NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001.

Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc.
Show detailsSUMMARY
Iodine is an essential component of the thyroid hormones that are involved in the regulation of various enzymes and metabolic processes. Thyroid iodine accumulation and turnover were used to set the Estimated Average Requirement. The Recommended Dietary Allowance (RDA) for adult men and women is 150 μg/day. The median intake of iodine from food in the United States is approximately 240 to 300 μg/day for men and 190 to 210 μg/day for women. The Tolerable Upper Intake Level (UL) for adults is 1,100 μg/day (1.1 mg/day), a value based on serum thyroptropin concentration in response to varying levels of ingested iodine.
BACKGROUND INFORMATION
Function
Iodine is an essential component of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3), comprising 65 and 59 percent of their respective weights. Thyroid hormones, and therefore iodine, are essential for mammalian life. They regulate many key biochemical reactions, especially protein synthesis and enzymatic activity. Major target organs are the developing brain, muscle, heart, pituitary, and kidney.
Observations in several areas have suggested possible additional roles for iodine. Iodine may have beneficial roles in mammary dysplasia and fibrocystic breast disease (Eskin, 1977; Ghent et al., 1993). In vitro studies show that iodine can work with myeloperoxidase from white cells to inactivate bacteria (Klebanoff, 1967). Other brief reports have suggested that inadequate iodine nutrition impairs immune response and may be associated with an increased incidence of gastric cancer (Venturi et al., 1993). While these other possibilities deserve further investigation, the overwhelming importance of nutritional iodine is as a component of the thyroid hormones.
Physiology of Absorption, Metabolism, and Excretion
Iodine is ingested in a variety of chemical forms. Most ingested iodine is reduced in the gut and absorbed almost completely (Nath et al., 1992). Some iodine-containing compounds (e.g., thyroid hormones and amiodarone) are absorbed intact. The metabolic pathway of iodinated radiocontrast media, such as Lipiodol, is not entirely clear. The oral administration of Lipiodol increases the iodine stores of the organism and has been successfully used in the correction of iodine deficiency (Benmiloud et al., 1994). Iodate, widely used in many countries as an additive to salt, is rapidly reduced to iodide and completely absorbed.
Once in the circulation, iodide is removed principally by the thyroid gland and the kidney. The thyroid selectively concentrates iodide in amounts required for adequate thyroid hormone synthesis, and most of the remaining iodine is excreted in urine. Several other tissues can also concentrate iodine, including salivary glands, breast, choroid plexus, and gastric mucosa. Other than the lactating breast, these are minor pathways of uncertain significance.
A sodium/iodide transporter in the thyroidal basal membrane is responsible for iodine concentration. It transfers iodide from the circulation into the thyroid gland at a concentration gradient of about 20 to 50 times that of the plasma to ensure that the thyroid gland obtains adequate amounts of iodine for hormone synthesis. During iodine deficiency, the thyroid gland concentrates a majority of the iodine available from the plasma (Wayne et al., 1964).
Iodide in the thyroid gland participates in a complex series of reactions to produce thyroid hormones. Thyroglobulin, a large glycoprotein of molecular weight 660,000, is synthesized within the thyroid cell and serves as a vehicle for iodination. Iodide and thyroglobulin meet at the apical surface of the thyroid cell. There thyroperoxidase and hydrogen peroxide promote the oxidation of the iodide and its simultaneous attachment to tyrosyl residues within the thyroglobulin molecule to produce the hormone precursors diiodotyrosine and monoiodotyrosine. Thyroperoxidase further catalyzes the intramolecular coupling of two molecules of diiodotyrosine to produce tetraiodothyronine (T4). A similar coupling of one monoiodotyrosine and one diiodotyrosine molecule produces triiodothyronine (T3). Mature iodinated thyroglobulin is stored extra-cellularly in the lumen of thyroid follicles, each consisting of a central space rimmed by the apical membranes of thyrocytes. Typically, thyroglobulin contains from 0.1 to 1.0 percent of its weight as iodine. About one-third of its iodine is in the form of thyroid hormone, the rest as the precursors. An average adult thyroid in an iodine-sufficient geographic region contains about 15 mg iodine (Fisher and Oddie, 1969b).
Thyroglobulin, which contains the thyroid hormones, is stored in the follicular lumen until needed. Then endosomal and lysosomal proteases digest thyroglobulin and release the hormones into the circulation. About two-thirds of thyroglobulin's iodine is in the form of the inactive precursors, monoiodotyrosine and diiodotyrosine. This iodine is not released into the circulation, but instead is removed from the tyrosine moiety by a specific deiodinase and then recycled within the thyroid gland. This process is an important mechanism for iodine conservation, and individuals with impaired or genetically absent deiodinase activity risk iodine deficiency.
Once in the circulation, T4 and T3 rapidly attach to several binding proteins synthesized in the liver, including thyroxine-binding globulin, transthyretin, and albumin. The bound hormone then migrates to target tissues where T4 is deiodinated to T3, the metabolically active form. The responsible deiodinase contains selenium, and selenium deficiency may impair T4 conversion and hormone action. The iodine of T4 returns to the serum iodine pool and follows again the cycle of iodine or is excreted in the urine.
Thyrotropin (TSH) is the major regulator of thyroid function. The pituitary secretes this protein hormone (molecular weight about 28,000) in response to circulating concentrations of thyroid hormone, with TSH secretion increasing when circulating thyroid hormone decreases. TSH affects several sites within the thyrocyte, the principal actions being to increase thyroidal uptake of iodine and to break down thyroglobulin in order to release thyroid hormone into the circulation. An elevated serum TSH concentration indicates primary hypothyroidism, and a decreased TSH concentration shows hyperthyroidism.
The urine contains the fraction of the serum iodine pool that is not concentrated by the thyroid gland. Typically, urine contains more than 90 percent of all ingested iodine (Nath et al., 1992). Most of the remainder is excreted in feces. A small amount may be in sweat.
Clinical Effects of Inadequate Intake
The so-called iodine deficiency disorders (IDD) include mental retardation, hypothyroidism, goiter, cretinism, and varying degrees of other growth and developmental abnormalities. These result from inadequate thyroid hormone production from lack of sufficient iodine. Most countries in the world currently have some degree of iodine deficiency, including some industrialized countries in Western Europe (Stanbury et al., 1998). Iodine deficiency was a significant problem in the United States and Canada, particularly in the interior, the Great Lakes region, and the Pacific Northwest, during the early part of the 20th century (Trowbridge et al., 1975). The Third National Nutrition and Health Examination Survey study of samples collected from 1988 to 1994 showed a median urinary iodine excretion of 145 μg/L, well above the lower level considered to reflect adequate intake (100 μg/L) (WHO Nutrition Unit, 1994), but this is a decrease from the value of 321 μg/L found in a similar survey in the 1970s (Hollowell et al., 1998). Estimated iodine intakes for Canadians are in excess of 1 mg/day (Fischer and Giroux, 1987). Both countries iodize salt with potassium iodide at 100 ppm (76 mg iodine/kg salt). Iodized salt is mandatory in Canada and used optionally by about 50 percent of the U.S. population.
The most damaging effect of iodine deficiency is on the developing brain. Thyroid hormone is particularly important for myelination of the central nervous system, which is most active in the perinatal period and during fetal and early postnatal development. Numerous population studies have correlated an iodine-deficient diet with increased incidence of mental retardation. A meta-analysis of 18 studies concluded that iodine deficiency alone lowered mean IQ scores by 13.5 points (Bleichrodt and Born, 1994).
The effects of iodine deficiency on brain development are similar to those of hypothyroidism from any other cause. The United States, Canada, and most developed countries have routine screening of all neonates by blood spot for TSH or T4 to detect among iodine-sufficient children the approximately one in 4,000 who will be hypothyroid, usually from thyroid aplasia. Iodine treatment can reverse cretinism especially when the treatment is begun early (Klein et al., 1972).
Cretinism is an extreme form of neurological damage from fetal hypothyroidism. It occurs in severe iodine deficiency and is characterized by gross mental retardation along with varying degrees of short stature, deaf mutism, and spasticity. As many as one in ten of some populations with very severe iodine deficiency may be cretins. Correction of iodine deficiency in Switzerland completely eliminated the appearance of new cases of cretinism, and a similar experience has occurred in other countries (Stanbury et al., 1998).
Thyroid enlargement (goiter) is usually the earliest clinical feature of iodine deficiency. It reflects an attempt to adapt the thyroid to the increased need, brought on by iodine deficiency, to produce thyroid hormones. Initially, goiters are diffuse but become nodular over time. In later stages they may be associated with hyperthyroidism from autonomous nodules or with thyroid follicular cancer. Goiter can be assessed approximately by palpation and more precisely by field ultrasonography. The International Council for the Control of Iodine Deficiency Disorders (WHO/UNICEF/ICCIDD, 1993) and the World Health Organization (WHO Nutrition Unit, 1994) have recommended surveying schoolchildren for thyroid size as one of the most practical indicators of iodine deficiency, and many reports on iodine nutrition are based primarily on such goiter surveys.
