NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014.
12.1. INTRODUCTION
The peripheral nervous system (PNS) is designed to receive inputs from the environment and transduce these into signals that are sent to the central nervous system (CNS). While most PNS neurons carry some receptors for classical neurotransmitters like glutamate, they are only weakly sensitive to these substances (or are not nearly as sensitive to them as CNS neurons are). Instead, as their primary function is to detect changes in external cues—for instance, heat and foreign chemicals—they are thought to be activated mostly through a broad range of receptors that recognize such environmental signals. These receptors can be divided into several broad groups. The groups comprising the olfactory and taste receptors represent the classic examples of families that, taken together, can detect an extraordinary range of substances from the environment. Both groups are members of the GPCR superfamily and are coupled to various G-proteins, through which they transduce their signals in second messenger mediated intracellular pathways. Each receptor is “tuned” to respond to varying degrees to chemicals with specific structures or properties. Many of these receptors exist—several dozen olfactory receptors in humans and several hundred in mice—and each is tuned differently. The circuitry underlying detection of odors is complex and knowledge of it is incomplete, though the field is moving forward rapidly, but it is thought that specific odors are detected by CNS processing of the signals sent from many different olfactory neurons that respond differently to the same odor. The ensemble input is different for every odor; in such a way, this library of receptors is capable of detecting a vast array of substances not ever made endogenously.
Among the sensations detected by the PNS, arguably the ones that induce the quickest behavioral (as opposed to motor) responses are those of pain and itch. These unpleasant sensations direct the organism to avoid a harmful situation or to remove a dangerous animal like a parasite. Like olfaction and taste, they are critical for survival, though extended experience of these sensations, often occurring in pathological states, dramatically lowers quality of life. It is known that mechanical stimulation and a wide range of chemicals can induce these sensations. While receptors have been discovered for several individual chemicals—one example is the TrpV1 channel for the neurotoxin capsaicin—other mechanisms must be discovered to account for the effects of most painful and itchy substances. One attractive hypothesis is that the neurons innervating the epithelia employ a family of receptors, analogous to the olfactory system, that serve to detect these noxious stimuli.
The Mrgpr family of receptors was discovered in 2001 and comprises 18 genes and pseudogenes in humans and 50 in mice (Dong et al. 2001) (Figure 12.1a). Many members are expressed exclusively in the dorsal root ganglia (DRG) and trigeminal ganglia (TG), which extend neurites into several layers of the skin and are responsible for most peripheral sensations, including noxious mechanical stimuli and temperature (Dong et al. 2001) (Figure 12.1b). This expression pattern raises the exciting possibility that they are specialized for somatosensation. In 2009, one of these receptors was found to be critical for the itch induced by the antimalarial drug chloroquine (Liu et al. 2009). In this and other studies, other pruritic substances were also shown to activate Mrgpr family members, further linking them to itch sensation (Liu et al. 2009, 2011). At the moment, it is unclear whether most pruritic stimuli act through these receptors, at least indirectly, but they have helped clarify the neural mechanisms underlying itch, and to a lesser extent, pain sensation. The history of research on Mrgprs is presented here, including what is known currently about this interesting family of receptors.

FIGURE 12.1
Overview of the Mrgpr family and generation of cluster knockout mice. (a) Protein phylogenetic tree of human and mouse Mrgpr family members. Ψ denotes pseudogenes. L1 denotes genes with transposon elements within ~650 bases of the 3′ end (more...)
12.2. DISCOVERY OF THE Mrgprs
The Mrgprs were cloned around the turn of the millennium, before the human and mouse genome projects were completed, in a screen performed using a knockout mouse with a striking and fortuitous phenotype (Dong et al. 2001). In the late 1990s, a basic helix-loop-helix transcription factor called Neurogenin 1 (Ngn1) was found to be critical for the development of a subset of neurons in the DRG that express TrkA, the receptor for nerve growth factor (Ma et al. 1999). This subset includes most neurons that detect nociceptive, or noxious, stimuli. Ngn1, which is expressed transiently in the DRG during embryogenesis, was shown to act as a master regulator of neurogenesis in these neurons, controlling the expression of a host of genes including the widely used developmental neuronal marker NeuroD (Ma et al. 1999). Without Ngn1, most nociceptive DRG neurons failed to develop, while others involved in types of low threshold mechanical input were still present (Ma et al. 1999). This opened up an opportunity to identify nociceptor-specific genes, by performing subtractive hybridization of the cDNAs in newborn wild-type and Ngn1-/- mice. The transcripts absent from the Ngn1-/- mice were likely to be exclusive to nociceptors.
Several genes already known to be involved in nociceptor function were identified from the screen, verifying the approach (Dong et al. 2001). Multiple unknown transcripts were also recovered, including one that strongly resembled a GPCR (Dong et al. 2001). This was found to have ~30% to 35% identity with Mas1, the putative receptor for angiotensin (1–7), a peptide involved in blood pressure and control of osmolarity (Santos et al. 2003). Interestingly, Mas1 is not enriched in the DRG and is not known to participate in nociception, indicating that this new receptor had a different endogenous ligand. This founding member was named Mas-related gene A1 (MrgprA1). Additional screens of DRG cDNA, and BAC libraries turned up several dozen members that could be divided into three groups, the MrgprA, MrgprB, and MrgprC families (Dong et al. 2001) (Figure 12.1a). Over 50 distinct sequences were found in mice, although it is unclear how many are physiologically relevant, as many do not have open reading frames and only a few have been shown to be expressed (Dong et al. 2001). Of the originally discovered members, 21 can be annotated to current editions of the mouse genome and are not predicted to be pseudogenes (Figure 12.1a). In addition to these, a more distantly related group of receptors was discovered and named MrgprD-H (Dong et al. 2001).
Human Mrgpr family members were discovered in searches of contemporary databases (Dong et al. 2001; Lembo et al. 2002). Human orthologs of the MrgprD-H members were fairly clear, though, interestingly, only four other family members were found. These members were somewhat more closely related to the mouse MrgprA family members than to other families, though no clear orthologs existed, and these members were given the names MrgprX1-4 or SNSRs (sensory neuron specific receptors) called by different groups (Dong et al. 2001; Lembo et al. 2002). The mouse MrgprA, MrgprB, and MrgprC family genes are all found in a tight cluster on chromosome 7 (Figure 12.1c). Likewise, the human MrgprX family is found clustered on chromosome 11. Both mouse and human genomic regions are characterized by multiple repetitive transposon elements, to the point that very little in the region except for the Mrgprs is unique to the genome (Zylka et al. 2003). Four studies have reported that the human genomic region is duplicated, with up to six copies detected in some individuals (Hindson et al. 2011; Kato et al. 2008; Redon et al. 2006; Wong et al. 2007). The sequences reported for the MrgprX family are slightly different (Dong et al. 2001; Lembo et al. 2002), provoking the question of whether these represent polymorphisms between individuals, or perhaps a different situation in which each individual actually has different, highly related MrgprX genes in the duplicated DNA.
