NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006.
Ophthalmological manifestations are common in Fabry disease and result from the progressive deposition of glycosphingolipids in various ocular structures. The most specific ocular manifestations of Fabry disease are conjunctival vascular abnormalities, corneal opacities (cornea verticillata), lens opacities and retinal vascular abnormalities. These do not usually cause significant visual impairment or other ocular symptoms, but can nevertheless be important because they can act as markers of the disease, with diagnostic and prognostic implications. Being an external organ and easily investigated with minimally invasive technologies, the eye may be useful for monitoring the natural history of Fabry disease and the response to enzyme replacement therapy. FOS – the Fabry Outcome Survey –provides comprehensive data on the ocular manifestations of Fabry disease. As of March 2005, 173 of the 688 patients enrolled in FOS have undergone a detailed ophthalmological examination. Cornea verticillata was the most frequently reported ophthalmic abnormality in both hemizygous males and heterozygous females, and may represent a useful diagnostic marker. Tortuous vessels and Fabry cataracts were more frequent in males than in females. Vessel tortuosity was associated with a more rapid progression of the disease and may have some value in predicting systemic involvement.
Introduction
Ophthalmological manifestations are common in Fabry disease, affecting various ocular structures. They do not usually cause significant visual impairment or other ocular symptoms, but can nevertheless be important because some manifestations act as markers of the disease, with diagnostic and prognostic implications. Because the eye is an external organ, easily investigated with minimally invasive technologies, ocular abnormalities may also provide a useful means of monitoring the natural history of the disease and patients' response to enzyme replacement therapy (ERT).
Cornea verticillata, the most typical ocular sign in Fabry disease, was first described by Fleischer in 1910 [1], but it was only in 1925 that Weicksel recognized cornea verticillata as being related to Fabry disease [2]. In 1968, while studying a family with various cases of cornea verticillata, Franceschetti found that several members were affected by Fabry disease and reported an X-linked recessive model of inheritance for this metabolic disorder [3]. Subsequent investigators have reported that Fabry disease has significant clinical effects in female heterozygotes [4–6]. This is possibly due to the process of random X-inactivation.
Common ocular findings
Eye abnormalities in Fabry disease result from the deficient activity of the lysosomal hydrolase, α-galactosidase A. This deficiency leads to a progressive deposition of glycosphingolipids in some ocular structures [7–9]. The most specific ocular manifestations of Fabry disease are:
- conjunctival vascular abnormalities
- corneal opacities (cornea verticillata)
- lens opacities
- retinal vascular abnormalities.
Some other ophthalmological features have been described anecdotally in association with Fabry disease, but a possible correlation between these features and the metabolic impairment is unclear.
Conjunctival vessel abnormalities
The most characteristic ophthalmological manifestations are increased vessel tortuosity (Figure 1), venous vascular aneurysmal dilation and 'sludging' of the blood in the small blood vessels. These changes can be seen in any conjunctival area, but they are most commonly located in the inferior bulbar conjunctiva.
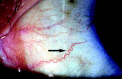
Figure 1
Conjunctival vessel tortuosity (arrowed).
The histopathological abnormalities underlying the conjunctival vessel tortuosity are seen as electron-dense deposits, often with a lamellar structure, within the endothelial cells, pericytes and smooth muscle cells of the conjunctival vessel walls. This abnormal storage process induces degenerative changes that are responsible for the weak mechanical resistance of the vessel walls to blood pressure; after several years, this results in typical irregularities of the vessel course [10]. Similar deposits have been reported in all layers of the conjunctival epithelial cells and in the goblet cells [11–13].
Corneal opacities
Cornea verticillata consist of bilateral whorl-like opacities located in the superficial corneal layers, most commonly in the inferior corneal area. These opacities are typically cream coloured, ranging from whitish to golden-brown. They are termed cornea verticillata because the deposits are distributed in a vortex pattern. In the early stages, the opacities may form fine horizontal lines, but they later develop into curving lines, radiating from a point below the centre of the cornea and forming small whorls, before becoming almost straight at the periphery (Figure 2). They show some resemblance to the corneal opacities found after chronic administration of drugs, such as chloroquine or amiodarone, but the amiodarone-related opacities are slightly different as they consist of horizontal lines with some arborization at their extremities, giving a "cats' whiskers" appearance [14].

