NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on the Economics of Antimalarial Drugs; Arrow KJ, Panosian C, Gelband H, editors. Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance. Washington (DC): National Academies Press (US); 2004.

Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance.
Show detailsINTRODUCTION TO THE STUDY
Chloroquine, after decades of use as the first-line drug for uncomplicated falciparum malaria, is failing. But it is still the most widely used antimalarial because a newer safe and effective alternative—artemisinin combination therapy (ACT) at a few dollars per course—has been considered unaffordable by national governments. As a result, illness and deaths from malaria are on the rise. Most fatalities occur among children in Africa, but failing drugs also account for a vast and largely unrecorded morbidity in people of other ages and demographics, not only in Africa, but in Asia and to some extent, everywhere that falciparum malaria (the most severe form) occurs. There also is evidence that drug resistance is leading to epidemics in fringe areas where malaria was previously well controlled when chloroquine was effective.
The IOM Committee on the Economics of Antimalarial Drugs was constituted to study the economics of making effective antimalarials accessible to those who could benefit from them. The Committee had two main tasks: 1) to recommend global actions to ensure the broadest possible access to effective antimalarial drugs, thus halting the accelerating loss of life (and malaria's attendant health and economic burdens) as quickly as possible; and 2) to consider some economic aspects of longer-term global malaria drug policy.
Genesis of the Project
The U.S. Agency for International Development (USAID) has a strong history and current portfolio in bilateral and multilateral assistance projects in malaria. USAID represents the United States in the “Roll Back Malaria” (RBM) partnership, centered in the World Health Organization (WHO), and including malaria-endemic countries, their bilateral and multilateral development partners, the private sector, nongovernmental and community-based organizations, foundations, and research and academic institutions. It was largely in connection with its leadership role in RBM that USAID approached the Institute of Medicine for independent advice and economic recommendations to counter the current global crisis in antimalarial drug resistance. The Bill and Melinda Gates Foundation later joined as a study cosponsor.
Charge to the Committee
The charge to the committee from USAID was the following:
The committee will recommend steps that could be taken to maximize the influence of both new and established antimalarial drugs while postponing the development of drug resistance. The immediate focus will be on the class of artemisinin derivatives and other drugs with which they are (or could be) coformulated or paired, but the methodology developed should be generally applicable to new agents still in the pipeline.
The statement of task emphasized the fact that that influence would be maximized to the extent that: 1) new and established antimalarial drugs were affordable to the people who needed them; and 2) the antimalarial drugs were engineered and packaged, and delivered in ways that encouraged adherence to prescribed regimens. The Committee applied its energy mainly to the first point, while reviewing relevant information and making some recommendations on the second.
About This Report
The crisis of spreading resistance to antimalarial drugs has biomedical origins, but the inability of affected countries to respond by changing national treatment policies and making effective artemisinin-based drugs widely available is rooted in economics. The recommendations in this report were developed in light of the biomedical, social and cultural, and economic realities that define the burden of malaria and how it is addressed in endemic countries today. We attempt to draw together all these threads to present the reader with a complete and coherent set of facts from which to draw conclusions.
The report is in three parts (Box 1-1). Part 1 focuses on the immediate financing problem and proposes a global solution. Part 2 gives a foundation for understanding malaria as a disease, the tools we have to control it, and the burden it imposes on affected populations. The chapters that make up Part 2 are intended as both background for the nonspecialist and updates for those with a basic knowledge of malaria. They lay groundwork for the policy discussions and recommendations, but the reader may choose to pass by some of this material. Part 3 stresses the need to make further inroads against malaria by investing in the development of better antimalarial drugs and finding ways to better use new and existing tools.
BOX 1-1
Structure of This Report.
The remainder of this chapter describes the extent of malaria in the world (with the greatest focus on Africa), how people deal with the disease on an individual level, how nations deal with it as a public health problem, and major global initiatives dealing with malaria control. It begins with a discussion of why this report focuses on artemisinin derivatives and artemisinin combination therapies (ACTs), and the roles of other antimalarial drugs.
THE ROLE OF THE ARTEMISININS IN MALARIA CONTROL
Artemisinins are the focus of international attention, and the centerpiece of the recommendations in this report. Their full story—from discovery onward—is recounted in Chapter 9, as are the properties, advantages, and disadvantages of other currently effective antimalarials, and those of historic importance. Because the artemisinins figure so prominently, a few lines of explanation are in order to justify the primacy given to this family of compounds in the current circumstances.
It already has been said that chloroquine—after a remarkable period of effectiveness—no longer works against falciparum malaria in much of the world. The only other drug that has had relatively widespread use in Africa—sulfadoxine-pyrimethamine (SP)—worked as first-line therapy for a much shorter period than chloroquine before being evaded by resistant malaria organisms in many places. Continued use of SP would foster additional resistant strains and the drug would become largely ineffective, probably within several years. This should not be allowed to happen because SP has a very specific and important role, at least for the time being. It is the agent of choice for intermittent preventive therapy (IPT) for pregnant women. Next to children, they are the individuals most vulnerable to malaria, particularly in Africa.
One further idea must be introduced, and this is the global shift in strategy toward simultaneous treatment with two different antimalarials, adopted as a means to delay the development and spread of drug-resistant malaria. Combination treatment is not specific to artemisnin combinations but will become the standard henceforth, involving other drugs in the future.
The question remains as to why artemisinins are considered essential components of combinations, over the other currently effective antimalarials with which they would be paired: amodiaquine, mefloquine, and the most recent addition, Lapdap.1 In fact, other combinations of drugs from different classes could be developed, but they are unlikely to be better than ACTs and would lack some of the benefits of a combination with an artemisinin. The main reasons for endorsing artemisinin-containing combinations are:
- the artemisinins represent a new family of compounds, with a novel mode of action, and faster antimalarial activity than any of the other drugs;
- they have proven themselves robust over at least a decade of consistent use in Asia, both in terms of effectiveness and safety and in the lack of any documented drug resistance (at least in part because they have a remarkably short half-life, so subtherapeutic levels in the body, which promote resistance, are fleeting);
- in places where the level of transmission is low, they may play a role in reducing transmission because they act against the gametocyte (sexual) stage of the malaria parasite, as well as the asexual forms responsible for malaria symptoms; and finally,
- they have proven effective at the individual level in every transmission zone in Africa and elsewhere.
In contrast, the “companion” drugs are all related to earlier drugs, and there is either already documented resistance to them in some places, or the presumption that cross-resistance to the related drugs could compromise them. (The choice of which companion drug should be used in a given area will depend largely on the existing or potential resistance to each one.) In addition, any cautions applied to ACTs because of the lack of widespread use in Africa apply equally to these drugs, since none of them has been used extensively in high-transmission settings.
Can we be absolutely sure that widespread use of ACTs will halt the rising malaria mortality in Africa now resulting from the use of ineffective drugs? Not by direct evidence, although current evidence points in that direction. The truth is that they have not been deployed on a large scale in the highly endemic parts of Africa. Determining their overall impact will require years of surveillance, which should be carried out as conscientiously as possible. But there is no rationale for delaying actions to move ACTs into the hands of the greatest number of consumers, as quickly as possible, to replace the failing drugs now being used.
MALARIA TODAY
The Extent and Effects of Malaria
There is no time in memory when malaria was not a global health problem. It was common in many parts of the world until well into the 20th century. It was eliminated in Europe, North America, and parts of other continents through deliberate programs of mosquito control and clinical treatment, as well as through generally improved social and living conditions. The muscle behind eradication efforts elsewhere was never applied in Africa's highly endemic areas, however (Breman, et al., 2001). Today, sub-Saharan Africa remains the area of greatest malaria concentration, but significant problems exist in Asia, in Latin America, and focally in other areas. At least 85 percent of deaths from malaria occur in Africa, 8 percent in Southeast Asia, 5 percent in the Eastern Mediterranean region, 1 percent in the Western Pacific, and 0.1 percent in the Americas.
Now, at the beginning of the 21st century, there is good reason to believe that inroads against malaria can be made in Africa and elsewhere, using the control measures described in this report, if effective combination antimalarials are made widely available.
Malaria in Africa
The extent of the challenge is illustrated by the map of Africa (Figure 1-1), showing a vast swath between the northern coastal countries and the most southerly ones (excluding most of the horn of Africa) where malaria transmission is intense and malaria is ever-present. This area is ringed by a region prone to seasonal malaria transmission or periodic epidemics—with often devastatingly high mortality rates. More than half a billion people— more than two-thirds of the African population—live with malaria year in, year out, most of them where transmission is intense. The vast majority of the million or so deaths from malaria occur in Africa, mostly among children who have yet to acquire sufficient immunity to protect them from heavy malaria infections. The prevalence of malaria varies with levels of endemicity, but it averages to at least one acute clinical episode per year for which some treatment is sought, or on the order of half a billion treated episodes.

FIGURE 1-1
Distribution of Endemic Malaria in Africa. SOURCE: Reprinted with permission from MARA/ARMA, Copyright 2001. Taken from Craig, MH, Snow RW, le Sueur D. 1999. A Climate-Based Distribution Model of Malaria Transmission in Sub-Saharan Africa. Parasitology (more...)
