By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003.

Holland-Frei Cancer Medicine. 6th edition.
Show detailsMalnutrition and cachexia may be assessed simply in office practice by (1) measurement of weight loss as a percentage of the patient's usual body weight, accounting for edema, if any; (2) current weight as it compares to ideal body weight; and (3) a history of decreased appetite and/or decreased food intake. Anthropometric studies (weight, weight/height ratio, calf circumference, and mid-arm circumference) can be useful when performed serially by the same professional to assess the impact of nutritional intervention. Biochemical measurements such as transferrin, albumin, prealbumin, creatinine-height index, and several others have been used to assess protein-caloric undernutrition. These values are subject to changes related to the underlying malignancy and its treatments, however, and may be unreliable as indicators of nutritional state.147 Quantitative measurement of body composition should be a reliable means of nutritional assessment that can be applied for sequential research studies with a considerable degree of accuracy.148
The subjective global assessment (SGA) is a validated tool that measures nutritional status based on the features of a medical history (weight changes, dietary intake changes, gastrointestinal symptoms that have persisted for more than 2 weeks, changes in functional capacity) and physical examination (loss of subcutaneous fat, muscle wasting, ankle/sacral edema, and ascites). The SGA has been applied successfully as a method of assessing nutritional status and predicting complications in a number of different patient groups including cancer patients.149 A simplified method called malnutrition screening test (MST), which is based on only two parameters, appetite and recent unintentional weight loss, is useful in the assessments of malnutrition in cancer patients undergoing radiotherapy.150,151
Clinical Manifestations ((Figure 144-2))
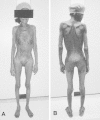
Figure 144-2
This 74-year-old woman with squamous cell carcinoma of the esophagus presented to Mount Sinai Hospital with a 46-1b weight loss over 1 year. (Four-color version of figure on CD-ROM)
Clinically, cachexia manifests as asthenia; anorexia; early satiety; nausea; taste change; significant loss of body fat, muscle, and other components; anemia; and hormonal aberration.152,153 The patients affected appear chronically ill and emaciated. The patient may be normothermic, febrile, or hypothermic. The skin is atrophic, shiny, or flaky and has loose folds. The temporal fossae are sunken as are the cheeks, leaving prominent zygomatic protuberances. The eyes may appear to bulge. Old denture plates become too large because of gum atrophy. The anatomy of the fat-depleted neck is revealed with prominent borders of the sternocleidomastoid muscles, larynx, and clavicles. The breasts sag and are shriveled from loss of fat. The ribs are visible and the interspaces sunken. The sternum and xiphoid are often visible. Vertebral spines are prominent. Heart rates are often high. The abdomen is scaphoid or protuberant, the pubis prominent. The buttocks lose their roundness, and large folds of skin hang around the prominent ischia. The elbows and knees look large compared to the sinewy atrophic muscle masses. The sacrum and greater trochanters, and sometimes the scapulae, lateral malleoli, and heels, are the sites of erythema and bulla formation, which may evolve into decubitus ulceration with its dry eschar or undermining necrosis of the entire skin. Fluid accumulation may be prominent in the bulbar conjunctivae, and along the chest and abdominal walls posterolaterally as an eccentric bulging, where it is compressed out of the fully dependent back by the patient's lying supine. At other times the entire lower back area contains fluid in patients who roll more from side to side. Bizarre patterns of fluid accumulation occur in patients who assume unusual constant postures, usually to avoid pain. The hair and nails grow more slowly, and if the patient recovers, transverse striae of the fingernails provide a historical record of the period.
Malnutrition not only affects morbidity and mortality but also can lead to lower quality of life and a depressing change in self-image.