Other consequences of iodine deficiency are impaired reproductive outcome, increased childhood mortality, decreased educability, and economic stagnation. Major international efforts have produced dramatic improvements in the correction of iodine deficiency in the 1990s, mainly through use of iodized salt in iodine-deficient countries.
SELECTION OF INDICATORS FOR ESTIMATING THE REQUIREMENT FOR IODINE
Iodine Accumulation and Turnover
The normal thyroid gland takes up the amount of circulating iodine necessary to make the proper amount of thyroid hormone for the body's needs. The affinity of the thyroid gland for iodine is estimated by the fraction of an orally administered dose of radioactive iodine (123I, 131I) that is concentrated in the thyroid gland (Wayne et al., 1964). The thyroid gland concentrates more radioactive iodine in iodine deficiency and less in iodine excess. Thus, values for euthyroid individuals in Western Europe, where some iodine deficiency exists, are higher than in the iodine-sufficient United States and Canada, where typical values are in the range of 5 to 20 percent at 24 hours. Other factors can influence the radioactive iodine uptake, including thyroidal overproduction of hormone (hyperthyroidism), hypothyroidism, subacute thyroiditis, and many chemical and medicinal products. Assuming iodine equilibrium, the mean daily thyroid iodine accumulation and release are similar. Thus, the average daily uptake and release (turnover) of iodine in the body can be used to estimate the average requirement of iodine, provided that the subjects tested have adequate iodine status and are euthyroid.
Such turnover studies have been conducted in euthyroid adults in the United States (Fisher and Oddie, 1969a, 1969b; Oddie et al., 1964). Turnover studies are based on the intravenous administration of 131I and the calculation of thyroid iodine accumulation from measurements of thyroidal and renal radioiodine clearances, urinary iodine excretion, and fractional thyroidal release rate.
Urinary Iodine
Over 90 percent of dietary iodine eventually appears in the urine (Nath et al., 1992; Vought and London, 1967). Data on urinary iodine excretion are variously expressed as a concentration (μg/L), in relationship to creatinine excretion (μg iodine/g creatinine), or as 24-hour urine collections (μg/day). Most studies have used the concentration in casual samples because of the obvious ease of collection. In populations with adequate general nutrition, urinary iodine concentration correlates well with the urine iodine/creatinine ratio. Urinary iodine excretion is recommended by the World Health Organization, the International Council for the Control of Iodine Deficiency Disorders, and the United Nations Children's Fund (WHO Nutrition Unit, 1994) for assessing iodine nutrition worldwide.
In the Third National Health and Nutrition Examination Survey (NHANES III), the urinary iodine concentration (μg/L) was 1.16 times the urinary iodine excretion expressed as μg/g creatinine (Hollowell et al., 1998). In NHANES I, this ratio was 1.09. Some population groups, particularly those with compromised general nutrition, have low creatinine excretion; therefore the urinary iodine to creatinine ratio is misleading (Bourdoux, 1998). The concentration of iodine in 24-hour urine samples correlates well with that in casual samples (Bourdoux, 1998). Information from NHANES III on urinary iodine excretion is provided in Appendix Table G-6. The median urinary iodine excretion was 1.38 to 1.55 μg/L for men and 1.1 to 1.29 μg/L for women. Data are not available on 24-hour urinary excretion of iodine.
Daily iodine intake can be extrapolated from urinary concentration as follows. The median 24-hour urine volume for ages 7 through 15 years is approximately 0.9 mL/hr/kg (or 0.0009 L/hr/kg) (Mattsson and Lindstrom, 1995). The 24-hour urine volume for adults is approximately 1.5 L (Larsson and Victor, 1988), a value in general agreement with an extrapolation of the calculation for children and adolescents. Urine volume among individuals and over time can vary considerably, but these numbers for daily volume appear reasonable for population estimates.
From the above information and assuming an average bioavailability of 92 percent, the daily iodine intake is calculated from urinary iodine concentration by the following formula:
Urinary iodine (μg/L) ÷ 0.92 × (0.0009 L/h/kg × 24 h/d) × wt (kg) = daily iodine intake;
or simplified,
Urinary iodine (μg/L) × 0.0235 × wt (kg) = daily iodine intake.
As an example, urinary iodine excretion of 100 μg/L in a 57-kg girl would indicate a daily iodine intake of 134 μg.
Simple methods for measuring urinary iodine exist (Dunn et al., 1993). Casual samples are easy to collect and have been the main-stay for biological monitoring in global studies of iodine nutrition. The urinary iodine concentration reflects very recent iodine nutrition (days) in contrast to indicators such as thyroid size and serum thyroid stimulating hormone (TSH) and thyroglobulin concentrations.
Thyroid Size
The size of the thyroid gland increases in response to iodine deficiency, mediated at least in part by increased serum TSH concentration. This earliest clinical response to impaired iodine nutrition reflects an adaptation to the threat of hypothyroidism. Excess iodine can also produce goiter because large amounts inhibit intrathyroidal hormone production, again leading to increased TSH stimulation and thyroid growth. Traditionally, goiter was assessed by neck palpation with each lobe of the normal thyroid being regarded as no larger than the terminal phalanx of the subject's thumb. Thyroid size is recommended by WHO/UNICEF/ICCIDD (WHO Nutrition Unit, 1994) for assessing iodine nutrition worldwide. The WHO/ UNICEF/ICCIDD classification (WHO Nutrition Unit, 1994) describes grade 1 goiter as palpable but not visible with the neck extended and grade 2 as visible with the neck in the normal position.
Ultrasonography defines thyroid size much more precisely and reliably. The technology—safe, practical, and easily performed in the field—is replacing palpation in most studies. Reference values related to body surface area and to age exist for iodine-sufficient children in the United States (Xu et al., 1999), in Europe (Delange et al., 1993), and in some other countries. Most data come from surveys in school-age children, who are easily available and whose thyroids reflect recent iodine nutrition. Individuals may continue to have thyroid enlargement permanently, even after iodine deficiency has been corrected (Delange and Burgi, 1989; Jooste et al., 2000).
Iodine Balance
Several attempts at iodine balance studies were published in the 1960s (Dworkin et al., 1966; Harrison, 1968; Harrison et al., 1965; Malamos et al., 1967; Vought and London, 1967). Because most iodine in the body is concentrated in the thyroid gland, the ability to determine balance within a short time is more realistic than for most other trace elements. But, as for many trace elements, there are serious limitations for deriving a daily iodine requirement based on balance studies. One limitation is that the baseline iodine intake at the study site and the long-range iodine intake of the subjects before the studies were likely different from current conditions in the United States. This applies particularly to the study of Harrison and coworkers (1965). Second, iodine balance is complicated by the need to consider the thyroidal compartment in addition to iodine intake and excretion (Dworkin et al., 1966). Thus, even in prolonged studies of several months, equilibrium is not clearly established, and in fact negative iodine balance has been reported (Dworkin et al., 1966). Third, techniques for assessment were crude by today's standards and key indicators, such as serum TSH, were not available. A fourth limitation is that while studies such as these try to control intake, iodine appears in many unidentified or unrecognized substances that are ingested; therefore control of iodine intake in these studies would have been limited. Despite the limitations of balance studies, data from them were used for estimating the average requirement for iodine in children.
Serum Thyroid Stimulating Hormone Concentration
Because serum TSH concentration responds to circulating levels of thyroid hormone, which in turn reflect adequate production of thyroid hormone, it is an excellent indicator of altered thyroid function in individuals. Sensitive assays have been widely available for about two decades, and serum TSH concentration is now the preferred test for assessing thyroid function in individuals. It is also used on blood spots by filter paper methodology in most countries for the routine screening of neonates to detect congenital hypothyroidism (WHO Nutrition Unit, 1994). The normal serum TSH concentration range in most assays is approximately 0.5 to 6.0 mU/ L, although each individual assay system needs to be standardized for euthyroid subjects. Studies of groups with differing iodine intakes, as reflected in urinary iodine concentrations, show different mean serum TSH concentrations, although they may remain within the normal range. The sensitivity of TSH can be enhanced by previous stimulation with TSH-releasing hormone (TRH) (Jackson, 1982). The latter is a hypothalamic tripeptide that stimulates release of TSH and prolactin. It is used clinically for individuals with borderline or confusing static TSH measurements; an exaggerated response to TRH suggests the threat of inadequate thyroid hormone availability and hypothyroidism. Several studies have shown that the mean serum TSH concentration and its response to TRH are increased in iodine deficiency, although absolute values may remain within the normal range (Benmiloud et al., 1994; Buchinger et al., 1997; Emrich et al., 1982; Moulopoulos et al., 1988).