It is worth remembering that the malaria map would have looked very different half a century ago, and that control measures are largely responsible for shrinking the highly-endemic zone. The low- and no-transmission areas of southern Africa (including Namibia, Swaziland, South Africa, Botswana, and Zimbabwe) were previously highly endemic—and could become so again, if control measures fail.
The map lays out the boundaries of a very large problem, but does not tell the whole story. Who becomes sick and who dies is what is important. Once the discussion moves beyond acknowledging that malaria is a “big” problem, however, estimates of the relevant numbers—malaria cases, deaths from malaria—and the distribution among the population, geographically and by age, vary so widely that they can appear unusable for policy and planning. This lack of specificity is at least part of what makes tackling the problem so unsettling.
Malaria in Asia
There are about 27 million cases and 30,000 deaths from falciparum malaria in Southeast Asia each year, with no perceptible decline over the last decade (WHO Regional Office for South-East Asia,). Unlike Africa, where acquired semi-immunity protects most adults from severe clinical disease and death, intermittent infections in Asian endemic areas are usually symptomatic, not uncommonly with very high parasitemias (parasite numbers in the blood) and a risk of death. Apart from the sheer numbers— not approaching African levels, but substantial—Southeast Asia has been the epicenter of drug-resistant malaria precisely because of this pattern of infection and treatment of nonimmune individuals with heavy parasite loads. Some of the most difficult problems arise in border areas, where refugees have gathered after political conflicts. Isolated tribal groups in various countries, with little or no access to health care, also suffer disproportionately from malaria.
Malaria in the New World
North America and most of the Caribbean are free from malaria transmission, a result of eradication efforts that began in earnest in the 1950s. But 200 million people live where there is still some risk of transmission, in 21 countries of Central and South America, and the Caribbean, about 20 percent of them in areas considered “high risk” for the region. About one million cases and 200 deaths were recorded in 2001. Drug resistance is becoming widespread, and changes in drug policy have already been made in some places. And the same factors that can disrupt control in Africa and Asia—migration, civil unrest, poor economic conditions—occur in the Americas.
The Varied and Far-Reaching Effects of Malaria2
Malaria is not simply a matter of episodes of illness and deaths, as enormous as those burdens are. Effects vary from place to place, but nearly all people living in endemic areas will at some stage in their lives become infected with malaria through the bite of a mosquito, and after the parasite has multiplied in numbers, they will suffer an attack of fever and other symptoms when large numbers of parasites are released into the bloodstream. Untreated, these episodes may progress in severity and naturally resolve, or the person may become overwhelmed and die. Or antimalarial drugs may be used to quell the infection. Functional immunity develops in those who survive the early episodes, if they are continually exposed to malaria from birth, preventing most symptomatic recurrences at older ages.
But that is not the whole story. There are less obvious, but equally serious, consequences of the chronic infections and repeated reinfections that characterize life in high-transmission areas, including most of sub-Saharan Africa. Chronic, subclinical infections can cause anemia and pre-dispose to undernutrition. These processes may further increase the chances of severe malaria developing with a subsequent infection, and possibly of more severe outcomes of infections with other pathogens. Unlike other adults in endemic areas, pregnant women are themselves more susceptible to malaria's most severe effects—including death—and asymptomatic infection of the placenta significantly reduces the weights of their newborn children, reducing their chances of surviving infancy.
Some proportion of those who survive severe malaria—with or without effective treatment—are left with permanent, serious effects, including epilepsy and spasticity. More subtle consequences have also been described and include behavioral disturbances and cognitive impairment. Currently, it is not possible to do much more than mention this spectrum of effects. They cannot yet be quantified, which is not surprising when even the most concrete of end points—the number of deaths from malaria—is only roughly estimated (see Chapter 7).
What People Do When They Suspect Malaria
Where malaria is common, a child may have four or five bouts of fever, and an adult one or two each year, all presumed to be malaria. People live with malaria in their midst in a variety of ways, but not infrequently with a compromised quality of life. Families do make efforts and spend precious resources to prevent malaria (in part, by eliminating nuisance insects), but most of the readily accessible methods, such as burning mosquito coils or leaves, are only partially effective. Insecticide-treated bednets—the most effective single preventive intervention—are still relatively rare, though coverage is increasing (see Chapter 8). In the absence of effective prevention, treatment of symptomatic cases is the most common form of malaria control.
Decisions about whether and what kind of treatment to seek depend, first, on whether the patient or caregiver thinks that malaria is the cause of the illness. A host of considerations—specific symptoms, personal and family history, season of the year—factor into this judgment. Unfortunately, the symptoms of severe malaria—convulsions, loss of consciousness—often are ascribed to other causes, so malaria treatment is not sought, at least initially. When a decision is made to seek treatment for what is presumed to be malaria, what actually happens reflects both the demand side—what people want—and the supply side—what is available to them. It is, in many ways, more complex than a parallel decision to seek treatment for a febrile illness in the United States or Europe, where there would be no need to worry, for instance, about whether a clinic or hospital actually has the drug needed to treat the disease. The costs of treatment in money, time, travel, and otherwise, in general, are more onerous for a poor rural African, Asian, or South American family than for their poor counterparts in richer countries. Decisions may be delayed while options are weighed, funds gathered, and arrangements made. People know that children die of malaria, but they also know from experience that most episodes resolve uneventfully. An unfortunate reality is that in the cases when children do die from malaria, it can happen very quickly, especially for the very young: one day the child has a mild fever and then one of a number of serious symptoms appear, and the child is dead. In those cases, the consequences of waiting are severe and irreversible.
The topic of this report demands some understanding of where patients go for malaria treatment (if they go), and in the case of drug treatment, the path drugs follow to get to the varied outlets in the public and private sectors. As is the case for a number of the key issues (e.g., the quantitative burden of malaria), there are many generalizations but frustratingly little solid information. What we really need to know is how to intervene to reduce the morbidity, shrink the overall malaria burden, and prevent the deaths. A starting point is knowing what people do when they or their children have malaria, whether they think it is malaria or something else. When this has been studied it has been almost exclusively among the survivors. Certain patterns emerge, as reviewed later in this chapter. The question that remains almost entirely untouched is what chain of treatment-seeking events took place for those who eventually died from malaria. A preliminary analysis of the most extensive such study undertaken has been completed recently by the Tanzania Essential Health Interventions Project (TEHIP) (de Savigny et al., 2003). (Appendix 1A is a detailed account of the study and its results to date.)
The TEHIP Study: Preliminary Findings
Study Description
TEHIP studied the circumstances leading to deaths from malaria in the stable perennial malaria transmission belt that runs along the coast of Tanzania and up the Rufiji and Kilombero River basins, from January 1999 through the end of 2001. The Rufiji Demographic Surveillance Site (DSS) covers 85,000 people in 17,000 households in 31 villages, registering all births, deaths, in-migrations, out-migrations, pregnancies and other vital events. A “verbal autopsy” is conducted for each person who dies in the population. Following routine DSS procedures for verbal autopsy,3 a trained health officer interviews the next of kin (or other close “informant”) about the person who died and the events leading to the death. Questions are asked about the use of health facilities during the fatal illness, reasons for using or not using a particular health facility, and confirmatory evidence of cause of death, if available. The interviewer assesses the information and assigns a tentative cause of death. A physician reviews the entire record and assigns the final cause.
From the full details of each case, treatment can be divided broadly into “modern care”—which includes biomedical, Western, pharmaceutical, professional, official or formal health care—and “traditional care”—which includes traditional medicine, traditional healers, traditional providers, lay providers, traditional practices, or folk care. (Use of “modern care” does not necessarily mean that the care was appropriate, or used correctly.)
Results
Over the 3-year period, 3,023 people died and 2,953 (97.7 percent) verbal autopsies were conducted. Out of these, 722 were suspected to be directly or indirectly due to malaria. Just under half (46 percent) of the 722 who died were male, 44 percent were under 5 years of age, and 39 (5 percent) had had convulsions indicating possible cerebral malaria. Findings of particular interest include:
- Treatment most often started at home using antipyretics and antimalarials from local shops or left over from previous episodes.
- 626 (87 percent) sought care at least once before death, while 96 (13 percent) did not, or could not, seek care. These figures were similar for those under and over age 5.
- Of the group that did not have convulsions, 91 percent used modern care first, and by the second treatment, more than 99 percent had used modern care. Hence, traditional care delayed modern care for 9 percent of cases.
- Of those who did have convulsions, a similar proportion (90 percent) used modern care first, but only 63 percent continued with modern care for a second treatment when the first had failed.
This pattern is considerably better than was seen in the mid-1980s in Bagamoyo, when 55 percent of children who died had not utilized any modern care (Mtango et al., 1992).
TEHIP is continuing to refine the information from this study and will be analyzing the narrative portion of the verbal autopsy questionnaires to look at some specific aspects of the health care received, including:
- reasons for delay in seeking modern care (e.g., tried to treat at home without antimalarials, no transport, poor recognition of severity, lack of confidence in modern care, no power to decide, insufficient finances);
- reasons for delay in receiving modern care (e.g., arrived after working hours or on the weekend, long queues); and
- reasons modern care was ineffective (e.g., poor communication, no referral, drugs not available, abusive health worker, noncompliant providers, poor adherence of patients).
