By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003.

Holland-Frei Cancer Medicine. 6th edition.
Show detailsAmeloblastoma
The ameloblastoma is the most common and important of the odontogenic tumors, representing approximately 1% of all jaw cysts and tumors according to an early definitive report by Small and Waldron.2 However, a more recent analysis suggests a rate varying from 3% to 19%. When considered as a subset only of odontogenic tumors, the incidence of ameloblastoma is more than 11% based on a review by Reichart and colleagues whose report was based on literature data from more than 3000 ameloblastomas.3 About 70% of all ameloblastomas occur in the molar-ramus region of the mandible. In the maxilla the molar region is also the most common site of occurrence. It is also the most dangerous area since invasion of the maxillary sinus and nasal cavity is common in large lesions.3,4 It is a benign but aggressive epithelial tumor whose cell of origin is derived from remnants of the enamel organ from which teeth are formed. Monoclonal antibody and immunofluorescent staining techniques have demonstrated positive staining for CD 56 and CD147 confirming that the cell of origin is from the stellate reticulum of the enamel organ and is of neural crest cell origin.5
Ameloblastomas have been divided into three groups based upon their radiographic appearance and location: unicystic, solid and multicystic, and peripheral.
The unicystic ameloblastoma arises from the odontogenic epithelium within a cyst or de novo as a neoplasm.6 Radiographically in its most common presentation it resembles a dentigerous cyst, presenting as a radiolytic lesion with a well-defined cortical border surrounding the crown of an unerupted tooth (Figure 91-1).

Figure 91-1
Unicystic ameloblastoma demonstrating ameloblastomatous cells in the wall of the “cyst” (hematoxylin and eosin ×100 magnification). (Four-color version of figure on CD-ROM)
Clinical
Most unicystic ameloblastomas occur in people younger than 40. There is no sexpredilection and almost 90% occur in the mandible, usually posteriorly, where they are associated with an impacted tooth.7 If large it presents as a painless swelling. If small it will be discovered as an incidental radiographic finding without symptoms. The differential diagnosis will be that of a radiolucency in a dentigerous relationship to an impacted tooth. Therefore, one must consider dentigerous cyst, (OKC), ameloblastoma, (COC), a small odontogenic myxoma, and ameloblastic fibroma.
Differential Diagnosis
Based predominantly upon the radiographic assessment the differential diagnosis includes an odontogenic cyst, a fibro-osseous lesion and possibly a small odontogenic myxoma.
Histopathology
The gross specimen appears to be an odontogenic cyst but histopathologically the epithelial lining is that of an ameloblastoma (see Figure 91-1). The essential finding is that of epithelial islands bordered by palisading columnar epithelial cells that exhibit reverse nuclear polarity. It accounts for about 15% of all ameloblastomas. Histologically there are three forms: luminal, in which the ameloblastomatous epithelial lining is confined to the epithelial surface of the cyst; intraluminal, in which nodules of tumor extend into the cystic lumen; and mural, in which the lesion extends into the connective tissue wall of the cyst.3
Treatment
Simple enucleation and curettage is probably sufficient treatment for the luminal variant. Generally, this diagnosis is rendered as an unwelcome surprise after removal of what appears to have been a dentigerous cyst. After thorough reexamination of the specimen from the newly established point of view that it is a unicystic ameloblastoma rather than a dentigerous cyst, one must then decide whether to observe or to curette the bone cavity with hand instruments or a bur. If observation is chosen there is no upper limit to surveillance. The mural variant shows infiltration of the cyst wall and should be treated by peripheral ostectomy or resection depending on size and location. When treated by simple enucleation and curettage, the recurrence rate has been reported to be between 10% and 33%.7,8
Peripheral Ameloblastoma
This is the appearance of ameloblastoma at an extrabony site. It is purely a lesion of the soft tissue located in a tooth-bearing region and represents a small minority of all ameloblastomas. It is presumed to arise from epithelial rests from the dental lamina or from basal epithelial cells of the gingival epithelium. There are approximately 100 cases reported in the English and Japanese language literature.9
Clinical
It usually occurs in middle age as a painless mass located on the gingiva of the posterior mandible.
Differential Diagnosis
The differential includes peripheral giant cell lesion, peripheral ossifying fibroma and gingival (periodontal) cyst.
