Patients were randomised between May 2006 and July 2008 and the final follow-up data collection took place in January 2012.
Unblinding of randomised treatments
The treatment allocation was unblinded six times during the course of the trial at the request of the sponsor organisation to assist in the management of suspected unexpected serious adverse reactions (SUSARs). In addition, unblinding of four participants at four separate sites was carried out at the request of the local investigator, on clinical grounds.
Telephone-based assessment of Expanded Disability Status Scale score
Of 3812 assessments of EDSS score over the study period, 42 (1.1%) were by telephone, rather than face to face. These telephone assessments were carried out on a total of 39 patients (25 assigned to active; 14 assigned to placebo).
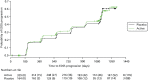
Figure 1 shows the trial profile and Table 3 shows discontinuations of trial medication and losses to follow-up. A total of 498 patients were randomly assigned to active treatment (n = 332) or matching placebo (n = 166). The data from three patients (two randomised to active, one to placebo) were removed from the trial because they withdrew their consent after randomisation. A further two patients (one randomised to active, one to placebo) were found to be ineligible after randomisation. Four hundred and ninety-three (329 active, 164 placebo) received their allocated intervention and were therefore included in an ITT analysis. Of the 493 randomised and treated participants, 415 (84%) completed follow-up, of whom 119 (29%) had prematurely discontinued trial medication (Figure 1).

FIGURE 1
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram. a, Nine patients initially did not fulfil entry criteria, but did so on subsequent rescreening. DNA, did not attend; PEP, primary end point of EDSS score progression.
TABLE 3
Analysis set and study completion (all randomised patients)
Baseline comparability of randomised groups
Baseline patient and disease characteristics were similar in both treatment groups (Table 4). At baseline, 59% of participants were women, 61% had SPMS and 78% had an EDSS score of 6.0 or 6.5. There were no important differences in outcome measures assessed at baseline (Table 5).
TABLE 4
Baseline characteristics of patients by treatment group and overall
TABLE 5
Outcome variables at baseline for patients randomised to the two treatment groups
Prescribed dose of trial medication
Prescribed daily doses of trial medication at each 6-monthly follow-up are summarised in Table 6 for those patients not discontinuing trial medication and for all patients. Among those patients not withdrawing from trial medication (n = 178 active, n = 118 placebo), median prescribed daily dose during final year of follow-up was four capsules (25th–75th percentiles 2–6 capsules) in the active group compared with six (25th–75th percentiles 4–8 capsules) in the placebo group. Final year medians among all patients were four capsules (25th–75th percentiles 2.0–5.5) for active and six capsules (25th–75th percentiles 4–8 capsules) for placebo. Percentiles of prescribed daily dose among non-withdrawals, by treatment group and weight group, are shown in Figure 2.
TABLE 6
Prescribed daily dose of trial medication at each 6-monthly follow-up, by treatment group, among non-withdrawals and overall
Random urine testing to determine any illicit cannabis use
Results from urinalyses throughout the study are given in Table 7. These results showed little illicit cannabis use in the placebo group and an increasing proportion of negative test results within the active group over time.
TABLE 7
Frequency and relative frequency (%) of patients in each treatment group with a urine sample tested positive or negative for cannabinoid presence. The frequency and relative frequency of tests yielding no result is also given
The main results are summarised in Table 8 and detailed below.
TABLE 8
Summary of main results
Pre-specified analyses of primary clinical outcomes
Primary analysis of time to first confirmed Expanded Disability Status Scale score progression
Primary analysis using a Cox regression model showed no evidence of an effect of age (p = 0.36), disease type (p = 0.12), sex (p = 0.56), weight (p = 0.11) or treatment (p = 0.57; see Table 8) on time to confirmed EDSS score progression. The HR for first EDSS score progression event in patients randomly assigned to dronabinol compared with those assigned to placebo was 0.92 [95% confidence interval (CI) 0.68 to 1.23; see Table 8]. At trial completion, Kaplan–Meier estimates of the probability of EDSS score progression were 0.55 (95% CI 0.46 to 0.63) in the dronabinol group compared with 0.60 (95% CI 0.44 to 0.71) in the placebo group (Figure 3).