Decisions to Seek Malaria Treatment and Beliefs about the Disease: The Knowledge Base4
Adults sense when they have a fever, and mothers know when their children are sick. Where malaria is common, people generally associate it with febrile illness, but this varies from place to place depending on cultural practices and beliefs, education and experience, and individual characteristics.
When malaria is suspected, people may go to one of a wide range of places (although in a given place, the choices may be few), including modern health providers in public clinics or health centers of various sizes in private facilities run by religious groups or other nongovernmental organizations; or the commercial private sector, which includes traditional healers, pharmacies, shops, markets, and drug peddlers. It is these latter outlets—mainly shops and drug peddlers, often referred to as the “informal private sector”—that provide antimalarial drugs for more than half of all treated episodes in sub-Saharan Africa (Mwabu, 1986; Deming et al., 1989; Ejezie et al., 1990; Snow et al., 1992; Mnyika et al., 1995). It also is common for people to get drugs from more than one source: people often begin with self-treatment using drugs from the informal sector, and then seek care from formal providers (McCombie, 1996).
But people have varied beliefs about the causes of disease and what treatments are appropriate. In the few places where it has been studied both in Africa and Asia, mild and severe malaria often are considered distinct diseases, with different words to describe them and different beliefs about how to treat them. Severe malaria with convulsions may be perceived as involving supernatural intervention, such as spirit possession or magic spells (Mwenesi et al., 1995; Winch et al., 1996; Ahorlu et al., 1997; Muela and Ribera, 2000), and as a consequence, people may be more likely to go to a traditional healer for treatment, such that the most dangerous cases are least likely to get effective antimalarial drugs. It is not uncommon in Africa for mothers to believe that “modern” treatment, injections in particular, are dangerous for children with convulsions (Mwenesi et al., 1995; Makemba et al., 1996; Ahorlu et al., 1997) even though injections often are preferred over tablets for other conditions. Alternatively, both traditional and Western medical providers may be consulted in turn.
Treatment through the Formal Sector
Most malaria treatment takes place at home with drugs from the informal sector, but formal facilities bear a substantial burden. In sub-Saharan Africa and rural Asia, fever is the reason for 20 to 40 percent of all clinic and dispensary visits. These outpatient facilities generally do not offer the life-saving emergency treatments required when severe malaria develops, however. Only hospitals or health centers with inpatient facilities—where malaria's share of admissions ranges between 0.5 and 50 percent (Chima et al., 2003)—are able to provide this care.
The pattern of treatment-seeking and treatment itself is different in countries outside of Africa—mainly Asia and the Latin America/Caribbean region. Malaria is a significant problem in a number of countries, but it is generally more localized to specific areas, and everywhere is much less common than in Africa. Therefore, the burden on individuals as well as the health care system is much less. In Asia and Latin America, people with malaria are treated in general medical facilities, or in special malaria clinics. Laboratory diagnosis is much more widely used, and where these facilities are not available on-site, patients with suspected malaria often are treated presumptively for the immediate symptoms, and given further treatment if their malaria is confirmed.
Treatment through the Informal Sector
Use of the informal sector for malaria treatment is very common throughout the world, even where malaria is less common. Most of this involves the purchase of antimalarials from shops, where people may ask for a “product” by name, rather than a “service” including diagnosis and advice. In Thailand, for example, “ya chud”—a packet with a mixture of medicines including antimalarial drugs sold in private outlets—is used frequently (one study found that around 90 percent of respondents had used ya chud at least once in their lifetime) (McCombie, 1996).
The role of traditional healers and the use of traditional medicines for uncomplicated malaria varies, but generally is considered to be of less importance than other sources of care (McCombie, 1996), though people may underreport use of these services because of perceived disapproval. However, in various parts of Africa, traditional healers often may be consulted for severe malaria. In one site in rural Tanzania in the early 1990s, 90 percent of the children under 5 who died from acute febrile illness with seizures, died at home. Most (85 percent) had been seen by some kind of traditional healer, and only about half had been to a formal health facility before they died (de Savigny et al., 1999).
Weaknesses of Malaria Treatment
People seeking treatment for malaria through any of the channels described here often are frustrated by their experiences. In both the public and private sectors, patients may be faced with low quality of care, lack of drugs or poor quality drugs, and unpredictable costs, to name just a few of the problems. All of these problems affect patients, but they also are concerns for health care systems. Some of the key problems are enumerated below.
Issues in Diagnosis
- Where diagnosis is based on symptoms alone, overdiagnosis, with resultant overuse of antimalarial drugs and real causes of illness possibly left untreated (Stein and Gelfand, 1985; Olivar et al., 1991; Guiguemde et al., 1997).
- Where parasitologic diagnosis is attempted, poor equipment, lack of supplies, limited training and supervision of laboratory staff, and inapropriate use of diagnostic results in treatment choices (Palmer et al., 1999; Barat et al., 1999).
- Lack of accurate microscopy, and for rapid diagnostic tests, the difficulty of determining whether a parasitic infection that is detected is the cause of the current illness. Especially in highly endemic areas, diagnosis based on symptoms may be equally valid.
Lack of Effective Drugs and Inappropriate Use of Drugs
- Chloroquine remains the first-line drug, and sometimes the only antimalarial stocked in health centers and hospitals, even in some countries with very high rates of chloroquine resistance.
- Health facilities frequently are out of stock of essential drugs including antimalarials, and the malaria peak in some countries coincides with the end of the financial year, exacerbating the problem at a key time.
- Second- and third-line drugs may not be made available to lower level health facilities, despite the high frequency of treatment failure with the first-line remedy.
- “Polypharmacy”—prescribing of multiple, unnecessary drugs in addition to the antimalarial is common. As well as being wasteful, it may increase the risk of side effects.
- Where patients have to pay for drugs, they may buy only some of the products prescribed, or incomplete doses, including the antimalarial itself. They may buy a complete course, but take only the first part (particularly if they feel better), storing the remainder for a subsequent episode, leading to systematic underdosing and encouraging the emergence of resistance.
- Expensive (and possibly more dangerous) formulations may be used in place of cheaper ones: in a study in Ghana, 42 percent of chloroquine prescribed to outpatients was administered by injection rather than orally (Ofori-Adjei and Arhinful, 1996).
- Poor quality drugs are common in retail pharmacies in Africa and Asia, due both to lack of quality control in manufacture and to degradation during storage (Shakoor et al., 1997; Maponga and Ondari, 2003).
- Counterfeit drugs are an enormous problem in tropical countries. Fake antimalarials have been a particular problem recently in Southeast Asia. For example, until a recent publicity campaign, more than half of the antimalarial drugs available in the private sector in Cambodia were fake. Widespread counterfeiting of artesunate has led to erroneous reports of resistance (Newton et al., 2001).
When patients buy their own drugs through shops or drug sellers, they may use their own judgment about how much to take (or how much they can afford to buy), and they also may get information from the sellers which may or may not be accurate. As an example of what takes place:
- In a survey in Kenya, only 4 percent of children given store-bought chloroquine got an adequate dose, and only half of those children received this dose over the recommended 3-day period (Marsh et al., 1999).
- In the same survey, aspirin was widely used and 22 percent of children received potentially toxic doses (Marsh et al., 1999).
How Quality Issues Affect Patients
Patients factor in what they know from past experience and general community knowledge when they decide on malaria treatment. In one rural area in Tanzania, the most common reason people gave for not using government services was the poor drug supply. At the same time, people report that public clinic staff are often rude and insensitive, and there are long waiting times to be seen. The facilities themselves often are in poor condition, discouraging people from coming (Gilson et al., 1994).
Costs are sometimes unpredictable in public facilities. Providers may add on charges for drugs that ought to be covered by the consultation fee. If there are no drugs in the clinic, the patient may have to pay for the consultation, and then be told to purchase the drugs privately.
Private clinics and other outlets have their problems too. While staff attitudes may be better and waiting times shorter, private facilities may not have as wide a range of equipment or trained staff as in the public sector (Silva et al., 1997; Mutizwa-Mangiza, 1997). And patients are aware they are paying higher prices for private services, which may make them skeptical about the motivations of private providers, balancing the welfare of patients against their own profits (Silva et al., 1997; Smithson et al., 1997).
DRUG FLOWS AND PRICING OF ANTIMALARIALS
Examples from Senegal, Zambia, and Cambodia
It is easy to find out that the prices people pay for drugs in many countries vary tremendously, but not so easy to know the pathways that drugs travel and the costs incurred along the way. Descriptions of the flow of drugs from manufacturer to consumer, and the price mark-ups that accompany each transaction, are scarce or nonexistent for Africa and Asia. The IOM Committee asked Management Sciences for Health (MSH) to document the flow and prices of antimalarial drugs in a sample of countries. The information summarized here is taken from their report (Shretta and Guimier, 2003).
MSH gathered information directly for this report in Senegal, Zambia, and Cambodia, all poor countries where malaria is a major health problem (see Table 1-1 for basic economic and health indicators for the three countries).
TABLE 1-1
Economic and Health Indicators in Senegal, Zambia, and Cambodia.
Malaria in Senegal, Zambia, and Cambodia
The entire population in Senegal is considered to be at risk of malaria; the disease is mesoendemic in some areas to hyperendemic in others. Malaria is the leading cause of morbidity, in 1996, the reason for 32 percent of outpatient consultations (27 percent for those under 5 years age, 39 percent for those over 5 years).
