By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsCleavage in Amphibians
Cleavage in most frog and salamander embryos is radially symmetrical and holoblastic, just like echinoderm cleavage. The amphibian egg, however, contains much more yolk. This yolk, which is concentrated in the vegetal hemisphere, is an impediment to cleavage. Thus, the first division begins at the animal pole and slowly extends down into the vegetal region (Figure 10.1; see also Figures 2.2D and 8.4). In the axolotl salamander, the cleavage furrow extends through the animal hemisphere at a rate close to 1 mm per minute. The cleavage furrow bisects the gray crescent and then slows down to a mere 0.02–0.03 mm per minute as it approaches the vegetal pole (Hara 1977).

Figure 10.1
Cleavage of a frog egg. Cleavage furrows, designated by Roman numerals, are numbered in order of appearance. (A, B) Because the vegetal yolk impedes cleavage, the second division begins in the animal region of the egg before the first division has divided (more...)
Figure 10.2A is a scanning electron micrograph showing the first cleavage in a frog egg. One can see the difference in the furrow between the animal and the vegetal hemispheres. Figure 10.2B shows that while the first cleavage furrow is still cleaving the yolky cytoplasm of the vegetal hemisphere, the second cleavage has already started near the animal pole. This cleavage is at right angles to the first one and is also meridional. The third cleavage, as expected, is equatorial. However, because of the vegetally placed yolk, this cleavage furrow in amphibian eggs is not actually at the equator, but is displaced toward the animal pole. It divides the frog embryo into four small animal blastomeres (micromeres) and four large blastomeres (macromeres) in the vegetal region. This unequal holoblastic cleavage establishes two major embryonic regions: a rapidly dividing region of micromeres near the animal pole and a more slowly dividing vegetal macromere area (Figure 10.2C; Figure 2.2E). As cleavage progresses, the animal region becomes packed with numerous small cells, while the vegetal region contains only a relatively small number of large, yolk-laden macromeres.

Figure 10.2
Scanning electron micrographs of the cleavage of a frog egg. (A) First cleavage. (B) Second cleavage (4 cells). (C) Fourth cleavage (16 cells), showing the size discrepancy between the animal and vegetal cells after the third division. (A from Beams and (more...)
An amphibian embryo containing 16 to 64 cells is commonly called a morula (plural: morulae; from the Latin, “mulberry,” whose shape it vaguely resembles). At the 128-cell stage, the blastocoel becomes apparent, and the embryo is considered a blastula. Actually, the formation of the blastocoel has been traced back to the very first cleavage furrow. Kalt (1971) demonstrated that in the frog Xenopus laevis, the first cleavage furrow widens in the animal hemisphere to create a small intercellular cavity that is sealed off from the outside by tight intercellular junctions (Figure 10.3). This cavity expands during subsequent cleavages to become the blastocoel.
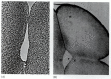
Figure 10.3
Formation of the blastocoel in a frog egg. (A) First cleavage furrow, showing a small cleft, which later develops into the blastocoel. (B) 8-cell embryo showing a small blastocoel (arrow) at the junction of the three cleavagefurrows. (From Kalt 1971; (more...)
The blastocoel probably serves two major functions in frog embryos: (1) it permits cell migration during gastrulation, and (2) it prevents the cells beneath it from interacting prematurely with the cells above it. When Nieuwkoop (1973) took embryonic newt cells from the roof of the blastocoel, in the animal hemisphere, and placed them next to the yolky vegetal cells from the base of the blastocoel, these animal cells differentiated into mesodermal tissue instead of ectoderm. Because mesodermal tissue is normally formed from those animal cells that are adjacent to the vegetal endoderm precursors, it seems plausible that the vegetal cells influence adjacent cells to differentiate into mesodermal tissues. Thus, the blastocoel appears to prevent the contact of the vegetal cells destined to become endoderm with those cells fated to give rise to the skin and nerves.