Serum Thyroglobulin Concentration
Although principally an intrathyroidal and follicular resident, some thyroglobulin (Tg) is normally secreted into the circulation and is detectable by standardized commercially available immunoassays. The largest clinical use of the serum Tg concentration is in detecting metastases of differentiated thyroid cancer, but it is typically elevated in thyroidal hyperplasia from any cause, including the endemic goiter of iodine deficiency. Many studies have shown a correlation between serum Tg concentration and degree of iodine deficiency as shown by urinary iodine excretion or other parameters (Benmiloud et al., 1994; Gutekunst et al., 1986). It is applicable to blood spot filter paper technology (Missler et al., 1994). Individuals with adequate iodine intake have a median serum Tg concentration of 10 ng/mL (WHO Nutrition Unit, 1994; WHO/UNICEF/ICCIDD, 1993). There are insufficient dose-response data on dietary iodine intake and serum Tg concentrations to estimate iodine requirements.
Thyroxine and Triiodothyronine Concentration
Assays for both thyroxine (T4) and triiodothyronine (T3) concentrations are standard clinical tools for measuring thyroid function, although they are not as sensitive as TSH. In iodine deficiency, serum T4 concentration is decreased and serum T3 concentration is normal or increased, relative to iodine-sufficient controls. This increased T3 concentration is an adaptive response of the thyroid to iodine deficiency. Fasting and malnutrition are associated with low T3 concentrations (Croxson et al., 1977; Gardner et al., 1979). However, most changes take place within the normal range, and the overlap with the iodine-sufficient normal population is large enough to make this a relatively insensitive and unreliable means for assessing iodine nutrition.
FACTORS AFFECTING THE IODINE REQUIREMENT
Bioavailability
Under normal conditions, the absorption of dietary iodine is greater than 90 percent (Albert and Keating, 1949; Nath et al., 1992; Vought and London, 1967). The fate of organic compounds of iodine in the intestine is different from that of iodine. When thyroxine is orally administered, the bioavailability is approximately 75 percent (Hays, 1991).
Soya flour has been shown to inhibit iodine absorption (Pinchera et al., 1965), and goiter and hypothyroidism were reported in several infants consuming infant formula containing soya flour (Shepard et al., 1960). If iodine was added to this formula, goiter did not appear.
Goitrogens
Some foods contain goitrogens, that is, substances that interfere with thyroid hormone production or utilization (Gaitan, 1989). Examples include cassava, which may contain linamarin and is metabolized to thiocyanate which in turn can block thyroidal uptake of iodine; millet, some species of which contain goitrogenic substances; water, particularly from shallow or polluted streams and wells, which may contain humic substances that block thyroidal iodination; and crucifera vegetables (e.g., cabbage). Most of these substances are not of major clinical importance unless there is coexisting iodine deficiency. Deficiencies of vitamin A, selenium, or iron can each exacerbate the effects of iodine deficiency.
Other Factors
Many ingested substances contain large amounts of iodine that can interfere with proper thyroid function. These include radiocontrast media, food coloring, certain medicines (e.g., amiodarone), water purification tablets, and skin and dental disinfectants. Erythrosine is a coloring agent widely used in foods, cosmetics, and pharmaceutical products, and contains high amounts of iodine. Data suggest that the increased thyroid stimulating hormone levels found following erythrosine ingestion is related to antithyroid effects of increased serum iodide concentrations, rather than a direct effect of erythrosine on thyroid hormones (Gardner et al., 1987). Similar to erythrosine, amiodarone, a highly effective antiarrhythmic drug that contains high levels of iodine, may alter thyroid gland function (Loh, 2000). Radiographic contrast media, following intravascular administration, results in the formation of iodinated serum proteins, which alter thyroid metabolism (Nilsson et al., 1987).
FINDINGS BY LIFE STAGE AND GENDER GROUP
Infants Ages 0 through 12 Months
Method Used to Set the Adequate Intake
No functional criteria of iodine status have been demonstrated that reflect response to dietary intake in infants. Thus, recommended intakes of iodine are based on an Adequate Intake (AI) that reflects the observed mean iodine intake of infants exclusively fed human milk.
Ages 0 through 6 Months. An AI is used as the recommended intake level for infants as determined by the method described in Chapter 2. The AI reflects the observed mean iodine intake of infants fed human milk. Iodine concentrations in human milk are influenced by maternal iodine intake (Gushurst et al., 1984). The median iodine concentration in human milk of American women who consumed noniodized salt was 113 μg/L, whereas the concentration in breast milk of women who consumed low or high amounts of iodized salt was 143 or 270 μg/L, respectively (Gushurst et al., 1984), and within the range observed by Etling and coworkers (1986) and Johnson and coworkers (1990) (Table 8-1). The median concentration of iodine in human milk for all women was 146 μg/L for 14 days to 3.5 years postpartum. Based on an average milk excretion of 0.78 L/day (Chapter 2) and an average concentration of 146 μg/L, the mean amount of iodine secreted in human milk is 114 μg/day.
TABLE 8-1
Iodine Concentration in Human Milk.
Iodine balance studies by Delange and coworkers (1984) showed that for full-term infants, aged 1 month and fed 20 μg/kg/day of iodine, total excretion was 12.7 μg/kg/day and iodine retention was 7.3 μg/kg/day. Thus, if the mean body weight at 6 months is 7 kg, then the infant in positive iodine balance excretes 90 μg/day.
Based on the median intake of iodine consumed from human milk and the average urinary iodine excretion of the infant, the AI for infants ages 0 through 6 months has been set at 110 μg/day.
Ages 7 though 12 Months. The AI for infants ages 7 through 12 months is 130 μg/day as determined by the method described in Chapter 2 to extrapolate from the younger infants. The AI for infants is greater than the Recommended Dietary Allowances (RDAs) for children and adolescents because the latter are based on extrapolation of adult data or on balance data for a specific age group (see “Children and Adolescents Ages 1 through 18 Years”).
Iodine AI Summary, Ages 0 through 12 months
| AI for Infants | |
| 0–6 months | 110 μg/day of iodine |
| 7–12 months | 130 μg/day of iodine |
Special Considerations
The iodine content in cow milk is dependent on the amount of iodine consumed by the animal (Swanson et al., 1990). As a result, the amount of iodine in cow milk increased by 300 to 500 percent from 1965 to 1980, partly because of the addition of organic iodine to animal feed (Hemken, 1980). There have been no studies in which the bioavailability of iodine in infant formulas and human milk have been compared.
Children and Adolescents Ages 1 through 18 Years
Evidence Considered in Estimating the Average Requirement
Ages 1 through 3 Years. A 4-day balance study was conducted by Ingenbleek and Malvaux (1974) on children aged 1.5 to 2.5 years who were previously malnourished and then nutritionally rehabilitated. The median iodine intake of the seven rehabilitated children was 63.5 μg/day, and the average iodine balance was +19 μg/day. The coefficient of variation (CV) was approximately 20 percent. No other studies assessing iodine requirements for this age group have been conducted. If the Estimated Average Requirement (EAR) for adults is extrapolated down on the basis of body weight (see Chapter 2), the EAR would be 36 μg/day. However, because an average intake of 63.5 μg/day resulted in a positive iodine balance, an EAR of 65 μg/day is set.
Ages 4 through 8 Years. Children 8 years of age who consumed 20 to 40 μg/day of iodine were in negative iodine balance (–23 to –26 μg/day) (Malvaux et al., 1969), indicating that the average minimum requirement is approximately 65 μg/day (40 + 26). If the EAR for adults is extrapolated down on the basis of body weight (see Chapter 2), the EAR would be 47 μg/day. No other studies for assessing iodine requirements for this age group have been conducted; therefore an EAR of 65 μg/day is set, using the higher estimate.
Ages 9 through 13 Years. The prevalence of goiter was estimated in European boys and girls aged 6 to 15 years (Delange et al., 1997). Goiter prevalence in a population increases inversely with iodine intake. Because iodine deficiency is rare in the United States, data from Europe are used to relate goiter, as determined by ultrasound, to urinary iodine excretion. As urinary iodine excretion increases, the goiter prevalence decreases and eventually changes only slightly (Figure 8-1). Although data from this figure are not available for estimating a 50 percent prevalence of goiter, the level of urinary iodine concentration at which there is only a 2 percent prevalence of goiter is approximately 100 μg/L. This approach can be used for estimating the RDA because it estimates the requirement for approximately 98 percent of the population. As described earlier, the daily iodine intake can be estimated from the urinary iodine concentration as follows:
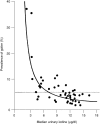
FIGURE 8-1
Inverse relationship between median urinary iodine concentrations and the prevalence of goiter in schoolchildren. The dotted line represents the upper limit of the prevalence of goiter (WHO Nutrition Unit, 1994). Adapted from Delange et al. (1997).
- 1.
Median urine volume is 1.2 mL/hour/kg for a 10-year-old child (the median excretion rate for all children aged 7 to 15 years was 0.9 mL/hour/kg) (Mattsson and Lindstrom, 1995) and the median weight is 40 kg (Chapter 1); therefore the urine volume is about 1.15 L/day (1.2 × 40 × 24 hr).
- 2.
Approximately 92 percent of dietary iodine is excreted in the urine (Nath et al., 1992; Vought and London, 1967).
- 3.
Therefore, for a 10-year-old child weighing 40 kg, the urinary iodine concentration of 100 μg/L approximates a daily iodine intake of 125 μg (1.15 ÷ 0.92 × 100). This value suggests an RDA of approximately 125 μg/day.