Malaria is endemic throughout Zambia and continues to be the leading cause of morbidity and mortality, especially among pregnant women and children under age 5. Malaria accounts for 36.7 percent of all outpatient attendance, 62.1 percent of inpatient admissions, and 10.3 percent of pregnant women seen at health facilities.
About two million of Cambodia's 12 million population are at risk of malaria. The worst affected are ethnic minorities, temporary migrants, settlers in forested areas, plantation workers and others who live in the country's hilly forested environments and forest fringes. In 1995, malaria and fever accounted for a quarter of outpatient attendances (42 percent in highly malarious areas), 6 percent of hospital admission (over 30 percent in malarious areas), and 15 percent of reported deaths (over 50 percent in endemic areas).
Drug Flows
In all three countries—and typical of most other malaria-endemic countries in Africa and Asia—drugs flow into the country and to consumers through the usual public and private sectors, and also through various illegal channels. In each sector, drugs flow into the system from large importers and some local manufacturers, who supply outlets lower down in the chains. Drug flows in each country are depicted schematically in Figures 1-2, 1-3, and 1-4.
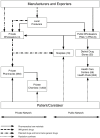
FIGURE 1-2
Organization of distribution networks for antimalarial drugs in Senegal, excluding the parallel market.
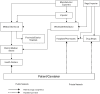
FIGURE 1-3
The distribution of pharmaceuticals in Zambia.

FIGURE 1-4
Flow of drugs in Cambodia, including public and private sectors.
Public Sector Drug Flows
Senegal
The Senegalese Pharmacie Nationale d'Approvisionnement (PNA), a state-owned enterprise, is responsible for importing, storing, and distributing the drugs on the national essential drug list. The drugs flow from the PNA downward through several levels of the public sector and are accessible to consumers in a variety of places, but always involve interactions with health care providers (physicians, midwives, nurses, or health workers.
The PNA supplies five regional stores (Pharmacie Regionale d'Approvisionnement [PRA]), which in turn supply the district depots, which then provide drugs to the health facilities. Similarly, health centers and health posts are supplied by the district depot. Health huts obtain their supplies from the posts. Overall, the public sector accounts for about 35 percent of the sales value of antimalarials.
Zambia
Most pharmaceuticals are imported from India (some also come from Zimbabwe, Tanzania, Kenya, and South Africa) and most come in as finished products, which are exempt from import duties and taxes. Six local companies manufacture generic essential drugs in Zambia (one meets international Good Manufacturing Practice standards). No multinational pharmaceutical companies produce drugs in Zambia.
All drugs on the national essential drug list, including antimalarials, are procured by the Central Board of Health, either by international competitive bidding or by selective tender. Preference (including a 15 percent price advantage) is given to locally manufactured or procured drugs. Local companies are rarely awarded large tenders, however, because they cannot meet some of the conditions.
All drugs procured by, or on behalf of, the government are delivered to Medical Stores Ltd. (the country's Central Medical Stores). Public sector drugs and medical supplies are delivered to all districts, nine general hospitals, and four specialized hospitals. When Medical Stores Ltd. cannot supply all the needs of districts, they purchase their own drugs to fill gaps.
The public sector is more important in Zambia than in many other countries. Up to 70 percent of people seeking treatment for malaria go first to the public sector. However, private sector use is increasing and use of public health facilities is decreasing, in part due to only partially effective exemption mechanisms for the poor and frequent drug shortages in the public sector.
Cambodia
Most pharmaceuticals (public and private sector) are imported, with some manufactured locally. About 25 percent of all legal pharmaceuticals imports come in through the public sector (through 14 companies in 2001) (SEAM, 2001). Six local manufacturers produce more than 26 essential drugs, including chloroquine. Blister packaging of A+M tablets and Malarine (a coformulation of the same two drugs, artesunate and mefloquine) is carried out by Cambodian Pharmaceutical Enterprise with support from WHO.
Public sector drug procurement and distribution are centralized and, since 2002, procurement has been through an open tender system. Drug needs for malaria are determined by the National Malaria Center (CNM), which informs the Central Medical Stores how much of each drug to send to each province. Drugs are supplied quarterly to all 73 referral hospitals and more than 700 health centers across the country. They are distributed by motor vehicles and in areas isolated during seasonal flooding, by boat. Road conditions generally are poor, which impedes distribution.
Private Sector Drug Flows
Senegal
Four private wholesalers feed into 650 pharmacies that sell drugs to consumers and also, in rural areas, to private chemists who sell, officially, a limited line of medications to consumers (however, much more variety is usually available). These networks represent nearly 65 percent of the total sales value of antimalarials in the country (excluding the illegal market). The private market is efficient at maintaining stocks, especially for urban pharmacies, which may be supplied daily.
Most of the products in the private sector come from France and are marketed under brand names, but essential generic medications—mostly locally produced—are sold under standard International Nonproprietary Names (INNs). Since June 2003, private networks also have distributed generic medications under INNs that they purchase at the PNA, which appear on the limited list of thirty medications, including three antimalarials (chloroquine tablets and two dosages of injectable quinine).
Zambia
About 50 registered pharmaceutical wholesalers and distributors import drugs into Zambia to supply the private market, which consists of private clinics, hospitals, and retail pharmacies. Unlike the situation in Senegal, the private sector cannot purchase drugs from the public sector to sell in private outlets.
Private distributors deliver drugs only within Lusaka, the capital, and to towns on the railway line to the Copper Belt and the Southern province. Depending on the location of the provider, a drug may go through two or three wholesalers before it arrives at its final place of sale.
Drug Flow through the Churches Health Association of Zambia
The Churches Health Association of Zambia (CHAZ)—the largest private not-for-profit organization in Zambia—includes more than 100 church-administered hospitals, health centers, and community programs, which operate as part of the public health system. Mission hospitals serve as district hospitals where there is no Ministry of Health hospital, mostly in poor rural areas.
CHAZ procures drugs quarterly, using a restricted tender system from suppliers pre-qualified by WHO. CHAZ is exempt from registration fees and various duties on imported drugs and medical equipment. The main suppliers are the International Dispensary Association in the Netherlands and Mission Pharma, in Denmark. CHAZ handles about 90 products, all of them on the Zambian National Formulary. About 55 of the 100 mission hospitals and health centers in Zambia regularly procure drugs from CHAZ, which they purchase directly from the warehouse in Lusaka.
Cambodia
The private sector—particularly drug sellers—are the first point of contact for more than 70 percent of the population when they are ill (Ministry of Health of Kingdom of Cambodia, 1998), and 75 percent of legal antimalarials are sold through the private sector. Self-treatment is common among the poor, and for many people indigenous or traditional healers remain the first point of contact. The private sector is largely unregulated for price and quality, and there are no accurate records of volumes of antimalarials (or other drugs) sold.
Social Marketing
A social marketing program for blister-packed mefloquine plus artesunate, branded as “Malarine,” and a “dipstick” diagnostic test for P. falciparum, has been scaled up across the country (in all but two provinces), following a European Union pilot program in selected areas. Vendors who have signed a franchise agreement are provided with promotional posters, package dispensers, and a start-up supply of free packages and tests. A suggested retail price of R7,900 (price printed on the package) includes a profit of R1,000 for the franchiser. In practice, higher prices are charged. The blister-packed drugs are sold on the basis of a positive dipstick test available for US$0.55. The dipstick price was designed to be included in the price for Malarine, but it is sold separately by retail outlets.
Drug Flows through the Illegal Sector
Senegal
Parallel to the public and private legal networks is a large, thriving illegal market, believed to account for millions of dollars worth of drugs. The components of the illegal market are the traditional market (“sidewalk medication”) found in cities in the interior and the Touba market, a highly organized illegal market providing pharmaceuticals at a wholesale and retail level. The illegal market is allegedly supplied from many sources, including organized smuggling networks, the private sector, leakage from the public sector, occasional diversions from the Dakar port or airport, illegal or declared imports of donations collected in France and in other countries in Europe by nongovernmental organizations (NGOs), and resale of prescribed medications not taken by patients (full or partial doses). Prices in the illegal market can be 30 percent lower than in the private sector, which makes them attractive to middle class as well as poorer customers, with or without a prescription.
Zambia
Apart from the registered suppliers, drugs are also supplied by some “cross border” traders (usually illegally) within the Common Market for Eastern and Southern Africa (COMESA) and the Southern African Development Cooperation Conference (SADCC) region.
Cambodia
The illegal market is supplied largely by pharmaceuticals smuggled across the border from neighboring countries, sold by an estimated 2,800 illegal drug sellers nationwide (Ministry of Health of Kingdom of Cambodia, 2001b). In Phnom Penh, there are an estimated 495 illegal drug sellers, compared with 298 registered pharmacies and drug sellers.
Availability of Antimalarials
Senegal
The public network provides four antimalarial drugs (amodiaquine, chloroquine, quinine, and SP), which come in nine different presentations, forms, and dosages. In the private sector, there are 13 compounds sold in 89 presentations, forms, and dosages.
