NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Breman JG, Alilio MS, White NJ, editors. Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. Northbrook (IL): American Society of Tropical Medicine and Hygiene; 2007 Dec.

Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene.
Show detailsGenetic modification (GM) of mosquitoes (which renders them genetically modified organisms, GMOs) offers opportunities for controlling malaria. Transgenic strains of mosquitoes have been developed and evaluation of these to 1) replace or suppress wild vector populations and 2) reduce transmission and deliver public health gains are an imminent prospect. The transition of this approach from confined laboratory settings to open field trials in disease-endemic countries (DECs) is a staged process that aims to maximize the likelihood of epidemiologic benefits while minimizing potential pitfalls during implementation. Unlike conventional approaches to vector control, application of GM mosquitoes will face contrasting expectations of multiple stakeholders, the management of which will prove critical to safeguard support and avoid antagonism, so that potential public health benefits can be fully evaluated. Inclusion of key stakeholders in decision-making processes, transfer of problem-ownership to DECs, and increased support from the wider malaria research community are important prerequisites for this. It is argued that the many developments in this field require coordination by an international entity to serve as a guiding coalition to stimulate collaborative research and facilitate stakeholder involvement. Contemporary developments in the field of modern biotechnology, and in particular GM, requires competencies beyond the field of biology, and the future of transgenic mosquitoes will hinge on the ability to govern the process of their introduction in societies in which perceived risks may outweigh rational and responsible involvement.
Introduction
The history of malaria vector control is typified by the search for compounds that interfere with vital physiologic processes in the mosquito.1 Ever since Ross’ first transmission models,2 it became clear that adult mosquito survival represents an Achilles heel in the epidemiology of malaria.3 This fundamental understanding led to the search for compounds with insecticidal activity that would persist in the environment in which these were applied. Most, if not all, new developments in the control of anophelines presented incremental improvements of this concept. Resistance was, and is still today, viewed as an unavoidable consequence of widespread insecticide use that can either be managed or overcome by the discovery of new compounds with desirable (i.e., long-term mosquitocidal) characteristics.4,5 Based on phenomenal historical successes around the globe, the huge efforts underway to scale-up use of insecticide-treated bednets (ITNs)6,7 and the renewed acceptability of DDT for vector control following the agreement to the Stockholm Convention on Persistent Organic Pollutants,8–11 it is hardly surprising that the mainstay of malaria vector control remains focused on chemical control. Disturbingly, all of these methods depend on the ability to reach target communities, acquisition of consent or willingness to use them, and cost and correct use. Both the use of ITNs and indoor residual spraying (IRS) target mosquito vectors in the domestic environment, and interest in peri-domestic control strategies (e.g., larval control) is slowly reviving.12,13
The above contrasts sharply with the more radical concept to render mosquito populations refractory to infection with Plasmodium parasites.14 The focus here is on the ability to modify the genome of Anopheles in such a manner that the acquired phenotypical traits are inherited in a non-Mendelian fashion and spread through a population with the aim to eventually replace a susceptible with a refractory population. Unlike the aim to shorten the daily survival of the vector, it is now the competence to transmit disease that serves as the intervention target. Alternatively, genetic engineering may convey conditional lethality,15 but focus here will be on engineered refractoriness. In contrast with insecticide-based strategies, however, there are no precedents (yet) of successful application, leading to the classification of this approach as linear technology-push.16 In the absence of market pull mechanisms, present in some circumstances for ITNs, furtherance of this approach depends on basic scientific developments and proof-of-principle experimentation, followed by stepwise introduction in suitable disease-endemic country (DEC) settings. Using the same process, genetic control strategies such as the sterile insect technique (SIT),17 in which large numbers of radiation-sterilized males confer sterility to the pest population on release, have created new market opportunities for the control of insect pests, which are now expanding.18 Although the concept here is population suppression and/or local elimination, it is probably the only benchmark available at present for vector control strategies based on modern biotechnological tools.19 The novelty of the approach thus poses challenges on several fronts. First, there are various technical hurdles affecting progress, posing challenges to the scientific community. Second, the transition from the laboratory to the field requires careful planning to manage the risk of project failure or premature termination. Third (and unknown at this stage), it remains to be seen under what ethical, legal, and societal frameworks (ELSI) adoption in DECs can be secured. In this article, we aim to shed light on all three challenges, with emphasis on the latter two, which inevitably will prove vital in the medium to long term.
Technological Challenges
Fifteen years ago, a report was published by the World Health Organization that focused on prospects of using genetic modification of anopheline mosquitoes for malaria control.20 Building on concepts posed in the late 1960s21 and genetic modification of Drosophila,22 a strategic roadmap was developed, with the following targets: a transgenic mosquito by 2000, a refractory mosquito by 2005, and field trials by 2010.23 Dramatic advances in modern biotechnology resulted in stable germ line transformation of Anopheles stephensi,24 An. gambiae,25 and An. albimanus26 as planned, with the first refractoriness to Plasmodium berghei engineered in An. stephensi ahead of schedule.27 However, by 2006, engineering of refractoriness to P. falciparum has not yet been reported. Current availability of the An. gambiae genome sequence enables functional studies on human malarias and anophelines in the search for novel control strategies.28 These successes heightened enthusiasm beyond those directly involved29,30 and resulted in the formation of new perspectives within related disciplines. Ecologic aspects of the approach have been reviewed,31–33 besides issues related to the transition of the approach from the bench to the field.34 Reviews and books on the subject continue to be published as it matures.35–37
These encouraging developments were presented to the broader malaria research and control community during a plenary debate at the 4th MIM Pan-African Malaria Conference, held in Yaoundé, Cameroon, in November 2005. After explanatory remarks on contemporary issues in the field of genetically modified (GM) mosquitoes,38 196 participants expressed their views on 10 statements (Figure 1). More than one half of the respondents viewed the above successes as indicative for future successful implementation of the approach (Figure 1, Q1) and were confident that systems to target human malarias will be developed (Figure 1, Q2). Three of four respondents believed that the lessons learned from previous genetic control trials, coupled with a vast increase in knowledge of the target organisms, will increase the likelihood of success in future trials (Figure 1, Q3). However, a variety of technical hurdles, related to biologic constraints remain, the most salient of which are listed below.