Histopathology
The histology is that of ameloblastoma, often demonstrating a connection to the surface epithelium from which the lesion arose. Originally some cases were described as intraoral basal cell carcinoma. It does not have an infiltrative pattern of growth but it may cause passive bone resorption without invasion of trabeculae. The lesion is not encapsulated.10 It may be differentiated from the similar peripheral odontogenic fibroma by the presence of dysplastic hard tissue and the absence of reverse polarity in palisaded columnar cells at the periphery of the epithelial islands.
Solid Ameloblastoma
This solid tumor is also an epithelial tumor whose origin is from the odontogenic rests of Serres and Malassez of the enamel organ and reduced enamel epithelium from the dental follicle. It is the most common subtype of ameloblastoma.
Clinical
Most frequently the presentation is that of a painless swelling causing expansion in the posterior mandible. When large the cortical boundaries of the mandible may be breached resulting in secondary infection of the soft tissue thereby causing pain. It is most common in the fourth and fifth decades but may occur at any age. There is no sex predilection. Either jawbone may be affected but involvement of the molar region of the mandible is most common. The exception to this pattern is for the desmoplastic variant, which occurs with equal likelihood in either jaw but with a marked preference for the anterior regions. In the mandible the most likely site is the anterior alveolar process.4 This is a tumor with a persistently aggressive pattern of growth for which recurrence rates of over 50% have been reported after conservative treatment. Aggressive treatment in the form of marginal mandibulectomy or segmental resection for lower jaw lesions and partial maxillectomy for upper jaw tumors has brought the recurrence rate down from 67% in 1937, to 33% in 1955 to about 20% in 1995. Half of the cases that ultimately recurred did so within 5 years with the longest recurrence being reported at 33 years.2,3,12 Others have reported a recurrence rate of less than 10% with recurrences developing more quickly after enucleation and curettage than after resection.13–16 Therefore, a period of surveillance exceeding 5 years is mandatory for proper management.
Radiographic
The appearance on imaging varies with size. Smaller lesions tend to be clearly defined radiolucencies with cortical borders. As they enlarge, the periphery becomes more indistinct, multilocularity occurs, tooth roots resorb and teeth become displaced. Large areas of loculation may demonstrate a “honeycombed” appearance (Figures 91-2 to 91-4). The unusual desmoplastic variant (5.3% of 64 cases reported as of 1998) lacks the typical radiographic appearance of a loculated, radiolytic lesion. Instead, these lesions tend not to resorb roots or move teeth and have ill-defined borders. Six of ten lesions reported by Kishino demonstrated a pattern of mixed radiopacity/radiolucency.4

Figure 91-2
Radiograph of ameloblastoma demonstrating a “honeycombed” pattern.
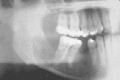
Figure 91-4
Ameloblastoma showing extensive destruction of the mandible and root resorption.

Figure 91-3
Ameloblastoma showing root resorption and expansion of the mandible.
Differential Diagnosis
The differential diagnosis is largely that of a radiolucency. This may include odontogenic cysts: OKC, dentigerous and COC; odontogenic tumors: CEOT, odontogenic myxoma, ameloblastic fibroma, clear cell odontogenic tumor; and nonodontogenic tumors: giant cell lesions, ossifying fibroma, and aneurysmal bone cyst.