While these cells are dividing, numerous cell adhesion molecules keep the blastomeres together. One of the most important of these molecules is EP-cadherin. The mRNA for this protein is supplied in the oocyte cytoplasm. If this message is destroyed (by injecting antisense oligonucleotides complementary to this mRNA into the oocyte), the EP-cadherin is not made, and the adhesion between the blastomeres is dramatically reduced (Heasman et al. 1994a,b), resulting in the obliteration of the blastocoel (Figure 10.4).
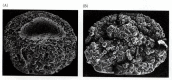
Figure 10.4
Depletion of EP-cadherin mRNA in the Xenopus oocyte results in the loss of adhesion between blastomeres and the obliteration of the blastocoel. (A) control embryo; (B) EP-cadherin-depleted embryo. (From Heasman et al. 1994b; photographs courtesy of J. (more...)
Amphibian Gastrulation
The study of amphibian gastrulation is both one of the oldest and one of the newest areas of experimental embryology. Even though amphibian gastrulation has been extensively studied for the past century, most of our theories concerning the mechanisms of these developmental movements have been revised over the past decade. The study of amphibian gastrulation has been complicated by the fact that there is no single way amphibians gastrulate. Different species employ different means toward the same goal (Smith and Malacinski 1983; Minsuk and Keller 1996). In recent years, the most intensive investigations have focused on the frog Xenopus laevis, so we will concentrate on its mode of gastrulation.
The fate map of Xenopus
Amphibian blastulae are faced with the same tasks as the invertebrate blastulae we followed in Chapters 8 and 9—namely, to bring inside the embryo those areas destined to form the endodermal organs, to surround the embryo with cells capable of forming the ectoderm, and to place the mesodermal cells in the proper positions between them. The movements whereby this is accomplished can be visualized by the technique of vital dye staining (see Chapter 1). Fate mapping by Løvtrup (1975; Landstrom and Løvtrup 1979) and by Keller (1975,1976) has shown that cells of the Xenopus blastula have different fates depending on whether they are located in the deep or the superficial layers of the embryo (Figure 10.5). In Xenopus, the mesodermal precursors exist mostly in the deep layer of cells, while the ectoderm and endoderm arise from the superficial layer on the surface of the embryo. Most of the precursors for the notochord and other mesodermal tissues are located beneath the surface in the equatorial (marginal) region of the embryo. In urodeles (salamanders such as Triturus and Ambystoma) and in some frogs other than Xenopus, many more of the notochord and mesoderm precursors are among the surface cells (Purcell and Keller 1993).

Figure 10.5
Fate maps of the blastula of the frog Xenopus laevis: (A) exterior; (B) interior. Most of the mesodermal derivatives are formed from the interior cells. (After Lane and Smith 1999; Newman and Krieg 1999.)
As we have seen, the unfertilized egg has a polarity along the animal-vegetal axis. Thus, the germ layers can be mapped onto the oocyte even before fertilization. The surface of the animal hemisphere will become the cells of the ectoderm (skin and nerves), the vegetal hemisphere surface will form the cells of the gut and associated organs (endoderm), and the mesodermal cells will form from the internal cytoplasm around the equator. This general fate map is thought to be imposed upon the egg by the transcription factor VegT and the paracrine factor Vg1. The mRNAs for these proteins are located in the cortex of the vegetal hemisphere of Xenopus oocytes, and they are apportioned to the vegetal cells during cleavage (see Figure 5.33). By using antisense oligonucleotides, Zhang and colleagues (1998) were able to deplete maternal VegT protein in early embryos. The resulting embryos lacked the normal fate map. The animal third of the embryo produced only ventral epidermis, while the marginal cells (which normally produced mesoderm) generated epidermal and neural tissue. The vegetal third (which usually produces endoderm) produced a mixture of ectoderm and mesoderm (Figure 10.6). Joseph and Melton (1998) demonstrated that embryos that lacked functional Vg1 lacked endoderm and dorsal mesoderm.