Figure 2
Cornea verticillata (arrowed).
Cornea verticillata have been described in almost all patients with Fabry disease, both in hemizygous males and heterozygous females. Hence, they are usually considered to be the most reliable ophthalmological marker of Fabry disease. However, a few patients with a genetic, biochemical and clinical diagnosis of Fabry disease do not show cornea verticillata, even after several years.
The cause of the vortex pattern of the corneal deposits is unknown. Various hypotheses cite the influence of ocular hydrodynamics, periodic blinking or ocular magnetic fields. Another possible cause is the centripetal movement of the renewing epithelial cells from the periphery towards the centre of the cornea [15–17].
There have been some reports of a sub-epithelial corneal haze, usually associated with the more typical whorl-like opacities. In most of these patients, the haze is diffuse and involves the whole cornea, but in some individuals it is limited to the central or limbal corneal area. It is generally brownish, or more rarely grey or whitish [18, 19]. The haze has been suggested to be an early manifestation of Fabry disease [20], although it has also been suggested to be a natural evolution of the vortex opacities [18].
Corneal pathology has been investigated both in hemizygous and heterozygous patients with Fabry disease [21–25]. The most relevant finding is the presence of intra-epithelial deposits consisting of dense laminated cytoplasmic inclusions, both membrane-bound and lying freely in the cytoplasm. In a histopathological study of the cornea of a woman with Fabry disease, Weingeist and Blodi described subepithelial ridges composed of re-duplicated basement membrane and amorphous electron-dense material between the basement membrane and Bowman's layer. They suggested that the diffuse accumulation of sphingolipids in the corneal epithelium might be responsible for the diffuse corneal haze, while the whorl-like pattern might be determined by a series of subepithelial ridges [26]. However, these ridges have not been consistently reported in either heterozygous women or hemizygous men [11].
A more recent ultrastructural investigation revealed a disruption of the normal pattern of the basement membrane without any evident re-duplication of the basal lamina [27]. Possible corneal endothelium involvement in Fabry disease has been suggested by the finding of pigment and corneal guttae on the endothelium in two patients [28], but this has not been confirmed by other investigators.
Lens opacities
Two specific types of lens opacities have been reported in patients with Fabry disease: an anterior capsular or subcapsular cataract, and a radial posterior subcapsular cataract. Anterior capsular and subcapsular opacities are generally bilateral and wedge-shaped, with a radial distribution, and have their bases near the equator and their apices toward the centre of the anterior capsule. Posterior subcapsular cataracts are rare but very specific for the disease (and hence are called Fabry cataracts). They consist of linear whitish opacities located near the posterior capsule, and have a spoke-like appearance (Figure 3).

Figure 3
Posterior subcapsular 'spoke-like' cataract (Fabry cataract; arrowed).
Fabry cataracts are not easily detected by direct observation using a slit lamp, and can often be missed on routine examination. They are best seen and imaged by retro-illumination, using the light reflected by the ocular fundus.
Laminated bodies have been described in the lens epithelium and stroma of patients with Fabry disease [11], but a histopathological study of a Fabry cataract has yet to be undertaken.
Retinal and choroidal vessel abnormalities
Retinal and choroidal vessel abnormalities are mainly represented by an increased tortuosity of the retinal vessels (sometimes with a 'corkscrew' appearance) associated with segmental venous dilation, arteriolar narrowing and arteriovenous nicking (localized constriction).
Retinal vessel tortuosity can be easily detected by simple ophthalmoscopy of the posterior segment of the eye. However, it is better appreciated using fluorescein angiography (Figure 4).
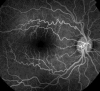
Figure 4
Retinal vessel tortuosity (fluorescein angiography).
Electron microscopy reveals the presence of dense laminated cytoplasmic inclusions in the endothelial cells and pericytes of retinal vessels, closely resembling the lesions described in the conjunctiva [11, 16]. Similar lesions have been reported in all of the major vessels of the eye [11, 24, 25]. In choroidal arterioles and iris vessels, they are associated with similar accumulations in the smooth muscle cells of the vessel walls [11, 24]; inclusions were also found in both iris and retinal pigment epithelium [11, 24].