12.3. EXPRESSION OF Mrgprs
Expression of MrgprA1-8 was detected by in situ hybridization in a subset of DRG and trigeminal neurons, but nowhere else in the body (Dong et al. 2001) (Figure 12.1b). Among members of the MrgprA-C families expressed at birth, MrgprA1 was, by far, the most widely expressed, seen in 13.5% of all DRG neurons. MrgprD also had a fairly wide expression pattern, but most other Mrgpr family members were observed only in a few neurons (~1%) or not at all at birth (Dong et al. 2001). Notably, several of the MrgprA members colocalized in the same neurons, indicating specialization of those neurons, and a partial overlap was seen between MrgprA1 and MrgprD (Dong et al. 2001). Importantly, Mrgpr expression was observed almost exclusively in neurons that were TrkA+ and could be stained with the plant lectin IB4, a marker of nociceptors (Dong et al. 2001). Most Mrgpr+ neurons did not overlap with proinflammatory peptides Substance P or CGRP labeling, indicating that most were nonpeptidergic, though low expression of these peptides could not be ruled out (Dong et al. 2001).
Some aspects of Mrgpr family expression were notably different in adult tissues. While Mrgpr-expressing neurons were IB4+, as in newborns, Mrgpr expression patterns largely became nonoverlapping within this neuronal subset (Dong et al. 2001). Specifically, Mrgpr+ neurons could be segregated into MrgprA3+, MrgprD+, and MrgprB4+ neurons, with (~5, 30, and 1% of total DRG neurons, respectively) (Dong et al. 2001). MrgprA1 expression was strongly reduced; other MrgprA family members could be detected in MrgprA3+ neurons at lower levels, and, interestingly, MrgprC11, the only coding member of the MrgprC family, was expressed in a pattern closely overlapping the MrgprA3 signal (Dong et al. 2001; Liu et al. 2009). In addition, nearly half of the MrgprA+ neurons now expressed CGRP, while Substance P expression was still undetectable both in MrgprA+ and MrgprD+ neurons (Dong et al. 2001).
The expression patterns of the human MrgprX family and MrgprD have not been characterized at the same level of detail as in mice. Human MrgprX1, MrgprX3, and MrgprX 4 were found in a human DRG cDNA library (Lembo et al. 2002), though separate studies have reported MrgprX2 expression in the DRG, as well (Kamohara et al. 2005; Robas et al. 2003; Zhang et al. 2005). No MrgprX family members were found in a panel of human tissues that did not include the DRG or TG (Dong et al. 2001), and a more complete panel confirmed expression only in the DRG and TG (Lembo et al. 2002), indicating that expression is spatially restricted, as in mice. MrgprX2, and to a much lesser extent, MrgprX1, has been detected in mast cells, which is to date the only reported cellular localization of Mrgprs outside of neurons (Subramanian et al. 2011; Tatemoto et al. 2006).
The highly restricted tissue expression pattern—exclusive to nociceptors and divided segregated within this class—raised the possibility that the Mrgprs were involved in some aspect of somatosensation, perhaps analogous to olfactory or taste receptors. This was bolstered by a pair of studies in which genetically encoded cellular labels were knocked into the MrgprB4 and MrgprD loci (Liu et al. 2007; Zylka et al. 2005). These modifications enabled easy detection of the complex morphology of neurons that normally express these receptors. Knocking in PLAP and, separately, eGFP into the MrgprD locus revealed that neurites extending to the skin exhibited extensive branching and termination into all layers of the epidermis except the dead cell layer of the stratum corneum (Zylka et al. 2005). MrgprB4+ neurons, labeled with PLAP, were also shown to penetrate into the shallow layers of the epidermis and undergo branching (Liu et al. 2007). The morphological characteristics of these neurons poised them for detection of subtle environmental stimuli; however, the exact function(s) of these receptors and the neurons that expressed them were not obvious from morphological data alone.
12.4. GENERATION OF Mrgpr KNOCKOUT ANIMALS
A knockout approach was undertaken for members of the MrgprA-C cluster (Liu et al. 2009). Generation of knockout animals turned out not to be a trivial task, owing to the extensive repetitive sequence that blanketed the entire cluster. The repetitive elements meant that very little DNA sequence in the locus was specific to the area, a serious impediment to conventional homologous recombination techniques that require unique sequences flanking the targeted gene to align the knockout/knockin construct to the locus during mitosis. After screening more than 10,000 embryonic stem cell clones, one knockout each was obtained for MrgprA1 and MrgprB4, genes selected for their high expression and presumed importance (Liu et al. 2009).
Given the dramatically expanded number of family members in mice, it seemed plausible that some of the genes are redundant and single knockouts might not show much of a phenotype. Therefore, an unusual approach was taken to knock out the genes between and including MrgprA1 and MrgprB4 (Mrgpr-clusterΔ-/- mice) (Liu et al. 2009) (Figure 12.1c). This took advantage of the remaining LoxP site in each knockout and was achieved by transient expression of Cre recombinase in stem cells harboring a copy of both knockouts to splice out the intermediate DNA (Liu et al. 2009). A total of 845 kb was deleted, containing 12 of the estimated 24 genes with complete open reading frames (MrgprA1-4, A10, A12, A14, A16, A19, B4, B5, and C11) (Liu et al. 2009) (Figure 12.1c). This includes most of the MrgprA family, and all of the genes that had been shown to be expressed at a high level in the DRG and TG. Importantly, no non-Mrgpr ORF exists in the deleted region.
12.5. ROLE OF Mrgprs IN ITCH IN MICE
The list of pruritogens used in animal studies is quite long. A landmark study in the itch field, published not long after the Mrgpr-cluster Δ-/- mice were generated, demonstrated that itch sensation induced by several of these well-established pruritogens was dependent on the receptor for gastrin-releasing peptide (Sun and Chen 2007). One of the substances used in the paper, an antimalarial drug called chloroquine (CQ), stood out because it was the least expensive by a large margin. In addition, study of its itch-inducing mechanism is medically important, as CQ is still in therapeutic use. Unfortunately, oral CQ evokes strong itch sensation in black Africans, which can be so strong that it limits compliance (Sowunmi et al. 1989). Researchers in Africa have undertaken many studies on the mechanism of such a highly undesirable side effect. They found that this type of itch does not fit the profile of an allergic response and that antihistamines are largely ineffective, indicating that another way to induce itch exists alongside the classical, histamine-dependent mechanism associated with allergic responses (Ekpechi and Okoro 1964; Salako 1984).
Mrgpr-cluster Δ-/- mice did not show general deficits in nociception. Multiple tests of pain sensation showed that Mrgpr-cluster Δ-/- mice responded similar to wild type (Liu et al. 2009). Histamine and compound 48/80-induced itch sensation were not reduced in Mrgpr-cluster Δ-/- mice, demonstrating that the ability to perceive itch was retained (Liu et al. 2009) (Figure 12.2a and b). A subcutaneous dose of CQ injected into the nape of the neck of wild-type mice induced strong scratching behavior within a couple of minutes, and which lasted for tens of minutes (Liu et al. 2009). Strikingly, CQ-induced scratching in Mrgpr-cluster Δ-/- mice was reduced by over 65%, indicating that an Mrgpr family member mediated most of this effect (Liu et al. 2009) (Figure 12.2c). The residual effect may be mast cell dependent, as scratching was reduced by ~35% in mice lacking mast cells, consistent with studies showing that CQ can activate mast cells (Liu et al. 2009).