Figure 10.6
The fates of the three regions of the Xenopus blastula are altered by the depletion of VegT. In normal embryos, the animal third forms epidermal and neural ectoderm, the equatorial third forms mesoderm, and the vegetal third contains the VegT protein (more...)
These findings tell us nothing, however, about which part of the egg will form the belly and which the back. The anterior-posterior, dorsal-ventral, and left-right axes are specified by the events of fertilization and are realized during gastrulation.
VADE MECUM
Amphibian development. The events of cleavage and gastrulation are difficult to envision without three-dimensional models. You can see movies of such 3-D models, as well as footage of a living Xenopus embryo, in the segments on amphibian development. [Click on Amphibian]
Cell movements during amphibian gastrulation
Before we look at the process of gastrulation in detail, let us first trace the movement patterns of the germ layers. Gastrulation in frog embryos is initiated on the future dorsal side of the embryo, just below the equator in the region of the gray crescent (Figure 10.7). Here, the cells invaginate to form a slitlike blastopore. These cells change their shape dramatically. The main body of each cell is displaced toward the inside of the embryo while the cell maintains contact with the outside surface by way of a slender neck (Figure 10.8). These bottle cells line the archenteron as it forms. Thus, as in the gastrulating sea urchin, an invagination of cells initiates archenteron formation. However, unlike gastrulation in sea urchins, gastrulation in the frog begins not at the most vegetal region, but in the marginal zone: the zone surrounding the equator of the blastula, where the animal and vegetal hemispheres meet. Here the endodermal cells are not as large or as yolky as the most vegetal blastomeres.
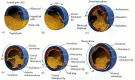
Figure 10.7
Cell movements during frog gastrulation. The meridional sections are cut through the middle of the embryo and positioned so that the vegetal pole is tilted toward the observer and slightly to the left. The major cell movements are indicated by arrows, (more...)
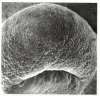
Figure 10.8
Surface view of an early dorsal blastopore lip of Xenopus. The size difference between the animal and vegetal blastomeres is readily apparent. (Micrograph courtesy of C. Phillips.)
The next phase of gastrulation involves the involution of the marginal zone cells while the animal cells undergo epiboly and converge at the blastopore (Figure 10.7C, D). When the migrating marginal cells reach the dorsal lip of the blastopore, they turn inward and travel along the inner surface of the outer animal hemisphere cells. Thus, the cells constituting the lip of the blastopore are constantly changing. The first cells to compose the dorsal blastopore lip are the bottle cells that invaginated to form the leading edge of the archenteron. These cells later become the pharyngeal cells of the foregut. As these first cells pass into the interior of the embryo, the dorsal blastopore lip becomes composed of cells that involute into the embryo to become the prechordal plate (the precursor of the head mesoderm). The next cells involuting into the embryo through the dorsal blastopore lip are called the chordamesoderm cells. These cells will form the notochord, a transient mesodermal “backbone” that plays an important role in distinguishing and patterning the nervous system.
As the new cells enter the embryo, the blastocoel is displaced to the side opposite the dorsal lip of the blastopore. Meanwhile, the blastopore lip expands laterally and ventrally as the processes of bottle cell formation and involution continue about the blastopore. The widening blastopore “crescent” develops lateral lips and finally a ventral lip over which additional mesodermal and endodermal precursor cells pass. With the formation of the ventral lip, the blastopore has formed a ring around the large endodermal cells that remain exposed on the vegetal surface. This remaining patch of endoderm is called the yolk plug; it, too, is eventually internalized (Figure 10.9). At that point, all the endodermal precursors have been brought into the interior of the embryo, the ectoderm has encircled the surface, and the mesoderm has been brought between them.
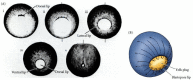
Figure 10.9
Epiboly of the ectoderm. (A) Changes in the region around the blastopore as the dorsal, lateral, and ventral lips are formed in succession. When the ventral lip completes the circle, the endoderm becomes progressively internalized. Numbers ii-v correspond (more...)
