NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014.
17.1. INTRODUCTION
Itch (or pruritus) has been defined as an unpleasant sensation that provokes the desire to scratch. Itch is also believed to signal danger from various environmental factors or physiological abnormalities. Therefore, it frequently accompanies a variety of inflammatory skin conditions and systemic diseases.
Histamine is the best-known pruritogen in humans and also acts as an experimental itch-causing substance. Clinically, antihistamines, i.e., histamine H1-receptor blockers, are commonly used to treat all types of itching resulting from renal and liver diseases, as well as from serious skin diseases such as atopic dermatitis. However, antihistamines often lack efficacy in patients with chronic itch that may involve other agonists, including proteases, neuropeptides, cytokines, and opioids, and their cognate receptors, such as thermoreceptors, PARs, Mrgprs, and opioid receptors. Such pruritogenic mediators and modulators released in the periphery may directly activate itch-sensitive fibers, especially C-fibers, by binding to specific receptors on the nerve terminals (Ikoma et al. 2006; Paus et al. 2006; Xiao and Patapoutian 2011). Nerve fibers are also activated by exogenous mechanical, chemical, and biological stimuli, resulting in itch responses (Akiyama et al. 2010; Tominaga and Takamori 2010).
Histological analyses have shown that epidermal nerve densities are increased in patients with atopic dermatitis and xerosis, suggesting that the higher density is partly responsible for itch sensitization in the periphery. Such hyperinnervation is probably caused by an imbalance of nerve elongation factors, such as nerve growth factor (NGF), and nerve repulsion factors, such as semaphorin 3A (Sema3A), produced by keratinocytes (Tominaga and Takamori 2010). These axonal guidance molecules may also act on keratinocytes, immune cells and vascular endothelial cells, and be indirectly involved in the modulation of itching. This chapter presents recent knowledge regarding itch sensitization associated with epidermal nerve density controlled by NGF and Sema3A, especially in atopic dermatitis.
17.2. SKIN DISEASES WITH ITCH INVOLVING EPIDERMAL NERVE FIBERS
Sensory nerve fibers are acceptors of itch sensation as well as pain in the skin. The neuronal mechanisms underlying intractable itch in the periphery have been partly identified. Histological investigations have shown that the density of epidermal nerve fibers is higher in the skin of patients with atopic dermatitis, contact dermatitis, and xerosis than in healthy controls (Figure 17.1) (Ikoma et al. 2006; Tominaga and Takamori 2010), although the nerve density in patients with pruigo nodularis and psoriasis remains debatable (Schuhknecht et al. 2011; Taneda et al. 2011; Kou et al. 2012). Similar findings have been observed in animal models, such as NC/Nga mice, an atopic dermatitis model (Tominaga et al. 2007a, 2009a), and dry skin model mice (Miyamoto et al. 2002; Tominaga et al. 2007b). These findings are indicative of increases in sensory nerve fibers responsive to exogenous trigger factors and to various endogenous pruritogens from immune cells and keratinocytes, suggesting that hyperinnervation is partly responsible for itch sensitization.
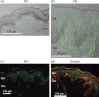
FIGURE 17.1
Distribution of epidermal nerve fibers in healthy and pruritic skin samples. Immunohistochemical staining of the skin of healthy controls (HC), patients with atopic dermatitis (AD), and xerotic patients with antibody to protein gene product 9.5 (anti-PGP9.5). (more...)
Several recent studies have found that epidermal innervation density is reduced in various pruritic skin conditions, such as lichen amyloidosis (Maddison et al. 2008), prurigo nodularis (Schuhknecht et al. 2011), nummular dermatitis (Maddison et al. 2011) and keloids (Tey et al. 2012). Chronic stimulation of itch-transmitting nerve fibers may result in a self-regulated hypoplasia that modulates the intensity and persistence of sensory input. This hypothesis is supported by the results of one study, which showed diminished skin innervation in the skin of patients with neuropathic itch (Wallengren et al. 2002).
