NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Research Council (US) Committee on Toxicology. Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents. Washington (DC): National Academies Press (US); 1997.

Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents.
Show detailsGB (Sarin or isopropyl methylphosphonofluoridate) is an organophosphate nerve agent. The physical and chemical properties, toxico-kinetics, and toxicity of GB are discussed in detail by CDEPAT (1994), Marrs et al. (1996), and Somani (1994). Only a few toxicity end points were considered (for example, lethality in animals, incapacitation, changes in cholinesterase (ChE) activity, and ocular effects). The subcommittee's assessment of the scientific validity of CDEPAT's proposed human-toxicity estimates for GB is discussed below.
Percutaneous Vapor Exposure
Lethal Effects (LCt50)
After reviewing the available animal lethality data, CDEPAT proposed a human LCt50 estimate of 10,000 mg-min/m3 following percutaneous exposure to GB vapor, assuming light clothing and exposure durations of 30 to 50 min for the soldiers. The existing LCt50 estimate is 15,000 mg-min/m3 (CDEPAT 1994).
The Army's proposed estimate is supported by an LCt50 of 9,700 mgmin/m3 in monkeys exposed to hot temperatures (80–95°F) and moderate relative humidity (35% to 55%) (Oberst et al. 1952). On the basis of the monkey and human studies, a lethal Ct (concentration × time) for humans was estimated to be slightly higher than 12,800 mg-min/m 3 (McGrath et al. 1951). Silver (1953) estimated the LCt50 to be about 15,000 mg-min/m3; the estimate was based on studies of McGrath et al. (1951) who investigated the inhibition of cholinesterase (ChE) in monkeys and men exposed to GB. The subcommittee believes that, on the basis of available data, 10,000 mg-min/m3 is a conservative estimate of the human LCt50.
The preliminary studies involved exposure to the right forearm, and data from these studies were used to determine the whole-body exposures. Therefore, the subcommittee concludes that CDEPAT's LCt50 estimate of 10,000 mg-min/m3 for GB is scientifically valid.
ECt50 for Threshold Effects
CDEPAT's proposed ECt50 estimate for threshold (minimal) effects following percutaneous exposure to GB vapor is 1,200 mg-min/m3, assuming light clothing and exposure durations of 30 to 50 min. There is no existing ECt50 estimate for threshold effects (CDEPAT 1994).
The Army's proposed estimate of 1,200 mg-min/m3 is based on human data using the most appropriate study (McGrath et al. 1951). Exposures of 190 to 1,010 mg-min/m3 (concentrations of 21 to 92 mg/m3; durations of 9 to 11 min) resulted in ChE levels of 95% to 108% of baseline; all subjects were asymptomatic, supporting a no-effect level of < 1,000 mg-min/m3 (McGrath et al. 1951). Humans exposed at 1,255 to 1,850 mg-min/m3 (concentrations of 81 to 109 mg/m3; exposure durations of 11.5 to 20 min) had ChE activities ranging from 31% to 90%. Two of nine individuals were asymptomatic, and the other seven experienced sweating that persisted for a minimum of 24 hr and a maximum of 30 days (McGrath et al. 1951). Therefore, the subcommittee concludes that CDEPAT's proposed ECt50 estimate for threshold effects is scientifically valid.
Inhalation Vapor Exposure
Lethal Effects (LCt50)
CDEPAT's proposed LCt50 estimate following inhalation exposure to GB vapor is 35 mg-min/m3, assuming minute volumes of 15 liters and exposure durations of 2 to 10 min. The existing LCt50 estimate is 70 mg-min/m 3 (CDEPAT 1994).
The LCt50 data for inhalation exposure for several animal species (mouse, rat, primate, dog, rabbit, cat, and pig) provide an LCt50 estimate for humans of 60 mg-min/m3 for 10-min exposures. The average ratio (of LCt50 for GA, GB, and GF) for 10-min and 2-min exposures was calculated to be 0.6 (CDEPAT 1994) and that ratio was also supported by a classified study. Using a factor of 0.6 to estimate the 2-min LCt50 from the 10-min LCt50, CDEPAT obtained a value of 35 mg-min/m 3 (60 × 0.5 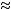 35).
35).
Human data from the Adamek Report (as cited in Wills and DeArmon 1954) showed deaths in four of four subjects exposed at 4 mg/m3 for 10 min (a Ct (concentration × time) of 40 mg-min/m3). Data were available for 48 other subjects, all of whom received some type of post-exposure therapy. Using data from exposed and unexposed individuals, the authors calculated an LCt50 of 24 mg-min/m3 (Wills and DeArmon 1954). However, on the basis of the 100% lethality observed in humans exposed at 40 mg-min/m3, the subcommittee recommends that the CDEPAT's proposed LCt50 estimate of 35 mg-min/m3 be lowered. The subcommittee also recommends that further research be conducted to establish the LCt 50 estimate for inhalation with a greater degree of confidence.
ECt50 for Severe Effects
CDEPAT's proposed ECt50 estimate for severe effects following inhalation exposure to GB vapor is 25 mg-min/m3, assuming minute volumes of 15 liters and exposure durations of 2 to 10 min. The existing ECt 50 estimate is 35 mg-min/m3 (CDEPAT 1994).
