Apart from any fair dealing for the purposes of research or private study, criticism or review, as permitted under the Copyright, Designs and Patents Act, 1988, no part of this publication may be reproduced, stored or transmitted in any form or by any means, without the prior written permission of the publisher or, in the case of reprographic reproduction, in accordance with the terms of licences issued by the Copyright Licensing Agency in the UK. Enquiries concerning reproduction outside the terms stated here should be sent to the publisher at the UK address printed on this page.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Clinical Guideline Centre (UK). Alcohol Use Disorders: Diagnosis and Clinical Management of Alcohol-Related Physical Complications [Internet]. London: Royal College of Physicians (UK); 2010. (NICE Clinical Guidelines, No. 100.)
August 2019: Some glossary terms were updated by NICE, and the recommended alcohol units for men and women were updated in line with advice from the UK Chief Medical Officer.

Alcohol Use Disorders: Diagnosis and Clinical Management of Alcohol-Related Physical Complications [Internet].
Show details2.1. Admission to hospital
2.1.1. Clinical Introduction
Some drinkers that consume alcohol in quantities outside healthy limits will develop an acute alcohol withdrawal syndrome when they abruptly stop or substantially reduce their alcohol consumption. Most patients manifest a minor symptom complex or syndrome, which may start as early as six to eight hours after an abrupt reduction in alcohol intake. It may include any combination of generalized hyperactivity, anxiety, tremor, sweating, nausea, retching, tachycardia, hypertension and mild pyrexia. These symptoms usually peak between 10 to 30 hours and subside by 40 to 50 hours. Seizures may occur in the first 12 to 48 hours and only rarely after this. Auditory and visual hallucinations may develop; these are characteristically frightening and may last for five to six days.
Delirium tremens (DTs) occurs uncommonly, perhaps in less than 5% of individuals withdrawing from alcohol. The syndrome usually starts some 48 to 72 hours after cessation of drinking and is characterized by coarse tremor, agitation, fever, tachycardia, profound confusion, delusions and hallucinations. Convulsions may herald the onset of the syndrome but are not part of the symptom complex. Hyperpyrexia, ketoacidosis, and profound circulatory collapse may develop.
Minor degrees of alcohol withdrawal are commonly encountered and individuals can be managed without recourse to specific therapy. However, patients with moderate or severe alcohol withdrawal symptoms often require sedation to prevent exhaustion and injury.
Evidence of physical dependence should always be sought because of the management implications; early morning retching, tremor, anxiety and irritability, ingestion of alcohol before midday, amnesia and "blackouts" are all suggestive. A history of previous withdrawal seizures and the development of delirium tremens clearly indicate a history of dependence. Guidance regarding diagnosis of dependence will be included in ‘Alcohol use disorders: diagnosis and clinical management of harmful drinking and alcohol dependence’ (NICE clinical guideline in development). Individuals who are known or are suspected of being dependent on alcohol may require help to withdraw from alcohol.
For the purposes of this guideline, medically-assisted withdrawal from alcohol with be referred to as (i) planned, which as the name implies is an elective process which is usually undertaken in the community or else as part of a planned programme within addiction services; or (ii) unplanned which occurs when patients stop or suddenly reduce their alcohol intake either inadvertently because of an intercurrent illness, because they make a conscious decision to stop or were inadvertently deprived of alcohol, for example, following an accident. These patients may present to their GP or to acute hospital or mental health services.
Making the decision about whether a person presenting with alcohol withdrawal needs admission to hospital is impacted by the severity of the syndrome, the person’s co-morbidities and the reason for the presentation. The severity of the syndrome can be assessed by experienced clinical staff. There are also well-recognised validated scoring systems to aid assessment of alcohol withdrawal. The most widely recognized is the CIWA-Ar (Clinical Institute of Withdrawal Assessment for Alcohol scale) which is used in the clinical setting and in research studies where a validated score is useful8. If the reason for presentation is an intercurrent illness that of itself requires admission, then the decision is made and the management of the withdrawal will occur in tandem. Very often however, the withdrawal symptoms are not life threatening and are the sole reason for presentation and there exists variation in admission practices for this cohort across the United Kingdom.
There is no doubt that some patients who wish to stop drinking but who have difficulty accessing the required services will deliberately stop drinking in order to gain admission to hospital to complete the process.