Zambia
In the face of widespread chloroquine resistance, in 2003, Coartem was named as the official first-line drug. Coartem has been introduced into pilot districts, but because of cost, SP (which is cheaper), is available in most other places. Although no longer provided through the public sector, there are still considerable sales of chloroquine in the private sector. In addition, more than a hundred different brands, presentations, and forms of antimalarials are available in the private market as branded products, generics, and branded generics, representing about 14 different compounds. Artemisinin products are freely available without prescription in this sector, as monotherapies and combinations.
Cambodia
More than 100 brands and forms of antimalarials are found in Cambodia's private sector outlets, where patients receive treatment based on their ability to pay. The public sector distributes mainly generic products, about 8 antimalarials in various forms and strengths. In total, 30 antimalarials are officially registered.
A combination of artesunate and mefloquine (A+M) was introduced as the first-line drug in 1999, with a recommendation that all first-line drugs be based on a diagnosis by either microscopy or a rapid dipstick test. The combination is available as “A+M” in the public sector and is socially marketed in the private sector as “Malarine.” Dipsticks also are socially marketed in the private sector.
Drug Pricing
In all three countries, in addition to transport, clearing charges and any taxes that may be added to manufacturing prices (in Senegal this is 10 percent and 1.5 percent), drug importers add a margin, which includes distribution costs. In Senegal, the markup by the public wholesaler was 13 percent, and by the private wholesaler, 9.9-24.6 percent, depending on the type of medication. District store markup in the public sector was typically 15 percent. In Zambia, wholesalers usually added a markup of 20-30 percent, and in Cambodia, this ranged from 1.82-49.57 percent. In Senegal, the Bamako pharmacies add margins of 30.5 percent in the public sector, while pharmacies and retailers in the private sector add margins of 9.9-56.2 percent. In Zambia, private pharmacies and retailers generally add a margin of 30-40 percent; in Cambodia these ranged from 1.85-2300 percent. These mechanisms, based on cumulative margins, lead to an almost doubling of the cost of entry prices (Table 1-2 lists consumer prices for selected antimalarials in the three countries, in some public and private sector outlets.)
TABLE 1-2
Consumer prices of selected antimalarials in three countries for a typical adult course of treatment (in US$).
Drug Financing
Senegal
More than 90 percent of drug costs are paid by consumers, either directly, in the private pharmacies or in public health facilities through the cost recovery system, or indirectly, through mandatory or voluntary contribution systems.
Zambia
Drugs in the public sector are financed by a combination of government funds and user fees. The fees vary according to the type of facility, the area of the country, and the patient. The poor and certain population groups (children under 5, people over 65), as well as certain services (e.g., antenatal care, tuberculosis treatment, childhood vaccination) are officially exempt from fees in all health facilities, but the system is not implemented well. Where fees are paid, the usual markup on drugs is about 30 percent, similar to the private sector.
In public hospitals, consultation fees vary from free to ZK5,000 (about US$1). In Lusaka and other urban areas, health centers charge membership (averaging ZK5,000 in Lusaka and ZK1500 in Kafue), which entitles the payer to treatment without further charges. Health centers elsewhere usually provide consultation services and drugs free of charge.
During 2000/1 CHAZ procured drugs, medical supplies, and vaccines valued at ZK379 million (US$95,000) with its own funds, and received donations valued at ZK9.55 billion (US$2.5 million). User fees, similar to those charged at public facilities, are charged at various mission hospitals.
Cambodia
More than 80 percent of health care is paid for directly by households and about 45 percent of households borrow money to pay medical bills. Formal user fees have been in place since 1997, with a required exemption for the poor. Since user fees were introduced, the quality of the service has improved, and use by the poor has increased. The exemptions function relatively well at the health center level, but not at referral hospitals, where staff discourage their use (Wilkinson et al., 2000).
The government accounts for less than 5 percent of health sector financing (Ministry of Health of Kingdom of Cambodia, 2002a,b). Pharmaceutical expenditures by the Ministry of Health Procurement Unit for the public sector in 2000 was approximately US$14.2 million (SEAM, 2001).
Donor funding, from governments and NGOs, amounts to 15 percent of total health financing (Ministry of Health of Kingdom of Cambodia, 2001b), and 25 percent of pharmaceuticals (SEAM, 2001). Donor resources (as are other public-sector resources) are allocated disproportionately to the capital area, where relatively few of the poor live (World Bank, 1999).
Common Themes and Contrasts
A wide variety of antimalarial drugs—more than those officially registered—can be bought for a wide variety of prices in these countries. One way or another (whether through a consultation fee or direct purchase), consumers pay a large proportion of the cost of antimalarial drugs. The private market prices are invariably higher than those in the public sector. Prices in the illegal sector may be among the lowest. A factor not mentioned earlier is that counterfeit drugs of all types are also common, and they are found not only in the private and illegal sectors, but in public sector facilities.
In Senegal and Cambodia, the private sector dominates the provision of antimalarial drugs, but in Zambia, the public sector is the main source of care for malaria, including provision of drugs. International donors, church-based clinics and hospitals, and other not-for-profit organizations all are established and play varying roles in the health care systems of these countries, making the flow of drugs even more fragmented and complex.
The extent of the illegal sector, the presence of many unregistered products even in the legal private sector, and the widespread problem of counterfeit drugs gives some sense of the difficulty of regulating drugs in these countries and their neighbors in Africa and Asia. While governments work toward greater control—with the aim of more rational drug use by their citizens—the current mix of distribution systems will not be changed quickly.
INTERNATIONAL ORGANIZATIONS AND MAJOR INITIATIVES IN MALARIA CONTROL
Global health programs have recognized malaria as a priority for decades. The most visible efforts have come through national malaria control programs with international support centered around the World Health Organization and its regional offices, but with considerable bilateral assistance from a number of countries. Particular lines of research have been driven consistently by funding from a select group of national science agencies including those in the United States, United Kingdom, France, and China; and from foundations, in particular, the Wellcome Trust and the Rockefeller Foundation. With sponsorship from several U.N. agencies, the WHO-housed Special Programme for Research and Training in Tropical Diseases (WHO/TDR) has worked for the past 28 years to develop and promote the best use of pharmaceuticals and other malaria control measures. In the last 10 years or so, the number of organizations and collaborations devoted to malaria has grown impressively (Table 1-3). The boost in funding from the Bill & Melinda Gates Foundation has had a major impact on existing initiatives, in taking new directions in malaria research and control, and in raising the public profile of malaria. The Global Fund to Fight AIDS, Tuberculosis and Malaria has also provided a central focus for international funding.
TABLE 1-3
Selected Organizations and Initiatives for Malaria Research and Control of Recent Origin.
The programs and initiatives most directly relevant to this study— related to the development and deployment of effective antimalarial drugs— are described briefly below.
TDR: The Special Programme on Research and Training in Tropical Diseases
Since it was started in 1975, the Special Programme on Research and Training in Tropical Diseases (TDR; a joint program of UNICEF, the World Bank, the U.N. Development Program and WHO, and housed at WHO) has organized research and development for new antimalarials, as well as supported research on the best ways to encourage the use of effective interventions of all types. TDR has leveraged its own modest budget by recruit ing collaborators and orchestrating larger projects. Among their accomplishments related to malaria are:
- several antimalarials brought through the R&D process, including key work on artemisinins and ACTs. TDR brought partners together, funded preclinical work, and orchestrated clinical trials;
- pivotal field trials for insecticide-treated bed nets, supporting research on better methods for treating nets, and encouraging industry to develop longer-lasting treated nets; and
- research on treatment-seeking and demonstrating the value of home treatment.
TDR remains active in malaria research (one of 10 diseases in its portfolio) and is a partner in many of the newer initiatives, including the Medicines for Malaria Venture (MMV), which has now taken the lead in R&D for new antimalarials (discussed in detail in chapter 10).
Roll Back Malaria (RBM)
Malaria was a top priority of Dr. Gro Harlem Brundtland when she assumed the leadership of WHO in 1998, convinced by African leaders that malaria was a heavy burden on the health and economies of many countries. The Global RBM Partnership was created to “reduce the burden of malaria throughout the world.” It was developed not as a WHO program, but as a loose confederation of endemic country governments, other UN organizations, bilateral aid donors, foundations, NGOs, community groups, research and teaching institutions, and others—all the relevant parties in malaria control. A small Secretariat within WHO was to serve the needs of RBM, with leadership drawn from the external partners. Although not without problems, RBM is the most significant development in consolidating worldwide efforts to control malaria since the end of the era of malaria “eradication” in the 1960s (which largely bypassed Africa).
The African summit on Roll Back Malaria, held in Abuja, Nigeria in April 2000 is an RBM milestone. The summit was attended by delegates from 44 of the 50 malaria endemic countries in Africa, including 19 heads of state and many other high-level officials of member countries. Top-level representation from WHO, the World Bank, the African Development Bank, and several other major donors helped to further raise the profile of the meeting and underscore the importance of tackling the malaria problem. Hundreds of millions of dollars in assistance were pledged (although most did not materialize). Ambitious, but potentially achievable targets for the year 2005 were adopted by the summit participants (Table 1-4).
TABLE 1-4
The Abuja Summit Targets, April 2002.
The RBM External Evaluation
In early 2002, several of the “core” RBM partners commissioned an evaluation of the organization and its progress by a seven-member team of experts, led by Richard Feachem (who shortly thereafter was named head of the Global Fund for AIDS, TB and Malaria). The evaluation, published in August 2002 (Feachem, 2002), documented both achievements and “serious constraints” that threaten progress in phase 2, defined as mid-2002 to 2007.
