NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009.
15.1. INTRODUCTION
The purpose of this chapter is to describe the use of optical methods to record, in a noninvasive fashion, intrinsic signals related to neuronal activity (i.e., fast optical signals). Fast signals have been used to study non-invasively the time course of activity in localized cortical areas [1–2], and, more recently, from the entire cortical surface [3]. In this chapter, we will first present a brief introduction to noninvasive functional imaging, and describe different approaches that can be used in this area of research. We will then describe the rationale and procedures related to the use of optical methods to create functional images of brain activity. We will then discuss examples of applications of fast optical imaging. We will conclude the chapter by discussing some advantages and limitations of the technique.
15.1.1. Fast Optical Signals and the Event-Related Optical Signal (EROS)
Fast optical signals are recently developed imaging methods whose purpose is to provide spatio-temporal maps of the transmission of near-infrared (NIR) light through the intact human brain. With this method, NIR light is shone through the scalp and skull, and changes in either the time taken by the photons to move through the head tissues [1] and/or in the amount of light reaching the detector [4] are observed. These two parameters (i.e., the photons’ time-of-flight and the light intensity) are influenced by the scattering and absorption properties of the tissue. Of particular importance here is the observation that the scattering properties of brain tissue vary concurrently with neural activity in the tissue [5–7]. Because optical measures are relatively localized (to volumes of just a few mm in diameter), it is possible to use these methods to derive estimates of the time course of activity in specific brain areas. The recording of fast optical signals can be time-locked to particular events (such as stimuli or responses), hence the name event-related optical signals, or EROS. The EROS technique has both advantages and limitations that will be described later in this chapter.
15.1.2. Why Non-invasive Functional Brain Imaging?
The past 20 years have seen a phenomenal expansion of noninvasive functional brain imaging [8]. One of the major advantages of neuroimaging is the possibility of measuring in vivo physiological signals from various brain areas in normal human subjects. This possibility is of great significance for scientists and clinicians alike. Of course, there are also limitations attached to the use of these measures: at present, noninvasive imaging techniques only allow us to measure the cumulative physiological response of macroscopic areas of the brain (thus summarizing the activity of thousands of neurons at a time), and cannot provide data about the activity of individual neurons. Further, experimental lesion studies are not possible, and it is therefore difficult to ascertain causal links between the activity observed in various brain areas and behavioral and physiological outcomes, although the use of transcranial magnetic stimulation (TMS [9]) may allow us to narrow down or experimentally test some of these links.
15.1.3. Types of Functional Imaging Methods
Several types of noninvasive functional methods are now available. These methods differ on a number of dimensions, most notably the type of signal that is imaged to obtain estimates of brain activity. Most of the experimental work in functional neuroimaging is based on imaging either hemodynamic/metabolic or neuronal signals (although other types of functional imaging signals, such as receptor–ligand distribution, have also been used [10]). Optical methods are unique in that they can be used to visualize both hemodynamic and neuronal signals [6–11]. In the next section we will describe some general principles of each of these two types of signals.
15.1.3.1. Hemodynamic/Metabolic Methods
This class of techniques aims at measuring changes in the concentration of metabolically significant substances during the course of an experiment. These changes in concentration can be inferred directly (e.g., by labeling the substance using radioactive, magnetic, or optical markers) or indirectly (e.g., by studying the effects of a target substance, such as deoxy-hemoglobin, on the magnetic resonance properties of the tissue where the substance occurs naturally). Techniques that allow us to measure hemodynamic/metabolic effects include positron emission tomography (PET [8]), functional magnetic resonance imaging (fMRI [12]), and near-infrared spectroscopy (NIRS [13]). In particular, fMRI has become widely used because of its excellent spatial resolution, its easily attainable coupling with anatomical imaging (structural MRI), and the wide diffusion of the required hardware (as MR scanners are available in most hospitals because of their clinical use). However, most fMRI data do not provide absolute estimates of the concentration of oxy- and deoxy-hemoglobin, but only a derived measure indexing their relative concentration (the blood-oxygenation-level-dependent, or BOLD, signal). Quantitative estimates can instead be obtained using optical spectroscopic techniques [13–14], although this requires solving several practical problems (see chapter on NIRS in this volume).
These hemodynamic/metabolic changes are of interest to neuroscientists and psychologists because they allow us to study not only metabolism and blood circulation in the brain, but also, indirectly, neuronal activity. In fact, it has been known for a long time that blood flow and metabolism increase in brain areas that are active during a particular task (e.g., Grinvald et al. [15]). Thus, hemodynamic/metabolic neuroimaging is based on the assumption that, by studying changes in these parameters, one can infer whether a particular brain area is involved in the neural activity associated with a particular task. Although this is a reasonable assumption in many cases, it also points to a limitation of these techniques as measures of neuronal activity. Namely, the inference of the occurrence of neuronal activity from hemodynamic/ metabolic data is indirect, being mediated by neurovascular coupling. [11,16] This term refers to the set of biochemical and biophysical steps that occur in between the onset of neuronal activity and the physiological (hemodynamic) events that follow it, and that are ultimately measured. Neurovascular coupling complicates the measures for two reasons: (1) it introduces an inherent delay in the physiological variable that is measured (e.g., change in the concentration of deoxy-hemoglobin) with respect to the phenomenon that is inferred (i.e., neuronal activity), which, in turn, limits the temporal resolution of the method, and (2) neurovascular coupling may not be constant, but may in fact vary as a function of the state of the organism, of the brain area from which the measures are taken, and of individual and group variables [17].
15.1.3.2. Neuronal Methods
Another type of signal used in functional neuroimaging is related to the movement of ions through and around the cell membranes, which occurs during neuronal activity. There are several methods that can be used to record ion-movement noninvasively. Some methods rely on the changes in electro-magnetic fields that can be detected from sensors located outside the head. Examples of these techniques are the electroencephalogram (EEG [18]), evoked and event-related potentials (EPs and ERPs [19]), and magnetoencephalography (MEG [20]). Note, however, that differently from single- and multiple-units activity measures obtained with implanted micro-electrodes, noninvasive electromagnetic measures taken at a distance (e.g., from the surface of the head) cannot provide information about the activity of individual neurons, but need to rely on the spatial and temporal summation of the fields generated by individual neurons. Spatial summation only occurs if the individual neurons are oriented in a consistent direction (with respect to their dendritic field–axon axis), generating an “open-field” configuration [21]. Thus, only a portion of the neuronal activity generated in the brain can be studied with these methods. In the case of the cerebral cortex, electromagnetic methods are most sensitive to the activity of pyramidal neurons (the output cells of the cortex), because of the size, prominence, and orientation of their dendritic trees. They are also most sensitive to the postsynaptic activity measurable in the dendrites rather than to the all-or-none activity typical of axons, because of the comparatively large size of the dendritic fields and the longer duration of post-synaptic potentials. Therefore, the effects observed with these methods differ from those observed with single-unit methods (which do not have a directional bias and are based mostly on action potential measures in axons and/or in the vicinity of cell bodies), but are more similar to field potentials recorded invasively. Further, the spatial resolution of general purpose 3D-reconstruction algorithms for electromagnetic measures, allowing investigators to reconstruct the distribution of the activity inside the head from surface measures, is limited [22] and/or needs to rely on assumptions that are often difficult to verify [23]. Optical methods, reviewed in the next section, provide a complementary approach for studying neuronal and hemodynamic activity and their coupling.