The decision whether patients with acute alcohol withdrawal need admission depends on a variety of factors. The first consideration would be the effectiveness of a hospital admission for medically-assisted withdrawal from alcohol; not only in managing the acute condition, but also in terms of facilitating long term abstinence. This will, in turn, depend on the local availability of, or liaison with, follow-up services aimed at relapse prevention. The second would be the risks involved with discharging the patient with a view to subsequent admission for elective withdrawal versus an immediate admission to complete the withdrawal process. This is of particular importance if it could be shown that elective or planned alcohol withdrawal is more effective. Given that many of these patients will undergo more than one medically-assisted withdrawal from alcohol, the risk of repeating this process is critical. One such proposed risk is the ‘kindling effect’; where the severity of the withdrawal symptoms increases after repeated withdrawal episodes. If this were shown to be the case, then the number of medically-assisted withdrawal episodes should perhaps be limited. Weighed up against these concerns is the sincere wish to do the best for an individual who wishes to stop drinking and the need to prevent them from developing severe withdrawal symptoms. It is also important to recognize that these patients may have other alcohol-related conditions and that the opportunity should not be lost, whether the patient is admitted or not, to diagnose these and manage the patient appropriately.
Therefore, the clinical questions asked, and upon which a literature search was undertaken, were:
‘What are the benefits and risks of unplanned ‘emergency’ withdrawal from alcohol in acute medical settings versus discharge?
What criteria (e.g. previous treatment, homelessness, levels of home support, age group) should be used to admit a patient with acute alcohol withdrawal for unplanned emergency withdrawal from alcohol?’
2.1.2. Clinical Methodological Introduction
No studies were identified that looked at the benefits and harms of unplanned medically-assisted withdrawal compared with planned medically-assisted withdrawal. With respect to the question of whether unplanned medically-assisted withdrawal is ‘safe’, studies were included that looked at the association between the number of previous medically-assisted withdrawals and the incidence of seizures, risk of developing DTs or severity of withdrawal. The severity of withdrawal was measured using the CIWA-Ar score in some studies. This is further described in the section on supportive care. Because there were a large number of potentially confounding variables, only studies that applied multivariate, covariate, regression or discriminant function analyses were included. Nine studies were excluded because they reported the results of univariate analysis only. Studies with a sample size of 50 or fewer were excluded from the evidence review.
For the question of what criteria should be used to admit a patient with acute alcohol withdrawal for unplanned ‘emergency’ withdrawal from alcohol, studies were included if they looked at factors that were potential predictors of severe withdrawal, seizure incidence or the development of DT, namely: age, history of a seizure, history of DTs, history of severe withdrawal, previous drinking history and breath or blood alcohol level.
Studies were included if they reported on individuals admitted for planned or unplanned medically-assisted withdrawals, but restricted to acute, inpatient settings only. Only one study specifically stated that people were recruited through a registry of trauma patients (and therefore represent a population of patients who may require unplanned emergency medically-assisted withdrawal in the general hospital setting) 9.
Very few studies described how they operationally defined ‘detoxification’, for example whether they included medically-assisted withdrawals only. One important methodological limitation is the retrospective nature of the data collection regarding the number of previous episodes of medically assisted withdrawals. Also the majority of studies obtained this information from hospital notes and thus the information may be of questionable accuracy. The table below summarises the methodological characteristics of the studies included in parts (a) and (b) of the question.
In one study the effect of multiple withdrawal episodes on cognitive function was assessed using a task of frontal lobe function (the Stroop task), a maze learning and vigilance task10. Cognition was compared in individuals who had undergone two or fewer medically-supervised detoxifications (LO, N=36) with those who had undergone two or more (HIGH, N=6) and a control group of ‘mild to moderate’ drinkers (CON, N=43). The patients were undergoing inpatient treatment and had been off treatment for alcohol withdrawal for at least two weeks prior to testing.
See Table 2-1 for a summary of study characteristics.
Table 2-1
Summary of the study design, patient population, incidence of previous detoxifications and incidence of withdrawal problems, seizures and DTs.