17.3. ROLES OF NGF AND Sema3A IN EPIDERMAL NERVE FIBERS
17.3.1. NGF
NGF is a neurotrophin that affects neurite outgrowth and neuronal survival (Lewin and Mendell 1993). Keratinocyte-derived NGF is a major mediator of cutaneous innervation density, in that local NGF concentrations are higher in the lesional skin of patients with prurigo nodularis, atopic dermatitis, psoriasis, contact dermatitis, and xerosis than in normal skin (Figure 17.2a) (Ikoma et al. 2006). In adult rat primary sensory neurons, NGF has been shown to upregulate neuropeptides, especially substance P (SP) and calcitonin gene-related peptide (CGRP) (Verge et al. 1995), both of which are involved in the hypersensitivity of itch sensation and neurogenic inflammation (Steinhoff et al. 2003). A more recent study demonstrated that human atopic keratinocytes produced elevated levels of NGF and mediated an increased outgrowth of CGRP-immunoreactive sensory fibers, whereas human atopic fibroblasts did not mediate this outgrowth (Roggenkamp et al. 2012). This result indicates that keratinocytes are key factors in hyperinnervation in individuals with atopic dermatitis. Intradermal injection of NGF was shown to sensitize nociceptors for cowhage- but not histamine-induced itch in human skin (Rukwied et al. 2012). Thus, increased NGF in the skin may also sensitize primary afferents, thereby contributing to chronic itch such as atopic dermatitis.
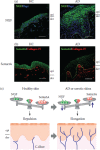
FIGURE 17.2
Epidermal NGF and Sema3A levels in pruritic skin samples. (a) Skin biopsies from HC and patients with AD were stained with anti-NGF antibody. Epidermal NGF levels (green) were higher in AD patients than in HC. Nuclei were counterstained with DAPI (blue). (more...)
Tumor necrosis factor (TNF)-α has also been found to enhance NGF production by human keratinocytes (Takaoka et al. 2009). TNF-α is a pivotal proinflammatory cytokine in the innate immune response and a key molecule for skin inflammation. Mast cells have been identified as important potential sources of TNF-α (Steinhoff et al. 2003). Plasma TNF-α concentration is higher in individuals with atopic dermatitis than without atopic dermatitis (Sumimoto et al. 1992), and both TNF-α and its receptors are upregulated in the dermal blood vessels of patients with psoriasis (Kristensen et al. 1993). In addition, skin barrier disruption induces the upregulation of TNF-α in the epidermis of acetone-treated mice, an acute dry skin model (Wood et al. 1992). These findings suggest that TNF-α is an important upregulator of NGF in the epidermis, a finding supported by results showing that TNF produced by mast cells promotes the elongation of cutaneous nerve fibers in a mouse model of contact dermatitis (Kakurai et al. 2006).
17.3.2. Semaphorin 3A (Sema3A)
Class 3 semaphorins, a family of secreted proteins, have been implicated in a variety of biological functions and were originally identified as axonal guidance cues during neural development. Sema3A is the first member of this protein family shown to cause growth cone collapse in neurons, i.e., to function as a nerve repulsion factor, through its interaction with a neuropilin-1 (Nrp-1)/plexin-A receptor complex (Fujisawa 2004; Sharma et al. 2012). We previously reported that Sema3A transcripts are also expressed in cultured normal human epidermal keratinocytes (Tominaga et al. 2008). The proteins are mainly distributed in the suprabasal layer of normal human skin, consistent with findings showing that Sema3A is expressed in differentiated keratinocyte cultures (Fukamachi et al. 2011). Moreover, epidermal Sema3A levels were lower in patients with atopic dermatitis than in healthy controls (Figure 17.2b), concomitant with an increase in epidermal nerve density (Tominaga et al. 2008). Increased epidermal nerve density in an acute dry skin model was recently shown to be associated with decreased levels of Sema3A expression (Kamo et al. 2011a, 2011b). Although the mechanism regulating the Sema3A gene has not yet been determined, these indicate good correlations between epidermal innervation and Sema3A levels. Interestingly, Sema3A has been found to inhibit NGF-induced sprouting of sensory afferents in adult rat spinal cord (Tang et al. 2004), whereas elevated levels of NGF reduced the Sema3A-induced collapse of sensory growth cones (Dontchev and Letourneau 2002). These findings suggest that decreasing the expression of Sema3A can accelerate epidermal nerve growth in patients with atopic dermatitis and xerosis. Thus, epidermal innervation may be regulated by a fine balance between NGF and Sema3A (Figure 17.2c).
