By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsThe Progressive Determination of the Amphibian Axes
Vertebrate axes do not form from localized determinants in the various blastomeres, as in Drosophila. Rather, they arise progressively through a sequence of interactions between neighboring cells. Amphibian axis formation is an example of regulative development. In Chapter 3, we discussed the concept of regulative development, wherein (1) an isolated blastomere has a potency greater than its normal embryonic fate, and (2) a cell's fate is determined by interactions between neighboring cells. Such interactions are called inductions (see Chapter 6). That such inductive interactions were responsible for amphibian axis determination was demonstrated by the laboratory of Hans Spemann at the University of Freiburg. The experiments of Spemann and his students framed the questions that experimental embryologists asked for most of the twentieth century, and they resulted in a Nobel Prize for Spemann in 1935. More recently, the discoveries of the molecules associated with these inductive processes have provided some of the most exciting moments in contemporary science.
The experiment that began this research program was performed in 1903, when Spemann demonstrated that early newt blastomeres have identical nuclei, each capable of producing an entire larva. His procedure was ingenious: Shortly after fertilizing a newt egg, Spemann used a baby's hair taken from his daughter to lasso the zygote in the plane of the first cleavage. He then partially constricted the egg, causing all the nuclear divisions to remain on one side of the constriction. Eventually, often as late as the 16-cell stage, a nucleus would escape across the constriction into the non-nucleated side. Cleavage then began on this side, too, whereupon Spemann tightened the lasso until the two halves were completely separated. Twin larvae developed, one slightly older than the other (Figure 10.17). Spemann concluded from this experiment that early amphibian nuclei were genetically identical and that each cell was capable of giving rise to an entire organism.

Figure 10.17
Spemann's demonstration of nuclear equivalence in newt cleavage. (A) When the fertilized egg of the newt Triturus taeniatus was constricted by a ligature, the nucleus was restricted to one-half of the embryo. The cleavage on that side of the embryo reached (more...)
However, when Spemann performed a similar experiment with the constriction still longitudinal, but perpendicular to the plane of the first cleavage (separating the future dorsal and ventral regions rather than the right and left sides), he obtained a different result altogether. The nuclei continued to divide on both sides of the constriction, but only one side—the future dorsal side of the embryo—gave rise to a normal larva. The other side produced an unorganized tissue mass of ventral cells, which Spemann called the Bauchstück—the belly piece. This tissue mass was a ball of epidermal cells (ectoderm) containing blood and mesenchyme (mesoderm) and gut cells (endoderm), but no dorsal structures such as nervous system, notochord, or somites (Figure 10.18).

Figure 10.18
Asymmetry in the amphibian egg. (A) When the egg is divided along the plane of first cleavage into two blastomeres, each of which gets one-half of the gray crescent, each experimentally separated cell develops into a normal embryo. (B) When only one of (more...)
Why should these two experiments give different results? One possibility was that when the egg was divided perpendicular to the first cleavage plane, some cytoplasmic substance was not equally distributed into the two halves. Fortunately, the salamander egg was a good place to test that hypothesis. As we have seen in Chapter 7 and above, there are dramatic movements in the cytoplasm following the fertilization of amphibian eggs, and in some amphibians these movements expose a gray, crescent-shaped area of cytoplasm in the region directly opposite the point of sperm entry. This area has been called the gray crescent. Moreover, the first cleavage plane normally splits the gray crescent equally into the two blastomeres. If these cells are then separated, two complete larvae develop. However, should this cleavage plane be aberrant (either in the rare natural event or in an experiment), the gray crescent material passes into only one of the two blastomeres. Spemann found that when these two blastomeres are separated, only the blastomere containing the gray crescent develops normally.
WEBSITE
10.3 Embryology and individuality. One egg usually makes only one adult. However, there are exceptions to this rule, and Spemann was drawn into embryology through the paradoxes of creating more than one individual from a single egg. http://www.devbio.com/chap10/link1003.shtml
It appeared, then, that something in the gray crescent region was essential for proper embryonic development. But how did it function? What role did it play in normal development? The most important clue came from the fate map of this area of the egg, for it showed that the gray crescent region gives rise to the cells that initiate gastrulation. These cells form the dorsal lip of the blastopore. The cells of the dorsal lip are committed to invaginate into the blastula, thus initiating gastrulation and the formation of the notochord. Because all future amphibian development depends on the interaction of cells rearranged during gastrulation, Spemann speculated that the importance of the gray crescent material lies in its ability to initiate gastrulation, and that crucial developmental changes occur during gastrulation.
In 1918, Spemann demonstrated that enormous changes in cell potency do indeed take place during gastrulation. He found that the cells of the early gastrula were uncommitted, but that the fates of late gastrula cells were determined. Spemann demonstrated this by exchanging tissues between the gastrulae of two species of newts whose embryos were differently pigmented (Figure 10.19). When a region of prospective epidermal cells from an early gastrula was transplanted into an area in another early gastrula where the neural tissue normally formed, the transplanted cells gave rise to neural tissue. When prospective neural tissue from early gastrulae was transplanted to the region fated to become belly skin, the neural tissue became epidermal (Table 10.1). Thus, these early newt gastrula cells were not yet committed to a specific fate. Such cells are said to exhibit conditional (i.e., regulative or dependent) development because their ultimate fates depend on their location in the embryo. However, when the same interspecies transplantation experiments were performed on late gastrulae, Spemann obtained completely different results. Rather than differentiating in accordance with their new location, the transplanted cells exhibited autonomous (or independent, or mosaic) development. Their prospective fate was determined, and the cells developed independently of their new embryonic location. Specifically, prospective neural cells now developed into brain tissue even when placed in the region of prospective epidermis, and prospective epidermis formed skin even in the region of the prospective neural tube. Within the time separating early and late gastrulation, the potencies of these groups of cells had become restricted to their eventual paths of differentiation. Something was causing them to become determined to epidermal and neural fates. What was happening?

Figure 10.19
Determination of ectoderm during newt gastrulation. Presumptive neural ectoderm from one newt embryo is transplanted into a region in another embryo that normally becomes epidermis. (A) When the tissues are transferred between early gastrulas, the presumptive (more...)
Table 10.1
Results of tissue transplantation during early- and late-gastrula stages in the newt.
Hans Spemann and Hilde Mangold: Primary Embryonic Induction
The most spectacular transplantation experiments were published by Hans Spemann and Hilde Mangold in 1924.* They showed that, of all the tissues in the early gastrula, only one has its fate determined. This self-differentiating tissue is the dorsal lip of the blastopore, the tissue derived from the gray crescent cytoplasm. When this dorsal lip tissue was transplanted into the presumptive belly skin region of another gastrula, it not only continued to be blastopore lip, but also initiated gastrulation and embryogenesis in the surrounding tissue (Figure 10.20). Two conjoined embryos were formed instead of one!