The midblastula transition: preparing for gastrulation
Now that we have seen an overview of amphibian gastrulation, we can look more deeply into its mechanisms. The first precondition for gastrulation is the activation of the genome. In Xenopus, the nuclear genes are not transcribed until late in the twelfth cell cycle (Newport and Kirschner 1982a,b). At that time, different genes begin to be transcribed in different cells, and the blastomeres acquire the capacity to become motile. This dramatic change is called the midblastula transition (see Chapters 8 and 9). It is thought that different transcription factors (such as the VegT protein, mentioned above) become active in different cells at this time, giving the cells new properties. For instance, the vegetal cells (probably under the direction of the maternal VegT protein) become the endoderm and begin secreting the factors that cause the cells above them to become the mesoderm (Wylie et al. 1996).
Positioning the blastopore
The vegetal cells are critical in determining the location of the blastopore, as is the point of sperm entry. The microtubules of the sperm direct cytoplasmic movements that empower the vegetal cells opposite the point of sperm entry to induce the blastopore in the mesoderm above them. This region of cells opposite the point of sperm entry will form the blastopore and become the dorsal portion of the body.
In Chapter 7, we saw that the internal cytoplasm of the fertilized egg remains oriented with respect to gravity because of its dense yolk accumulation, while the cortical cytoplasm actively rotates 30 degrees animally (“upward”), toward the point of sperm entry (see Figure 7.33). In this way, a new state of symmetry is acquired. Whereas the unfertilized egg was radially symmetrical about the animal-vegetal axis, the fertilized egg now has a dorsal-ventral axis. It has become bilaterally symmetrical (having right and left sides). The inner cytoplasm moves as well. Fluorescence microscopy of early embryos has shown that the cytoplasm of the presumptive dorsal cells differs from that of the presumptive ventral cells (see Figure 7.35; Danilchik and Denegre 1991). These cytoplasmic movements activate the cytoplasm opposite the point of sperm entry, enabling it to initiate gastrulation. The side where the sperm enters marks the future ventral surface of the embryo; the opposite side, where gastrulation is initiated, marks the future dorsum of the embryo (Gerhart et al. 1981, 1986; Vincent et al. 1986). If cortical rotation is blocked, there is no dorsal development, and the embryo dies as a mass of ventral (primarily gut) cells (Vincent and Gerhart 1987).
Although the sperm is not needed to induce these movements in the egg cytoplasm, it is important in determining the direction of the rotation. If an egg is artificially activated, the cortical rotation still takes place at the correct time. However, the direction of this movement is unpredictable. The directional bias provided by the point of sperm entry can be overridden by mechanically redirecting the spatial relationship between the cortical and internal cytoplasms. When a Xenopus egg is turned 90 degrees, so that the point of sperm entry faces upward, the cytoplasm rotates such that the embryo initiates gastrulation on the same side as sperm entry (Gerhart et al. 1981; Cooke 1986). One can even produce two gastrulation initiation sites by combining the natural sperm-oriented rotation with an artificially induced rotation of the egg. Black and Gerhart (1985, 1986) let the initial sperm-directed rotation occur, but then immobilized eggs in gelatin and gently centrifuged them so that the internal cytoplasm would flow toward the point of sperm entry. When the centrifuged eggs were then allowed to develop in normal water, two sites of gastrulation emerged, leading to conjoined twin larvae (Figure 10.10). Black and Gerhart hypothesized that the twinning was caused by the formation of two areas of interaction: one axis formed where the normal cortical rotation caused the normal cytoplasmic interactions in the vegetal region of the cell, the other where the centrifugation-driven cytoplasm interacted with the vegetal components.

Figure 10.10
Twin blastopores produced by rotating dejellied Xenopus eggs ventral side (sperm entry point) up at the time of first cleavage. (A) Two blastopores are instructed to form: the original one (opposite the point of sperm entry) and the new one created by (more...)