2.1.3. Clinical Evidence Statements
Previous detoxifications and severity of alcohol withdrawal
The following measures of severity of withdrawal were significantly associated with the number of previous detoxifications or were reported to be significantly different between patients with no or a small number of previous detoxifications and those with a high number:
- A slower rate of decline on the CIWA-Ar day 0 to 4 of withdrawal associated with multiple detoxifications (multiple versus 0 to 1 detoxifications; p<0.05).11Level 2++
- Severe withdrawal (requirement for 600 mg or more, total, cumulative benzodiazepine (expressed in chlordiazepoxide equivalents) was significantly associated with participation in two or more prior alcohol treatment programs (OR 2.6 [95%CI 1.3 to 5.6]; p=0.01).21Level 3
The following measures of severity of withdrawal were not significantly associated with the number of previous detoxifications or were not significantly different between patients with a low and those with a high number of detoxifications:
- The CIWA-Ar score on admission was not significantly related to the number of previous admissions (not significant).11Level 2++
- The severity of alcohol withdrawal (alcohol withdrawal syndrome scale) was not significantly related to the number of previous prior inpatients detoxifications or prior withdrawal delirium (not significant).13Level 2++
- The frequency of alcohol-specific hospitalisations was not significantly associated with withdrawal problems (DT, alcoholic hallucinations and alcoholic dementia during hospitalisation) (withdrawal problems versus no withdrawal problems mean 0.95 (SE0.10) versus 0.82 [0.03] not significant).15Level 2+
Previous detoxifications and incidence of seizures
Four studies report that patients with a history of previous detoxifications or withdrawals were significantly more likely to experience a seizure:
- There was a significant difference between those patients who had unspecified seizures in the index hospitalisation and those who did not and the mean number of previous alcohol-specific hospitalizations (with a primary diagnoses of alcohol dependence and acute alcohol intoxification) (in the previous 3 years) (mean 1.48 [SE0.23] versus 0.81 [SE0.03]; MD 0.67; p<0.01). 15Level 2+
- Two studies reported a significant association between the history of a seizure and the total number of previous detoxification admissions (mean 2, R2-Ad 0.035, F=13.2; p<0.001) 17(mean 2, R2-Ad 0.041, F=15.1; p<0.0001) 18.Level 3
- A history of DTs and/or convulsions compared with no history of DTs and/or convulsions was significantly associated with a history of more withdrawal episodes (28 versus 16) (OR 1.01, 95%CI 1.00 to 1.02; p<0.01) 12.Level 2++
Previous detoxifications and incidence of DTs
One study reported no significant association between previous detoxification history and the development of DTs (0.94; 95%CI 0.68 to 1.29;p=0.70) 20.
Level 2++
Cognitive impairments
There were no significant differences (ANCOVA) reported between patients with a high number of previous detoxifications and those with a low number on the Stroop task (errors 2.67 [SE1.73] versus 2.62 [0.55]; MD 0.05; ns, maze learning [errors 1.73 {SE0.34} versus 1.47 {0.41}]; MD 0.26; not significant) or vigilance tasks (number correct 0.67 (SE0.07 versus 0.79 [0.02]; MD 0.12; ns)10.
Level 2++
Factors associated with the incidence of seizures
Previous history of a seizure
No studies reported on this outcome.
Previous history of DT
No studies reported on this outcome.
Age
Two studies reported that:
Alcohol consumption/history
The following were not correlated with prevalence of seizure history:
Alcohol level on admission
No studies reported on this variable in relationship to the incidence of seizures.
Factors associated with the risk of developing DT
One study developed a model for identifying patients with a high risk of developing delirium tremens after assessment in the emergency department. Five risk factors were significantly associated with its occurrence, (of relevance to those factors included in this evidence review):
- a history of previous withdrawal seizures (R2=0.068, t=2.35; p=0.019). A previous history of withdrawal seizures independently contributed 6.8% to the risk of developing DTs 19.Level 3
- a history of previous episodes of DTs (R2=060, t=2.07; p=0.039). A previous history of alcohol–related DTs contributed 6% to the risk of developing DTs 19.Level 3
- Signs of overactivity of the autonomic nervous system accompanied by an alcohol concentration of more than 1 gram per litre of body fluid (R2=0.129 t=3.11; p=0.002) 19.Level 3
- alcohol concentration of more than 1 gram per litre of body fluid not accompanied by signs of autonomic hyperactivity was not associated with the risk of developing DTs (ns in univariate analysis and therefore not entered into the regression model) 19Level 3
Age
One study on trauma patients reported that:
Alcohol consumption/history
One study reported that:
Alcohol level on admission
One study reported that:
- blood alcohol concentration ≥ 43 mmol/L (200 mg/dL) was a significant predictor of the development of DTs (DT present versus DT absent 52/104 [60%] versus 833/1751 [48%]; OR 1.69 [95%CI 1.08 to 2.62]; p=0.02)9.Level 2++
Factors associated with severe alcohol withdrawal
Previous history of a seizure
One study reported that:
- a history of withdrawal seizures was not a significant predictor of severe withdrawal (symptom-triggered regimen, 600 mg or more, total, cumulative benzodiazepine [expressed in chlordiazepoxide equivalents]) 21.Level 3
Previous history of DT
One study reported that:
- a history of DTs was a significant predictors of severe withdrawal (600 mg or more, total, cumulative benzodiazepine (expressed in chlordiazepoxide equivalents) (OR 2.9; 95%CI 1.3 to 6.2; p=0.007) 21.Level 3
Age
Two studies reported no significant associations between age:
- Level 2++
- Level 3
- Level 3
Alcohol consumption/history
Two studies reported no significant associations between drinking consumption and drinking history and:
- Withdrawal severity (maximum AWS score) and alcohol duration, alcohol intake/drinking day (not significant) 13.Level 2++
There was no significant association between severity of withdrawal (600 mg or more, total, cumulative benzodiazepine [expressed in chlordiazepoxide equivalents]) and:
- number of drinking days over past month (not significant) 21.Level 3
Alcohol level on admission
One study reported on the association between breath alcohol level on admission and the severity of withdrawal. The results were reported separately for admission to a non-medical setting and a medical setting 23.