Histopathology
The histologic pattern is fascinating, there being six recognized variations. The most common pattern is follicular, with the others being plexiform, acanthomatous, granular, basaloid and desmoplastic (Figures 91-5, 91-6). Despite the wide variation in appearance there is no difference in clinical behavior among the subtypes although some consideration has been given to the idea that the desmoplastic subtype is more aggressive. However, this latter concept may be more likely related to the larger size at the time of diagnosis (4.3 cm) compared with other forms of ameloblastoma whose average size at presentation is 3.0 cm. The acanthomatous variant tends to occur in older patients but does not have a different biological behavior.3 Most reviews do not support a higher recurrence rate for any of the different histologic types of these lesions when they are treated by resection.4,17
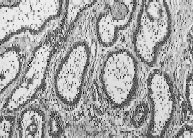
Figure 91-5
Photomicrograph of a follicular ameloblastoma ( ×100 magnification) showing typical pattern of palisading of epithelial cells. Courtesy of James Geist, DDS, MS. (Four-color version of figure on CD-ROM)
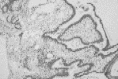
Figure 91-6
Photomicrograph of a plexiform ameloblastoma (hematoxylin and eosin ×100). Courtesy of James Geist, DDS, MS. (Four-color version of figure on CD-ROM)
In all forms there are islands, strands and sometimes sheets of epithelial cells in an invasive pattern of growth destroying trabeculae and forming cysts of various sizes but not inducing metaplastic bone formation. Within epithelial clusters the central cells resemble stellate reticulum when the growth pattern is follicular, squamous cells in the acanthomatous pattern, granular cells in the granular cell pattern, anastomosing cords of epithelium in the plexiform pattern and basaloid cells in the basal pattern. The distinctive microscopic finding is that of a single layer of columnar cells palisaded at the periphery of the epithelial islands. These cells demonstrate nuclei located away from the basement membrane in a pattern of reversed nuclear polarity as is seen during normal amelogenesis within the developing tooth follicle.18 This unique pattern seen normally during tooth morphogenesis occurs in the ameloblastoma and is unique (see Figure 91-5).
It would be worthwhile to understand the basic biology of the epithelium of this tumor in order to improve conceptualization of treatment and therefore enhance treatment results. During the past decade some of the techniques used for the investigation of epithelial malignancy have been applied to this tumor making available new information about the nature of its epithelial lining. Perhaps, in time, this information will result in improved treatment outcomes.
The odontogenic epithelium resembles that of the enamel organ or dental lamina, in particular the cells of the stellate reticulum. Although the detailed mechanism controlling differentiation and oncogenesis is unknown, some important points have been discovered in the past two decades. As summarized by Kumamoto there is a spectrum of altered immunoreactivity in the cellular apoptotic pathway of this epithelium that reaches maximum downregulation of Fas (apoptosis-related factors which are part of the tumor necrosis factor superfamily [TNF]) expression and upregulation of FasL (L = ligand) expression, an indication of escape from programmed cell death, that is, apoptosis.19,20
Caspase-3 is a member of the family of cysteine proteases of the interleukin-1B converting enzyme/cell death abnormal (CED)-3 family that are required for programmed cell death. Caspase-3 is the most downstream enzyme whose expression is associated with cell death. It has also been discovered that there is a progression of increased expression of Caspase-3 in the sequence: dental follicle-desmoplastic ameloblastoma-malignant ameloblastoma.
It was also noted that apoptosis was altered when apoptosis-related factors like the bcl-2 family of proteins and the p53 group were altered by mutation.
A host of other factors regulating cell cycle kinetics has also been examined in an attempt to unravel the biology of this fascinating epithelium. Cyclin D1 has been identified as a possible protooncogene named PRAD1, CCND1 or Bcl-1 gene. Cell kinetic inhibitors, particularly those acting in concert with the p16 protein, may have a significant role in transforming this epithelium into a malignant one.20
The cell adhesion molecule CD 56 has been found in ameloblastic epithelium suggesting that this lesion develops from proliferation of odontogenic cells of neural crest origin. It has also been found in squamous cell carcinomas of the head and neck and in adenoid cystic carcinoma with attribution of a positive influence on metastatic spread in the former but not in the latter.21,22 Another cell surface receptor, CD 147 has been found both in nests of odontogenic epithelium and in the fibroblasts surrounding those nests within ameloblastomas.5 It has been suggested that this indicates a collagenasestimulating effect that could influence the aggressive behavior of the ameloblastoma. However, this has not been proven.
There are two malignant entities associated with ameloblastoma, the malignant ameloblastoma and the ameloblastic carcinoma. The latter will be discussed under the heading of primary intraosseous carcinoma and the former will conclude this section. (v.i.)
Treatment
Wide excision of the intraosseous ameloblastoma is required. This implies maxillectomy for the maxillary lesion and either marginal or segmental resection of the mandible for mandibular lesions. When the bony confines of the mandible are breached the soft tissue must also be excised. This must include the periosteum and a reasonable amount of soft tissue beyond it. Particularly in the maxilla this may be a very difficult maneuver. Bony margins of one centimeter are recommended. A helpful adjunct for determining adequacy of resection in the operating theater is to obtain an intraoperative radiograph to assess marginal status since frozen section control on bone margins is not available (illustrated in Figure 91-14 demonstrating the same principle in regard to an odontogenic keratocyst).