Figure 10.20
Organization of a secondary axis by dorsal blastopore lip tissue. (A) Dorsal lip tissue from an early gastrula is transplanted into another early gastrula in the region that normally becomes ventral epidermis. (B) The donor tissue invaginates and forms (more...)
In these experiments, Spemann and Mangold used differently pigmented embryos from two newt species: the darkly pigmented Triturus taeniatus and the nonpigmented Triturus cristatus. So when Spemann and Mangold prepared these transplants, they were able to readily identify host and donor tissues on the basis of color.† When the dorsal lip of an early T. taeniatus gastrula was removed and implanted into the region of an early T. cristatus gastrula fated to become ventral epidermis (belly skin), the dorsal lip tissue invaginated just as it would normally have done (showing self-determination), and disappeared beneath the vegetal cells. The pigmented donor tissue then continued to self-differentiate into the chordamesoderm (notochord) and other mesodermal structures that normally form from the dorsal lip. As the new donor-derived mesodermal cells moved forward, host cells began to participate in the production of the new embryo, becoming organs that normally they never would have formed. In this secondary embryo, a somite could be seen containing both pigmented (donor) and unpigmented (host) tissue. Even more spectacularly, the dorsal lip cells were able to interact with the host tissues to form a complete neural plate from host ectoderm. Eventually, a secondary embryo formed, face to face with its host. These technically difficult experiments have been repeated using nuclear markers, and the results of Spemann and Mangold have been confirmed (Smith and Slack 1983; Recanzone and Harris 1985).
WEBSITE
10.4 Spemann, Mangold, and the organizer. Spemann did not see the importance of this work the first time they did it. This website provides a more detailed account of why Spemann and Mangold did this experiment. http://www.devbio.com/chap10/link1004.shtml
Spemann (1938) referred to the dorsal lip cells and their derivatives (notochord, prechordal mesoderm) as the organizer because (1) they induced the host's ventral tissues to change their fates to form a neural tube and dorsal mesodermal tissue (such as somites), and (2) they organized host and donor tissues into a secondary embryo with clear anterior-posterior and dorsal-ventral axes. He proposed that during normal development, these cells organize the dorsal ectoderm into a neural tube and transform the flanking mesoderm into the anterior-posterior body axis. It is now known (thanks largely to Spemann and his students) that the interaction of the chordamesoderm and ectoderm is not sufficient to “organize” the entire embryo. Rather, it initiates a series of sequential inductive events. As discussed in Chapter 6, the process by which one embryonic region interacts with a second region to influence that second region's differentiation or behavior is called induction. Because there are numerous inductions during embryonic development, this key induction wherein the progeny of dorsal lip cells induce the dorsal axis and the neural tube is traditionally called primary embryonic induction.‡
The Mechanisms of Axis Formation in Amphibians
The experiments of Spemann and Mangold showed that the dorsal lip of the blastopore, and the notochord that forms from it, constituted an “organizer” that could instruct the formation of new embryonic axes. But the mechanisms by which the organizer was constructed and through which it operated were totally unknown. Indeed, it is said that Spemann and Mangold's paper posed more questions than it answered. Among these questions were:
- How did the organizer get its properties? What caused the dorsal blastopore lip to differ from any other region of the embryo?
- What factors were being secreted from the organizer to cause the formation of the neural tube and to create the anterior-posterior, dorsal-ventral, and left-right axes?
- How did the different parts of the neural tube become established, with the most anterior becoming the sensory organs and forebrain, and the most posterior becoming spinal cord?
We will take up each of these questions in turn.
The origin of the Nieuwkoop center
The major clue in determining how the dorsal blastopore lip obtained its properties came from the experiments of Pieter Nieuwkoop (1969, 1973, 1977). He and his colleagues in the Netherlands demonstrated that the properties of this newly formed mesoderm were induced by the vegetal (presumptive endoderm) cells underlying them. He removed the equatorial cells (i.e., presumptive mesoderm) from a blastula and showed that neither the animal cap (presumptive ectoderm) nor the vegetal cap (presumptive endoderm) produced any mesodermal tissue. However, when the two caps were recombined, the animal cap cells were induced to form mesodermal structures such as notochord, muscles, kidney cells, and blood cells (Figure 10.21). The polarity of this induction (whether the animal cells formed dorsal mesoderm or ventral mesoderm) depended on the dorsal-ventral polarity of the endodermal (vegetal) fragment. While the ventral and lateral vegetal cells (those closer to the side of sperm entry) induced ventral (mesenchyme, blood) and intermediate (muscle, kidney) mesoderm, the dorsalmost vegetal cells specified dorsal mesoderm components (somites, notochord), including those having the properties of the organizer. The dorsalmost vegetal cells of the blastula, which are capable of inducing the organizer, have been called the Nieuwkoop center (Gerhart et al. 1989).
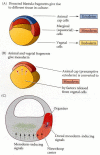
Figure 10.21
Summary of experiments by Nieuwkoop and by Nakamura and Takasaki (1970), showing mesodermal induction by vegetal endoderm. (A) Isolated animal cap cells become a mass of ciliated epidermis, isolated vegetal cells generate gutlike tissue, and isolated (more...)
The Nieuwkoop center was demonstrated in the 32-cell Xenopus embryo by transplantation and recombination experiments. First, Gimlich and Gerhart (Gimlich and Gerhart 1984; Gimlich 1985, 1986) performed an experiment analogous to the Spemann and Mangold studies, except that they used blastulae rather than gastrulae. When they transplanted the dorsalmost vegetal blastomere from one blastula into the ventral vegetal side of another blastula, two embryonic axes were formed (see Figure 10.11B). Second, Dale and Slack (1987) recombined single vegetal blastomeres from a 32-cell Xenopus embryo with the uppermost animal tier of a fluorescently labeled embryo of the same stage. The dorsalmost vegetal cell, as expected, induced the animal pole cells to become dorsal mesoderm. The remaining vegetal cells usually induced the animal cells to produce either intermediate or ventral mesodermal tissues (Figure 10.22). Thus, dorsal vegetal cells can induce animal cells to become dorsal mesodermal tissue.
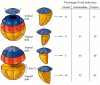
Figure 10.22
The regional specificity of mesoderm induction can be demonstrated by recombining cells of 32-cell Xenopus embryos. Animal pole cells were labeled with fluorescent polymers so that their descendants could be identified, then combined with individual vegetal (more...)
The Nieuwkoop center is created by the cytoplasmic rotation that occurs during fertilization (see Chapter 7). When this rotation is inhibited by UV light, the resulting embryo will not form dorsal-anterior structures such as the head or neural tube (Vincent and Gerhart 1987). However, these UV-treated embryos can be rescued by transplantation of the dorsalmost vegetal blastomeres from a normal embryo at the 32-cell stage (Dale and Slack 1987; see Figure 10.11A). If eggs are rotated toward the end of the first cell cycle so that the future ventral side is upward, two Nieuwkoop centers are formed, leading to two dorsal blastopore lips and two embryonic axes (see Figure 10.10). Therefore, the specification of the dorsal-ventral axis begins at the moment of sperm entry.
The molecular biology of the Nieuwkoop center
In Xenopus, the endoderm is able to induce the formation of mesoderm by causing the presumptive mesodermal cells to express the Xenopus Brachyury (Xbra) gene. The mechanism of this induction is not well understood (see Harland and Gerhart 1997), but the Xbra protein is a transcription factor that activates the genes that produce mesoderm-specific proteins. While all the vegetal cells appear to be able to induce the overlying marginal cells to become mesoderm, only the dorsalmost vegetal cells can instruct the overlying dorsal marginal cells to become the organizer. The major candidate for the factor that forms the Nieuwkoop center in these dorsalmost vegetal cells is β-catenin.
WEBSITE
10.5 Mesoderm induction. There are numerous theories concerning how the generic mesoderm is induced by the endoderm. Evidence points to three molecules as possible mesoderm inducers: bFGF, Vg1, and an activin-like protein. These proteins can activate Xbra as well as other mesodermal proteins. http://www.devbio.com/chap10/link1005.shtml
β-catenin is a multifunctional protein that can act as an anchor for cell membrane cadherins (see Chapter 3) or as a nuclear transcription factor (see Chapter 6). In Xenopus embryos, β-catenin begins to accumulate in the dorsal region of the egg during the cytoplasmic movements of fertilization. β-catenin continues to accumulate preferentially at the dorsal side throughout early cleavage, and this accumulation is seen in the nuclei of the dorsal cells (Figure 10.23A-D; Schneider et al. 1996; Larabell et al. 1997). This region of β-catenin accumulation originally appears to cover both the Nieuwkoop center and organizer regions. During later cleavage, the cells containing β-catenin may reside specifically in the Nieuwkoop center (Heasman et al. 1994a; Guger and Gumbiner 1995).