Mosquito Fitness
The introduction of novel phenotypic traits in transgenic mosquitoes may induce a fitness cost that could impede the effectiveness of genetic drive mechanisms and thus the spread of transgenes in target field populations.39 Expression of marker proteins and effector molecules, particularly when expressed using ubiquitous promoters, may be detrimental to the mosquito.40 In addition, transgene insertion may interfere with gene expression of the mosquito strain because of the essentially random nature of genomic insertion. This so-called insertional mutagenesis may be naturally selected against or be overcome through selection of lines with higher fitness.41 However, the inability to control insertion site location using conventional vectors has led to the development of vectors that can provide a stable docking site for site-targeted transgene insertion. This, coupled with the use of suicide vectors, will increase safety and make transgene expression more predictable.42 However, it will still be necessary to select, from a series of insertions with docking sites, the most fit. Finally, the genetic make-up of transgenic lines and inbreeding have been posed as additional factors affecting the fitness of transgenic lines.39,43 This latter factor is probably not a serious concern and can probably be dealt with using a well-designed breeding scheme. These shortcomings may be overcome by circumventing the mosquito genome altogether and instead focus on genetic modification of symbiotic (midgut) bacteria as a means to deliver anti-pathogen products.44 Whatever approach is adopted in the coming years, compensation for fitness loss, preferably by conferring fitness advantages to transgenics that outweigh cost-benefit equilibria in susceptible wild-type mosquitoes,45 will remain a critical challenge. The measurement of fitness in the laboratory with laboratory strains is at best inadequate and at worst misleading. The only meaningful measure of fitness will be when the GM mosquitoes are competed against wild mosquitoes in the field. As a concept to address this, Scott and others46 called for the development of consensus methodology in fitness studies based on stepwise progression from laboratory to semi-field settings (see below), with competitiveness of GM mosquitoes as a key criterion.
Genetic Drive
On the premise that transgenic lines with acceptable fitness can be developed, the next challenge will be to drive the desirable attributes to fixation in field populations. In contrast with SIT campaigns, in which massive repeated releases of sterile males are practiced, the use of males able to drive a transgene into a target population may require the production and release of much smaller numbers, thereby gradually altering its susceptibility in favor of refractoriness. Reviews on genetic drive mechanisms47 and desirable attributes thereof48,49 focus on the possible fitness costs associated with the drive mechanism itself (as described above), the rate at which introgression of transgenes in populations occurs, and the ability of the mechanism to drive large genetic constructs. Models have been developed to study these phenomena.50,51 These indicate that the complete linkage between genes delivering anti-pathogen traits, and the genetic drive mechanism deployed, besides stable and reliable levels of phenotypic expression of transgenes, is mandatory to accomplish adequate penetration of the target population and impact on disease transmission. If there is a fitness cost associated with a refractoriness effector gene, recombination between it and the driver will eventually lead to fixation of the driver alone without the effector gene, with no long-term impact on vector competence.
A second major issue to take into account is the response of the target organism (i.e., the Plasmodium), to the anti-pathogen trait. This “trait” will be a protein that either prevents development of the parasite or kills it. The pressure on the parasite population will undoubtedly elicit a response given the time that the population replacement process may take. A mutational event that enables the parasite to negate the effects of the anti-pathogen trait will rapidly be selected for despite the ongoing gene driving process. Unfortunately, in contrast to the other two factors mentioned above, parasite response to selection pressure in the field is not a “researchable topic” and may only be studied under artificial laboratory conditions. Hopefully, targeting the Plasmodium population with parasiticidal compounds will not result in the development of resistance as occurred when the vector population was targeted with insecticidal compounds.
Fitness of GM mosquitoes, the search for appropriate genetic drive mechanisms, and the response of the parasite will remain challenges for the foreseeable future. However, in line with the confidence expressed by the majority of the respondents during the debate in Yaoundé, we are of the opinion that improved understanding of insect transformation and mosquito genetics, behavior, and ecology will enable the development of GM mosquito strains worthy of testing beyond the confines of the laboratory.
Transitional Challenges
Given such accomplishments, a new array of challenges emerges.52 The complexity of these form the interface between the technological and the ELSI described below.53 A stepwise approach has been proposed for fitness evaluations,46 and this model can be expanded to include components of the transitional change to bridge laboratory and field research (Figure 2).34 In furtherance to previous studies on transgenic insects, such as the pink bollworm,54 which were limited by tethering the insects and cutting off their wings, the endpoint of studies on GM mosquitoes will be their full evaluation in contained semi-field environments in a DEC setting. At least two of the four Grand Challenges in Global Health projects on genetic control of disease vectors (although these focus on Aedes rather than anopheline vectors)55,56 aim for this goal before 2010. Although this target is clear and restrictive in the sense that concerns related to open-field releases can be controlled, it does pose additional issues, particularly the choice of DEC setting.