We noted evidence of some study site effects and of an effect of baseline EDSS score on time to confirmed progression (Figure 4). Most notably, relative to a baseline EDSS score of 4.0, there was an increased hazard of disease progression among those with a baseline EDSS score of 5.5 (HR 3.17, 95% CI 1.45 to 6.93; p = 0.004) and a reduced hazard among those with a baseline EDSS score of 6.5 (HR 0.49, 95% CI 0.24 to 0.98; p = 0.04). However, the numbers of participants in the individual EDSS groups are small (Figure 5), as are the numbers in some study sites (see Table 16 and Appendix 3).
TABLE 16
Premature discontinuations of trial medication and losses to follow-up, by study site
The global PH test gave no evidence that the PH assumption was violated (χ2 = 36, 36 degrees of freedom; p = 0.47).
Sensitivity analyses of time to first confirmed Expanded Disability Status Scale score progression
Results of sensitivity analysis showed that when losses to follow-up were treated as progression events rather than censored observations, the estimated HR (active : placebo) for EDSS score progression changed to 1.11 (95% CI 0.86 to 1.44; see Table 8), but the estimated effect of treatment remained non-significant (p = 0.41). This change in HR might be because the dronabinol group had a higher proportion of losses to follow-up for EDSS assessment [56 of 71 (79%)] than the placebo group [15 of 71 (21%)] and represents a worst-case scenario in terms of patient deterioration and hence the potential benefit of dronabinol.
The EPC reviewed data on 95 patients [71 active (74.7%); 24 placebo (25.3%)], for which there were ambiguities regarding EDSS scores. The EPC considered 22 (12 active; 10 placebo) of these patients to have progressed. These patients had no confirmed progression according to the data collected from the trial schedule. A further four patients (three active; one placebo) were considered to have progressed prior to the time of progression determined from the trial schedule. Clinical information on the remaining 69 patients reviewed by the EPC either confirmed non-progression or was insufficient to draw any further conclusions over those made on the primary data. As a result, data derived following EPC review consisted of a total of 240 first progression events compared with 218 in the primary data (with losses to follow-up considered as censored observations in both).
Conclusions from the main analyses of time to first EDSS score progression were robust to sensitivity analyses in terms of whether or not conclusions from the EPC were considered in defining EDSS progressions under both approaches to dealing with losses to follow-up, that is treated as censored observations or as progression events (see Table 8).
Furthermore, estimated HRs (active : placebo) for EDSS score progression remained similar after sequential removal of study sites with high loss to follow-up rates, under each of the two ways of treating losses to follow-up and each of the two data sets, that is according to trial schedule or following EPC review (Figure 6).
Pre-specified subgroup analyses of time to first confirmed Expanded Disability Status Scale score progression
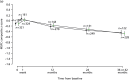
Pre-specified subgroup analyses of time to first EDSS score progression suggested a differential effect of treatment between participants with lower (4.0–5.5) and higher (6.0–6.5) baseline EDSS scores (Figure 7). There was little evidence of differential effects of treatment among subgroups defined in terms of sex, disease type, or age or weight at registration.

FIGURE 7
Estimated HRs (active : placebo) and 95% CIs for EDSS score progression by subgroup.
Primary analysis of change in Multiple Sclerosis Impact Scale-29 20-point physical subscale
A multilevel model fitted to repeated measures of MSIS-29phys score showed no evidence of an effect of treatment [estimated between-group difference (active–placebo) −0.91 points, 95% CI −2.01 to 0.19 points; p = 0.11; see Table 8], or of disease type, sex, weight or study site (data not shown; p > 0.05 for all).
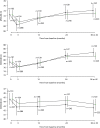
It was estimated that MSIS-29phys score reduced by a mean of 1.4 points (95% CI 0.3 to 2.5 points; p = 0.02) for every 10-year increase in age. In both treatment groups, mean MSIS-29phys score decreased from baseline to month 3, after which it tended to increase (Figure 8).
With the exception of a small reduction in MSIS-29phys score in patients with a baseline EDSS score of 5.0 compared with those with a score of 4.0, MSIS-29phys score tended to increase with increasing baseline EDSS score (data not shown).
Results from the primary analysis of repeated measures of MSIS-29phys remained unchanged after removal of non-significant terms from the fitted model and under an alternative analysis based on comparison of treatment groups in terms of change from baseline to last valid observation [estimated between-group difference (active–placebo) –1.4 points, 95% CI –3.3 to 0.4 points; p = 0.13].