Figure 10.23
The role of Wnt pathway proteins in dorsal-ventral axis specification. (A-D) Differential translocation of β-catenin into Xenopus blastomere nuclei. (A) Early 2-cell stage of Xenopus, showing β-catenin (orange) predominantly at the dorsal (more...)
β-catenin is necessary for forming the dorsal axis, since experimental depletion of β-catenin transcripts with antisense oligonucleotides results in the lack of dorsal structures (Heasman et al. 1994a). Moreover, the injection of exogenous β-catenin into the ventral side of the embryo produces a secondary axis (Funayama et al. 1995; Guger and Gumbiner 1995). β-catenin is part of the Wnt signal transduction pathway and is negatively regulated by the glycogen synthase kinase 3 (GSK-3; see Chapter 6). GSK-3 also plays a critical role in axis formation by suppressing dorsal fates. Activated GSK-3 blocks axis formation when added to the egg (Pierce and Kimelman 1995; He et al. 1995; Yost et al. 1996). If endogenous GSK-3 is knocked out by a dominant negative protein in the ventral cells of the early embryo, a second axis forms (Figure 10.23E).
So how can β-catenin become localized to the future dorsal cells of the blastula? Labeling experiments (Yost et al. 1996; Larabell et al. 1997) suggest that β-catenin is initially synthesized (from maternal messages) throughout the embryo, but is degraded by GSK-3-mediated phosphorylation specifically in the ventral cells. The critical event for axis determination may be the movement of an inhibitor of GSK-3 to the cytoplasm opposite the point of sperm entry (i.e., to the future dorsal cells). One candidate for this agent is the Disheveled protein. This protein is the normal suppressor of GSK-3 in the Wnt pathway (see Figure 6.23), and it is originally found in the vegetal cortex of the unfertilized Xenopus egg. However, upon fertilization, Disheveled is translocated along the microtubular array to the dorsal side of the embryo (Figure 10.24; Miller et al. 1999). Thus, on the dorsal side of the embryo, β-catenin should be stable, since GSK-3 is not able to degrade it; while in the ventral portion of the embryo, GSK-3 should initiate the degradation of β-catenin.
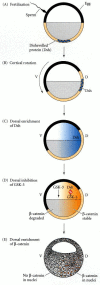
Figure 10.24
Model of the mechanism by which the Disheveled protein stabilizes β-catenin in the dorsal portion of the amphibian egg. (A) Disheveled (Dsh) associates with a particular set of proteins at the vegetal pole of the unfertilized egg. (B) Upon fertilization, (more...)
WEBSITE
10.6 GBP. In addition to Disheveled, a second inhibitor of GSK-3 has been identified in Xenopus eggs. This protein, GBP, can rescue axis formation in UV-treated eggs. http://www.devbio.com/chap10/link1006.shtml
β-catenin is a transcription factor that can associate with other transcription factors to give them new properties. It is known that Xenopus β-catenin can combine with a ubiquitous transcription factor known as Tcf3, and that a mutant form of Tcf3 lacking a β-catenin binding domain results in embryos without dorsal axes (Molenaar et al. 1996). The β-catenin/Tcf3 complex appears to bind to the promoters of several genes whose activity is critical for axis formation. One of these genes is siamois, which is expressed in the Nieuwkoop center immediately following the midblastula transition. If this gene is ectopically expressed in the ventral vegetal cells, a secondary axis emerges on the former ventral side of the embryo, and if cortical rotation is prevented, siamois expression is eliminated (Lemaire et al. 1995; Brannon and Kimelman 1996). The Tcf3 protein is thought to inhibit siamois transcription when it binds to that gene's promoters in the absence of β-catenin. However, when the Tcf3/β-catenin complex binds to its promoter, siamois is activated (Figure 10.25; Brannon et al. 1997).