Figure 91-14
Radiograph of resection specimen of patient in Figure 91-13 when he finally presented for treatment 8 years later.
Treatment by enucleation and curettage is unacceptable with recurrence rates of 60% to 90% in the mandible and 100% with a mortality rate of 60% in the maxilla.23
Even with resection recurrences in the mandible may be anticipated at a rate of up to 20.6%.3
Recurrence outside of bone can become a lifetime affliction of an incurable disease or result in death. There have been many soft tissue recurrences reported in the literature and our head and neck registry reports one case of a woman who has suffered from persistent and recurrent ameloblastoma for nearly 50 years. It was originally treated by maxillectomy with clear margins and then extra bony recurrence developed which has resulted in many operations, loss of the zygoma and part of the frontal bone and ultimately direct extension along the base of the skull into the brain.
Management of the inferior alveolar neurovascular bundle becomes an issue in treating mandibular lesions. Since most ameloblastomas are located in the molar to ramus area, one must evaluate encroachment on the inferior alveolar nerve to determine whether excision of the nerve as part of the specimen is necessary. For very small unicystic ameloblastomas the concept of nerve preservation has been brought up in a study by Nakamura and colleagues in which it was noted that tumor did not infiltrate around the nerve in unicystic tumors.24 In multicystic lesions there was consistent infiltration of perineural tissue. In general, since large lesions encroach upon the nerve and displace it inferiorly the nerve should be removed. If nerve reconstruction is chosen one has to decide whether to reconstruct the nerve with an autogenous sural nerve graft or some other form of conduit. Nerve reconstruction is performed most appropriately at the time of resection.
Reconstruction of bone may be accomplished either primarily or secondarily. Since the oral mucosa is nearly always violated, the chance for postoperative dehiscence and partial or complete loss of the bone graft is always a threat. However, corticocancellous blocks securely fixed with wire or miniplate osteosynthesis coupled with intermaxillary fixation or a rigid reconstruction plate will generally survive despite dehiscence. Absolute minimization of the risk of dehiscence and/or postoperative infection requires placement of the bone graft on a secondary basis using either blocks or condensed cancellous grafts. Resection using a reconstruction plate to maintain the occlusion and primary reconstruction of the inferior alveolar nerve followed by secondary bone grafting is illustrated (Figure 91-7).

Figure 91-7
Resection-reconstruction for ameloblastoma showing a reconstruction plate to maintain occlusion and autogenous nerve graft to reconstruct inferior alveolar nerve. (Four-color version of figure on CD-ROM)
Malignant Variant
The malignant ameloblastoma is indeed a strange tumor. It is a tumor histopathologically identical to ameloblastoma but it metastasizes. Histopathologically there are no features of the nuclei, altered patterns of growth, or dysplastic architecture that would lead one to suspect malignancy. Yet, some ameloblastomas (particularly follicular) have recurred persistently at the index site with demonstrated distant metastases. The metastatic lesions themselves have the same histopathologic features as do the primary tumor. Lung and lymph nodes have been the two most common sites for metastases in the tumor registry of Sinai Grace Hospital (Detroit) although one case of brain metastasis occurred. The literature suggests that lymph nodes and lung are the most likely sites for metastases followed by bone.2,25,26
Calcifying Epithelial Odontogenic Tumor (Pindborg Tumor)
This epithelial odontogenic neoplasm, first described by Jens Pindborg in 1956 closely resembles the ameloblastoma in its clinical and radiographic appearance.27 It probably arises from neoplastic cells derived from the stratum intermedium of the enamel organ.