Increased tortuosity of the retinal vessels is very common in patients with Fabry disease, but is not specific to this disease; the same abnormality can be observed in other retinal disorders, such as hypertensive retinopathy.
In addition to involvement of the larger retinal vessels, some authors have reported microvascular changes imaged using fluorescein angiography of the retina [29, 30]. These angiographic findings are not consistently observed in patients with Fabry disease and therefore cannot be considered typical of the disease.
Occasional ocular findings
Several other ocular manifestations have been described in association with Fabry disease, but have been reported only in isolated patients or in very small series. Sometimes the pathophysiological link with abnormal glycosphingolipid metabolism is unclear. The association of some of these vascular occlusive disorders with Fabry disease, although probably due to the compromised choroidal and retinal vasculature, is therefore hypothetical and requires further investigation.
Posterior segment of the eye
Most of the occasional ophthalmological findings involve the posterior segment of the eye. There have been some cases of retinal artery and vein occlusions reported in association with Fabry disease [31–33], usually in patients under 30 years of age. An anterior ischaemic optic neuropathy has been observed in association with cilioretinal artery occlusion in a female carrier of Fabry disease [34]. A sudden decrease of visual acuity was reported in a young man with Fabry disease, together with pale areas in a lobular distribution on the eye fundus; this clinical picture is suggestive of choroidal ischaemia [35]. Fabry disease should therefore be considered in the differential diagnosis of ocular vascular occlusive disorders, especially in young patients.
Optic atrophy or papilloedema has been reported in several patients with Fabry disease, but the pathophysiology remains unclear [36]. Detection of papilloedema is facilitated by angiographic examination which, in the later phases, will show leakage of the dye from the borders of the disc. Myelinated nerve fibres have also been reported in association with Fabry disease [37].
Abnormalities of the peripheral retinal pigment epithelium have been described in an 18-year-old man with skin and kidney lesions typical of Fabry disease and a mutation of the GLA gene. Possible explanations for this uncommon finding are that there had been localized areas of vitreo-retinal traction, previous neuro-retinal detachment or choroidal ischaemia [38].
Anterior segment of the eye
Some rare ocular findings have also been reported that affect the anterior segment of the eye in patients with Fabry disease. Lid oedema has been described in several patients, usually in hemizygotes [19, 20], and angiokeratomas can occasionally be located on the skin of the lids. A dry eye syndrome has also been reported [39], sometimes in association with altered pupillary motility (reduced constriction with pilocarpine), suggesting that there is an impairment of autonomic function [40].
Visual field defects
The visual field was assessed in 27 patients with Fabry disease using Goldmann perimetry [18]. An enlargement of the blind spot was noted in 37% of the tested eyes. The enlargement was bilateral in most cases and was not associated with colour perception abnormalities. This defect might reflect subclinical involvement of the optic pathways, probably resulting from localized ischaemic events. Moreover, abnormalities of pattern-reversal visual-evoked responses have recently been reported in a child with Fabry disease [41].
Prevalence and clinical significance of ocular manifestations
The prevalence of the most significant eye abnormalities in Fabry disease are summarized in Table 1 [18, 20, 42–45]. Possible explanations for the differences in the reported prevalence of specific eye abnormalities in the various studies include underlying differences in demographic features (mainly age, sex and ethnic origin), genotype (determining different phenotypes) and technologies used to detect the eye signs, as well as the subjective evaluation of the investigators and, in a few cases, the influence of ERT.
Table 1
Prevalence of ocular manifestations of Fabry disease in published studies.
An ophthalmological evaluation should be carried out in every patient in whom there is a clinical suspicion of Fabry disease. Some eye abnormalities are present in most individuals with Fabry disease and can be helpful in confirming the diagnosis in patients or in apparently healthy relatives, while other manifestations may be less specific. For example, conjunctival vessel abnormalities and cornea verticillata are both relatively common in Fabry disease. Cornea verticillata are highly sensitive for the diagnosis of Fabry disease (i.e. are present in almost all patients) as well as being highly specific (i.e. rarely found in non-affected subjects). By contrast, conjunctival vessel tortuosity is encountered in a number of other diseases and its evaluation is subjective.