15.2. PRINCIPLES OF FAST OPTICAL IMAGING
15.2.1. The Scattering Signal
This book reviews techniques that use optical methods for studying brain function. Several optical signals can be used. This chapter focuses on signals that are temporally concurrent with neuronal changes. Changes of this type have been reported elsewhere in this book. They can generally be described in terms of changes of the scattering coefficient of neural tissue when the tissue is active. These changes have been demonstrated in individual axons [5], brain slices [6], and depth optical recording in animals [7]. For instance, Rector et al. [7] developed a methodology for recording changes in the transparency of rat hippocampus or other deep brain structures in vivo. They showed that stimulation of the Schaeffer’s collateral elicits a change in the transparency of the CA1 field whose latency is consistent with that of the evoked potential from CA1 recorded with electrophysiological methods. They attributed these effects to changes in the scattering properties of this area, presumably associated with the migration of ions through the neuronal membrane. Similarly, the same group reported fast and localized intrinsic optical signals from the barrel cortex of rats in response to single-whisker mechanical stimulation [24].
Although there is strong evidence for the existence of scattering changes concurrent with electrically recorded neuronal activity, the exact causes of these changes are still under investigation. Two phenomena are considered as potential causes: (a) changes in neuronal membrane conformation related to the difference in potential between intra- and extra-cellular compartments; [25] and (b) volumetric changes in the intra- and extra-cellular compartments due to the movement of ions and associated water molecules during cell depolarization or hyperpolarization. Recent data suggest that the former mechanism is probably strictly related to birefringence changes, whereas the scattering changes are more likely related to the volumetric phenomena [26].
15.2.2. Non-Invasive Measurement of Optical Properties
15.2.2.1. Continuous vs. Time-Resolved Measures
Although scattering changes accompanying neuronal activity have been demonstrated for quite some time using invasive techniques, only recently methods have become available for their non-invasive measurement. Because of the high absorption by hemoglobin and water at other wavelengths, noninvasive measurements of the human brain are restricted to the near-infrared range. Further, the scattering-based functional changes are relatively small, and require appropriate instrumentation for their measurement. Two types of measures have been used: Measures of delay in the flight of NIR photons through active areas (delay measures [1]), and measures of the amount of light moving through active areas (intensity measures [4]). In heterogeneous surface-bound media such as the head, both delay and intensity measures are sensitive to both absorption and scattering changes, although in a different manner [1,27]. In addition, these measures differ in terms of spatial resolution, relative sensitivity to deep and superficial events, and type of noise or artifact contamination.
Intensity measures are simpler to obtain and model than photon-delay measures. The simplest recording apparatus (the optode) consists of a source of NIR light, which can be connected to the head using an optic fiber, and a light measurement instrument, which can also be connected to the head using an optic fiber. Several types of light sources can be used, including incandescent lamps, light emitting diodes (LEDs), laser diodes, and regular lasers. Several types of detectors can also be used, including light-sensitive diodes, photo-multiplier tubes, CCD-cameras, and so on. Irrespective of the sources and detectors used, it is important that environmental light sources be controlled or excluded. This can be achieved either by insulating the detector instrument (and the head) from environmental light sources (e.g., Steinbrink et al. [4]) or, preferably, by labeling the instrumental light source in a special manner. This can be achieved, for example, by modulating the light source at a particular frequency (e.g., Wolf et al. [28]).
Photon-delay measures are more complex, because the delays to be measured (and their changes) are of the order of picoseconds or fractions thereof. The measurement requires that the light-source vary over time. This can be achieved by making the light-sources either pulsate or oscillate in intensity at a very high frequency. The latter measures are more convenient in terms of cost.
Time-resolved measures are used in NIR spectroscopy because they make the computation of the absolute concentration of oxy- and deoxy-hemoglobin easier [29]. However, if the main interest of the investigation is in detecting changes (such as those related to neuronal events), this particular advantage of time-resolved measures may be less important. Another differentiation between measures obtained with continuous light (i.e., measures of tissue transparency, usually labeled intensity measures) and time-resolved measures (i.e., measures of modulation and delay of the light waves or pulses when passing through the head tissue) is their relative sensitivity (or lack thereof) to environmental light, movement artifacts, and to activity in superficial (i.e., skin and skull) compared to deep (i.e., intracranial) head layers. Continuous measures cannot distinguish between light coming from the instrumental source and light coming from other environmental sources. Modulated or pulsated sources make this distinction possible because they produce light that changes with a particular frequency and/or time course. Time-of-flight measures are relatively insensitive to movements, which may cause changes in the coupling between the optodes and the head, and may have strong effects on intensity measures [30]. Finally, time-of-flight measures are more sensitive to deep effects [27,31–32] than intensity measures.
15.2.2.2. Penetration and 3D Reconstruction
Noninvasive measures are obtained from instruments placed on the surface of the head. A central issue for this research is to determine which parts of the brain are responsible for the effects that are observed. Photons injected in a particular point of the head (a scattering medium) propagate randomly through the tissue. However, because a large number of photons are always involved, the propagation of the photon population can in principle be expressed mathematically, using equations that describe the statistical volume explored by each source-detector pair. Any change in the measurement parameters can then be probabilistically ascribed to this volume. These equations are relatively simple for surface-bound homogenous media. In this case, photons moving between a particular source and detector (both located on the surface of the medium at a certain distance from each other) are most likely to be contained within a volume shaped as a “curved spindle” whose extremes are located at the source and detector (see Figure 15.1). The spatial gradients defining this spindle (which is important in defining the spatial resolution of the technique) depend on various factors, including the parameter (intensity or photon delay) used for the measurement. In fact, these gradients are sharper for measures of photon delay than for measures of the amount of light transmitted (continuous measures). Thus, photon delay measures possess a higher spatial resolution. In addition, they may have effects that are of opposite sign for very superficial events (occurring in the skin) rather than in deep layers (occurring in the brain [27]).
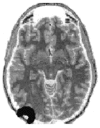
FIGURE 15.1
Schematic representation of the volume in the brain that is explored by a particular source-detector pair located in left occipital areas. (From Gratton, G. and Fabiani, M. Int. J. Psychophysiol., 42, 109–121, 2001. With permission.)
An important consideration is that head tissues vary considerably with respect to their optical properties (scattering and absorption). For instance, bone has a relatively high scattering coefficient but a low absorption coefficient compared to the gray matter. The cerebrospinal fluid is almost transparent to light, but the subarachnoid space in which it is contained is traversed by a dense web of blood vessels. Thus, within this space, NIR light can travel without much resistance in a direction perpendicular to the surface, but can only travel very little in a direction parallel to the surface, as it will be absorbed. For this reason, the subarachnoid space has little influence on the transmission of light through the head [32]. The opposite occurs with the white matter, which has a very high scattering coefficient, and whose effect is to reflect most of the light reaching it. The heterogeneity of the optical properties of the head tissues has the consequence of deforming the volume investigated by a particular source-detector pair [27,32]. The effects of this distortions appear to be relatively moderate (a few mm) at source-detector distances of less than 5 cm [33], but could potentially be larger at longer source-detector distances.
The maximum depth at which fast (scattering-dominant) optical effects can be studied noninvasively is largely determined by the source-detector distance. For homogenous media, the maximum depth is between half and a quarter of the source-detector distance [34]. However, the sensitivity to phenomena occurring at different depths varies depending on the type of measure used. Measures of amount of light transmission are more sensitive to superficial phenomena, whereas measures of photon-delay are more sensitive to deep phenomena [27,32,34]. This may lead to discrepancies in the results obtained with these two sets of measures.
Various investigators have reported methods for the 3D reconstruction of photon migration data [35–43]. Most methods are based on finite element models of the propagation of photons through tissue, and use an iterative process to determine the scattering and absorption coefficients of various compartments of the tissue itself. These models are therefore computation-intensive.