Fever
Recurrent unexplained fever is a common manifestation of certain tumors in man.154 Lymphomas, especially Hodgkin disease, acute leukemias, renal cell carcinoma, osteogenic sarcoma, and atrial myxoma, have a high frequency of associated fever. The possible pathogenesis of tumor-associated fever includes undiagnosed infection, release of pyrogen from tumor and of inflammatory cytokines from tumor and/or monocytes and macrophages from host, tissue necrosis with leukocytic infiltration, abnormalities of liver function with altered conjugation of steroids, or excessive heat production by tumor cells. Patients with Hodgkin disease and lymphoma have high levels of IL-6 and IL-10.155,156 Serum levels of IL-6 correlated with B symptoms. IL-6 is also elevated in renal cell carcinoma. Fever depletes energy states rapidly, accelerating the advent of cachexia.
Fatigue
Fatigue is a common symptom in patients with advanced cancer and cachexia. Factors contributing to fatigue are probably multifactorial and may include anemia, weight loss, fever, pain, medication, and infection.157 In cancer patients, many of these factors are influenced by a frequently disrupted balance between endogenous cytokine levels and their natural antagonists. Indeed, cancer cells and the immune system appear to overexpress a range of cytokines in patients with cancer. Some of these cytokines act as autocrine or paracrine growth factors for the neoplastic tissue while simultaneously causing secondary symptoms related to fatigue. For instance, cancer-associated anemia may be due to a blunted erythropoietin response and/or cytokines (IL-1, IL-6, TNF-α), which suppress erythropoiesis.
Specific Nutritional Deficiencies
In patients undergoing cancer chemotherapy, deficiencies can be diagnosed almost exclusively based on recognition of clinical signs and symptoms, and based on the patient's response to a precise, potent, and prolonged therapeutic trial.158 Vitamin levels in the blood are often nondiagnostic in such cases. Vitamin B1 deficiency in a form of wet beriberi, vitamin B2 deficiency manifested as bilateral angular cheilosis and cheilitis, and niacin deficiency with the skin lesions of pellagra on the feet and legs have been described in patients with cancer and leukemia during treatment. Prompt response to treatment with the respective vitamins was confirmatory of the clinical impression. Clinical manifestations of folic acid deficiency are frequently seen in patients treated with methotrexate. The toxicity produced by methotrexate includes ulcerative stomatitis and pharyngitis, gastroenteritis, and proctitis. Marrow aplasia or megaloblastic dysplasia with macrocytic anemia and leukopenia may occur. Once tissue damage takes effect, the specific antidote leucovorin is not effective. Vitamin K deficiency with pronounced bleeding tendency occurs in patients with severe malnutrition, intestinal malabsorption, or obstructive jaundice and after long-term treatment with antibiotics that are active against the intestinal flora and/or compete with vitamin K for intestinal absorption sites. Treatment with vitamin K1 oxide, and in severe cases with fresh-frozen plasma or a concentrate of vitamin K-dependent coagulation factors, is effective.
Specific Organ Dysfunction
There are many other clinical manifestations that are related to cachexia that develop with or without direct effects of the tumor on certain organs of cancer patients. Patients with advanced cancer often have gastric stasis and delayed gastric emptying, which may cause early satiety.6 Idiopathic steatorrhea or villous atrophy has been associated with a variety of cancers in humans. Refractory sprue comprises a heterogeneous group of patients with diverse underlying causes. A large group of patients with refractory sprue harbor occult intestinal T-cell lymphoma.159 In an autopsy series, cancer patients comprised 59% of cases with nonbacterial thrombotic (marantic) endocarditis.160 Tumors most frequently associated with this condition were carcinoma of the ovaries, biliary system, pancreas, lung, and stomach. Forty percent of all patients at autopsy were cachectic. Deposits of antigen-antibody complexes in the renal glomeruli can be a cause of nephrotic syndrome seen in patients with cancer or leukemia.161,162 Progressive multifocal leukoencephalopathy, a rare demyelinating syndrome that usually occurs in patients who have leukemia, lymphoma, carcinomatosis, acquired immunodeficiency syndrome, or in patients who have received chemotherapy or immunosuppressive therapy, has been associated with simian virus 40 or, more persuasively, with JC virus.163,164 Diencephalic syndrome is characterized by progressive emaciation, loss of adipose tissue, pallor without anemia, euphoria, alertness, and hyperactivity.62 Neurologic signs are usually absent but the optic pathway may be involved. Patients usually have a good appetite and a food intake at times in excess of normal requirements. In other patients, intermittent vomiting occurs. This syndrome occurs from tumors of the third ventricle and the anterior hypothalamus, 85% of which are gliomas. The pathogenesis of lipolysis remains undetermined. Excessive energy expenditure secondary to constant physical overactivity may be a cause of emaciation.