Field Site Selection
It seems logical to select field sites on the assumption that, given successful evaluation of novel strains in semi-field environments, GM mosquitoes will be released in the country and the environment in which such evaluations took place. Beyond the stipulated criteria that should govern site selection,57,58 such as geographic isolation, genetic make-up of the vector population,59 disease transmission intensity, etc., which in essence match those of other genetic control approaches such as SIT, broader concerns related to future open-field releases emerge. First, it must be considered that population replacement strategies leave a population capable of vectoring other pathogens (e.g., filarial worms). More than 40% of the respondents considered this scenario unjustifiable (Figure 1, Q4). Second, these prerequisites limit the scale and scope of opportunities. There are very few sites in Africa with sufficient ecological or physical (e.g., islands) isolation and hypoendemic malaria transmission by a single vector species where evaluation may take place. Our effort to develop SIT against An. arabiensis has (thus far) led to the identification of just two sites with appropriate conditions (Northern Sudan and the island of La Réunion). However, despite the high levels of investment in genetic control strategies (still a fraction of the global investment in drugs and vaccines R&D)60 and the (possible) limited applicability of these in sub-Saharan Africa, 45% of the respondents consider the endeavor worthwhile (Figure 1, Q5). We share this view, because experiences in proof-of-principle settings may serve as the basis for expansion of the approach to more intricate settings, as has been experienced with SIT against tsetse flies (with the initial elimination of Glossina austeni from the island of Unguja, Zanzibar,61 followed by current expansion of activities in more complex settings on mainland Africa).
The key point addressed here is that the selection of field sites, even with the intermediate aim of semi-field evaluation of GM strains, requires commitment planning based on wider issues (e.g., biotechnology policies, pubic opinion) that may be present in the DEC setting of choice (Figure 2). One half the respondents shared this view (Figure 1, Q6).
Problem-Ownership Transfer
Given the novelty of the approach, commitment planning becomes essential. Commitment here refers to the willingness of DECs to first evaluate and ultimately adopt the approach as a possible tool for malaria control to augment their established strategic portfolio of disease control methods (e.g., ITNs, IRS). Clearly this will depend on the effectiveness of the proposed strategy and outcome of laboratory evaluations. The likelihood of adoption, in turn, will increase with investment in knowledge and skills acquisition (or capacity and capability building) in the DEC with regard to the GM mosquito approach.34,62 The ultimate aim is to transfer problem-ownership to the DEC. This model is now being used by the IAEA when developing area-wide integrated SIT programs against tsetse flies in collaboration with its Member States (MSs). Beyond the technical support provided in the form of expert advice, training (through fellowships), and provision of equipment, MSs bear full responsibility for the development and implementation of the programs. The inherent implication here is that DECs, ad interim, will engage their often limited human and financial resources towards research rather than control efforts. Interestingly, one half the respondents favor this (Figure 1, Q7) and apparently see maturation of equality in terms of competence and partnership as beneficial.
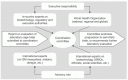
Beyond problem-ownership transfer and, given the controversial nature of genetically modified organisms (GMOs), the establishment of an international and independent coordinating entity has been called for repeatedly.53,57,63,64 This body could coordinate research efforts, focus on the broader dissemination of scientific progress to multiple stakeholders, and facilitate collaborative efforts and partnership strengthening within and beyond the scientific community. The establishment of a guiding coalition may safeguard against potential antagonism and ensure integrity of all those involved during the transitional and implementation phases of the GM mosquito endeavor. The proposed constituency of this coordinating entity is shown in Figure 3. Executive responsibility, in accordance with problem-ownership transfer, lies with experts, regulatory and executive authorities, and WHO officials in DECs, with additional (international) expertise serving in an advisory capacity. As transition from the laboratory to the field proceeds, the committee evaluates scientific progress and gives recommendations for subsequent research. Although this committee is not yet in existence, nearly 70% of the respondents agreed that its absence in this or any other suitable format and constituency could lead to failure of the GM mosquito endeavor (Figure 1, Q9).
Containment and Risk Management
With robust and suitable transgenic strains available, appropriate DEC settings and problem-ownership transfer, and a guiding coalition in place, trials in contained semi-field systems pose the next set of challenges. Although guidelines for the handling, transportation, and laboratory confinement for transgenic mosquitoes have been developed,65 no such guidelines are currently available for similar activities (and semi-field research) in DECs. It is to be expected that research on GM mosquitoes in their endemic environment will require heightened levels of containment, and a task force is currently drafting such guidelines (A James, personal communication). Clearly, such guidelines will have to amalgamate international, national, and perhaps local guidelines.66
Semi-field research (i.e., strain evaluation in large outdoor enclosures) has been undertaken since the early days of genetic mosquito control67 but has only recently been used for research on African anophelines.68 A clear challenge, required for the evaluation of mosquito fitness, will be the establishment of self-perpetuating populations with overlapping generations in such enclosures. The concomitant exposure of such populations to Plasmodium infections to evaluate their refractoriness will pose additional hurdles and will have to use an artificial bloodfeeding system for this purpose.69 Experience with this type of research is still limited, as are issues related to the physical (i.e., structural) and biologic containment (e.g., release of sterilized or otherwise reproductively impaired specimens) or inadvertent release of GM mosquitoes (i.e., the response to accidents).
Risk, which is the product of an identified hazard (i.e., the degree of severity of the adverse consequence) and the likelihood of that hazard actually occurring,70 and risk management (reducing the likelihood of hazards or mitigating the impact of adverse consequences) form a crucial part of research on genetically engineered organisms. Such risks can be probabilistic, hypothetical, or speculative. Only the former two classes are amenable to study, because at least the hazard is known or can be assumed to exist on scientific grounds. Only in such cases can risk assessment progress through scientific research. We have recently developed a risk classification matrix for research on transgenic mosquitoes in semi-field systems based on 1) the reproductive ability of the strain tested, 2) the genetic drive system used, and 3) the nature of the effector molecule (M Benedict, H Bossin, and B Knols, unpublished data). This indicates that fertile insects carrying genetically engineered transposable elements to drive novel anti-pathogen traits will require the highest containment levels. The nature of the vector used for gene transfer and its mobility properties both intra-genomic and inter-genomic (i.e., lateral or horizontal transfer) are thus of primary importance in risk assessment.71
Stakeholder Involvement
Most publications that focus on ELSI (see below) advocate the involvement of the broader stakeholder community at a time when open-field releases are foreseen.72–74 Here we also underscore the importance of initiating this process earlier, during the transitional stages, when planning semi-field systems research. Even if this research will be undertaken in isolated areas in DECs, it will not go unnoticed in the community, press, etc., and requires careful management. Research on (non-transgenic) mosquitoes in large outdoor enclosures in Kenya and Tanzania56 has raised considerable concern (if not anxiety) in nearby communities (H Ferguson and G Killeen, personal communication), and this is likely to be more so in research involving GM mosquitoes (Figure 1, Q8 and Q9). Potential risks involved, even when considered relatively small (such as the ability of transgenic mosquitoes to vector other diseases), may be perceived and viewed in a different light outside of the scientific community.
