By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003.

Holland-Frei Cancer Medicine. 6th edition.
Show detailsNormal Histology and Anatomy
The prostate sits in the pelvis, surrounded by the rectum posteriorly and the bladder superiorly (Figure 111-3). Its normal function is not well understood; it produces some seminal fluid and may facilitate sperm motility. The prostate is composed of branching glands, with ducts that are lined with secretory epithelial cells and basal cells.64 Scattered neuroendocrine cells are also present and are thought to provide a paracrine function in the gland.65 Secretory epithelial cells represent the major cell type in the gland, are androgen-dependent for growth, and produce PSA and prostatic acid phosphatase.66 The basal cell layer is not dependent on androgen for growth and is believed to contain the stem cell population for the epithelial prostate cells. Surrounding the gland is a stroma that includes fibroblasts, smooth muscle, nerves, and lymphatics. Stromal-epithelial interactions remain poorly understood, but recent insights suggest that the stroma produces multiple growth factors important for growth and development of normal prostate as well as prostate cancer.67–69
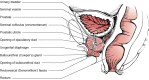
Figure 111-3
Normal prostate anatomy.
Early anatomists suggested that the prostate developed in a lobar pattern, despite the fact that normal adult prostates do not have discernible lobes. More recently, McNeal and colleagues conducted detailed studies of the normal and pathologic anatomy of the prostate and defined a concept of anatomic zones, rather than lobes, to describe the prostate.70, 71 There are four major zones within the normal prostate: the peripheral zone (70% of glandular tissue), the central zone (20% of glandular tissue), the transition zone (75% of glandular tissue), and the anterior fibromuscular stroma (Figure 111-4). The peripheral zone extends posterolaterally around the gland from the apex to the base and represents the most common site in the prostate for developing prostate carcinomas. The central zone surrounds the ejaculatory duct apparatus and makes up the majority of the prostatic base. The transition zone constitutes two small lobules that abut the prostatic urethra and represent the region where benign prostatic hyperplasia (BPH) primarily originates. Compared to peripheral zone cancers, carcinomas that originate in the transition zone have been suggested to be of lower malignant potential; however, other studies have suggested that there is no difference in outcome when controlled for grade and stage.72, 73
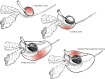
Figure 111-4
Zonal anatomy of the prostate: the three glandular zones of the prostate and the anterior fibromuscular stroma.
There is no true anatomic capsule that surrounds the prostate, similar to that seen in the kidney. The smooth muscle of the prostatic stroma gradually extends into fibrous tissue that then ends in loose connective and adipose tissue.74 At the apex and anteriorly, even this minimally distinct border is lost.
Although differences exist in how “extracapsular disease” is defined, extension of cancer outside of the prostate gland has been demonstrated in many studies to be an important prognostic factor for recurrence. Even more recent anatomic studies performed by Walsh and Donker delineated the presence of the two neurovascular bundles that pass adjacent to the gland posterolaterally.75 Acknowledgment of the role that the neurovascular bundles play in normal erectile function and defining their presence outside of Denonvillier fascia allowed Walsh to develop a “nerve-sparing” radical retropubic prostatectomy procedure, which significantly improved the odds of preserving potency postoperatively.76
Premalignant Lesions
The development of solid tumors is generally thought to be a multistep process, whereby successive genetic events occur in a normal cell to render it increasingly malignant. The prototype for this model of carcinogenesis in solid tumors is colon cancer, but similar studies have confirmed this model in other diseases including breast and cervical cancers.77 In prostate cancer, the genetic and epigenetic phenomena that occur that result in cancer are not well understood, but there is evidence that premalignant lesions in the prostate may precede the development of cancer by many years.
Although many terms have been used to describe prostatic epithelial dysplasia or atypia,78 these heterogeneous morphologic lesions now are considered under the single term “prostatic intraepithelial neoplasia” (PIN) (Figure 111-5).79 PIN is defined as the presence of cytologically atypical or dysplastic epithelial cells within architecturally benign-appearing glands and acini. Three different grades have been described: grade 1 (mild), grade 2 (moderate), and grade 3 (severe). Grades 2 and 3 PIN often are combined as “high grade.” PIN is presumed to be a premalignant lesion based on its common presence adjacent to prostate adenocarcinomas.80 High-grade PIN lesions are more often seen in multifocal cancers as well. Autopsy studies suggest that PIN precedes the development of cancer by at least 10 years.
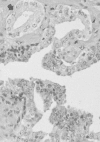
Figure 111-5
Photomicrograph of high-grade prostatic intraepithelial neoplasm (PIN) with basal cell layer (open arrows) with budding microacinus lacking basal cells (curved solid arrows). A microacinus of invasive Gleason pattern 3 adenocarcinoma is seen in the adjacent (more...)
A recent retrospective review compared 61 patients with high-grade PIN alone on their initial prostate biopsy to a matched control of 47 patients with negative biopsies.81 After a median follow-up of 6.8 years, prostate cancer was diagnosed in 49% of the PIN group and 21% of controls (p = .003). Despite this, cancers were generally detected early, and no differences in survival were noted between the two groups. These data suggest that the presence of high-grade PIN can predict subsequent development of cancer, perhaps through the multistep carcinogenesis process noted earlier. However, close routine clinical follow-up remains the standard of care after diagnosis of high-grade PIN alone.