Laboratory Manifestations
Energy Expenditure
In cancer patients, resting energy expenditure and metabolic rates are elevated and the increases are regarded as a contributing factor to weight loss.165 An elevation of 12% in the metabolic rate was estimated to account for the loss of body weight of 1 to 2 kg per month.166 In other studies minimal or no elevation in metabolic rates were observed in many cancer patients. Measurements of resting energy expenditure in cancer patients showed that only one-third of them were hypermetabolic, one-half were hypometabolic, and the rest were normometabolic.167,168 Following curative resection resting energy expenditure normalized or remained normal, but after palliative resection, resting energy expenditure remained hypermetabolic or significantly increased toward hypermetabolism. There are substantial metabolic differences depending on the anatomic site and histologic type of cancer. Thus, patients with lung cancer showed an increase in resting energy expenditure compared to healthy controls, whereas patients with gastrointestinal cancer had no elevation in resting energy expenditure.169 Increases in resting energy expenditures were more pronounced in patients with an acute-phase response.170 Patients with lower gastrointestinal tract cancer were metabolically similar to normal volunteers, while patients with upper gastrointestinal cancer had elevated rates of lipolysis, glucose turnover, and an increased reliance on glucose as an energy substrate.171,172 Patients with advanced head and neck cancer as well as with sarcoma were also metabolically abnormal, having accelerated rates of glucose turnover, glucose recycling, and a loss of the protein-sparing response to starvation.173,174
Carbohydrate Metabolism
Abnormalities of carbohydrate metabolism in tumor-bearing animals may include abnormal glucose tolerance, elevated blood lactate, and changes in glucose turnover, pool size, and half-life. In cancer patients with progressive weight loss, enhanced glucose use with increases in both complete oxidation and the Cori cycle, and “diabetic glucose” tolerance have been described.175,176 Glucose uptake and lactate release by human colon carcinomas have been found to exceed the peripheral tissue exchange rate by 30-fold and 43-fold, respectively.177 A positive correlation was found between tumor bulk and rate of plasma glucose appearance, plasma glucose clearance, the percent of tissue glucose uptake recycled to lactate, and oxygen consumption.178 In these patients insulin:glucose ratios were either increased, decreased, or unchanged, but the insulin: glucagon ratio was always decreased.179 Hyperglucagonemia may be an important hormonal alteration in the tumor-bearing host. Increases in glucagon levels caused anorexia, enhanced tumor protein synthesis, and stimulated tumor growth.180 Inhibition of glucagon secretion and reversal of insulin:glucose ratios were associated with increased carcass weight, increased muscle weight, increased liver cellular protein, and decreased tumor protein contents, as compared to tumor-bearing untreated controls.181
In patients with advanced cancer, hypoglycemia is also occasionally found.182 The explanation for hypoglycemia in islet cell tumors is the production of ectopic insulin or proinsulin. In patients with large hepatic carcinoma hypoglycemia occurs as a consequence of poor hepatic glycogen reserves and liver failure. Possible pathogenesis of hypoglycemia in large abdominal sarcomas, adrenocortical carcinoma, and gastrointestinal carcinomas includes excessive consumption of glucose by the neoplasms, elaboration of tryptophan derivatives, which inhibit hepatic gluconeogenesis, and production of insulin or insulin-like growth factor I and II.183–186
Fat Metabolism