Ethical, Legal, and Social Issues
It is noteworthy that the ethical, legal, and social issues surrounding the application of GM mosquitoes are nearly always featured last in publications on the topic (including this one).32–37 Given the controversies on GMOs, this is hardly surprising, and it is often argued that basic research should provide the answers needed to dampen the debate and the risk of polarization.75 Research is the only route through which risk assessment and future evaluation of the public health benefits of the approach can progress. The view, held by 34% of the malaria research and control community, that the approach “will never fly” (Figure 1, Q10) is therefore both surprising and worrying at the same time. It indicates that, even in an informed group of malariologists, skepticism remains strong over acceptance and tolerance of ambiguity and uncertainty that accompanies (any) scientific endeavor. It has taken four decades of research to develop promising malaria vaccines and 20 years from the first application of insecticide on a bednet to the currently available long-lasting impregnated nets that are being introduced in Africa on a large scale. What matters here is that views on GM mosquitoes are not likely to be viewed in isolation, but as part of a wider debate over developments in biotechnology (i.e., GM plants,76 stem cell research, animal cloning, etc.) and the perception of these, fueling skepticism and/or antagonism. Seeking coherence and agreement within the malaria community to avoid further polarization is thus called for. The coordination committee described above (Figure 3) could well undertake this task.
Beyond internal stakeholders (i.e., the malaria research and control community), a much broader framework of stakeholder groups, affected by or able to affect the GM mosquito endeavor, can or might exert their influence in the foreseeable future. Their importance and necessary inclusion in various capacities cannot be overemphasized and yet has received only marginal attention to date. Stakeholder influence has increased dramatically over the last few decades, enforced by the internet and other modern communication tools, enabling criticism to emerge from all corners of society and not just through the press. With beliefs and values already stretched by the global GMO debate, the pace with which government-imposed, novel, yet controversial, initiatives in several instances is taking place in DECs is likely to be affected. Failure to involve key stakeholders at a sufficiently early stage may lead to the disruption, or worse, to the termination of contained and open-field trials of GM mosquitoes. The history of genetic control trials against culicine mosquitoes in India in the mid-1970s77,78 shows how opposition can have far-reaching consequences. After several years of work on field testing of the mating competitiveness of sterile male mosquitoes,79 accusations that the project was meant to obtain data for biologic warfare using yellow fever were launched in the press and taken up by opposition politicians. Shortly afterward, a well-prepared attempt to eradicate an urban Ae. aegypti population by sterile male releases was banned by the government of India 2 days before its launch.80 Given that only (non-biting) males were to be released, no data relevant to biologic warfare could have been collected, yet such rumors about this project still persist.81 Reluctance of WHO at the time to intervene in what they viewed as an internal Indian political matter has been listed as a reason why the situation escalated,82 hinting once more at the need for an impartial global coordinating entity for genetic control trials. A clear task would be to develop stakeholder power matrices83 to estimate the power and legitimacy each stakeholder has in terms of influence and claims, from which the potential impact on the purpose of research trials can be deducted. Figure 4 uses this approach to understand the opposition Monsanto faced when intending to enter the European market with GM crops.84 Similarly, it addresses the position of key stakeholders in a GM mosquito project whereby the likely position of non-governmental organizations (NGOs), the general public, etc., may change over time in search for increased power. The role of DEC partner institutions and their governments will become critical in managing the position of these groups, highlighting the need for problem-ownership transfer and empowerment of DEC partners.
The role of (currently disempowered) target communities inhabiting areas earmarked for possible future releases of GM mosquitoes requires special attention. Several challenges emerge here. First, some societies or parts thereof may be more willing to accept evaluation of the approach in their surroundings than others. This results in a paradox: should biologic criteria (see above) outweigh social acceptance or vice versa? Congruence between the governing variables of these criteria is preferred but may not be assumed to be in existence at present. Second, the nature of genetic control interventions (per definition area-wide) will affect all citizens in release areas, requiring informed community consent.85 The widely advocated involvement of target communities in field evaluation of the approach is likely to be influenced by a debate on the uncertainties and ambiguities related to it in view of existing alternative vector control tools with proven efficacy. If malaria eradication in isolated settings (e.g., islands) can be accomplished through integrated disease management (e.g., mass chemotherapy combined with ITNs),86 application of alternative approaches with unknown efficacy will require careful evaluation from an ethical perspective. The boundaries for this consent, moreover, are blurred. The ability of vectors to disperse actively and/or passively (e.g., on cars, boats, aircraft) places any trial beyond the confines of a given spatial dimension and must be fully recognized. It is likely that acquisition of consent for open-field releases will be initiated centrally and follow international and country-specific guidelines. Subsequently, community consent may be obtained through community consultation of inhabitants of ear-marked areas and take the form of intense dialog with elected representatives of those communities, based on local governance frameworks.
Of particular concern is the epidemiologic consequences of reductions in transmission intensity and how this may affect the development of immunity to malaria and the age at which infections are acquired.87 Discussions on this issue have been widespread with regard to currently used vector control interventions (such as ITNs)88 and center around pre-intervention levels of transmission (and related levels of immunity), incomplete efficiency of refractoriness,89 and/or collapse of the transgene system. In the latter case, or with the introduction of competent vectors into areas freed of malaria for extended periods of time, the likelihood of epidemics is real. It is clear, therefore, that the introduction of GM mosquitoes will necessitate long-term monitoring of the impact on malaria epidemiology.