There are two variants of this lesion, the central and the peripheral. The central lesion occurs predominantly in middle age, peaking at age 40, with most occurring between age 30 and 50 but may occur at any age with equal distribution between the sexes. There is a predilection for occurrence within the mandible (75%) with the premolar-molar area being most commonly affected. The usual presentation is as a slow-growing and painless swelling of the jaw without an attendant paresthesia. About half of these lesions are associated with impacted teeth.8,28 Unusual variations include the presence of clear cells that may represent clear cell odontogenic carcinoma.29 Rarely, malignant transformation to squamous cell carcinoma has been reported.30
A peripheral lesion exists that arises from the gingiva as a soft tissue lesion covered by normal mucosa. Probably the cells of origin are epithelial rests within the gingiva. These are not aggressive and can be removed by simple excision.
The central lesion arises from the stratum intermedium of the enamel organ or from the reduced enamel epithelium of the embedded tooth.31 The peripheral lesion arises from epithelial rests in gingivae or basal cells of gingival surface epithelium and is most common in the anterior jaw region.29
Radiographic Features
The radiographic presentation is highly variable depending upon the degree of production of calcified material within the lesion. When radiolucent it resembles the pattern of ameloblastoma, frequently being multilocular with scalloped margins. Thinning and expansion of the cortical bone along with resorption of tooth roots or displacement of teeth is common. Almost half are associated with an impacted tooth often in a dentigerous relationship. The radiolucent form may be indistinguishable from a dentigerous cyst. In the mixed phase radiopaque material is scattered throughout the radiolucent mass. Even in densely radiopaque lesions there is a thin band of radiolucency around the periphery of the lesion (Figure 91-8).

Figure 91-8
Radiograph of calcifying epithelial odontogenic tumor demonstrating a pattern of radiodensity. Courtesy of James Geist, DDS, MS
Differential Diagnosis
Considering the usual location in the posterior mandible and a radiolytic presentation, the differential diagnosis should include ameloblastoma, dentigerous cyst, odontogenic keratocyst and odontogenic myxoma. When radiopacities are present one must also consider COC, odontomas, adenomatoid odontogenic tumor (AOT) and ameloblastic fibroodontoma.
Histopathology
The eosinophilic epithelial cells are polyhedral, occur in sheets or small islands, are often pleomorphic, and occasionally multinucleated. Desmosomes clearly identify them as epithelial. The stroma is fibrous. Some of the epithelial cells enclose an eosinophilic homogeneous extracellular material that stains positively for amyloid. This latter material may calcify in the shape of concentric rings (Liesegang rings), or as spherical dystrophic calcifications within the amyloid bodies as psammoma bodies similar to those found in carcinomas of the thyroid and in meningiomas. These give rise to the radiopacities often seen on radiography. These calcifications appear to increase with age of the tumor.32 Occasionally the CEOT may be found in combination with an AOT.
Treatment
Case reports do not include any series of significant size with long-term surveillance. However, invasion of marrow spaces does not seem to occur as commonly as is the case for ameloblastoma.30 The implication frequently drawn from this histopathologic observation is that its behavior is less aggressive than is that of the ameloblastoma. On a clinical basis this de-escalation of treatment is not warranted particularly since recurrence rates after conservative resection have been reported as being in the range of 15%, which is not satisfactory. In addition, recurrences have developed long after resection in a pattern similar to that of ameloblastoma.33 A 1 cm bony margin should be obtained and the reconstruction may be accomplished either primarily or secondarily according to the conditions found at operation. However, the peripheral CEOT may be treated by simple excision.
Calcifying Odontogenic Cyst (Gorlin Cyst, Keratinizing and Calcifying Odontogenic Cyst)
The COC, although named a cyst, is more properly considered to be an odontogenic tumor of ectodermal origin. The distinguishing histologic characteristic of this lesion is its ability to form “ghost” cells. The ghost cells themselves to do not impart aggressive biologic behavior to these lesions. Some controversy persists regarding proper treatment. Most lesions are cystic and are associated with either impacted teeth or odontomas, are quite well differentiated and respond well to enucleation and curettage. Many respond to treatment in a way similar to odontogenic cysts. However, solid varieties that contain a true tumor-like proliferation of epithelial cells, producing both ghost cells and osteoid or dentinoid, may be more aggressive and require limited resection. The COC may be found in association with four odontogenic lesions: ameloblastoma, ameloblastic odontoma, ameloblastic fibroodontoma and complex odontoma.34 The traditional maxim that they should be treated like ameloblastomas is still valid.
