By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsExperimental studies
The angiosperm zygote is embedded within the ovule and ovary and thus is not readily accessible for experimental manipulation. The following approaches, however, can yield information on the formation of the plant embryo:
- Histological studies of embryos at different stages show how carefully regulated cell division results in the construction of an organism, even without the ability to move cells and tissues to shape the embryo.
- Culture experiments using embryos isolated from ovules and embryos developing de novo from cultured sporophytic tissue provide information on the interactions between the embryo and surrounding sporophytic and endosperm tissue.
- In vitro fertilization experiments provide information on gamete interactions.
- Biochemical analyses of embryos at different stages of development provides information on such things as the stage-specific gene products necessary for patterning and establishing food reserves.
- Genetic and molecular analyses of developmental mutants characterized using the above approaches have greatly enhanced our understanding of embryonic development.
- Clonal analysis involves marking individual cells and following their fate in development (see Poethig 1987 for details on the methodology). For example, seeds heterozygous for a pigmentation gene may be irradiated so that a certain cell loses the ability to produce pigment. Its descendants will form a colorless sector that can be identified and related to the overall body pattern.
Embryogenesis
In plants, the term embryogenesis covers development from the time of fertilization until dormancy occurs. The basic body plan of the sporophyte is established during embryogenesis; however, this plan is reiterated and elaborated after dormancy is broken. The major challenges of embryogenesis are
- 1.
To establish the basic body plan. Radial patterning produces three tissue systems, and axial patterning establishes the apical-basal (shoot-root) axis.
- 2.
To set aside meristematic tissue for postembryonic elaboration of the body structure (leaves, roots, flowers, etc.).
- 3.
To establish an accessible food reserve for the germinating embryo until it becomes autotrophic.
Embryogenesis is similar in all angiosperms in terms of the establishment of the basic body plan (Steeves and Sussex 1989) (see Figure 20.15). There are differences in pattern elaboration, however, including differences in the precision of cell division patterns, the extent of endosperm development, cotyledon development, and the extent of shoot meristem development (Esau 1977; Johri et al. 1992).
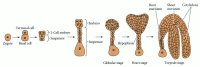
Figure 20.15
Angiosperm embryogenesis. A representative dicot is shown; a monocot would develop only a single cotyledon. While there are basic patterns of embryogenesis in angiosperms, there is tremendous morphological variation among species.
Polarity is established in the first cell division following fertilization. The establishment of polarity has been investigated using brown algae as a model system (Belanger and Quatrano 2000). The zygotes of these plants are independent of other tissues and amenable to manipulation. The initial cell division results in one smaller cell, which will form the rhizoid (root homologue) and anchor the rest of the plant, and one larger cell, which gives rise to the thallus (main body of the sporophyte). The point of sperm entry fixes the position of the rhizoid end of the apical-basal axis. This axis is perpendicular to the plane of the first cell division. F-actin accumulates at the rhizoid pole (Kropf et al. 1999). However, light or gravity can override this fixing of the axis and establish a new position for cell division (Figure 20.13; Alessa and Kropf 1999). Once the apical-basal axis is established, secretory vesicles are targeted to the rhizoid pole of the zygote (Figure 20.14). These vesicles contain material for rhizoid outgrowth, with a cell wall of distinct macromolecular composition. Targeted secretion may also help orient the first plane of cell division. Maintenance of rhizoid versus thallus fate early in development depends on information in the cell walls (Brownlee and Berger 1995). Cell wall information also appears to be important in angiosperms (reviewed in Scheres and Benfey 1999).

Figure 20.13
Axis formation in the brown alga Pelvetia compressa. (A) An F-actin patch (orange) is first formed at the point of sperm entry (the blue spot marks the sperm pronucleus). (B) Later, light was shone in the direction of the arrow. The sperm-induced axis (more...)

Figure 20.14
Asymmetrical cell division in brown algae. Time course from 8 to 25 hours after fertilization, showing algal cells stained with a vital membrane dye to visualize secretory vesicles, which appear first, and the cell plate, which begins to appear about (more...)