With only few surveys having been undertaken to assess the viewpoints from communities regarding the use of GM mosquitoes,90 the current and future attitudes toward the approach may be influenced by global developments in the field of biotechnology and require careful analysis to avoid repetition of negative past experiences.
Future Perspectives
In the above sections, we described multiple challenges that the GM mosquito endeavor will face in the coming years. Open-field releases are not anticipated in the next 5–10 years, during which time the arena of malaria and its control will undergo changes that will affect the likelihood of implementation. Although forecasting is often inaccurate, some salient points may be emphasized that can either positively or negatively affect GM mosquito strategies. For instance, by 2025, 90% of the world’s population will live in developing countries,91 and 52% of the African population will live in urban environments, of which 300 million will be in slums.92 Environmental modification and/or degradation may lead to reduction of malaria, and high population densities may result in a shift of interest toward case management, personal protection measures, or larval control.93 On the other hand, genetic control strategies may become particularly attractive as operations over relatively small areas can achieve maximum impact in number of people protected.94 Moreover, it has been observed that urban areas harbor island populations of one species (e.g., An. stephensi and An. arabiensis) surrounded by another species in rural settings (An. culicifacies and An. gambiae s.s., respectively), essentially rendering the urban species an isolated population.95
Over the last 15 years, dramatic progress has been made in the development of malaria vaccines,96 and availability of these will probably affect the future of vector control interventions negatively. Given the efforts to increase uptake of ITNs in much of sub-Saharan Africa, problems with insecticide resistance will presumably increase, necessitating alternative vector control strategies. At present, there seems to be little to offer beyond the ITN/IRS era, which may influence the perception and willingness to apply genetic control strategies favorably. Whether ongoing globalization will dissolve the current differences in perception of GMOs remains to be seen, yet it seems unlikely that complacency and acceptance will prevail in all corners of society. Preparedness for antagonism to avoid another incident like the India example described above is essential, yet little has been done at present in this direction. Any genetic control trial receiving bad press and opposition is likely to affect similar efforts worldwide, with the real risk of delaying implementation of strategies that potentially may save thousands of lives every year. Holistic and integrative approaches, although tedious, time-consuming, and resource-intensive, are needed to move genetic control trials forward in a manner that maximizes support of all stakeholders involved, with the greatest chance to properly assess the merits in terms of public health benefits. Current efforts focus mainly on the technical challenges listed above, and good science and persistence are key ingredients to face these. Resolving transitional and implementation challenges may prove much more complex and time consuming.97 However, these will ultimately determine whether “transgenic mosquitoes will ever fly to control malaria.”
Acknowledgments: The authors thank Prof Chris Curtis for helpful comments on the manuscript
Financial support: The IAEA provided support to Bart G. J. Knols for attending the 4th Pan-African MIM conference. Bart G. J. Knols receives financial support through a VIDI grant from the Dutch Scientific Organization.
References
- 1.
- Spielman A, d’Antonio M. Mosquito: The Story of Man’s Deadliest Foe. London: Faber and Faber; 2002.
- 2.
- Ross R. The Prevention of Malaria. London: Murray; 1911.
- 3.
- MacDonald G. The Epidemiology and Control of Malaria. London: Oxford University Press; 1957.
- 4.
- Hemingway J, Field L, Vontas J. An overview of insecticide resistance. Science. 2002;298:96–97. [PubMed: 12364782]
- 5.
- Hemingway J. Taking aim at mosquitoes. Nature. 2004;430:936. [PubMed: 15318236]
- 6.
- Hill J, Lines J, Rowland M. Insecticide-treated nets. Adv Parasitol. 2006;61:77–128. [PubMed: 16735163]
- 7.
- Grabowsky M, Nobiya T, Ahun M, Donna R, Lengor M, Zimmerman D, Ladd H, Hoekstra E, Bello A, Baffoe-Wilmot A, Amofah G. Distributing insecticide-treated bednets during measles vaccination: a low-cost means of achieving high and equitable coverage. Bull World Health Organ. 2005;83:195–201. [PMC free article: PMC2624203] [PubMed: 15798843]
- 8.
- United Nations Environment Programme. Stockholm Convention on Persistent Organic Pollutants (POPs). 2004. [Accessed July 31, 2007]. Available at: http://chm
.pops.int/ - 9.
- Curtis CF. Should the use of DDT be revived for malaria vector control? Biomedica (Bogota). 2004;22:455–461. [PubMed: 12596442]
- 10.
- Maharaj R, Mthembu DJ, Sharp BL. Impact of DDT reintroduction on malaria transmission in KwaZulu-Natal. S Afr Med J. 2005;95:871–874. [PubMed: 16344885]
- 11.
- Kaiser J, Enserink M. Environmental toxicology. Treaty takes a POP at the dirty dozen. Science. 2000;290:2053. [PubMed: 11187821]
- 12.
- Killeen GF, Seyoum A, Knols BGJ. Rationalizing historical successes of malaria control in Africa in terms of mosquito resource availability management. Am J Trop Med Hyg. 2004;71(Suppl 2):87–93. [PubMed: 15331823]
- 13.
- Gu W, Novak RJ. Habitat-based modeling of impacts of mosquito larval interventions on entomological inoculation rates, incidence, and prevalence of malaria. Am J Trop Med Hyg. 2005;73:546–552. [PubMed: 16172479]
- 14.
- Christophides GK. Transgenic mosquitoes and malaria transmission. Cell Microbiol. 2005;7:325–333. [PubMed: 15679836]
- 15.
- Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287:2474–2476. [PubMed: 10741964]
- 16.
- Rothwell R. Towards the fifth-generation innovation process. In: Henry J, Mayle D, editors. Managing Innovation and Change. 2. London: Sage Publications; 1991. pp. 115–135.
