NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Erectile dysfunction (ED) is the inability to achieve and maintain an erection sufficient to permit satisfactory sexual intercourse. Approximately 23% of men aged 40-80 years worldwide have symptoms of ED. The prevalence of ED is higher in older men but this condition can occur in men of any age. The prevalence of ED ranges between 30-90% in men with diabetes and may be the presenting symptom for the disease in almost a third of cases. In this chapter, we review the physiology of penile erection and pathophysiology of ED. We review contemporary medical treatments for ED, surgical options for patients that fail medical management, and novel therapies in development. We also briefly review priapism, the condition of prolonged penile erection not associated with sexual desire. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Erectile dysfunction (ED) is defined as the inability to achieve and maintain an erection sufficient to permit satisfactory sexual intercourse (1). ED may result from psychological, neurologic, hormonal, vascular, or medication-induced causes. Approximately 23% of men aged 40-80 years worldwide have symptoms of ED (2). ED occurs in men of all ages; the prevalence increases with age (3, 4). Diabetes is a common and important case of ED due to the diseases impact on both neurological and vascular factors germane to penile erection (5).
PHYSIOLOGY OF PENILE ERECTIONS
Penile erection is a neurovascular event modulated by psychological and hormonal status. The penis is innervated by autonomic and somatic nerves. The autonomic nervous innervation of the penis consists of sympathetic fibers (derived from the T11-L2 spinal cord level) and parasympathetic fibers (derived from the S2-4 spinal cord level) which merge to form the cavernous nerves in the pelvis. The autonomic nervous innervation of the penis regulates corporal smooth muscle and arterial contraction and relaxation, which are the primary drivers of penile tumescence. The somatic portion of the pudendal nerve is responsible for penile sensation and the contraction and relaxation of the bulbocavernosus and ischiocavernosus striated muscles. These muscles are important for maximal penile erection and for expulsion of semen during ejaculation (6, 7).
The penis is flaccid in the resting state. Suppression of penile erection and maintenance of the flaccid state is mediated by the sympathetic nervous system and adrenergic nerve terminals. Vascular factors such as endothelin may also contribute to resting smooth muscle tone in the penis. Smooth muscle tone is ultimately mediated by activation of the myosin light chain, which tends to increase contraction. The RhoA/Rho kinase pathway also plays a critical role in smooth muscle contraction by suppressing myosin light chain phosphatase. A depiction of contractile molecules which mediate penile flaccidity is presented in Figure 1.
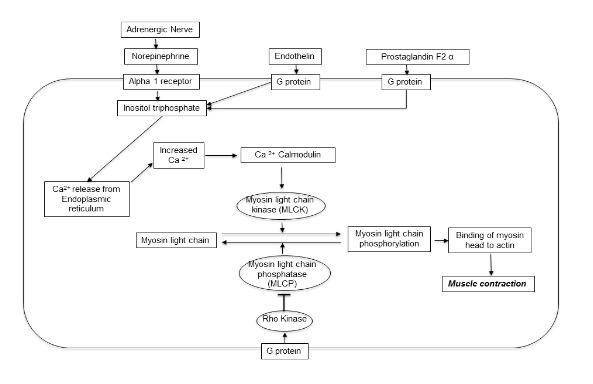
Figure 1.
Pathways that Promote Contraction and Oppose Erection
Penile erection is induced in part by suppression of sympathetic tone. A number of brain centers integrate erotic stimuli and contribute to erection by suppressing the sympathetic nervous system. The neurotransmitter dopamine appears to play a critical role in central nervous system-based stimulation of erection whereas serotonin plays a mostly (but not universally) inhibitory role (8, 9).
Erection also requires activation of the parasympathetic nervous system; this is mediated in large part by release of nitric oxide (NO) from nonadrenergic-noncholinergic cavernous nerve terminals in the genital vascular system. NO is produced in nerve tissue by neuronal nitric oxide synthase (nNOS). NO activates soluble guanylyl cyclase, which cleaves guanosine triphosphate (GTP) to produce cyclic guanosine monophosphate (cGMP). cGMP in turn activates cGMP-specific protein kinases, which phosphorylates certain proteins and ion channels, resulting in: opening of the potassium channels and hyperpolarization; sequestration of intracellular calcium by the endoplasmic reticulum; and inhibition of calcium channels, blocking calcium influx. The consequence is a drop in cytosolic calcium content (10). Decline in calcium content results in smooth muscle relaxation via relaxation of the actin myosin cross-bridges. There is resultant vasodilation in the arteries and arterioles supplying the erectile tissue. A several-fold increase in blood flow occurs, with a concomitant increase in compliance of the corporeal sinusoids from relaxed cavernous smooth muscle. The increase in blood flow triggers activation of endothelial nitric oxide synthase (eNOS) that further increases NO production and maintenance/enhancement of vasodilation (11). A graphical depiction of the NO/cGMP relaxant pathway is depicted in Figure 2. Additional vasodilation may be accomplished through the cyclic adenosine monophosphate pathway, depicted in Figure 3.

Figure 2.
The NO cGMP Pathway in Erection

Figure 3.
The cAMP Pathway in Erection
With enhancement of corporal blood flow there is rapid filling and expansion of the corporal sinusoidal system against the tunica albuginea. The subtunical venular plexuses are compressed between the corporeal sinusoids and the tunica albuginea, resulting in almost total occlusion of venous outflow (12, 13). With trapping of blood within the corpora cavernosa the flaccid penis becomes erect; during the full erection phase intracavernous pressures increases to approximately 100 mm Hg. With increasing sexual arousal, the bulbocavernosus reflex is triggered, causing the ischiocavernosus muscles to forcefully compress the base of the blood-filled corpora cavernosa and the penis. During this rigid erection phase the penis (including the corpus spongiosum and glans) becomes very hard, with intracavernous pressure reaching several hundred mm Hg in some cases. During this phase, arterial inflow may cease or even reverse (i.e., arterial blood flows retrograde towards the heart) (14)
With sexual climax or cessation of arousal, the penis returns to the flaccid state. Flaccidity is initiated in large part by hydrolysis of cGMP to guanosine monophosphate by phosphodiesterase type 5 (PDE5). Other phosphodiesterases are found in the corpus cavernosum, but they do not appear to play a major role in detumescence. Detumescence is also mediated by sympathetic discharge during ejaculation, leading to vasoconstriction and cessation of NO release from the cavernous nerves and corporal endothelium. The sub-tunical venous channels open with contraction of the trabecular smooth muscle, permitting egress of trapped blood and restoring flaccidity (10).
CLASSIFICATION OF ERECTILE DYSFUNCTION
ED may be mediated by defects in the neuronal, hormonal, arterial, or cavernosal systems. ED is commonly associated with specific medications. ED is also strongly associated with psychological distress. ED is in most cases multifactorial, with at least some element of a psychogenic component as a primary cause or as secondary development in reaction to the ED state. Some disease states (e.g., diabetes) may cause ED via multiple mechanisms (Table 1).
Table 1.
Classification and Common Causes of Erectile Dysfunction
| Category of Erectile dysfunction | Common disorders | Pathophysiology |
|---|---|---|
| Neurogenic | -Stroke -Alzheimer’s disease -Spinal cord injury -Radical pelvic surgeries -Diabetic neuropathy -Pelvic injury | -Interrupted neuronal innervation -Failure to initiate NO release |
| Psychogenic | -Depression -Psychological stress -Performance anxiety -Relationship problems | -Impaired nitric oxide (NO) release -Loss of libido -Sympathetic nervous system activation |
| Hormonal | -Androgen deficiency -Hyperprolactinemia -Diabetes -Chronic opioid use | -Loss of libido -Inadequate NO release -Morphological changes in penis (atrophy) |
| Vasculogenic (arterial and cavernosal) | -Hypertension -Atherosclerosis -Hyperlipidemia -Diabetes mellitus -Obesity -Trauma/pelvic fracture -Tobacco use -Peyronie’s disease | -Impaired penile veno-occlusion -Inadequate arterial inflow |
| Drug-induced | -Antihypertensives -Antiandrogens -Antidepressants -Alcohol abuse | -Central nervous system suppression -Decreased libido -Alcoholic neuropathy -Vascular insufficiency |
| Systemic diseases | -Aging -Diabetes mellitus -Chronic renal failure -Generalized atherosclerotic disease | -Multifactorial -Neuronal and vascular dysfunction |
Sexual function progressively declines as men age, although this decline need not be considered any more “natural” than the development of diabetes, hypercholesterolemia, hypertension, or any other disease state more common in the aged. With age, the latency period between sexual stimulation and erection increases, erections are less turgid, ejaculation is less forceful, ejaculatory volume decreases, and the refractory period between erections lengthens (15). In most cases aging is also associated with a decrease in penile sensitivity to tactile stimulation, a decrease in serum testosterone concentration, and an increase in cavernous muscle tone (16).
Erectile dysfunction is more common in patients with neurologic disorders such as Parkinson's and Alzheimer's diseases, stroke and cerebral trauma (17). This may be due to both a decrease in libido and/or inability to initiate the erectile process. Spinal cord injury patients have varying degrees of erectile dysfunction largely dependent on the location and extent of the lesion. Men with lesions of the sacral spine may have disruption of the sacral reflex arc and may not respond to genital stimulation. Men with spinal cord injury that preserves the sacral segments may retain reflexogenic erections but may struggle to obtain erection related to mental arousal, which is generated by cortical suppression of sympathetic tone (6). Although erections of some form are often possible in men with spinal cord injury, they are typically less rigid and of shorter duration compared to erections in men without spinal cord injury. Even in men without spinal cord injury, sensory input from the genitalia remains essential to achieving and maintaining reflexogenic erection. This input may be compromised due to peripheral neuropathy in men with diabetes (18).
About 50 percent of men with chronic diabetes mellitus are reported to have ED, although this number dramatically increases with age. In addition to the disease's effect on small vessels, it may also affect the cavernous nerve terminals and endothelial cells, resulting in deficiency of neurotransmitters (10). Additionally, corporal smooth muscle relaxation in response to neuronal- and endothelial-derived nitric oxide (NO) is impaired in men with diabetes, possibly due to the accumulation of glycosylation products (19-21).
Chronic renal insufficiency (CRI) has frequently been associated with diminished erectile function, impaired libido, and infertility (22, 23).The mechanism of ED in CRI is likely multifactorial and includes low serum testosterone, vascular insufficiency, medication-related, depressed libido, and autonomic and somatic neuropathy (24-26). Diabetes is a major risk factor for CRI and hence CRI is a relevant consideration in men with diabetes.
Psychogenic ED may relate to performance anxiety, strained relationship, lack of sexual arousal, and overt psychiatric disorders such as depression and schizophrenia (27). The strong relationship between psychogenic stress and sexual dysfunction is well established (28).
Androgen deficiency is associated with diminished libido and less frequent nocturnal erections (29). However, erection response is preserved in many men with low serum testosterone, suggesting that androgens are beneficial but not essential for erection (30). Men with diabetes are at increased risk of androgen deficiency; serum testosterone testing is often warranted in diabetic men with or without ED (29, 31). Prolactin is another pituitary hormone that has inhibitory activity on central dopaminergic activity and gonadotropin-releasing hormone secretion. Hyperprolactinemia has been linked to reproductive and sexual dysfunction.