Figure 10.25
Summary of events hypothesized to bring about the induction of the organizer in the dorsal mesoderm. Cortical rotation causes the translocation of Disheveled protein to the dorsal side of the embryo. Dsh binds GSK-3, thereby allowing β-catenin (more...)
The Siamois protein is critical for the expression of organizer-specific genes (Fan and Sokol 1997; Kessler 1997). Siamois protein binds to the promoter of the goosecoid gene and activates its expression (Laurent et al. 1997). The protein product of goosecoid appears to be essential for activating numerous genes in the Spemann organizer. So one could expect that the dorsal side of the embryo would contain β-catenin, that β-catenin would allow this region to express Siamois, and that Siamois would initiate the formation of the organizer. However, Siamois alone is not sufficient for generating the organizer; another protein also appears to be critical in the activation of goosecoid and the formation of the organizer. Recent studies suggest that maximum goosecoid expression occurs when there is synergism between the Siamois protein and a vegetally expressed TGF-β signal (see Chapter 6) (Brannon and Kimelman 1996). While the cortical rotation may activate the β-catenins and allow the expression of siamois in the dorsal region of the embryo, the translation of vegetally localized messages encoding a factor of the TGF-β family may generate a protein that permits the activation of goosecoid best in the cells that will become the organizer. The TGF-β family protein in the Nieuwkoop center could induce the cells in the dorsal marginal zone above them to express some transcription factor that would also bind to the promoter of the goosecoid gene and cooperate with siamois to activate it (see Figure 10.25).
The candidates for this TGF-β factor include Vg1, VegT, and Nodal-related proteins. Each of these proteins is made in the endoderm (see Figure 5.33). Agius and colleagues (2000) have provided evidence that all of these proteins may act in a pathway, and that the critical proteins are the Nodal-related factors. When they repeated the Nieuwkoop animal-vegetal recombination experiments (see Figure 10.21) but included a specific inhibitor of Nodal-related proteins, the induction by the vegetal cells failed to occur. (The inhibitor did not inhibit Vg1, VegT, or activin.) Moreover, they found that during the late blastula stages, three Nodal-related proteins (Xnr1, Xnr2, and Xnr4) are expressed in a dorsal-to-ventral gradient in the endoderm. This gradient is formed by the activation of Xenopus Nodal-related gene expression by the synergistic action of VegT and Vg1 with β-catenin. Agius and his colleagues present a model, shown in Figure 10.26, in which the dorsally located β-catenin and the vegetally located VegT and Vg1 signals interact to create a gradient of Nodal-related proteins (Xnr1, 2, 4) across the endoderm. These Nodal-related proteins specify the mesoderm such that those regions with little or no Nodal-related protein become ventral mesoderm, those regions with some Nodal protein become lateral mesoderm, and those regions with a great deal of Nodal protein become the organizer. These Nodal-related proteins will activate the goosecoid gene, and the specific inhibitor of Nodal-related proteins prevents this activation.
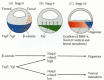
Figure 10.26
Model for mesoderm induction and organizer formation by the interaction of β-catenin and TGF-β proteins. (A) At late blastula stages, Vg1 and VegT are found in the vegetal hemisphere, while β-catenin is located in the dorsal region. (more...)
The Functions of the Organizer
While the Nieuwkoop center cells remain endodermal, the cells of the organizer become the dorsal mesoderm and migrate underneath the dorsal ectoderm. There, the dorsal mesoderm induces the central nervous system to form. The properties of the organizer tissue can be divided into five major functions:
- 1.
The ability to become dorsal mesoderm (prechordal plate, chordamesoderm, etc.)
- 2.
The ability to dorsalize the surrounding mesoderm into lateral mesoderm (when it would otherwise form ventral mesoderm)
- 3.
The ability to dorsalize the ectoderm into neural ectoderm
- 4.
The ability to initiate the movements of gastrulation
- 5.
The ability to cause the neural plate (the induced neural ectoderm) to become the neural tube
In Xenopus (and other vertebrates), the formation of the anterior-posterior axis follows the formation of the dorsal-ventral axis. Once the dorsal portion of the embryo is established, the movement of the involuting mesoderm establishes the anterior-posterior axis. The mesoderm that migrates first through the dorsal blastopore lip gives rise to the anterior structures; the mesoderm migrating through the lateral and ventral lips forms the posterior structures.
It is now thought that the cells of the organizer ultimately contribute to four cell types—pharyngeal endoderm, head mesoderm (prechordal plate), dorsal mesoderm (primarily the notochord), and the dorsal blastopore lip (Keller 1976; Gont et al. 1993). The pharyngeal endoderm and prechordal plate lead the migration of the organizer tissue and appear to induce the forebrain and midbrain. The dorsal mesoderm induces the hindbrain and trunk. The dorsal blastopore lip forms the dorsal mesoderm and eventually becomes the chordaneural hinge that induces the tip of the tail.
When the organizer was first described, it started one of the first truly international scientific research programs: the search for the organizer molecules. Researchers from Britain, Germany, France, the United States, Belgium, Finland, Japan, and the Soviet Union all tried to find these remarkable substances (see Gilbert and Saxén 1993). R. G. Harrison (quoted by Twitty 1966, p. 39) referred to the amphibian gastrula as the “new Yukon to which eager miners were now rushing to dig for gold around the blastopore.” Unfortunately, their picks and shovels proved too blunt to uncover the molecules involved. The analysis of organizer molecules had to wait until recombinant DNA technologies enabled investigators to make cDNA clones from blastopore lip mRNA and to see which of these clones encoded factors that could dorsalize the embryo.
WEBSITE
10.7 Early attempts to locate the organizer molecules. While Spemann did not believe that molecules alone could organize the embryo, his students began a long quest for these factors. http://www.devbio.com/chap10/link1007.shtml
The formation of the dorsal (organizer) mesoderm involves the activation of several genes. The secreted proteins of the Nieuwkoop center are thought to activate a set of transcription factors in the mesodermal cells above them. These transcription factors then activate the genes encoding the secreted products of the organizer. Several organizer-specific transcription factors have been found and are listed in Table 10.2.
Table 10.2
Proteins expressed solely or almost exclusively in the organizer (partial list).
As mentioned earlier, one of the important targets of the Nieuwkoop center appears to be the goosecoid gene. The area of expression of goosecoid mRNA correlates with the organizer domain in both normal and experimentally treated animals. When lithium chloride treatment is used to increase the organizer mesoderm throughout the marginal zone, the expression of goosecoid likewise is expanded. Conversely, when eggs are treated with UV light prior to first cleavage, both dorsal-anterior induction and goosecoid expression are significantly inhibited. Injection of the full-length goosecoid message into the two ventral blastomeres of a 4-cell Xenopus embryo causes the progeny of those blastomeres to involute, undergo convergent extension, and form the dorsal mesoderm and head endoderm of a secondary axis (Figure 10.27; Niehrs et al. 1993). Labeling experiments (Niehrs et al. 1993) have shown that such goosecoid-injected cells are able to recruit neighboring host cells into the dorsal axis as well. Thus, the Nieuwkoop center activates the goosecoid gene in the organizer tissues, and this gene encodes a DNA-binding protein that (1) activates the migration properties (involution and convergent extension) of the dorsal blastopore lip cells, (2) autonomously determines the dorsal mesodermal fates of those cells expressing it, and (3) enables the goosecoid-expressing cells to recruit neighboring cells into the dorsal axis. Goosecoid also has been found to activate Xotx2, a gene that is critical for brain formation, in the anterior mesoderm and in the presumptive brain ectoderm (Blitz and Cho 1995).

Figure 10.27
Ability of goosecoid mRNA to induce a new axis. (A) At the gastrula stage, a control embryo (either uninjected or given an injection of goosecoid-like mRNA but lacking the homeobox) has one dorsal blastopore lip. (B) An embryo whose ventral vegetal blastomeres (more...)
The diffusible proteins of the organizer I: the BMP inhibitors
Goosecoid protein works in the nucleus. It must activate (either directly or indirectly) those genes encoding the soluble proteins that function to organize the dorsal-ventral and anterior-posterior axis. Early evidence for diffusible signals from the notochord came from several sources. First, Hans Holtfreter (1933) showed that if amphibian embryos are placed in a high salt solution, the mesoderm will evaginate rather than invaginate, and will not underlie the ectoderm. Such ectoderm is not underlain by the notochord, and it does not form neural structures. Further evidence for soluble factors came from the transfilter studies of Finnish investigators (Saxén 1961; Toivonen et al. 1975; Toivonen and Wartiovaara 1976). Newt dorsal lip tissue was placed on one side of a filter thin enough so that no processes could fit through the pores, and competent gastrula ectoderm was placed on the other side. After several hours, neural structures were observed in the ectodermal tissue (Figure 10.28). The identities of the factors diffusing from the organizer, however, took another quarter of a century to identify.
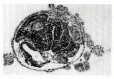
Figure 10.28
Neural structures induced in presumptive ectoderm by newt dorsal lip tissue, separated from the ectoderm by a Nucleopore filter with an average pore diameter of 0.05 mm. Anterior neural tissues are evident, including some induced eyes. (From Toivonen (more...)
Recent studies on induction have resulted in a remarkable and non-obvious conclusion: The ectoderm is actually induced to become epidermal. The agents of this induction are bone morphogenetic proteins (BMPs). The nervous system forms from that region of the ectoderm that is protected from this epidermal induction (Hemmati-Brivanlou and Melton 1997). In other words, (1) the “default fate” of the ectoderm is to become neural; (2) certain parts of the embryo induce the ectoderm to become epidermal tissue, and (3) the organizer tissues act by secreting molecules that block this induction, thereby allowing the ectoderm “protected” by these factors to become neural.
Noggin
In 1992, the first of the soluble organizer molecules was isolated. Smith and Harland (1992) constructed a cDNA plasmid library from dorsalized (lithium chloride-treated) gastrulae. RNAs synthesized from sets of these plasmids were injected into ventralized embryos (having no neural tube) produced by irradiating early embryos with ultraviolet light. Those sets of plasmids whose RNAs rescued the dorsal axis in these embryos were split into smaller sets, and so on, until single-plasmid clones were isolated whose mRNAs were able to restore the dorsal axis in such embryos. One of these clones contained the noggin gene (Figure 10.29). Smith and Harland (1992) have shown that newly transcribed (as opposed to maternal) noggin mRNA is first localized in the dorsal blastopore lip region and then becomes expressed in the notochord (Figure 10.30). Injection of noggin mRNA into 1-cell, UV-irradiated embryos completely rescued the dorsal axis and allowed the formation of a complete embryo. The mRNA sequence for the Noggin protein suggests strongly that it is a secreted protein. In 1993, Smith and his colleagues found that Noggin could accomplish two of the major functions of the Spemann-Mangold organizer: it induced dorsal ectoderm to form neural tissue, and it dorsalized mesoderm cells that would otherwise contribute to the ventral mesoderm. Noggin binds to BMP4 and BMP2 and inhibits their binding to receptors (Zimmerman et al. 1996).