- 17.
- Dyck VA, Hendrichs J, Robinson AS. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Dordrecht: Springer; 2005.
- 18.
- Esteva L, Mo Yang H. Mathematical model to assess the control of Aedes aegypti mosquitoes by the sterile insect technique. Math Biosci. 2005;198:132–147. [PubMed: 16125739]
- 19.
- Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. [PubMed: 12901936]
- 20.
- Anonymous,. Geneva: World Health Organization; 1991. Prospects for Malaria Control by Genetic Manipulation of Its Vectors.
- 21.
- Curtis CF. Possible use of translocations to fix desirable genes in insect pest populations. Nature. 1968;218:368–369. [PubMed: 5649682]
- 22.
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. [PubMed: 6289435]
- 23.
- Morel CM, Touré YT, Dobrokhotov B, Oduola AM. The mosquito genome—a breakthrough for public health. Science. 2002;298:79. [PubMed: 12364774]
- 24.
- Catteruccia F, Nolan T, Loukeris TG, Blass C, Savakis C, Kafatos FC, Crisanti A. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405:959–962. [PubMed: 10879538]
- 25.
- Grossman GL, Rafferty CS, Clayton JR, Stevens TK, Mukabayire O, Benedict MQ. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Mol Biol. 2001;10:597–604. [PubMed: 11903629]
- 26.
- Perera OP, Harrell RA II, Handler AM. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol Biol. 2002;11:291–297. [PubMed: 12144693]
- 27.
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. [PubMed: 12024215]
- 28.
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O’Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. [PubMed: 12364791]
- 29.
- Alphey L, Beard CB, Billingsley P, Coetzee M, Crisanti A, Curtis C, Eggleston P, Godfray C, Hemingway J, Jacobs-Lorena M, James AA, Kafatos FC, Mukwaya LG, Paton M, Powell JR, Schneider W, Scott TW, Sina B, Sinden R, Sinkins S, Spielman A, Toure Y, Collins FH. Malaria control with genetically manipulated insect vectors. Science. 2002;298:119–121. [PubMed: 12364786]
- 30.
- Coleman PG, Alphey L. Genetic control of vector populations: an imminent prospect. Trop Med Int Health. 2004;9:433–437. [PubMed: 15078260]
- 31.
- Scott TW, Takken W, Knols BGJ, Boëte C. The ecology of genetically modified mosquitoes. Science. 2002;298:117–119. [PubMed: 12364785]
- 32.
- Takken W, Scott TW, editors. Ecological Aspects for Application of Genetically Modified Mosquitoes. Dordrecht: Springer; 2003.
- 33.
- Ferguson HM, John B, Ng’habi K, Knols BGJ. Redressing the sex imbalance in knowledge of vector biology. Trends Ecol Evol. 2005;20:202–209. [PubMed: 16701369]
- 34.
- Knols BGJ, Louis C, editors. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Dordrecht: Springer; 2006.
- 35.
- Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. [PubMed: 15703759]
- 36.
- Gould F, Magori K, Huang X. Genetic strategies for controlling mosquito-borne diseases. Am Sci. 2006;94:238–246.
- 37.
- Boëte C, editor. Genetically Modified Mosquitoes for Malaria Control. Georgetown: Eurekah/Landes Bioscience; 2006.
- 38.
- Knols BGJ. Current controversies: is the transgenic mosquito as a weapon against malaria ever going to fly? Available at: http://www
.kaisernetwork .org/health_cast/hcast_index .cfm?display =detail&hc=1567. Accessed July 31, 2007. - 39.
- Marrelli MT, Moreira CK, Kelly D, Alphey L, Jacobs-Lorena M. Mosquito transgenesis: what is the fitness cost? Trends Parasitol. 2006;22:197–202. [PubMed: 16564223]
- 40.
- Moreira LA, Wang J, Collins FH, Jacobs-Lorena M. Fitness of anopheline mosquitoes expressing transgenes that inhibit Plasmodium development. Genetics. 2004;166:1337–1341. [PMC free article: PMC1470781] [PubMed: 15082552]
- 41.
- Lyman RF, Lawrence F, Nuzhdin SV, Mackay TF. Effects of single P-element insertions on bristle number and viability in Drosophila melanogaster. Genetics. 1996;143:277–292. [PMC free article: PMC1207261] [PubMed: 8722781]
- 42.
- Horn C, Handler AM. Site-specific genomic targeting in Drosophila. Proc Natl Acad Sci USA. 2005;102:12483–12488. [PMC free article: PMC1194931] [PubMed: 16116081]
- 43.
- Catteruccia F, Godfray HC, Crisanti A. Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science. 2003;299:1225–1227. [PubMed: 12595691]
- 44.
- Riehle MA, Jacobs-Lorena M. Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochem Mol Biol. 2005;35:699–707. [PubMed: 15894187]
- 45.
- Ferguson H, Gandon S, Mackinnon M, Read A. Malaria parasite virulence in mosquitoes and its implications for the introduction and efficacy of GMM malaria control programmes. In: Boëte C, editor. Genetically Modified Mosquitoes for Malaria Control. Georgetown: Eurekah/Landes Bioscience; 2006. pp. 103–116.
- 46.
- Scott TW, Rasgon JL, Black WC IV, Gould F. Fitness studies: developing a consensus methodology. In: Knols BGJ, Louis C, editors. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Dordrecht: Springer; 2006. pp. 171–181.
- 47.
- James AA. Gene drive systems in mosquitoes: rules of the road. Trends Parasitol. 2005;21:64–67. [PubMed: 15664528]
- 48.
- Braig HR, Yan G. The spread of genetic constructs in natural insect populations. In: Letourneau DK, Burrows BE, editors. Genetically Engineered Organisms: Assessing Environmental and Human Health Effects. Boca Raton: CRC Press; 2001. pp. 251–314.
- 49.