17.3.3. Mechanisms by Which Nerve Fibers Penetrate Basement Membranes
NGF stimulation of nerve fiber elongation in pruritic skin may result in the accumulation at the growth cone of integrins, which interact with a variety of extracellular matrix (ECM) components (Grabham and Goldberg 1997; Gardiner 2011). During this process, matrix metalloproteinases (MMPs) would be required for the growth cone to abrogate the three-dimensional ECM barriers. Using an in vitro model of basement membrane, consisting of Boyden chambers and Matrigel, and constituting a unique culture system of rat dorsal root ganglion (DRG) neurons, we recently showed that MMP-2 localized on the growth cone was involved in the penetration mechanism (Figure 17.3) (Tominaga et al. 2009b). In Boyden chamber, cultures using type I collagen gels and cultured DRG neurons, MMP-8 secreted by the nerve fibers was shown to be involved in nerve growth within the gels (Figure 17.3) (Tominaga et al. 2011). The expression of MMP-2 and MMP-8 was upregulated by NGF and downregulated by Sema3A. Interestingly, MMP2 expression was induced by its enzymatic substrates, including type IV, collagen, laminin, and fibronectin, as well as by MG. Moreover, MMP-8 expression was upregulated by its substrates types I and III collagens. The expression of both MMP genes was not altered by nonsubstrate molecules. The selection and upregulation of MMPs corresponding to the ECM components surrounding the growing nerve fibers are required for efficient nerve fiber penetration, suggesting that the coordinated activation of neurotrophin and ECM-integrin signaling is necessary for efficient and long-distance axon extension (Grabham and Goldberg 1997; Gardiner et al. 2011). Because class 3 semaphorin signaling inhibits integrin-mediated adhesion signaling, Sema3A stimulation of the growing nerve fibers may provide a reverse signaling pathway for these events (Zhou et al. 2008). Thus, although the integrin-mediated regulatory system remains unclear in our in vitro studies, this mechanism might be applicable to pruritic skin diseases involving skin hyperinnervation.

FIGURE 17.3
Possible models of nerve fiber growth into basement membrane or within the dermis. (a) NGF, which is produced by cutaneous cells such as epidermal keratinocytes, immune cells, and fibroblasts, promotes the production of MMP-2 and MMP-8 in sensory nerve (more...)
17.4. NGF AND Sema3A AS ANTIPRURITIC TARGETS
17.4.1. Anti-NGF Antibody and Its Receptor Inhibitors
The effects of NGF are mediated by its binding to two classes of transmembrane receptors, a high-affinity receptor (tropomyosin-related kinase A, TrkA) and a low-affinity receptor (p75) (Lewin and Mendell 1993). To date, two anti-NGF approaches have been used to treat pruritus of atopic dermatitis in NC/Nga mice. One study showed that the intraperitoneal administration of anti-NGF neutralizing antibody to atopic NC/Nga mice significantly suppresed both epidermal nerve growth and scratching behavior, but did not ameliorate scratching that had already developed (Takano et al. 2005). Similarly, application of the TrkA inhibitors AG879 and K252a to the backs of the necks of atopic NC/Nga mice five times per week significantly improved established dermatitis and scratching behavior, and decreased the numbers of nerve fibers in the epidermis, suggesting that NGF plays important roles in the pathogenesis of atopic dermatitis-like skin lesions (Takano et al. 2007). Thus, NGF and its receptors may be therapeutic targets in patients with pruritic skin diseases.