Figure 10.29
Rescue of dorsal structures by Noggin protein. When Xenopus eggs are exposed to ultraviolet radiation, cortical rotation fails to occur, and the embryos lack dorsal structures (top). If such an embryo is injected with noggin mRNA, it develops dorsal structures (more...)

Figure 10.30
Localization of the noggin mRNA in the organizer tissue, shown by in situ hybridization. (A) At gastrulation, noggin message (dark areas) accumulates in the dorsal marginal zone. (B) When cells involute, noggin mRNA is seen in the dorsal blastopore lip. (more...)
Chordin and nodal-related 3
The second organizer protein found was chordin. It was isolated from clones of cDNA whose mRNAs were present in dorsalized, but not in ventralized, embryos (Sasai et al. 1994). These clones were tested by injecting them into ventral blastomeres and seeing whether they induced secondary axes. One of the clones capable of inducing a secondary neural tube contained the chordin gene. The chordin mRNA was found to be localized in the dorsal blastopore lip and later in the dorsal mesoderm of the notochord (Figure 10.31). Like Noggin, chordin binds directly to BMP4 and BMP2 and prevents their complexing with their receptors (Piccolo et al. 1996). In zebrafish, a loss-of-function mutation of chordin (the “chordino” mutant) has a greatly reduced neural plate and an enlarged region of ventral mesoderm (Hammerschmidt et al. 1996). Nodal-related-3 (Xnr-3) is synthesized by the superficial cells of the organizer and is also able to block BMP4 (Smith et al. 1995; Hansen et al. 1997).

Figure 10.31
Chordin mRNA localization. (A) Whole-mount in situ hybridization shows that just prior to gastrulation, chordin message (dark area) is expressed in the region that will become the dorsal blastopore lip. (B) As gastrulation begins, Chordin is expressed (more...)
Follistatin
The fourth organizer-secreted protein, follistatin, was found in the organizer through an unexpected result of an experiment that was looking for something else. Ali Hemmati-Brivanlou and Douglas Melton (1992, 1994) wanted to see whether the protein activin was crucial for mesoderm induction, so they constructed a dominant negative activin receptor and injected it into Xenopus embryos. Remarkably, the ectoderm of these embryos began to express neural-specific proteins. It appeared that the activin receptor (which also binds other structurally similar molecules such as the bone morphogenetic proteins) normally functioned to bind an inhibitor of neurulation. When its function was blocked, all the ectoderm became neural. In 1994, Hemmati-Brivanlou and Melton proposed a “default model of neurulation” whereby the organizer functioned by producing inhibitors of whatever was blocking neurulation. That is to say, the “normal” fate of an ectodermal cell was to become a neuron; it had to be induced to become an epidermal skin cell. The organizer somehow prevented the ectodermal cells from being induced. This model was supported by, and explained, some cell dissociation experiments that had also produced odd results. Three studies, by Grunz and Tacke (1989), Sato and Sargent (1989), and Godsave and Slack (1989) had shown that when whole embryos or their animal caps were dissociated, they formed neural tissue. This result would be explainable if the “default state” of the ectoderm was not epidermal, but neural, and the tissue had to be induced to have an epidermal phenotype. The organizer, then, would block this epidermalizing induction.
Since the naturally occurring protein follistatin binds to and inhibits activin (and other related proteins), it was hypothesized that it might be one of the factors secreted by the organizer. Using in situ hybridization, Hemmati-Brivanlou and Melton (1994) found the mRNA for follistatin in the dorsal blastopore lip and notochord.
So it appeared that there might be a neural default state and an actively induced epidermal fate. This hypothesis was counter to the neural induction model that had preceded it for 70 years. But what proteins were inducing the epidermis, and were they really being blocked by the molecules secreted by the organizer?
The leading candidate for the epidermal inducer is bone morphogenesis protein-4 (BMP4). It was known that there is an antagonistic relationship between BMP4 and the organizer. If the mRNA for BMP4 is injected into Xenopus eggs, all the mesoderm in the embryo becomes ventrolateral mesoderm, and no involution occurs at the blastopore lip (Dale et al. 1992; Jones et al. 1992). Conversely, overexpression of a dominant negative BMP4 receptor resulted in the formation of two dorsal axes (Graff et al. 1994; Suzuki et al. 1994). In 1995, Wilson and Hemmati-Brivanlou demonstrated that BMP4 induced ectodermal cells to become epidermal. By 1996, several laboratories had demonstrated that Noggin, chordin, and follistatin each was secreted by the organizer and that each prevented BMP from binding to the ectoderm and mesoderm near the organizer (Piccolo et al. 1996; Zimmerman et al. 1996; Iemura et al. 1998).
When BMP binds to ectodermal cells, it activates the expression of genes such as msx1, which induce the expression of epidermal-specific genes, while inhibiting those genes that would produce a neural phenotype (Suzuki et al. 1997b). In the mesoderm, BMP4 activates genes such as Xvent1, which give the mesoderm a ventral phenotype. Low doses of BMP4 appear to activate muscle formation; intermediate levels instruct cells to become kidney; and high doses activate those genes that instruct the mesoderm to become blood cells (Hemmati-Brivanlou and Thomsen 1995; Gawantka et al. 1995; Dosch et al. 1997). The varying doses are created by the interaction of BMP4 (coming from the ventral and lateral mesoderm) with the BMP antagonists coming from the organizer§ (Figure 10.32).