- Rasgon JL, Gould F. Transposable element insertion location bias and the dynamics of gene drive in mosquito populations. Insect Mol Biol. 2005;14:493–500. [PubMed: 16164605]
- 50.
- Curtis CF. Models to investigate some issues regarding the feasibility of driving refractoriness genes into mosquito vector populations. In: Knols BGJ, Louis C, editors. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Dordrecht: Springer; 2006. pp. 199–202.
- 51.
- Curtis CF, Coleman PG, Kelly DW, Campbell-Lendrum DH. Advantages and limitations of transgenic vector control: sterile males versus gene drivers. In: Boëte C, editor. Georgetown: Eurekah/Landes Bioscience; 2006. Genetically Modified Mosquitoes for Malaria Control. pp. 60–78.
- 52.
- Jacobs-Lorena M, James AA. Genetic Modification of Insects of Medical Importance: Past, Present and Future. Geneva: World Health Organization; 2003.
- 53.
- Touré YT, Knols BGJ. Genetically-modified mosquitoes for malaria control: requirements to be considered before field releases. In: Boëte C, editor. Georgetown: Eurekah/Landes Bioscience; 2006. Genetically Modified Mosquitoes for Malaria Control. pp. 146–151.
- 54.
- USDA (United States Department of Agriculture). 2002. http://www
.aphis.usda .gov/biotech/arthropod. - 55.
- Grand Challenges in Global Health. 2006. http://www
.gcgh.org/subcontent .aspx?SecID=392. - 56.
- Clayton J. Scientists plan field tests for GM mosquitoes. Lancet Infect Dis. 2006;6:191–192. [PubMed: 16583503]
- 57.
- Knols BGJ, Bossin H. Identification and characterization of field sites for genetic control of mosquitoes. In: Knols BGJ, Louis C, editors. Dordrecht: Springer; 2006. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. pp. 203–209.
- 58.
- Spielman A, Beier JC, Kiszewski AE. Ecological and community considerations in engineering arthropods to suppress vector-borne disease. In: Letourneau DK, Burrows BE, editors. Genetically Engineered Organisms: Assessing Environmental and Human Health Effects. Boca Raton: CRC Press; 2002. pp. 315–329.
- 59.
- Zhong D, Temu EA, Guda T, Gouagna L, Menge D, Pai A, Githure J, Beier JC, Yan G. Dynamics of gene introgression in the African malaria vector Anopheles gambiae. Genetics. 2006;172:2359–2365. [PMC free article: PMC1456378] [PubMed: 16452145]
- 60.
- PATH. Malaria Research and Development: An Assessment of Global Investment. Seattle: Malaria R&D Alliance; 2005. [Accessed July 31, 2007]. Available at http://www
.malariaalliance .org/PDFs/RD_Report_complete.pdf. - 61.
- Vreysen MJB, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, Juma KG, Dyck VA, Msangi AR, Mkonyi PA, Feldmann HU. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J Econ Entomol. 2000;93:123–135. [PubMed: 14658522]
- 62.
- Mshinda H, Killeen GF, Mukabana WR, Mathenge EM, Mboera LEG, Knols BGJ. Development of genetically modified mosquitoes in Africa. Lancet Infect Dis. 2004;4:264–265. [PubMed: 15120341]
- 63.
- Touré YT, Manga L. Ethical, legal and social issues in the use of genetically modified vectors for disease control. In: Knols BGJ, Louis C, editors. Dordrecht: Springer; 2006. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. pp. 221–225.
- 64.
- Pew Initiative on Food and Biotechnology. Biotech bugs: a look at the science and public policy surrounding the release of genetically modified insects; Washington, DC. September 20–21, 2004. 2005.
- 65.
- ACME (American Committee of Medical Entomology). Arthropod containment guidelines. 2002. [Accessed July 31, 2007]. Available at: http://www
.astmh.org/SIC/files/ACGv31 .pdf. - 66.
- International Atomic Energy Agency. Status and Risk Assessment of the Use of Transgenic Arthropods in Plant Protection. FAO/IAEA; Vienna, Austria: 2006.
- 67.
- Curtis CF. Population replacement in Culex fatigans by means of cytoplasmic incompatibility. 2 Field cage experiments with overlapping generations. Bull World Health Organ. 1976;53:107–119. [PMC free article: PMC2366410] [PubMed: 1085660]
- 68.
- Knols BGJ, Njiru BN, Mathenge EM, Mukabana WR, Beier JC, Killeen GF. MalariaSphere: a greenhouse-enclosed simulation of a natural Anopheles gambiae (Diptera: Culicidae) ecosystem in western Kenya. Malar J. 2002;1:19. [PMC free article: PMC149390] [PubMed: 12537599]
- 69.
- Knols BGJ, Njiru BN, Mukabana WR, Mathenge EM, Killeen GF. Contained semi-field environments for ecological studies on transgenic African malaria vectors: benefits and constraints. In: Takken W, Scott TW, editors. Dordrecht: Springer; 2003. Ecological Aspects for Application of Genetically Modified Mosquitoes. pp. 91–106.
- 70.
- Fiksel J, Covello VT. Biotechnology Risk Assessment: Issues and Methods for Environmental Introductions. New York: Pergamon Press; 1986.
- 71.
- Handler AM, Atkinson PW. Status and Risk Assessment of the Use of Transgenic Arthropods in Plant Protection. FAO/IAEA; Vienna, Austria: 2006. Areas of concern for the evaluation of transgenic arthropods. International Atomic Energy Agency; pp. 45–56.
- 72.
- Macer DRJ. Ethical, Legal and Social Issues of Genetically Modified Disease Vectors in Public Health. Geneva: UNDP/World Bank/WHO; 2003.
- 73.
- Macer D. Ethical, legal and social issues of genetically modifying insect vectors for public health. Insect Biochem Mol Biol. 2005;35:649–660. [PubMed: 15894183]
- 74.