Level 2+
- Non-medical settingLinear regression analysis showed a significant relationship between breath alcohol levels on admission and severity of withdrawal (amount of chlordiazepoxide used in first 48 hours) (R2=0.26;p<0.0001). When patients were classified in to two groups based on the median level of breath alcohol on admission (≤33 mmol/L [150 mg/dL versus > 33 mmol/L]) higher levels were associated with more severe adverse outcomes, including transfer to acute care hospital for medical detoxification and a maximum withdrawal assessment score of greater than 6 (indicating medical consultation is required). When the same threshold was applied to the medical setting, the threshold distinguished between those patients who required a total of 50 mg chlordiazepoxide or less and those who required more 23.Level 2+
- Medical settingLinear regression analysis showed a significant relationship between breath alcohol levels on admission and severity of withdrawal (R2=0.41; p<0.0001)23.Level 2+
2.1.4. Health Economic methodological Introduction
One UK cost-effectiveness analysis was identified and was presented to the GDG.
Parrot 2006 24 presented a cost-utility analysis (reporting cost per QALY gained) based on a case series (n = 54) from a direct-access alcohol detoxification service in Manchester (Smithfield Centre). This service offered a 10-day detoxification including three to four days for the management of withdrawal. The following six to seven days involved social care interventions. All non-referred admissions for alcohol detoxification from April to November 1998 were prospectively followed for a 6-month period to collect quality of life and resource use data (non-direct-access patients formally referred from other services or professionals were excluded). Retrospective resource use data were collected for the 6-month period before the admission by interview/questionnaire.
The costs incorporated in the analysis were the 10-day treatment cost at the centre, and the costs related to health services, alcohol services, criminal justice services, and social services. Patient-level quality of life data were collected on admission to the centre and 6 month later using the EuroQol (EQ-5D) questionnaire25. No sensitivity analysis was undertaken.
2.1.5. Health Economic Evidence Statements
Results of the Parrot 2006 study24 were calculated comparing data from the case series pre- and post-detoxification. Two cost-effectiveness ratios were presented. The first cost-effectiveness ratio considered the QALY gain from admission to 6 months post-discharge (0.033), and the 10-days detoxification cost only. The result indicated a cost of £33,727 per QALY gained. The second cost-effectiveness ratio presented considered the same QALY difference (0.033), but estimated the impact on costs by comparing 6-month costs pre- and post-detoxification from a broader perspective including health service costs, alcohol service costs, criminal justice service costs, and social service costs. The result indicated a cost of £65,454 per QALY gained. If the costs relating to the criminal justice services are excluded, then the costs would be £69,090 per QALY gained – this would be the usual NICE reference case.
The Parrot analysis24 was based on outcomes collected from a case series pre- and post-treatment. This method might be more biased than a cohort study comparing an intervention with a control group. However, the magnitude and direction of this bias is unknown. The small size of the case series (n=54) is another limitation of this study. Finally, results from this analysis need to be considered carefully as the study was undertaken on a specialist alcohol unit with a potentially different caseload to that of a general hospital.
2.1.6. From evidence to recommendations
The GDG recognised this is a very difficult area in which to produce guidance as each individual is different and the clinical problem is often compounded by social problems. It was emphasised that these clinical decisions must be made with compassion and with the patient’s best interests in mind.
People with a co-incident medical problem requiring admission were excluded from the review as these individuals will be admitted for the co-incident problem and started on a regimen to manage their withdrawal from alcohol.
The majority of the studies collated data retrospectively which raises questions about the accuracy of reporting.
The GDG noted the evidence review did not find that repeated unplanned medically assisted withdrawals from alcohol caused harm. Some low quality studies supported an association, but there were as many studies showing no association. While the kindling hypothesis was not disproved, the group agreed there was not enough clinical evidence in favour of the hypothesis to support a recommendation.
As there were no studies comparing the efficacy of hospital admission for an unplanned medically assisted withdrawal from alcohol with either a planned admission or planned out-patient management it was not possible to make an evidence-based recommendation regarding the efficacy of unplanned medically assisted withdrawal from alcohol. Nevertheless, consensus opinion based on experience within the group was that unplanned medically assisted withdrawal from alcohol in isolation is rarely an effective long-term treatment for alcohol dependence. It may be the case that patients who have planned to stop drinking and present to general hospitals may have good long-term outcomes with regard to abstinence if the appropriate follow up services focusing on relapse prevention are provided on discharge. At present, however, there is often a delay between discharge and the institution of relapse prevention treatment. It was felt that, on balance, these patients were likely to get better long-term benefits by undergoing a planned withdrawal in an elective manner, organised through addiction services, with the relevant and appropriate follow-up.