Figure 10.32
Model for the action of the organizer. (A) BMP4 (and certain other molecules) is a powerful ventralizing factor. Organizer proteins such as Chordin, Noggin, and Follistatin block the action of BMP4. The antagonistic effects of these proteins can be seen (more...)
Thus, by 1996, there was a consensus that BMP4 was the active inducer of ventral ectoderm (epidermis) and the ventralizer of the mesoderm (blood cells and connective tissue), and that Noggin, chordin, and follistatin could prevent its function. The organizer worked by secreting inhibitors of BMP4, not by directly inducing neurons.
The diffusible proteins of the organizer II: the Wnt inhibitors
It had been thought that all the neural tissue induced by the organizer was induced to become forebrain, and that the notochord represented the most anterior portion of the organizer. However, the most anterior regions of the head and brain are underlain not by notochord, but by pharyngeal endoderm and head (prechordal) mesoderm. This “endomesoderm” constitutes the leading edge of the dorsal blastopore lip. Recent studies have shown that these cells not only induce the most anterior head structures, but that they do it by blocking the Wnt pathway as well as blocking BMP4.
Cerberus
In 1993, Christian and Moon showed that Xwnt8, a member of the Wnt family of growth and differentiation factors, inhibited neural induction. It was found to be synthesized throughout the marginal mesoderm—except in the region forming the dorsal lip. Thus, in addition to BMP4, there was a second anti-neuralizing protein being secreted from the nonorganizer mesoderm. Were there any proteins in the organizer that countered this activity?
In 1996, Bouwmeester and colleagues showed that the induction of the most anterior head structures could be accomplished by a secreted protein called Cerberus.¶ Unlike the other proteins secreted by the organizer, Cerberus promotes the formation of the cement gland (the most anterior region of tadpole ectoderm), eyes, and olfactory (nasal) placodes. When cerberus mRNA was injected into a vegetal ventral Xenopus blastomere at the 32-cell stage, ectopic head structures were formed (Figure 10.34). These head structures were made from the injected cell as well as from neighboring cells. The cerberus gene is expressed in the pharyngeal endomesoderm cells that arise from the deep cells of the early dorsal lip, and the Cerberus protein can bind both BMPs and Xwnt8 (Glinka et al. 1997; Piccolo et al. 1999).
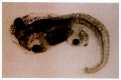
Figure 10.34
Cerberus mRNA injected into a single D4 (ventral vegetal) blastomere of a 32-cell Xenopus embryo induces head structures as well as a duplicated heart and liver. The secondary eye (a single cyclopic eye) and olfactory placode can be readily seen. (From (more...)
Frzb and Dickkopf
Shortly after the attributes of Cerebus were demonstrated, two other proteins, Frzb and Dickkopf, were discovered to be synthesized in the involuting endomesoderm. Frzb (pronounced “frisbee”) is a small, soluble form of Frizzled, the Wnt receptor, that is capable of binding Wnt proteins in solution (Figure 10.35; Leyns et al. 1997; Wang et al. 1997). It is synthesized predominantly in the endomesoderm cells beneath the head (Figure 10.36A). If embryos are made to synthesize excess Frzb, Wnt signaling fails to occur, and the embryos lack ventral posterior structures, becoming solely head (Figure 10.36B). The Dickkopf (German; “big head,” “stubborn”) protein also appears to interact directly with Wnt proteins extracellularly. Injection of antibodies against Dickkopf protein into the blastocoel causes the resulting embryos to have small, deformed heads with no forebrain (Glinka et al. 1998).
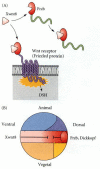
Figure 10.35
Xwnt8 is capable of ventralizing the mesoderm and preventing anterior head formation in the ectoderm. (A) Frzb is a protein secreted by the anterior region of the organizer. It binds to Xwnt8 before that inducer can bind to its receptor. Frzb resembles (more...)

Figure 10.36
Frzb expression and function. (A) Double in situ hybridization localizing frzb (dark blue) and chordin (brown) messages. frzb mRNA can be seen to be transcribed in the head endomesoderm of the organizer, but not in the notochord (where chordin is expressed). (more...)
Glinka and colleagues (1997) have thus proposed a new model for embryonic induction. The induction of trunk structures may be caused by the blockade of BMP signaling from the notochord. However, to produce a head, both the BMP signal and the Wnt signal must be blocked. This blockade comes from the endomesoderm, now considered the most anterior portion of the organizer.
Conversion of the ectoderm into neural plate cells
So far, we have discussed the factors that prevent the dorsal ectoderm from becoming epidermis. Obviously, once that is accomplished, other genes must transform the ectoderm into neural tissue. The key protein involved in activating the neural phenotype in the ectoderm appears to be neurogenin (Ma et al. 1996). The transcription factors that appear in the ectoderm in the absence of BMP are able to induce the expression of neurogenin, and the transcription factors (such as Msx1) induced in the ectoderm by BMP signals are able to repress neurogenin expression (Figure 10.37; see Sasai 1998). Neurogenin is itself a transcription factor, and it activates a series of genes whose products are responsible for the neural phenotype. One of the genes activated by neurogenin is the gene for NeuroD, a transcription factor that activates the genes producing the structural neural-specific proteins (Lee et al. 1995). In addition, Noggin or Cerberus can induce another transcription factor in the ectoderm. This protein is called Xenopus brain factor-2 (XBF-2), and it appears to repress the epidermal genes (Mariani and Harland 1998). By these pushes and pulls, the dorsal ectoderm is converted into neural plate tissue.
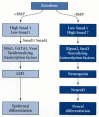
Figure 10.37
Hypothetical pathways differentiating ectoderm into epidermis or neural ectoderm. In the presence of BMP signaling, epidermalizing transcription factors are generated, leading to the activation of the pathway enabling the cell to become an epidermal keratinocyte. (more...)
WEBSITE
10.8 The autonomous specification of the endoderm. While the mesoderm is induced, and the differences between neural and epidermal ectoderm are induced, the endoderm appears to be specified autonomously. Recent studies are investigating how this is done. http://www.devbio.com/chap10/link1008.shtml
The Regional Specificity of Induction
The determination of regional differences
One of the most fascinating phenomena in neural induction is the regional specificity of the neural structures that are produced. Forebrain, hindbrain, and spinocaudal regions of the neural tube must all be properly organized in an anterior-to-posterior direction. The organizer tissue not only induces the neural tube, but also specifies the regions of the neural tube. This region-specific induction was demonstrated by Hilde Mangold's husband, Otto Mangold (1933). He transplanted four successive regions of the archenteron roof of late-gastrula newt embryos into the blastocoels of early-gastrula embryos (Figure 10.39). The most anterior portion of the archenteron roof induced balancers and portions of the oral apparatus (Figure 10.39A); the next most anterior section induced the formation of various head structures, including nose, eyes, balancers, and otic vesicles (Figure 10.39B); the third section induced the hindbrain structure (Figure 10.39C); and the most posterior section induced the formation of dorsal trunk and tail mesoderm‖ (Figure 10.39D). Moreover, when dorsal blastopore lips from early salamander gastrulae were transplanted into other early salamander gastrulae, they formed secondary heads. When dorsal lips from later gastrulas were transplanted into early salamander gastrulae, however, they induced the formation of secondary tails (Figure 10.40; Mangold 1933). These results show that the first cells of the organizer to enter the embryo induce the formation of brains and heads, while those cells that form the dorsal lip of later-stage embryos induce the cells above them to become spinal cords and tails.

Figure 10.39
Regional specificity of induction can be demonstrated by implanting different regions (color) of the archenteron roof into early Triturus gastrulae. The resulting embryos develop secondary dorsal structures. (A) Head with balancers. (B) Head with balancers, (more...)