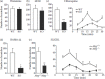
FIGURE 12.2
Scratching response to select pruritogens is drastically reduced in Mrgprcluster Δ-/- mice. (a, b) Scratching bouts in response to histamine (10 μmol) and the mast cell degranulator Compound 48/80 (100 μg) are comparable in WT (more...)
Cultured DRG neurons were used to determine whether this effect correlated with DRG activation. Approximately 5% of wild-type neurons, mostly small-to-medium diameter, responded to CQ with an increase in intracellular calcium and generation of action potentials (Liu et al. 2009) (Figure 12.3a and b). This effect was completely abolished in Mrgpr-cluster Δ-/- neurons (Liu et al. 2009) (Figure 12.3a and b). Because 12 possibly functioning receptors were deleted, it became imperative to find out which one(s) responded to CQ. Testing in heterologous cells revealed that MrgprA3, a highly expressed member in adult mouse DRG, had the strongest response, and that no other deleted member could be activated except for a very weak response by MrgprA1 (Figure 12.4a left panel). MrgprA3 expression in Mrgpr-cluster Δ-/- mice restored sensitivity to CQ, and siRNA-mediated knockdown blocked the response in wild-type neurons, firmly establishing that MrgprA3 is the receptor for CQ in mouse DRG (Liu et al. 2009). Importantly, related chemicals were not active, establishing the specificity of the ligand–receptor relationship (Liu et al. 2009) (Figure 12.4b and c).

FIGURE 12.3
DRG neurons responsive to CQ and BAM8-22 define a subset of the nocioceptive population. (a) Cultured DRG neurons from young adult Mrgprcluster Δ-/- mice show no response to 1 mM CQ but respond normally to 50 μM histamine (n = 3 experiments (more...)

FIGURE 12.4
Responses to various pruritogens in Mrgpr-transfected cell lines. (a) Representative traces of the rise in intracellular calcium evoked by Mrgpr agonists in transfected HEK cells. Cells were loaded with the calcium indicator Fura-2 and imaged at 340- (more...)
The discovery of CQ as an MrgprA3 agonist served as a tool to find a human ortholog, as none clearly exists from sequence homology. MrgprX1 was found to be activated by CQ, a striking finding, since MrgprX1 is also found in the DRG, and the gene rests in the same part of the human genome as MrgprA3 is in the mouse (Liu et al. 2009) (Figure 12.4a and c). A variety of peptide libraries had already been tested on MrgprX1, and the most specific ligand found was a fragment of Bovine Adrenal Medulla peptide (BAM8-22) (Lembo et al. 2002). Full length BAM is a ligand for opioid receptors and for MrgprX1; however, BAM activates the opioid receptors at its N-terminus only, and removing this to generate BAM8-22 abolished this activity but did not affect MrgprX1 activation (Lembo et al. 2002). In light of the link between Mrgprs and itch, BAM8-22 was tested for pruritogenic activity by subcutaneous injection into the nape of the neck. As with CQ, BAM8-22 induced itch in wild-type mice and a response in DRG neurons, while Mrgpr-cluster Δ-/- mice had almost no behavioral or neuronal response (Liu et al. 2009) (Figures 12.2d and 12.3c). Interestingly, BAM8-22 was found not to activate MrgprA3, but MrgprC11, illustrating a possible complicated relationship between Mrgprs in rodents and those in other species (Liu et al. 2009) (Figure 12.4a).
An unexpected discovery in a subsequent study perhaps hints at a much broader role for the Mrgprs in itch mediation. Over 10 years ago, a GPCR called PAR2 was linked to inflammation and itch (Steinhoff et al. 2000, 2003). PARs, or protease-activated receptors, are a unique family of GPCRs whose ligands come from their own N-termini. Upon exposure to serine proteases (e.g., trypsin and tryptase), mouse PAR2 is cleaved at a residue that is just N-terminal to the amino acid sequence SLIGRL, such that the unmasked sequence can now activate PAR2 itself (Ramachandran and Hollenberg 2008). For each PAR, a synthetic peptide has been designed, on the basis of the exposed sequence after protease cleavage, and these so-called activating peptides are widely used as specific agonists for their corresponding PARs. SLIGRL-NH2 is the activating peptide for PAR2 and induces itch in wild-type mice, but had not been tested in PAR2 -/- mice to confirm that it actually worked through its parent receptor (Shimada et al. 2006). When this test was performed, the PAR2-/- mice actually scratched more than wild-type mice, ruling out a role for the receptor in inducing itch (Liu et al. 2011). Instead, when tested in the Mrgpr-cluster Δ-/- mice, the scratching response was nearly abolished (Figure 12.2d and e), as was the response in DRG neurons (Figure 12.3d) (Liu et al. 2011). SLIGRL-NH2 was found to activate MrgprC11, like BAM8-22 (Liu et al. 2011) (Figure 12.4d and e). The corresponding human peptide, SLIGKV-NH2, was found to activate MrgprX2, not MrgprX1, underscoring the need to account for species differences in the ligand-binding profiles (Liu et al. 2011).
This finding invites speculation that a relationship exists between Mrgprs and proteases. If the SLIGRL/SLIGKV sequence becomes untethered by further proteolysis, the free peptide can activate Mrgprs in the vicinity, which would be an unprecedented form of cross-activation between receptors. Because PAR2 has been detected on small diameter DRG neurons, colocalization close enough for cross-activation is plausible. Another unexplored possibility is that, because Mrgprs can be activated by PAR ligands, they might have a similar binding pocket and might themselves be activated by proteases, though no sequences orthologous to SLIGRL/SLIGKV exist in the extracellular regions.
In a fascinating finding, DRG neurons responding to BAM8-22, CQ, and SLIGRL almost completely overlapped, consistent with in situ studies showing overlap of Mrgprc11 and Mrgpra3 mRNA, and were also responsive to histamine and capsaicin (Liu et al. 2009; Zylka et al. 2003) (Figure 12.3e and h). This implicated this subset of neurons as specialized, itch-selective nociceptors, though the exact significance is still unclear (and may be species specific, as described in the Questions section).