17.4.2. Sema3A Replacement Therapy
Decreasing the expression of Sema3A accelerates epidermal nerve growth in patients with some pruritic skin diseases, such as atopic dermatitis and dry skin (Tominaga et al. 2008; Tominaga and Takamori 2010). Therefore, replacement by exogenous Sema3A may have antipruritic effects. Recently, recombinant Sema3A replacement approaches (intradermal injection or ointment application) were found to significantly inhibit scratching behavior and to improve dermatitis score in atopic NC/Nga mice compared with controls (Yamaguchi et al. 2008; Negi et al. 2012). The therapeutic efficacy of exogenous Sema3A on the atopic dermatitis-like symptoms was greater than that of current agents, such as betamethasone and tacrolimus (Negi et al. 2012). Moreover, histological examination showed decreases in (1) the numbers of epidermal PGP9.5- or SP-immunoreactive fibers; (2) the numbers of inflammatory infiltrates, including mast cells, eosinophils and CD4+ T cells; (3) the production of IL-4; (4) the density of dermal blood vessels; and (5) epidermal thickness in Sema3A-treated lesional skin (Yamaguchi et al. 2008; Negi et al. 2012). These observations suggest that exogenous Sema3A not only affects sensory nerve fibers but also immune cells or other cells, such as endothelial cells and keratinocytes, that express Nrp-1, a coreceptor for Sema3A (Romeo et al. 2002). Therefore, replacement of Sema3A may be useful in therapeutic strategies for patients with pruritic skin conditions such as atopic dermatitis.
17.4.3. Others
Several existing therapies may normalize abnormal levels of NGF and Sema3A in pruritic skin associated with a reduction in epidermal nerve density. Oral administration of olopatadine hydrochloride, a histamine H1-receptor antagonist, significantly suppressed scratching behavior, improved dermatitis score, and inhibited neurite outgrowth in the lesional skin of mice with atopic dermatitis. Notably, olopatadine treatment increased Sema3A expression in the epidermis (Murota et al. 2010; Ohsawa and Hirasawa 2012). Although it is unclear whether these effects are caused by specific blocking of histamine H1-receptor signaling, olopatadine may in part improve imbalances of NGF and Sema3A in the epidermis.
Our recent study using dry skin mice showed that application of heparinoid cream (emollient) resulted in greater improvements in epidermal nerve density and epidermal NGF levels than application of petrolatum, although heparinoid cream had no effect on epidermal Sema3A levels (Kamo et al. 2011a). In addition, various types of ultraviolet (UV)-based therapy, including psoralen-ultraviolet A (PUVA) and narrow-band UVB, have been widely used to treat patients with atopic dermatitis (Krutmann 2000). These UV-based therapies were shown to reduce the number of cutaneous nerve fibers, especially in the epidermis, in patients with atopic dermatitis and psoriasis, and to inhibit pruritus (Wallengren and Sundler 2004; Tominaga et al. 2009c). Similar effects of UV-based therapy on epidermal nerve fibers were observed in dry skin mice (Kamo et al. 2011b). The abnormal expression of Sema3A and NGF in the epidermis was normalized by PUVA or narrowband UVB therapy (Tominaga et al. 2009c; Kamo et al. 2011b).
17.5. CONCLUSION
Nerve density in the epidermis may be involved in itch sensitization in pruritic skin diseases. Epidermal innervation is thought to be regulated by a fine balance between nerve elongation factors and nerve repulsion factors through the regulation of expression of MMPs. Treatment with anti-NGF agents, Sema3A replacement and other treatments such as UV-based therapies may normalize epidermal nerve fiber density. These findings may expand the knowledge of potential therapeutic strategies for ameliorating pruritus associated with epidermal nerve density, including patients with atopic dermatitis and xerosis.
ACKNOWLEDGMENTS
This work was supported by a Health Labor Sciences Research Grant for Research on Allergic Disease and Immunology from the Japanese Ministry of Health, Labor and Welfare, by a KAKENHI (20591354 and 2079081) and a “High-Tech Research Center” Project for Private Universities. Matching fund subsidy from MEXT and by a JSPS Research Fellow.