As such, the GDG emphasised the need to direct people presenting with withdrawal towards alcohol addiction services and encourage them to undergo planned withdrawal (to be covered in ‘Alcohol use disorders: diagnosis and clinical management of harmful drinking and alcohol dependence’ [NICE clinical guideline in development]). The risks of sudden withdrawal from alcohol should be made clear to the person and advice should be given about how best to engage with the most appropriate local addiction services. Advice about reducing and stopping drinking may be given at this point, but what this advice should be was outside the scope of this guidance. It is important to recognize, however, that we are, by definition, referring to a dependent population in withdrawal and that the most acute concerns are the assessment and management of the acute withdrawal episode. If the patient does not require admission, this will usually involve drinking and then slowly reducing alcohol consumption or undergoing a planned medically assisted withdrawal of alcohol.
The GDG agreed, by expert consensus, that individuals may also need admission due to the severity or predicted severity of the syndrome. More specifically, if a person presents following or in a withdrawal seizure or delirium tremens they should be admitted for medical care. In addition the evidence was examined to identify which factors confer a high risk of the withdrawal episode progressing to either seizure or delirium tremens. Factors increasing the risk of DTs have been investigated 19 and have been identified as:
- history of alcohol withdrawal seizures
- a history of DTs
- signs and symptoms of autonomic over-activity with blood ethanol concentration greater than 100mg/100ml
The GDG considered that these factors should be used as predictors of a severe withdrawal episode and accepted as an indication that the person should be admitted for medically assisted withdrawal. While some of these features may not mandate admission if the current withdrawal episode is mild, it was agreed they each have predictive utility in a clinical setting. Without stronger evidence it was not felt appropriate to give guidance about the severity of autonomic symptoms and BAC that would constitute high risk. This will be dictated by the clinical setting with each of the above predictors being of relevance.
All of the studies reviewed were in adult populations although age was not restricted when undertaking the literature search. As such, the GDG agreed that while the presentation of a young person with alcohol withdrawal is rare it is associated with a unique set of problems and management should always include addressing any underlying long-term psychosocial issues. The GDG agreed that this population is particularly vulnerable and that admission should be considered at a lower threshold in those under 18 and advised in those under 16. The GDG recognises that intoxication is a more common problem than withdrawal in this age group.
No correlation was found between age and the severity of withdrawal: however, it was noted that frail people may be more susceptible to post-discharge injury from falls, slips and the like. The GDG agreed there should be a lower threshold for admission for the medical management of alcohol withdrawal in this population. They recognised that biological is more important than chronological age.
The GDG noted that a person’s level of social support outside the hospital setting can make a considerable difference to the outcome and may impact upon the decision as to whether they will require admission or not.
2.1.7. Recommendations
- R1.
For people in acute alcohol withdrawal with, or who are assessed to be at high risk of developing, alcohol withdrawal seizures or delirium tremens, offer admission to hospital for medically assisted alcohol withdrawal.
- R2.
For young people under 16 years who are in acute alcohol withdrawal, offer admission to hospital for physical and psychosocial assessment, in addition to medically assisted alcohol withdrawal.
- R3.
For certain vulnerable people who are in acute alcohol withdrawal (for example, those who are frail, have cognitive impairment or multiple comorbidities, lack social support, have learning difficulties or are 16 or 17 years), consider a lower threshold for admission to hospital for medically assisted alcohol withdrawal.
- R4.
For people who are alcohol dependent but not admitted to hospital, offer advice to avoid a sudden reduction in alcohol intakea and information about how to contact local alcohol support services.
2.1.8. Research Recommendation
- RR1.
What is the clinical and cost effectiveness of admitting people who attend hospital in mild or moderate acute alcohol withdrawal for unplanned medically assisted alcohol withdrawal compared with no admission and a planned medically assisted alcohol withdrawal with regard to the outcome of long-term abstinence?
2.2. Treatment for acute alcohol withdrawal
2.2.1. Clinical Introduction
Often, alcohol withdrawal requires no drug management. Whether drugs are required or not, it is important that the patients are comfortable, in a well lit room and well hydrated. This is particularly important when delirium is present. It is also important to maintain the dignity of the patient.
Several classes of drug can be used to treat the symptoms of alcohol withdrawal. The most widely used are the benzodiazepines, but within this class there are many drugs, each with a different bioavailability and half life. In addition, other agents such as anticonvulsants and antipsychotics have been used. While the application of these drugs is often “off-label”, there has been a lot of experience with their use in withdrawal. In general, drugs are prescribed through the oral route unless they have been refused. Then intramuscular or intravenous routes are used.