Figure 10.40
Regionally specific inducing action of the dorsal blastopore lip. (A) Young dorsal lips (which will form the anterior portion of the organizer) induce anterior dorsal structures when transplanted into early newt gastrulae. (B) Older dorsal lips transplanted (more...)
Molecular correlates of neural caudalization
In the 1950s, evidence accumulated for the existence of two gradients in amphibian embryos: a dorsal gradient of “neuralizing” activity and a caudal gradient of posteriorizing (“mesodermalizing”) activity (Nieuwkoop 1952; Toivonen and Saxén 1955). The neuralizing activity came from the organizer and induced the ectoderm to be neural. The posteriorizing activity originated in the posterior of the embryo and weakened anteriorly. Recent studies have extended this model and provided candidates for the posteriorizing molecules. As predicted, the two signaling systems—neuralizing and posteriorizing—work independently (Kolm and Sive 1997). Chordin, Noggin, and the other molecules discussed above constitute the neuralizing factors secreted by the organizer. The candidates for the posteriorizing factor include eFGF, retinoic acid, and Wnt3a.
WEBSITE
10.10 Regional specification. The research into regional specification has been a fascinating endeavor involving scientists from all over the world. Before molecular biology gave us the tools to uncover morphogenetic proteins, embryologists developed ingenious ways of finding out what those proteins were doing. http://www.devbio.com/chap10/link1010.shtml
eFGF
Fibroblast growth factors are able to turn anterior neural tissue into posterior neural tissue. When early-gastrula ectoderm (which has not yet been underlain by dorsal mesoderm) was isolated and neuralized by Noggin, Chordin, or Follistatin, anterior-type neural markers were found in that tissue. However, when isolated early-gastrula ectoderm was incubated with a neural inducer plus FGF2, it expressed more posterior neural markers. FGF2 will also induce forebrain tissue to express hindbrain-specific genes (Cox and Hemmati-Brivanlou 1995; Lamb and Harland 1995). Furthermore, when FGF signaling is blocked in vivo by a dominant negative FGF receptor, the resulting tadpoles lack their posterior segments (Amaya et al. 1991). FGF2 is probably not the natural posteriorizing FGF in Xenopus, since it is not secreted by the embryo at this site, and it is not localized to any side of the embryo. However, embryonic FGF (eFGF, a Xenopus FGF similar to mammalian FGF4) is found in Xenopus posterior and tailbud mesoderm, and it has the same effects as FGF2 (Isaacs et al. 1992). Overexpression of eFGF up-regulates several posteriorly expressed genes, including the Xenopus homologue of caudal. These genes, in turn, encode proteins that appear to regulate the Hox genes controlling the specification of body segments along the anterior-posterior axis. This leads to the more posterior specification of the caudal nervous system (Pownall et al. 1996). Interestingly, eFGF may be induced by the posterior notochord (Taira et al. 1997).
Retinoic acid
Retinoic acid is also likely to play a role in posteriorizing the neural tube. If Xenopus neurulae are treated with nanomolar to micromolar concentrations of retinoic acid (RA), their forebrain and midbrain development is impaired in a concentration-dependent fashion (Figure 10.41; Papalopulu et al. 1991; Sharpe 1991). When lower concentrations are used, the actual induction of neural tissue does not appear to be inhibited, but fewer forebrain messages and structures are produced (Durston et al. 1989; Sive et al. 1990). Retinoic acid appears to affect both the mesoderm and the ectoderm. Ruiz i Altaba and Jessell (1991) found that anterior dorsal mesoderm from RA-treated gastrulae was unable to induce head structures in host embryos, and Sive and Cheng (1991) found that RA-treated ectoderm was unable to respond to the anterior-inducing mesoderm of untreated gastrulae. An RA gradient (tenfold higher in the posterior than in the anterior) has been detected in the dorsal mesoderm of early Xenopus neurulae (Chen et al. 1994). Like eFGF, retinoic acid has been shown to activate the expression of more posterior Hox genes (Cho et al. 1991b; Sive and Cheng 1991; Kolm and Sive 1997).
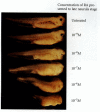
Figure 10.41
Effects of RA and FGF on the anterior-posterior axis. Late neurula embryos exposed to progressively higher concentrations of retinoic acid (and allowed to grow until the controls reached tadpole stage) lose posterior regions of the embryo (as well as (more...)
Wnt3a
Another candidate for the caudalizing factor is Xenopus Wnt3a (McGrew et al. 1995). This protein is found in the neural ectoderm of the early neurula. When ectoderm is isolated from Xenopus gastrulae but remains connected to the dorsal blastopore lip, the ectoderm develops an anterior-posterior array of neural markers. If the embryo is first injected with Xwnt3a RNA (causing the overexpression of this protein), the anterior markers are lost. The basic model of neural induction, then, looks like the diagram in Figure 10.42.