Clinical
This lesion may occur in any age group although the mean age at presentation is 33 years. The mandible is more commonly affected than the maxilla and the usual site is the incisor-canine area. A slow growing and painless expansion of the dentoalveolus is the most common physical finding.35
Radiographic
The typical finding is that of a radiolytic lesion with smooth cortical borders usually presenting in a dentigerous relationship to an impacted tooth or odontoma. It may contain focal areas of calcification. The multilocular radiolucency is more common than the unilocular.
Histopathology
The COC is usually cystic although it may present as a solid tumor. The central jaw lesion is much more common than is the peripheral soft tissue variant. In the cystic type, the epithelial lining is usually thin. In the solid, neoplastic type, there is a proliferation of epithelial cells into the connective tissue capsule and also into the cyst lumen. The epithelial cells may be squamous, cuboidal or columnar. These cells, arranged in the form of sheets of pale eosinophilic cells, blend with epithelial cells whose nuclei have degenerated leaving remnant cell outlines. They then become keratinized and calcify thereby completing the transformation from epithelial to ghost cell (Figure 91-9). These ghost cells are the signature cells of the COC.36 There may be areas of loose connective tissue resembling the stellate reticulum. These are epithelial cells that lose their nuclei, may keratinize and then calcify. They are often surrounded by giant cells.

Figure 91-9
Photomicrograph of calcifying odontogenic cyst showing “ghost” cells (hematoxylin and eosin ×100). Courtesy of James Geist, DDS, MS. (Four-color version of figure on CD-ROM)
Based upon the subtleties of the histopathology, Praetorius and colleagues of the Royal Dental College in Copenhagen created the following classification system.37
Type I
These are the cystic variants all of which behave indolently and are treated by enucleation and curettage with a low recurrence rate. They are subdivided as follows:
A. Simple Unicystic
Histopathologic criteria consist of thin epithelial lining, focal areas of connective tissue resembling the stellate reticulum and the presence of ghost cells. Production of dysplastic dentin may also occur.
B. Odontoma Type
Histology follows the same general pattern as for the simple unicystic but calcified tissue is also present within the wall of the cyst. This calcified tissue may take the form of a composite odontoma. Another variant has an ameloblastic fibroma present within the wall of the cyst (intramural ameloblastic fibroma).
C. Ameloblastomatous Type
In this variation the capsule contains a proliferation of epithelial cells in a pattern of ameloblastoma that extends both into the cyst wall (capsule) and within the cyst lumen.
Type II: Neoplastic
The type II variant is actually a tumor and this accounts for the dilemma in assigning proper treatment of this lesion. This variant behaves more aggressively than does the type I since it is really a tumor and not a cyst. However, the name COC lumps both variants within a single named entity. This lesion contains strands and islands of ameloblastoma-like epithelium that infiltrates the connective tissue. Both ghost cells and dentinoid are present along with giant cells particularly where the ghost cells are entwined with the supporting stroma. Malignant transformation has not been reported.
Treatment
Type I lesions are all treated by enucleation and curettage with expectation of a very low recurrence rate. The type II lesion is a true tumor with aggressive characteristics and it should therefore be treated following the same principles as outlined for ameloblastoma. These cases are very rare and the question arises as to whether they are really ameloblastomas that happen to produce ghost cells.
Clinical
Most lesions occur in the mandible, predominantly in adults and over a wide range of ages. Usually the presentation is as a painless swelling. Extraosseous occurrence has been reported.
Radiographic
The usual finding is a well-defined radiolucency with a well corticated border in which radiopacities may be found. It is often found in a dentigerous relationship to an impacted tooth.
Odontogenic Keratocyst
Although not classified as a neoplasm, the OKC remains a controversial entity because of the very high recurrence rates reported after simple enucleation (9% to 60%) a treatment that would be sufficient for the dentigerous cyst that it so closely resembles on imaging.38,39 Correct treatment is extremely important since recurrence is highly likely after simple enucleation. It is also associated with the multiple basal cell nevus syndrome of Gorlin and Goltz.
Clinical
The mean age for occurrence is 35 years and there is no sex predilection. Painless expansion of the cortex in the posterior body and ramus area of the mandible is the most typical presentation. Although it may occur in any area of either jaw, 79% occur in the mandible.38 In large lesions the possibility exists of spread through bone and into the soft tissues often with secondary infection causing pain.