The basic body plan of the angiosperm laid down during embryogenesis also begins with an asymmetrical* cell division, giving rise to a terminal cell and a basal cell (Figure 20.15). The terminal cell gives rise to the embryo proper. The basal cell forms closest to the micropyle and gives rise to the suspensor. The hypophysis is found at the interface between the suspensor and the embryo proper. In many species it gives rise to some of the root cells. (The suspensor cells divide to form a filamentous or spherical organ that degenerates later in embryogenesis.) In both gymnosperms and angiosperms, the suspensor orients the absorptive surface of the embryo toward its food source; in angiosperms, it also appears to serve as a nutrient conduit for the developing embryo. Culturing isolated embryos of scarlet runner beans with and without the suspensor has demonstrated the need for a suspensor through the heart stage in dicots (Figure 20.16; Yeung and Sussex 1979). Embryos cultured with a suspensor are twice as likely to survive as embryos cultured without an attached suspensor at this stage. The suspensor may be a source of hormones. In scarlet runner beans, younger embryos without a suspensor can survive in culture if they are supplemented with the growth hormone gibberellic acid (Cionini et al. 1976).

Figure 20.16
Role of the suspensor in dicot embryogenesis. Culturing scarlet runner bean embryos with and without their suspensors has demonstrated that the suspensor is essential at the heart-shaped stage, but not later. (After Yeung and Sussex 1979.)
As the establishment of apical-basal polarity is one of the key achievements of embryogenesis, it is useful to consider why the suspensor and embryo proper develop unique morphologies. Here the study of embryo mutants in maize and Arabidopsis has been particularly helpful. Investigations of suspensor mutants (sus1, sus2, and raspberry1) of Arabidopsis have provided genetic evidence that the suspensor has the capacity to develop embryo-like structures (Figure 20.17; Schwartz et al. 1994; Yadegari et al. 1994). In these mutants, abnormalities in the embryo proper appear prior to suspensor abnormalities.† Earlier experiments in which the embryo proper was removed also demonstrated that suspensors could develop like embryos (Haccius 1963). A signal from the embryo proper to the suspensor may be important in maintaining suspensor identity and blocking the development of the suspensor as an embryo. Molecular analyses of these and other genes are providing insight into the mechanisms of communication between the suspensor and the embryo proper.

Figure 20.17
The SUS gene suppresses embryonic development in the suspensor. (A) Wild-type embryo and suspensor. (B) sus mutant with suspensor developing like an embryo (arrow). (C) Model showing how the embryo proper suppresses embryonic development in the suspensor (more...)
Maternal effect genes play a key role in establishing embryonic pattern in animals (see Chapter 9). The role of extrazygotic genes in plant embryogenesis is less clear, and the question is complicated by at least three potential sources of influence: sporophytic tissue, gametophytic tissue, and the polyploid endosperm. All of these tissues are in close association with the egg/zygote (Ray 1998). Endosperm development could also be affected by maternal genes. Sporophytic and gametophytic maternal effect genes have been identified in Arabidopsis, and it is probable that the endosperm genome influences the zygote as well. The first maternal effect gene identified, SHORT INTEGUMENTS 1 (SIN1), must be expressed in the sporophyte for normal embryonic development (Ray et al. 1996). Two transcription factors (FBP7 and FBP11) are needed in the petunia sporophyte for normal endosperm development (Columbo et al. 1997). A female gametophytic maternal effect gene, MEDEA (after Euripides’ Medea, who killed her own children), has protein domains similar to those of a Drosophila maternal effect gene (Grossniklaus et al. 1998). Curiously, MEDEA is in the Polycomb gene group (see Chapter 9), whose products alter chromatin, directly or indirectly, and affect transcription. MEDEA affects an imprinted gene (see Chapter 5) that is expressed by the female gametophyte and by maternally inherited alleles in the zygote, but not by paternally inherited alleles (Vielle-Calzada et al. 1999). How significant maternal effect genes are in establishing the sporophyte body plan is still an unanswered question.
Radial and axial patterns develop as cell division and differentiation continue (Figure 20.18; see also Bowman 1994 for detailed light micrographs of Arabidopsis embryogenesis). The cells of the embryo proper divide in transverse and longitudinal planes to form a globular stage embryo with several tiers of cells. Superficially, this stage bears some resemblance to cleavage in animals, but the nuclear/cytoplasmic ratio does not necessarily increase. The emerging shape of the embryo depends on regulation of the planes of cell division and expansion, since the cells are not able to move and reshape the embryo. Cell division planes in the outer layer of cells become restricted, and this layer, called the protoderm, becomes distinct. Radial patterning emerges at the globular stage as the three tissue systems (dermal, ground, and vascular) of the plant are initiated. The dermal tissue (epidermis) will form from the protoderm and contribute to the outer protective layers of the plant. Ground tissue (cortex and pith) forms from the ground meristem, which lies beneath the protoderm. The procambium, which forms at the core of the embryo will give rise to the vascular tissue (xylem and phloem), which will function in support and transport. The differentiation of each tissue system is at least partially independent. For example, in the keule mutant of Arabidopsis, the dermal system is defective while the inner tissue systems develop normally (Mayer et al. 1991).