In the absence of adequate data on GB for this effect, CDEPAT's proposed estimate is based on the assumption that the ratio of ICt50 (incapacitation dose for 50% of a given population) and LCt50 is about 0.7. The ratio is supported by a study conducted in monkeys (Cresthull et al. 1957). The proposed ECt50 estimate of 25 mg-min/m3 was calculated by multiplying the LCt50 of 35 mg-min/m3 by 0.7, which equals 25 mg-min/m3. The subcommittee believes this approach is reasonable. However, the subcommittee recommended that the LCt50 for inhalation exposure be lowered; therefore, the ECt50 should be lowered correspondingly. The subcommittee recommends that further research be conducted to establish the ECt50 for severe effects with a greater degree of confidence.
ECt50 for Mild Effects
CDEPAT's recommended ECt50 estimate for mild effects (miosis or rhinorrhea) after exposure to GB vapor is 0.5 mg-min/m3, assuming exposure durations of 2 to 10 min. That estimate is independent of minute volume. The existing ECt50 estimate is 2 mg-min/m3 (CDEPAT 1994). A question that needs to be addressed is the degree of miosis and rhinorrhea that should be used to estimate the ECt50 for mild effects. The data listed in the CDEPAT report do not support CDEPAT's (1994) conclusion that ''Review of other human data indicated that miosis and rhinorrhea probably occur in at least 50% of the population at GB doses of ≤ 1.0 mg-min/m3." Further, some reports suggest that neither miosis nor rhinorrhea occurred at 0.5 mg-min/m3. The threshold symptoms appear to occur at exposures of approximately 2 mg-min/m3. Thus, the ECt50 value could be slightly higher (because of a steep dose-response curve) for the nerve agents.
The subcommittee concludes that CDEPAT's estimate of 0.5 mg-min/m3 is not supported by the available data, which indicate that the value is higher; therefore, the subcommittee recommends that the proposed estimate be raised. The subcommittee also recommends that further research be conducted to establish the ECt50 for mild effects with a greater degree of confidence.
Percutaneous Liquid Exposure
Lethal Effects (LD50)
CDEPAT's proposed LD50 estimate for percutaneous exposure to GB liquid on bare skin is 1,700 mg for a 70-kg man. That estimate is the same as the existing estimate (CDEPAT 1994). The proposed value was calculated using a ratio of ChE50 inhibition levels in rabbits (whole blood) to ChE50 inhibition levels in humans (red blood cells) (CDEPAT 1994). In rabbits, a reduction of 88.8% ChE resulted in 53% deaths. In humans, ChE50 inhibition was based on exposure of bare skin and was estimated to be between 350 and 400 mg for a 70-kg man. One of three subjects who died was exposed to a concentration of 20 mg of GB liquid under one layer of serge plus one layer of flannel and showed a 96% inhibition of ChE. The two other subjects showed 82% and 87% ChE inhibition but had no clinical symptoms. On the basis of the limited and inconsistent data in humans, the subcommittee concludes that the degree of confidence in CDEPAT's estimate is low. The subcommittee recommends that the proposed LD 50 estimate of 1,700 mg for a 70-kg man be considered an interim value until further research is done. The subcommittee also recommends that further research be conducted to establish the LD50 estimate with a greater degree of confidence.
ED50 for Severe Effects
CDEPAT's proposed ED50 for severe effects after percutaneous exposure to GB liquid on bare skin is 1,000 mg for a 70-kg man. There is no existing toxicity estimate for GB via this route (CDEPAT 1994).
Data are not sufficient to estimate the human ED50 for severe effects after percutaneous exposure to liquid GB, and there are few estimates. The proposed estimate of 1,000 mg per a 70-kg man is based on data assuming that the ID50-to-LD50 ratio of 0.6 is valid. The data were extrapolated from relative similarities in ChE inhibition in pigs and humans; the human LD50 was estimated to be 2,500 mg for a 70-kg man, and the ID50 was estimated to be 1,500 mg for a 70-kg man (Silver 1953). Thus, the ratio of 0.6 (1,500 mg for a 70-kg man to 2,500 mg for a 70-kg man = 0.6) was used to estimate the ED50 for severe effects from percutaneous liquid exposure to GB (Reutter et al. 1992). The subcommittee supports CDEPAT's proposed ED50 estimate of 1,000 mg for a 70-kg man (0.6 × 1,700 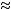 1,000) as an interim value. The subcommittee recommends that further research be conducted to establish the ED50 estimate for severe effects with a greater degree of confidence.
1,000) as an interim value. The subcommittee recommends that further research be conducted to establish the ED50 estimate for severe effects with a greater degree of confidence.
Conclusions and Recommendations
The subcommittee's conclusions concerning CDEPAT's proposed estimates for GB are summarized in Table 3-1.
TABLE 3-1
Evaluation of Human-Toxicity Estimates for GB.
Of the seven human-toxicity estimates for GB proposed by CDEPAT, the subcommittee agrees that two estimates are scientifically valid for protecting soldiers. The subcommittee recommends that two serve as interim estimates, two be lowered, and one raised. The subcommittee recommends further research for most of the adverse health effects to establish the estimates with a greater degree of confidence.
- Review of Acute Human-Toxicity Estimates for GB (Sarin) - Review of Acute Human-...Review of Acute Human-Toxicity Estimates for GB (Sarin) - Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents
Your browsing activity is empty.
Activity recording is turned off.
See more...