Furthermore, cornea verticillata can be easily detected on routine ophthalmological examination using a slit lamp. This is a non-invasive, inexpensive and simple procedure. Hence, cornea verticillata can be used as an ophthalmological marker for Fabry disease. The only drawbacks are that cornea verticillata (similar to other ophthalmological manifestatons of Fabry disease) do not result in visual symptoms and therefore do not cause patients to consult their doctor, and that general ophthalmologists are unfamiliar with this marker. For these reasons, ophthalmological screening programmes for Fabry disease have a low efficacy [46]. A campaign to inform general ophthalmologists about the recognition and clinical implications of cornea verticillata might increase the number of early diagnoses of Fabry disease.
It is difficult to follow-up ophthalmological features of Fabry disease with a standard examination. Assessing the degree of vessel tortuosity in the conjunctiva and fundus is highly subjective and non-specific. Lens opacities are either non-specific (e.g. anterior subcapsular cataracts) or very rare (e.g. 'spoke-like' Fabry cataracts). Cornea verticillata are useful for diagnosis, but changes over time are difficult to detect; clinical evaluation may be highly subjective, as corneal imaging is poorly reproducible and unreliable due to multiple technical photographic problems.
Based on a standard ophthalmological examination, changes in the ophthalmological features of Fabry disease can be detected reliably only when they become clinically obvious. They are therefore seldom useful in clinical practice for patient follow-up. Furthermore, ophthalmological abnormalities have not been observed in some patients.
Enzyme replacement therapy
One of the authors (AS) has seen a few instances of apparent regression of corneal opacities after ERT, detected using a slit lamp. However, quantification of the vortex opacities is highly subjective, and reliable methods of imaging cornea verticillata are still awaited. Hence, there are still questions about the use of cornea verticillata to monitor individual responses to therapy.
Future developments
Future developments may include an internationally agreed classification of the eye abnormalities that would allow 'scoring' of the severity of eye involvement. This, coupled with more sophisticated investigations (e.g. confocal corneal microscopy or colour Doppler imaging of orbital vessels), could enable the natural history of ocular manifestations to be elucidated as well as allowing the response to ERT to be monitored.
Ocular manifestations in FOS –the Fabry Outcome Survey
Assessment of eye abnormalities in patients with Fabry disease forms a significant part of FOS, an international database for all patients with Fabry disease who are receiving, or are candidates for, ERT with agalsidase alfa. As of March 2005, data have been collected from a total of 688 patients, of whom 173 (82 males, 91 females; about 25% of the FOS population) have undergone a detailed ophthalmological examination. This examination has paid particular attention to cornea and lens opacities (Fabry cataracts), and to the course of vessels in the conjunctiva and retina (grouped together as 'vessel tortuosity').
Data have been reported using a specific ophthalmological form (the eye examination form). The various signs have been classified simply as either present or absent, according to the subjective judgement of the examiner, without any attempt to grade the severity of the abnormalities. Data analysis has focused particularly on prevalence (in the total FOS cohort and according to age and gender), concurrence of specific ocular manifestations in the same patient, and associations with systemic findings.
Overall prevalence
The presence of cornea verticillata has been reported in 76.9% of females and 73.1% of males; vessel tortuosity has been observed in 21.9% of females and 48.7% of males; and Fabry cataracts have been noted in 9.8% of females and 23.1% of males. Hence, in agreement with data from other studies [20, 42–45], cornea verticillata are the most common ophthalmic abnormality in Fabry disease in both hemizygous males and heterozygous females.
The prevalence of vessel tortuosity in FOS is lower than reported in previous smaller series [18, 20, 42–45]. This might be accounted for by the difficulties in evaluating the vessels objectively and by the lack of a clear distinction between tortuosity in the conjunctival and in the retinal vascular areas.
Tortuous vessels and Fabry cataracts (but not cornea verticillata ) are more frequent in male than in female patients in the FOS database. This might be due to more severe clinical involvement in hemizygous males, or to some hormonal influence on ocular haemodynamics [47, 48] or lens structure.
Age- and gender-related prevalence
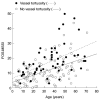
The prevalence of cornea verticillata was similar in males and females, as well as in different age groups. The prevalence of tortuous vessels and Fabry cataract, however, was lower in females than in males, and the prevalence of tortuous vessels increased significantly with age in males (Figure 5).