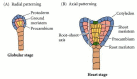
Figure 20.18
Radial and axial patterning. (A) Radial patterning in angiosperms begins in the globular stage and results in the establishment of three tissue systems. (B) The axial pattern (shoot-root axis) is established by the heart stage.
The globular shape of the embryo is lost as cotyledons (“first leaves”) begin to form. Dicots have two cotyledons, which give the embryo a heart-shaped appearance as they form. The axial body plan is evident by this heart stage of development. Hormones (specifically, auxins) may mediate the transition from radial to bilateral symmetry (Liu et al. 1993). In monocots, such as maize, only a single cotyledon emerges.
In many plants, the cotyledons aid in nourishing the plant by becoming photosynthetic after germination (although those of some species never emerge from the ground). In some cases—peas, for example—the food reserve in the endosperm is used up before germination, and the cotyledons serve as the nutrient source for the germinating seedling.‡ Even in the presence of a persistent endosperm (as in maize), the cotyledons store food reserves such as starch, lipids, and proteins. In many monocots, the cotyledon grows into a large organ pressed against the endosperm and aids in nutrient transfer to the seedling. Upright cotyledons can give the embryo a torpedo shape. In some plants, the cotyledons grow sufficiently long that they must bend to fit within the confines of the seed coat. The embryo then looks like a walking stick. By this point, the suspensor is degenerating.
The shoot apical meristem and root apical meristem are clusters of stem cells that will persist in the postembryonic plant and give rise to most of the sporophyte body. The root meristem is partially derived from the hypophysis in some species. All other parts of the sporophyte body are derived from the embryo proper. Genetic evidence indicates that the formation of the shoot and root meristems is regulated independently. This independence is demonstrated by the dek23 maize mutant and the shootmeristemless (STM) mutant of Arabidopsis, both of which form a root meristem but fail to initiate a shoot meristem (Clark and Sheridan 1986; Barton and Poethig 1993). The STM gene, which has been cloned, is expressed in the late globular stage, before cotyledons form. Genes have also been identified that specifically affect the development of the root axis during embryogenesis. Mutations of the HOBBIT gene in Arabidopsis (Willemsen et al. 1998), for example, affect the hypophysis derivatives and eliminate root meristem function.
The shoot apical meristem will initiate leaves after germination and, ultimately, the transition to reproductive development. In Arabidopsis, the cotyledons are produced from general embryonic tissue, not from the shoot meristem (Barton and Poethig 1993). In many angiosperms, a few leaves are initiated during embryogenesis. In the case of Arabidopsis, clonal analysis points to the presence of leaves in the mature embryo, even though they are not morphologically well developed (Irish and Sussex 1992). Clonal analysis has demonstrated that the cotyledons and the first two true leaves of cotton are derived from embryonic tissue rather than an organized meristem (Christianson 1986).
Clonal analysis experiments provide information on cell fates, but do not necessarily indicate whether or not cells are determined for a particular fate. Cells, tissues, and organs are shown to be determined when they have the same fate in situ, in isolation, and at a new position in the organism (see McDaniel et al. 1992 for more information on developmental states in plants). Clonal analysis has demonstrated that cells that divide in the wrong plane and “move” to a different tissue layer often differentiate according to their new position. Position, rather than clonal origin, appears to be the critical factor in embryo pattern formation, suggesting some type of cell-cell communication (Laux and Jurgens 1994). Microsurgery experiments on somatic carrot embryos demonstrate that isolated pieces of embryo can often replace the missing complement of parts (Schiavone and Racusen 1990; Scheres and Heidstra 1999). A cotyledon removed from the shoot apex will be replaced. Isolated embryonic shoots can regenerate a new root; isolated root tissue regenerates cotyledons, but is less likely to regenerate the shoot axis. Although most embryonic cells are pluripotent and can generate organs such as cotyledons and leaves, only meristems retain this capacity in the postembryonic plant body.
Footnotes
- *
Asymmetrical cell division is also important in later angiosperm development, including the formation of guard cells of leaf stomata and of different cell types in the ground and vascular tissue systems.
- †
Another intriguing characteristic of these mutants is that cell differentiation occurs in the absence of morphogenesis. Thus, cell differentiation and morphogenesis can be uncoupled in plant development.
- ‡
Mendel's famous wrinkled-seed mutant (the rugosus or r allele) has a defect in a starch branching enzyme that affects starch, lipid, and protein biosynthesis in the seed and leads to defective cotyledons (Bhattacharyya et al. 1990).
- Embryonic Development - Developmental BiologyEmbryonic Development - Developmental Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...