Vascular disease is a common and important cause of ED (32). Common vascular risk factors associated with generalized penile arterial insufficiency include hypertension, hyperlipidemia, cigarette smoking, diabetes mellitus, trauma, and pelvic irradiation (33). Focal stenosis of the common penile artery is not common but can be a cause of ED in men who have sustained repetitive and/or severe pelvic or perineal trauma (e.g. biking accidents, pelvic fractures) (34).
Veno-occlusive dysfunction (VOD) results from disruption of the blood trapping capacity of the tunica albuginea of the corpora. In this situation there may be inadequate compression of the subtunical venules during the full erection phase (35). Venous leak ED may also be the result of degenerative changes that affect the penis including Peyronie's disease, penile scarring, and diabetes mellitus. A patient may develop VOD from traumatic injury to the tunica albuginea such as a penile fracture. Venous leak can also be seen in anxious men with excessive adrenergic tone causing structural alterations of the cavernous smooth muscle and endothelium and insufficient trabecular smooth muscle relaxation (36).
Virtually all drugs have been associated with ED although some classes are more prone to sexual side effects than others. Central neurotransmitter pathways, including serotonergic, noradrenergic, and dopaminergic pathways involved in sexual function, may be disturbed by antipsychotics and antidepressants (27). Although any antihypertensive agent could theoretically cause ED by decreasing the availability of blood to the corporal arteries (i.e., a pressure-head phenomenon), differences are noted between various classes of medications (37). Angiotensin converting enzyme inhibitors and angiotensin receptor blockers are relatively less likely to cause ED. Beta-adrenergic blocking drugs may lead to erectile dysfunction by potentiating a-1 adrenergic activity in the penis, although more modern beta blockers with eNOS activity (such as nebivolol) may help minimize this effect (38) . Thiazide diuretics have been reported to cause erectile dysfunction by an unknown mechanism (39). Anti-androgens are another common drug type that may contribute to ED(40).
Recreational drugs including cigarettes and alcohol affect erectile function. Cigarette smoking may induce vasoconstriction and penile venous leakage because of its contractile effect on the cavernous smooth muscle. More importantly, chronic use may accelerate atherosclerotic changes in penile microvasculature (41). Alcohol in small amounts may improve erections and increase libido because of its vasodilatory effect and the suppression of performance anxiety. However, large amounts of alcohol can cause central sedation, decreased libido and transient erectile dysfunction. Chronic alcoholism may cause hypogonadism and polyneuropathy; this may in turn affect penile nerve function (42).
EVALUATION OF ERECTILE DYSFUNCTION
Erectile dysfunction may be the first manifestation of many disease states, including diabetes mellitus, coronary artery disease, hyperlipidemia, hypertension, spinal-cord compression, pituitary tumors, and pelvic malignancies. ED is a sentinel event for major adverse cardiac events and is therefore an indication for formal cardiovascular evaluation (32). The evaluation of a patient with ED requires a thorough history (medical, sexual, and psycho-social), physical examination and appropriate laboratory tests (creatinine, fasting glucose, lipid profile, total testosterone, and bioavailable or free testosterone) aimed at detecting underlying metabolic disease states (1). If the man's testosterone concentration (free, total, or bioavailable) is low, serum prolactin and luteinizing hormone should be assayed to detect abnormalities of the hypothalamic-pituitary axis (29, 31).
After assessing the therapeutic needs and goals of the patient, further diagnostic and treatment options can be offered. The patient’s partner(s) should be involved in treatment planning and decision making (1). In men who do not have a sexual partner, there should be discussion about goals in terms of future sexual relationships. Decisions on treatment should be based on patient goals and risk tolerance. If the patient has a regular partner, it is beneficial to involve them in conversations about goals and treatment options. The most recent AUA guideline on ED recommends that all treatments be considered first line. Most men are best served by a progression from minimally to maximally invasive options; however, a properly counseled man may elect to bypass less invasive options if he so desires and if deemed medically appropriate (1).
The patient's performance status and cardiovascular health should be evaluated, in consultation with a cardiologist, if necessary, in order to assess the patient's ability to tolerate sexual activity (43). Patients who have poor exercise tolerance should consider physical conditioning prior to resuming sexual activity (43). Exercise, healthy diet, and weight loss may be sufficient to partially restore erection response in some men (44)
Men with complex ED may benefit from a referral for further testing and treatment by a sexual medicine specialist, sex therapist, or medical provider with expertise in a specific health condition germane to sexual responses (Table 2). The indications for specialty referral include complex gonadal or other endocrine disorders, neurologic deficit suggestive of brain or spinal cord disease, deep-seated psychologic or psychiatric problems, post-traumatic or lifelong erectile dysfunction and unstable cardiovascular disease (1).
Table 2.
Medical Workup of Erectile Dysfunction
| Test | Indications |
|---|---|
| Combined injection and stimulation (CIS), Intracavernous injection of vasodilator followed by penile self-stimulation) | Assessment of penile vascular function and presence of penile deformities with erection. Therapeutic test in men who choose intracavernous therapy for ED |
| CIS with color Duplex ultrasonography (CDU) | Assessment of arterial inflow and veno-occlusive potential of the penis Assess for fibrosis/plaque formation/calcification of the corpora |
| Cavernosography | Assessment for congenital or traumatic venous leakage in men with veno-occlusive ED by CDU (rarely utilized in contemporary practice) |
| Pelvic arteriography | Assess for arterial lesions in men with arterial ED by CDU (rarely utilized in contemporary practice) |
| Ambulatory nocturnal penile tumescence and rigidity (Rigiscan®) | Assess nocturnal erection responses (Historically used to differentiate psychogenic from organic erectile dysfunction but prone to unreliability and seldom utilized in contemporary practice) |
Peyronie’s disease (PD) is an acquired fibrotic disorder of the penis that merits specific mention in the context of managing ED (45). The condition remains poorly understood but is thought to involve a genetic predisposition to collagen deposition and inflammation in connective tissues after minor traumas as may be experienced during sexual activity. Men with PD are at increased risk of other fibrotic conditions including Dupuytren’s contractures and tympanosclerosis (45).
PD was once thought to be quite rare; with the introduction of highly effective therapy for ED many cases of PD that might have once gone unrecognized are now diagnosed. Prevalence estimates vary but signs of PD may be detected by experienced examiners in approximately 9% of men presenting for evaluation by an experienced urologist (46).
The classical manifestation of PD is penile curvature, but the disorder may also be associated with pain, narrowing, corporal fibrosis, and hinging (45). The single US-FDA approved medical therapy for PD is injections of Clostridial collagenase into the tunica albuginea of the corporal bodies followed by a course of penile modeling. This therapy has been associated with modest but significant improvements in penile curvature compared to placebo injections (47). In cases of severe deformity and/or failure of medical therapy, surgical intervention may be required. Surgery for PD can take the form of 1) plication of the convex side of the penis with sutures to induce straightening, 2) incision with grafting of the concave or narrowed portion of the corpora, or 3) placement of a penile prosthetic device in cases where there is concomitant severe ED (45, 48).
MEDICAL MANAGEMENT OF ERECTILE DYSFUNCTION
Medical management options with demonstrated efficacy in management of ED are presented in table 3.
Table 3.
Treatment Options for Erectile Dysfunction
| Treatment | Cost | Advantage | Disadvantage | Recommendation |
|---|---|---|---|---|
| Psychosexual Therapy | Variable | -Non-invasive -Partner involved -Potentially curative for psychogenic ED | -Time consuming -Costly -Knowledgeable experts may not be available in all geographic areas | Useful as primary or adjunct therapy in almost all cases |
| Oral: PDE 5 inhibitor (sildenafil, tadalafil, vardenafil, avanafil) | $1-50 /dose Oral | -Effective in 1-2 hours -Up to 24-hour action (tadalafil) -May be taken on demand or as daily supplement (tadalafil only) | -Strict contraindication in men on nitrates and relative contraindication with some other drugs -Potential for side effects | First-line treatment for the majority of patients |
| Vacuum Constriction Device | $150- 450 /device | No systemic side effects | -May compromise ejaculation -May cause discomfort and/or numbness -Marginal penile rigidity | Low risk but marginal efficacy in producing erections sufficient for sexual activity |
| Transurethral: MUSE®1 | $25 /dose | -Local therapy -Few systemic side effects | -Moderately effective -Requires training -May cause penile pain | Marginal clinical response in many cases and pain with administration limit efficacy |
| Penile injection (Caverject®, Edex®, or compounded drugs)2 | $5-30 /dose | -Highly effective -Few systemic side effects | -Requires injection -May cause penile pain -High dropout rate. -Risk of priapism or fibrosis | Highly effective in men who fail or are not candidates for PDE5I |
| Penile Prosthesis (all types) | $8,000- 15,000 | -Produces a reliably rigid penile shaft | -Risk of device infection -Requires surgery -Requires replacement after mechanical failure | For men dissatisfied with medical management who are willing to have surgery |
| Vascular Surgery | $10,000- 15,000 | -Potentially curative | -Requires surgery -Poor results in older men with generalized vascular disease | Restrict to arterial bypass surgery in specialized centers for select healthy young men with traumatic arterial disruption as cause of ED. Surgery for venous leak is no longer recommended as a standard of care in any patient. |
- 1.
MUSE® signifies Medicated Urethral System for Erection. It contains alprostadil pellet.
- 2.
Caverject® and Edex® both contain injectable alprostadil. Drug mixtures contain two or three of the following drugs: papaverine, phentolamine and prostaglandin, atropine.
Androgens
Historically, androgens were thought to enhance male sexual function. However, androgen therapy has only been shown to be of clinical benefit in men with low serum testosterone and symptoms potentially referable to hypogonadism (29). There is controversy over what constitutes a “low” level of testosterone with cut points for normal ranging between 264-350 ng/dL depending on definitions based on population norms and/or increased odds of symptoms potentially referable to low serum testosterone. (29, 31, 49, 50). Assessment of serum free testosterone (ideally with equilibrium dialysis or other highly precise methodology) is controversial and recommended in select cases by some experts (51) but not by others (31, 52). The sexual benefits of supplementation in men with low serum testosterone primarily pertains to libido but mild improvements in erectile function in patients may occur with testosterone supplementation alone (53). The United States Testosterone Trial demonstrated moderate improvement of erectile function with testosterone gel therapy compared to placebo in men ≥ 65 years and serum total testosterone concentrations ≤ 275 ng/dl; these men were not a priori screened for erectile dysfunction.(54)
Testosterone cypionate, enanthate, and undecanoate may be used for intramuscular replacement therapy. A standard dosing regimen is 200 mg intramuscularly every two weeks although different dosing regimens have been described. Lower doses at shorter intervals may produce less pronounced variation in serum testosterone levels between doses.
Several daily transdermal testosterone preparations (testosterone patches or gels) are available. Daily application of these preparations raises serum testosterone concentrations to within the normal range in over 90 percent of men. The most common adverse effects of testosterone patches are skin irritation and contact dermatitis. Gel formulations are less prone to skin irritation but care must be taken to avoid skin-to-skin contact and transfer of testosterone to others (e.g., spouse, children) for at least 2 hours post dosing (29).