Figure 5
Prevalence of (a) cornea verticillata, (b) tortuous vessels and (c) Fabry cataract according to age and gender in FOS – the Fabry Outcome Survey. Numbers in columns indicate numbers of patients.
Ocular manifestations of Fabry disease can be detected in young children (to date, the youngest child in whom ocular manifestations have been found was 3 years old). This is consistent with previous reports of cornea verticillata in very young patients, including a 6-month-old child [20] and a fetus [22], and supports the case for ophthalmological examination of paediatric patients. At 20 years of age, approximately 30% of males and 25% of females had tortuous vessels, and approximately 20% of males and 10% of females had Fabry cataract. The frequency after 40 years of age was approximately 60% in males and 25% in females for tortuous vessels, and more than 30% in males and 15% in females for Fabry cataract.
Associations between eye abnormalities
Cornea verticillata may be the only eye abnormality observed in a patient with Fabry disease, and is not necessarily associated with vessel tortuosity. By contrast, vessel tortuosity rarely occurs as the only ocular sign in patients with Fabry disease.
Association between eye abnormalities and other signs and symptoms
Differences in disease progression were analysed using linear regression of disease severity, adjusting for age and sex. Disease severity was assessed by the Mainz Severity Score Index (MSSI) [49], as adapted for use in FOS. This is a scoring system developed to measure the severity of Fabry disease and to monitor its clinical course in response to ERT. There was no relationship between the presence of cornea verticillata and disease severity, as measured by the adapted MSSI. However, a significant relationship could be seen between the presence of vessel tortuosity and disease severity (p = 0.01): progression was more rapid in patients with tortuous vessels than in those without vessel tortuosity (Figure 6). Patients with vessel tortuosity also showed a more rapid deterioration in renal function, based on the estimated glomerular filtration rate (p = 0.012). Furthermore, the relationship between the increase in cardiac size (mean wall thickness) with age and vessel tortuosity was even more significant (p < 0.0001) (Figure 7). For Fabry cataracts, the numbers were too small to enable any valid conclusions to be drawn.
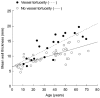
Figure 7
Linear regression analysis showing the correlation between the heart mean wall thickness and increasing age (i.e. disease severity) in patients in FOS – the Fabry Outcome Survey – with and without tortuous vessels.
Conclusions
A review of published literature and analysis of the FOS database suggest the following conclusions regarding ocular manifestations of Fabry disease.
- The presence of cornea verticillata is highly sensitive and specific for Fabry disease in both male and female patients. Cornea verticillata can therefore be considered a useful ophthalmological marker for Fabry disease, although it should be borne in mind that corneal opacities may also occur in patients taking certain drugs.
- In some cases, cornea verticillata can be an isolated occurrence, without the presence of other eye abnormalities.
- Tortuous vessels are common in Fabry disease, but are relatively non-specific for the disease.
- Posterior subcapsular cataracts with a spoke-like appearance are rare but, when present, may suggest a diagnosis of Fabry disease.
- The prevalence of cornea verticillata was similar in males and females as well as in different age groups; however, the frequency of tortuous vessels and Fabry cataracts was lower in females than in males.
- Eye abnormalities can also be detected in very young children.
- The presence of vessel tortuosity appears to be associated with a more rapid progression of Fabry disease.
- The study of eye abnormalities can aid diagnosis but does not significantly improve the accuracy of monitoring progression of the disease and its response to treatment. Technological developments are required before eye signs can be quantified and used successfully to monitor patients affected by Fabry disease.
References
- 1.
- Fleischer B. Uber eine eigenartige bisher nicht bekannte Hornhauttrubung [On an unusual thus far unknown type of corneal cloudiness] Graefes Arch Ophthalmol. 1910;77:136–40.
- 2.
- Weicksel J. Angiomatosis bzw Angiokeratosis universalis (eine sehr seltene Haut-und Gefasserkrankung) [Angiomatosis or angiokeratosis universalis (a very rare disease of the skin and blood and lymphatic vessels)] Deutsch Med Wschr. 1925;51:898.
- 3.
- Franceschetti AT. La cornea verticillata at ses relations avec la maladie de Fabry [Cornea verticillata (Gruber) and its relation to Fabry disease (angiokeratoma corporis diffusum)] Ophthalmologica. 1968;156:232–8. [PubMed: 5304462]
- 4.