- Aultman KS, Walker ED, Gifford F, Severson DW, Beard CB, Scott TW. Research ethics. Managing risks of arthropod vector research. Science. 2000;288:2321–2322. [PubMed: 10917830]
- 75.
- Robinson AS, Franz G, Atkinson PW. Insect transgenesis and its potential role in agriculture and human health. Insect Biochem Mol Biol. 2004;34:113–120. [PubMed: 14871607]
- 76.
- Knols BGJ, Dicke M. Bt crop risk assessment in the Netherlands. Nat Biotechnol. 2003;21:973–974. [PubMed: 12949550]
- 77.
- Oh New Delhi, Oh Geneva (editorial). Nature. 1975;256:355–357.
- 78.
- World Health Organization. WHO-supported collaborative research projects in India: the facts. WHO Chron. 1976;30:131–139. [PubMed: 1266199]
- 79.
- Grover KK, Suguna SG, Uppal DK, Singh KRP, Ansari MA. Field experiments on the competitiveness of males carrying genetic control systems for Aedes aegypti. Entomol Exp Appl. 1976;20:8–18.
- 80.
- Reuben R, Rahman SJ, Panicker KN, Das PK, Brooks GD. The development of a strategy for large-scale releases of sterile males of Aedes aegypti (L.). J Commun Dis. 1975;7:313–326.
- 81.
- Powell K, Jayaraman KS. Mosquito researchers deny plotting secret biowarfare test. Nature. 2002;419:867. [PubMed: 12410269]
- 82.
- Curtis CF. Review of previous applications of genetics to vector control. In: Knols BGJ, Louis C, editors. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Dordrecht: Springer; 2006. pp. 33–43.
- 83.
- Winstanley DD, Sorabji S, Dawson S. When the pieces don’t fit: a stakeholder power matrix to analyse public sector restructuring. Publ Money Manag. 1995 April–June:19–26.
- 84.
- Chan Kim W, Mauborgne R. Blue Ocean Strategy: How to Create Uncontested Market Space and Make Competition Irrelevant. Cambridge, MA: Harvard Business School Press; 2005.
- 85.
- Macer D. Ethical, legal and social issues of genetically modifying insect vectors for public health. Insect Biochem Mol Biol. 2005;35:649–660. [PubMed: 15894183]
- 86.
- Kaneko A, Taleo G, Kalkoa M, Yamar S, Kobayakawa T, Bjorkman A. Malaria eradication on islands. Lancet. 2000;356:1560–1564. [PubMed: 11075770]
- 87.
- Reyburn H, Drakeley C. The epidemiological consequences of reducing the transmission intensity of P. falciparum. In: Boëte C, editor. Genetically Modified Mosquitoes for Malaria Control. Georgetown: Eurekah/Landes Bioscience; 2006. pp. 89–102.
- 88.
- Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–264. [PubMed: 12521262]
- 89.
- Boëte C, Koella JC. A theoretical approach to predicting the success of genetic manipulation of malaria mosquitoes in malaria control. Malar J. 2002;1:3. [PMC free article: PMC111501] [PubMed: 12057019]
- 90.
- Inaba M, Macer DRJ. Attitudes to biotechnology in Japan in 2003. Eubios J Asian Int Bioethics. 2003;13:78–89. [PubMed: 16273712]
- 91.
- Mercer D. Future Revolutions. London: Orion Business; 1998.
- 92.
- World Bank Regional Reports—Africa Region. 2001. http://web
.mit.edu/urbanupgrading /upgrading /case-examples/overview-africa /regional-overview.html. - 93.
- Donnelly MJ, McCall PJ, Lengeler C, Bates I, D’Alessandro U, Barnish G, Konradsen F, Klinkenberg E, Townson H, Trape JF, Hastings IM, Mutero C. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005;4:12. [PMC free article: PMC552321] [PubMed: 15720713]
- 94.
- Curtis CF, Andreasen M. Large scale control of mosquito vectors of disease. In: Tan KH, editor. Area-Wide Control of Fruit Flies and Other Insect Pests. Palau Penang: Penerbit Universiti Sains Malaysia; 2000. pp. 135–142.
- 95.
- Kristan M, Fleischmann H, della Torre A, Stich A, Curtis CF. Pyrethroid resistance/susceptibility and differential urban/rural distribution of Anopheles arabiensis and An. gambiae s.s. malaria vectors in Nigeria and Ghana. Med Vet Entomol. 2003;17:326–332. [PubMed: 12941018]
- 96.
- Reed ZH, Friede M, Kieny MP. Malaria vaccine development: progress and challenges. Curr Mol Med. 2006;6:231–245. [PubMed: 16515513]
- 97.
- Knols BGJ, Hood-Nowotny RC, Bossin H, Franz G, Robinson A, Mukabana WR, Kemboi SK. GM sterile mosquitoes—a cautionary note. Nat Biotechnol. 2006;24:1067–1068. [PubMed: 16964206]
Authors’ addresses: Bart G. J. Knols, Entomology Unit, FAO/IAEA Agriculture and Biotechnology Laboratory, A-2444 Seibersdorf, Austria and Laboratory of Entomology, Wageningen University and Research Centre, PO Box 8031, 6700 EH Wageningen, The Netherlands, E-mail: ln.ruw@slonK.traB. Hervé C. Bossin and Alan S. Robinson, Entomology Unit, FAO/IAEA Agriculture and Biotechnology Laboratory, A-2444 Seibersdorf, Austria. Wolfgang R. Mukabana, Department of Zoology, University of Nairobi, PO Box 30197-00100 GPO, Nairobi, Kenya.
- Transgenic Mosquitoes and the Fight against Malaria: Managing Technology Push in...Transgenic Mosquitoes and the Fight against Malaria: Managing Technology Push in a Turbulent GMO World - Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives
Your browsing activity is empty.
Activity recording is turned off.
See more...