Malvaux and coworkers (1969) conducted a balance study on 16 boys and girls (aged 9 to 13 years) in Belgium. The average iodine intake was 31 μg/day, and the average balance was –24 μg/day. This finding would suggest a minimum average requirement of approximately 55 μg/day (31 + 24).
The iodine requirement has not been determined based on energy expenditure; however, the thyroid hormones, which contain iodine, are involved with metabolic rate. Therefore, the EAR is extrapolated from adults by using metabolic body weight (kg0.75) and the method described in Chapter 2 to set an EAR at 73 μg/day.
Ages 14 through 18 Years. Malvaux and colleagues (1969) reported that the average iodine balance of 10 children (aged 14 to 16 years) was –24 μg/day when they consumed an average 34 μg/day of iodine, which would give 58 μg/day as an average requirement. No other data are available for estimating an average requirement for this age group. However, extrapolating down from adult data as described in Chapter 2 and using metabolic weight gives an EAR of 95 μg/ day, which is used to set the RDA as it is a higher estimate.
Iodine EAR and RDA Summary, Ages 1 through 18 Years
| EAR for Children | |
| 1–3 years | 65 μg/day of iodine |
| 4–8 years | 65 μg/day of iodine |
| EAR for Boys | |
| 9–13 years | 73 μg/day of iodine |
| 14–18 years | 95 μg/day of iodine |
| EAR for Girls | |
| 9–13 years | 73 μg/day of iodine |
| 14–18 years | 95 μg/day of iodine |
The RDA for iodine is set by using a CV of 20 percent (see “Adults Ages 19 Years and Older”). The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for iodine the RDA is 140 percent of the EAR). The calculated values for RDAs have been rounded, and are in the range of 125 μg/day for a 10-year-old child as presented on the previous page.
| RDA for Children | |
| 1–3 years | 90 μg/day of iodine |
| 4–8 years | 90 μg/day of iodine |
| RDA for Boys | |
| 9–13 years | 120 μg/day of iodine |
| 14–18 years | 150 μg/day of iodine |
| RDA for Girls | |
| 9–13 years | 120 μg/day of iodine |
| 14–18 years | 150 μg/day of iodine |
Adults Ages 19 Years and Older
Evidence Considered in Estimating the Average Requirement
Thyroid Iodine Accumulation and Turnover. Thyroidal radioiodine accumulation is used to estimate the average requirement. Turnover studies have been conducted in euthyroid adults (Fisher and Oddie, 1969a, 1969b). In one of these studies, the average accumulation of radioiodine by the thyroid gland for 18 men and women aged 21 to 48 years was 96.5 μg/day (Fisher and Oddie, 1969a). The second study involved 274 euthyroid subjects from Arkansas. The calculated uptake and turnover was 91.2 μg/day (Fisher and Oddie, 1969b). The accumulation of radioidine by the thyroid gland correlated well with urinary radioidine excretion. DeGroot (1966) measured iodine turnover in four normal subjects by three methods: absolute iodine uptake (21 to 97 μg/day) determined by using the method of Riggs (1952), thyroid hormone secretion (69 to 171 μg/ day) determined by using the method of Berson and Yalow (1954), and thyroid hormone secretion (49 to 147 μg/day) determined by using the method of Ermans and coworkers (1963). There is no evidence to suggest that the average iodine requirement is altered with aging, or to have differences based on gender in adults.
Supporting Data. Other considerations support an EAR in the general range of 95 μg/day for adults (Delange, 1993; Dunn et al., 1998). A study by Vought and London (1967) demonstrated that the obligatory amount of iodine excreted was 57 μg/day. Despite the methodologic limitations of balance studies, when 100 μg/day of iodine was provided to 13 subjects, an average slight positive balance (13 μg) was observed (Harrison, 1968). In a study of five pregnant and four nonpregnant women, balance was calculated at about 160 μg/day (Dworkin et al., 1966). Given this higher estimate in women, adjusting for smaller body weight in women was not justified.
Iodine EAR and RDA Summary, Ages 19 Years and Older
| EAR for Men | |
| 19–30 years | 95 μg/day of iodine |
| 31–50 years | 95 μg/day of iodine |
| 50–70 years | 95 μg/day of iodine |
| > 70 years | 95 μg/day of iodine |
| EAR for Women | |
| 19–30 years | 95 μg/day of iodine |
| 31–50 years | 95 μg/day of iodine |
| 50–70 years | 95 μg/day of iodine |
| > 70 years | 95 μg/day of iodine |
The CV was calculated to be 40 percent by using the data of Fisher and Oddie (1969a). Part of this variation is due to the complexity of the experimental design and calculations used to estimate turnover. Assuming that half of the variation is due to experimental design, a CV of 20 percent, rather than 10 percent based on energy (see Chapter 1), is used to set the RDA. The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for iodine the RDA is 140 percent of the EAR). The calculated values for RDAs were rounded to the nearest 50 μg.
| RDA for Men | |
| 19–30 years | 150 μg/day of iodine |
| 31–50 years | 150 μg/day of iodine |
| 50–70 years | 150 μg/day of iodine |
| > 70 years | 150 μg/day of iodine |
| RDA for Women | |
| 19–30 years | 150 μg/day of iodine |
| 31–50 years | 150 μg/day of iodine |
| 50–70 years | 150 μg/day of iodine |
| > 70 years | 150 μg/day of iodine |
Pregnancy
Evidence Considered in Estimating the Average Requirement
Thyroid Iodine Content of the Newborn. The daily accumulation of iodine by the newborn can be used to estimate the daily fetal iodine uptake. It is estimated that the average iodine content of the newborn thyroid gland is 50 to 100 μg with close to 100 percent being turned over daily (Delange, 1989; Delange and Ermans, 1991). An estimated daily thyroid iodine uptake of approximately 75 μg/day by the fetus and an EAR of 95 μg/day for nonpregnant women would yield an EAR of 170 μg/day during pregnancy.
Iodine Balance. Iodine balance studies by Delange and coworkers (1984) showed that the average iodine retention of full-term infants was 6.7 μg/kg/day. With an average fetal weight of 3 kg, the mean retention of a fully developed fetus would be approximately 22 μg/ day. A study demonstrated that pregnant women were at balance when consuming approximately 160 μg/day (Dworkin et al., 1966). Based on balance studies, the EAR ranges from 117 (22 + 95) (Delange et al., 1984) to 160 μg/day (Dworkin et al., 1966).
Iodine Supplementation During Pregnancy. In iodine deficiency, the size of the thyroid gland increases during pregnancy. Studies have measured thyroid volume by ultrasound and correlated it with urinary iodine excretion and the effects of iodine supplementation during pregnancy (Berghout and Wiersinga, 1998). Pregnant women in an iodine-deficient area of Italy were given iodized salt estimated to add 120 to 180 μg/day of iodine (Romano et al., 1991). Their urinary iodine increased from 37 to 154 μg/day during the second trimester and was 100 μg/day during the third trimester. Untreated control subjects showed little change. The initial thyroid volume of 9.8 mL did not change in those treated with iodine, but increased by 16 percent in the controls. Thus, the total daily iodine intake of about 200 μg prevented goiter. In another study from Denmark (Pedersen et al., 1993), 54 pregnant women were given 200 μg/day of iodine as potassium iodide drops beginning the second trimester. Urinary iodine increased from 55 to 105 μg/L, their thyroid volume (initially 9.6 mL) increased by 15.5 percent, and serum thyroid stimulating hormone (TSH) and serum thyroglobulin (Tg) concentrations did not change. Untreated control subjects showed increases of 31 percent in thyroid volume, 75 percent in serum Tg concentration, and 21 percent in serum TSH concentration. Thus, approximately 250 to 280 μg/day of iodine prevented goiter during pregnancy. In a third study (Glinoer, 1998), pregnant women with an initial urinary iodine of 36 μg/L were treated with an additional 100 μg/day. Their median urinary iodine concentration increased to 100 μg/L at 33 weeks, and their thyroid volume increased by 15 percent, compared with 30 percent in control subjects. Thus, a supplement of 100 μg iodine, bringing the total daily iodine intake to about 150 μg/day, was insufficient to prevent increased thyroid size.
On the basis of the above data, the EAR is set at 160 μg/day.
Iodine EAR and RDA Summary, Pregnancy
| EAR for Pregnancy | |
| 14–18 years | 160 μg/day of iodine |
| 19–30 years | 160 μg/day of iodine |
| 31–50 years | 160 μg/day of iodine |
The RDA for iodine is set by using a CV of 20 percent (see “Adults Ages 19 Years and Older”). The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of individuals in the group (therefore, for iodine the RDA is 140 percent of the EAR). The calculated values for RDAs were rounded to the nearest 10 μg.
| RDA for Pregnancy | |
| 14–18 years | 220 μg/day of iodine |
| 19–30 years | 220 μg/day of iodine |
| 31–50 years | 220 μg/day of iodine |
Lactation
Method Used to Estimate the Average Requirement
The EAR during lactation is based on the average requirement of adolescent girls and nonpregnant women plus the average daily loss of iodine in human milk. The EAR for adolescent girls and adult women is 95 μg/day, and the average daily loss of iodine in human milk is approximately 114 μg/day (Gushurst et al., 1984). Therefore, the EAR for lactating women is 209 μg/day.
