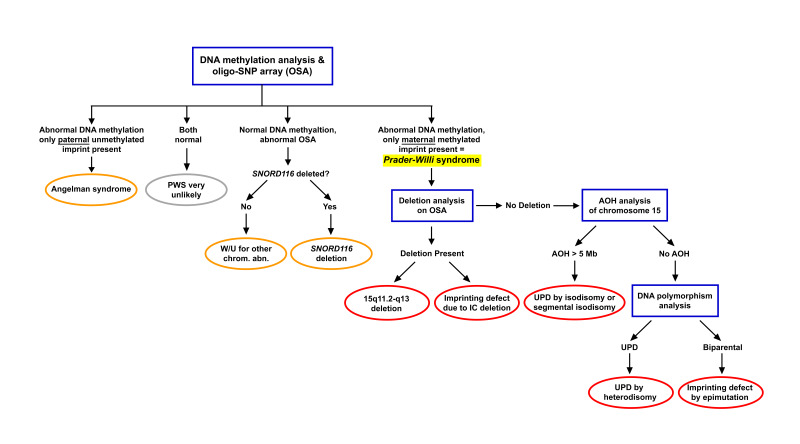
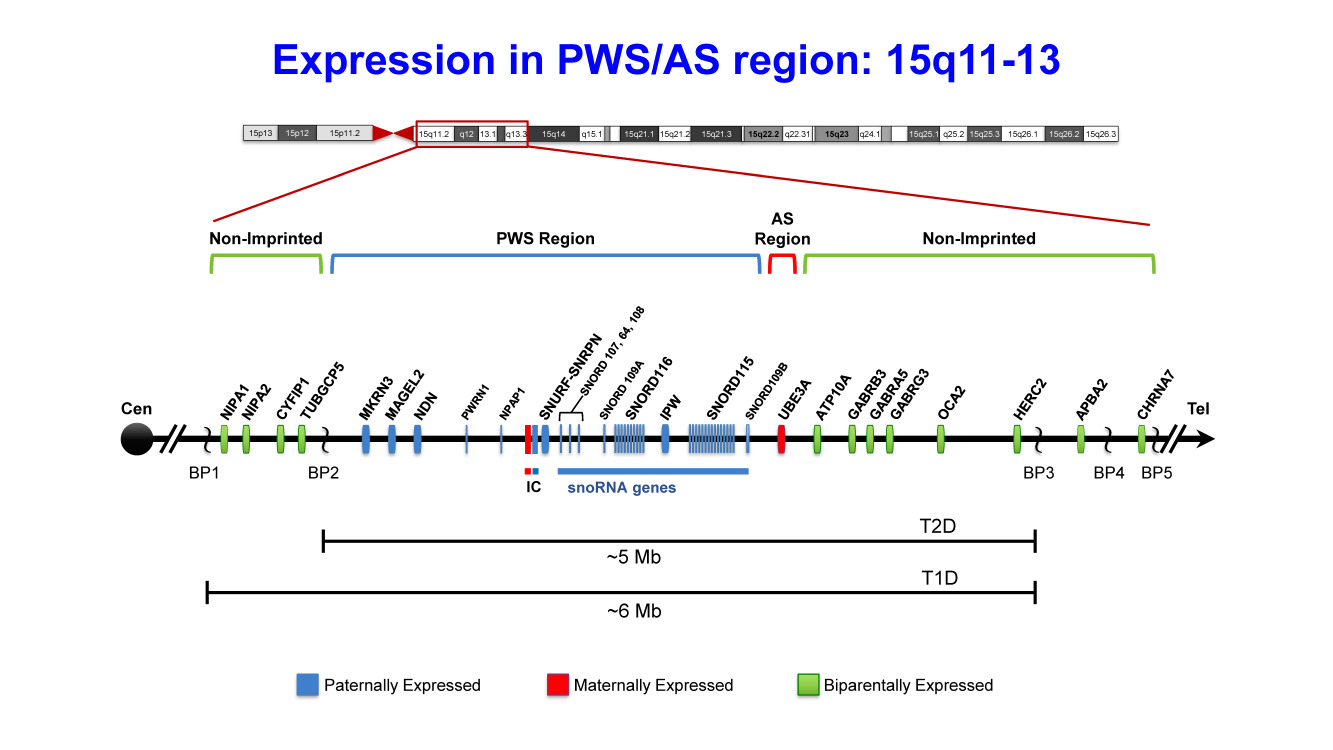
Clinical Description
Prader-Willi syndrome (PWS) is a complex, multisystem disorder characterized by neonatal hypotonia with poor suck and poor weight gain without nutritional support, developmental delay, mild cognitive impairment, hypogonadism leading to genital hypoplasia and pubertal insufficiency, short stature if untreated with growth hormone (GH), childhood-onset obesity if excessive eating is not limited, behavioral findings, and typically a characteristic facial appearance. Less consistent but common features include decreased fetal movements, small hands and/or feet, hypopigmentation compared to the affected individual's family members, skin picking, strabismus and visual acuity abnormalities, sleep disturbance (including daytime sleepiness and sometimes sleep apnea), thick, viscous saliva, and articulation differences. There is some variability in clinical findings depending on the molecular cause of PWS.