REFERENCES
- Akiyama T, Carstens M.I, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–83. [PMC free article: PMC2955821] [PubMed: 20709455]
- Dontchev V.D, Letourneau P.C. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci. 2002;22:6659–69. [PMC free article: PMC6758175] [PubMed: 12151545]
- Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59:24–33. [PubMed: 15007824]
- Fukamachi S, Bito T, Shiraishi N. et al. Modulation of semaphorin 3A expression by calcium concentration and histamine in human keratinocytes and fibroblasts. J Dermatol Sci. 2011;61:118–23. [PubMed: 21176873]
- Gardiner N.J. Integrins and the extracellular matrix: Key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol. 2011;71:1054–72. [PubMed: 21761574]
- Grabham P.W, Goldberg D.J. Nerve growth factor stimulates the accumulation of beta1 integrin at the tips of filopodia in the growth cones of sympathetic neurons. J Neurosci. 1997;17:5455–65. [PMC free article: PMC6793820] [PubMed: 9204928]
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–47. [PubMed: 16791143]
- Kakurai M, Monteforte R, Suto H, Tsai M, Nakae S, Galli S.J. Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am J Pathol. 2006;169:1713–21. [PMC free article: PMC1780201] [PubMed: 17071594]
- Kamo A, Tominaga M, Negi O, Tengara S, Ogawa H, Takamori K. Topical application of emollients prevents dry skin-inducible intraepidermal nerve growth in acetone-treated mice. J Dermatol Sci. 2011a;62:64–6. [PubMed: 21316926]
- Kamo A, Tominaga M, Tengara S, Ogawa H, Takamori K. Inhibitory effects of UV-based therapy on dry skin-inducible nerve growth in acetone-treated mice. J Dermatol Sci. 2011b;62:91–7. [PubMed: 21458246]
- Kou K, Nakamura F, Aihara M. et al. Decreased expression of semaphorin-3A, a neurite-collapsing factor, is associated with itch in psoriatic skin. Acta Derm Venereol. 2012;92:521–8. doi:10.2340/00015555-1350. [PubMed: 22565412]
- Kristensen M, Chu C.Q, Eedy J, Feldmann M, Brennan F.M, Breathnach S.M. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: Epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 1993;94:354–62. [PMC free article: PMC1534247] [PubMed: 8222328]
- Krutmann J. Phototherapy for atopic dermatitis. Clin Exp Dermatol. 2000;25:552–8. [PubMed: 11122227]
- Lewin G.R, Mendell L.M. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–9. [PubMed: 7694405]
- Maddison B, Namazi M.R, Samuel L.S. et al. Unexpected diminished innervation of epidermis and dermoepidermal junction in lichen amyloidosus. Br J Dermatol. 2008;159:403–6. [PubMed: 18547301]
- Maddison B, Parsons A, Sangueza O, Sheehan D.J, Yosipovitch G. Retrospective study of intraepidermal nerve fiber distribution in biopsies of patients with nummular eczema. Am J Dermatopathol. 2011;33:621–3. [PubMed: 21712693]
- Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–92. [PubMed: 11949883]
- Murota H, El-latif M.A, Tamura T, Amano T, Katayama I. Olopatadine hydrochloride improves dermatitis score and inhibits scratch behavior in NC/Nga mice. Int Arch Allergy Immunol. 2010;153:121–32. [PubMed: 20407268]
- Negi O, Tominaga M, Tengara S. et al. Topically applied semaphorin 3A ointment inhibits scratching behavior and improves skin inflammation in NC/Nga mice with atopic dermatitis. J Dermatol Sci. 2012;66:37–43. [PubMed: 22381253]
- Ohsawa Y, Hirasawa N. The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via anti-pruritic and anti-inflammatory effects in NC/Nga mice. Allergy. 2012;67:1014–22. [PubMed: 22686688]