Iodine EAR and RDA Summary, Lactation
| EAR for Lactation | |
| 14–18 years | 209 μg/day of iodine |
| 19–30 years | 209 μg/day of iodine |
| 31–50 years | 209 μg/day of iodine |
The RDA for iodine is set by using a CV of 20 percent (see “Adults Ages 19 Years and Older”). The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for iodine the RDA is 140 percent of the EAR). The calculated RDA value is rounded to the nearest 10 μg.
| RDA for Lactation | |
| 14–18 years | 290 μg/day of iodine |
| 19–30 years | 290 μg/day of iodine |
| 31–50 years | 290 μg/day of iodine |
INTAKE OF IODINE
Food Sources
The iodine content in most food sources is low and can be affected by content of soil, irrigation, and fertilizers. Most foods provide 3 to 75 μg per serving. Foods of marine origin have higher concentrations of iodine because marine animals concentrate iodine from seawater. Processed foods may also contain higher levels of iodine due to the addition of iodized salt or additives such as calcium iodate, potassium iodate, potassium iodide, and cuprous iodide.
Dietary Intake
Based on analysis of 234 core foods conducted by the Food and Drug Administration (1982–1991 (Pennington et al., 1995) and analysis of 60 additional core foods and intake data by the U.S. Department of Agriculture Continuing Survey of Food Intakes by Individuals (1994–1996), the median intake of iodine from food in the United States is approximately 240 to 300 μg/day for men and 190 to 210 μg/day for women (Appendix Table E-4). For all life stage and gender groups, less than 25 percent of individuals had intakes below the Estimated Average Requirement.
Intake from Supplements
Information from the Third National Health and Nutrition Examination Survey (NHANES III) on the use of supplements containing iodine is given in Appendix Table C-17. The median intake of iodine from supplements was approximately 140 μg/day for adult men and women. In 1986, approximately 12 percent of men and 15 percent of nonpregnant women took a supplement that contained iodine (Moss et al., 1989; see Table 2-2).
TOLERABLE UPPER INTAKE LEVELS
The Tolerable Upper Intake Level (UL) is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects in almost all individuals. Although members of the general population should be advised not to routinely exceed the UL, intake above the UL may be appropriate for investigation within well-controlled clinical trials. Clinical trials of doses above the UL should not be discouraged, as long as subjects participating in these trials have signed informed consent documents regarding possible toxicity and as long as these trials employ appropriate safety monitoring of trial subjects. In addition, the UL is not meant to apply to individuals who are receiving iodine under medical supervision.
Hazard Identification
Most people are very tolerant of excess iodine intake from food (Pennington, 1990). Certain subpopulations, such as those with autoimmune thyroid disease and iodine deficiency, respond adversely to intakes considered safe for the general population. For the general population, high iodine intakes from food, water, and supplements have been associated with thyroiditis, goiter, hypothyroidism, hyperthyroidism, sensitivity reactions, thyroid papillary cancer, and acute responses in some individuals. There may be other unrecognized sources of iodine that increase the risk of adverse effects. Because of significant species differences in basal metabolic rates and iodine metabolism (Hetzel and Maberly, 1986), animal data were of limited use in setting a UL.
Adverse Effects
Acute Responses. Among human cases of acute iodine poisoning, there are reports of burning of the mouth, throat, and stomach, abdominal pain, fever, nausea, vomiting, diarrhea, weak pulse, cardiac irritability, coma, cyanosis, and other symptoms (Finkelstein and Jacobi, 1937; Tresch et al., 1974; Wexler et al., 1998). These are quite rare and are usually associated with doses of many grams.
Hypothyroidism and Elevated Thyroid Stimulating Hormone (TSH). Clinical hypothyroidism occurs when thyroid hormone production is inadequate. Subclinical hypothyroidism is defined as an elevation in TSH concentration while a normal serum thyroid hormone concentration is maintained. An elevation or increase over baseline (prior to iodine intake) in serum TSH concentration is considered an initial marker for hypothyroidism, although clinical hypothyroidism has not occurred. Laurberg and coworkers (1998) showed that in populations with high iodine intake, impaired thyroid function (i.e., elevated TSH concentration) is increased. Intervention studies looking for the earliest effects in iodine-sufficient populations show an increase in serum TSH concentration, or in TSH response to TSH-releasing hormone (TRH), without the TSH increasing to the abnormal range (Gardner et al., 1988; Paul et al., 1988). A randomized, controlled clinical trial in Wales by Chow and coworkers (1991) showed significantly elevated TSH concentrations associated with total iodide intakes of 750 μg/day or more. The study involved supplemental intake of 500 μg/day of iodide or placebo by 225 adult women (aged 25 to 54 years) for 28 days in addition to the estimated dietary intake of 250 μg/day. The baseline urinary iodide concentrations, however, suggest that many subjects probably had borderline iodine deficiency. Thus their conclusions may not apply to an iodine-sufficient population, such as that of the United States.
Goiter. Excess iodine may produce thyroid enlargement (goiter), mostly from increased TSH stimulation. Evidence of iodine-induced goiter comes from studies involving pharmacological doses (Wolff, 1969) and population groups with high, chronic iodine intakes (50,000 to 80,000 μg/day) in Japan and China (Suzuki and Mashimo, 1973; Suzuki et al., 1965). Wolff (1969) reported that prolonged intakes greater than 18,000 μg/day increased the risk of goiter.
Thyroid Papillary Cancer. Chronic stimulation of the thyroid gland by TSH is known to produce thyroid neoplasms (Money and Rawson, 1950). High iodine intake has also been associated with increased risk of thyroid papillary cancer in humans (Franceschi, 1998; Lind et al., 1998). Such evidence is lacking in experimental animals (Delange and Lecomte, 2000).
Thyroid Effects in Newborn Infants. Iodine goiter and hypothyroidism have been observed in newborns after prenatal exposure to excess iodine (Ayromlooi, 1972; Carswell et al., 1970; LaFranchi et al., 1977; Senior and Chernoff, 1971; Wolff, 1969). Rectal irrigation with povidone-iodine, a topical antiseptic, has been shown to be toxic to infants (Kurt et al., 1996; Means et al., 1990).
Other Adverse Effects. Other adverse effects of excess iodine intake include iodermia, a rare dermatological reaction to iodine intake. These dermatoses may consist of acneiform eruptions, pruritic red rashes, and urticaria (Parsad and Saini, 1998). In its most severe form, iodermia has resulted in death (Sulzberger and Witten, 1952). Iodine-induced hyperthyroidism occurs most frequently with iodine administration to patients with underlying thyroid disease and with iodine supplementation in areas of deficiency (Delange et al., 1999; Stanbury et al., 1998). Seasonal variations in thyrotoxicosis have been related to variations in daily iodine intake from 126 to 195 μg to 236 to 306 μg (Nelson and Phillips, 1985).
Summary
Challenged thyroid function shown by TSH concentrations elevated over baseline is the first effect observed in iodine excess. While an elevated TSH concentration may not be a clinically significant adverse effect, it is an indicator for increased risk of developing clinical hypothyroidism. Therefore, an elevated TSH concentration above baseline was selected as the critical adverse effect on which to base a UL.
Dose-Response Assessment
Adults
Data Selection. The appropriate data for derivation of a UL for adults are those relating intake to thyroid dysfunction shown by elevated TSH concentrations. Studies conducted in countries with a history of inadequate iodine intake were not included in this review because of the altered response of TSH to iodine intake.
Identification of No-Observed-Adverse-Effect Level (NOAEL) and Lowest-Observed-Adverse-Effect Level (LOAEL). Gardner and coworkers (1988) evaluated TSH concentrations in 30 adult men aged 22 to 40 years who received 500, 1,500, or 4,500 μg/day of supplemental iodide for 2 weeks. Baseline urinary iodine excretion was 287 μg/day; therefore baseline iodine intake from food is estimated to be approximately 300 μg/day. The mean basal serum TSH concentration increased significantly in those receiving the two higher doses, although it remained within the normal range. This study shows a LOAEL of 1,500 plus 300 μg/day, for a total of 1,800 μg/day.
In a similar study (Paul et al., 1988), nine men aged 26 to 56 years and 23 women aged 23 to 44 years received iodine supplements of 250, 500, or 1,500 μg/day for 14 days. Baseline urinary iodine excretion was 191 μg/day. Because greater than 90 percent of dietary iodine is excreted in urine (Nath et al., 1992), it was estimated that the baseline iodine intake was approximately 200 μg. Those receiving 1,500 μg/day of iodide showed a significant increase in baseline and TRH-stimulated serum TSH, effects not seen in the two lower doses. No subjects in this study had detectable antithyroid antibodies. The conclusion would be that an iodine intake of about 1,700 μg/ day increased TSH secretion. Both of the above studies support a LOAEL between 1,700 and 1,800 μg/day. Thus, the lowest LOAEL of 1,700 μg/day was selected.