Table 2.
Prader-Willi Syndrome: Frequency of Select Features
View in own window
| Feature | % of Persons w/Feature | Comment |
|---|
|
Infantile hypotonia
| 95%-100% | Assoc w/poor suck; results in poor weight gain w/o feeding support |
|
Dysphagia
| 90%-100% | Typically present at birth & persists to adulthood |
|
Motor delay
| 90%-100% | |
|
Language delay
| 90%-100% | Incl abnormal speech articulation, speech apraxia |
|
Intellectual disability
| 90%-100% | Most w/mild disability; ranges from severe learning disabilities to significant cognitive disability |
|
Endocrine manifestations
| 90%-100% | Hypogonadism, abnormal pubertal development, growth deficiency, diabetes mellitus, hypothyroidism |
|
Hyperphagia & obesity
| 90%-100% | |
|
Characteristic behavior profile
| 70%-90% | Anxiety, tantrums, rigidity, OCD, manipulative behavior, autistic features, ADHD; psychosis becomes evident in young adults, esp those w/UPD 15 |
|
Increased pain threshold
| 60%-80% | May mask urgent medical issues |
|
Dysmorphic facial features
| 50%-70% | May attenuate w/GH therapy; more common in persons w/15q deletion |
|
Hypopigmentation
| 50%-70% | Primarily in those w/15q deletion |
|
Skin picking
| 50%-60% | Decreases in older adults |
|
Strabismus
| 40%-60% | |
|
Sleep abnormalities
| 30%-40% | Central apnea (in infants), obstructive sleep apnea, daytime sleepiness, narcolepsy |
|
Scoliosis
| 40%-80% | |
|
Seizures
| 10%-20% | Typically generalized & treatable |
ADHD = attention-deficit/hyperactivity disorder; GH = growth hormone; OCD = obsessive-compulsive disorder; UPD = uniparental disomy
Perinatal findings. A retrospective study of prenatal ultrasounds of 47 individuals with PWS younger than age ten years found that affected individuals had decreased fetal movements (88%), were small for gestational age (65%), had asymmetrical intrauterine growth with increased head-to-abdomen circumference ratio (43%), and had polyhydramnios (34%) when compared to controls [Gross et al 2015]. Prenatal hypotonia usually results in decreased fetal movements, abnormal fetal position at delivery, and increased incidence of assisted delivery or cesarean section. Fetal size is generally within the normal range, but the birth weight and body mass index (BMI) are on average 15% lower than in typically developing sibs [Miller et al 2011].
Hypotonia. Infantile hypotonia is a nearly universal finding, causing decreased movement and lethargy with decreased spontaneous arousal, weak cry, and poor reflexes, including poor suck. Hypotonia is central in origin, and neuromuscular studies including muscle biopsy, when done for diagnostic purposes, are generally normal or show nonspecific signs of disuse.
Poor suck, dysphagia, lethargy, and poor appetite result in poor weight gain in early infancy without assisted feeding. Feeding difficulties are reported in 99% of infants; nasogastric tube feeding (a gastrostomy tube is rarely needed) or the use of special nipples is generally required for a variable period of time, usually weeks to months. By the time the child is drinking from a cup or eating solids, a period of approximately normal eating behavior occurs.
The hypotonia improves over time. However, children and adults remain mildly hypotonic, with decreased muscle bulk and tone.
Developmental delay. Early motor milestones are achieved at approximately double the normal age (e.g., sitting at 12 months, walking at 24 months). Language milestones are also typically delayed and speech is impaired [Dimitropoulos et al 2013]. Speech articulation is abnormal in many individuals with PWS. Speech apraxia has been reported in 7%-10% and is more common in those with 15q deletion. Although a small proportion of affected individuals have extremely impaired language development, verbal ability is a relative strength for most.