- Paus R, Schmelz M, Bíró T, Steinhoff M. Frontiers in pruritus research: Scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–86. [PMC free article: PMC1451220] [PubMed: 16670758]
- Roggenkamp D, Falkner S, Stäb F, Petersen M, Schmelz M, Neufang G. Atopic keratinocytes induce increased neurite outgrowth in a coculture model of porcine dorsal root ganglia neurons and human skin cells. J Invest Dermatol. 2012;132:1892–900. [PubMed: 22418869]
- Romeo P.H, Lemarchandel V, Tordjman R. Neuropilin-1 in the immune system. Adv Exp Med Biol. 2002;515:49–54. [PubMed: 12613542]
- Rukwied R.R, Main M, Weinkauf B, Schmelz M. NGF sensitizes nociceptors for cowhage- but not histamine-induced itch in human skin. J Invest Dermatol. 2012;133:268–70. doi:10.1038/jid.2012.242. [PubMed: 22832487]
- Schuhknecht B, Marziniak M, Wissel A. et al. Reduced intraepidermal nerve fibre density in lesional and nonlesional prurigo nodularis skin as a potential sign of subclinical cutaneous neuropathy. Br J Dermatol. 2011;165:85–91. [PubMed: 21410670]
- Sharma A, Verhaagen J, Harvey A.R. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci. 2012;6:28. [PMC free article: PMC3389612] [PubMed: 22783168]
- Steinhoff M, Ständer S, Seeliger S, Ansel J.C, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–88. [PubMed: 14623709]
- Sumimoto S, Kawai M, Kasajima Y, Hamamoto T. Increased plasma tumour necrosis factor-alpha concentration in atopic dermatitis. Arch Dis Child. 1992;67:277–9. [PMC free article: PMC1793658] [PubMed: 1575548]
- Takano N, Sakurai T, Kurachi M. Effects of anti-nerve growth factor antibody on symptoms in the NC/Nga mouse, an atopic dermatitis model. J Pharmacol Sci. 2005;99:277–86. [PubMed: 16276037]
- Takano N, Sakurai T, Ohashi Y, Kurachi M. Effects of high-affinity nerve growth factor receptor inhibitors on symptoms in the NC/Nga mouse atopic dermatitis model. Br J Dermatol. 2007;156:241–6. [PubMed: 17223862]
- Takaoka K, Shirai Y, Saito N. Inflammatory cytokine tumor necrosis factor-alpha enhances nerve growth factor production in human keratinocytes, HaCaT cells. J Pharmacol Sci. 2009;111:381–91. [PubMed: 19942804]
- Taneda K, Tominaga M, Negi O. et al. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br J Dermatol. 2011;165:277–84. [PubMed: 21457210]
- Tang X.Q, Tanelian D.L, Smith G.M. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–27. [PMC free article: PMC6729810] [PubMed: 14749426]
- Tey H.L, Maddison B, Wang H. et al. Cutaneous innervation and itch in keloids. Acta Derm Venereol. 2012;92:529–31. doi:10.2340/00015555-1336. [PubMed: 22378106]
- Tominaga M, Kamo A, Tengara S, Ogawa H, Takamori K. In vitro model for penetration of sensory nerve fibres on a Matrigel basement membrane: Implications for possible application to intractable pruritus. Br J Dermatol. 2009b;161:1028–37. [PubMed: 19857208]
- Tominaga M, Ogawa H, Takamori K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br J Dermatol. 2008;158:842–4. [PubMed: 18241279]
- Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: An atopic dermatitis model. J Invest Dermatol. 2009a;129:2901–5. [PubMed: 19571818]
- Tominaga M, Ozawa S, Ogawa H, Takamori K. A hypothetical mechanism of intraepidermal neurite formation in NC/Nga mice with atopic dermatitis. J Dermatol Sci. 2007a;46:199–210. [PubMed: 17350228]
- Tominaga M, Ozawa S, Tengara S, Ogawa H, Takamori K. Intraepidermal nerve fibers increase in dry skin of acetone-treated mice. J Dermatol Sci. 2007b;48:103–11. [PubMed: 17643268]
- Tominaga M, Takamori K. Recent advances in pathophysiological mechanisms of itch. Expert Rev Dermatol. 2010;5:197–212.