- MacDermot KD, Holmes A, Miners AH. Anderson–Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38:769–75. [PMC free article: PMC1734754] [PubMed: 11732485]
- 5.
- Whybra C, Kampmann C, Willers I, Davies J, Winchester B, Kriegsmann J. et al. Anderson–Fabry disease: clinical manifestations of disease in female heterozygotes. J Inherit Metab Dis. 2001;24:715–24. [PubMed: 11804208]
- 6.
- Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C. et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34:236–42. [PubMed: 15025684]
- 7.
- Germain DP. Maladie de Fabry (déficit en α-galactosidase A): physiopathologie, signes cliniques et aspects génétiques [Fabry's disease (α-galactosidase-A deficiency): physiopathology, clinical signs, and genetic aspects] J Soc Biol. 2002;196:161–73. [PubMed: 12360745]
- 8.
- Hauser AC, Lorenz M, Sunder-Plassmann G. The expanding clinical spectrum of Anderson–Fabry disease: a challenge to diagnosis in the novel era of enzyme replacement therapy. J Intern Med. 2004;255:629–36. [PubMed: 15147526]
- 9.
- Masson C, Cisse I, Simon V, Insalaco P, Audran M. Fabry disease: a review. Joint Bone Spine. 2004;71:381–3. [PubMed: 15474388]
- 10.
- Libert J, Toussaint D. Tortuosities of retinal and conjunctival vessels in lysosomal storage diseases. Birth Defects Orig Artic Ser. 1982;18:347–58. [PubMed: 6816307]
- 11.
- Font RL, Fine BS. Ocular pathology in Fabry's disease. Histochemical and electron microscopic observations. Am J Ophthalmol. 1972;73:419–30. [PubMed: 4335185]
- 12.
- Libert J, Tondeur M, Van Hoof F. The use of conjunctival biopsy and enzyme analysis in tears for the diagnosis of homozygotes and heterozygotes with Fabry disease. Birth Defects Orig Artic Ser. 1976;12:221–39. [PubMed: 821559]
- 13.
- McCulloch C, Ghosh M. Ultrastructural changes in the cornea and conjunctiva of a heterozygous woman with Fabry's disease. Can J Ophthalmol. 1984;19:192–8. [PubMed: 6430531]
- 14.
- Mantyjarvi M, Tuppurainen K, Ikaheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998;42:360–6. [PubMed: 9493278]
- 15.
- Dufier JL, Gubler MC, Dhermy P, Lenoir G, Paupe J, Haye C. La maladie de Fabry et ses manifestations ophtalmologique [Fabry's disease in ophthalmology] J Fr Ophtalmol. 1980;3:625–30. [PubMed: 6259247]
- 16.
- Roussel T, Grutzmacher R, Coster D. Patterns of superficial keratopathy. Aust J Ophthalmol. 1984;12:301–16. [PubMed: 6442135]
- 17.
- Bron AJ. Vortex patterns of the corneal epithelium. Trans Ophthalmol Soc UK. 1973;93:455–72. [PubMed: 4210604]
- 18.
- Orssaud C, Dufier J, Germain D. Ocular manifestations in Fabry disease: a survey of 32 hemizygous male patients. Ophthalmic Genet. 2003;24:129–39. [PubMed: 12868031]
- 19.
- Rahman A. The ocular manifestations of hereditary dystopic lipidosis (angiokeratoma corporis diffusum universale). Arch Ophthalmol. 1963;69:708–16. [PubMed: 13990468]
- 20.
- Sher NA, Letson RD, Desnick RJ. The ocular manifestations in Fabry's disease. Arch Ophthalmol. 1979;97:671–6. [PubMed: 106811]
- 21.
- Tuppurainen K, Collan Y, Rantanen T, Hollmen A. Fabry's disease and cornea verticillata. A report of 3 cases. Acta Ophthalmol (Copenh). 1981;59:674–82. [PubMed: 6797229]
- 22.
- Tsutsumi A, Uchida Y, Kanai T, Tsutsumi O, Satoh K, Sakamoto S. Corneal findings in a foetus with Fabry's disease. Acta Ophthalmol (Copenh). 1984;62:923–31. [PubMed: 6098121]
- 23.