Recent refinements in testosterone supplementation include novel testosterone pellets, which are implanted in a minor office procedure and provide 2-4 months of therapeutic testosterone levels (55). A ten week depot testosterone preparation was recently approved for use in the United States; this long acting formulation may obviate the need for frequent injections (56). These long-acting formulations may be considered for patients who have tolerated shorter acting formulations and reported significant benefit. Additional recent innovations that have achieved FDA approval include subcutaneous injectable testosterone and nasal testosterone (31).
Testosterone treatment may contribute to polycythemia, acne, edema, and decreased HDL cholesterol. Testosterone may also lead to estrogen by aromatization in adipose tissue; this may lead to gynecomastia and theoretically to higher risk of deep venous thrombosis. Men receiving androgen replacement therapy require routine follow up appointments with measurement of hematocrit, serum testosterone, and PSA. It is prudent to have more frequent checks in the early phase after initiation of therapy (29).
Due to the well-established benefits of surgical or medical castration for the management of advanced prostate cancer there has been a long-standing concern that testosterone supplementation may increase the risk of prostate cancer (31, 57). This relationship is based primarily on conjecture; there is an emerging body of literature which suggests that testosterone supplementation does not materially alter the risk of prostate cancer development (58). There is limited evidence that men with low serum testosterone may be at greater risk of aggressive prostate cancer (59), although there is currently no evidence that supplementation moderates this risk. Numerous reports have been published of men with successfully treated prostate cancer using testosterone supplements with no increase in risk for PSA recurrence (60). Some clinicians have also reported continued testosterone supplementation in men with untreated prostate cancer; until more data are available providers in general practice should exercise caution with respect to testosterone supplementation in men with untreated prostate cancer (57, 61).
The issue of cardiac safety and testosterone received a great deal of media attention in the mid 2010s. High profile publications in prestigious journals reported increased rates of cardiovascular events and mortality in men using testosterone supplements (62, 63). Testosterone is known to exert several effects that may increase cardiac risk. At the same time, the known benefits of testosterone include increased lean body mass, decreased adiposity, and improved insulin sensitivity (64). Men with low serum testosterone are known to be at greater risk of all-cause mortality (65). Higher rates of cardiac events in men taking testosterone supplement may be due to baseline risk rather than additional risk from supplementation.
Type 5 Phosphodiesterase Inhibitors (PDE5I)
Oral therapy with a type 5 phosphodiesterase inhibitor (PDE5I, e.g., sildenafil, vardenafil, tadalafil, and avanafil) is in the most frequently utilized first-line therapy men with ED (1). PDE5I block the inactivation of cGMP and result in increased smooth muscle relaxation and penile arterial blood flow. In the absence of sexual stimulation these medications have minimal effect on penile blood flow; this relationship may explain frequent PDE5I “failure” in men not properly counseled on proper use of this class of drugs.
Numerous placebo-controlled studies have been conducted on the safety and efficacy of sildenafil since it was approved for clinical use in 1998 (66). Sildenafil has been consistently shown to increase the number of erections, penile rigidity, orgasmic function, and overall sexual satisfaction compared to placebo in men with ED of every etiology, including diabetes and radical prostatectomy. Similar data exist for the other drugs in this class.
Most clinical trials of PDE-5 inhibitors show only mild to moderate, self-limited adverse events associated with all of the PDE-5 inhibitors (70). The most common complaints in men using PDE-5 inhibitors are headache (16 percent), flushing (10 percent), dyspepsia (7 percent), nasal congestion (4 percent), and visual disturbances/ color sensitivity (3 percent). Tadalafil distinguishes itself from vardenafil and sildenafil by the relative lack of visual side effects. It does however have the possible adverse effect of back pain and/or myalgia (71).
PDE5I have an excellent track record of cardiac safety (72). To date, tens of millions of men in over 100 countries have used sildenafil. Since the release of the drug, over 200 deaths temporally associated with sildenafil therapy were reported to the Food and Drug Administration in the United States of America. Sexual activity is a likely contributor to myocardial infarction in men with heart disease, with sildenafil acting to enable men not previously active to engage in sexual activity (73, 74). PDE5I therapy is safe for most men, although men with cardiovascular disease should consult with their cardiologist prior to engaging in sexual activity. The Princeton III guidelines provide recommendations for management of ED in men with cardiovascular disease. Princeton III recommends risk stratification before initiation of therapy; men at low-risk may be treated whereas men with high-risk (e.g., recent MI, unstable angina, NYHA Class III or IV heart failure, unstable arrythmia) should not be treated until their situation stabilizes. Men who do not fall neatly into low- or high-risk categories should undergo cardiac evaluation (43).
A lower starting dose (25 mg of sildenafil, 5 mg of vardenafil or tadalafil, 50 mg of avanafil) is recommended in patients who may attain and maintain higher plasma levels of PDE5I. These include patients who are older than 65, have severe renal impairment, or take potent CYP450 3A4 inhibitors. Patients who take ritonavir should not take more than 25 mg of Sildenafil in a 48-hour period.
Patients using PDE5I and requiring alpha-blocker therapy for hypertension or benign prostate hyperplasia should start at low doses of PDE5 inhibitor, which can be titrated to affect. To avoid symptomatic hypotension, PDE5 inhibitors should not be taken within 4 hours of an alpha-blocker. One study found a significant rate of hypotension (28% versus 6% with placebo) in patients taking concomitant doxazosin and tadalafil (75). The rate of hypotension matched that seen in placebo-treated patients in patients taking tamsulosin and tadalafil, and some studies suggest that the interaction has less clinical relevance in patients who have undergone long-term alpha-blocker therapy. It is likely that all four PDE5I commercially available in the US interact to some degree with alpha-blockers and that concurrent use of alpha-blockers and PDE5I may cause patients to develop orthostatic hypotension. Other antihypertensive agents appear to be well tolerated by men concurrently taking PDE5I.
PDE5I have been associated with spontaneous non-arteritic ischemic optic neuropathy (NAION), the most common acute optic neuropathy. Estimated annual incidence is 2.3 to 10.3 per 100,000 and is more common in Caucasians than African Americans, Asians, or Latinx persons. Most NAION patients do not become legally blind, but the degree of visual acuity and visual field loss is typically significant.
Risk factors common to NAION and ED include hypertension, diabetes mellitus, hypercholesterolemia, age over 50 years, coronary artery disease, and smoking (76). A large review of ophthalmology records in the United States and Europe reported a very slight but statistically significant relationship between NAION and PDE5I use within the last 30 days. The authors concluded that use of PDE5I may be associated with an increase of approximately three NAION cases per 100,000 men over the age of 50 (77, 78). Men using PDE5I should be counseled to stop treatment and contact their physician immediately should visual changes or loss occur. Men with a history of NAION should not use PDE5 inhibitors (79).
PDE5I have also been implicated in several cases of hearing loss. Several dozen cases of PDE5 inhibitor associated hearing loss have been reported and some research has indicated mechanisms by which hearing loss may be attributable to action of these drugs (80, 81). Men who experience hearing impairment while using PDE5 inhibitors should halt treatment until they are able to speak with their doctors regarding long-term risk of hearing loss.
SILDENAFIL
Sildenafil (Viagra®, Pfizer) works best when taken on an empty stomach (especially avoiding high lipid foods) and reaches maximum plasma concentrations within 30 to 120 minutes (mean 60 minutes). It is eliminated predominantly by hepatic metabolism, and the terminal half-life is about 4 hours. The recommended starting dose is 50 mg taken one hour before sexual activity. The maximal recommended frequency is once per day. The efficacy of sildenafil has been extensively studied in patients with other coexisting diseases (82). No significant difference in response rate was noted comparing a normal cohort of patients with patients with hypertension (83), spinal cord injury (84), depression (85), and the elderly (86). Patients with a neurogenic etiology for ED (e.g., radical prostatectomy, diabetes) have a lower response rate to sildenafil compared to patients without a neurogenic cause; the drug is still effective for many of these men, however (87, 88) (Table 4).
Table 4.
Success of Sildenafil* in Men with Erectile Dysfunction
| Response | Cause of Erectile Dysfunction | ||||
|---|---|---|---|---|---|
| Diabetes Mellitus | Spinal Cord Injury | Radical Prostatectomy | Psychogenic | Depression | |
| (N = 268) | (N = 178) | (N = 107) | (N = 179) | (N = 151) | |
| Improved erection | |||||
| Placebo | 10% | 12% | 15% | 26% | 18% |
| Sildenafil | 57% | 83% | 43% | 84% | 76% |
| Successful intercourse | |||||
| Placebo | 12% | 13% | N/A | 29% | NA |
| Sildenafil | 48% | 59% | 43% | 70% | N/A |
- *
Sildenafil dosage 50 to 100 mg (source: Sildenafil package insert (Pfizer Inc. New York, NY., 1998))
VARDENAFIL
Vardenafil (Levitra® and Staxyn®, Bayer/GSK) is a potent and highly selective PDE5I. Its chemical structure is quite similar to sildenafil however in in vitro studies the selectivity and potency of vardenafil are superior. The medication can be given in either 10 or 20 mg oral dosage (Levitra®) or a 10 mg sublingual dissolving lozenge (Staxyn®). The time to peak plasma concentration is 40-55 minutes. Vardenafil has been shown to be highly efficacious in a wide range of clinical indications (89, 90). In one multicenter phase III trial patients with diabetes (type I and II) were found to respond to 20 mg dosage of vardenafil significantly better than a similar control group. The effect also seemed to improve after 12 weeks of treatment (91). Although similar in chemical structure, the in vitro potency and selectivity of vardenafil are superior to that of sildenafil. Evidence that these in vitro effects translate to superior results in vivo are lacking.
Several newer studies have demonstrated vardenafil to have a faster onset of action than seen with other medications of the same class. In particular, one study (ONTIME) found that 21% of men with moderate to severe erectile dysfunction obtained erections of sufficient firmness for sexual intercourse at 11 minutes after using 20 mg of Vardenafil. At 25 minutes, 53% of patients obtained erections sufficient enough for penetration as compared to placebo (26%). The statistically superior response to vardenafil versus placebo was observed in all times from 11-25 minutes (92). Like sildenafil, absorption of vardenafil is impaired when the medication is taken after a high fat meal (93). The side effects most frequently seen with vardenafil include flushing, dyspepsia, headache, and visual disturbances (94) Adverse events reported in men taking vardenafil closely resemble those in men taking sildenafil and tadalafil. Headache (21%), flushing (13%), and dyspepsia (6%) are seen at various frequencies depending on dosages used.
TADALAFIL
Tadalafil (Cialis®, Eli Lilly, USA) is a PDE5I which has a distinctly different chemical structure from vardenafil and sildenafil (71). Because tadalafil has lower affinity for PDE-6 (localized to the eye) it is associated with a low incidence of visual side effects. Tadalafil is dosed at 5, 10 and 20 mg on demand and is also available as a daily dose medication at 2.5 and 5 mg. Tadalafil’s erection potentiating effect may onset within 30-45 minutes of dosing but may last as long as 24-48 hours (95). Similar to other PDE5I, the risk of serious adverse events with tadalafil is low. In addition to the lack of visual side effects, tadalafil also tends to have a reduced incidence of facial flushing compared to the other PDE5I. The incidence of back/muscle pain is generally higher with tadalafil as compared to other PDE5I.