Uncertainty Assessment. There is little uncertainty regarding the range of iodine intakes that are likely to induce elevated TSH concentration over baseline. A LOAEL of 1,700 μg/day and a NOAEL of 1,000 to 1,200 μg/day are estimated for adult humans. This results in an uncertainty factor (UF) of 1.5 to derive a NOAEL from a LOAEL. A higher uncertainty factor was not considered because of the mild, reversible nature of elevated TSH over baseline.
Derivation of a UL. The LOAEL of 1,700 μg/day was divided by a UF of 1.5 to obtain a UL of 1,133 μg/day of iodine, which was rounded down to 1,100 μg/day.
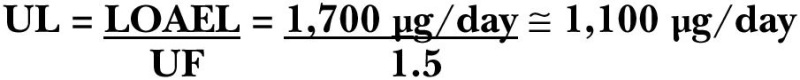
Iodine UL Summary, Ages 19 Years and Older
| UL for Adults | |
| ≥ 19 years | 1,100 μg/day of iodine |
Other Life Stage Groups
Infants. For infants, the UL was judged not determinable because of insufficient data on adverse effects in this age group and concern about the infant's ability to handle excess amounts. To prevent high intake, the only source of intake for infants should be from food and formula.
Children and Adolescents. Given the dearth of information, the UL values for children and adolescents are extrapolated from those established for adults. Thus, the adult UL of 1,100 μg/day of iodine was adjusted for children and adolescents on the basis of body weight as described in Chapter 2 and using reference weights from Chapter 1 (Table 1-1). Values have been rounded down.
Pregnancy and Lactation. No altered susceptibility of pregnant or lactating women to excess iodine has been noted. Therefore, the UL for pregnant and lactating females is the same as that for nonpregnant and nonlactating females.
Iodine UL Summary, Ages 0 through 18 Years, Pregnancy, Lactation
| UL for Infants | |
| 0–12 months | Not possible to establish; source of intake should be from food and formula only |
| UL for Children | |
| 1–3 years | 200 μg/day of iodine |
| 4–8 years | 300 μg/day of iodine |
| 9–13 years | 600 μg/day of iodine |
| UL for Adolescents | |
| 14–18 years | 900 μg/day of iodine |
| UL for Pregnancy | |
| 14–18 years | 900 μg/day of iodine |
| 19–50 years | 1,100 μg/day of iodine |
| UL for Lactation | |
| 14–18 years | 900 μg/day of iodine |
| 19–50 years | 1,100 μg/day of iodine |
Special Considerations
Autoimmune thyroid disease (AITD) is common in the U.S. population and particularly in older adult women. Individuals with AITD who are treated for iodine deficiency or nodular goiter (Carnell and Valente, 1998; Foley, 1992; Massoudi et al., 1995) may have increased sensitivity to adverse effects of iodine intake. Some young adults with simple goiter and iodine deficiency who were supplemented with 200 μg/day of iodine developed either mild transient hyperthyroidism or hypothyroidism, positive antibodies, and reversible histological changes of lymphocytic thyroiditis (Kahaly et al., 1997). The sensitivities of these distinct subgroups do not fall within the range of sensitivities expected for the healthy population.
Studies have correlated an increase in the incidence of AITD with a population's higher intake of iodine (Foley, 1992). Additional data provide some correlation between the incidence of circulating antithyroid antibodies (a marker for AITD) and dietary iodine intake (Schuppert et al., 2000). At this time there is not sufficient data to determine a UL for this subpopulation. Therefore, a UL could not be set for individuals with AITD.
Intake Assessment
Iodine is secreted in human and cow's milk and is present in dairy products, marine fish, and a variety of foods grown in iodide-rich soils. It is especially high in some foods, such as certain seaweed. Normal diets are unlikely to supply more than 1 mg/day. Also, a variety of environmental and therapeutic exposures are adventitious sources of iodine (Farwell and Braverman, 1996). Intake of 10 g of 0.001 percent iodized salt results in an intake of 770 μg/day. Based on the Food and Drug Administration Total Diet Study (Appendix Table E-4), the highest intake of dietary iodine for any life stage or gender group at the ninety-fifth percentile was approximately 1.14 mg/day, which is equivalent to the UL for adults. The iodine intake from the diet (Appendix Table E-4) and supplements (Appendix Table C-17) at the ninety-fifth percentile is approximately 1.15 mg/ day.
Risk Characterization
For most people, iodine intake from usual foods and supplements is unlikely to exceed the UL. In North America, where much of the iodine consumed is from salt iodized with potassium iodide, symptoms of iodine deficiency are rare. In certain regions of the world where goiter is present, therapeutic doses may exceed the UL. The UL is not meant to apply to individuals who are being treated with iodine under close medical supervision.
RESEARCH RECOMMENDATIONS FOR IODINE
- Correlation of community iodine intake with autoimmune thyroid disease and papillary thyroid cancer.
- Continual monitoring of U.S. urinary iodine by the National Health and Nutrition Examination Survey and inclusion of data on thyroid size in children, determined by ultrasound.
- Role of iodine in fibrocystic breast disease.
- Iodine nutrition and immune response.
- Iodine nutrition in relation to other nutrients, particularly vitamin A, iron, and selenium.
- Effects of iodine concentration in water purification.
- Further standardization of thyroid volume by ultrasound and urinary iodine excretion in areas with different iodine intake.
REFERENCES
- Albert A, Keating FR Jr. 1949. Metabolic studies with I131 labeled thyroid compounds. J Clin Endocrinol 9:1406–1421. [PubMed: 15399558]
- Ayromlooi J. 1972. Congenital goiter due to maternal ingestion of iodides. Obstet Gynecol 39:818–822. [PubMed: 5068335]
- Benmiloud M, Chaouki ML, Gutekunst R, Teichert HM, Wood WG, Dunn JT. 1994. Oral iodized oil for correcting iodine deficiency: Optimal dosing and outcome indicator selection. J Clin Endocrinol Metab 79:20–24. [PubMed: 8027227]
- Berghout A, Wiersinga W. 1998. Thyroid size and thyroid function during pregnancy: An analysis. Eur J Endocrinol 138:536–542. [PubMed: 9625365]
- Berson SA, Yalow RS. 1954. Quantitative aspects of iodine metabolism. The exchangeable organic iodine pool, and the rates of thyroidal secretion, peripheral degradation and fecal excretion of endogenously synthesized organically bound iodine. J Clin Invest 1533–1552. [PMC free article: PMC1072579] [PubMed: 13211808]
- Bleichrodt N, Born MP. 1994. A meta-analysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, editor. , ed. The Damaged Brain of Iodine Deficiency: Cogitive, Behavioral, Neuromotor, Educative Aspects . NY: Cognizant Communication. Pp.195–200.
- Bourdoux P. 1998. Evaluation of the iodine intake: Problems of the iodine/creatinine ratio—Comparison with iodine excretion and daily fluctuations of iodine concentration. Exp Clin Endocrinol Diabetes 106:S17–S20. [PubMed: 9865547]
- Buchinger W, Lorenz-Wawschinek O, Semlitsch G, Langsteger W, Binter G, Bonelli RM, Eber O. 1997. Thyrotropin and thyroglobulin as an index of optimal iodine intake: Correlation with iodine excretion of 39,913 euthyroid patients. Thyroid 7:593–597. [PubMed: 9292948]
- Carnell NE, Valente WA. 1998. Thyroid nodules in Graves' disease: Classification, characterization, and response to treatment. Thyroid 8:647–652. [PubMed: 9737358]
- Carswell F, Kerr MM, Hutchison JH. 1970. Congenital goitre and hypothyroidism produced by maternal ingestion of iodides. Lancet 1:1241–1243. [PubMed: 4192491]
- Chow CC, Phillips DI, Lazarus JH, Parkes AB. 1991. Effect of low dose iodide supplementation on thyroid function in potentially susceptible subjects: Are dietary iodide levels in Britain acceptable? Clin Endocrinol 34:413–416. [PubMed: 2060151]
- Croxson MS, Hall TD, Kletzky OA, Jaramillo JE, Nicoloff JT. 1977. Decreased serum thyrotropin induced by fasting. J Clin Endrocrinol Metab 45:560–568. [PubMed: 903402]
- DeGroot LJ. 1966. Kinetic analysis of iodine metabolism. J Clin Endocrinol Metab 26:149–173. [PubMed: 4159447]
- Delange F. 1989. Iodine nutrition and congenital hypothyroidism. In: Delange F, editor; , Fisher DA, editor; , Glinoer D, editor. , eds. Research in Congential Hypothyroidism . New York: Plenum Press.
- Delange F. 1993. Requirements of iodine in humans. In: Delange F, editor; , Dunn JT, editor; , Glinoer D, editor. , eds. Iodine Deficiency in Europe: A Continuing Concern . New York: Plenum Press. Pp.5–13.