Lipid metabolism in animal systems is altered by cancer, including loss of body fat early in tumor growth; induction of hyperlipidemia; changes in a variety of serum lipid and lipoprotein fractions; suppression of the postprandial rise in fatty acid synthesis in the liver; and reduction in the rate of excretion of 14CO2 after intraperitoneal injections of 14C-tripalmitin.187–189 Hyperlipidemia is a common characteristic in cancer patients. Hyperlipidemia can develop as the result of a decline in the activity of lipoprotein lipase in the adipose tissue that impairs uptake of exogenous fatty acid and/or increased hepatic lipogenesis.190 Both the decreases in lipoprotein lipase and increases in hepatic lipogenesis are mediated by TNF-αand IL-1, the latter via IL-6. Likewise, lipolysis occurs via tumor-derived lipolytic factor(s).1,24,112 In patients with hyperlipidemia plasma triglyceride levels are elevated, high-density lipoprotein levels are decreased, and very-low-density lipoprotein levels are elevated. Plasma total lipoprotein lipase activity in cancer patients with varying degrees of weight loss was decreased by 35%, as compared to that of normal volunteers.191 Both hepatic and peripheral lipoprotein lipase activities were equally depressed. In addition, cancer patients were found to have higher rates of fat oxidation when compared to control subjects with equal weight loss.165,192 The magnitude of the increase in fat oxidation suggests that this occurs in the host rather than in tumor tissue, contributing to fat loss in cancer patients. The inability of glucose loading to suppress fatty acid oxidation in cachectic patients suggests that there is a disturbance in normal homeostatic mechanisms involving the integration of glucose.66
Patients with ovarian and endometrial tumors were found to have lower concentrations of linoleic acid in subcutaneous adipose tissue than did cancer-free controls, suggesting mobilization of structural lipid to supply to tumor.192 Linoleic acid is also a stimulator of tumor growth.193 Lipolysis of host fat, hyperlipidemia, and resynthesis of the fatty acid within a tumor cell may represent another futile cycle contributing to cancer cachexia.
In a study of autopsy samples, brown adipose tissue comprised 80% of periadrenal tissue in cachectic cancer patients, as compared to 13% in age-matched controls.194 Brown adipocytes express abundant amounts of β3-adrenergic receptors. The most unique feature of brown adipocytes is their expression of UCP-1, a 32-kDa protein of the inner mitochondrial membrane, which functions to uncouple mitochondrial respiration, that is, the failure to make high-energy phosphate bonds. The physiologic consequence of uncoupling protein activity is unrestrained oxidation of fuels with the sole byproduct being the generation of heat, leading to increased energy expenditure. Brown adipose tissue also plays an important role in regulating total-body fat stores.119
Protein Metabolism
Protein-calorie undernutrition is universally prevalent in patients with advanced cancer.195 Whole-body protein turnover is increased in most patients with advanced cancer, as compared with starved normal individuals and weight-losing noncancer patients.196 The energy cost of this increased protein turnover has been estimated to be 100 Kcal/d.198 Loss of skeletal-muscle protein, a hallmark of cancer cachexia, occurs through increased rate of skeletal muscle protein breakdown and a reduction in the rate of muscle protein synthesis.197,198
Three major proteolytic pathways responsible for the catabolism of protein in skeletal muscle are known: (1) the cytosolic calcium-activated pathway, which is responsible mainly for tissue injury, necrosis, and autolysis; (2) the lysosomal system, which is involved mainly in the proteolysis of extracellular proteins and cell surface receptors; and (3) the ATP-ubiquitin-dependent proteolytic system, which is involved in breakdown of myofibrillar proteins in starvation, sepsis, metabolic acidosis, and cancer cachexia.1,199
Protein degradation in cachectic cancer patients is mediated primarily through the ubiquitin-proteasome proteolytic pathway. It has been suggested that glucocorticoids activate this system in sepsis, while in cancer cachexia, a tumor-derived glycoprotein, proteolysis-inducing factor, induces protein catabolism in skeletal muscle by increasing expression of proteasome subunits and the ubiquitin carrier protein, E214k.1,200,201 In this system, ubiquitin is bound covalently to the protein substrate, which acts as a signal for degradation by the multisubunit proteasome. Because this process requires ATP, this may be another process contributing to the elevated energy expenditure observed in cancer cachexia.202 Induction of proteasome expression by glucocorticoids appears to be a direct result of the downregulation of the activity of nuclear factor-kappaB, while proteolysis-inducing factor acts through 15-hydroxyeicosatetraenoic acid as an intracellular transducer.1,200,201 The mechanism behind overexpression of the ubiquitin gene under these conditions may be related to β2-adrenoreceptor overactivity.203