During a planned medically-assisted withdrawal (to be covered in ‘Alcohol use disorders: diagnosis and clinical management of harmful drinking and alcohol dependence’ [NICE clinical guideline in development]), the aim is to prevent symptoms of withdrawal. In the acute, unplanned setting patients may present with withdrawal of varying severity which may include seizures or delirium.
The goals of treatment when managing withdrawal are to minimize the symptoms, promote the comfort and dignity of the patient and prevent complications such as seizures and delirium tremens. Care must be taken not to over-sedate the patient, and certain groups are more susceptible to complications than others; most notably those with respiratory illness or liver failure.
In current UK practice, benzodiazepines are the most commonly used agents, with chlordiazepoxide and diazepam favoured in many places. Others favour clomethiazole or carbamazepine.
The clinical question asked, and upon which the literature search was undertaken, was:
‘What is the safety and efficacy of a benzodiazepine (chlordiazepoxide or diazepam, alprazolam, oxazepam, clobazam, lorazepam) versus a) placebo b) other benzodiazepines (chlordiazepoxide or diazepam, alprazolam, oxazepam, clobazam, lorazepam) c) other agents (clomethiazole or carbamazepine) d) other agents (clomethiazole or carbamazepine) versus placebo for patients in acute alcohol withdrawal?’
2.2.2. Clinical Methodological Introduction
For this question, studies were restricted to systematic reviews/ meta-analysis of RCTs or individual RCTs. One Cochrane systematic review on benzodiazepines for alcohol withdrawal was identified and appraised26. This reported on the efficacy and safety of benzodiazepines in comparison with placebo or other pharmacological intervention or other benzodiazepines.
Level 1++
The Cochrane systematic review included studies on patients who were not in acute alcohol withdrawal. In addition, some studies were on pharmacological interventions that were not relevant for the clinical question under consideration here. In addition, the drug clomethiazole was classified as an anticonvulsant in the Cochrane and re-classified as a hypnotic (other agents) for the meta-analysis presented. After these studies had been removed, 21 out of the 56 studies were included in the meta-analysis. However, not all studies reported on the outcomes reported here. The follow-up period ranged from eight hours to 14 days.
The outcome ‘therapeutic success’ included measures of severity of withdrawal syndrome (for example, the CIWA-Ar score).
There was a large degree of heterogeneity in the trials with respect to sample size, patient population (for example including severity of alcohol withdrawal, inclusion/exclusion criteria) and dosage and scheduling of pharmacological agents.
No relevant papers were identified for any of the drug comparisons that reported on safety and efficacy for specific patient populations, for example older adults or adolescents.
2.2.3. Clinical Evidence Statements
See Table 2-2 for a summary of results.
Table 2-2
Summary of results.
Benzodiazepines versus placebo
Alcohol withdrawal seizures
A meta-analysis of three studies (Chlordiazepoxide N=2, Lorazepam N=1) found that benzodiazepines were significantly more effective than placebo (RR: 0.16 [95% CI: 0.04 to 0.69] p=0.01). See Figure 2-1 for the forest plot extracted from the Cochrane systematic review 26.
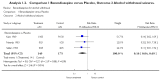
Figure 2-1
Forest plot extracted from Cochrane review.
Level 1++
There were no significant differences between benzodiazepines and placebo for 26:
- therapeutic success
- mortality
- side effects
- discontinuation due to side effects .
Level 1++
Benzodiazepines versus benzodiazepines
There were non-significant differences when one benzodiazepine was compared with another benzodiazepine for 26:
- alcohol withdrawal seizures
- therapeutic success
- mortality
- side effects
- life threatening side effects
- discontinuation due to side effects
- alcohol withdrawal delirium
- Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) score (change from baseline) at 48 hours
Level 1++
Benzodiazepines versus carbamazepine
There were no significant differences when benzodiazepines were compared with anticonvulsants for 26:
- alcohol withdrawal seizures
- mortality
- side effects
- discontinuation due to side effects
- alcohol withdrawal delirium
- CIWA-Ar score (change from baseline) at 48 hours
Level 1++
Benzodiazepines versus clomethiazole
There were non-significant differences when benzodiazepines was compared with clomethiazole for 26:
- alcohol withdrawal seizures
- therapeutic success
- mortality
- side effects
- life threatening side effects
- discontinuation due to side effects.
Level 1++
Clomethiazole versus placebo
There were no results reported in the Cochrane systematic review for the outcomes specified 26.
Level 1++
Carbamazepine versus placebo
No relevant papers were identified.