- Tominaga M, Tengara S, Kamo A, Ogawa H, Takamori K. Psoralen-ultraviolet A therapy alters epidermal Sema3A and NGF levels and modulates epidermal innervation in atopic dermatitis. J Dermatol Sci. 2009c;55:40–6. [PubMed: 19443185]
- Tominaga M, Tengara S, Kamo A, Ogawa H, Takamori K. Matrix metalloproteinase-8 is involved in dermal nerve growth: Implications for possible application to pruritus from in vitro models. J Invest Dermatol. 2011;131:2105–12. [PubMed: 21697883]
- Verge V.M, Richardson P.M, Wiesenfeld-Hallin Z, Hokfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: A novel role in peptide suppression in adult sensory neurons. J Neurosci. 1995;15:2081–96. [PMC free article: PMC6578124] [PubMed: 7534343]
- Wallengren J, Sundler F. Phototherapy reduces the number of epidermal and CGRP-positive dermal nerve fibres. Acta Derm Venereol. 2004;84:111–5. [PubMed: 15206689]
- Wallengren J, Tegner E, Sundler F. Cutaneous sensory nerve fibers are decreased in number after peripheral and central nerve damage. J Am Acad Dermatol. 2002;46:215–7. [PubMed: 11807432]
- Wood L.C, Jackson S.M, Elias P.M, Grunfeld C, Feingold K.R. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–7. [PMC free article: PMC443124] [PubMed: 1644919]
- Xiao B, Patapoutian A. Scratching the surface: A role of pain-sensing TRPA1 in itch. Nat Neurosci. 2011;14:540–2. [PubMed: 21522144]
- Yamaguchi J, Nakamura F, Aihara M. et al. Semaphorin3A alleviates skin lesions and scratching behavior in NC/Nga mice, an atopic dermatitis model. J Invest Dermatol. 2008;128:2842–9. [PubMed: 18615113]
- Zhou Y, Gunput R.A, Pasterkamp R.J. Semaphorin signaling: Progress made and promises ahead. Trends Biochem Sci. 2008;33:161–70. [PubMed: 18374575]
- Review An update on peripheral mechanisms and treatments of itch.[Biol Pharm Bull. 2013]Review An update on peripheral mechanisms and treatments of itch.Tominaga M, Takamori K. Biol Pharm Bull. 2013; 36(8):1241-7.
- Review Intractable Itch in Atopic Dermatitis: Causes and Treatments.[Biomedicines. 2021]Review Intractable Itch in Atopic Dermatitis: Causes and Treatments.Umehara Y, Kiatsurayanon C, Trujillo-Paez JV, Chieosilapatham P, Peng G, Yue H, Nguyen HLT, Song P, Okumura K, Ogawa H, et al. Biomedicines. 2021 Feb 25; 9(3). Epub 2021 Feb 25.
- Review Itch and nerve fibers with special reference to atopic dermatitis: therapeutic implications.[J Dermatol. 2014]Review Itch and nerve fibers with special reference to atopic dermatitis: therapeutic implications.Tominaga M, Takamori K. J Dermatol. 2014 Mar; 41(3):205-12.
- Review Role of PAR-2 in Neuroimmune Communication and Itch.[Itch: Mechanisms and Treatment...]Review Role of PAR-2 in Neuroimmune Communication and Itch.Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Itch: Mechanisms and Treatment. 2014
- Review Peripheral Opioids.[Itch: Mechanisms and Treatment...]Review Peripheral Opioids.Bigliardi PL, Bigliardi-Qi M. Itch: Mechanisms and Treatment. 2014
- Sensitization of Itch Signaling - ItchSensitization of Itch Signaling - Itch
- Probe Reports from the NIH Molecular Libraries ProgramProbe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...