We have so far used a simpler approach [44] consisting of approximating the path between a particular source-detector pair using a spindle-shaped volume (to be scaled in size depending on the source-detector distance). This approach introduces approximations in the 3D reconstruction of the order of several mm, but may be adequate for many practical applications and is computationally very simple. Gratton et al. [33] reported data comparing estimates of the depth of regions of brain activity obtained using this approach and fMRI. The study was based on manipulating the eccentricity of visual stimuli, which influences the depth of the region of medial occipital cortex where activity should be observed (i.e., more eccentric visual stimuli are processed in deeper areas of the calcarine fissure than more central visual stimuli). Optical imaging data were recorded using several source-detector distances, and the distance at which the largest effect was observed was used to estimate the depth of the optical effect (estimated as half of this distance). Functional MRI data were also recorded from the same subjects, using the same paradigm. Results indicate that the two estimates differ, on average, by less than 1 mm. This provides support for the simplified approach to the estimation of the depth of the optical effects (and therefore to 3D reconstruction) described above.
15.2.2.3. Data Collection and Artifact Correction
There are at present several companies that produce recording instruments suitable for recording fast optical data. These instruments are capable of collecting measures of the intensity and/or delay of the photons reaching the detector at a very fast pace (up to more than 1 kHz). Multiple-channel systems are available (up to 64 sources and 32 detectors). The use of multiple channels may involve time-multiplexing to distinguish the signals associated with different source-detector pairs. Time-multiplexing may reduce the temporal resolution of the instrument. All systems are designed to be movable (and are therefore potentially portable) and use low-energy nonionizing radiation, which makes them safe and suitable for extended and/or repeated recordings with the same subject. Also, most systems allow for an external event channel or at least for external triggering, so as to enable synchronization with the system producing the stimulation and/or recording the subject response. This allows for the recording of event-related brain activity (EROS).
In addition to the fast neuronal signals, the raw data obtained with optical recording systems (and in particular the intensity data) carry a number of other signals. These signals are related to (a) arterial and capillary pulse (about 1 Hz); (b) respiration (about 0.25 Hz); and (c) pressure waves in the blood vessels (about 0.1 Hz). These signals (which can be of interest in a number of cases) are considerably larger than the neuronal signal and can in fact obscure it. Thus, they should be treated as artifacts if the research focuses on the recording of neuronal signals. These artifacts are quite evident for intensity data, but less so for time delay data, which are less sensitive to superficial phenomena (and therefore less sensitive to capillary pulse). Some of these artifacts (as well as slow drifts related to heating or cooling of the apparatus) can be eliminated using appropriate high-pass filters. The pulse artifact is more problematic, because its frequency is closer to that of the neuronal signals. However, this artifact (which is ubiquitous in intensity recordings) generates very characteristic sawtooth waves. The shape regularity of this artifact can be used to devise algorithms for its correction. Gratton and Corballis [45] proposed one such method, which is now used in several laboratories.
Other types of artifacts are due to movements of the subjects or of the optical apparatus. These movements produce large signals, due to variations in the interface between the measurement instruments (e.g., optic fibers) and the head, which are temporally well localized and can be discarded using automatic procedures [30]. In addition, these artifacts are most evident in intensity measures, but produce very limited effects on photon delay measures, most likely because the latter reflect the differential proportion of photons with long and short diffusive paths within the head, rather than their total amount. In Figure 15.2 we report an example of the head gear (a modified motorcycle helmet) that we use to hold the source and recording fiber into place, so that the coupling between the optic fibers, and the head is firmly in place.

FIGURE 15.2
Example of head gear (a modified motorcycle helmet) that is used to hold in place the source and detector fibers.
15.2.2.4. Signal Processing and Statistical Analysis
The recording apparatus described above yields time series that can be time-locked to particular events (i.e., to stimuli and/or to the subject’s responses to the stimuli). These time series can be processed with the standard methodologies used for analyzing other types of time series, such as those obtained with ERPs or fMRI recordings. A simple way of extracting the fast optical signal is by averaging the time-locked waveforms obtained for particular stimuli or response conditions. More complex analysis procedures can also be used, such as cross correlation, wavelet analysis, combined time-frequency analysis, and so on. In addition, low- and high-pass filtering can be used to improve the signal-to-noise ratio [46]. Statistics about the reliability of the observed effects can then be computed either within or across subjects, using standard statistical methods. Fabiani et al. [47] have shown that nonparametric statistics, based on bootstrap methods, can also be useful to determine the reliability of effects.
As mentioned above, data from a single source-detector pair (or channel) refer to a curved spindle-shaped volume with vertices at the source and detector. These data, therefore, provide an estimate of the origin of the signal. Given the variability in cortical anatomy across individuals, it is necessary to record from multiple surface locations to make sure to “hit” the active area in a particular subject, as well as to refer the functional data to the underlying structural anatomy of the individual (see Whalen et al. [48]). Whenever multiple recordings are obtained, it is important to establish that effects are not due to chance, as the probability of capitalizing on chance of course increases with the number of locations. This can be achieved in several ways:
- Bonferroni-corrected confidence intervals or p-values can be computed. Our current software uses a more refined version of this correction procedure, based on the application of Gaussian field theory to the computation of number of independent comparisons [49]. This, in turn, is used to compute p-values corrected for multiple comparisons.
- An alternative approach is to first compute an omnibus statistics, across all locations, and then, if the omnibus effect is significant, to determine the exact location at which the effect is maximum. Bootstrap methods may be useful for this second step [47].
- Finally, a multivariate approach can be used to deal with the problem of multiple comparisons, or at least to minimize the number of locations investigated [50].
When collapsing data across subjects, it is important to consider that recordings from the same surface location (identified using scalp landmarks) in different subjects may not correspond to the same functional cortical area in all subjects. The earliest studies using optical imaging data by and large ignored this issue, which may however lead to a marked reduction of the signal-to-noise ratio (because active locations from one subject may be averaged with inactive locations from another subject). This problem is not unique to optical imaging, as it is typical of any brain imaging technique with a spatial resolution higher than a few cm. Several investigators have proposed a number of tools for realigning and rescaling brain and cortical areas in different subjects. In principle, all of these tools can also be applied to EROS data. However, this requires that (1) the exact locations used for each source and detectors be recorded within a fiducial space (this can be achieved by using a magnetic 3D digitizer, such as the Polhemus [7] 3D digitizer system), (2) the same fiducial locations (e.g., nasion and preauricular points) be marked on structural MR images (e.g., by using vitamin E pills or other markers), (3) a method is used to co-register the digitized data about the location of the sources and detectors and the subject’s head (see Whalen et al. [48]), (4) a 3D reconstruction method be used to determine the volume from which data are recorded from, separately for each individual subject (see description above), (5) data from different source-detector pairs whose investigated volume overlap at least in part be combined to improve spatial resolution and signal quality (an example of this approach is given by the pi-detector proposed by Wolf et al. [28]), and (6) the 3D reconstructed data from different subjects be aligned and rescaled using one of the procedures described in the literature (the simplest of which is the Talairach transformation [51]). Once these transformations have been obtained, data from different subjects can be pooled together, and between-subjects statistics can be computed. Figure 15.3 shows digitized sources and detectors overlaid on a subject’s structural MRI (A), projected onto the brain with scalp and skull removed (B) and showing overlapping “spindle” volumes used for 3D reconstruction (C).
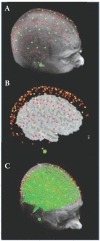
FIGURE 15.3
(A) Digitized sources (red dots) and detectors (yellow dots) overlaid on a subject’s structural MRI; (B) Sources and detector projected onto the brain with scalp and skull removed; (C) Overlapping “spindle” volumes used for 3D (more...)