2.2.4. Health Economic Methodological Introduction
No relevant economic evidence was identified that assessed the cost-effectiveness of giving benzodiazepines, clomethiazole or other agents as a treatment for acute alcohol withdrawal. GDG members received a list of costs for the different drugs appraised by the clinical literature review, in association with the specific dosages as recommended for use in England and Wales.
2.2.5. Health economic evidence statement
The cost of medications for treating patients with acute alcohol withdrawal (AAW) is relatively low27 (See Table 2-3), and this treatment is given for a short period (mean duration of treatment for AAW was reported to be between 9 hours to 101 hours28–30). The cost-impact related to this therapy is therefore likely to be small.
Table 2-3
Drug treatment indications and cost.
2.2.6. From evidence to recommendation
The research studies considered in this review assessed short-term outcomes for safety and efficacy of agents used for the prevention and treatment of symptoms of alcohol withdrawal including seizures. The trials did not capture any qualitative aspects of the patient experience (for example, safety, dignity and comfort) and the number of events recorded for each outcome was small. The incidence of reported side-effects of medication was low. No deaths were reported in any of the studies.
The GDG noted that the study sizes were small and heterogeneous with respect to inclusion / exclusion criteria and none included young people or older adults in their samples. Therefore, the study populations may not be representative of those presenting to clinical practice especially as patients with a history of substance misuse or a concurrent medical or psychiatric condition were excluded.
The cost to the NHS for each of the agents was low and no information was available about how any of the agents affects length of hospital stay or other elements of resource use. The cost-effectiveness is therefore uncertain but given the low cost the GDG suspected that these therapies would be considered cost-effective.
The evidence showed benzodiazepines to be more effective than placebo for the prevention of alcohol withdrawal seizures. No other significant differences were found within and across the agents considered (benzodiazepines, carbamazepine and clomethiazole). In particular, there was no evidence to support the widely held view that clomethiazole is less safe than the other agents, although the GDG were concerned about use of this agent outside a closely monitored inpatient setting. The trial evidence available was not sufficient to reassure the GDG regarding the use of this agent outside these circumstances. The GDG noted that there is wide variation in the choice of agent used in clinical practice, which reflects the lack of evidence supporting a particular agent.
In older adults and people with compromised liver function, long-acting agents are known to accumulate. In the absence of clinical evidence supporting one agent over another, the GDG agreed on consensus that a shorter-acting agent (e.g. oxazepam or lorazepam) could be offered to the elderly or if there was evidence of encephalopathy. Patients with decompensated liver disease and alcohol withdrawal can be very challenging to manage. While not necessarily requiring management on liver units, it was felt that these patients would benefit from the input of a clinician experienced in the management of liver disease and encephalopathy as well as withdrawal. Specific recommendations for the management of these patients have not been made as treatment will depend on the severity of the liver disease as well as the severity of the withdrawal. In general, shorter acting agents should be used with closer monitoring. Lorazepam has the benefit of being short acting, and not being metabolized in the liver. Longer acting benzodiazepines can be used with the knowledge that less wil be required, accumulation will be greater and metabolism will be slower.
No recommendation has been made about the setting of the management of withdrawal. If patients are discharged form hospital to finish their withdrawal in the community, howver, it is very important to co-ordinate the care with the care giver in the community.
2.2.7. Recommendations
- R5.
Offer pharmacotherapy to treat the symptoms of acute alcohol withdrawal as follows:
- Clomethiazoled may be offered as an alternative to a benzodiazepine or carbamazepine. However, it should be used with caution, in inpatient settings only and according to the summary of product characteristics.
- R6.
People with decompensated liver disease who are being treated for acute alcohol withdrawal should be offered advice from a healthcare professional experienced in the management of patients with liver disease.
- R7.
Offer information about how to contact local alcohol support services to people who are being treated for acute alcohol withdrawal.
2.2.8. Research Recommendations
- RR2.
What is the efficacy and cost effectiveness of clomethiazole compared with chlordiazepoxide or carbamazepine or benzodiazepines for the treatment of acute alcohol withdrawal with regard to the outcomes of withdrawal severity, risk of seizures, risk of delirium tremens, length of treatment and patient satisfaction?
2.3. Dosing regimens
2.3.1. Clinical Introduction
People with acute alcohol withdrawal will respond differently to the drugs used to treat this condition. This variability is dictated partly by the severity of the withdrawal, but also by the person’s age and co-morbidities. As such, it is very important to deliver the appropriate dose of drugs at the right time to control the withdrawal and keep them comfortable, but not over-sedated.
Many centres across the UK have protocols recommending fixed dose regimen of drugs. However, this is only one of three possible treatment regimens (see Table 2-3 for an example of these) and the GDG’s aim was to determine which is the safest and most effective for achieving the goals of therapy for acute alcohol withdrawal:
Table 2-3
Example of dosing regimens for acute alcohol withdrawal.