Intellectual disabilities are generally evident by the time the child reaches preschool age. Testing indicates that most individuals with PWS have mild intellectual disability (mean IQ: 60-70), with approximately 40% having borderline disability or low-normal intelligence and approximately 20% having moderate disability. Regardless of measured IQ, most children with PWS have multiple severe learning disabilities and poor academic performance for their intellectual abilities [Whittington & Holland 2017]. Based on the authors' experiences, a small percentage of individuals with PWS are able to attend and graduate from college. Increased skill with jigsaw puzzles is reported, particularly in individuals with a 15q deletion.
Endocrine manifestations
Hypogonadism is present in both sexes and manifests as genital hypoplasia, incomplete pubertal development, and infertility in the vast majority. Genital hypoplasia is evident at birth and throughout life.
Males. The penis may be small, and most characteristic is a hypoplastic scrotum with poorly rugated scrotal skin and decreased pigmentation. Unilateral or bilateral cryptorchidism is present in 80%-90% of males.
Females. Genital hypoplasia is often overlooked; however, the labia majora, labia minora, and clitoris are often small from birth.
Hypogonadism is usually associated with low serum concentration of gonadotropins and causes incomplete, delayed, and sometimes disordered pubertal development. Infertility is almost universal, although a few instances of reproduction in females have been reported [
Cassidy et al 2012]. Although hypogonadism in PWS has long been believed to be entirely hypothalamic in origin, studies have suggested a combination of hypothalamic and primary gonadal deficiencies [
Eldar-Geva et al 2009,
Hirsch et al 2009,
Eldar-Geva et al 2010,
Gross-Tsur et al 2012], a conclusion largely based on the absence of hypogonadotropism and abnormally low inhibin B levels in some affected individuals of both sexes.
Male infants with PWS and cryptorchidism can be treated with human chorionic gonadotropin (hCG), which results in anatomically lower testes as well as improvement in the size of the penis and scrotal sac, prior to urologic surgery. Undergoing orchiopexy at a younger age, as well as higher levels of inhibin B and testosterone after hCG treatment, have been associated with a greater number of germ cell-containing tubules on testicular histology [
Bakker et al 2015].
Premature pubarche has been reported in 15%-20% of males and 30% of females. Premature adrenarche was reported in 15%-20% of individuals with PWS. Premature pubarche and adrenarche have been associated with elevated dehydroepiandrosterone sulfate levels. Advanced bone age is also reported. Central precocious puberty, typically idiopathic, has been reported in 5% of individuals.
Growth deficiency. Data from at least 15 studies involving more than 300 affected children document reduced GH secretion in individuals with PWS [
Burman et al 2001]. Short stature is reported in 60%-70% of untreated individuals; if not apparent in childhood, short stature is almost always present during the second decade in the absence of GH treatment. The lack of a pubertal growth spurt results in an average untreated height of 155 cm for males and 148 cm for females. GH deficiency is also seen in adults with PWS [
Grugni et al 2006,
Höybye 2007]. Treatment with GH is beneficial in individuals with PWS regardless of GH sufficiency status [
Alves & Franco 2020,
Höybye et al 2021]. Growth charts for affected infants and children not treated with GH have been published [
Butler et al 2011,
Butler et al 2015], and growth charts for GH-treated children with PWS have been developed [
Butler et al 2016].
The hands and feet grow slowly and are generally below the fifth centile by age ten years (70%-90% of individuals) in the absence of GH treatment, with an average adult female foot size of 20.3 cm and average adult male foot size of 22.3 cm.
Hypothalamic pituitary dysfunction. Impaired hypothalamic development and function results in multiple hormonal deficiencies, including GH deficiency, hypogonadism, hypothyroidism, corticotropin deficiency, and abnormal oxytocin neurons [
Tauber & Hoybye 2021].
Type II diabetes. Up to 25% of adults with PWS (particularly those with significant obesity) have type II diabetes [
Tauber & Hoybye 2021], with a mean age of onset of 20 years. Earlier diagnosis, education of parents, GH therapy, and the availability of group homes specific for adults with PWS have led to a reduction in the development of morbid obesity and type II diabetes.
Central hypothyroidism, with a normal thyroid-stimulating hormone and low free thyroxine, has been documented in up to 25% of individuals with PWS. The mean age of diagnosis is two years [
Miller et al 2008,
Diene et al 2010].