The accomplishments include raising the profile of malaria and increasing global funding—funding for malaria control doubled in RBM's first 3 years. The constraints centered on a lack of progress in getting activities under way quickly at the country level. RBM may not be able to achieve a “significant reduction in the global burden of malaria by 2007” given the pace of progress, which could undermine the partnership if not rectified.
Three major reforms and two tactical changes were recommended:
Major Reforms
- Reorganization of the RBM Secretariat
- Creation of an independent governance board
- Reconstitution of the Technical Support Network
Tactical Changes
- Selection of 8 to 12 focus countries that show a high degree of commitment and can make rapid progress in the next 3 years
- Appointment of Country Champions to provide dynamic leadership in these focus countries
Even before the final report was issued in August 2002, RBM was reorganized along the lines recommended. It is too soon to know whether these changes will lead to the success envisioned by the evaluation team, but major efforts are being made toward that end. If the recommendations in this report are implemented, RBM's ability to make rapid progress will get a large boost, particularly in increasing access to effective drugs.
The United Nations Millennium Development Goals
At the United Nations Millennium Summit in September 2000, world leaders agreed to a set of goals and targets to combat the world's ills: poverty, hunger, disease, illiteracy, environmental degradation, and discrimination against women. All 189 Member States of the United Nations adopted the eight broad “Millennium Development Goals” (MDGs), with timetables for achieving them by 2015. One of the goals names malaria specifically, and two others are directly relevant. These MDGs are:
- Reverse the spread of diseases, especially HIV/AIDS and malaria.
- Reduce under-5 mortality by two-thirds.
- Reduce maternal mortality by three-quarters.
The UN secretary general and the administrator of the UN Development Programme created the Millennium Project to recommend the best strategies for achieving the MDGs. Ten task forces of global experts were established to identify operational priorities, organizational means of implementation, and financing structures necessary to achieve the MDGs. Task Force 5 is focused on HIV/AIDS, malaria, TB, other major diseases and access to essential medicines, and a subgroup focused specifically on malaria. Each task force is to complete its report by the end of 2004, and a final synthesis of recommendations will be presented to the UN secretary general and the chair of the UN Development Group by June 30, 2005.
The issues addressed by the IOM Committee on the Economics of Antimalarial Drugs are directly relevant to the MDGs, and should complement the work of the malaria subgroup of Task Force 5.
The Global Fund
The Global Fund to Fight AIDS, Tuberculosis and Malaria (the “Global Fund”) began operation just 2 years ago, in 2002. The idea of a centralized fund to help combat the three most devastating infectious diseases in countries without the resources to do so was first raised at a meeting in July 2000 of the Group of Eight (G8) leading industrialized countries. It gained currency through further discussions and meetings, and was solidified by United Nations Secretary-General Kofi Annan at the African Summit on HIV/AIDS in Abuja, Nigeria, in April 2001, who called for the creation of a global trust fund for the three diseases.
As of July 2003, total pledges by donors to the Fund amounted to US$4.7 billion. Forty governments have pledged 98 percent of the funds, and 2 percent come from the private sector. By February 2004, a total of US$245 million in Global Fund resources had been disbursed to about 120 countries. In the first two rounds of grants, 21 percent went for malaria programs (59 percent for HIV/AIDS, 19 percent for tuberculosis). Over the first three rounds, a minority of funding was destined for purchase of ACTs—less than US$20 million, and less than the amount allocated for purchases of chloroquine and SP (Attaran et al., 2004). This reflects the requests of countries to the Global Fund, which does not dictate how countries should approach malaria control. However, the international health financing community—of which the Global Fund is a major part— has significant influence on what countries plan by way of disease control and what they ask for. Given the level of funding of the Global Fund itself, countries have made realistic requests, which may not include large quantitites of ACTs, because the price would make such requests nonviable. Countries also are concerned about how sustainable a system built on ACTs would be after Global Fund grants run out (if not renewed).
A principle by which the Global Fund operates is that their grants are not fungible: they must augment existing funding for the three diseases and not supplant them. Evidence that allocations for malaria from other sources are being reduced is grounds for the Global Fund to terminate a malaria grant. This principle acknowledges the historic underinvestment in malaria and the other diseases. Strictly speaking, it also can be seen as limiting the endemic countries' ability to freely allocate resources according to their own priorities, while signalling the global acceptance—of both donor and recipient countries—that only with greater resources is there hope of making progress against these diseases. With continued and increasing donor contributions, the Global Fund has the potential to be a major positive force for malaria control.
REFERENCES
- Ahorlu CK, Dunyo SK, Afari EA, Koram KA, Nkrumah FK. 1997. Malaria-related beliefs and behaviour in Southern Ghana: Implications for treatment, prevention and control. Tropical Medicine and International Health 2(5):488-499. [PubMed: 9217705]
- Attaran A, Barnes KI, Curtis C, d'Alessandro U, Fanello CI, Galinski MR, Kokwaro G, Looareesuwan S, Makanga M, Mutabingwa TK, Talisuna A, Trape JF, Watkins WM. 2004. WHO, the Global Fund, and medical malpractice in malaria treatment. Lancet 363(9404):237-240. [PubMed: 14738799]
- Barat L, Chipipa J, Kolczak M, Sukwa T. 1999. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? American Journal of Tropical Medicine and Hygiene 60(6):1024-1030. [PubMed: 10403337]
- Berman P, Nwuke K, Rannan-Eliya R, Mwanza A. 1995. Zambia: Non-Governmental Health Care Provision . Boston: Harvard University International Health Systems Program.
- Breman JG, Egan A, Keusch GT. 2001. Introduction and summary: The intolerable burden of malaria: A new look at the numbers. American Journal of Tropical Medicine and Hygiene 64(1-2 Suppl):iv-vii. [PubMed: 11425185]
- Brugha R, Chandramohan D, Zwi A. 1999. Viewpoint: Management of malaria—working with the private sector. Tropical Medicine and International Health 4(5):402-406. [PubMed: 10402978]
- Chima RI, Goodman CA, Mills A. 2003. The economic impact of malaria in Africa: A critical review of the evidence. Health Policy 63(1):17-36. [PubMed: 12468115]
- De Savigny D, Setel P, Kasale H, Whiting D, Reid G, Kitange H, Mbuya C, Mgalula L, Machibya H, Kilima P. 1999. Linking demographic surveillance and health service needs: The AMMP/TEHIP experience in Morogoro, Tanzania . Presentation at MIM African Malaria Conference, Durban, South Africa.
- De Savigny D, Mwageni E, Mayombana C, Masanja H, Minhaj A, Momburi D, Mkilindi Y, Mbuya C, Kasale H, and Reid G. 2003. Care seeking patterns in fatal malaria: Evidence from Tanzania. Paper commissioned by the Institute of Medicine, Washington DC.
- Deming MS, Gayibor A, Murphy K, Jones TS, Karsa T. 1989. Home treatment of febrile children with antimalarial drugs in Togo. Bulletin of the World Health Organization 67(6):695-700. [PMC free article: PMC2491321] [PubMed: 2633884]
- Ejezie GC, Ezedinachi ENU, Usanga EA, Gemade EII, Ikpatt NW, Alaribe AAA. 1990. Malaria and its treatment in rural villages of Aboh Mbaise, Imo State, Nigeria. Acta Tropica 48(1):17-24. [PubMed: 1980800]
- Feachem R. 2002. Final Report of the External Evaluation of Roll Back Malaria . Liverpool: Malaria Consortium.
- Gilson L, Alilio M, Heggenhougen K. 1994. Community satisfaction with primary health care services: An evaluation undertaken in the Morogoro region of Tanzania. Social Science and Medicine 39(6):767-780. [PubMed: 7973873]
- Guiguemde TR, Ouedraogo I, Ouedraogo JB, Coulibaly SO, Gbary AR. 1997. [Malaria morbidity in adults living in urban Burkina Faso]. [French]. Medecine Tropicale 57(2): 165-168. [PubMed: 9304011]
- Guimier JM, Candau D, Garenne M. 2001. Etude Sur L'Accessibilité Aux Médicaments Au Sénégal—LEEM (France)-Ministere De La Santé Du Sénégal . Dakar: Ministry of Health.
- Hanson K, Goodman C, Lines J, Meeks S, Bradley D, Mills A. 2004. The Economics of Malaria Control Interventions. Geneva: Global Forum for Health Research, World Health Organization;
- Makemba AM, Winch PJ, Makame VM, Mehl GL, Premji Z, Minjas JN, Shiff CJ. 1996. Treatment practices for degedege, a locally recognized febrile illness, and implications for strategies to decrease mortality from severe malaria in Bagamoyo district, Tanzania. Tropical Medicine and International Health 1(3):305-313. [PubMed: 8673832]
- Maponga C, Ondari, C. 2003. The Quality of Antimalarials: A Study in Selected African Countries WHO/EDM/PAR/2003.4 . Geneva: World Health Organization.
