By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001.

Neuroscience. 2nd edition.
Show detailsAcetylcholine is the neurotransmitter at neuromuscular junctions, at synapses in the ganglia of the visceral motor system, and at a variety of sites within the central nervous system. Whereas a great deal is known about the function of cholinergic transmission at the neuromuscular junction and at ganglionic synapses, the actions of ACh in the central nervous system are not as well understood.
Acetylcholine is synthesized in nerve terminals from acetyl coenzyme A (acetyl CoA, which is synthesized from glucose) and choline, in a reaction catalyzed by choline acetyltransferase (CAT) (Figure 6.8). The presence of CAT in a neuron is thus a strong indication that ACh is used as one of its transmitters. Choline is present in plasma at a concentration of about 10 mM, and is taken up into cholinergic neurons by a high-affinity Na+/choline transporter. About 10,000 molecules of ACh are packaged into each vesicle by a vesicular ACh transporter.
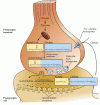
Figure 6.8
Acetylcholine metabolism in cholinergic nerve terminals. The synthesis of acetylcholine from choline and acetyl CoA requires choline acetyltransferase. Acetyl CoA is derived from pyruvate generated by glycolysis, while choline is transported into the (more...)
In contrast to most other small-molecule neurotransmitters, the postsynaptic action of ACh at many cholinergic synapses (the neuromuscular junction in particular) are not terminated by reuptake but by a powerful hydrolytic enzyme, acetylcholinesterase (AChE). This enzyme is concentrated in the synaptic cleft, ensuring a rapid decrease in ACh concentration after its release from the presynaptic terminal. AChE has a very high catalytic activity (about 5000 molecules of ACh per AChE molecule per second) and hydrolyzes ACh into acetate and choline. As already mentioned, cholinergic nerve terminals typically contain a high-affinity, Na+-choline transporter that takes up the choline produced by ACh hydrolysis.
Among the many interesting drugs that interact with cholinergic enzymes are the organophosphates. Compounds such as diphenyl trichloroethane (DTT) and the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) were originally developed as insecticides. This group also includes some potent chemical warfare agents. One such compound is the nerve gas “Sarin,” which was made notorious a few years ago after a group of terrorists released this gas in Tokyo's underground rail system. Organophosphates can be lethal to humans (and insects) because they inhibit AChE, causing ACh to accumulate at cholinergic synapses. This build-up of ACh depolarizes the postsynaptic cell and renders it refractory to subsequent ACh release, causing, among other effects, neuromuscular paralysis.
- Acetylcholine - NeuroscienceAcetylcholine - Neuroscience
Your browsing activity is empty.
Activity recording is turned off.
See more...