Atypical adenomatous hyperplasia is another lesion that may represent a premalignant change, although data for this are more scant than for PIN. The lesion's characteristic appearance fulfills the architectural criteria for malignancy—disruption of the basal cell layer, mainly in the transition zone—but without the cytologic changes diagnostic of cancer.82
Histology of Prostate Cancer
Over 95% of prostate cancers are adenocarcinomas that arise from prostatic epithelial cells (Figure 111-6).83 Other rare histologies have been described, including mucinous or signet-ring cell carcinomas, adenoid cystic carcinomas, carcinoid tumors, large prostatic duct carcinomas (including the endometriod typeadenocarcinomas), and small-cell undifferentiated cancers. It is important to recognize these unusual variants of prostate cancer since standard hormonal therapies may be less effective with some of them.84 Tumors with a neuroendocrine appearance (carcinoid and small-cell undifferentiated) may arise from Kulchitsky cells, which are found in the basal regions of the prostatic epithelium.65 Small-cell carcinomas of the prostate have similar histologic and clinical features to other extrapulmonary small-cell carcinomas. Transitional cell carcinomas involving the substance of the prostate may also be mistaken for prostate adenocarcinoma. Even when the transitional cell features of these tumors are recognized, it may be difficult to distinguish a transitional cell carcinoma arising in the transitional epithelium of the distal prostatic ducts from a tumor arising from the bladder epithelium and spreading contiguously into the prostatic ducts.85
Many studies have confirmed the primary prognostic importance of the degree of histologic differentiation of prostate adenocarcinoma. The degree of differentiation is typically graded by patterns of gland formation and, less importantly, by cytologic detail. The most widely accepted grading scheme for adenocarcinoma of the prostate is that developed by Gleason (Figure 111-7).86 Recognizing the heterogeneous differentiation in prostate carcinomas, Gleason described a system for classifying prostate tumors based on two levels of scoring. The primary pattern of differentiation is assigned a Gleason grade of 1 to 5 based on the dominant morphology of the specimen and its departure from normal appearance; the secondary pattern (the next most common pattern) is also assigned a grade. This results in a two-digit score: for example, 3 + 4 = 7. The Gleason system has been criticized for inadequate recognition of the proportion of the tumor that is composed of the secondary pattern and for a lack of adequate distinction between good and poor prognoses in cancers with scores of 5 to 7, which represent the majority of patients. However, its reproducibility and reliability between pathologists have been shown consistently to be excellent. Gleason's original work demonstrated a clear association between a higher Gleason score and higher mortality, which others have confirmed.87 Alternative systems have been proposed based on the cytologic characteristics of the tumor or the proportion of the tumor that forms glands. In addition, many other predictors of clinical behavior have been explored, including PSA, clinical stage, tumor volume, deoxyribonucleic acid (DNA) ploidy, and nuclear morphometry. Many molecular markers have also been explored, but currently the Gleason score remains the most broadly applicable and prognostically useful histologic grading system.88

Figure 111-7
Histologic grading scheme for adenocarcinomas of the prostate. Source, Gleason DF.
Molecular Pathogenesis
A greater understanding of the molecular determinants of prostate cancer progression is essential for the further delineation of the malignant potential of this disease.89 The molecular mechanisms of carcinogenesis in the prostate are under intensive investigation in the laboratory, and several promising areas of investigation have been identified.
Alterations in molecules that regulate the cell cycle and apoptosis offer promising avenues for further investigation. P53,90 p27,91 p21, and Rb92 have been studied, with resulting variable levels of evidence that they participate in the pathogenesis of prostate cancer. In one study, loss of expression of the tumor-suppressor protein p27 was strongly associated with the development of BPH, whereas variable levels of p27 expression were noted in prostate cancers.93 In addition, there is a high frequency of somatic mutations in PTEN, a tumor-suppressor gene, which suggests that this may be a frequent target for inactivation in sporadic prostate cancers.94 One study documented a 60% rate of PTEN mutations; most of these were found in cell lines from metastatic disease, although mutations were also seen in primary cancers.95 In fact, another group demonstrated higher rates of PTEN loss or mutation in advanced stage and grade tumors.96 This growing body of evidence suggests that alterations in PTEN may be an important step in the development of prostate cancer.
Growth factors and their receptors also play an important role in the growth regulation of normal and cancerous cells. There have been numerous specific growth factors implicated in the growth and survival of prostate cancer (Table 111-4).67 Although the mechanisms of the progression of prostate cancer are poorly understood, many of these growth factor genes can be amplified in this setting. Although initially regulated by androgen, it is possible that, through genetic changes, genes encoding one or more of these growth factors could be dysregulated from androgen control. Further, in the androgen-independent state, there may be cross-talk between the signal transduction pathways of peptide growth factors and the androgen receptor (AR) pathway.69
Table 111-4
Growth Factors Implicated in Prostate Cancer.
Insulin-like growth factors I and II (IGF-I and -II), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor (TGF)-β have each been shown to be dysregulated in the development of prostate cancer. IGF is thought to play an important role in the development of the prostate. In prostate cancer, several lines of evidence suggest that IGF-II may be important in prostate cancer growth. First, several prostate xenograft models express both IGF-I and II as well as receptors for each. PC-3, an androgen-independent prostate cancer cell line, secretes IGF-II. In addition, experiments suggest that IGF-II levels are increased in adenocarcinoma cells, suggesting a role in prostate carcinogenesis. However, the most compelling evidence of the functional importance of IGF-I comes from population studies. Chan and colleagues reported on the relationship between plasma IGF-1 levels and prostate cancer, citing a relative risk of 4.3 for men in the highest quartile compared to men in the lowest quartile. This association was independent of PSA levels. Further, IGF levels increased the detection rate of prostate cancer over PSA alone.97
- Biology of Prostate Cancer - Holland-Frei Cancer MedicineBiology of Prostate Cancer - Holland-Frei Cancer Medicine
Your browsing activity is empty.
Activity recording is turned off.
See more...