- Delange F, Burgi H. 1989. Iodine deficiency disorders in Europe. Bull World Health Organ 67:317–325. [PMC free article: PMC2491245] [PubMed: 2670299]
- Delange F, Ermans AM. 1991. Iodine deficiency. In: Braverman LE, editor; , Utiger RD, editor. , eds. Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text , 6th ed. Philadelphia: JD Lippincott.
- Delange F, Lecomte P. 2000. Iodine supplementation: Benefits outweigh risks. Drug Safety 22:89–95. [PubMed: 10672891]
- Delange F, Bourdoux P, Vo Thi LD, Ermans AM, Senterre J. 1984. Negative iodine balance in preterm infants. Ann Endocrinol 45:77.
- Delange F, Dunn JT, Glinoer D. 1993. In: Iodine Deficiency in Europe. A Continuing Concern . New York: Plenum Press.
- Delange F, Benker G, Caron P, Eber O, Ott W, Peter F, Podoba J, Simescu M, Szybinsky Z, Vertongen F, Vitti P, Wiersinga W, Zamrazil V. 1997. Thyroid volume and urinary iodine in European schoolchildren: Standardization of values for assessment of iodine deficiency. Eur J Endocrinol 136:180–187. [PubMed: 9116913]
- Delange F, de Benoist B, Alnwick D. 1999. Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt. Thyroid 9:545–556. [PubMed: 10411116]
- Dunn JT, Crutchfield HE, Gutekunst R, Dunn AD. 1993. Two simple methods for measuring iodine in urine. Thyroid 3:119–123. [PubMed: 8369650]
- Dunn JT, Semigran MJ, Delange F. 1998. The prevention and management of iodine-induced hyperthyroidism and its cardiac features. Thyroid 8:101–106. [PubMed: 9492159]
- Dworkin HJ, Jacquez JA, Beierwaltes WH. 1966. Relationship of iodine ingestion to iodine excretion in pregnancy. J Clin Endocrinol Metab 26:1329–1342. [PubMed: 5959526]
- Emrich D, Karkavitsas N, Facorro U, Schurnbrand P, Schreivogel I, Schicha H, Dirks H. 1982. Influence of increasing iodine intake on thyroid function in euthyroid and hyperthyroid states. J Clin Endocrinol Metab 54:1236–1241. [PubMed: 6804478]
- Ermans AM, Dumont JE, Bastenie PA. 1963. Thyroid function in a goiter endemic: I. Impairment of hormone synthesis and secretion in the goitrous gland. J Clin Endocrinol 23:539–549.
- Etling N, Padovani E, Fouque F, Tato L. 1986. First-month variations in total iodine content of human breast milks. Early Hum Dev 13:81–85. [PubMed: 3956424]
- Farwell AP, Braverman LE. 1996. Thyroid and Antithyroid Drugs. In: Hardman JG, editor; , Limbird LE, editor; , Molinoff PB, editor; , Ruddon RW, editor; , Gilman AG, editor. , eds. Goodman and Gilman's The Pharmacological Basis of Therapeutics , 9th ed. New York: McGraw-Hill. Pp.1383–1409.
- Finkelstein R, Jacobi M. 1937. Fatal iodine poisoning: A clinicopathologic and experimental study. Ann Intern Med 10:1283–1296.
- Fischer PW, Giroux A. 1987. Iodine content of a representative Canadian diet. J Can Diet Assoc 48:24–27.
- Fisher DA, Oddie TH. 1969. a. Thyroidal radioiodine clearance and thyroid iodine accumulation: Contrast between random daily variation and population data. J Clin Endocrinol Metab 29:111–115. [PubMed: 5762312]
- Fisher DA, Oddie TH. 1969. b. Thyroid iodine content and turnover in euthyroid subjects: Validity of estimation of thyroid iodine accumulation from short-term clearance studies. J Clin Endocrinol Metab 29:721–727. [PubMed: 4305619]
- Foley TP Jr. 1992. The relationship between autoimmune thyroid disease and iodine intake: A review. Endokrynol Pol 43:53–69. [PubMed: 1345585]
- Franceschi S. 1998. Iodine intake and thyroid carcinoma—A potential risk factor. Exp Clin Endocrinol Diabetes 106:S38–S44. [PubMed: 9865553]
- Gaitan E. 1989. Environmental Goitrogenesis . Boca Raton: CRC Press.
- Gardner DF, Kaplan MM, Stanley CA, Utiger RD. 1979. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med 300:579–584. [PubMed: 105290]
- Gardner DF, Utiger RD, Schwartz SL, Witorsch P, Myers B, Braverman LA, Witorsch RJ. 1987. Effects of oral erythrosine (2′,4′,5′,7′-tetraiodofluorescein) on thyroid function in normal men. Toxicol Appl Pharmacol 91:299-304. [PubMed: 2447681]
- Gardner DF, Centor RM, Utiger RD. 1988. Effects of low dose oral iodide supplementation on thyroid function in normal men. Clin Endocrinol 28:283–288. [PubMed: 3139337]
- Ghent WR, Eskin BA, Low DA, Hill LP. 1993. Iodine replacement in fibrocystic disease of the breast. Can J Surg 36:453–460. [PubMed: 8221402]
- Glinoer D. 1998. Iodine supplementation during pregnancy: Importance and biochemical assessment. Exp Clin Endocrinol Diabetes 106:S21. [PubMed: 9865548]
- Gushurst CA, Mueller JA, Green JA, Sedor F. 1984. Breast milk iodine: Reassessment in the 1980s. Pediatrics 73:354–357. [PubMed: 6546615]
- Gutekunst R, Smolarek H, Hasenpusch U, Stubbe P, Friedrich HJ, Wood WG, Scriba PC. 1986. Goitre epidemiology: Thyroid volume, iodine excretion, thyroglobulin and thyrotropin in Germany and Sweden. Acta Endocrinol 112:494–501. [PubMed: 3529785]
- Harrison MT. 1968. Iodine balance in man. Postgrad Med J 44:69–71. [PMC free article: PMC2466447] [PubMed: 5639233]
- Harrison MT, Harden R, Alexander WD, Wayne E. 1965. Iodine balance studies in patients with normal and abnormal thyroid function. J Clin Endocrinol 25:1077–1084. [PubMed: 14328378]
- Hemken RW. 1980. Milk and meat iodine content: Relation to human health. J Am Vet Med Assoc 176:1119–1121. [PubMed: 7216884]
- Hetzel BS, Maberly GF. 1986. Iodine. In: Mertz W, editor. , ed. Trace Elements in Human and Animal Nutrition , Vol. 2. Orlando: Academic Press. Pp.139–208.
- Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter EW, Maberly GF, Braverman LE, Pino S, Miller DT, Garbe PL, DeLozier DM, Jackson RJ. 1998. Iodine nutrition in the United States. Trends and public health implications: Iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab 83:3401–3408. [PubMed: 9768638]
- Ingenbleek Y, Malvaux P. 1974. Iodine balance studies in protein-calorie malnutrition. Arch Dis Child 49:305–309. [PMC free article: PMC1648751] [PubMed: 4208456]
- Jackson IM. 1982. Thyrotropin-releasing hormone. New Engl J Med 306:145–155. Johnson LA, Ford HC, Doran J, Richardson VF. 1990. A survey of the iodide concentration of human milk. N Z Med J 103: 393–394.