It appears that cortical rotation enables the vegetal blastomeres opposite the point of sperm entry to induce the cells above them to initiate gastrulation. Gimlich and Gerhart (1984), using transplantation experiments on 64-cell Xenopus embryos, showed that the three vegetal blastomeres opposite the point of sperm entry are able to induce the formation of the dorsal lip of the blastopore and of a complete dorsal axis when transplanted into UV-irradiated embryos (which otherwise would have failed to properly initiate gastrulation: Figure 10.11A). Moreover, these three blastomeres, which underlie the prospective dorsal lip region, can also induce a secondary blastopore and axis when transplanted into the ventral side of a normal, unirradiated embryo (Figure 10.11B). Holowacz and Elinson (1993) found that cortical cytoplasm from the dorsal vegetal cells of the 16-cell Xenopus embryo was able to induce the formation of secondary axes when injected into ventral vegetal cells. Neither cortical cytoplasm from animal cells nor the deep cytoplasm from ventral cells could induce such axes. Later in this chapter, we will provide evidence that this dorsal signal is the transcription factor β-catenin (Wylie et al. 1996; Larabell et al. 1997).
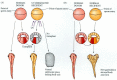
Figure 10.11
Transplantation experiments on 64-cell amphibian embryos demonstrating that the vegetal cells underlying the prospective dorsal blastopore lip region are responsible for causing the initiation of gastrulation. (A) Rescue of irradiated embryos by transplanting (more...)
Invagination and involution
Amphibian gastrulation is first visible when a group of marginal endoderm cells on the dorsal surface of the blastula sinks into the embryo. The outer (apical) surfaces of these cells contract dramatically, while their inner (basal) ends expand. The apical-basal length of these cells greatly increases to yield the characteristic “bottle” shape. In salamanders, these bottle cells appear to have an active role in the early movements of gastrulation. Johannes Holtfreter (1943,1944) found that bottle cells from early salamander gastrulae could attach to glass coverslips and lead the movement of those cells attached to them. Even more convincing were Holtfreter's recombination experiments. When dorsal marginal zone cells (which would normally give rise to the dorsal lip of the blastopore) were excised and placed on inner prospective endoderm tissue, they formed bottle cells and sank below the surface of the inner endoderm (Figure 10.12). Moreover, as they sank, they created a depression reminiscent of the early blastopore. Thus, Holtfreter claimed that the ability to invaginate into the deep endoderm is an innate property of the dorsal marginal zone cells.
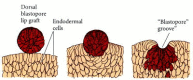
Figure 10.12
A graft of cells from the dorsal marginal zone of a salamander embryo sinks into a layer of endodermal cells and forms a blastopore-like groove. (After Holtfreter 1944.)
WEBSITE
10.1 Demostrating tissue affinities. The tissue affinities that Holtfreter predicted have been demonstrated quantitatively by new studies that measure the surface tensions of different cell layers. http://www.devbio.com/chap10/link1001.shtml
The situation in the frog embryo is somewhat different. R. E. Keller and his students (Keller 1981; Hardin and Keller 1988) have shown that although the bottle cells of Xenopus may play a role in initiating the involution of the marginal zone as they become bottle-shaped, they are not essential for gastrulation to continue. The peculiar shape change of the bottle cells is needed to initiate gastrulation; it is the constriction of these cells that first forms the slit-like blastopore. However, after starting these movements, the Xenopus bottle cells are no longer needed for gastrulation. When bottle cells are removed after their formation, involution and blastopore formation and closure continue.