Absorption of tadalafil is minimally impacted upon by food intake. An integrated analysis from five randomized control double blind placebo trials revealed that men with varying severities of ED significantly improved with Tadalafil therapy at 10 and 20 mg dosages. The mean IIEF score (International Index of Erectile Dysfunction score) increased by 6.5 and 7.9 at the 10 and 20 mg dosage of tadalafil. This increase was statistically significant compared to placebo (96).
In pharmacological studies tadalafil appears to be rapidly absorbed and reaches a peak serum concentration by 2 hours. The most salient unique property of tadalafil is its half-life of approximately 17.5 hours. The absorption and excretion of tadalafil does not appear to be affected by food or alcohol. In a study of the delayed efficacy of tadalafil, at 36 hours post dose 62% of men taking the 20 mg treatment dose reported successful sexual intercourse compared to 33% of men who had taken placebo (97).
Like sildanafil and vardenafil, tadalafil potentiates the hypotensive effects of nitric oxide and it is therefore contraindicated in patients taking nitrate medications. Tadalafil appears to be very well tolerated in most studies. The most frequently reported adverse events are headache and dyspepsia. Back pain, nasal congestion, myalgia, and nasal congestion have also been reported but tend to be mild. The rate of treatment discontinuation from adverse events is low at 2.2% compared with the placebo discontinuation rate of 1.3% (96).
AVANAFIL
A fourth PDE5 inhibitor called avanafil (Stendra®, Vivus) was approved for use in the United States in May of 2012 and became commercially available in 2014 (98). The chemical structure of avanafil differs from that of the three other drugs from the PDE5 inhibitor class. Avanafil is available in 50, 100, and 200 mg dosing and is efficacious in men with diabetes related ED (98). Avanafil is distinguished by having a rapid onset of action and a half-life of approximately 5-10 hours (99). The side effect profile is relatively similar to other drugs from the PDE5 inhibitor class (100).
STRATEGIES FOR MEN WHO FAIL TO RESPOND TO PDE5 INHHBITORS
Several strategies may salvage men who report failure with PDE5 inhibitors. An important first step is re-education on the correct use of the medications. Many patients need to be reminded that these medications are reliant on central mechanisms and that they will not work well without erotic stimulation. Up to 55% of sildenafil initial non-responders will respond after education (101). Dose titration or selection of an alternative PDE5I may be of benefit. Sildenafil, vardenafil, and avanafil tend to absorb more slowly (and hence have lower efficacy) after a high fat meal; tadalafil absorption is less dependent on timing of meals.
Assay of serum testosterone should also be considered in PDE5I failures. Testosterone supplementation has also been associated with improvements in response to PDE5I (102). A randomized controlled trial of testosterone plus PDE5I therapy vs. testosterone alone in men with ED did not show additive benefit from supplementation (103). However, this study was limited in that, during the pre-testosterone treatment run-in phase of the trial with administration of a PDE5I drug, mean serum testosterone levels improved to above the enrollment criterion of 330 mg/dL in both the placebo and testosterone treatment arms (103). The mechanism of the testosterone increase during the run-in in these cohorts is unclear but complicates interpretation of these data.
PDE-5 INHIBITORS AND CARDIOVASCULAR SAFETY
There has been concern about the cardiovascular safety, but controlled and post-marketing studies of the four FDA-approved PDE5I have demonstrated no increase in myocardial infarction or death rates in either double-blind, placebo-controlled trials or open-label studies when compared with expected rates in the study populations. Patients with known coronary artery disease or heart failure receiving PDE5I did not exhibit worsening ischemia, coronary vasoconstriction, or worsening hemodynamics on exercise testing or cardiac catheterization (43). Sexual activity, regardless of PDE5I use, has been associated with incremental increase in risk of cardiac events, an effect that is attenuated in patients who regularly engage in physical exertion.(104) An ability to perform exertion equivalent to 3-4 metabolic equivalents (METS) is consistent with cardiac reserve sufficient for most sexual activity; demands of up to 6 METs may be required for more exertional sex (105).
The vasodilator effects of PDE5 inhibitors may be more marked in patients with hypertension or coronary artery disease. As with all vasodilators, caution is advised in certain conditions: aortic stenosis, left ventricular outflow obstruction, hypotension, and hypovolemia (43). Caution is advised when an alpha-blocker and a PDE-5 inhibitor are taken within a close time frame, as a drug interaction can lead to excessive vasodilation and hypotension (106).
Nitrates are absolutely contraindicated in patients taking PDE5I (table 5) (1). These include organic nitrates, including sublingual nitroglycerin, isosorbide mononitrate, isosorbide dinitrate and other nitrate preparations used to treat angina, as well as amyl nitrite or amyl nitrate (so-called “poppers,” a recreational drug). Past use of nitrates, e.g., more than two weeks before the use of PDE5I, is not considered a contraindication. Patients who develop angina during sexual activity with a PDE5I or any ED medical therapy should be instructed to discontinue sexual activity and consider seeking emergency medical care. The patient should inform emergency medical personnel that a PDE5I was taken. Sublingual nitroglycerin should not be taken in this context as this may lead to serious hypotension.
Table 5.
Recommendations for PDE5I in Men With Cardiac Disease*
|
|
|
|
|
|
- *
Adapted from materials provided by the Princeton III Consensus Conference (43)
Other Treatments of Erectile Dysfunction
In patients for whom PDE5I or are contraindicated, alternative management options include the vacuum constriction device (VCD), trans-urethral suppositories (MUSE®), and intra-cavernous injection (ICI) therapy (1).
VACUUM CONSTRICTION DEVICES (VCD)
VCD produces erection via vacuum suctioning of venous blood into the corporal spaces. Blood is subsequently trapped with a constriction device placed at the base of the phallus. Its potential side effects include discomfort, petechiae, numbness, and interference with ejaculation (1). The erection induced by the VCD is based on trapping of venous blood and therefore the erection may be colder and less firm than natural erections. Some men also experience pain and/or difficulty with operating the device. No constrictive device should be left on the penis for more than 60 minutes and the device should be promptly removed if there are signs of numbness or ischemia.
MUSE®
The Medicated Urethral Suppository for Erections (MUSE®) is a transurethral prostaglandin suppository that has several advantages including local application, minimal systemic effects, and the rarity of drug interaction. However, this type of secondary treatment has failed to gain popularity due to its major drawbacks including moderate to severe penile pain, low response rate, and inconsistent efficacy (107) It has been extensively studied in Europe and the United States and was found to be effective in 43 percent of men with erectile dysfunction of various organic causes. The most common side effects were penile pain (32 percent) and urethral pain or burning (12 percent) (108). Using an adjustable constriction device placed at the base of the penis after MUSE® administration resulted in an increase in successful sexual intercourse in 69 percent of men (109). The standard starting dose for MUSE® is 500-μg dosed in the office. Depending on the patient's response, this dose can be titrated from 250-1000 μg. It is important to administer the test dose in the office due to the risks of urethral bleeding, vasovagal reflex, hypotension, and priapism that can occur with this medication. There is a theoretical risk of prostaglandin transfer during vaginal intercourse after use of MUSE®; for this reason, MUSE® should not be used to facilitate vaginal intercourse in a partner who is pregnant.
INTRACAVERNOUS MEDICATIONS
There are several intracavernous medications available for the treatment of ED including papaverine, prostaglandin E1 (PgE1), and phentolamine (1, 110). These medications are commonly utilized in combinations which yield superior results and lower risk profiles due to synergistic properties between drugs. Of these, prostaglandin E1 is the only drug that is FDA approved as a single agent monotherapy injection for ED. The most common intracavernosal therapies used in the U.S. are two or three-drug mixtures containing papaverine, phentolamine and/or alprostadil (typically known as Bimix if contains the first two or Trimix if it contains all three) in varying concentrations. Some experts utilize atropine for injection-based ED therapy, which is compounded with trimix to form so called quadmix. The usual dose of mixed solution ranges from 0.1 to 0.5 ml. These solutions have demonstrated efficacy and have been in clinical use for almost four decades.
Due to risk of priapism or needle injury, men must receive appropriate training and education by medical personnel before beginning injection therapy for ED. The goal is to achieve an erection that is adequate for sexual intercourse but does not last for more than four hours. The two major side effects of intracavernous injection are priapism and fibrosis (penile deviation, nodules or plaque). Priapism is typically preventable through careful dose titration. Post-injection compression is recommended to reduce the likelihood of bleeding or bruising.
In the U.S., the only FDA approved intracavernosal drug for ED is PgE1 (i.e., trade names Edex® and Caverject®). PgE1 results in erections usable for intercourse in more than 70 percent of treated men after appropriate dose titration (110). The usual dose ranges from 5 to 40 μg as a monotherapy. The most frequent side effect is painful erections that occur in 17 to 34 percent of men (110, 111). This hyperalgesic effect is most prominent in men with partial nerve injury, such as those with diabetic neuropathy and those who have undergone radical pelvic surgery. PgE1 has been associated with risk for priapism and corporal fibrosis as well (112, 113).
Papaverine is a non-specific phosphodiesterase inhibitor that increases cAMP and cGMP concentrations in penile erectile tissue (114). It is usually dosed at 15 to 60 mg. Specific side effects from papaverine include priapism and corporal fibrosis.
Phentolamine is a competitive alpha-adrenergic receptor antagonist. It is used in combination with papaverine (115, 116). Standard doses of phentolamine range from 0.5 to 3mg. The potential side effects of phentolamine include hypotension and reflex tachycardia.
Although the response rate to injection therapy is high, in long-term studies 38 to 80 percent of men cease to use the treatment over time (117). To avoid the cumbersome nature of injection therapy, some men alternate injection therapy with sildenafil or MUSE®, preferring injection in circumstances when an erection of longer duration is desired. Alternatively, in men whom injection therapy alone fails or is insufficient, combination of injections with PDE5I has been utilized in some settings (118). The risk of priapism is magnified in these cases so this option should be considered only in men with severe ED who require intensive stimulation to induce erection response.
SURGICAL MANAGEMENT OF ERECTILE DYSFUNCTION
Surgery is indicated for the management of ED refractory to medical management. One of the earliest contemporary surgical approaches to ED was described by Wooten in 1902, who recommended ligation of the dorsal vein of the penis as a means for restoring erections (122). The superficially placed rigid prosthesis made from synthetic material soon followed (123). These early prostheses tended to shift under the penile skin and were generally unsatisfactory.
Truly modern penile prostheses originated in the early 1970s; these devices were revolutionary in that they were designed to be placed within the corpora cavernosa. Although early models had substantial limitations, these devices were the first penile prosthetics to provide acceptable functional and cosmetic results (124, 125).
Penile arterial bypass surgery was also first reported as a treatment for ED in the 1970s (126). Venous surgery for ED was re-introduced with modifications in the early 1980s to treat corporal veno-occlusive dysfunction (127).
While penile prosthetic devices are generally effective for a wide range of patients, vascular surgery (both arterial and venous) for ED has been effective only in very select patients, typically healthy young men with either congenital or traumatic ED. The 2018 AUA Guidelines on Erectile Dysfunction recommend against venous surgery for ED and recommend arterial revascularization only in highly select patients in centers of excellence (1). Penile prosthesis placement is the standard surgical treatment for ED.