12.6. Mrgpr SIGNALING PATHWAYS
Recent studies have implicated transient receptor potential (Trp) channels as intracellular mediators of multiple receptors involved in nociception (Bandell et al. 2004; Imamachi et al. 2009; Jordt et al. 2004). This exciting adjustment to classical modes of GPCR signaling is expanding to a diverse collection of GPCRs and is in line with experiments showing abundant expression of multiple Trp channels in nociceptive neurons (Moran et al. 2011; Wu et al. 2010). The mouse DRG neuron response to CQ was blocked by ruthenium red, a nonspecific blocker of Trp channels, and also by extracellular EGTA, a calcium chelator, implicating that calcium influx via Trp channels is a crucial part of MrgprA3 signaling (Liu et al. 2009). A further set of experiments showed that TrpA1 was required for CQ and BAM8-22 induced rises in intracellular calcium and action potentials in DRG neurons (Wilson et al. 2011). No scratching was observed in TrpA1-/- mice, and both MrgprA3 and MrgprC11 were shown to couple to TrpA1 in heterologous cells (Wilson et al. 2011). The pathways were not identical, however. MrgprC11 signaling was shown to require phospholipase C (PLC) as an intermediate, while MrgprA3 signaling did not require PLC but instead involved an active Gbeta/gamma complex (Wilson et al. 2011). This in itself is another interesting modification of the classical GPCR models, in which the alpha subunits are the active messenger proteins. Importantly, MrgprC11 did not require active Gbeta/gamma signaling, indicating at least partially exclusive pathways to the same mediator (Wilson et al. 2011).
A surprising finding from this series of studies is that TrpV1, an abundant channel in nociceptors and one present in MrgprA3+/MrgprC11+ neurons, was not required for signaling (Wilson et al. 2011). Very little is known about how these receptors specifically couple to TrpA1 while “avoiding” TrpV1, though there is some indication that MrgprC11 actually can couple to TrpV1 when the two are overexpressed (Wilson et al. 2011). Among the unresolved questions are whether the receptors form separate complexes with Trp channels or act on a common pool, and whether other Trp channels are involved, perhaps as heterodimers with TrpA1.
MrgprX1 may have an inhibitory role in some neurons, or at least in specific neuronal compartments. BAM8-22 strongly inhibited the high voltage activated calcium current in the somata of primary neurons heterologous expressing MrgprX1 (Chen and Ikeda 2004). This is difficult to reconcile with the observed BAM8-22 induced rise in intracellular calcium, and action potential generation, in mouse DRG neurons, but it is possible that MrgprX1 and MrgprC11 can couple to different effectors in different cell types or in different parts of the same cell. Interestingly, BAM8-22 was shown to inhibit pain responses in mouse models of several types of pathological pain (Guan et al. 2010). Perhaps this ability, which is Mrgpr dependent, is due to selective inhibition of the pain circuit.
12.7. BAM8-22, AN Mrgpr AGONIST, INDUCES ITCH IN HUMANS
Mouse studies indicated that skin injection of BAM8-22 induces itch (Liu et al. 2009). If this is the case, it could be an endogenous transmitter for itch and would make MrgprX1 a potentially useful therapeutic target for more than just CQ-induced itch. To test whether BAM8-22 can induce itch in humans, Robert LaMotte’s laboratory applied BAM8-22 onto the skin of healthy volunteers by inactivated cowhage spicules loaded with the peptide (Sikand et al. 2011). Cowhage spicules penetrate the shallow layers of skin and act as an epidermal and intradermal applicator. All individuals reported strong itch sensation at a level similar to histamine application (Sikand et al. 2011). Importantly, a truncated form of BAM8-22, BAM8-18, which lacks the Mrgpr-interacting motif, failed to elicit any sensation in these people (Sikand et al. 2011). In addition, application of an antihistamine cream, which abolished histamine-evoked itch, had no effect on BAM8-22 induced itch (Sikand et al. 2011). Together these data confirm what was found in mice and demonstrate that BAM8-22 may be an endogenous itch mediator in humans and that its action likely goes through MrgprX1. Interestingly, an animal model of liver disease, which often leads to itch symptoms in humans, has elevated BAM22 levels in blood (Swain et al. 1994).
As mentioned briefly above, extensive copy number variation (CNV) in the MrgprX1 gene has been observed, first in a pair of studies of CNV across the human genome and later shown in two independent studies (Hindson et al. 2011; Kato et al. 2008; Redon et al. 2006; Wong et al. 2007). In fact, as many as six copies were found in some individuals (Hindson et al. 2011). The Mrgpr cluster contains a tremendous number of repetitive elements, and as described earlier in this chapter, duplication of a genomic region including a presumed MrgprX ancestor has been proposed to allow for the expansion of family members (Zylka et al. 2003). It seems plausible that genomic instability in this region has led to these repeats in humans. As of this writing, such extensive duplication in other Mrgprs has not been discovered, limiting studies to MrgprX1. If copy number is correlated with mRNA and protein expression levels, it may indicate a striking heterogeneity in the human population in its sensitivity to MrgprX1-recognized pruritogens.
A further possible source of variation in chloroquine sensitivity may come from polymorphisms. Numerous mouse Mrgprs have been shown to carry polymorphisms in different strains (Dong et al. 2001); some coding differences exist between the MrgprX1 genes reported by the first two labs to clone the gene (Dong et al. 2001; Lembo et al. 2002), and dbSNP at NCBI has archived many missense mutations in the human population, though how these might affect sensitivity has not been studied. Given the multiple copies of MrgprX1 in many individuals, it may be that one individual could have several different SNPs and that particular combinations may result in widely varying sensitivities. It is interesting that macaques have been shown to carry the canonical receptor genes, along with additional copies of several of the MrgprXs that differ only in a few amino acids (Zhang et al. 2005). These are slightly more divergent than single SNPs and may be the predicted additional copies on duplicated DNA. Their activation profiles actually are somewhat different from the canonical receptors, giving some validity to these possibilities (Zhang et al. 2005).
12.8. DIFFERENCES BETWEEN ITCH RECEPTORS AND OLFACTORY RECEPTORS
Olfactory neurons, like nociceptors, have numerous receptors designed to detect information from the environment. Each olfactory neuron expresses only one of these receptors, and odorous substances activate a subset of receptors, and thus a subset of neurons, to varying degrees (Gottfried 2010). It is thought that each smell activates a different subset and at different intensities, resulting in a unique signature (Gottfried 2010). The data from Mrgprs indicate that itch sensation is not as sensitive as this. Unlike olfactory neurons, a single nociceptor can express multiple Mrgprs implicated in itch, as well as histamine receptors. Thus, several different pruritogens can activate the same neurons that could carry mostly the same information. Perhaps the intensity of activation encodes additional information, but it is unclear how the signal could be more than whether something is itchy and how itchy it is.
Three findings hint that itch sensation might be more complex. First, recent data indicate that a subset of myelinated neurons, which would not include the majority of Mrgpr+ neurons, can also transmit itch signals (Ringkamp et al. 2011). Little is known about these neurons, but it opens up the possibility that different pruritogens can be perceived differently. Second, some pruritogens induce more than just itch. If some pruritogen receptors are on multiple types of neurons or other cell types involved in nociception, the ligands for those receptors might elicit a mixed sense profile. One example of such a profile, actually, is BAM8-22, which induces a stinging sensation along with itch, though it is not known why (Sikand et al. 2011). Third, BAM8-22 may be able to elicit different sensations, depending on what part of the body is exposed to it. BAM8-22 induces itch and some stinging sensation when applied to human forearms and scratching behavior when administered subcutaneously to the mouse rostral back (Liu et al. 2009; Sikand et al. 2011). However, when injected into the mouse hind paw, it induces activity more consistent with pain (and similar to the response to capsaicin) (Grazzini et al. 2004). Because no model exists for animal itch in nonhairy skin, the licking and biting observed may indeed be a response to itch, though hyperalgesia to heat was also observed, more consistent with pain (Grazzini et al. 2004). Another study showed that spinal application of BAM8-22 actually reduced pain behaviors, demonstrating a clearly complex role for MrgprC11 in mouse nociception (Guan et al. 2010). Regardless, this raises the possibility that pruritogen receptors like MrgprC11 may exist on different subtypes of neurons, depending on where they terminate in the body, or, alternatively, that the same types of neurons are part of different circuits.