Pre-specified analyses of secondary outcomes
Results of multilevel models fitted to data on the secondary outcomes MSWS-12v2, MSFC, RMI, SF-36(PH) and MSSS-88 are summarised in Table 8 and detailed below.
Multiple Sclerosis Walking Scale-12
A multilevel model fitted to repeated measures of MSWS-12v2 score showed no evidence of an effect of treatment [estimated effect –0.19 (95% CI –0.97 to 0.60); p = 0.74; see Table 8], or of disease type, sex or weight (data not shown; p > 0.05 for all). There was some evidence of study site effects (data not shown) and of effects of baseline EDSS score. Compared with those with a baseline EDSS score of 4.0, MSWS-12v2 was estimated to be, on average, 5.7 (95% CI 2.3 to 9.0), 6.1 (95% CI 3.7 to 8.5) and 9.3 (95% CI 6.8 to 11.8) points higher in those with a baseline EDSS score 5.5, 6.0 and 6.5, respectively. In both treatment groups, mean MSWS-12v2 score decreased from baseline to month 3, after which it tended to increase (Figure 9).
Multiple Sclerosis Functional Composite
A multilevel model fitted to repeated measures of MSFC composite z-score showed no evidence of an effect of treatment; estimated between-group difference (active–placebo) –0.03 (95% CI –0.19 to 0.09; p = 0.72; see Table 8). Multilevel models fitted to the MSFC component-wise z-scores each showed no evidence of an effect of treatment. Estimated between-group differences (active–placebo) were: T25-FW –0.08 (95% CI –0.25 to 0.09; p = 0.37); 9-HPT 0.05 (95% CI –0.04 to 0.13; p = 0.28); and PASAT –0.01 (95% CI –0.10 to 0.09; p = 0.92). Across both treatment groups, mean T25-FW, 9-HPT and composite z-scores increased from baseline to week 1, after which they tended to decrease. After an initial increase at week 1, PASAT z-scores remained relatively constant over the 3-year study period (Figure 10).
Rivermead Mobility Index
A multilevel model fitted to repeated measures of RMI showed no evidence of an effect of treatment [the estimated between-group difference (active–placebo) was 0.04 (95% CI –0.24 to 0.32; p = 0.76; see Table 8)] or of disease type, sex or weight (data not shown; p > 0.05 for all). There was some evidence of study site effects (data not shown) and of effects of baseline EDSS score. Compared with those with a baseline EDSS score of 4.0, RMI was estimated to be, on average, 1.57 points (95% CI 0.38 to 2.76 points), 2.44 points (95% CI 1.59 to 3.30 points) and 4.99 points (95% CI 4.10 to 5.88 points) lower in those with a baseline EDSS score of 5.5, 6.0 and 6.5, respectively. In both treatment groups, RMI decreased from baseline to 30 months, after which it remained fairly constant (Figure 11).
Short Form questionnaire-36 items (physical health subscale)
A multilevel model fitted to repeated measures of SF-36(PH) showed no evidence of an effect of treatment [the estimated between-group difference (active–placebo) was –0.15 (95% CI –0.83 to 0.53; p = 0.67; see Table 8)], or of disease type, sex or weight (data not shown; p > 0.05 for all). In both treatment groups, mean SF-36(PH) score increased from baseline to month 3, after which it tended to decrease (Figure 12).
With the exception of a small increase in SF-36(PH) score in patients with a baseline EDSS score of 5.0 compared with those with a score of 4.0, SF-36(PH) score tended to decrease with increasing baseline EDSS score (data not shown).
Multiple Sclerosis Spasticity Scale-88
Figure 13 shows estimated mean MSSS-88 scores, with 95% CIs, by visit and treatment group, for each of the eight subscales.
For each of the physical components of the MSSS-88, subscales 1 to 6 inclusive, after an initial decrease from baseline to month 3, mean scores tended to increase. Estimated means were consistent across treatment groups, as seen by the overlapping CIs. For the two psychological components, subscales 7 and 8, after an initial decrease from baseline to month 3, mean scores remained relatively constant over the study period. Estimated mean scores for these components tended to be higher in the active group than in placebo, but any differences failed to reach statistical significance.