Arterial Revascularization
Arterial revascularization offers patients the possibility of restoration of normal erectile function without the necessity of medications, injections or devices. Penile arterial bypass surgery was first described in the early 1970s (126) and has undergone many modifications since its early description (128). Penile arterial insufficiency is most frequently the result of general arteriosclerosis associated with medical conditions such as hypercholesterolemia, hypertension, cigarette smoking, and diabetes mellitus. In patients with arterial insufficiency from these entities, penile revascularization is unlikely to be of benefit as penile small vessel disease is likely to be present. Healthy young men with traumatic disruption of a discrete segment of the penile vasculature are the population who has the greatest chance of benefit from penile arterial revascularization (1, 128).
Penile arterial insufficiency is diagnosed by performing an intracavernous injection test followed by self-stimulation (audiovisual or manual). Duplex ultrasonography of the penis performed during injection and stimulation test can help delineate echogenicity of corporal tissues, peak flow velocity of cavernosal arteries, thickness of tunica albuginea and cavernosal arteries, diameter and wave form of arteries. If arterial insufficiency is confirmed, selective penile/pudendal arteriogram is necessary to identify penile vascular anatomy, demonstrate communication between cavernosal and dorsal arteries and confirm location of obstructive/traumatized arterial lesion (129).
Several techniques of revascularization have been described (128). Anastomosis of the epigastric artery to the dorsal penile artery (revascularization) or the deep dorsal vein (arterialization) is a common approach. Long term results in these patients are highly variable. Careful selection and refinements in technique can improve success rates. Young men with arterial insufficiency secondary to pelvic trauma are the ideal patients for this procedure (128). The most frequent side effect with penile arterialization is glans hyperemia. This can occur in up to 13% of patients who have undergone epigastric artery-deep dorsal vein anastomosis. Other potential complications include infection, hematoma and thrombosis of anastomosis.
Venous Surgery
Venogenic dysfunction is often suspected during evaluation by the finding of a sub-optimal erectile response to intracavernosal injection despite a normal arterial response on duplex Doppler sonography (129). The presence of persistent end-diastolic flow of greater than 5 cm/sec on duplex sonography may signify venous leak ED. Appropriate conduct of duplex sonography requires repeat dosing of erectogenic agents; this is omitted by some practitioners and may contribute to a false positive finding of venous leak in a patient who would have a normal response to repeat dosing (129).
The gold standard confirmatory test for the diagnosis of venous leak ED is dynamic infusion cavernosometry and cavernosography (DICC). Cavernosometry and cavernosography can be used to document the severity of venous leakage as well as visualize the sites of leakage (130). Like duplex Doppler ultrasound of the penis, these procedures require administration of an erectogenic agent. An abnormal cavernosometry result is 1) an intracavernosal infusion rate of greater than 10cc/minute of saline to maintain the erection or 2) a drop of intracavernosal pressure of greater than 50 mmHg within 30 seconds of terminating the saline infusion (131, 132). An abnormal cavernosogram (performed immediately after cavernosometry) shows visualization of penile veins or venous leakage from the crura on films taken immediately after intracavernous injection of diluted contrast.
As venous surgery for ED is no longer a recommended standard of care, these tests are primarily of historical interest outside of a clinical trial setting.
Prosthetic Surgery
When there is lack of efficacy or dissatisfaction with medical management of ED, penile prosthesis placement is the procedure of choice for restoration of penile rigidity (48). Penile prosthesis surgery is irreversible in that corporal tissue is permanently altered such that physiologic erections are no longer possible. Contemporary prostheses include two or three- piece hydraulic pumps and semi-rigid/malleable rods.
Most malleable prostheses are made of silicone rubber with a central intertwined metallic core. The advantages of malleable devices are that they are easy to implant, easy to operate, and have few mechanical parts with minimal risk for mechanical failure. The major disadvantage of the semi-rigid devices is that the penis is never fully rigid nor fully flaccid. These devices may interfere with urination, are difficult to conceal, and have a higher likelihood of device erosion. Malleable prosthesis is most commonly utilized in resource poor settings or for patients who want a device of minimal complexity (48, 133). Examples of malleable prostheses are presented in Figures 4 and 5.

Figure 4.
Genesis® from Coloplast

Figure 5.
Spectra™ from Boston Scientific
Two-piece inflatable prostheses consist of a pair of cylinders attached to a scrotal pump. The reservoir is in the proximal portion of the cylinders. The prosthesis can be deflated by bending the penis at mid-shaft. While these devices permit some appearance of flaccidity, the lack of a separate reservoir does limit the amount of detumescence that can be attained. These devices represent a compromise between ease of use/placement and potential for expansion (48). A two-piece penile prosthesis is shown in Figure 6.

Figure 6.
Ambicor™ Two-piece Prosthesis from Boston Scientific
Three-piece inflatable prostheses are the gold standard for treatment of medically refractory ED. These devices consist of paired penile cylinders, a scrotal pump, and a reservoir for saline that is placed in the suprapubic space or posterior to the rectus fascia. Three-piece prostheses provide excellent rigidity when erect and a more natural appearance when flaccid. When fully erect, they are as rigid as the two-piece device. In the flaccid state, they surpass flaccidity of two-piece prostheses (48). Hydraulic three-piece penile implants account for about 85% of the US market. Examples of three-piece penile prosthesis are presented in Figures 7 and 8.

Figure 7.
AMS 700™ Three-piece Prosthesis from Boston Scientific

Figure 8.
Titan® Three-piece Prosthesis from Coloplast
Prostheses have very high satisfaction rates (>90%) in management of ED and modern prosthetics are very durable with mean lifespans of 10-15 years (134, 135). This high satisfaction rate is likely due to the capacity of prostheses to allow for spontaneous and repeated reliable erections without external medications or devices. However, men willing to undergo implantation of a penile prosthetic are a self-selecting group and may not be representative of the general male population with ED.
Infection remains the most devastating and feared complication of penile implant surgery. Modern prostheses allow for antibiotic impregnation and elution (136). In the setting of high volume surgeons, infection rates are less than 2% for first-time prosthesis (137). Although some studies suggest that elevated HbA1c levels may predict a higher rate of infections in diabetics having penile prosthesis surgery, more recent studies refute this. Elevated HbA1c may not be a risk factor for infection but poor short-term blood sugar control (defined by morning fasting glucose levels >200 ng/ml) was associated with higher infection risk (138).
Patients must be counseled that it may take a significant amount of time to become comfortable operating penile implants. Many men express dissatisfaction with penile length after prosthesis placement; careful counseling and setting reasonable expectations may help mitigate post-operative concerns.
PRIAPISM
Priapism is a prolonged penile erection in the absence of sexual stimulation. Two subtypes of priapism have been described; current nomenclature differentiates these as non-ischemic and ischemic (139).
Non-Ischemic Priapism
Non-ischemic (formerly known as high-flow) priapism, is relatively rare. In this scenario, injury to a cavernosal artery, usually after perineal or direct penile trauma, results in uncontrolled high arterial inflow within the corpora cavernosa. Non-ischemic priapism is typically painless and the penis only semi-erect. Patients with arterial priapism typically seek medical attention later than those with ischemic priapism, due to the fact that non-ischemic priapism causes less pain and discomfort (139).
Non-ischemic priapism is not considered a medical emergency. In some series men have lived for many years with the condition (140). In such cases men obtain additional tumescence with sexual arousal. In the setting where treatment is desired, conservative management (e.g., observation, ice packs, etc.) may allow the injured cavernous artery to seal off and restore normal blood flow to the corporal bodies. Androgen blockade with ketoconazole or gonadotropin-releasing hormone agonists have been reported as successful in management of non-ischemic priapism, possibly by reducing spontaneous erections and enabling healing of the fistula tract (141). In refractory cases, super selective arteriography allows for the exact determination of the site of injury and treatment through embolization (142). Surgical exploration and ligation of the fistula tract may also be considered by experienced surgeons. There is a risk of ED associated with aggressive treatment of high flow priapism, especially with the use of permanent embolic agents (143).
Ischemic Priapism
Ischemic (formerly known as low-flow) priapism is much more common and is characterized by inadequate venous outflow from the penis; this restricts arterial inflow and creates an acidotic hypoxic environment leading to a painful prolonged erection(139). Ischemic priapism is accurately conceptualized as compartment syndrome of the penis. This more common type of priapism has become a well-recognized clinical entity because of the widespread use of intracavernous agents for erections. Ischemic priapism is also commonly associated with blood dyscrasias (e.g., sickle cell disease, thalassemia, leukemia), advanced pelvic malignancies, and medications such as trazodone (139).
Ischemic priapism is a serious medical condition and can lead to permanent penile fibrosis and ED. When effectively treated early (within 6-12 hours of onset) little risk of permanent injury exists. Tissue injury is visible after 12 hours of ischemia, characterized as interstitial edema. Within 24 hours, there is destruction of the sinusoidal endothelium and adherence. Men with priapism of over 36 hours duration rarely if ever recover normal erectile function (139, 144). Men using vasoactive drugs for penile self-injection should be instructed to seek medical attention if they experience an erection persisting longer than 3-4 hours.
Aspiration of corporal blood via a large bore needle is the procedure of choice to facilitate detumescence in ischemic priapism that is not mediate by intracavernous injection therapy. (139). In most cases, aspiration is followed by installation of a sympathomimetic agent; the agent of choice for the treatment of ischemic priapism is phenylephrine at a dosage of 100-250 ug per mL. The sympathomimetic agent is injected into the cavernous body via the same needle in 2-5 minute intervals until the penis is no longer rigid. At this concentration, little impact on the systemic BP is seen in most men although blood pressure and heart rate should be monitored.
The recent AUA Guideline on Priapism suggests that aspiration may be omitted and the clinician may proceed immediately to phenylephrine injection in the setting of priapism induced by erectogenic injections (139). The AUA priapism guideline does not recommend the use of over-the-counter remedies for priapism such as pseudoephedrine, terbutaline, exercise, or ice packs because these have marginal efficacy and may delay initiation of effective therapy. These remedies may be tried by patients, but patients should come promptly to a clinic or ER for definitive treatment (139).
Detumescence often occurs with initial injection and will first be noted with return of the arterial pulsations of the penis. Cases refractory to aspiration and sympathomimetic agents (typically those of longer duration) may require surgical shunting procedures to return the penis to a flaccid state (145, 146).
Although exchange transfusion and oxygenation is recommended by some for management of sickle cell related ischemic priapism (147), definitive management by phenylephrine irrigation with or without aspiration should not be delayed (148).
The long-term sequelae of ischemic priapism may include penile scarring and fibrosis, penile deformity, and ED. Strategies to avoid these complications include education in men using erectogenic agents and rapid treatment of priapism as it occurs. Some highly reliable patients with recurrent priapism related to sickle cell disease or other blood dyscrasia can be instructed on self-administration of phenylephrine (149).