Central adrenal insufficiency (CAI). A Dutch study initially suggested that CAI was common in individuals with PWS [
de Lind van Wijngaarden et al 2008], but subsequent studies, including one large international study, found that CAI is rare (1.2%) in adults with PWS [
Rosenberg et al 2020]. The international study concluded that there is no need for hydrocortisone supplementation in individuals with PWS in the absence of clinical manifestations and confirmation of adrenal insufficiency.
Appetite, obesity, and gastrointestinal manifestations. In contrast to the long-held view that there are only two distinct nutritional phases in PWS (i.e., poor weight gain followed by hyperphagia leading to obesity), a multicenter study found that the transition between nutritional phases is much more complex, with seven different nutritional phases through which individuals with PWS typically progress [Miller et al 2011] (see Table 3).
Table 3.
Nutritional Phases in Prader-Willi Syndrome
View in own window
| Phase | Median Ages | Clinical Characteristics |
|---|
|
0
| Prenatal to birth | ↓ fetal movements & lower birth weight than sibs |
|
1a
| Birth to 9 mos | Hypotonia w/difficulty feeding & ↓ appetite |
|
1b
| 9 to 25 mos | Improved feeding & appetite; growing appropriately |
|
2a
| 2.1 to 4.5 yrs | Weight ↑ w/o appetite ↑ or excess calories |
|
2b
| 4.5 to 8 yrs | ↑ appetite & calories but can feel full |
|
3
| 8 yrs to adulthood | Hyperphagic, rarely feels full |
|
4
| Adulthood | Appetite no longer insatiable for some |
Hyperphagia in PWS is believed to be caused by a hypothalamic abnormality resulting in lack of satiety. Food-seeking behaviors such as hoarding or foraging for food, eating of inedible objects, and stealing of food or money to buy food are common. At this time there are no consistently identified hormonal abnormalities to explain the hyperphagia, and the metabolic correlates of hyperphagia in PWS remain uncertain.
Obesity results from these hyperphagic behaviors and from decreased total caloric requirement. The latter is due to decreased resting energy expenditure resulting from decreased activity and decreased lean body mass (primarily muscle) compared with unaffected individuals. Obesity in individuals with PWS is primarily central (abdomen, buttocks, and thighs) in both sexes. Interestingly, there is less visceral fat in obese individuals than would be expected for the degree of obesity. Obesity and its complications are the major causes of morbidity and mortality (see Life span at the end of this section).
Early diagnosis allows the clinician to begin anticipatory guidance concerning the natural history of PWS, and in particular the nutritional phases (see
Table 3), informing the family about the risks of obesity and the need to monitor weight gain and restrict calories beginning around age 18 to 36 months. Obesity may be prevented if the diet, exercise, and supervision program described in
Treatment of Manifestations is instituted.
If started at a young age, GH treatment, along with good dietary control, may prevent obesity and the high proportion of fat mass. It may also modify the typical PWS facial appearance, improve motor milestones, and improve some cognitive abilities [
Butler et al 2019b,
Ayet-Roger et al 2022].
Acute gastrointestinal manifestations. In most affected individuals, gastric emptying is delayed (60%-80% of individuals), and vomiting is rare; decreased vomiting is reported in 80%-90%, increasing the risk of gastric necrosis with significant hyperphagia. Abdominal distention, bloating, pain, lethargy, loss of appetite, and vomiting may be signs of life-threatening gastric inflammation or necrosis. Individuals with these symptoms should be urgently evaluated by a medical professional and may require hospitalization. Radiographic imaging and possibly emergency surgery may be required. Use of antidiarrheal medications can lead to severe colonic distention, necrosis, and rupture.
Behavior. A characteristic behavior profile with anxiety, temper tantrums (outbursts), rigidity/resistance to change, obsessive-compulsive behaviors, and social cognition deficits becomes evident in early childhood in individuals with PWS [Ishii et al 2017, Schwartz et al 2021]. These behaviors increase with age and BMI in childhood and adolescence, although it was noted by Dykens [2013] that externalizing behavioral issues (e.g., aggression, impulsivity) tend to decline in older adults (age >40 years).
Behavioral and psychiatric issues interfere most with the quality of life in adolescence and adulthood, including affecting ability to live independently.
Pain insensitivity. Diminished typical pain perception is common and may mask the presence of infection, injury, or fractures. Individuals with PWS may not report pain until the condition is severe, and they may have difficulty localizing pain.
Dysmorphic features. Characteristic facial features (narrow bifrontal diameter, almond-shaped palpebral fissures, narrow nasal bridge, thin vermilion of the upper lip with down-turned corners of the mouth) may or may not be apparent at birth and may slowly evolve over time [Cassidy & Driscoll 2009, Cassidy et al 2012]. Treatment with GH may attenuate the dysmorphic features over time.