- Jooste PL, Weight MJ, Lombard CJ. 2000. Short-term effectiveness of mandatory iodization of table salt, at an elevated iodine concentration, on the iodine and goiter status of school children with endemic goiter. Am J Clin Nutr 71:75–80. [PubMed: 10617949]
- Kahaly G, Dienes HP, Beyer J, Hommel G. 1997. Randomized, double blind, placebo-controlled trial of low dose iodide in endemic goiter. J Clin Endocrinol Metab 82:4049–4053. [PubMed: 9398711]
- Klebanoff SJ. 1967. Iodination of bacteria: A bacterial mechanism. J Exp Med 126:1063–1078. [PMC free article: PMC2138423] [PubMed: 4964565]
- Klein AH, Meltzer S, Kenny FM. 1972. Improved prognosis in congenital hypothyroidism treated before age three months. J Pediatr 81:912–915. [PubMed: 5086719]
- Kurt TL, Morgan ML, Hnilica V, Bost R, Petty CS. 1996. Fatal iatrogenic iodine toxicity in a nine-week old infant. J Toxicol Clin Toxicol 34:231–234. [PubMed: 8618260]
- LaFranchi SH, Buist NR, Murphey WH, Larsen PR, Foley TP Jr. 1977. Transient neonatal hypothyroidism detected by newborn screening program. Pediatrics 60:539–541. [PubMed: 905020]
- Larsson G, Victor A. 1988. Micturition patterns in a healthy female population, studied with a frequency/volume chart. Scand J Urol Nephrol 114:53–57. [PubMed: 3201170]
- Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR. 1998. Iodine intake and the pattern of thyroid disorders: A comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab 83:765–769. [PubMed: 9506723]
- Lind P, Langsteger W, Molnar M, Gallowitsch HJ, Mikosch P, Gomez I. 1998. Epidemiology of thyroid diseases in iodine sufficiency. Thyroid 8:1179–1183. [PubMed: 9920375]
- Loh KC. 2000. Amiodarone-induced thyroid disorders: A clinical review. Postgrad Med J 76:133–140. [PMC free article: PMC1741517] [PubMed: 10684321]
- Malamos B, Koutras DA, Marketos SG, Rigopoulos GA, Yataganas XA, Binopoulos D, Sfontouris J, Pharmakiotis AD, Vought RL, London WT. 1967. Endemic goiter in Greece: An iodine balance study in the field. J Clin Endocrinol Metab 27:1372–1380. [PubMed: 4167506]
- Malvaux P, Beckers C, de Visscher M. 1969. Iodine balance studies in nongoitrous children and in adolescents on low iodine intake. J Clin Endocrinol Metab 29:79–84. [PubMed: 5762324]
- Massoudi MS, Meilahn EN, Orchard TJ, Foley TP Jr, Kuller LH, Constantino JP, Buhari AM. 1995. Prevalence of thyroid antibodies among healthy middle-aged women. Findings from the thyroid study in healthy women. Ann Epidemiol 5:229–233. [PubMed: 7606312]
- Mattsson S, Lindstrom S. 1995. Diuresis and voiding pattern in healthy schoolchildren. Br J Urol 76:783–789. [PubMed: 8535727]
- Means LJ, Rescorla FJ, Grosfeld JL. 1990. Iodine toxicity: An unusual cause of cardiovascular collapse during anesthesia in an infant with Hirschsprung's disease. J Pediatr Surg 25:1278–1279. [PubMed: 2286907]
- Missler U, Gutekunst R, Wood WG. 1994. Thyroglobulin is a more sensitive indicator of iodine deficiency than thyrotropin: Development and evaluation of dry blood spot assays for thyrotropin and thyroglobulin in iodine-deficient geographical areas. Eur J Clin Chem 32:137–143. [PubMed: 8031964]
- Money WL, Rawson RW. 1950. The experimental production of thyroid tumors in the rat exposed to prolonged treatment with thiouracil. Cancer 3:321–335.
- Moss AJ, Levy AS, Kim I, Park YK. 1989. Use of Vitamin and Mineral Supplements in the United States: Current Users, Types of Products, and Nutrients . Advance Data, Vital and Health Statistics of the National Center for Health Statistics, Number 174. Hyattsville, MD: National Center for Health Statistics.
- Moulopoulos DS, Koutras DA, Mantzos J, Souvatzoglou A, Piperingos GD, Karaiskos KS, Makriyannis D, Sfontouris J, Moulopoulos SD. 1988. The relation of serum T4 and TSH with the urinary iodine excretion. J Endocrinol Invest 11:437–439. [PubMed: 3209822]
- Nath SK, Moinier B, Thuillier F, Rongier M, Desjeux JF. 1992. Urinary excretion of iodide and fluoride from supplemented food grade salt. Int J Vitam Nutr Res 62:66–72. [PubMed: 1587711]
- Nelson M, Phillips DI. 1985. Seasonal variations in dietary iodine intake and thyrotoxicosis. Hum Nutr Appl Nutr 39:213–216. [PubMed: 3840142]
- Nilsson R, Ehrenberg L, Fedoresak I. 1987. Formation of potential antigens from radiographic contrast media. Acta Radiol 28:473–477. [PubMed: 2958066]
- Oddie TH, Fisher DA, Long JM. 1964. Factors affecting the estimation of iodine entering the normal thyroid gland using short-term clearance studies. J Clin Endocrinol 24:924–933. [PubMed: 14216483]
- Parsad D, Saini R. 1998. Acneform eruption with iodized salt. Int J Dermatol 37:478. [PubMed: 9646145]
- Paul T, Meyers B, Witorsch RJ, Pino S, Chipkin S, Ingbar SH, Braverman LE. 1988. The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metabolism 37:121–124. [PubMed: 3340004]
- Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS, Larsen KR, Eriksen GM, Johannesen PL. 1993. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab 77:1078–1083. [PubMed: 8408456]
- Pennington JA. 1990. A review of iodine toxicity reports. J Am Diet Assoc 90:1571–1581. [PubMed: 2229854]
- Pennington JAT, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. 1995. Composition of core foods in the U.S. food supply, 1982-1991. J Food Comp and Anal 8:171-217.
- Pinchera A, MacGillivray MH, Crawford JD, Freeman AG. 1965. Thyroid refractoriness in an athyreotic cretin fed soybean formula. N Engl J Med 273:83–87. [PubMed: 14301203]
- Riggs DS. 1952. Quantitative aspects of iodine metabolism in man. Pharmacol Rev 4:284–370. [PubMed: 12993583]
- Romano R, Jannini EA, Pepe M, Grimaldi A, Olivieri M, Spennati P, Cappa F, D'Armiento M. 1991. The effects of iodoprophylaxis on thyroid size during pregnancy. Am J Obstet Gynecol 164:482–485. [PubMed: 1992688]
- Schuppert F, Ehrenthal D, Frilling A, Suzuki K, Napolitano G, Kohn LD. 2000. Increased major histocompatibility complex (MHC) expression in nontoxic goiters is associated with iodine depletion, enhanced ability of the follicular thyroglobulin to increase MHC gene expression, and thyroid antibodies. J Clin Endocrinol Metab 85:858–867. [PubMed: 10690902]
- Shepard TH, Pyne GE, Kirschvink JF, McLean M. 1960. Soybean goiter: Report of three cases. N Engl J Med 262:1099–1103.
- Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Vidor G, Braverman LE, Medeiros-Neto G. 1998. Iodine-induced hyperthyroidism: Occurrence and epidemiology. Thyroid 8:83–100. [PubMed: 9492158]
- Sulzberger MB, Witten VH. 1952. Allergic dermatoses due to drugs. Postgrad Med 11:549–557. [PubMed: 14948699]
- Suzuki H, Mashimo K. 1973. Further studies of “endemic goiter” in Hokkaido, Japan. In: Mashimo K, editor; , Suzuki H, editor. , eds. Iodine Metabolism and Thyroid Function , Vol. 6. Sapporo, Japan: Hokkaido University School of Medicine. P. 143.
- Suzuki H, Higuchi T, Sawa K, Ohtaki S, Horiuchi Y. 1965. “Endemic coast goiter” in Hokkaido, Japan. Acta Endocrinol 50:161–176. [PubMed: 4158495]
- Swanson EW, Miller JK, Mueller FJ, Patton CS, Bacon JA, Ramsey N. 1990. Iodine in milk and meat of dairy cows fed different amounts of potassium iodide or ethylenediamine dihydroiodide. J Dairy Sci 73:398–405. [PubMed: 2329204]
- Tresch DD, Sweet DL, Keelan MH, Lange RL. 1974. Acute iodide intoxication with cardiac irritability. Arch Intern Med 134:760–762. [PubMed: 4411847]
- Trowbridge FL, Hand KE., Nichaman MZ. 1975. Findings relating to goiter and iodine in the Ten-State Nutrition Survey. Am J Clin Nutr 28:712–716. [PubMed: 1146723]
- Venturi S, Venturi A, Cimini D, Arduini C, Venturi M, Guidi A. 1993. A new hypothesis: Iodine and gastric cancer. Eur J Cancer Prev 2:17–23. [PubMed: 8428171]
- Vought RL, London WT. 1967. Iodine intake, excretion and thyroidal accumulation in healthy subjects. J Clin Endocrinol Metab 27:913–919. [PubMed: 4165697]
- Wayne EJ, Koutras DA, Alexander WD. 1964. Clinical Aspects of Iodine Metabolism . Oxford: Blackwell Scientific.
- Wexler P, Gad SC, Hartung R, Henderson RF, Krenzelok EP, Locey BJ, Mehendale HM, Plaa GL, Pope C, Witschi H. 1998. Encyclopedia of Toxicology , Vol. 2. San Diego: Academic Press. Pp.186–187.
- WHO (World Health Organization) Nutrition Unit. 1994. Indicators for Assessing Iodine Deficiency Disorders and their Control through Salt Iodization . Geneva: WHO.
- WHO/UNICEF/ICCIDD (United Nations Childrens Fund/International Council for Control of Iodine Deficiency Disorders). 1993. Indicators for Assessing Iodine Deficiency Disorders and their Control Programmes . Report of a joint WHO/ UNICEF/ICCIDD consultation (review version). Geneva: WHO.
- Wolff J. 1969. Iodide goiter and the pharmacologic effects of excess iodide. Am J Med 47:101–124. [PubMed: 4307521]
- Xu F, Sullivan K, Houston R, Zhao J, May W, Maberly G. 1999. Thyroid volumes in U.S. and Bangladeshi schoolchildren: Comparison with European schoolchildren. Eur J Endocrinol 140: 498–504. [PubMed: 10366405]
- Iodine - Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chr...Iodine - Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc
Your browsing activity is empty.
Activity recording is turned off.
See more...