CONCLUSIONS
As our understanding of the basic mechanisms of erectile function continues to grow, new and improved therapies for the management of ED will continue to emerge. We have witnessed a dramatic change in the treatment of men with erectile dysfunction over the past 30 years. Treatment options have progressed from psychosexual therapy and penile prostheses in the 1970s, through arterial revascularization, vacuum constriction devices, and intracavernous injection therapy in the 1980s, to transurethral and oral drug therapy in the 1990s. Restoration of erectile capacity and facilitation of satisfying sexuality will remain an important goal for men and their sexual partners in the future.
ACKNOWLEDGEMENT
This manuscript was adapted from a prior version which included contributions from the following authors; William O. Brant, MD, Derek Bochinski, MD, Anthony J. Bella, MD.
REFERENCES
- 1.
- Burnett A.L., et al. Erectile Dysfunction: AUA Guideline. J Urol. 2018;200(3):633–641. [PubMed: 29746858]
- 2.
- Laumann E.O., et al. A population-based survey of sexual activity, sexual problems and associated help-seeking behavior patterns in mature adults in the United States of America. Int J Impot Res. 2009;21(3):171–8. [PubMed: 19242482]
- 3.
- Laumann E.O., et al. Sexual problems among women and men aged 40-80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17(1):39–57. [PubMed: 15215881]
- 4.
- Nguyen H.M.T., Gabrielson A.T., Hellstrom W.J.G. Erectile Dysfunction in Young Men-A Review of the Prevalence and Risk Factors. Sex Med Rev. 2017;5(4):508–520. [PubMed: 28642047]
- 5.
- Kamenov Z.A. A comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diabetes. 2015;123(3):141–58. [PubMed: 25502583]
- 6.
- Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med. 2011;8 Suppl 4:310–5. [PubMed: 21967393]
- 7.
- Steers W.D. Neural pathways and central sites involved in penile erection: neuroanatomy and clinical implications. Neurosci Biobehav Rev. 2000;24(5):507–16. [PubMed: 10880817]
- 8.
- Giuliano F., Rampin O. Neural control of erection. Physiology &. Behavior. 2004;83(2):189–201. [PubMed: 15488539]
- 9.
- Peeters M., Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci Biobehav Rev. 2008;32(3):438–53. [PubMed: 17919726]
- 10.
- Dean R.C., Lue T.F. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32(4):379–95. p. v. [PMC free article: PMC1351051] [PubMed: 16291031]
- 11.
- Musicki B., Burnett A.L. eNOS Function and Dysfunction in the Penis. Experimental Biology and Medicine. 2006;231(2):154–165. [PubMed: 16446491]
- 12.
- Banya Y., et al. Two Circulatory Routes Within the Human Corpus Cavernosum Penis: A Scanning Electron Microscopic Study of Corrosion Casts. Journal of Urology. 1989;142(3):879–883. [PubMed: 2769885]
- 13.
- Fournier G.R., et al. Mechanisms of Venous Occlusion During Canine Penile Erection: An Anatomic Demonstration. Journal of Urology. 1987;137(1):163–167. [PubMed: 3795360]
- 14.
- Aboseif S.R., Lue T.F. Hemodynamics of penile erection. Urol Clin North Am. 1988;15(1):1–7. [PubMed: 3278471]
- 15.
- Araujo A.B., Mohr B.A., McKinlay J.B. Changes in sexual function in middle-aged and older men: longitudinal data from the Massachusetts Male Aging Study. J Am Geriatr Soc. 2004;52(9):1502–9. [PubMed: 15341552]
- 16.
- Echeverri Tirado L.C., Ferrer J.E., Herrera A.M. Aging and Erectile Dysfunction. Sex Med Rev. 2016;4(1):63–73. [PubMed: 27872006]
- 17.
- Thomas C., Konstantinidis C. Neurogenic Erectile Dysfunction. Where Do We Stand? Medicines (Basel). 2021;8(1) [PMC free article: PMC7825654] [PubMed: 33430218]
- 18.
- Fukui M., et al. Andropausal symptoms in men with Type 2 diabetes. Diabetic Medicine. 2012;29(8):1036–1042. [PubMed: 22248017]
- 19.
- Defeudis G., et al. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab Res Rev. 2022;38(2):e3494. p. [PMC free article: PMC9286480] [PubMed: 34514697]
- 20.
- Angulo J., et al. Enhancement of both EDHF and NO/cGMP pathways is necessary to reverse erectile dysfunction in diabetic rats. J Sex Med. 2005;2(3):341–6. [PubMed: 16422865]
- 21.
- Ryu J.-K., et al. Erectile Dysfunction Precedes Other Systemic Vascular Diseases Due to Incompetent Cavernous Endothelial Cell-Cell Junctions. Journal of Urology. 2013;190(2):779–789. [PubMed: 23454152]
- 22.
- Lai C.-F., et al. Sexual Dysfunction in Peritoneal Dialysis Patients. American Journal of Nephrology. 2007;27(6):615–621. [PubMed: 17851229]
- 23.
- Lew-Starowicz M., Gellert R. The Sexuality and Quality of Life of Hemodialyzed Patients—ASED Multicenter Study. The Journal of Sexual Medicine. 2009;6(4):1062–1071. [PubMed: 19175866]
- 24.
- Bagcivan I., et al. The evaluation of the effects of renal failure on erectile dysfunction in a rabbit model of chronic renal failure. BJU International. 2003;91(7):697–701. [PubMed: 12699488]
- 25.
- Kielstein J.T., Zoccali C. Asymmetric Dimethylarginine: A Cardiovascular Risk Factor and a Uremic Toxin Coming of Age? American Journal of Kidney Diseases. 2005;46(2):186–202. [PubMed: 16112037]
- 26.
- Maio M.T., et al. Calcification of the Internal Pudendal Artery and Development of Erectile Dysfunction in Adenine‐Induced Chronic Kidney Disease: A Sentinel of Systemic Vascular Changes. The Journal of Sexual Medicine. 2014;11(10):2449–2465. [PubMed: 25138987]
- 27.
- Waldinger, M.D., Psychiatric disorders and sexual dysfunction, in Neurology of Sexual and Bladder Disorders. 2015, Elsevier. p. 469-489. [PubMed: 26003261]
- 28.
- Makhlouf A., Kparker A., Niederberger C.S. Depression and erectile dysfunction. Urol Clin North Am. 2007;34(4):565–74. p. vii. [PubMed: 17983896]
- 29.
- Bhasin S., et al. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. [PubMed: 29562364]
- 30.
- Boloña E.R., et al. Testosterone Use in Men With Sexual Dysfunction: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Trials. Mayo Clinic Proceedings. 2007;82(1):20–28. [PubMed: 17285782]
- 31.
- Mulhall J.P., et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol. 2018;200(2):423–432. [PubMed: 29601923]
- 32.
- Gandaglia G., et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65(5):968–78. [PubMed: 24011423]
- 33.
- Andersson K.-E. Erectile Physiological and Pathophysiological Pathways Involved in Erectile Dysfunction. Journal of Urology. 2003;170(2S) [PubMed: 12853766]
- 34.
- Levine F.J., Greenfield A.J., Goldstein I. Arteriographically determined occlusive disease within the hypogastric-cavernous bed in impotent patients following blunt perineal and pelvic trauma. J Urol. 1990;144(5):1147–53. [PubMed: 2231888]
- 35.
- Rajfer J., Rosciszewski A., Mehringer M. Prevalence of corporeal venous leakage in impotent men. J Urol. 1988;140(1):69–71. [PubMed: 3379701]
- 36.
- Christ G.J., et al. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoceptor responsiveness with age and disease in isolated tissues. Br J Pharmacol. 1990;101(2):375–81. [PMC free article: PMC1917674] [PubMed: 1701678]
- 37.
- La Torre A., et al. Sexual Dysfunction Related to Drugs: A Critical Review. Part IV: Cardiovascular Drugs. Pharmacopsychiatry. 2014;48(01):1–6. [PubMed: 25405774]
- 38.
- Shindel A., Kishore S., Lue T. Drugs Designed to Improve Endothelial Function: Effects on Erectile Dysfunction. Current Pharmaceutical Design. 2008;14(35):3758–3767. [PubMed: 19128228]
- 39.
- Chang S.W., et al. The impact of diuretic therapy on reported sexual function. Arch Intern Med. 1991;151(12):2402–8. [PubMed: 1746997]
- 40.
- Nguyen P.L., et al. Adverse Effects of Androgen Deprivation Therapy and Strategies to Mitigate Them. European Urology. 2015;67(5):825–836. [PubMed: 25097095]
- 41.
- Biebel M.G., Burnett A.L., Sadeghi-Nejad H. Male Sexual Function and Smoking. Sexual Medicine Reviews. 2016;4(4):366–375. [PubMed: 27872030]
- 42.
- Grover S., et al. Sexual dysfunction in patients with alcohol and opioid dependence. Indian journal of psychological medicine. 2014;36(4):355–365. [PMC free article: PMC4201785] [PubMed: 25336765]
- 43.
- Nehra A., et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87(8):766–78. [PMC free article: PMC3498391] [PubMed: 22862865]
- 44.
- Glina S., Sharlip I.D., Hellstrom W.J.G. Modifying Risk Factors to Prevent and Treat Erectile Dysfunction. The Journal of Sexual Medicine. 2013;10(1):115–119. [PubMed: 22971247]
- 45.
- Nehra A., et al. Peyronie's Disease: AUA Guideline. The Journal of urology. 2015;194(3):745–753. [PMC free article: PMC5027990] [PubMed: 26066402]
- 46.
- Mulhall J.P., et al. SUBJECTIVE AND OBJECTIVE ANALYSIS OF THE PREVALENCE OF PEYRONIE’S DISEASE IN A POPULATION OF MEN PRESENTING FOR PROSTATE CANCER SCREENING. Journal of Urology. 2004;171(6 Part 1):2350–2353. [PubMed: 15126819]
- 47.
- Gelbard M.K., Chagan L., Tursi J.P. Collagenase Clostridium histolyticum for the Treatment of Peyronie's Disease: The Development of This Novel Pharmacologic Approach. The Journal of Sexual Medicine. 2015;12(6):1481–1489. [PubMed: 25940867]
- 48.
- Levine L.A., et al. Penile Prosthesis Surgery: Current Recommendations From the International Consultation on Sexual Medicine. The Journal of Sexual Medicine. 2016;13(4):489–518. [PubMed: 27045255]
- 49.
- Travison T.G., et al. Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161–1173. [PMC free article: PMC5460736] [PubMed: 28324103]
- 50.
- Wu F.C., et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–35. [PubMed: 20554979]
- 51.
- Bhasin S., Ozimek N. Optimizing Diagnostic Accuracy and Treatment Decisions in Men With Testosterone Deficiency. Endocr Pract. 2021;27(12):1252–1259. [PubMed: 34390882]
- 52.
- Yeap B.B., et al. Endocrine Society of Australia position statement on male hypogonadism (part 1): assessment and indications for testosterone therapy. Med J Aust. 2016;205(4):173–8. [PubMed: 27510348]
- 53.
- Corona G., et al. Meta-analysis of Results of Testosterone Therapy on Sexual Function Based on International Index of Erectile Function Scores. European Urology. 2017;72(6):1000–1011. [PubMed: 28434676]
- 54.
