Acne: Two Phase 3 Randomized Clinical Trials. JAMA Dermatol. 2020;156(6):621–630. [PMC free article: PMC7177662] [PubMed: 32320027]
- 196.
- Dhillon S. Clascoterone: First Approval. Drugs. 2020;80(16):1745–1750. [PubMed: 33030710]
- 197.
- Rosette C, Rosette N, Mazzetti A, Moro L, Gerloni M. Cortexolone 17α-Propionate (Clascoterone) is an Androgen Receptor Antagonist in Dermal Papilla Cells In Vitro. J. Drugs Dermatol. 2019;18(2):197–201. [PubMed: 30811143]
- 198.
- Wolf R, Matz H, Zalish M, Pollack A, Orion E. Prostaglandin analogs for hair growth: Great Expectations. Dermatology Online Journal. 2003;9(3) [PubMed: 12952754] [CrossRef]
- 199.
- Blume-Peytavi U, Lönnfors S, Hillmann K, Bartels NG. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. Journal of the American Academy of Dermatology. 2012;66(5):794–800. [PubMed: 21875758]
- 200.
- Piérard G, Piérard-Franchimont C, Nikkels-Tassoudji N, Nikkels A, Saint Léger D. Improvement in the inflammatory aspect of androgenetic alopecia. A pilot study with an antimicrobial lotion. Journal of Dermatological Treatment. 1996;7(3):153–157.
- 201.
- Inui S, Itami S. Reversal of androgenetic alopecia by topical ketoconzole: relevance of anti-androgenic activity. J. Dermatol. Sci. 2007;45(1):66–68. [PubMed: 16997533]
- 202.
- Perez BSH, Hugo Perez BS. Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Medical Hypotheses. 2004;62(1):112–115. [PubMed: 14729013]
- 203.
- Ziering CL, Perez-Meza D, Zimber M, Zeigler F, Hubka M, Mansbridge J, Hubka K, Naughton GK. Hair regrowth following treatment with hypoxic cell-derived hair follicle signaling molecules; safety and efficacy in a first-in-man clinical trial. International Society of Hair Restoration Surgery. 2011;21(5):162–165.
- 204.
- Rose PT. The latest innovations in hair transplantation. Facial Plast. Surg. 2011;27(4):366–377. [PubMed: 21792780]
- 205.
- Takikawa M, Nakamura S, Nakamura S, Ishirara M, Kishimoto S, Sasaki K, Yanagibayashi S, Azuma R, Yamamoto N, Kiyosawa T. Enhanced Effect of Platelet-Rich Plasma Containing a New Carrier on Hair Growth. Dermatologic Surgery. 2011;37(12):1721–1729. [PubMed: 21883644]
- 206.
- Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl. Med. 2015;4(11):1317–1323. [PMC free article: PMC4622412] [PubMed: 26400925]
- 207.
- Li ZJ, Choi H-I, Choi D-K, Sohn K-C, Im M, Seo Y-J, Lee Y-H, Lee J-H, Lee Y. Autologous Platelet-Rich Plasma: A Potential Therapeutic Tool for Promoting Hair Growth. Dermatologic Surgery. 2012;38(7):1040–1046. [PubMed: 22455565]
- 208.
- Lee G-Y, Lee S-J, Kim W-S. The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J. Eur. Acad. Dermatol. Venereol. 2011;25(12):1450–1454. [PubMed: 21812832]
- 209.
- Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin. Drug Investig. 2009;29(5):283–292. [PubMed: 19366270]
- 210.
- Lanzafame RJ, Blanche RR, Chiacchierini RP, Kazmirek ER, Sklar JA. The growth of human scalp hair in females using visible red light laser and LED sources. Lasers Surg. Med. 2014;46(8):601–607. [PMC free article: PMC4265291] [PubMed: 25124964]
- 211.
- Hamblin MR, Agrawal T, de Sousa M. Handbook of Low-Level Laser Therapy. CRC Press; 2016.
- 212.
- Bae JM, Jung HM, Goo B, Park YM. Hair regrowth through wound healing process after ablative fractional laser treatment in a murine model. Lasers in Surgery and Medicine. 2015;47(5):433–440. [PubMed: 25945952]
- 213.
- Haedersdal M, Erlendsson AM, Paasch U, Anderson RR. Translational medicine in the field of ablative fractional laser (AFXL)-assisted drug delivery: A critical review from basics to current clinical status. J. Am. Acad. Dermatol. 2016;74(5):981–1004. [PubMed: 26936299]
- 214.
- Suchonwanit P, Rojhirunsakool S, Khunkhet S. A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med. Sci. 2019;34(9):1857–1864. [PubMed: 30982177]
- 215.