The major factor in the movement of cells into the embryo appears to be the involution of the subsurface marginal cells, rather than the superficial ones. The movements of the vegetal endoderm place the prospective pharyngeal endoderm adjacent to the roof of the blastocoel. This places the prospective pharyngeal endoderm immediately ahead of the migrating mesoderm. The cells then migrate along the basal surface of the blastocoel roof (Figure 10.13A-D; Winklbauer and Schürfeld 1999). The superficial layer of marginal cells is pulled inward to form the endodermal lining of the archenteron, merely because it is attached to the actively migrating deep cells. While experimental removal of the bottle cells does not affect the involution of the deep or superficial marginal zone cells into the embryo, the removal of the deep involuting marginal zone (IMZ) cells and their replacement with animal region cells (which do not normally undergo involution) stops archenteron formation.

Figure 10.13
Early movements of Xenopus gastrulation. The yellow is vegetal endoderm. Orange represents the prospective pharyngeal endoderm (as seen by Cerberus expression). Dark orange represents the prospective head mesoderm (as seen by goosecoid expression), and (more...)
The convergent extension of the dorsal mesoderm
Involution begins dorsally, led by the pharyngeal endomesoderm* and the prechordal plate. These tissues will migrate most anteriorly beneath the surface ectoderm. The next tissues to enter the dorsal blastopore lip contain notochord and somite precursors. Meanwhile, as the lip of the blastopore expands to have dorsolateral, lateral, and ventral sides, the prosepective heart mesoderm, kidney mesoderm, and ventral mesoderm enter into the embryo.
Figures 10.13D-F depict the behavior of the IMZ cells at successive stages of Xenopus gastrulation (Keller and Schoenwolf 1977; Keller 1980, 1981; Hardin and Keller 1988). The IMZ is originally several layers thick. Shortly before their involution through the blastopore lip, the several layers of deep IMZ cells intercalate radially to form one thin, broad layer. This intercalation further extends the IMZ vegetally. At the same time, the superficial cells spread out by dividing and flattening. When the deep cells reach the blastopore lip, they involute into the embryo and initiate a second type of intercalation. This intercalation causes a convergent extension along the mediolateral axis (Figure 10.13F) that integrates several mesodermal streams to form a long, narrow band. This is reminiscent of traffic on a highway when several lanes must merge to form a single lane. The anterior part of this band migrates toward the animal cap. Thus, the mesodermal stream continues to migrate toward the animal pole, and the overlying layer of superficial cells (including the bottle cells) is passively pulled toward the animal pole, thereby forming the endodermal roof of the archenteron (see Figures 10.7 and 10.13E). The radial and mediolateral intercalations of the deep layer of cells appear to be responsible for the continued movement of mesoderm into the embryo.
The adhesive changes driving convergent extension appear to be directed by two cell adhesion molecules, paraxial protocadherin and axial protocadherin. The former is initially found throughout the dorsal mesoderm, but then is turned off in the precursors of the notochord. At that time, axial protocadherin becomes expressed in the notochordal tissue (Figure 10.14). An experimental dominant negative form of paraxial protocadherin (which is secreted instead of being bound to the cell membrane) prevents convergent extension† (Kim et al. 1998). Moreover, the expression domain of paraxial protocadherin separates the trunk mesodermal cells, which undergo convergent extension, from the head mesodermal cells, which do not.

Figure 10.14
The expression of paraxial protocadherin. (A) Expression of paraxial protocadherin during late gastrulation shows the distinct down-regulation in the notochord and the absence of expression in the head region. (B) Double-stained cross section through (more...)
Migration of the involuting mesoderm
As mesodermal movement progresses, convergent extension continues to narrow and lengthen the involuting marginal zone. The IMZ contains the prospective endodermal roof of the archenteron in its superficial layer (IMZS) and the prospective mesodermal cells, including those of the notochord, in its deep region (IMZD). During the middle third of gastrulation, the expanding sheet of mesoderm converges toward the midline of the embryo. This process is driven by the continued mediolateral intercalation of cells along the anterior-posterior axis, thereby further narrowing the band. Toward the end of gastrulation, the centrally located notochord separates from the somitic mesoderm on either side of it, and the notochord cells elongate separately (Wilson and Keller 1991). This may in part be a consequence of the different protocadherins in the axial and paraxial mesoderms (Kim et al. 1998). This convergent extension of the mesoderm appears to be autonomous, because the movements of these cells occur even if this region of the embryo is experimentally isolated from the rest of the embryo (Keller 1986).