- Macrae WG, Ghosh M, McCulloch C. Corneal changes in Fabry's disease: a clinicopathologic case report of a heterozygote. Ophthalmic Paediatr Genet. 1985;5:185–90. [PubMed: 3934620]
- 24.
- Riegel EM, Pokorny KS, Friedman AH, Suhan J, Ritch RH, Desnick RJ. Ocular pathology of Fabry's disease in a hemizygous male following renal transplantation. Surv Ophthalmol. 1982;26:247–52. [PubMed: 6806927]
- 25.
- Francois J, Hanssens M, Teuchy H. Corneal ultra-structural changes in Fabry's disease. Ophthalmologica. 1978;176:313–30.
- 26.
- Weingeist TA, Blodi FC. Fabry's disease: ocular findings in a female carrier. A light and electron microscopy study. Arch Ophthalmol. 1971;85:169–76. [PubMed: 5545717]
- 27.
- Hirano K, Murata K, Miyagawa A, Terasaki H, Saigusa J, Nagasaka T. et al. Histopathologic findings of cornea verticillata in a woman heterozygous for Fabry's disease. Cornea. 2001;20:233–6. [PubMed: 11248839]
- 28.
- Grace EV. Diffuse angiokeratosis (Fabry's disease). Am J Ophthalmol. 1966;62:139–45. [PubMed: 5296018]
- 29.
- Dantas MA, Fonseca RA, Kaga T, Yannuzzi LA, Spaide RF. Retinal and choroidal vascular changes in heterozygous Fabry disease. Retina. 2001;21:87–9. [PubMed: 11217945]
- 30.
- Ohkubo H. Several functional and fluorescein fundus angiographic findings in Fabry's disease. Ophthalmologica. 1988;196:132–6. [PubMed: 3136413]
- 31.
- Sher NA, Reiff W, Letson RD, Desnick RJ. Central retinal artery occlusion complicating Fabry's disease. Arch Ophthalmol. 1978;96:815–7. [PubMed: 207248]
- 32.
- Andersen MV, Dahl H, Fledelius H, Nielsen NV. Central retinal artery occlusion in a patient with Fabry's disease documented by scanning laser ophthalmoscopy. Acta Ophthalmol (Copenh). 1994;72:635–8. [PubMed: 7887166]
- 33.
- Oto S, Kart H, Kadayifcilar S, Ozdemir N, Aydin P. Retinal vein occlusion in a woman with heterozygous Fabry's disease. Eur J Ophthalmol. 1998;8:265–7. [PubMed: 9891901]
- 34.
- Abe H, Sakai T, Sawaguchi S, Hasegawa S, Takagi M, Yoshizawa T. et al. Ischemic optic neuropathy in a female carrier with Fabry's disease. Ophthalmologica. 1992;205:83–8. [PubMed: 1475086]
- 35.
- Guenoun JM, Parc C, Monnet D, Brezin AP. [Loss of visual acuity due to choroidal ischemia in Fabry's disease] J Fr Ophtalmol. 2003;26:842–4. [PubMed: 14586228]
- 36.
- Velzeboer CM, de Groot WP. Ocular manifestations in angiokeratoma corporis diffusum (Fabry). Br J Ophthalmol. 1971;55:683–92. [PMC free article: PMC1208523] [PubMed: 5124844]
- 37.
- Calmettes L, Deodati F, Suc JP, Bec P, Conte J, Pasternac A. et al. Au sujet d'un noveau cas de maladie de Fabry [A new case of Fabry's disease] Bull Soc Ophtalmol Fr. 1967;67:1025–8. [PubMed: 5622647]
- 38.
- Jourdel D, Defoort-Dhellemmes S, Labalette P, Ryckewaert M, Hache JC. Anomalies pigmentaires rétiniennes associées à une maladie de Fabry [Retinal pigment anomalies associated with Fabry's disease] J Fr Ophtalmol. 1998;21:755–60. [PubMed: 10052049]
- 39.
- Klein P. Ocular manifestations of Fabry's disease. J Am Optom Assoc. 1986;57:672–4. [PubMed: 3095413]
- 40.
- Cable WJ, Kolodny EH, Adams RD. Fabry disease: impaired autonomic function. Neurology. 1982;32:498–502. [PubMed: 6803189]
- 41.