- To Evaluate the Safety, Tolerability and Efficacy in Male and Female With AGA Treated With HMI-115 Over a 24-week Treatment Period. Available at: https://clinicaltrials.gov/ct2/show/NCT05324293. Accessed September 5, 2022.
- 216.
- Hope Medicine Inc. Hope Medicine announced global license agreement with Bayer AG to advance the development and commercialization of the monoclonal antibody directed against prolactin (PRL) receptor. PR Newswire 2019. Available at: https://www
.prnewswire .com/news-releases /hope-medicine-announced-global-license-agreement-with-bayer-ag-to-advance-the-development-and-commercialization-of-the-monoclonal-antibody-directed-against-prolactin-prl-receptor-300824750.html. Accessed September 5, 2022. - 217.
- Sundberg JP, Beamer WG, Uno H, Van Neste D, King LE. Androgenetic Alopecia: In Vivo Models. Experimental and Molecular Pathology. 1999;67(2):118–130. [PubMed: 10527763]
- 218.
- Twitty VC, Bodenstein D. Correlated genetic and embryological experiments on Triturus. III. Further transplantation experiments on pigment development. IV. The study of pigment cell behavior in vitro. Journal of Experimental Zoology. 1939;81(3):357–398.
- 219.
- Rassman WR, Bernstein RM, McClellan R, Jones R, Worton E, Uyttendaele H. Follicular Unit Extraction: Minimally Invasive Surgery for Hair Transplantation. Dermatologic Surgery. 2002;28(8):720–728. [PubMed: 12174065]
- 220.
- Norwood OT. Scalp reduction in the treatment of androgenic alopecia. Dermatol. Clin. 1987;5(3):531–544. [PubMed: 3301111]
- 221.
- Khandpur S, Suman M, Reddy BS. Comparative Efficacy of Various Treatment Regimens for Androgenetic Alopecia in Men. The Journal of Dermatology. 2002;29(8):489–498. [PubMed: 12227482]
- 222.
- Boyapati A, Sinclair R. Combination therapy with finasteride and low-dose dutasteride in the treatment of androgenetic alopecia. Australas. J. Dermatol. 2013;54(1):49–51. [PubMed: 22686691]
- 223.
- Jimenez F, Alam M, Vogel JE, Avram M. Hair transplantation: Basic overview. J. Am. Acad. Dermatol. 2021;85(4):803–814. [PubMed: 33905785]
- 224.
- Kassimir JJ. Use of topical minoxidil as a possible adjunct to hair transplant surgery. Journal of the American Academy of Dermatology. 1987;16(3):685–687. [PubMed: 3558912]
- 225.
- Inc. KN, Kernel Networks Inc. Comparison of Topical Minoxidil 5% in Ethanol Plus Propylene Glycol Versus Minoxidil 5% in Ethanol Alone in Treatment of Women With Female Pattern Hair Loss. Case Medical Research 2019. doi: 10.31525/ct1-nct04090801. [CrossRef]
- 226.
- Leavitt M, David P-M, Rao NA, Barusco M, Kaufman KD, Ziering C. Effects of Finasteride (1 mg) on Hair Transplant. Dermatologic Surgery. 2005;31(10):1268–1276. [PubMed: 16188178]
- Review Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness.[Br J Dermatol. 2011]Review Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness.Sinclair R, Patel M, Dawson TL Jr, Yazdabadi A, Yip L, Perez A, Rufaut NW. Br J Dermatol. 2011 Dec; 165 Suppl 3:12-8.
- What's New in Therapy for Male Androgenetic Alopecia?[Am J Clin Dermatol. 2023]What's New in Therapy for Male Androgenetic Alopecia?Saceda-Corralo D, Domínguez-Santas M, Vañó-Galván S, Grimalt R. Am J Clin Dermatol. 2023 Jan; 24(1):15-24. Epub 2022 Sep 28.
- Review Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia.[J Dermatolog Treat. 2022]Review Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia.Gupta AK, Talukder M, Williams G. J Dermatolog Treat. 2022 Nov; 33(7):2946-2962. Epub 2022 Aug 15.
- Review A review of the treatment of male pattern hair loss.[Expert Opin Pharmacother. 2020]Review A review of the treatment of male pattern hair loss.York K, Meah N, Bhoyrul B, Sinclair R. Expert Opin Pharmacother. 2020 Apr; 21(5):603-612. Epub 2020 Feb 17.
- Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study.[Indian J Dermatol Venereol Lep...]Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study.Shanshanwal SJ, Dhurat RS. Indian J Dermatol Venereol Leprol. 2017 Jan-Feb; 83(1):47-54.
- Male Androgenetic Alopecia - EndotextMale Androgenetic Alopecia - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