Radiographic
The usual pattern is that of a unilocular or multilocular lesion with smooth or irregular borders. Root resorption and displacement of impacted teeth may occur. Often the lucency is located in a dentigerous relationship with the crown of an unerupted tooth and it may not be distinguishable from a simple dentigerous cyst (Figures 91-10 to 91-14). Multiple OKCs are the rule in the basal cell nevus syndrome and the presence of multiple OKCs should mandate an evaluation of the patient for the presence of this syndrome.

Figure 91-13
Odontogenic keratocyst extending into the superior aspect of ramus in a pattern of discontinuity.
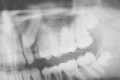
Figure 91-10
Odontogenic keratocyst in dentigerous relationship to unerupted tooth.

Figure 91-11
Odontogenic cyst (not odontogenic keratocyst) in the sister of the patient in Figure 91-10.

Figure 91-12
Orthopantomogram of odontogenic keratocyst with wide displacement of teeth, root resorption, and extensive bony destruction.
Histopathology
The classic presentation is that of a thin uniform epithelium four to six cells thick without rete pegs, in a corrugated format. The surface usually produces a corrugated layer of parakeratin although orthokeratinization may also occur. The basal cells are polarized with hyperchromatic nuclei. The lumen may contain keratin debris and within the walls of the cyst there may be proliferating islands of odontogenic epithelium or areas of cystic degeneration. (Figure 91-15) Inflammation, when present, may alter the histologic appearance and render diagnosis more difficult. The lining is very thin and this accounts for the difficulty in ensuring its complete removal during surgery. Daughter or satellite cysts are sometimes found in adjacent soft tissue and this may relate to the high recurrence rates from limited surgical treatment.

Figure 91-15
Photomicrograph of the epithelial lining of an odontogenic keratocyst showing typical pattern of keratin production and lack of rete pegs (hematoxylin and eosin ×20 magnification). Courtesy of Sara Gordon, DDS, MS. (Four-color version of figure (more...)
Treatment
Enucleation and curettage with removal of at least an additional millimeter of bone either by mechanical or chemical means is often recommended. This may be sufficient if the entire epithelium is removed in a single piece, which is very difficult to accomplish. Large cysts require removal of involved teeth and more commonly marginal or en bloc resection. With enucleation and curettage recurrence rates still hover at around 10%.40 If the lesion recurs resection is the treatment of choice for the second surgery (see Figure 91-14). Long term surveillance is mandatory.
Glandular Odontogenic Cyst
This very rare cyst, originally named sialo-odontogenic cyst, has recently been reviewed based upon 13 cases.41 In brief, this lesion presents as jaw swelling, with pain in about 25% of cases. More than half occurs in patients between the ages of 40 and 60 with equal prevalence in males and females. Almost 90% occur in the anterior mandible. Radiographically, the smaller lesions are unilocular and the larger are multilocular with corticated borders. Histologically, the epithelium is nonkeratinizing and the cells may be cuboidal or columnar, some forming mucus-secreting glandular elements within the cyst wall. Occasionally cilia are present. The epithelium may form cystic elements within the marrow spaces that are not accompanied by inflammation. This finding may contribute to the difficulty in achieving successful treatment after simple enucleation and curettage. The differential diagnosis includes odontogenic cysts, aneurysmal bone cyst, giant cell lesions, myxoma, ameloblastoma and central mucoepidermoid carcinoma of the jaws. Treatment by enucleation and curettage has resulted in recurrence rates of between 30% and 55%.41,42 Consequently, this lesion should be treated in a manner similar to that for the odontogenic keratocyst.