Hypopigmentation of hair, eyes, and skin is frequently found in individuals with 15q deletion due to the deletion of OCA2 located at 15q.
Dermatologic manifestations. Skin and mucosal picking, often leaving chronic open sores, is one of the more difficult issues. Picking at the nose, rectum, or vagina is common and often unknown to caregivers. Picking can result in pigmentary changes, scarring, and infections.
Peripheral edema is not uncommon in obese individuals with PWS and may lead to chronic changes of the legs.
Ophthalmologic manifestations. Strabismus in those with PWS is often diagnosed by age five years. Myopia and hyperopia are common [Bohonowych et al 2021].
Sleep abnormalities are well documented and include reduced rapid eye movement (REM) latency, altered sleep architecture, oxygen desaturation, and both central and obstructive apnea [Festen et al 2006, Priano et al 2006]. Primary hypothalamic dysfunction is thought to be the cause of the alterations in sleep microstructure and abnormalities in ventilation during sleep. Some individuals with PWS have excessive daytime sleepiness, which resembles narcolepsy, with rapid onset of REM sleep and decrease in non-REM sleep instability [Bruni et al 2010]. Narcolepsy, reported in 10%-35%, is often undiagnosed in individuals with PWS due to the prevalence of excessive daytime somnolence. Multiple sleep latency tests must be done to diagnose narcolepsy. Cataplexy has been known to occur in individuals with PWS, but the prevalence is not known.
Skeletal findings. Scoliosis, present in 40%-80% of affected individuals, varies in age of onset and severity, and may be present from infancy. It is presumed to be related to hypotonia, since there are no underlying structural anomalies. Kyphosis occurs commonly in adolescents and adults with PWS. Hip dysplasia occurs in approximately 20%-30% [Trizno et al 2018]. The incidence of osteopenia and osteoporosis is increased in individuals with PWS. Frequency of bone fractures, particularly of long bones, seems increased, although no well-designed study has been published.
Seizures, typically generalized seizures, may occur in early childhood. They tend to be infrequent and often resolve after a few years of anti-seizure medication [Takeshita et al 2013].
Dental issues. Saliva flow is decreased in people with PWS and can lead to increased dental caries and speech articulation difficulties. Dried material on the lips is common. Dental crowding and enamel hypoplasia are also more common.
Life span. The mortality rate in individuals with PWS is higher than in controls with intellectual disability. With improved management, the death rate has declined to 1.25% per annum [Whittington et al 2015]. Several large studies have indicated that respiratory failure and other febrile illnesses are the most frequent causes of death in children, and cardiac disease and failure, pulmonary thromboembolism, obesity-related complications, and gastric causes are most frequent in adults [Butler et al 2017, Pacoricona Alfaro et al 2019]. Of note, a family questionnaire-based survey of more than 2,000 individuals known to the Prader-Willi Syndrome Association (USA), of whom 114 were deceased, showed that those who were living had a significantly higher rate of GH treatment (threefold higher) than those who were deceased, even after adjusting for the greater age of those who were deceased [Proffitt et al 2019].
Acute gastric distention with gastric rupture and necrosis has been reported as a cause of death in several individuals with PWS, particularly following an eating binge among those who are thin but were previously obese. It may be unrecognized because of a high pain threshold.
Choking, especially on hot dogs, has been reported as cause of death in approximately 8% of deaths in individuals with PWS. Disordered pharyngeal and esophageal swallowing and lack of attempt to clear residue or coughing is common in PWS [Gross et al 2017], and combined with rapid eating may increase the risk of aspiration-related mortality.
Concern about the possible contribution of GH administration to unexpected death has been raised by reported deaths of individuals within a few months of starting GH therapy. The few reported deaths were mostly in obese individuals who had preexisting respiratory or cardiac disorders with evidence of upper airway obstruction and uncorrected tonsillar and adenoidal hypertrophy. In other studies, the rate of death in affected individuals on and off GH therapy did not differ; thus, the relationship of GH administration to unexpected death remains unclear. See Management for recommended evaluations prior to starting GH therapy.
Neuroimaging. Reported abnormalities on brain imaging include white matter lesions, ventriculomegaly, decreased volume of brain tissue in the parietal and occipital lobes, Sylvian fissure polymicrogyria, incomplete insular closure, gray matter volume changes, and reduced pituitary height. Their relationship to clinical manifestations remains unclear.