12.9. OTHER Mrgprs
Much less is known about Mrgpr family members that are not orthologs of MrgprX1. The only other members linked to putative ligands are MrgprD, MrgprA9, and MrgprX2. The locations of most family members are incompletely characterized, and, except for MrgprD-G, it is not even clear whether orthologs exist across species. In this section, summaries are provided of information regarding the better characterized family members.
12.9.1. MrgprD
MrgprD, as discussed above, is present in the majority of IB4+, nonpeptidergic DRG neurons, meaning that most nociceptors express this gene (Zylka et al. 2005). RT-PCR assays turned up evidence for lower levels of expression in the bladder, uterus, testes, and arteries in a panel of rat mRNA pools (Shinohara et al. 2004). MrgprD orthologs from humans, rats, and mice respond to beta alanine, a natural cellular metabolite, at low micromolar concentrations in heterologous cells (Shinohara et al. 2004). The most pronounced effect both in mouse and rat DRG neurons was to enhance their excitability by inhibiting potassium channel M-currents via a Gi/o dependent pathway (Crozier et al. 2007; Rau et al. 2009). The enhancement was such that a stimulus that normally produced a single action potential now produced a short train (Crozier et al. 2007; Rau et al. 2009). Thus, the observed effect was to modulate neurons so that they were more sensitive to other stimuli. DRG neurons from MrgprD-/- mice were less sensitive than wild-type neurons to heat, cold, and mechanical stimulation (Rau et al. 2009). Because beta-alanine levels in the body are high enough to activate MrgprD, it may be that MrgprD is constitutively active and that nociceptors adjust their overall excitability when they sense extreme changes in beta-alanine levels.
MrgprD+ neurons do not appear to be linked to a single sensation. Mice with genetically encoded cell tracers in the MrgprD locus revealed that these neurons make connections in all parts of Lamina II in the dorsal horn of the spinal cord, the target of most nociceptors (Wang and Zylka 2009). No specific type of neuron was enriched, and no area in Lamina II was obviously more targeted. One study showed that the cells were highly responsive to ATP and almost totally unresponsive to other noxious substances (Dussor et al. 2008). It thus appears that the function of MrgprD is considerably different from MrgprA3 and MrgprC11.
12.9.2. MrgprA9
The rat ortholog of mouse MrgprA9 was found primarily in small diameter DRG neurons, and in a separate study, mouse MrgprA9 was found in the brain and spleen, though DRG tissue was not examined (Bender et al. 2002; von Kugelgen et al. 2008). Both orthologs were activated by low nanomolar concentrations of adenine, a cell metabolite and potential transmitter, resulting primarily in Gi/o activity (Bender et al. 2002; von Kugelgen et al. 2008). The effects of adenine in neurons were not examined, and because human MrgprX family members are unresponsive to adenine (Bender et al. 2002), it appears that this receptor is rodent specific.
12.9.3. MrgprE and MrgprF
MrgprE and MrgprF appear to be linked somehow, as mouse MrgprE knockouts have strongly reduced expression of MrgprF but no other tested family member (Cox et al. 2008). MrgprE was found in multiple brain regions in mice and humans, with expression appearing to be somewhat more restricted in humans (Zhang et al. 2005). Human MrgprE also was found in the placenta (Zhang et al. 2005). Human MrgprE was detected in ~85% of DRG neurons and a subset of enteric neurons (Avula et al. 2011; Zhang et al. 2005). MrgprE knockout mice responded normally to all tested pain assays, and the function and ligands of this receptor have yet to be discovered (Cox et al. 2008).
MrgprF expression is poorly characterized, though it, too, was found in enteric neurons in mice (Avula et al. 2011). Interestingly, despite the putative link between the two receptors, MrgprE and MrgprF were only rarely detected in the same enteric neurons (Avula et al. 2011). Both receptors were downregulated in inflammatory intestinal conditions (Avula et al. 2011). The ligand(s) for MrgprF, too, awaits discovery.
12.9.4. MrgprX2
MrgprX2 is a unique member of the family in that it is the only member shown to be expressed in a specific cell type outside of the nervous system. The mRNAs for MrgprD and MrgprE have been detected in nonnervous tissue, but the cell types are undetermined (Shinohara et al. 2004; Zhang et al. 2005). While MrgprX2 has been detected by several laboratories in the DRG (Kamohara et al. 2005; Robas et al. 2003; Zhang et al. 2005), it also is expressed in mast cells, a part of the innate immune system (Subramanian et al. 2011; Tatemoto et al. 2006). Mast cells are found in most tissues but are enriched in areas at the interface between the inside and outside of the organism, like the skin, lung, and intestine. They are thought of as “first response” cells, activated by pathogens shortly after they breach the tissue, and release immunomodulatory factors, proteases, and massive amounts of histamine and other transmitters that together help inactivate or kill the pathogens (Theoharides et al. 2012). Their classical activation pathway involves cross-linking of IgE receptors, but they also can be activated by numerous positively charged peptides, including exogenous peptides in venoms and toxins, and peptides released from neurons, like substance P and members of the bradykinin family (Ferry et al. 2002; Pundir and Kulka 2010). They occupy a prominent role in itch, because they are the major source of histamine in the skin (Yamatodani et al. 1982), and pain, because they can induce acute inflammation (Theoharides et al. 2012).
Interestingly, MrgprX2 was shown to be activated by substance P and several other mast cell activators at micromolar levels, higher than required for their canonical receptors, if known, but in line with concentrations needed for mast cell activation in mice (Tatemoto et al. 2006). These substances are generally thought to be receptor independent, as they have been shown to intercalate into lipid bilayers and possibly mimic the G-protein activating domains of GPCRs to activate G proteins directly (Ferry et al. 2002). In the absence of knockout studies, the role of MrgprX2 is unknown, especially given the high concentrations of agonists required to activate the receptor. No confirmed orthologs in rodents have been identified to facilitate study of MrgprX2. Rat MrgprB3 was shown to respond to two mast cell activators, but the concentrations required and responses of other Mrgprs was not reported (Tatemoto et al. 2006). No examination has been reported for a mouse ortholog.
12.10. DIMERIZATION
Mrgprs have been considered as though they operate independently of each other, even though multiple family members have been found in the same cells. Two studies have shown that this view may have to be reconsidered. In one study, MrgprD and MrgprE were shown to interact when coexpressed in heterologous cells (Milasta et al. 2006). The presence of MrgprE enhanced the intracellular signaling ability of MrgprD after beta-alanine stimulation and slowed down its internalization rate (Milasta et al. 2006). Local subcellular regulation of MrgprE may thus modulate MrgprD signaling so that the same ligand can have dramatically different effects, depending on where it contacts the cell. If a ligand is found for MrgprE, the same experiment can be tested on it to see if the modulation is bidirectional. This also suggests that activation of one family member may be able to influence the surface fraction and activity of others in the cell.