While ubiquitin-proteasome-dependent proteolysis is the predominant mechanism of muscle protein loss, there is evidence that several other regulatory mechanisms may also be important in producing muscle cachexia induced by sepsis, severe injury, and cancer.204 Participation of the putrescine-spermine pathway in muscle tissue in tumor-bearing animals has been suggested.205 TNF-induced apoptosis has been observed in experimental cancer-associated cachexia.206 Many cytokines activate the transcriptional coactivator, peroxisome proliferator-activated receptor (PPAR)-gamma coactivator-1 (PGC-1) through phosphorylation by p38 kinase, resulting in stabilization and activation of PGC-1 protein. Cytokine or lipopolysaccharide (LPS)-induced activation of PGC-1 in cultured muscle cells or muscle tissue in vivo caused increased respiration and expression of genes linked to mitochondrial uncoupling and energy expenditure. These data illustrate a direct thermogenic action of cytokines and p38 mitogen-activated protein (MAP) kinase through the transcriptional coactivator PGC-1.207
There is an imbalance between the amino acid composition of skeletal muscle and acute-phase proteins.208 In the fasting state, the synthetic rate of fibrinogen, a positive acute-phase protein, is higher in cancer patients than in healthy controls. In cancer patients in the fed state, fibrinogen synthetic rate rose by a median of 38%, whereas in controls there was no significant change.209 These findings demonstrate significant upregulation by feeding of acute-phase protein synthesis in cachectic cancer patients. This may provide a mechanism whereby a proportion of supplied nutrients is diverted from anabolism in cancer cachexia.
Specific protein abnormalities in cancer patients are exemplified by anemia, hypoalbuminemia, acute-phase protein response, and hepatic catalase depression.
Anemia
More than 50% of all cancer patients are anemic. The causes of anemia include acute or chronic blood loss, marrow involvement by tumor cells, and marrow-suppressive effects of chemotherapy or radiation therapy. Less frequently, anemia results from red cell aplasia, folate or B12 deficiency, iron deficiency, hemolytic process, hypersplenism, or “pure red cell aplasia.” In many instances, the cause of anemia cannot be found in cancer patients. In these cases, anemia is described as “anemia of chronic disease,” where reuse of heme products within the marrow is inefficient.
Several negative regulators of erythropoiesis are involved in the pathogenesis of defective erythroid maturation. The erythropoietic precursors are acted on by apoptosis inducers, namely, transforming growth factor (TGF)-β1, TNF-α, IFN-γ, and Fas ligand (Fas-L).210,211 These proapoptogenic factors are physiologically involved in modulating the maturation of erythroid cells and are upregulated in the anemia of chronic disorders, as well as in many solid tumors and hematologic malignancies.212–214
Severe normochromic/normocytic anemia in patients with multiple myeloma is caused by the abnormal upregulation of apoptogenic receptors, including both Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL), by highly malignant myeloma cells as the pathogenesis of the ineffective erythropoiesis, and by chronic exhaustion of the erythroid matrix.215
A meta-analysis of earlier controlled clinical trials of epoetin treatment of anemia associated with cancer therapy has been reported.216 Epoetin reduced the odds of transfusion for cancer patients undergoing therapy; however, evidence was insufficient to determine whether initiating epoetin earlier spares more patients from transfusion or results in better quality of life than waiting until hemoglobin concentrations decline to nearly 10 g/dL.
Occasionally, patients become polycythemic because of erythropoietin-producing tumors such as renal adenocarcinoma, hepatocellular carcinoma, and cerebellar hemangioblastoma.