Multilevel models fitted to three groups of the MSSS-88, where MSSS-88 (1) combines subscales 1–3; MSSS-88 (2) combines subscales 4–6 and MSSS-88 (3) combines subscales 7 and 8 (as described in Chapter 2), each showed no evidence of an effect of treatment. Estimated between-group difference (active–placebo) for MSSS-88 (1) was 0.26 (95% CI –1.99 to 2.52; p = 0.82; see Table 8); for MSSS-88 (2) was –0.02 (95% CI –2.35 to 2.32; p = 0.99; see Table 8); and for MSSS-88 (3) was 1.00 (95% CI –0.70 to 2.70; p = 0.25; see Table 8). In both treatment groups, mean MSSS-88 (1) and mean MSSS-88 (2) decreased from baseline to month 3, after which they tended to increase (Figure 14). After an initial decrease from baseline to month 3, mean MSSS-88 (3) remained relatively constant over the study period (see Figure 14).
Investigation of adverse events and serious adverse events
The number of participants experiencing at least one SAE was 114 (35%) in the Δ9-THC group and 46 (28%) in the placebo group, the most common SAE being admission to hospital for MS-related events and infections. The number and nature of SAEs experienced was similar across treatment groups (Table 9).
TABLE 9
Occurrences of SAEs and the most common AEs
There were numerous non-serious AEs in both groups, consistent with the effects of MS and the known safety profile of cannabinoids. The median number of events per participant in the active group was 11 (25th–75th percentiles 7–17) compared with 10 (25th–75th percentiles 6–14) in the placebo group. Of those events judged to be either moderate or severe, the most frequent are documented in Table 9. Among these AEs, there was some suggestion that those participants on active treatment were more likely to experience dizziness or light-headedness and dissociative and thinking or perception disorders. On the other hand, a higher proportion of patients in the placebo group experienced musculoskeletal pain and aches than in the active group.
Six SAEs were classified as potential SUSARs in accordance with European clinical trials legislation. Three events occurred in each of the active and placebo groups. Trial treatment was discontinued in three participants as a result of the SAE. Three SAEs were classified as nervous system disorders: two were psychiatric events and one related to the gastrointestinal tract.
Category rating scales
Responses to questions 9–16 of the category rating scales, relating to how the patient felt at the time of completing the questionnaire, compared with just before the start of the study, have been grouped (as described in Chapter 2) and summarised, in terms of frequencies and relative frequencies in the two treatment groups, at each follow-up (Tables 10–13). Unadjusted p-values from chi-squared tests for trend are given.
TABLE 13
Frequencies and relative frequencies (%) of responses to category rating scale questions 9–16, by treatment group, at 3 years after baseline. Percentages are taken with respect to total number of patients in the corresponding treatment group
Generally, a higher proportion of patients on active treatment than on placebo reported being more forgetful at the time of follow-up compared with before the study. At 3 months from baseline, there was an approximately twofold increase in proportion of responses classified as ‘worse’ in the active group compared with placebo (32% active, 15% placebo; p = 0.0067; see Table 10). These proportions were similar across treatment groups at 1-year follow-up (49% active, 42% placebo; p = 0.25; see Table 11), at 2 years there was an approximately 30% increase in ‘worse’ responses in the active group compared with placebo (57% active, 44% placebo; p = 0.037; see Table 12) and similarly at 3 years, with a 33% increase (60% active, 45% placebo; p = 0.11; see Table 13). Responses to the remaining questions were similar across treatment groups.
TABLE 11
Frequencies and relative frequencies (%) of responses to category rating scale questions 9–16, by treatment group, at 1 year after baseline. Percentages are taken with respect to total number of patients in the corresponding treatment group
TABLE 12
Frequencies and relative frequencies (%) of responses to category rating scale questions 9–16, by treatment group, at 2 years after baseline. Percentages are taken with respect to total number of patients in the corresponding treatment group
Analysis of premature discontinuations of trial medication and losses to follow-up
Of the 493 patients included in the ITT analysis, 119 (24.1%; 89 active, 30 placebo) prematurely discontinued trial medication but remained in follow-up. Seventy-eight patients (15.8%; 62 active, 16 placebo) were lost to follow-up, meaning that 296 (60%) patients completed the study on trial treatment.