Another study found a possible interaction between MrgprX1 and delta opioid receptor (DOR) (Breit et al. 2006). When an agonist, BAM22, which could activate both receptors, was applied to cells overexpressing both receptors, MrgprX1 was activated at the expense of DOR signaling (Breit et al. 2006). Coapplication of agonists that activate each receptor independently resulted again in selective MrgprX1 signaling while inhibiting DOR signaling (Breit et al. 2006). The two receptors evidently could heterodimerize when overexpressed, indicating that they can form a complex and that MrgprX1 somehow dominates over DOR signaling when signals for both receptors are received (Breit et al. 2006). The physiological relevance of this is unclear, but considering that MrgprX1 agonists may inhibit pain sensation (Guan et al. 2010), a role for Mrgprs in modulation of pain pathways is an especially exciting idea. MrgprX2 can be activated directly by morphine (Akuzawa et al. 2007) MrgprX2 might be involved in morphine-induced itch.
12.11. QUESTIONS
The Mrgpr field, and indeed the entire field of itch, is exciting but immature. Even basic cell biology tools like reliable antibodies are not established, while the networks that define itch pathways in the nervous system are just beginning to be sketched out. Thus, perhaps it is not surprising that a number of fundamental questions have not been answered. In this section, we will cover some of the most outstanding issues that must be resolved to sharpen our view of the field.
12.11.1. Do Mrgpr Expression Patterns Change between Species?
In mice, studies have shown that MrgprA3, MrgprC11, and histamine receptors are colocalized in a subset of DRG neurons (Liu et al. 2009). This has led to the hypothesis that these neurons are itch selective. The story, however, becomes more complicated when data from other species are taken into account. A population of rat trigeminal and DRG neurons are responsive to chloroquine but not histamine, demonstrating that not all pruritogens activate chloroquine-sensitive neurons (Klein et al. 2011). The rat ortholog of MrgprC11, MrgprC, does not closely colocalize with other Mrgprs (Zylka et al. 2003). Humans have a mixed response to BAM8-22, reporting a stinging sensation, and thus do not experience pure itch (Sikand et al. 2011). This indicates that MrgprX1, if this is the only receptor for BAM8-22, is in a wider group of neurons than those involved in itch, though other explanations are possible.
These possible differences in expression patterns are hardly unique to Mrgprs. Expression of TrpV1 and Substance P has been reported to differ as well between rats and mice, and even between trigeminal and DRG neurons in the same species (Price and Flores 2007). It still may be the case that itch-selective neurons exist, but the markers that precisely define these neurons may not be the same between species. Also, more than one population of itch neurons may be present. In primates, different CNS neurons respond to histamine and cowhage, another pruritogen, indicating that at some point the nervous system detects them as distinct signals (Davidson et al. 2007). Myelinated neurons may also carry an itch signal, and some evidence exists in humans that neurons sensitive to pruritogens do not completely overlap (Johanek et al. 2008; Ma et al. 2012; Ringkamp et al. 2011).
12.11.2. Truly Orthologous Pairs?
The homology between putative orthologous Mrgprs in humans and mice is lower than for most GPCRs. Human MrgprX1 shares only 54% amino acid identity with the mouse MrgprC11 and less than 50% with mouse MrgprA3, its other putative ortholog. In contrast, other receptors associated with itch share much higher identity. For instance, the longest forms of the Histamine 1 receptor are 78% identical, and various splice variants of the mu opioid receptor exceed 90% identity. In fact, the relatively low sequence similarity and expansion in rodents has made pairing of orthologs between the MrgprX family and the mouse MrgprA-C cluster difficult. To date, only MrgprX1 has been shown to have mouse orthologs, and these were linked through similar ligand binding profiles and expression patterns (Han et al. 2002; Lembo et al. 2002; Liu et al. 2009). However, some divergences have been observed that complicate such a simple approach. One notable example is that MrgprX1 in humans responds to chloroquine and BAM8-22, while separate receptors are needed in mouse to detect these substances (Liu et al. 2009). SLIGRL-NH2, the canonical mouse PAR2 agonist, is detected by MrgprC11, while the comparable sequence in humans, SLIGKV-NH2, is recognized not by MrgprX1 but by its relative, MrgprX2 (Liu et al. 2011). The relevance of the differences in affinity is unknown because it is unclear which, if any, of these substances actually transmits an itch signal. It is reasonable to predict that some of these substances are unimportant to itch, that the receptors are not exposed to them, and thus that any differences in affinity are not relevant. However, it also is possible that the mouse and human receptors are not fully orthologous and may have some unique properties.
12.11.3. Ligands—Endogenous or Exogenous, or Both?
The existence of around 50 genes and pseudogenes in mice (with 30 of them potentially encoding full reading frames), compared to ~18 in humans (10 potentially coding), is reminiscent of vomeronasal and olfactory receptors (Dong et al. 2001; Zylka et al. 2003). Similarly to the Mrgpr family, most of these sensory receptors are located in gene clusters and have substantially more members in rodents than in humans (Mombaerts 2004). It is thought that they respond to external cues and that humans have fewer because the sense of smell is much more critical for survival and communication in rodents than in humans. Can a similar inference be made for the Mrgprs? Are most of the substances detected by the Mrgpr family found in the environment? Is the difference in receptor cohort between species related to differences in environmental threats? As more ligands are discovered for the Mrgpr family members, an evaluation can be made of this and other hypotheses, but for now no explanation suffices as to why the numbers vary so widely in different species.
Ligand binding profiles actually would argue against the Mrgprs as detectors of external substances, as most identified ligands are endogenous, except for chloroquine. MrgprX2 has been shown to be activated by high concentrations of several positively charged peptides found outside the body, some of which can also activate MrgprX1, but the relevance of these low-affinity interactions is not known (Tatemoto et al. 2006). The finding that any endogenous substance can activate Mrgpr family members is curious. Currently, it is not known whether most itch and pain sensation is similar to olfaction, in which the substances directly activate neurons. It is possible that pruritogens might act indirectly by inducing other cells, like keratinocytes and endothelial cells, to release transmitters that are recognized by neurons. More studies of known endogenous Mrgpr agonists may shed some light on this highly important issue.
As a final note, receptor orthologs that have such little homology, and yet the same ligands are rarely seen. An example to be considered is that of the cytokine receptors. These are mostly not GPCRs but may yet be instructive, as they show extensive divergence between orthologs, sometimes 40% or more at the amino acid level. One such case is the broadly important receptor for the cytokine IL-4. This receptor is only 52% identical between human and mouse. Interestingly, IL-4 itself also has diverged extensively, with the precursor sharing only ~40% identity. Another example is IL-31, whose orthologs share about 30% identity, and its coreceptor IL31RA, with about 60% identity between mouse and human. It is possible, then, that receptors can diverge extensively at the amino acid level and retain their relevant ligand binding properties, because the ligands changed, as well. Thus, when considering the basic properties of the Mrgprs of large families and extensive divergence, precedents can be found in other receptor families for external and internal ligands.