15.3. EXPERIMENTAL RESULTS
There are currently more than 30 published reports on peer-reviewed journals, coming from eight different laboratories of original studies investigating fast optical signals. All but one [52] reported the observation of a fast optical signal, although the methods used in different studies varied significantly. The majority of these studies has been conducted in our laboratory, and has used photon-delay measures as the main dependent variable. Our major goal has been to show that the fast optical signal can be used to study noninvasively the time course of activity in selected cortical areas, although our recent work has extended the measurement to progressively larger portions of the cortex, and most recently to the entire cortical surface [3]. To support this claim, we have run experiments demonstrating the following:
- Rapid changes in the optical signal can be recorded immediately after stimulation (within 100 ms or less) or immediately before a motor response. This is essential to demonstrate that a fast optical signal occurs. The short latency of the signal makes it very unlikely that it can be due to hemodynamic effects, which take several hundred milliseconds to develop [53].
- Similarly, when recording fast signals with NIR light of two wavelengths, such as 690 and 830 nm, which are located at opposite sides of the isosbestic point for oxy- and deoxy-hemoglobin, the EROS data show effects in the same direction [54]. This again indicates that the signal is due to scattering changes (and therefore of neuronal origin) rather than to rapid deoxy-genation phenomena (such as the initial dip reported with high-field fMRI by Ugurbil and colleagues [55]—and in exposed cortex optical imaging studies—e.g., Malonek and Grinvald [53]) as the latter should result in effects of opposite sign at these wavelengths.
- These changes only occur if a stimulus is used, which is appropriate for eliciting activity in that particular area of cortex, whereas other stimuli, even within the same modality but not expected to activate that particular cortical region do not elicit a response. (For instance, when stimulating the right visual field effects should be visible in the left occipital cortex.) This is important to demonstrate that the phenomenon is specific to particular cortical regions and not a general or artifactual response (such as one due to movements, global arousal phenomena, etc.).
- These changes are temporally overlapping with other neuronal signals, such as ERPs, recorded concurrently from the same subjects and conditions [56–58]. This is important if one intends to use these measures to study the time course of neuronal activity. The comparison can also help narrow down the time intervals of interest (much in the same way that regions of interest can be established in the spatial domain—see point E).
- The fast optical effects are localized to brain areas where hemodynamic measures with high spatial resolution (such as fMRI) indicate that neuronal activity occurs [59–60]. This is important if these measures are used as indices of local neuronal activity in selected cortical areas.
- Similar effects are recorded from different areas, when different stimulus or response modalities are used. This is important to demonstrate that fast optical signals are not the property of one particular cortical area, but are a general property of the cortex. This, in turn, allows us to generalize our studies to different domains.
We will now describe some of the findings obtained with EROS in various modalities and paradigms. Figures 15.4–15.7 show examples of data obtained in these studies.
![FIGURE 15.4. EROS recorded from medial occipital areas during two visual stimulation experiments using the same paradigm [1].](/books/NBK20223/bin/ch15f4.gif)
FIGURE 15.4
EROS recorded from medial occipital areas during two visual stimulation experiments using the same paradigm [1]. (A) Diagram of the stimulation conditions. The stimulation consisted of reversals of black-and-white grids occurring every 500 ms; only one (more...)
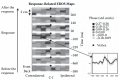
FIGURE 15.5
Maps of the optical activity preceding and following a motor response. The maps are presented as if viewed from the top of the head. The brain area contralateral to the responding hand is presented on the left, and that ipsilateral to the responding hand (more...)
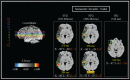
FIGURE 15.6
Statistical maps of EROS data (modified from Tse, C.-Y. et al., Imaging the cortical dynamics of language processing with the event-related optical signal, Proc. Natl. Acad. Sci. USA, 104, 17157, 2007. With permission). Left panel: z (top–bottom) (more...)
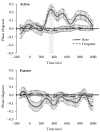
FIGURE 15.7
EROS waveforms (with standard error bars) for rare/deviant (solid) and frequent (dashed) tones under active (top) and passive (bottom) instruction conditions for the voxel of peak activity in frontal cortex (Talairach coordinates: x: 24, y: 42) in an (more...)
15.3.1. Visual Modality
A large number of studies on the fast signal have been conducted in the visual modality. With one exception [52], these studies indicate that a fast optical signal can be recorded in response to visual stimuli. Recordings have been made from both medial and more lateral occipital areas. The first study [1] was based on the reversal of one of four grids placed in different quadrants of the visual field. In this study, the reversals occurred every 500 ms. The original study was based on a small sample size (N = 3),* but the results were replicated in a subsequent study using a larger sample size (N = 8, Gratton and Fabiani [61]). A comparison of the results of these two studies is presented in Figure 15.4. Both studies indicated the existence of a fast optical effect consisting of an increase in the photon delay that occurred approximately 100 ms after stimulation (grid reversal). This effect was localized, in that it occurred at locations placed on the contralateral hemisphere to where the stimulation occurs. Further, stimulation of upper quadrants resulted in larger effects at lower locations in the occipital cortex (and vice versa), in a manner consistent with the well-known representation of the visual field in medial occipital cortex. The location of the response of our original study was found to correspond closely with that of the hemodynamic response measured with fMRI, whereas the time course was similar to that of the visual evoked potential (VEP [59]).
Our 1995 study, as well as the subsequent 2003 replication shown in Figure 15.4, were based on a fixed interstimulus interval (ISI). It is therefore theoretically possible that the observed response could be due to a time-locked response to some previous stimulation. However, two subsequent studies rule out this possible confound. In one of these studies [11] (see also Gratton et al. [54]) we systematically varied the ISI from 100 to 1000 ms; in each case, a response (characterized by an increase in photon delay) was observed with a latency between 60 and 100 ms from stimulation. In fact, the latency of the response increased slightly with the ISI. The same study also indicated that the fast optical response increased in amplitude with ISI, in a manner consistent with that of the concurrently recorded VEP. Increases in phase delay with similar peak latency (60–100 ms) were also reported in several other studies [33,62–63]. In all cases, when the stimulus was unilateral, this fast response was only observed in the hemisphere contralateral to stimulation, thus confirming the spatial localization of the response. Further, we found that the more lateral the area stimulated, the larger the source-detector distance at which the response (i.e., an increase in photon delay) was observed [33]. This is consistent with the idea that lateral stimuli elicit activity in deeper cortical areas than foveal stimuli, consistent both with fMRI findings obtained in the same study and with the well-known functional organization of medial occipital cortex. The latency of the optical effects is also generally consistent with what is known about the visual system. For instance, typically stimuli with abrupt onset tend to elicit activity with a shorter latency (about 60–80 ms) than isoluminant alternating stimuli (about 80–100 ms). This is also generally consistent with what is observed for VEPs. In some of our studies the recording was extended to more lateral occipital areas. Fast optical responses could also be recorded from these areas with a latency similar or only slightly longer than in more medial areas [50,59,64]. Finally, several studies showed optical responses with much longer latencies (200–300 ms [1,61,63,65]). These responses were often ipsilateral or bilateral, perhaps reflecting subsequent feedback processes from other cortical or subcortical areas.
In summary, a number of studies indicate the presence of a fast optical response to visual stimuli in medial occipital areas, characterized by an increase in phase delay with a latency of 60–100 ms from stimulation. The localization of this response is consistent with the well-known representation of the visual field in medial occipital cortex, and the temporal properties of the signal are consistent with the time course of neuronal signals, such as the VEP.