There was evidence of an increased risk of discontinuation of trial medication in the active group compared with placebo (p < 0.001, log-rank test) (Figure 15).
Reasons for discontinuation of trial medication or loss to follow-up were dominated by AEs, accounting for 65% of all early discontinuations (Table 14). Reasons for loss to follow-up are summarised in Table 15. The most common reasons were reported as ‘MS or other health issues’ and ‘other’, accounting for 50% (39 out of 78) of all losses to follow-up. ‘Travel or burden of the trial’ accounted for 22% (17 out of 78) of reasons for loss to follow-up and accounted for a larger proportion of losses in placebo patients [5 out of 16 (31%) compared with 12 out of 62 (19%) of the losses in the active group].
TABLE 14
Frequencies and relative frequencies (%) of reasons for discontinuation of trial medication or loss to follow-up, by treatment group, disease type, baseline EDSS, sex and overall
TABLE 15
Frequencies and relative frequencies (%) of reasons for loss to follow-up, by treatment group, disease type, baseline EDSS, sex and overall
Rates of discontinuations from trial medication or loss to follow-up varied across study sites (Table 16).
Following a forward selection procedure, a Cox regression model fitted to data on time to discontinuation of trial medication or loss to follow-up showed evidence of effects of treatment allocation, sex and study site on the risk of withdrawal or loss to follow-up. The risk of withdrawal or loss to follow-up was estimated to be higher in men than in women [HR (men : women) 1.37, 95% CI 1.02 to 1.84] and higher in the active group than in the placebo group [HR (active : placebo) 1.97, 95% CI 1.41 to 2.76].
Pre-specified analyses of magnetic resonance imaging substudy
Two hundred and seventy-four patients from 13 study sites were allocated to the MRI substudy. Of these, one patient was excluded at baseline visit (as baseline scan was deemed problematic, due to patient tremor).
Baseline data on demographic and disease characteristics were similar across treatment groups (Table 17). Fifty-nine per cent of patients were women, 64% had SPMS and 76% had an EDSS score of 6.0 or 6.5.
TABLE 17
Baseline characteristics of patients in the MRI substudy, by treatment group and overall
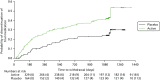
Forty-seven of the 182 patients (25.8%) on active treatment and 17 of the 91 patients (18.7%) on placebo were lost to follow-up during the study period. Figure 16 shows the flow of patients over the 3-year follow-up period.

FIGURE 16
Flow of participants through the MRI substudy.
Between-treatment-group comparisons of PBVC and numbers of new or enlarging T2 and new T1 lesions at each annual follow-up showed little evidence of an association between treatment allocation and these outcomes (Table 18).
TABLE 18
Descriptive statistics and between-treatment-group comparisons for MRI outcome measures at years 1, 2 and 3
A multilevel model fitted to cumulative PBVC showed no evidence of an effect of active treatment on brain atrophy compared with placebo over the course of the study [estimated between-group difference in PBVC (active–placebo) was −0.01%, 95% CI −0.26% to 0.24%; p = 0.94; see Table 8]. However, brain atrophy did change significantly over time (p < 0.0001); using a fitted model, cumulative PBVC was estimated to be a mean of −0.58% at year 1, −1.20% at year 2 and −2.02% at year 3 (Figure 17).
There was evidence of an effect of baseline normalised brain volume (NBV) on brain atrophy. Using a fitted model, it was estimated that, for a 100-unit reduction in baseline NBV, brain atrophy increased by a mean of 0.21% (95% CI 0.08% to 0.34%; p = 0.003).
Treatment did not significantly affect the occurrence of new or newly enlarging T2 lesions [estimated odds ratio (OR) (active : placebo) 0.89, 95% CI 0.50 to 1.58; p = 0.70; see Table 8] or new T1 lesions [estimated OR (active : placebo) 1.05, 95% CI 0.59 to 1.88; p = 0.87; see Table 8].
Publication Details
Copyright
Included under terms of UK Non-commercial Government License.
Publisher
NIHR Journals Library, Southampton (UK)
NLM Citation
Ball S, Vickery J, Hobart J, et al. The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Southampton (UK): NIHR Journals Library; 2015 Feb. (Health Technology Assessment, No. 19.12.) Chapter 3, Results: main study and magnetic resonance imaging substudy.