- Marsh VM, Mutemi WM, Muturi J, Haaland A, Watkins WM, Otieno G, Marsh K. 1999. Changing home treatment of childhood fevers by training shop keepers in rural Kenya. Tropical Medicine and International Health 4(5):383-389. [PubMed: 10402975]
- McCombie SC. 1996. Treatment seeking for malaria: A review of recent research. Social Science and Medicine 43(6):933-945. [PubMed: 8888463]
- Ministry of Health of Kingdom of Cambodia. 1996. Health Policy and Strategies 1996– 2000 . Presentation at the meeting of the Meeting on Malaria Programme. Brussels: Ministry of Health, Kingdom of Cambodia.
- Ministry of Health of Kingdom of Cambodia. 1998. National Health Statistics 1998 . Phnom Penh: Ministry of Health, Kingdom of Cambodia.
- Ministry of Health of Kingdom of Cambodia. 2000. Cambodia's Health Sector Performance Report 2000 . Phnom Penh: Ministry of Health, Kingdom of Cambodia.
- Ministry of Health of Kingdom of Cambodia. 2001. a. Country Update on Malaria Control, Kingdom of Cambodia . Presentation at the meeting of the 4th Global Partners Meeting, Washington, DC, April 18-19, 2001. Prepared by the National Malaria Control Programme, Ministry of Health, Kingdom of Cambodia.
- Ministry of Health of Kingdom of Cambodia. 2001. b. Joint Health Sector Review Report . Phnom Penh: Ministry of Health, Kingdom of Cambodia.
- Ministry of Health of Kingdom of Cambodia. 2002. a. Health Sector Strategic Plan 2003-7 . Phnom Penh: Ministry of Health, Kingdom of Cambodia.
- Ministry of Health of Kingdom of Cambodia. 2002. b. Ministry of Health Expenditure Report 2000 . Ministry of Economy and Finance statement to fiscal working group by Minister of Health, April 23, 2002; Cambodia Development Council (CDC) estimates of external aid, April 2002. Phnom Penh: Ministry of Health, Kingdom of Cambodia.
- Ministry of Health of Senegal. 2000. Senegalese Survey of Health Indicators. Dakar: Senegal Ministry of Health.
- Ministry of Health of Zambia. 2003. Request for Proposals for the Management of MSL. TB/ SP/029/03 . Lusaka: Zambia Ministry of Health.
- Mnyika KS, Killewo JZJ, Kabalimu TK. 1995. Self-medication with antimalarial drugs in Dar es Salaam, Tanzania. Tropical and Geographical Medicine 47(1):32-34. [PubMed: 7747329]
- Mtango FDE, Neuvians D, Broome CV, Hightower AW, Pio A. 1992. Risk factors for deaths in children under 5 years old in Bagamoyo District, Tanzania. Tropical Medicine and Parasitology 43(4):229-233. [PubMed: 1293726]
- Muela SH, Ribera MJ. 2000. Illness naming and home treatment practices for malaria—an example from Tanzania. Paper presented at workshop on People and Medicine in East Africa. Basel, Switzerland: Swiss Tropical Institute.
- Mutizwa-Mangiza D. 1997. The opinions of health and water service users in Zimbabwe. Working paper. International Development Department, University of Birmingham, Birmingham, UK. [Online]. Available: http://www
.idd.bham.ac .uk/research/Projects /Role_of_gov/workingpapers/paper24 .htm. - Mwabu GM. 1986. Health care decisions at the household level: Results of a rural health survey in Kenya. Social Science and Medicine 22(3):315-319. [PubMed: 3961547]
- Mwenesi H, Harpham T, Snow RW. 1995. Child malaria treatment practices among mothers in Kenya. Social Science and Medicine 40(9):1271-1277. [PubMed: 7610432]
- Newton P, Proux S, Green M, Smithuis F, Rozendaal J, Prakongpan S, Chotivanich K, Mayxay M, Looareesuwan S, Farrar J, Nosten F, White NJ. 2001. Fake artesunate in Southeast Asia. Lancet 357(9272):1948-1950. [PubMed: 11425421]
- Ofori-Adjei D, Arhinful DK. 1996. Effect of training on the clinical management of malaria by medical assistants in Ghana. Social Science and Medicine 42(8):1169-1176. [PubMed: 8737435]
- Olivar M, Develoux M, Chegou Abari A, Loutan L. 1991. Presumptive diagnosis of malaria results in a significant risk of mistreatment of children in Urban Sahel. Transactions of the Royal Society of Tropical Medicine and Hygiene 85(6):729-730. [PubMed: 1801337]
- Palmer K, Schapira A, Barat L. 1999. Draft position paper prepared for informal consultation on “Malaria diagnostics at the turn of the century.” Presentation at the meeting of the role of light microscopy and dipsticks in malaria diagnosis and treatment. A joint meeting of TDR, Roll Back Malaria and USAID. Geneva, Switzerland.
- SEAM. 2001. Strategies for Enhancing Access to Medicines. Country Assessment: Cambodia . Arlington, VA: Management Sciences for Health.
- Shakoor O, Taylor RB, Behrens RH. 1997. Assessment of the incidence of substandard drugs in developing countries. Tropical Medicine and International Health 2(9):839-845. [PubMed: 9315042]
- Shretta R, Guimier J-M. 2003. Flow of antimalarial drugs in the public and private sector, affordability and discussions of potential strategies to improve financial access. Paper commissioned by the Institute of Medicine.
- Silva T, Russell S, Rakodi C. 1997. The opinions of health and water service users in Sri Lanka. Working paper. International Development Department, University of Birmingham, Birmingham, UK. [Online]. Available: http://www
.idd.bham.ac .uk/research/Projects /Role_of_gov/workingpapers/paper25 .htm. - Smithson P, Asamoa-Baah A, Mills A. 1997. The case of the health sector in Ghana. Working paper. International Development Department, University of Birmingham, Birmingham, UK. [Online]. Available: http://www
.idd.bham.ac .uk/research/Projects /Role_of_gov/workingpapers/paper26 .htm. - Snow RW, Peshu N, Forster D, Mwenesi H, Marsh K. 1992. The role of shops in the treatment and prevention of childhood malaria on the coast of Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 86(3):237-239. [PubMed: 1412642]
- Stein CM, Gelfand M. 1985. The clinical features and laboratory findings in acute Plasmodium falciparum malaria in Harare, Zimbabwe. Central African Journal of Medicine 31(9):166-170. [PubMed: 3910259]
- United States Embassy. 2003. [Online]. Available: http://usembassy
.state.gov/Cambodia [accessed 2003]. - WHO (World Health Organization). 2003. More equitable pricing for essential drugs: What do we mean and what are the issues? In: WHO Commission on Macroeconomics and Health, Norway . Background paper for the WHO-WTO secretariat workshop on differential pricing and financing of essential drugs, Høsbjør, Norway, April 8-11, 2001. World Health Organization. [Online]. Available: www
.who.int/medicines /library/edm_general /who-wto-hosbjor/equitable_pricing.doc. - WHO Regional Office for South-East Asia. Malaria: Issues and Challenges. [Online]. Available: http://w3
.whosea.org/mal/issues.htm [accessed April 7, 2004]. - WHO/UNICEF. 2003. The Africa Malaria Report 2003 . Geneva: World Health Organization.
- Wilkinson D, Holloway J, Fallavier P. 2000. Findings from the Evaluation of User Fees, Categorized by Evaluation Objectives . Draft study to evaluate the impact of user fees on access, equity and health providers' practices. Phnom Penh, November 2000.
- Winch PJ, Makemba AM, Kamazima SR, Lurie M, Lwihula GK, Premji Z, Minjas JN, Shiff CJ. 1996. Local terminology for febrile illnesses in Bagamoyo district, Tanzania and its impact on the design of a community-based malaria control programme. Social Science and Medicine 42(7):1057-1067. [PubMed: 8730911]
- World Bank. 1999. Volume Two: Main Report. Poverty Reduction and Economic Management Sector Unit, East Asia and Pacific Region. Cambodia: Public Expenditure Review . Washington, DC: The World Bank.
- World Bank. 2002. World Development Indicators . Washington, DC: The World Bank.
- World Bank. 2003. World Development Indicators . Washington, DC: The World Bank.
APPENDIX 1A. THE TEHIP STUDY
Why Do They Die? An Inquiry into Treatments Preceding Deaths from Malaria5
A child in rural Tanzania dies from malaria. One can hardly help but envision the cascade of events—what might have happened or not happened—that led to such a sorrowful ending. But guesswork is not an adequate substitute for knowing. The solutions that will keep such children alive and malaria-free depend on understanding why and where the fatal failures of social transactions occurred.
The Research Area
TEHIP studied the circumstances leading to deaths from malaria in the stable, perennial malaria-transmission belt that runs along the coast of Tanzania and up the Rufiji and Kilombero River basins. The transmission risk there is typical of the majority (75 percent) of Tanzania and of sub-Saharan Africa in general (MARA Collaboration, 2003). The population of Rufiji District is 203,000, divided among several ethnic groups. The district occupies 14,500 km2 and is entirely rural, with 94 registered villages.
There are 57 formal health facilities: two hospitals (one government and one NGO), five health centers with inpatient facilities (all government), and 50 dispensaries (46 government). Over-the-counter drugs are available from many private shops and kiosks in the villages. People also consult traditional healers and traditional birth attendants.