ACKNOWLEDGMENT
The work was supported by grants from the NIH to X.D. (NS054791 and GM087369).
REFERENCES
- Akuzawa N, Obinata H, Izumi T, Takeda S. Morphine is an exogeneous ligand for MrgX2, a G protein-coupled receptor for cortistatin. JCAB. 2007;2:4–9.
- Avula L.R, Buckinx R, Alpaerts K, Costagliola A, Adriaensen D, Van Nassauw L, Timmermans J.P. The effect of inflammation on the expression and distribution of the MAS-related gene receptors MrgE and MrgF in the murine ileum. Histochem Cell Biol. 2011;136:569–585. [PubMed: 21912971]
- Bandell M, Story G.M, Hwang S.W, Viswanath V, Eid S.R, Petrus M.J, Earley T.J, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. [PubMed: 15046718]
- Bender E, Buist A, Jurzak M, Langlois X, Baggerman G, Verhasselt P, Ercken M. et al. Characterization of an orphan G protein-coupled receptor localized in the dorsal root ganglia reveals adenine as a signaling molecule. Proc Natl Acad Sci USA. 2002;99:8573–8578. [PMC free article: PMC124315] [PubMed: 12084918]
- Breit A, Gagnidze K, Devi L.A, Lagace M, Bouvier M. Simultaneous activation of the delta opioid receptor (deltaOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits deltaOR signaling. Mol Pharmacol. 2006;70:686–696. [PubMed: 16682504]
- Chen H, Ikeda S.R. Modulation of ion channels and synaptic transmission by a human sensory neuron-specific G-protein-coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons. J Neurosci. 2004;24:5044–5053. [PMC free article: PMC6729361] [PubMed: 15163697]
- Cox P.J, Pitcher T, Trim S.A, Bell C.H, Qin W, Kinloch R.A. The effect of deletion of the orphan G-protein coupled receptor (GPCR) gene MrgE on pain-like behaviours in mice. Mol Pain. 2008;4:2. [PMC free article: PMC2242784] [PubMed: 18197975]
- Crozier R.A, Ajit S.K, Kaftan E.J, Pausch M.H. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J Neurosci. 2007;27:4492–4496. [PMC free article: PMC6672314] [PubMed: 17442834]
- Davidson S, Zhang X, Yoon C.H, Khasabov S.G, Simone D.A, Giesler G.J Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. [PMC free article: PMC3008349] [PubMed: 17855615]
- Dong X, Han S, Zylka M.J, Simon M.I, Anderson D.J. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. [PubMed: 11551509]
- Dussor G, Zylka M.J, Anderson D.J, McCleskey E.W. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. J Neurophysiol. 2008;99:1581–1589. [PMC free article: PMC2438606] [PubMed: 18234974]
- Ekpechi O.L, Okoro A.N. A pattern of pruritus due to chloroquine. Arch Dermatol. 1964;89:631–632. [PubMed: 14107655]
- Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507–1515. [PubMed: 12182955]
- Gottfried J.A. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. [PMC free article: PMC3722866] [PubMed: 20700142]
- Grazzini E, Puma C, Roy M.O, Yu X.H, O’Donnell D, Schmidt R, Dautrey S. et al. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci USA. 2004;101:7175–7180. [PMC free article: PMC406485] [PubMed: 15118101]
- Guan Y, Liu Q, Tang Z, Raja S.N, Anderson D.J, Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci USA. 2010;107:15933–15938. [PMC free article: PMC2936626] [PubMed: 20724664]
- Han S.K, Dong X, Hwang J.I, Zylka M.J, Anderson D.J, Simon M.I. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci USA. 2002;99:14740–14745. [PMC free article: PMC137489] [PubMed: 12397184]
- Hindson B.J, Ness K.D, Masquelier D.A, Belgrader P, Heredia N.J, Makarewicz A.J, Bright I.J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–8610. [PMC free article: PMC3216358] [PubMed: 22035192]
- Imamachi N, Park G.H, Lee H, Anderson D.J, Simon M.I, Basbaum A.I, Han S.K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA. 2009;106:11330–11335. [PMC free article: PMC2708751] [PubMed: 19564617]
- Johanek L.M, Meyer R.A, Friedman R.M, Greenquist K.W, Shim B, Borzan J, Hartke T, LaMotte R.H, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. [PMC free article: PMC2564794] [PubMed: 18650342]
- Jordt S.E, Bautista D.M, Chuang H.H, McKemy D.D, Zygmunt P.M, Hogestatt E.D, Meng I.D, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. [PubMed: 14712238]
- Kamohara M, Matsuo A, Takasaki J, Kohda M, Matsumoto M, Matsumoto S, Soga T, Hiyama H, Kobori M, Katou M. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem Biophys Res Commun. 2005;330:1146–1152. [PubMed: 15823563]
- Kato M, Nakamura Y, Tsunoda T. An algorithm for inferring complex haplotypes in a region of copy-number variation. Am J Human Genetics. 2008;83:157–169. [PMC free article: PMC2495074] [PubMed: 18639202]
- Klein A, Carstens M.I, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011;106:1078–1088. [PMC free article: PMC3174811] [PubMed: 21653727]
- Lembo P.M, Grazzini E, Groblewski T, O’Donnell D, Roy M.O, Zhang J, Hoffert C. et al. Proenkephalin A gene products activate a new family of sensory neuron–specific GPCRs. Nat Neurosci. 2002;5:201–209. [PubMed: 11850634]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel K.N, Kim A, Ru F. et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. [PMC free article: PMC2989405] [PubMed: 20004959]
- Liu Q, Vrontou S, Rice F.L, Zylka M.J, Dong X, Anderson D.J. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–948. [PubMed: 17618277]
- Liu Q, Weng H.J, Patel K.N, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4:ra45. [PMC free article: PMC3144551] [PubMed: 21775281]
- Ma C, Nie H, Gu Q, Sikand P, Lamotte R.H. In vivo responses of cutaneous C-mechanosensitive neurons in mouse to punctate chemical stimuli that elicit itch and nociceptive sensations in humans. J Neurophysiol. 2012;107:357–363. [PMC free article: PMC3349700] [PubMed: 21994268]
- Ma Q, Fode C, Guillemot F, Anderson D.J. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. [PMC free article: PMC316844] [PubMed: 10398684]
- Milasta S, Pediani J, Appelbe S, Trim S, Wyatt M, Cox P, Fidock M, Milligan G. Interactions between the Mas-related receptors MrgD and MrgE alter signalling and trafficking of MrgD. Mol Pharmacol. 2006;69:479–491. [PubMed: 16282220]
- Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–278. [PubMed: 15034552]
- Moran M.M, McAlexander M.A, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. [PubMed: 21804597]
- Price T.J, Flores C.M. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. [PMC free article: PMC1899162] [PubMed: 17113352]