- Snyder P.J., et al. Effects of Testosterone Treatment in Older Men. N Engl J Med. 2016;374(7):611–24. [PMC free article: PMC5209754] [PubMed: 26886521]
- 55.
- McCullough A.R., et al. A multi-institutional observational study of testosterone levels after testosterone pellet (Testopel((R))) insertion. J Sex Med. 2012;9(2):594–601. [PubMed: 22240203]
- 56.
- Corona G., Maseroli E., Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014;15(13):1903–26. [PubMed: 25080279]
- 57.
- Khera M., et al. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115–23. [PubMed: 24011426]
- 58.
- Golla V., Kaplan A.L. Testosterone Therapy on Active Surveillance and Following Definitive Treatment for Prostate Cancer. Current urology reports. 2017;18(7):49–49. [PMC free article: PMC5486590] [PubMed: 28589395]
- 59.
- Léon P., et al. Low circulating free and bioavailable testosterone levels as predictors of high-grade tumors in patients undergoing radical prostatectomy for localized prostate cancer. Urologic Oncology: Seminars and Original Investigations. 2015;33(9):384.e21–384.e27. [PubMed: 25595576]
- 60.
- Natale C., et al. Testosterone Therapy After Prostate Cancer Treatment: A Review of Literature. Sex Med Rev. 2021;9(3):393–405. [PubMed: 33516741]
- 61.
- Dupree J.M., et al. The safety of testosterone supplementation therapy in prostate cancer. Nat Rev Urol. 2014;11(9):526–530. [PubMed: 25069737]
- 62.
- Basaria S., et al. Adverse events associated with testosterone administration. The New England journal of medicine. 2010;363(2):109–122. [PMC free article: PMC3440621] [PubMed: 20592293]
- 63.
- Vigen R., et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–36. [PubMed: 24193080]
- 64.
- Cittadini A., Isidori A.M., Salzano A. Testosterone therapy and cardiovascular diseases. Cardiovasc Res. 2021 [PubMed: 34293112]
- 65.
- Muraleedharan V., Hugh Jones T. Testosterone and mortality. Clinical Endocrinology. 2014;81(4):477–487. [PubMed: 25041142]
- 66.
- Goldstein I., et al. Oral Sildenafil in the Treatment of Erectile Dysfunction. New England Journal of Medicine. 1998;338(20):1397–1404. [PubMed: 9580646]
- 67.
- Hatzimouratidis K., Hatzichristou D. How to treat erectile dysfunction in men with diabetes: from pathophysiology to treatment. Curr Diab Rep. 2014;14(11):545. [PubMed: 25193347]
- 68.
- Madeira C.R., et al. Efficacy and safety of oral phosphodiesterase 5 inhibitors for erectile dysfunction: a network meta-analysis and multicriteria decision analysis. World J Urol. 2021;39(3):953–962. [PubMed: 32388784]
- 69.
- Phe V., Roupret M. Erectile dysfunction and diabetes: a review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab. 2012;38(1):1–13. [PubMed: 22056307]
- 70.
- Wright P.J. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int J Clin Pract. 2006;60(8):967–75. [PubMed: 16780568]
- 71.
- Padma-Nathan H. Efficacy and tolerability of tadalafil, a novel phosphodiesterase 5 inhibitor, in treatment of erectile dysfunction. The American Journal of Cardiology. 2003;92(9):19–25. [PubMed: 14609620]
- 72.
- Chrysant S.G. Effectiveness and safety of phosphodiesterase 5 inhibitors in patients with cardiovascular disease and hypertension. Curr Hypertens Rep. 2013;15(5):475–83. [PubMed: 23917809]
- 73.
- Herrmann H.C., et al. Hemodynamic Effects of Sildenafil in Men with Severe Coronary Artery Disease. New England Journal of Medicine. 2000;342(22):1622–1626. [PubMed: 10833207]
- 74.
- Muller J.E. Triggering myocardial infarction by sexual activity. Low absolute risk and prevention by regular physical exertion. Determinants of Myocardial Infarction Onset Study Investigators. JAMA: The Journal of the American Medical Association. 1996;275(18):1405–1409. [PubMed: 8618365]
- 75.
- Kloner R.A., et al. Effect of sildenafil in patients with erectile dysfunction taking antihypertensive therapy. Sildenafil Study Group. Am J Hypertens. 2001;14(1):70–3. [PubMed: 11206684]
- 76.
- Hattenhauer M.G., et al. Incidence of Nonarteritic Anteripr Ischemic Optic Neuropathy. American Journal of Ophthalmology. 1997;123(1):103–107. [PubMed: 9186104]
- 77.
- Campbell U.B., et al. Acute Nonarteritic Anterior Ischemic Optic Neuropathy and Exposure to Phosphodiesterase Type 5 Inhibitors. The Journal of Sexual Medicine. 2015;12(1):139–151. [PubMed: 25358826]
- 78.
- Flahavan E.M., et al. Prospective Case-crossover Study Investigating the Possible Association Between Nonarteritic Anterior Ischemic Optic Neuropathy and Phosphodiesterase Type 5 Inhibitor Exposure. Urology. 2017;105:76–84. [PubMed: 28336289]
- 79.
- Pomeranz H.D. The Relationship Between Phosphodiesterase-5 Inhibitors and Nonarteritic Anterior Ischemic Optic Neuropathy. Journal of Neuro-Ophthalmology. 2016;36(2):193–196. [PubMed: 26720519]
- 80.
- Hong B.N., et al. High dosage sildenafil induces hearing impairment in mice. Biol Pharm Bull. 2008;31(10):1981–4. [PubMed: 18827368]
- 81.
- Thakur J.S., et al. Hearing loss with phosphodiesterase-5 inhibitors: a prospective and objective analysis with tadalafil. Laryngoscope. 2013;123(6):1527–30. [PubMed: 23553123]
- 82.
- Carson C.C., et al. The efficacy of sildenafil citrate (Viagra) in clinical populations: an update. Urology. 2002;60(2) Suppl 2:12–27. [PubMed: 12414330]
- 83.
- Kloner R.A. Pharmacology and Drug Interaction Effects of the Phosphodiesterase 5 Inhibitors: Focus on α-Blocker Interactions. The American Journal of Cardiology. 2005;96(12):42–46. [PubMed: 16387566]
- 84.
- Lombardi G., et al. Ten-year follow-up of sildenafil use in spinal cord-injured patients with erectile dysfunction. J Sex Med. 2009;6(12):3449–57. [PubMed: 19686427]
- 85.
- Seidman S.N., et al. Treatment of erectile dysfunction in men with depressive symptoms: results of a placebo-controlled trial with sildenafil citrate. Am J Psychiatry. 2001;158(10):1623–30. [PubMed: 11578994]
- 86.
- Wagner G., et al. Sildenafil Citrate (VIAGRA(R)) Improves Erectile Function in Elderly Patients With Erectile Dysfunction: A Subgroup Analysis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(2):M113–M119. [PubMed: 11213274]
- 87.
- Blander D.S., et al. Efficacy of sildenafil in erectile dysfunction after radical prostatectomy. Int J Impot Res. 2000;12(3):165–8. [PubMed: 11045910]
- 88.
- Jarow J.P., Burnett A.L., Geringer A.M. Clinical efficacy of sildenafil citrate based on etiology and response to prior treatment. J Urol. 1999;162(3 Pt 1):722–5. [PubMed: 10458352]
- 89.
- van Ahlen H., et al. The Real-life Safety and Efficacy of Vardenafil: An International Post-marketing Surveillance Study - Results from 29 358 German Patients. Journal of International Medical Research. 2005;33(3):337–348. [PubMed: 15938595]
- 90.
- Young J.M. Vardenafil. Expert Opinion on Investigational Drugs. 2002;11(10):1487–1496. [PubMed: 12387708]
- 91.
- Goldstein I., et al. Vardenafil, a New Phosphodiesterase Type 5 Inhibitor, in the Treatment of Erectile Dysfunction in Men With Diabetes. Diabetes Care. 2003;26(3):777–783. [PubMed: 12610037]
- 92.
- Montorsi F., et al. Earliest Time to Onset of Action Leading to Successful Intercourse with Vardenafil Determined in an At‐Home Setting: A Randomized, Double‐Blind, Placebo‐Controlled Trial. The Journal of Sexual Medicine. 2004;1(2):168–178. [PubMed: 16422971]
- 93.
- Rajagopalan P., et al. Effect of High-Fat Breakfast and Moderate-Fat Evening Meal on the Pharmacokinetics of Vardenafil, an Oral Phosphodiesterase-5 Inhibitor for the Treatment of Erectile Dysfunction. The Journal of Clinical Pharmacology. 2003;43(3):260–267. [PubMed: 12638394]
- 94.
- Hellstrom W.J., et al. Vardenafil for treatment of men with erectile dysfunction: efficacy and safety in a randomized, double-blind, placebo-controlled trial. J Androl. 2002;23(6):763–71. [PubMed: 12399521]
- 95.
- Forgue S.T., et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61(3):280–8. [PMC free article: PMC1885023] [PubMed: 16487221]
- 96.
- Brock G.B., et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168(4 Pt 1):1332–6. [PubMed: 12352386]
- 97.
- Young J.M. Tadalafil Improved Erectile Function at Twenty-Four and Thirty-Six Hours After Dosing in Men With Erectile Dysfunction: US Trial. Journal of Andrology. 2005;26(3):310–318. [PubMed: 15866997]
- 98.
- Goldstein I., et al. Avanafil for the treatment of erectile dysfunction: a multicenter, randomized, double-blind study in men with diabetes mellitus. Mayo Clin Proc. 2012;87(9):843–52. [PMC free article: PMC3498142] [PubMed: 22857780]
- 99.
- Jung J., et al. Tolerability and pharmacokinetics of avanafil, a phosphodiesterase type 5 inhibitor: a single- and multiple-dose, double-blind, randomized, placebo-controlled, dose-escalation study in healthy Korean male volunteers. Clin Ther. 2010;32(6):1178–87. [PubMed: 20637970]
- 100.
- Kyle J.A., Brown D.A., Hill J.K. Avanafil for erectile dysfunction. Ann Pharmacother. 2013;47(10):1312–20. [PubMed: 24259695]
- 101.
- Hatzichristou D., et al. Sildenafil failures may be due to inadequate patient instructions and follow-up: a study on 100 non-responders. Eur Urol. 2005;47(4):518–22. [PubMed: 15774252]
- 102.
- Buvat J., et al. Hypogonadal Men Nonresponders to the PDE5 Inhibitor Tadalafil Benefit from Normalization of Testosterone Levels with a 1% Hydroalcoholic Testosterone Gel in the Treatment of Erectile Dysfunction (TADTEST Study). Journal of Sexual Medicine. 2011;8(1):284–293. [PubMed: 20704642]
- 103.
- Spitzer M., et al. Effect of Testosterone Replacement on Response to Sildenafil Citrate in Men With Erectile Dysfunction. Annals of Internal Medicine. 2012;157(10):681. [PubMed: 23165659]
- 104.
- Dahabreh I.J., Paulus J.K. Association of episodic physical and sexual activity with triggering of acute cardiac events: systematic review and meta-analysis. JAMA. 2011;305(12):1225–33. [PMC free article: PMC5479331] [PubMed: 21427375]
- 105.