Hypoalbuminemia
Depression of serum albumin level is a common manifestation in patients with advanced cancer. Three mechanisms of hypoalbuminemia can be considered: decreased synthesis, increased degradation, or an increased transcapillary escape rate.217 In hepatocytes in culture and in a murine model, decreases in albumin synthesis by TNF-α or IL-6 have been demonstrated.218,219 No significant differences in plasma volume or in albumin secretion rate were found in cancer patients when compared to healthy controls.220,221 Albumin intravascular mass was decreased but its fractional synthetic rate was increased in the cancer group. The changes in transcapillary escape rates observed in at least some advanced cancer patients with hypoalbuminemia were relatively small and overall did not correlate with circulating albumin concentrations. Reduced albumin degradation was reported in a heterogeneous group of patients with advanced cancer. Thus, exact mechanisms of cachexia-associated hypoalbuminemia have not been determined.
The nephrotic syndrome and protein-losing enteropathy, albeit rare, are known to occur in patients with cancer and when present contribute to the development of hypoalbuminemia.222 Hypoalbuminemia is the major side effect of certain chemotherapeutic agents.145,146
Acute-Phase Protein Response
In contrast to unchanged albumin synthesis in the liver of cancer patients with weight loss and hypoalbuminemia, fibrinogen synthesis rates are increased.223,224 These changes reflect an aspect of the acute-phase protein response, a reprioritization of liver protein synthesis often seen in trauma, inflammation, and infection.224,225 Elevated circulating IL-6 levels were associated with acute-phase response in these patients.226 Acute-phase protein response may be seen in a significant proportion of patients with a variety of cancers.227–229 The presence of such a response in cancer patients is strongly associated with a reduced quality of life in patients with gastrointestinal cancer and shortened survival in patients with renal, pancreatic, and colorectal cancers.230–234
Hepatic Catalase
Depression of hepatic catalase activity is another remote effect of cancer. Serial needle biopsies of the liver were performed and catalase activities were determined in patients with cancer undergoing chemotherapy and radiotherapy.235 Changes in hepatic catalase activity correlated with clinical response and with deterioration and subsequent death. However, the exact relationship between hepatic catalase depression and cachexia has not been established. Thus, prolonged administration of 3-amino-1,2,4-triazole, an inhibitor of catalase, was reported to have no effect on the growth rate of young rats.236 Acatalasemia has been found as a genetic defect in apparently healthy relatives of patients with oral gangrene.237
Neuroendocrine System
A variety of hormonal abnormalities have been reported in cancer patients. These abnormalities include increases in plasma cortisol, increase in urinary excretion of cortisol and its metabolites and catecholamines, decreases in testosterone levels or hypogonadism, and diabetic glucose tolerance secondary to insulin resistance and/or to increased rate of insulin clearance.133,134,238,239 The elevated basal metabolic rate observed in cancer patients is associated with an increase in heart rate and may be caused by an elevated adrenergic state.165
Water and Electrolytes
Hyponatremia is common in patients with advanced cancer. The cause of hyponatremia may include ectopic secretion of antidiuretic hormone (ADH) or an ADH-like substance by the tumor; cardiac, renal, or adrenal cortical insufficiency; excessive loss of sodium from gastrointestinal fistulae, diarrhea, salt-losing nephropathy, and diuretic use. Cyclophosphamide and vinca alkaloids are also known to produce hyponatremia either as a direct neurotoxic effect on the central nervous system with release of ADH from its site of formation and storage or as direct renal tubular toxicity. Hyponatremia occurs quite frequently in patients with intracranial tumors. This may result from excessive use of dextrose-water solutions, inadequate salt intake in obtunded patients under improper dietary supervision, and rarely from excessive ADH release. The search for a specific cause of hyponatremia in cancer patients is not always successful.
A subclinical or overt increase in total body water may occur in patients with cancer. Malnutrition, hypoalbuminemia, “nonspecific” adrenal response, and mechanical obstruction of venous or lymphatic return may play a role. Fluid overload may also be produced by zealous hydration as a part of treatment. New sodium-free water may arise from excessive loading by mouth or vein, oxidation of fat, and catabolism of cellular tissue.
- Manifestations of Cachexia - Holland-Frei Cancer MedicineManifestations of Cachexia - Holland-Frei Cancer Medicine
Your browsing activity is empty.
Activity recording is turned off.
See more...