Figure 10.42
Model for the organizer function and axis specification in Xenopus gastrula. (1) BMP inhibitors from organizer tissue (dorsal mesoderm and pharyngeal mesendoderm) block the formation of epidermis, ventrolateral mesoderm, and ventrolateral endoderm. (2) (more...)
The relationship between these three pathways of posteriorization has yet to be worked out. They may play different roles in different regions of the embryo, and they may work together. Retinoic acid has its major effect on hindbrain patterning, while eFGF is more important in the regionalization of the spinal cord (Blumberg et al. 1997; Kolm et al. 1997; Godsave et al. 1998). Wnt3a may suppress anterior genes and be a permissive factor allowing the other two proteins to function (McGrew et al. 1997).
Specifying the left-right axis
While the developing tadpole looks symmetrical from the outside, there are several internal organs, such as the heart and the gut tube, that are not evenly balanced on the right and left sides. In other words, in addition to its dorsal-ventral and anterior-posterior axes, the embryo has a left-right axis. Somehow, the body must be given clues as to which half is right and which half is left.
In all vertebrates studied so far, the crucial event in left-right axis formation is the expression of a nodal gene in the lateral plate mesoderm on the left side of the embryo. In Xenopus, this gene is Xnr-1 (Xenopus nodal-related-1). If the expression of this gene is also permitted to occur on the right-hand side, the position of the heart (which is normally on the left side) and the coiling of the gut are randomized.
But what causes the expression of Xnr-1 solely on the left-hand side? In Xenopus, it is possible that the first clue is given at fertilization. The microtubules involved in cytoplasmic rotation appear to be crucial, since if their formation is blocked, no left-right axis appears (Yost 1998). Moreover, the Vg1 protein, which appears to be expressed throughout the vegetal hemisphere, seems to be processed into its active form predominantly on the left-hand side of the embryo. Injecting active Vg1 protein into the left vegetal blastomeres has no effect, but adding it to the right vegetal blastomeres leads to the expression of Xnr-1 in both the right and left lateral plate and to the randomization of heart and gut positions (Hyatt et al. 1996). Moreover, when active Vg1 protein is injected into a particular right-side vegetal blastomere (the third vegetal cell to the right of the dorsal midline), the entire left-right axis is inverted. No other TGF-β family member is able to accomplish this reversal (Hyatt and Yost 1998). It is not yet known how the events at fertilization lead to the expression of Xnr-1 in the left lateral plate mesoderm during gastrulation.
The pathway by which the Xnr-1 protein instructs the heart and gut to fold properly is also unknown, but one of the key genes activated by Xnr-1 appears to be pitx2. Since this gene is activated by Xnr-1, it is normally expressed only on the left side of the embryo. However, if the Pitx2 protein is injected into the right side, too, the placement of the heart and the coiling of the gut are randomized (Figure 10.43; Ryan et al. 1998). Pitx2 persists on the left side of the embryo as the heart and gut develop, controlling their respective positions. Pitx2 may be at the “heart of the heart” (Strauss 1998).
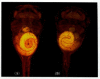
Figure 10.43
Pitx2 determines the direction of heart looping and gut coiling. (A) Wild-type (stage 45) Xenopus tadpole viewed from the ventral side, showing rightward heart looping and counterclockwise gut coiling. (B) If an embryo is injected with Pitx2 so that this (more...)
We are finally putting names to the “agents” and “soluble factors” of the experimental embryologists. We are finally delineating the intercellular pathways of paracrine factors and transcription factors that constitute the first steps in the processes of organogenesis. The international research program initiated by Spemann's laboratory in the 1920s is reaching toward a conclusion. But this research has found levels of complexity far deeper than Spemann would have conceived, and just as Spemann's experiments told us how much we didn't know, so today, we are faced with a whole new set of problems generated by our solutions to older ones.
Surveying the field in 1927, Spemann remarked:
We still stand in the presence of riddles, but not without hope of solving them. And riddles with the hope of solution—what more can a scientist desire?
The challenge still remains.
Snapshot Summary: Early Development and Axis Formation in Amphibians
- 1.
Amphibian cleavage is holoblastic, but unequal due to the presence of yolk in the vegetal hemisphere.
- 2.
Amphibian gastrulation begins with the invagination of the bottle cells, followed by the coordinated involution of the mesoderm and the epiboly of the ectoderm. Vegetal rotation plays a significant role in directing the involution.
- 3.
The driving forces for ectodermal epiboly and the convergent extension of the mesoderm are the intercalation events in which several tissue layers merge. Fibronectin plays a critical role in enabling the mesodermal cells to migrate into the embryo.
- 4.
The dorsal lip of the blastopore forms the organizer tissue of the amphibian gastrula. This tissue dorsalizes the ectoderm, transforming it into neural tissue, and it transforms ventral mesoderm into lateral mesoderm.
- 5.
The organizer consists of pharyngeal endoderm, head mesoderm, notochord, and dorsal blastopore lip. The organizer functions by secreting proteins (Noggin, chordin, and follistatin) that block the BMP signal that would otherwise ventralize the mesoderm and activate the epidermal genes in the ectoderm.
- 6.
In the head region, an addition set of proteins (Cerberus, Frzb, Dickkopf) block the Wnt signal from the ventral and lateral mesoderm.
- 7.
The organizer is itself induced by the Nieuwkoop center, located in the dorsalmost vegetal cells. This center is formed by cortical rotation during fertilization, which translocates the Dishevelled protein to the dorsal side of the egg.
- 8.
The Dishevelled protein stabilizes β-catenin in the dorsal cells of the embryo. Thus, the Nieuwkoop center is formed by the accumulation of β-catenin, which can complex with Tcf3 to form a transcription factor complex that can activate the transcription of the siamois gene.
- 9.
The siamois product and a TGF-β signal (perhaps from Vg1) can activate the goosecoid gene in the organizer. The goosecoid gene can activate other genes that cause the organizer to function.
- 10.
Other posteriorizing signals (Wnt3a, retinoic acid, eFGF) can influence the anterior-posterior specification of the neural tube.
- 11.
The left-right axis appears to be initiated at fertilization through the Vg1 protein. In a still unknown fashion, this protein activates a Nodal protein solely on the left side of the body. As in other vertebrates, the Nodal protein activates expression of Pitx2, which is critical in distinguishing left-sidedness from right-sidedness in the heart and gut tubes.
Box
BMP4 and Geoffroy's Lobster.
Competence, Bias, and Neurulation
In addition to the signals coming from the underlying chordal plate and dorsal mesoderm, there may also be a bias in the cells of the dorsal part of the embryo toward becoming neural. Phillips and colleagues (London et al. 1988; Savage and Phillips 1989) have shown that the dorsal and ventral animal pole cells of the early-cleavage embryo differ in their expression of the Epi1 protein. Not only do the presumptive epidermal cells express this protein, which is not expressed in the presumptive neural cells, but the region of cells failing to express Epi1 increases during gastrulation. Moreover, in the ventral mesoderm, proteins encoded by Xvex-1 and Xvex-2 block the expression of dorsal genes (Shapira et al. 2000). Other differences between dorsal and ventral ectoderm also become apparent at this time, prior to the notochord's movement beneath the ectoderm (Otte and Moon 1992). The cues for this “bias” toward neurulation may be provided by signals from the dorsal lip traveling in a planar (horizontal) fashion through the ectoderm (Figure 10.38; Sharpe et al. 1987; Dixon and Kintner 1989; Doniach et al. 1993). The molecular basis of this planar “ectodermal” signaling system is not known. It is possible that it is more important in some species than in others. ▪
WEBSITE
10.9 Planar induction. Several studies suggest that the planar mode of signal transduction—from the dorsal lip through the ectoderm—may also help pattern the ectoderm. Other studies argue against planar induction playing any role in patterning the ectoderm. http://www.devbio.com/chap10/link1009.shtml

Figure 10.38
Ectodermal bias toward neurulation. (A) Neural gene expression occurs more readily in dorsal ectoderm than in ventral ectoderm. Ectoderm fragments from the dorsal and ventral thirds of a Xenopus embryo were wrapped around dorsal mesoderm (notochord) tissue. (more...)
Footnotes
- *
Hilde Proescholdt Mangold died in a tragic accident in 1924, when her kitchen's gasoline heater exploded. At the time she was 26 years old, and her paper was just being published. Hers is one of the very few doctoral theses in biology that have directly resulted in the awarding of a Nobel Prize. For more information about Hilde Mangold and her times and the experiments that identified the organizer, see Hamburger 1984, 1988, and Fässler and Sander 1996.
- †
It was fortunate that Spemann's laboratory, and those of his students, usually used salamander embryos for their experiments. It turns out that frog ectoderm is much more difficult to induce than that of these urodeles.
- ‡
This classic term has been a source of confusion because the induction of the neural tube by the notochord is no longer considered the first inductive process in the embryo. We will soon discuss inductive events that precede this “primary” induction.
- §
The details of BMP4 production and degradation are discussed in Chapter 22.
- ¶
“Cerberus” is another name out of Greek mythology; the protein is named after the three-headed dog that guarded the entrance to Hades.
- ‖
The induction of dorsal mesoderm—rather than the dorsal ectoderm of the nervous system—by the posterior end of the notochord was confirmed by Bïjtel (1931) and Spofford (1945), who showed that the posterior fifth of the neural plate gives rise to tail somites and the posterior portions of the pronephric kidney duct.
- Axis Formation in Amphibians: The Phenomenon of the Organizer - Developmental Bi...Axis Formation in Amphibians: The Phenomenon of the Organizer - Developmental Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...