- Liasis A, Ioannidis A, Thompson D, Davey C, Nischal K. Visual electrophysiology in patients with Anderson–Fabry disease. Acta Paediatr Suppl. 2006;451:119.
- 42.
- Spaeth GL, Frost P. Fabry's disease. Its ocular manifestations. Arch Ophthalmol. 1965;74:760–9. [PubMed: 5846554]
- 43.
- Nguyen TT, Gin T, Nicholls K, Low M, Galanos J, Crawford A. Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin Experiment Ophthalmol. 2005;33:164–8. [PubMed: 15807825]
- 44.
- Vauthier L, Cobut O, Depasse F, Dehout F, Van Maldergem L. Ophthalmological findings in a series of 20 patients with Fabry disease: preliminary results. Acta Paediatr Suppl. 2006;451:118.
- 45.
- Fumex-Boizard L, Cochat P, Fouilhoux A, Guffon N, Denis P. Relation entre les manifestations ophtalmologiques et les atteintes générales chez dix patients atteints de la maladie de Fabry [Relation between ocular manifestations and organ involvement in ten patients with Fabry disease] J Fr Ophtalmol. 2005;28:45–50. [PubMed: 15767898]
- 46.
- Hauser AC, Lorenz M, Voigtlander T, Fodinger M, Sunder-Plassmann G. Results of an ophthalmologic screening programme for identification of cases with Anderson–Fabry disease. Ophthalmologica. 2004;218:207–9. [PubMed: 15103218]
- 47.
- Harris-Yitzhak M, Harris A, Ben-Refael Z, Zarfati D, Garzozi HJ, Martin BJ. Estrogen-replacement therapy: effects on retrobulbar hemodynamics. Am J Ophthalmol. 2000;129:623–8. [PubMed: 10844054]
- 48.
- Atalay E, Karaali K, Akar M, Ari ES, Simsek M, Atalay S. et al. Early impact of hormone replacement therapy on vascular hemodynamics detected via ocular colour Doppler analysis. Maturitas. 2005;50:282–8. [PubMed: 15780527]
- 49.
- Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E. et al. The Mainz Severity Score Index: a new instrument for quantifying the Anderson–Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet. 2004;65:299–307. [PubMed: 15025723]
- Ocular manifestations of Fabry's disease: data from the Fabry Outcome Survey.[Br J Ophthalmol. 2007]Ocular manifestations of Fabry's disease: data from the Fabry Outcome Survey.Sodi A, Ioannidis AS, Mehta A, Davey C, Beck M, Pitz S. Br J Ophthalmol. 2007 Feb; 91(2):210-4. Epub 2006 Sep 14.
- Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre.[Clin Exp Ophthalmol. 2005]Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre.Nguyen TT, Gin T, Nicholls K, Low M, Galanos J, Crawford A. Clin Exp Ophthalmol. 2005 Apr; 33(2):164-8.
- Review Ophthalmic Manifestations in Fabry Disease: Updated Review.[J Pers Med. 2023]Review Ophthalmic Manifestations in Fabry Disease: Updated Review.Gambini G, Scartozzi L, Giannuzzi F, Carlà MM, Boselli F, Caporossi T, De Vico U, Baldascino A, Rizzo S. J Pers Med. 2023 May 27; 13(6). Epub 2023 May 27.
- Ocular Manifestations of Fabry Disease: Report from a Tertiary Eye Care Center in Türkiye.[Turk J Ophthalmol. 2024]Ocular Manifestations of Fabry Disease: Report from a Tertiary Eye Care Center in Türkiye.Korkmaz İ, Kalkan Uçar S, Onay H, Yıldırım Sözmen E, Çoker M, Palamar M. Turk J Ophthalmol. 2024 Jun 28; 54(3):127-132.
- Review Ocular features of Fabry disease: diagnosis of a treatable life-threatening disorder.[Surv Ophthalmol. 2008]Review Ocular features of Fabry disease: diagnosis of a treatable life-threatening disorder.Samiy N. Surv Ophthalmol. 2008 Jul-Aug; 53(4):416-23.
- Ophthalmological manifestations of Fabry disease - Fabry DiseaseOphthalmological manifestations of Fabry disease - Fabry Disease
- ABAT [Mustela nigripes]ABAT [Mustela nigripes]Gene ID:132026915Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...