15.3.2. Auditory Modality
Several studies have been completed in the auditory modality by our lab and others [57–58,66–69]. Most of these studies are based on passive auditory oddball paradigms. In these studies, the subjects read books (or perform other tasks) while series of tones are presented to them via headphones. The tones vary along some dimension. For instance, in our first paper [66], a tone could be either long (75 ms duration) or short (25 ms duration). The long duration tone occurred 80% of the times, and was labeled the “standard” stimulus. The short duration tone occurred 20% of the times, and was labeled the “deviant” stimulus. The ERPs elicited in this paradigm have been the subject of extended investigation [70], and are typically characterized by two types of activities. First, there are some responses that are elicited by all stimuli (although their amplitude is larger for stimuli with higher overall intensity). They are labeled exogenous potentials, the most evident of which is the N1 (which is probably itself the sum of several components [71]). Second, there are some potentials that are typically generated only by deviant stimuli; among them is the Mismatch-Negativity (MMN [70]), an ERP component with a peak latency of approximately 120–200 ms. There is some uncertainty about whether the N1 and MMN components are generated in the same or in different cortical areas. The Rinne et al.’s study [66] was set up to show (1) that fast optical effects could be recorded from auditory areas, (2) that different optical responses with characteristics similar to those of the N1 and the MMN could be obtained, and (3) whether these two fast optical responses were generated in the same or different areas. The results confirmed the first two hypotheses, and showed that the optical response to deviant stimuli (corresponding to the electrical MMN) was systematically recorded approximately 1 cm below the location where the response to the high-energy standard stimuli (corresponding to the electrical N1) was maximal. The response to the deviant stimuli had a latency of 160–180 ms, whereas the response to the standard stimuli had a latency of 100 ms. These latencies closely correspond to the latencies of the electrical MMN and N1, respectively. Both responses consisted of an increase in the photon delay parameter. The results of subsequent work by our lab and others [57–58,67–69] confirmed and extended those of Rinne’s work [66], and also indicated that the responses to the deviant stimuli occurred later (160–180 ms) and at more ventral locations (1 cm) with respect the responses to the standard stimuli (whose peak latency was 100 ms). Further, anatomical 3D reconstruction of these subsequent studies indicated that the optical responses occurred in superior or medial temporal cortex.
In summary, the data from the auditory modality show that fast optical responses with latencies consistent with those of auditory evoked potentials can be recorded from temporal cortex. Several phenomena can be distinguished on the basis of their locations, time course, and response to experimental manipulations.
15.3.3. Somatosensory Modality
Fast optical imaging in the somatosensory modality has been conducted in several labs, including a study by Steinbrink and colleagues [4], one by Franceschini and Boaz [72], and one by Maclin and colleagues in our lab [56]. Steinbrink’s work was based on a different technique than that used by our group. They used a continuous light source (a lamp), instead of a radio-frequency modulated light source. The measures obtained were changes in the amount of light transmitted from the source to the detector in the period immediately following somatosensory stimulation. Stimulations were brief low-intensity electric shocks delivered to either arm (median nerve stimulation). Continuous measures were obtained from parietal locations. Rapid (60–160 ms from stimulation) changes were observed when the contralateral side was stimulated, but not when the ipsilateral side was stimulated. These data confirm that a fast optical signal can be recorded noninvasively, and that this signal is localized (at least to the contralateral hemisphere). Similar results were obtained by Franceschini and Boaz [72], and by Maclin et al. [56] In the latter study, however, we observed a faster response with a latency of approximately 20 ms, in addition to the 100–200 ms response reported by the other studies. Both of these responses are consistent with those obtained with other technologies, such as ERPs [56].
15.3.4. Motor Modality
We have conducted two studies investigating fast optical signals in the motor modality. One of them was an early study [27] that involved unilateral hand and/or foot tapping at a frequency of 0.8 Hz. In this study, there was no synchronization between the subjects’ movements and the optical recordings, and a single-channel system was used. The analysis was conducted in the frequency domain, and showed significant oscillations of the optical signal (photon delay) at the frequency of tapping and its harmonics. These oscillations were larger on the side contralateral to the tapping limb.
In a subsequent study [73], we recorded fast optical imaging data during a choice reaction time task. This experiment was based on a synchronized multichannel system, which allowed us to generate maps of brain activity at different times with respect to the response. Some of these maps are shown in Figure 15.5. They show that a fast optical signal (consisting of an increase in phase delay, analogous to those observed in the visual and auditory modalities) can be observed in the last 100 ms before the button press on the side contralateral to the responding hand. The time course of the optical response is very similar to that of the Lateralized Readiness Potential (LRP), an electrical measure used to index response preparation [74]. Similar data have been also obtained by Morren et al. [75] and Franceschini and Boas [72]. Morren and colleagues [75] showed that a fast optical signal shortly preceding the response can be observed using either photon delay or intensity as dependent variables, although the phase effects were observed in a much smaller number of channels than the intensity data, presumably reflecting their higher spatial resolution.
These data provide further support to the generality of the fast optical signal as a measure of localized cortical activity. They also indicate that different methodologies can be used to record fast optical signals.
15.3.5. Cognitive Studies
Many of the studies reported in the previous sections were conducted to assess the feasibility of using fast optical signals as a noninvasive index of neuronal function. As such, they used relatively simple sensory stimulation. However, there have been an increasing number of experiments using fast optical signals to investigate a variety of cognitive functions. For instance, EROS has been used to investigate attention effects in early visual cortex [62], visual memory in occipital areas [63,65], conflict conditions in motor cortex [73], sensory memory processes in the superior temporal gyrus [57–58,66–69], and inferior frontal gyrus [67–69], working memory load effects in the middle and superior frontal gyri [76], executive function in prefrontal cortex [3,50,77], and sentence processing in superior temporal and inferior frontal gyrus [78]. The general purpose of these studies has been to investigate variations in the time course of activity in these cortical regions as a function of various experimental manipulations. By and large, the latencies of the brain responses observed in these studies have been consistent with those of ERP responses, which were often recorded concurrently with the optical data. However, the optical data also provided detailed information about the localization of the cortical areas where the effects were observed. In some studies (e.g., Rykhlevsakaia et al. [50]), the data also provided the possibility of studying the relationships between the activities observed in different cortical regions—in other words, studying functional connectivity. We now provide two brief examples of recently published data.
Example 1. Application of EROS to the Study of Sentence Comprehension (Tse et al. [78])
Words that are unpredictable within the sentence context, either in terms of their meaning or their syntax, generate special types of brain responses, as identified with ERP measures. Specifically, Kutas and Hillyard [79] showed that semantically anomalous words (e.g., the last word of the sentence “the pizza is too hot to cry”) elicit a particular ERP component with a latency of 400 ms from word presentation, which they labeled the “N400.” This component was later shown to be elicited by any word providing unpredictable information about the semantic meaning of a particular sentence, and has been considered to be a measure of lexical access (for reviews see [19,80]). Although the N400 has been studied extensively, the exact location(s) in the brain where it is generated is still under some debate. As EROS can provide information about both the temporal and spatial properties of brain activity, we applied it to the analysis of anomalous sentences [78]. Specifically we compared the brain activity elicited by semantically anomalous words (such as the word “cry” in the example above) to that elicited by predictable words in the same sentence positions (such as the word “eat” appearing in the same position), or to words that were syntactically anomalous, although semantically appropriate (these are known to produce a different ERP response, labeled the “P600” [80]). We recorded optical activity from an extended montage covering all of the perisylvian structures in the left hemisphere (an area traditionally linked to both language production and language comprehension). The results (some of which are shown in Figure 15.6) showed that semantically anomalous words elicited a set of specific brain activities in both the superior temporal gyrus and the inferior frontal cortex. These activities were not observed for semantically appropriate words. One of these optical responses occurred with a latency of approximately 400 ms in the superior temporal gyrus. This activity was correlated across subjects with their electrical N400s, which was recorded simultaneously with the optical response. Syntactically anomalous words elicited a different pattern of activities, including activity with a somewhat longer latency in more dorsal areas of the superior temporal cortex. In this case the optical response also correlated both in time and in amplitude (across subjects) with the electrical scalp activity.
