From: How Genetic Switches Work

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
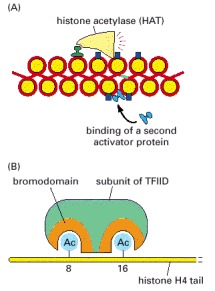
(A) Some gene activator proteins can bind directly to DNA that is packaged in unmodified chromatin. By attracting histone acetylases (and nucleosome remodeling complexes), these “pioneer” activators can facilitate the binding to DNA of additional activator proteins that cannot bind to unmodified chromatin. These additional proteins can in turn carry out additional modifications of chromatin or act directly on the transcription machinery as shown in Figures 7-43 and 7-44. (B) A subunit of the general transcription factor TFIID contains two 120-amino acid protein domains called bromodomains. Each bromodomain forms a binding pocket for an acetylated lysine side chain (designated Ac in the figure); in TFIID the two pockets are separated by 25 Å, which is the optimal spacing for recognizing a pair of acetylated lysines separated by six or seven amino acids on the N-terminal tail of histone H4. In addition to the pattern of acetylation shown, this subunit of TFIID also recognizes the histone H4 tail acetylated at positions 5 and 12 and the fully acetylated tail. It has no appreciable affinity for an unacetylated H4 tail and only low affinity for an H4 tail acetylated at a single lysine. As shown in Figure 4-35, certain patterns of histone H4 acetylation, including those recognized by TFIID, are associated with transcriptionally active regions of chromatin.
From: How Genetic Switches Work

NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.