Integrated management of childhood illness (IMCI) services, intermittent presumptive treatment (IPT) of malaria in pregnancy, and 1st, 2nd, and 3rd line antimalarial services are available at all formal health services. Within the District, insecticide treated nets (ITNs) are distributed through social marketing.
The Rufiji Demographic Surveillance Site (DSS) covers part of Rufiji District, including 1,800 km2 north of the Rufiji River and west of the Rufiji Delta, with a population of 85,000 in 17,000 households in 31 villages. The DSS registers all births, deaths, in-migrations, out-migrations, pregnancies, and other vital events. Events are detected by 150 village “key informants,” and verified by DSS staff. Twenty-eight full-time enumerators update the population register every 4 months, based on household surveys.
Verbal Autopsies
The information available for each death is gathered during the process of “verbal autopsy” (VA). Once a key informant documents a death in the study area, a DSS VA supervisor, who is also a trained clinical officer or health officer, conducts the VA interview using standard questionnaires that are designed specifically for deaths of: a) children under 31 days of age; b) children under 5 years but 31 days and older; and c) population aged 5 years and above. The questionnaires and responses are in Kiswahili, the common language of the area. Open-ended and closed questions are asked on the history of events leading to death, as well as about previously diagnosed medical conditions, and signs and symptoms before death. Use of health facilities prior to death, reasons for using or not using a particular health facility, and confirmatory evidence of cause of death (if available) are asked about and recorded. A typical bereavement interview for a VA takes 45 to 60 minutes.
The VA supervisor establishes a tentative cause of death from signs, symptoms, sequence, and severity, as well as any confirmatory evidence. A physician reviews the material and makes the final determination of cause of death that is subsequently entered in the database.
Malaria-Related Deaths
This study covered the period January 1999 through the end of December 2001, during which 3,023 deaths occurred and 2,953 (97.7 percent) verbal autopsies were conducted in the DSS. Of these deaths, 722 which fell under the following codings were suspected to be directly or indirectly due to malaria:
- acute febrile illness 1-4 weeks
- acute febrile illness ≤7days
- acute febrile illness including malaria
- acute febrile illness with seizures
- acute febrile illness with anemia
- cerebral malaria
- fever plus malnutrition
- malaria
- malaria confirmed
- unspecified acute febrile illness
Among these 722, 45.8 percent were male, 54.2 percent female; 44.3 percent were under 5 years of age, 55.7 percent were 5 years or older; 39 (5.4 percent) had convulsions and possible cerebral malaria, while 94.6 percent reported no convulsions.
As a cautionary note, VA methods have distinct limitations. Specifically, not all the cases identified as “malaria” in this series truly represent malaria, especially those with unspecified acute febrile illness at an older age. And undoubtedly, some malaria deaths were coded as nonmalaria. For example, life-threatening anemia, likely due to malaria, is prevalent in young children over 6 months of age in the study area, yet VA coded deaths due to anemia with malaria are infrequent among recorded malaria deaths.
Types of Care Sought
The term modern care describes what conventionally includes biomedical, Western, pharmaceutical, professional, official, or formal health care. Traditional care describes what conventionally includes traditional medicine, traditional healers, traditional providers, lay providers, traditional practices, or folk care. From the VAs, 13 possible sources of treatment were collapsed into six subcategories of provider type:
- 1.
Government
- 2.
Home/Shops
- 3.
Non-Government
- 4.
Traditional Medicine at Home
- 5.
Traditional Medicine at Practitioner
- 6.
No Care
and into three broader subcategories:
- 1.
Modern Care
- 2.
Traditional Care
- 3.
No Care
Of the 722 individuals whose deaths were ascribed to malaria, 626 (87 percent) sought care at least once before death, while 96 (13 percent) did not, or could not, seek care. Treatment usually starts at home using antipyretics and antimalarials from local shops or left over from previous episodes. Malaria is perceived by adult caregivers as a mild disease, and if it becomes serious or life threatening, the presumptive diagnosis changes from malaria to something that is more often treated with traditional medicine or practices. Yet these beliefs are not rigid. Every case is subject to a process of continuing debate and reevaluation within the family. As a result, modern pharmaceuticals also are sought, albeit with delay, when convulsions fail to resolve, or recur after traditional medicine (Oberlander and Elverdan, 2000). Significant switching from one provider to another occurs over time; this phenomenon is most apparent when comparing treatment of malaria without convulsions to malaria with convulsions.
Key Observations
- Cases with convulsions were significantly (p<0.05) more likely to receive care.
- Of the group that did not have convulsions, 91 percent used modern care first, and by the second treatment, more than 99 percent used modern care. Hence traditional care delayed modern care for 9 percent of cases.
- Of the group that had convulsions, 90 percent used modern care first, but only 63 percent continued with modern care for a second treatment.
- Government health facilities and shopkeepers were the main source of antimalarial drugs. This pattern held up over broad age groups, sex, and socioeconomic status. (At the time of the study, all government providers had adequate drug supplies, which could be a factor in their popularity. The first-line drug was chloroquine, however, at a time when drug resistance was already widespread in this area.)
Other Relevant Factors
In this study, modern care was more popular than previous reports would suggest. But some part of that modern care involved self-treatment and a variety of private providers. Knowledge of appropriate treatment regimens is lacking on the part of the public as well as the private providers (Brugha et al., 1999; Marsh et al., 1999), and under-dosing in home-based care is common. These may account for at least some treatment failures.
The only other study of health-seeking patterns in a large series of VAs is from the mid-1980s from the Bagamoyo District of Tanzania, another district in the Coast Region (Mtango et al., 1992). In that series, malaria deaths were not analyzed separately, but government providers were the choice in only 45 percent of all deaths. A preference for traditional healers was cited by 41 percent of mothers as the reason for not using government services. At that time, it was also true that government providers were often without adequate drug supply.
Why Did They Die?
The study results to date cannot answer that question, but they begin to narrow the possibilities. Some form of care was sought in the majority of malaria deaths (88 percent). No care was sought for 12 percent of child deaths. Included among these were sudden deaths following apparently mild illness, which may have been impossible to prevent under the circumstances. This pattern is considerably better than was seen in the mid-1980s in Bagamoyo, when 55 percent of children who died had not utilized any modern care (Mtango et al., 1992), and better than for all deaths in the same area during the same period when 20 percent of all-cause deaths had no prior health-seeking events (Tanzania Essential Health Interventions Project and Ministry of Health of Tanzania, 2002).
With belief systems for malaria treatment-seeking now firmly on the side of modern care, there is obviously something still failing in the transaction to obtain this care, or in the quality of the care and referral once it is accessed. TEHIP is continuing to refine the information from this study, and will be analyzing the narrative portion of the verbal autopsy questionnaires to look at some specific aspects of the health care received, including:
- delay in seeking modern care (e.g., tried to treat at home without antimalarials, no transport, beliefs, poor recognition of severity, lack of confidence in modern care, no power to decide, insufficient finances);
- delay in receiving modern care (e.g., after working hours, weekends, long queues, satisfaction); and
- ineffective modern care (e.g., poor communication, no referral, drugs not available, abusive health worker, non-compliant providers, poor adherence of patients).
All of these factors could have contributed to failed treatment. The study, as yet, can provide no direct information on the possible contribution of drug resistant malaria to these deaths.
The current results suggest that policies and efforts aimed at improving early recognition of symptoms and danger signs at home, prompt treatment or treatment-seeking, compliance with the full course of treatment, and the quality of the antimalarial available are highly justified.
REFERENCES
- De Savigny D, Mwageni E, Mayombana C, Masanja H, Minhaj A, Momburi D, Mkilindi Y, Mbuya C, Kasale H, and Reid G. 2003. Care seeking patterns in fatal malaria: Evidence from Tanzania. Paper commissioned by the Institute of Medicine.
- MARA Collaboration. 2003. MARA LITe for Africa CD version 3.0.0 . South Africa: Medical Research Council of South Africa.
- Mtango FDE, Neuvians D, Broome CV, Hightower AW, Pio A. 1992. Risk factors for deaths in children under 5 years old in Bagamoyo District, Tanzania. Tropical Medicine and Parasitology 43(4):229-233. [PubMed: 1293726]
- Oberlander L, Elverdan B. 2000. Malaria in the United Republic of Tanzania: Cultural considerations and health-seeking behaviour. Bulletin of the World Health Organization 78(11):1352-1357. [PMC free article: PMC2560636] [PubMed: 11143196]
- Tanzania Essential Health Interventions Project, Ministry of Health of Tanzania. 2002. Burden of Disease Profile 2001—Coastal Zone . Dar es Salaam: Ministry of Health of Tanzania.
Footnotes
- 1
SP and Lapdap both are made up of two drugs but are not considered combinations in the sense of ACTs because the mechanisms of action of the two drugs are linked, and the individual drugs are not fully effective antimalarials.
- 2
Chapter 6 gives a detailed account of the disease and its effects, which are summarized briefly here.
- 3
Not all verbal autopsies use the exact method described here. Procedures for gathering information and for assigning the recorded cause of death may differ.
- 4
This section is drawn largely from an in-depth review by Hanson et al. (2004).
- 5
Based on an analysis prepared for this study (de Savigny et al., 2003).
- Malaria Today - Saving Lives, Buying TimeMalaria Today - Saving Lives, Buying Time
Your browsing activity is empty.
Activity recording is turned off.
See more...