Example 2. Application of EROS to the Analysis of Frontal Activity in an Oddball Task (Low et al. [76])
The human brain is fine-tuned to analyze departures from regularities in the environment. For instance, when a series of identical tones are presented, the occurrence of a different tone elicits a very special brain response. However, when the tone series is unattended, this brain response is simpler: ERP studies have shown that this situation elicits a component labeled the Mismatch Negativity (MMN [70]), typically followed by another brain response labeled P3a. Optical imaging studies, some of which conducted in our laboratory, have also found that deviant auditory stimuli generate a response in superior temporal cortex followed by activation in inferior prefrontal cortex [58,66–69]. The brain response, however, is enhanced when the auditory stimuli are relevant to the subject’s task, and are therefore attended. In this case, ERP studies indicate the presence of a large response, labeled the P3 or P300 component (for a review see [19]). The brain origin of this scalp-recorded component is still under some debate, and it is likely that multiple structures may contribute to its generation. In one recently published study, we [76] examined frontal activity elicited by attended and unattended deviant auditory stimuli with EROS. Some of the results of this study are presented in Figure 15.7. They show two different types of responses for attended and unattended deviant items. Whereas a positive response is observed in right dorsolateral prefrontal cortex for task-relevant deviant items, the same area does not respond in the same way to unattended deviant items. The optical prefrontal activity elicited by attended deviant items is simultaneous with early parts of the electrical P300 response. This is consistent with various modeling efforts that suggest that this region may be related to the frontal P3, a subcomponents that is typically observed slightly before the posterior, parietal aspects of the P300. The prefrontal region where the EROS response was observed is also consistent with fMRI activity recorded in similar paradigms [81].
15.4. DISCUSSION
15.4.1. Summary of Empirical Data
In this chapter we have reviewed evidence indicating that it is possible to record fast optical signals using noninvasive procedures. Fast optical signals have been recorded in different modalities (including visual, auditory, somatosensory, and motor) in the context of simple sensory and motor paradigms, as well as more complex cognitive tasks. They are likely related to scattering changes associated with neuronal activity. In general, these responses are characterized by an increase in photon delay and a reduction in light transmission in active cortical areas.
15.4.2. Relationship with Other Techniques
Several studies show that the time course of the fast optical signal is similar to the event-related electrical activity recorded in the same conditions. Also, several studies show that the optical signal is localized in a manner consistent with the known functional anatomy of the cortical areas involved, and is also consistent with fMRI data recorded in similar conditions. These data provide support for the idea that fast optical imaging can be used to study the time course of activity in localized cortical areas.
15.4.3. Unresolved Issues and Future Research Directions
Several issues remain to be addressed. First, it has been hypothesized that the fast optical signal (or EROS) is due to changes in the scattering coefficient in active cortical areas determined by the movement of ions across the neuronal membrane. The research conducted using depth recordings with miniaturized optical instruments or implanted optic fibers [7] is clearly relevant for this purpose, as is the work by various groups on in vitro preparations [6,25]. However, additional investigation on the physiological basis of the signal is needed to determine the exact relationships between physical events in the brain and the noninvasive measures. Further, it would be very useful to determine whether these relationships hold equally for different types of neurons (i.e., pyramidal cells versus interneurons) and/or different neurotransmitter systems.
Second, more research is needed to determine which methods are most suitable for recording fast optical signals. Both photon delay and intensity signals have been used. We have carried out some comparisons between these two methods [54], but a more systematic analysis of the relationships between these two measures is needed. It is also possible that a combination of the two measures may be useful to increase the signal-to-noise ratio.
Third, although substantial advancements have been made in the area of 3D reconstruction, a single method still needs to obtain general acceptance. This area of research will gain from the developments of easy-to-use, general purpose analysis software. Comparisons with fMRI data, such as that carried out by [11,60] can provide validation to the various proposed methods.
Fourth, so far fast optical signals appear to be recordable non-invasively from relatively shallow areas (3 cm or so from the surface of the head), but the penetration to deeper structures is severely limited. However, the penetration may be improved by increases in signal-to-noise ratio and by other technological advancements (such as the use of the “pi detector,” [28] the use of photon delay measures compared to intensity measures, or by varying the modulation frequency [82]).
Fifth, raw data are at present noisy, often requiring data collection from a large number of trials and subjects. Several tools can be used to increase the signal-to-noise ratio, including instrumental improvements (such as the use of more powerful light sources and reduced time multiplexing) and analytical methods (such as the use of appropriate filtering methods [46], wavelet analysis, cross-correlation and autoregression methods [50], independent component analysis [75], etc.).
15.4.4. Conclusions
Fast optical imaging is a promising new tool for studying brain function, and in particular the time course of activity in selected cortical areas. Advantages of the technique include noninvasivity, portability, good combination of spatial and temporal resolution, relatively low cost, and easy integration with other techniques, such as fMRI, electrophysiological methods, and NIR spectroscopy. Main limitations are its reduced penetration and, at least at present, its low signal-to-noise ratio. The technique appears particularly well suited to provide a bridge between hemodynamic and neuronal methods, and can be used profitably both in the study of brain physiology and in cognitive neuroscience. In particular, in combination with anatomical methods such as diffusion tensor imaging (for a review see Rykhlevskaia et al. [83]) or structural MRI, fast optical signals recorded from the entire cortical surface can be used to test hypotheses about changes in structural and functional connectivity, as the temporal order of activation of various brain areas can be modeled together with anatomical information to provide constraints on models of cognitive processing [50,77].
ACKNOWLEDGMENTS
The work presented in this chapter was supported in part by NIMH grant MH ROI 80182 to G. Dratton and by NIA grant AGROI 21887 to M. Fabiani.
REFERENCES
- 1.
- Gratton G, et al. Shades of gray matter: Noninvasive optical images of human brain responses during visual stimulation. Psychophysiology. 1995;32:505. [PubMed: 7568645]
- 2.
- Gratton G, Fabiani M. Shedding light on brain function: The event-related optical signal. Trends Cogn. Sci. 2001;5:357. [PubMed: 11477005]
- 3.
- Agran J, et al. When the rules keep changing: The timing of activation of task-general and task-specific brain regions involved. in preparation, submitted.
- 4.
- Steinbrink J, et al. Somatosensory evoked fast optical intensity changes detected non-invasively in the adult human head. Neurosci. Lett. 2000;291:105. [PubMed: 10978585]
- 5.
- Cohen LB. Changes in neuron structure during action potential propagation and synaptic transmission. Physiol. Rev. 1972;53:373. [PubMed: 4349816]
- 6.
- Frostig RD, et al. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in-vivo high resolution optical imaging of intrinsic signals. Proc. Natl. Acad. Sci. USA. 1990;87:6082. [PMC free article: PMC54476] [PubMed: 2117272]
- 7.
- Rector DM, et al. Light scattering changes follow evoked potentials from hippocampal Schaeffer collateral stimulation. J. Neurophysiol. 1997;78:1707. [PubMed: 9310454]
- 8.
- Raichle ME. Visualizing the mind. Sci Amer. 1994 April;5:8. [PubMed: 8178115]
- 9.
- Pascual-Leone A. et al. Transcranial magnetic stimulation in cognitive neuroscience—virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10:232. [PubMed: 10753803]
- 10.
- Toga AW, Mazziotta JC, editors. Brain Mapping: The Methods. Academic Press; San Diego: 1996.
- 11.
- Gratton G, et al. Comparison of neuronal and hemodynamic measure of the brain response to visual stimulation: An optical imaging study. Hum. Brain Mapping. 2001;13:13. [PMC free article: PMC6872061] [PubMed: 11284043]
- 12.
- Ogawa S, et al. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA. 1992;89:5951. [PMC free article: PMC402116] [PubMed: 1631079]
- 13.
- Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trend. Neurosci. 1997;20:435. [PubMed: 9347608]
- 14.
- Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264. [PubMed: 929199]
- 15.
- Grinvald A, et al. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361. [PubMed: 3785405]
- 16.
- Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovasc. Brain Metab. Rev. 1995;7:240. [PubMed: 8519605]
- 17.
- Miller KL, et al. Non-linear transport dynamics of the cerebral blood flow response. Hum. Brain Mapping. 2001;13:1. [PMC free article: PMC6871988] [PubMed: 11284042]
- 18.
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of Psychophysiology. 3rd ed. Cambridge University Press; New York: 2007. p. 56.
- 19.
- Fabiani M, et al. Event related brain potentials. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of Psychophysiology. 3rd ed. Cambridge University Press; New York: 2007. p. 85.
- 20.
- Hari R, et al. Timing of human cortical functions during cognition: Role of MEG. Trends Cogn. Sci. 2000;4:455. [PubMed: 11115759]
- 21.
- Allison T, et al. The central nervous system. In: Coles MGH, Porges SW, Donchin E, editors. Psychophysiology: Systems, Processes, and Applications. Guilford; New York: 1986. p. 5.
- 22.
- Pascual-Marqui RD, et al. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994;18:49. [PubMed: 7876038]
- 23.
- Dale AM, et al. Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55. [PubMed: 10798392]
- 24.
- Rector DM, et al. Spatiotemporal mapping of rat whisker barrels with fast scattered light signals. Neuroimage. 2005;26:619. [PubMed: 15907319]
- 25.
- Stepnoski RA, et al. Noninvasive detection of changes in membrane potential in cultured neurons by light scattering. Proc. Natl. Acad. Sci. USA. 1991;88:9382. [PMC free article: PMC52721] [PubMed: 1946349]
- 26.
- Foust AJ, Rector DM. Optically teasing apart neural swelling depolarization. Neuroscience. 2007;145:887. [PMC free article: PMC1888560] [PubMed: 17303339]
- 27.
- Gratton G, et al. Rapid changes of optical parameters in the human brain during a tapping task. J. Cogn. Neurosci. 1995;7:446. [PubMed: 23961904]
- 28.
- Wolf U, et al. Detecting cerebral functional slow and fast signals by frequency-domain near-infrared spectroscopy using two different sensors. OSA Biomed. Top. Meeting, Tech. Dig. 2000:427.
- 29.
- Gratton E, et al. The possibility of a near-infrared optical imaging system using frequency-domain methods. Proc. III Int. Conf. Peace through Mind/Brain Sci; 1990. p. 183.
- 30.
- Maclin E, et al. Amelioration of movement artifacts in slow and fast optical recordings. presented at the Cogn. Neurosci. Soc; San Francisco. 2008.
- 31.
- Okada E, et al. Theoretical and experimental investigation of near-infrared light propagation in a model of the adult head. Appl. Opt. 1997;36:21. [PubMed: 18250644]
- 32.
- Firbank M, et al. A theoretical study of the signal contribution of regions of the adult head to near-infrared spectroscopy studies of visual evoked responses. Neuroimage. 1998;8:69. [PubMed: 9698577]
- 33.
- Gratton G, et al. Toward non-invasive 3-D imaging of the time course of cortical activity: Investigation of the depth of the event-related optical signal (EROS). NeuroImage. 2000;11:491. [PubMed: 10806035]
- 34.
- Gratton G, et al. Feasibility of intracranial near-infrared optical scanning. Psychophysiology. 1994;31:211. [PubMed: 8153259]
- 35.
- Alfano RR, et al. Advances in optical imaging of biomedical media. Ann. New York Acad. Sci. 1997;820:248. [PubMed: 9237460]
- 36.
- Arridge SR, Hebden JC. Optical imaging in medicine: II. Modeling and reconstruction. Phys. Med. Biol. 1997;1997;42:841. [PubMed: 9172263]
- 37.
- Arridge SR, Schweiger M. Image reconstruction in optical tomography. Phil. Trans. Royal Soc. London—Series B: Biol. Sci. 1997;352:717. [PMC free article: PMC1691961] [PubMed: 9232860]
- 38.
- Benaron DA, et al. Non-recursive linear algorithms for optical imaging in diffusive media. Adv. Exper. Med. Biol. 1994;361:215. [PubMed: 7597946]
- 39.
- Chang J, et al. Optical imaging of anatomical maps derived from magnetic resonance images using time-independent optical sources. IEEE Trans. Med. Imaging. 1997;16:68. [PubMed: 9050409]
- 40.
- Franceschini MA, et al. On-line optical imaging of the human brain with 160-ms temporal resolution. Opt. Expr. 2000;6:49. [PubMed: 19401744]
- 41.
- Jiang H, et al. Optical image reconstruction using DC data: Simulations and experiments. Phys. Med. Biol. 1996;41:1483. [PubMed: 8858732]
- 42.
- Paulsen KD, Jiang H. Spatially varying optical property reconstruction using a finite element diffusion equation approximation. Med. Phys. 1995;22:691. [PubMed: 7565358]
- 43.
- Zhu W, et al. Iterative total least-squares image reconstruction algorithm for optical tomography by the conjugate gradient method. J. Opt. Soc. Amer. A—Opt. Image Sci. 1997;14:799. [PubMed: 9088090]
- 44.
- Gratton G, AOptcont@ and, AOpt-3D@: A software suite for the analysis, 3D reconstruction of the event-related optical signal (EROS) Psychophysiology. 2000;37:S44.
- 45.
- Gratton G, Corballis PM. Removing the heart from the brain: Compensation for the pulse artifact in the photon migration signal. Psychophysiology. 1995;32:292. [PubMed: 7784538]
- 46.
- Maclin E, et al. Optimum filtering for EROS measurements. Psychophysiology. 2003;40:542. [PubMed: 14570162]
- 47.
- Fabiani M, et al. Bootstrap assessment of the reliability of maxima in surface maps of brain activity of individual subjects derived with electrophysiological and optical methods. Beh. Res. Meth., Instr., Comp. 1998;30:78.
- 48.
- Whalen C, Maclin EL, Fabiani M, Gratton G. Validation of the method for coregistering scalp recording locations with 3D structural MR images. Human Brain Mapping. 2008;29(11):1288–1301. [PMC free article: PMC6871211] [PubMed: 17894391]
- 49.
- Kiebel SJ, et al. Robust smoothness estimation in statistical parametric maps using standardized residuals from the general linear model. NeuroImage. 1999;10:756. [PubMed: 10600421]
- 50.
- Rykhlevskaia E, et al. Lagged covariance structure models for studying functional connectivity in the brain. NeuroImage. 2006;30:1203. [PubMed: 16414282]
- 51.
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportion System: An Approach to Cerebral Imaging. Thieme; New York: 1988.
- 52.
- Steinbrink J, et al. The fast optical signal—robust or elusive when non-invasively measured in the human adult? Neuroimage. 2005;26:996. [PubMed: 15961042]
- 53.
- Malonek D, Grinvald A. The spatial and temporal relationship between cortical electrical activity and responses of the microcirculation during sensory stimulation: implications for optical, PET, and MR functional brain imaging. Science. 1996;272:551–554. [PubMed: 8614805]
- 54.
- Gratton G, et al. Effects of measurement method, wavelength, and source-detector distance on the fast optical signal. NeuroImage. 2006;32:1576. [PubMed: 16872842]
- 55.
- Menon RS, et al. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: Echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn. Reson. Med. 1995;33:453. [PubMed: 7760717]
- 56.
- Maclin EL, et al. The Event Related Optical Signal (EROS) to electrical stimulation of the median nerve. NeuroImage. 2004;21:1798. [PubMed: 15050600]
- 57.