- Pundir P, Kulka M. The role of G protein-coupled receptors in mast cell activation by antimicrobial peptides: Is there a connection? Immunol Cell Biol. 2010;88:632–640. [PubMed: 20309008]
- Ramachandran R, Hollenberg M.D. Proteinases and signalling: Pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153 Suppl 1:S263–S282. [PMC free article: PMC2268078] [PubMed: 18059329]
- Rau K.K, McIlwrath S.L, Wang H, Lawson J.J, Jankowski M.P, Zylka M.J, Anderson D.J, Koerber H.R. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29:8612–8619. [PMC free article: PMC2756673] [PubMed: 19571152]
- Redon R, Ishikawa S, Fitch K.R, Feuk L, Perry G.H, Andrews T.D, Fiegler H. et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. [PMC free article: PMC2669898] [PubMed: 17122850]
- Ringkamp M, Schepers R.J, Shimada S.G, Johanek L.M, Hartke T.V, Borzan J, Shim B, LaMotte R.H, Meyer R.A. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–14849. [PMC free article: PMC3218799] [PubMed: 22016517]
- Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem. 2003;278:44400–44404. [PubMed: 12915402]
- Salako L.A. Toxicity and side-effects of antimalarials in Africa: A critical review. Bull World Health Org. 1984;62 Suppl:63–68. [PMC free article: PMC2536191] [PubMed: 6335683]
- Santos R.A, Simoes e Silva A.C, Maric C, Silva D.M, Machado R.P, de Buhr I, Heringer-Walther S. et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. [PMC free article: PMC166216] [PubMed: 12829792]
- Shimada S.G, Shimada K.A, Collins J.G. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol. 2006;530:281–283. [PubMed: 16356490]
- Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S. et al. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–23564. [PubMed: 15037633]
- Sikand P, Dong X, LaMotte R.H. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. 2011;31:7563–7567. [PMC free article: PMC3111068] [PubMed: 21593341]
- Sowunmi A, Walker O, Salako L.A. Pruritus and antimalarial drugs in Africans. Lancet. 1989;2:213. [PubMed: 2568535]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov P.S, Luger T.A, Schmelz M. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. [PMC free article: PMC6740542] [PubMed: 12867500]
- Steinhoff M, Vergnolle N, Young S.H, Tognetto M, Amadesi S, Ennes H.S, Trevisani M. et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. [PubMed: 10655102]
- Subramanian H, Kashem S.W, Collington S.J, Qu H, Lambris J.D, Ali H. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol Pharmacol. 2011;79:1005–1013. [PMC free article: PMC3102546] [PubMed: 21441599]
- Sun Y.G, Chen Z.F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. [PubMed: 17653196]
- Swain M.G, MacArthur L, Vergalla J, Jones E.A. Adrenal secretion of BAM-22P, a potent opioid peptide, is enhanced in rats with acute cholestasis. Am J Physiol. 1994;266:G201–G205. [PubMed: 8141292]
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H. et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. [PubMed: 16979137]
- Theoharides T.C, Alysandratos K.D, Angelidou A, Delivanis D.A, Sismanopoulos N, Zhang B, Asadi S. et al. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. [PMC free article: PMC3318920] [PubMed: 21185371]
- von Kugelgen I, Schiedel A.C, Hoffmann K, Alsdorf B.B, Abdelrahman A, Muller C.E. Cloning and functional expression of a novel Gi protein-coupled receptor for adenine from mouse brain. Mol Pharmacol. 2008;73:469–477. [PubMed: 17975009]
- Wang H, Zylka M.J. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–13209. [PMC free article: PMC2789299] [PubMed: 19846708]
- Wilson S.R, Gerhold K.A, Bifolck-Fisher A, Liu Q, Patel K.N, Dong X, Bautista D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. [PMC free article: PMC3181150] [PubMed: 21460831]
- Wong K.K, deLeeuw R.J, Dosanjh N.S, Kimm L.R, Cheng Z, Horsman D.E, MacAulay C. et al. A comprehensive analysis of common copy-number variations in the human genome. Am J Human Genetics. 2007;80:91–104. [PMC free article: PMC1785303] [PubMed: 17160897]
- Wu L.J, Sweet T.B, Clapham D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. [PMC free article: PMC2964900] [PubMed: 20716668]
- Yamatodani A, Maeyama K, Watanabe T, Wada H, Kitamura Y. Tissue distribution of histamine in a mutant mouse deficient in mast cells: Clear evidence for the presence of non-mast-cell histamine. Biochem Pharmacol. 1982;31:305–309. [PubMed: 7073763]
- Zhang L, Taylor N, Xie Y, Ford R, Johnson J, Paulsen J.E, Bates B. Cloning and expression of MRG receptors in macaque, mouse, and human. Brain Res Mol Brain Res. 2005;133:187–197. [PubMed: 15710235]
- Zylka M.J, Dong X, Southwell A.L, Anderson D.J. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci USA. 2003;100:10043–10048. [PMC free article: PMC187757] [PubMed: 12909716]
- Zylka M.J, Rice F.L, Anderson D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. [PubMed: 15629699]
- INTRODUCTION
- DISCOVERY OF THE Mrgprs
- EXPRESSION OF Mrgprs
- GENERATION OF Mrgpr KNOCKOUT ANIMALS
- ROLE OF Mrgprs IN ITCH IN MICE
- Mrgpr SIGNALING PATHWAYS
- BAM8-22, AN Mrgpr AGONIST, INDUCES ITCH IN HUMANS
- DIFFERENCES BETWEEN ITCH RECEPTORS AND OLFACTORY RECEPTORS
- OTHER Mrgprs
- DIMERIZATION
- QUESTIONS
- ACKNOWLEDGMENT
- REFERENCES
- Review Engineering Aspects of Olfaction.[Neuromorphic Olfaction. 2013]Review Engineering Aspects of Olfaction.Persaud KC. Neuromorphic Olfaction. 2013
- Review Peripheral Opioids.[Itch: Mechanisms and Treatment...]Review Peripheral Opioids.Bigliardi PL, Bigliardi-Qi M. Itch: Mechanisms and Treatment. 2014
- Review Pruriceptors.[Itch: Mechanisms and Treatment...]Review Pruriceptors.Ringkamp M, Meyer R. Itch: Mechanisms and Treatment. 2014
- Review Influence of Cat Odor on Reproductive Behavior and Physiology in the House Mouse: (Mus Musculus).[Neurobiology of Chemical Commu...]Review Influence of Cat Odor on Reproductive Behavior and Physiology in the House Mouse: (Mus Musculus).Voznessenskaya VV. Neurobiology of Chemical Communication. 2014
- Review Role of PAR-2 in Neuroimmune Communication and Itch.[Itch: Mechanisms and Treatment...]Review Role of PAR-2 in Neuroimmune Communication and Itch.Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Itch: Mechanisms and Treatment. 2014
- Mrgprs as Itch Receptors - ItchMrgprs as Itch Receptors - Itch
- Sensitization of Itch Signaling - ItchSensitization of Itch Signaling - Itch
- Probe Reports from the NIH Molecular Libraries ProgramProbe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...