During gastrulation, the animal cap and noninvoluting marginal zone (NIMZ) cells expand by epiboly to cover the entire embryo. The dorsal portion of the NIMZ extends more rapidly toward the blastopore than the ventral portion, thus causing the blastopore lips to move toward the ventral side. While those mesodermal cells entering through the dorsal lip of the blastopore give rise to the dorsal axial mesoderm (notochord and somites), the remainder of the body mesoderm (which forms the heart, kidneys, blood, bones, and parts of several other organs) enters through the ventral and lateral blastopore lips to create the mesodermal mantle. The endoderm is derived from the IMZS cells that form the lining of the archenteron roof and from the subblastoporal vegetal cells that become the archenteron floor (Keller 1986).
Epiboly of the ectoderm
While involution is occurring at the blastopore lips, the ectodermal precursors are expanding over the entire embryo. Keller (1980) and Keller and Schoenwolf (1977) have used scanning electron microscopy to observe the changes in both the superficial cells and the deep cells of the animal and marginal regions. The major mechanism of epiboly in Xenopus gastrulation appears to be an increase in cell number (through division) coupled with a concurrent integration of several deep layers into one (Figure 10.15). During early gastrulation, three rounds of cell division increase the number of the deep cell layers in the animal hemisphere. At the same time, complete integration of the numerous deep cells into one layer occurs. The most superficial layer expands by cell division and flattening. The spreading of cells in the dorsal and ventral marginal zones appears to proceed by the same mechanism, although changes in cell shape appear to play a greater role than in the animal region. The result of these expansions is the epiboly of the superficial and deep cells of the animal cap and NIMZ over the surface of the embryo (Keller and Danilchik 1988). Most of the marginal zone cells, as previously mentioned, involute to join the mesodermal cell stream within the embryo. As the ectoderm epibolizes over the entire embryo, it eventually internalizes all the endoderm within it. At this point, the ectoderm covers the embryo, the endoderm is located within the embryo, and the mesoderm is positioned between them.

Figure 10.15
Scanning electron micrographs of the Xenopus blastocoel roof, showing the changes in cell shape and arrangement. Stages 8 and 9 are blastulae; stages 10–11.5 represent progressively later gastrulae. (From Keller 1980; photographs courtesy of R. (more...)
WEBSITE
10.2 Migration of the mesodermal mantle. Different growth rates coupled with the intercalation of cell layers allows the mesoderm to expand in a tightly coordinated fashion. http://www.devbio.com/chap10/link1002.shtml
Box
Fibronectin and the Pathways for Mesodermal Migration.
Footnotes
- *
The pharyngeal endoderm and head mesoderm cannot be separated experimentally at this stage, so they are therefore sometimes referred to collectively as the pharyngeal endomesoderm. The notochord is the basic unit of the dorsal mesoderm, but it is thought that the dorsal portion of the somites may also have similar properties.
- †
Dominant negative proteins are mutated forms of the wild-type protein that interfere with the normal functioning of the wild-type protein. Thus, a dominant negative protein will have an effect similar to a loss-of-function mutation in the gene encoding the particular protein.
- Early Amphibian Development - Developmental BiologyEarly Amphibian Development - Developmental Biology
- GNL3 G protein nucleolar 3 [Homo sapiens]GNL3 G protein nucleolar 3 [Homo sapiens]Gene ID:26354Gene
- PREDICTED: Homo sapiens PWWP domain containing 2A (PWWP2A), transcript variant X...PREDICTED: Homo sapiens PWWP domain containing 2A (PWWP2A), transcript variant X1, mRNAgi|2217354323|ref|XM_011534424.4|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...