Adenomatoid Odontogenic Tumor
The adenomatoid odontogenic tumor is derived from odontogenic epithelium and has the property of inducing surrounding nonepithelial tissue to produce dentinoid. It is one of several odontogenic tumors, among them ameloblastic fibroodontoma, CEOT and COC that has this inductive capacity. Although this rare tumor was once thought to be a variant of ameloblastoma it has been recognized as a separate tumor that has a completely different biologic behavior pattern. Probably it develops as a proliferation of cells from the enamel organ occurring late in odontogenesis. It is not aggressive, does not invade marrow spaces and responds well to conservative treatment.43
Clinical
Most lesions occur in young people, usually below age 20 with a notable female predilection. The usual site of involvement is the anterior maxilla, most commonly in association with an impacted maxillary canine tooth. This causes a painless swelling of the anterior maxilla predominantly in the dentoalveolar component. Its usual position is pericoronal, that is, it is in a dentigerous relationship to the impacted tooth. The maxilla is more than twice as likely to be involved than is the mandible. Extraosseous cases have been reported: 64% occur in females, 65% in the maxilla, 74% associated with impacted teeth, two-thirds of which are associated with the maxillary or mandibular canine and 70% develop anterior to the canine in either jaw.44
Radiographic
The appearance of the AOT is that of a well-defined radiolucency with a clear cortical margin but there may be areas of radiopacities within the lesion. However, the predominant pattern is that of a radiolucency. It is usually unilocular and depending upon size, it may displace teeth. A unique feature is that it is in a dentigerous relationship to the crown of an unerupted tooth, most commonly a maxillary canine, and the point of attachment of the lesion to the tooth is usually below the level of the cemento-enamel junction, that is, it extends onto the root.45
Differential Diagnosis
When a dentigerous relationship is present one must consider dentigerous cyst, OKC, and unicystic ameloblastoma.
When not associated with the crown of a tooth any odontogenic cyst or tumor containing opacities should be considered namely, CEOT, COC, ossifying fibroma.
Histopathology
This lesion is surrounded by a thick fibrous capsule and appears to be contained within a cyst-like structure. The epithelial cells exist in two forms. Polyhedral or spindle shaped cells occur in nests, whorls and rosettes that surround central spaces that sometimes contain amyloid. Columnar or cuboidal cells may form duct-like structures. These latter cells resemble pre-ameloblasts, and the duct-like structures, when present, are a distinctive and characteristic microscopic feature. The surrounding stroma is sparse. There may be scattered calcifications that could represent preenamel or predentin and these account for the scattered densities often present on the radiograph.44
Treatment
Simple enucleation is sufficient with recurrence being almost unknown.
Squamous Odontogenic Tumor
This is a very rare odontogenic epithelial tumor whose cells of origin are probably epithelial rests present in the periodontal membrane or gingivae. It does not have potential for an aggressive growth pattern.
Clinical
This lesion usually presents as a slow-growing and painless mass associated with a loose but vital tooth. It occurs most commonly in young adults in the second or third decade of life. It does not seem to have a site predilection within the jaws and multisite occurrences have been reported. It may also present as an asymptomatic radiolucency discovered on routine dental radiographs.46
Radiography
The usual form is that of a well-circumscribed triangular radiolucency adjacent to the cervical portion of the root of an erupted tooth. It may not be distinguishable from periodontal bone loss. It may occur in multiple sites.
Differential Diagnosis
The common odontogenic cysts have to be considered first. Histologically one must consider differentiating this lesion from acanthomatous ameloblastoma, and the various forms of intraosseous carcinoma. The epithelium, however, is banal in the squamous odontogenic tumor. Other odontogenic cysts may contain islands of epithelium within their walls that do not represent SOT but are simply enclavements of odontogenic epithelium without aggressive growth potential.47
Histopathology
The epithelium is present in the form of islands and sheets of orderly squamous epithelial cells distributed within a mature connective tissue stroma. Intraepithelial calcifications and microcysts may be present.48
Treatment
The lesion is so rare that a definitive statement cannot be made. However, enucleation and curettage has led to few recurrences.
- Ameloblastoma
- Peripheral Ameloblastoma
- Solid Ameloblastoma
- Calcifying Epithelial Odontogenic Tumor (Pindborg Tumor)
- Calcifying Odontogenic Cyst (Gorlin Cyst, Keratinizing and Calcifying Odontogenic Cyst)
- Type I
- Type II: Neoplastic
- Odontogenic Keratocyst
- Glandular Odontogenic Cyst
- Adenomatoid Odontogenic Tumor
- Squamous Odontogenic Tumor
- Ectodermal Tumors - Holland-Frei Cancer MedicineEctodermal Tumors - Holland-Frei Cancer Medicine
Your browsing activity is empty.
Activity recording is turned off.
See more...