- Fabiani M, et al. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. J. Cogn. Neurosci. 2006;18:637. [PubMed: 16768366]
- 58.
- Sable JJ, et al. Optical imaging of perceptual grouping in human auditory cortex. Eur. J. Neurosci. 2007;25:298. [PubMed: 17241291]
- 59.
- Gratton G, et al. Fast and localized event-related optical signals (EROS) in the human occipital cortex: Comparison with the visual evoked potential and fMRI. NeuroImage. 1997;6:168. [PubMed: 9344821]
- 60.
- Zhang X, et al. Bartels KE, Bass LS, Riese WT de, Gregory KW, Hirschberg H, Katzir A, Kollias N, Madsen SJ, Malek RS, McNally-Heintzelman KM, Tate LP Jr, Trowers EA, Wong BJ-F, editors. The study of cerebral hemodynamic and neuronal response to visual stimulation using simultaneous NIR optical tomography and BOLD fMRI in humans. Proc. SPIE Vol. 5686, Photonic Therapeutics and Diagnostics. 2005:566. [PMC free article: PMC3138137] [PubMed: 21776185]
- 61.
- Gratton G, Fabiani M. The event related optical signal (EROS) in visual cortex: Replicability, consistency, localization and resolution. Psychophysiology. 2003;40:561. [PubMed: 14570164]
- 62.
- Gratton G. Attention and probability effects in the human occipital cortex: An optical imaging study. NeuroReport. 1997;8:1749. [PubMed: 9189926]
- 63.
- Gratton G, et al. Memory-driven processing in human medial occipital cortex: An event-related optical signal (EROS) study. Psychophysiology. 1998;38:348. [PubMed: 9564756]
- 64.
- Gratton G, et al. Biomedical Optics 2006 Technical Digest. Optical Society of America; Washington, DC: 2006. Time course of activation of human occipital cortex measured with the event-related optical signal (EROS) p. MD4.
- 65.
- Fabiani M, et al. Multiple visual memory phenomena in a memory search task. Psychophysiology. 2003;40:472. [PubMed: 12946120]
- 66.
- Rinne T, et al. Scalp-recorded optical signals make sound processing from the auditory cortex visible. NeuroImage. 1999;10:620. [PubMed: 10547339]
- 67.
- Tse CY, et al. Event-related optical imaging reveals the temporal dynamics of right temporal and frontal cortex activation in pre-attentive change detection. Neuroimage. 2006;29:314. [PubMed: 16095922]
- 68.
- Tse CY, Penney TB. Preattentive change detection using the event-related optical signal. IEEE Eng. Med. Biol. Mag. 2007;26:52. [PubMed: 17672232]
- 69.
- Tse CY, Penney TB. On the functional role of temporal and frontal cortex activation in passive detection of auditory deviance. Neuroimage. 2008 [PubMed: 18474433]
- 70.
- Näätänen R. Mismatch negativity (MMN): Perspectives for application. Int. J. Psychophysiol. 2000;37:3. [PubMed: 10828371]
- 71.
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375. [PubMed: 3615753]
- 72.
- Franceschini MA, Boas DA. Noninvasive measurement of neuronal activity with near-infrared optical imaging. Neuroimage. 2004;21:372. [PMC free article: PMC3786741] [PubMed: 14741675]
- 73.
- DeSoto MC, et al. When in doubt, do it both ways: Brain evidence of the simultaneous activation of conflicting responses in a spatial Stroop task. J. Cogn. Neurosci. 2001;13:523. [PubMed: 11388924]
- 74.
- Gratton G, et al. Pre- and poststimulus activation of response channels: A psychophysiological analysis. J. Exp. Psychol.: Hum Perc. Perf. 1988;11:331. [PubMed: 2971764]
- 75.
- Morren G, et al. Detection of fast neuronal signals in the motor cortex from functional near infrared spectroscopy measurements using independent component analysis. Med. Biol. Eng. Comput. 2004;42:92. [PubMed: 14977228]
- 76.
- Low KA, et al. Fast optical imaging of frontal cortex during active and passive oddball tasks. Psychophysiology. 2006;43:127. [PubMed: 16712583]
- 77.
- Gratton G, Rykhlevskaia E, Nee E, Leaver E, Fabiani M. Does white matter matter? Spatiotemporal dynamics of task switching in aging. J Cogn Neurosci. (in press) [PMC free article: PMC2917701] [PubMed: 18752402]
- 78.
- Tse C.-Y, et al. Imaging the cortical dynamics of language processing with the event-related Optical Signal. Proc. Natl. Acad. Sci. USA. 2007;104:17157. [PMC free article: PMC2040398] [PubMed: 17942677]
- 79.
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203. [PubMed: 7350657]
- 80.
- Kutas M, et al. Language. In: Cacioppo J, Tassinary L, Berntson G, editors. Handbook of Psychophysiology. 3rd ed. Cambridge University Press; New York: 2007. p. 555.
- 81.
- McCarthy G, et al. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J. Neurophysiol. 1997;77:1630. [PubMed: 9084626]
- 82.
- Maclin EL, et al. Improving the signal-to-noise ratio of Event Related Optical Signals (EROS) by manipulating wavelength and modulation frequency. IEEE EMBM. 2007;26:47. [PubMed: 17672231]
- 83.
- Rykhlevskaia EI, et al. Combining structural and functional neuroimaging data for studying brain connectivity: A review. Psychophysiology. 2008;45:173. [PubMed: 17995910]
- 84.
- Gratton G, Fabiani M. The event-related optical signal: A new tool for studying brain function. Int. J. Psychophysiol. 2001;42:109–121. [PubMed: 11587771]
Footnotes
- *
The small sample size was due to the necessity of repeating the measurement approximately 12 times per subject to create a functional map with a 1-channel (1 source, 1 detector) system.
- Review In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.[In Vivo Optical Imaging of Bra...]Review In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.Rector DM, Yao X, Harper RM, George JS. In Vivo Optical Imaging of Brain Function. 2009
- Review Visualizing Adult Cortical Plasticity Using Intrinsic Signal Optical Imaging.[In Vivo Optical Imaging of Bra...]Review Visualizing Adult Cortical Plasticity Using Intrinsic Signal Optical Imaging.Frostig RD, Chen-Bee CH. In Vivo Optical Imaging of Brain Function. 2009
- Review Quantitative In Vivo Imaging of Tissue Absorption, Scattering, and Hemoglobin Concentration in Rat Cortex Using Spatially Modulated Structured Light.[In Vivo Optical Imaging of Bra...]Review Quantitative In Vivo Imaging of Tissue Absorption, Scattering, and Hemoglobin Concentration in Rat Cortex Using Spatially Modulated Structured Light.Cuccia DJ, Abookasis D, Frostig RD, Tromberg BJ. In Vivo Optical Imaging of Brain Function. 2009
- Effects of measurement method, wavelength, and source-detector distance on the fast optical signal.[Neuroimage. 2006]Effects of measurement method, wavelength, and source-detector distance on the fast optical signal.Gratton G, Brumback CR, Gordon BA, Pearson MA, Low KA, Fabiani M. Neuroimage. 2006 Oct 1; 32(4):1576-90. Epub 2006 Jul 26.
- Review Optical Imaging of Brain Activity In Vivo Using Genetically Encoded Probes.[In Vivo Optical Imaging of Bra...]Review Optical Imaging of Brain Activity In Vivo Using Genetically Encoded Probes.Wachowiak M, Knöpfel T. In Vivo Optical Imaging of Brain Function. 2009
- Fast Optical Signals: Principles, Methods, and Experimental Results - In Vivo Op...Fast Optical Signals: Principles, Methods, and Experimental Results - In Vivo Optical Imaging of Brain Function
- txid906888[Organism:exp] AND "sequence from type"[Filter] (154)Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...