- Oliva-Lozano J.M., et al. What Are the Physical Demands of Sexual Intercourse? A Systematic Review of the Literature. Arch Sex Behav. 2022;51(3):1397–1417. [PMC free article: PMC8917001] [PubMed: 35147835]
- 106.
- Kloner R.A. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha-blocker interactions. Am J Cardiol. 2005;96(12B):42M–46M. [PubMed: 16387566]
- 107.
- Werthman P., Rajfer J. Muse therapy: preliminary clinical observations. Urology. 1997;50(5):809–811. [PubMed: 9372900]
- 108.
- Padma-Nathan H., et al. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med. 1997;336(1):1–7. [PubMed: 8970933]
- 109.
- Lewis R.W. Transurethral Alprostadil with MUSE TM (medicated urethral system for erection) vs intracavernous Alprostadil - a comparative study in 103 patients with erectile dysfunction. International Journal of Impotence Research. 1998;10(1):61–61. [PubMed: 9542692]
- 110.
- Linet O.I., Neff L.L. Intracavernous prostaglandin E1 in erectile dysfunction. Clin Investig. 1994;72(2):139–49. [PubMed: 8186662]
- 111.
- Porst H. The rationale for prostaglandin E1 in erectile failure: a survey of worldwide experience. J Urol. 1996;155(3):802–15. [PubMed: 8583582]
- 112.
- Canale D., et al. Long-term intracavernous self-injection with prostaglandin E1 for the treatment of erectile dysfunction. Int J Androl. 1996;19(1):28–32. [PubMed: 8698535]
- 113.
- Chew K.K., et al. Penile fibrosis in intracavernosal prostaglandin E1 injection therapy for erectile dysfunction. Int J Impot Res. 1997;9(4):225–9. [PubMed: 9442421]
- 114.
- Stief C.G., Wetterauer U. Erectile Responses to Intracavernous Papaverine and Phentolamine: Comparison of Single and Combined Delivery. Journal of Urology. 1988;140(6):1415–1416. [PubMed: 3193506]
- 115.
- Jeremy J.Y., et al. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol. 1997;79(6):958–63. [PubMed: 9202566]
- 116.
- Stief C.G., Wetterauer U. Erectile responses to intracavernous papaverine and phentolamine: comparison of single and combined delivery. J Urol. 1988;140(6):1415–6. [PubMed: 3193506]
- 117.
- Purvis K., Egdetveit I., Christiansen E. Intracavernosal therapy for erectile failure—Impact of treatment and reasons for drop-out and dissatisfaction. International Journal of Impotence Research. 1999;11(5):287–299. [PubMed: 10553808]
- 118.
- Moncada I., et al. Combination therapy for erectile dysfunction involving a PDE5 inhibitor and alprostadil. Int J Impot Res. 2018;30(5):203–208. [PubMed: 30050072]
- 119.
- Ernst E., Pittler M.H. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159(2):433–6. [PubMed: 9649257]
- 120.
- Heaton J.P., et al. Recovery of erectile function by the oral administration of apomorphine. Urology. 1995;45(2):200–6. [PubMed: 7855966]
- 121.
- Rosen R.C., et al. Evaluation of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in patients with an inadequate response to Viagra. Int J Impot Res. 2004;16(2):135–42. [PubMed: 14999221]
- 122.
- Wooten J.S. Ligation of the dorsal vein of the penis as a cure for atonic impotence. Texas Medical Journal. 1902;18:325–327. [PMC free article: PMC9591833] [PubMed: 36954936]
- 123.
- Simmons M., Montague D.K. Penile prosthesis implantation: past, present and future. International Journal of Impotence Research. 2008;20(5):437–444. [PubMed: 18385678]
- 124.
- Scott F.B., Bradley W.E., Timm G.W. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology. 1973;2(1):80–2. [PubMed: 4766860]
- 125.
- Small M.P. Small-Carrion penile prosthesis: a report on 160 cases and review of the literature. J Urol. 1978;119(3):365–8. [PubMed: 642091]
- 126.
- Michal V., Kramar R., Pospichal J. Femoro-pudendal by-pass, internal iliac thromboendarterectomy and direct arterial anastomosis to the cavernous body in the treatment of erectile impotence. Bull Soc Int Chir. 1974;33(4):343–50. [PubMed: 4434623]
- 127.
- Kim E.D., McVary K.T. Long-Term Results With Penile Vein Ligation for Venogenic Impotence. Journal of Urology. 1995;153(3):655–658. [PubMed: 7861508]
- 128.
- Munarriz R., Thirumavalavan N., Gross M.S. Is There a Role for Vascular Surgery in the Contemporary Management of Erectile Dysfunction? Urol Clin North Am. 2021;48(4):543–555. [PubMed: 34602174]
- 129.
- Sikka S.C., et al. Standardization of Vascular Assessment of Erectile Dysfunction. The Journal of Sexual Medicine. 2013;10(1):120–129. [PubMed: 22970798]
- 130.
- Delcour C., et al. Impotence: evaluation with cavernosography. Radiology. 1986;161(3):803–806. [PubMed: 3786737]
- 131.
- Hatzichristou D.G., et al. In Vivo Assessment of Trabecular Smooth Muscle Tone, its Application in Pharmaco-Cavernosometry and Analysis of Intracavernous Pressure Determinants. Journal of Urology. 1995;153(4):1126–1135. [PubMed: 7869480]
- 132.
- Nehra A., et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol. 1996;156(4):1320–9. [PubMed: 8808863]
- 133.
- Shah R. Twenty-five years of the low-cost, noninflatable, Shah Indian penile prosthesis: The history of its evolution. Indian J Urol. 2021;37(2):113–115. [PMC free article: PMC8173931] [PubMed: 34103792]
- 134.
- Kim D.S., et al. AMS 700CX/CXM Inflatable Penile Prosthesis Has High Mechanical Reliability at Long-Term Follow-Up. The Journal of Sexual Medicine. 2010;7(7):2602–2607. [PubMed: 20384938]
- 135.
- Ohl D.A., et al. Prospective evaluation of patient satisfaction, and surgeon and patient trainer assessment of the Coloplast titan one touch release three-piece inflatable penile prosthesis. J Sex Med. 2012;9(9):2467–74. [PubMed: 22759540]
- 136.
- Al Mohajer M., Darouiche R.O. Infections Associated with Inflatable Penile Prostheses. Sexual Medicine Reviews. 2014;2(3-4):134–140. [PubMed: 27784564]
- 137.
- Serefoglu E.C., et al. Long‐Term Revision Rate due to Infection in Hydrophilic‐Coated Inflatable Penile Prostheses: 11‐Year Follow‐up. The Journal of Sexual Medicine. 2012;9(8):2182–2186. [PubMed: 22759917]
- 138.
- Cakan M., et al. Risk factors for penile prosthetic infection. Int Urol Nephrol. 2003;35(2):209–13. [PubMed: 15072498]
- 139.
- Bivalacqua T.J., et al. Acute Ischemic Priapism: An AUA/SMSNA Guideline. J Urol. 2021;206(5):1114–1121. [PubMed: 34495686]
- 140.
- Hakim L.S., et al. Evolving concepts in the diagnosis and treatment of arterial high flow priapism. J Urol. 1996;155(2):541–8. [PubMed: 8558656]
- 141.
- Mwamukonda K.B., et al. Androgen Blockade for the Treatment of High-Flow Priapism. The Journal of Sexual Medicine. 2010;7(7):2532–2537. [PubMed: 20456623]
- 142.
- Kim K.R., et al. Treatment of High-flow Priapism with Superselective Transcatheter Embolization in 27 Patients: A Multicenter Study. Journal of Vascular and Interventional Radiology. 2007;18(10):1222–1226. [PubMed: 17911511]
- 143.
- Savoca G., et al. Sexual function after highly selective embolization of cavernous artery in patients with high flow priapism: long-term followup. J Urol. 2004;172(2):644–7. [PubMed: 15247752]
- 144.
- Bennett N., Mulhall J. Sickle cell disease status and outcomes of African-American men presenting with priapism. J Sex Med. 2008;5(5):1244–1250. [PubMed: 18312286]
- 145.
- Brant W.O., et al. T-Shaped Shunt and Intracavernous Tunneling for Prolonged Ischemic Priapism. Journal of Urology. 2009;181(4):1699–1705. [PubMed: 19233430]
- 146.
- Burnett A.L., Pierorazio P.M. Corporal “Snake” Maneuver: Corporoglanular Shunt Surgical Modification for Ischemic Priapism. The Journal of Sexual Medicine. 2009;6(4):1171–1176. [PubMed: 19207268]
- 147.
- Marouf R. Blood transfusion in sickle cell disease. Hemoglobin. 2011;35(5-6):495–502. [PubMed: 21981466]
- 148.
- Merritt A.L., Haiman C., Henderson S.O. Myth: blood transfusion is effective for sickle cell anemia-associated priapism. CJEM. 2006;8(2):119–22. [PubMed: 17175874]
- 149.
- Teloken C., et al. Intracavernosal etilefrine self-injection therapy for recurrent priapism: one decade of follow-up. Urology. 2005;65(5):1002. [PubMed: 15882751]
- Review New treatment options for erectile dysfunction in patients with diabetes mellitus.[Drugs. 2004]Review New treatment options for erectile dysfunction in patients with diabetes mellitus.Basu A, Ryder RE. Drugs. 2004; 64(23):2667-88.
- Review Sexual Dysfunction in Diabetes.[Endotext. 2000]Review Sexual Dysfunction in Diabetes.Shindel AW, Lue TF. Endotext. 2000
- Weaker masturbatory erection may be a sign of early cardiovascular risk associated with erectile dysfunction in young men without sexual intercourse.[J Sex Med. 2014]Weaker masturbatory erection may be a sign of early cardiovascular risk associated with erectile dysfunction in young men without sexual intercourse.Huang YP, Chen B, Yao FJ, Chen SF, Ouyang B, Deng CH, Huang YR. J Sex Med. 2014 Jun; 11(6):1519-26. Epub 2014 Mar 3.
- Review Anatomy, Pathophysiology, Molecular Mechanisms, and Clinical Management of Erectile Dysfunction in Patients Affected by Coronary Artery Disease: A Review.[Biomedicines. 2021]Review Anatomy, Pathophysiology, Molecular Mechanisms, and Clinical Management of Erectile Dysfunction in Patients Affected by Coronary Artery Disease: A Review.Sangiorgi G, Cereda A, Benedetto D, Bonanni M, Chiricolo G, Cota L, Martuscelli E, Greco F. Biomedicines. 2021 Apr 16; 9(4). Epub 2021 Apr 16.
- Review Endovascular therapy for erectile dysfunction: current knowledge and future perspectives.[Minerva Cardiol Angiol. 2021]Review Endovascular therapy for erectile dysfunction: current knowledge and future perspectives.Sangiorgi G, Pizzuto A, Diehm N, Greco F, Fusco F, Chiricolo G, Vismara A, Altieri VM, Cereda A, Bongo S. Minerva Cardiol Angiol. 2021 Oct; 69(5):579-595. Epub 2020 Jun 2.
- Medical and Surgical Therapy of Erectile Dysfunction - EndotextMedical and Surgical Therapy of Erectile Dysfunction - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