Fixed dose
In general, these regimens start with a standard dose, which is then reduced over the next several days. Most include an “as required” option to treat breakthrough symptoms.
Symptom-triggered
This type of regimen tailors treatment to the person’s requirements as determined by the severity of their withdrawal signs and symptoms. As such the patient is regularly assessed and monitored, either using clinical experience and questioning alone or with the help of a designated questionnaire such as the CIWA-Ar. Pharmacotherapy is provided if the patient needs it and treatment is withheld if there are no symptoms of withdrawal.
Front-loaded
The loading dose regimen provides a large dose of long-acting pharmacotherapy at the start of the treatment regimen and then provides it on an ‘as required’ basis after this.
When managing acute alcohol withdrawal it is important to correctly assess the person’s symptoms since they guide the use of the ‘as required’ treatment in all three dosing regimen. Clinical judgement can be supported by tools that have been developed specifically for this purpose; most notably the revised clinical institute withdrawal assessment from alcohol (CIWA-Ar) tool8. This 10 point tool has become the one of the widely used observer-rated measures of alcohol withdrawal severity. We aimed to determine whether an alcohol withdrawal assessment tool compared to clinical judgement alone improved outcomes in managing the treatment of people with acute alcohol withdrawal.
The clinical questions asked, and upon which a literature search was undertaken were:
‘In adults and young people in acute alcohol withdrawal, what is the clinical efficacy and safety of, and patient satisfaction associated with, a) a symptom-triggered compared with a fixed-schedule benzodiazepine dose regimen b) symptom triggered compared with loading-dose regimen c) loading-dose compared with fixed-schedule regimen?
What assessment tools, including clinical judgement, are associated with improved clinical and patient outcomes when using a symptom-triggered dose regimen in patients with acute alcohol withdrawal?’
2.3.2. Clinical Methodological Introduction
Four studies were identified that compared symptom-triggered with fixed-dosing regimens 28,29,31,30.
Level 3
Two studies compared symptom-triggered management with routine hospital detoxification practice 32,33.
Level 3
Four studies compared front-loading with fixed-dose treatment regimens 34,35,36,37.
Level 2+
One further study was identified that compared symptom-triggered bolus therapy with a continuous infusion of flunitrazepam, clonidine and haloperidol38.
Level 1+
Three of the studies comparing symptom-triggered with fixed-dosing were undertaken in patients admitted to specialised addiction service/dependency units 28,29,30. One study was undertaken in patients admitted to general medical wards with alcohol dependence and a comorbid medical condition31. One of the studies excluded patients with a history of alcohol withdrawal seizures 29 and two studies included these patients 28,30. Two of the studies almost exclusively include men 28,29.
Level 3
Of the two retrospective case series studies comparing symptom-triggered therapy with ‘routine’ hospital practice, one included patients with ‘uncomplicated’ alcohol withdrawal syndrome 33 and the other included patients admitted to a general medical service but excluded those presenting with seizure or admitted to ITU32. In one study routine hospital practice was defined as ‘patients received medication as ordered by the admitting provider, usually a medical or psychiatry resident. Only the addiction unit used a standardized withdrawal assessment tool. Other services used vital sign parameters or non specific terminology such as ‘alcohol withdrawal’ for PRN orders in a less standardized way, with or without a scheduled medication taper’33. In the remaining study routine hospital practice referred to ‘usual care - empiric benzodiazepine dosage usually on a tapering fixed-dose regimen or with as-needed doses at the discretion of medical staff but without a uniform pattern’32.
Level 3
All the studies comparing front-loading with fixed-dosing regimens were undertaken in patients admitted to specialised addiction service/dependency units 34,35,37,36.
Level 2+
The study comparing symptom-triggered bolus therapy with a continuous infusion was undertaken in patients with trauma or gastrointestinal surgery who subsequently developed alcohol withdrawal syndrome in the intensive care unit (ICU).38
Level 1+
The studies differed with respect to patient populations, intervention, CIWA-Ar criteria for treatment/no treatment, frequency of CIWA-Ar administration and treatment regimens. See table Table 2-4 below.
Table 2-4
Summary of included studies.
One retrospective case series looked at patients treated with front-loading diazepam who were given subsequent doses of diazepam with (N=133) or without (N=117) reference to the CIWA-Ar. The CIWA-Ar was administered hourly ‘during the early stages of withdrawal’ and then on an as-needed basis. If the score was greater than 10, 20 mg diazepam or 100 mg chlordiazepoxide were administered. In the comparison group patients were given additional medication without reference to the CIWA-Ar (the decision whether to use the scale was left to the staff i.e. non random) 39.
Level 3
Part b
What assessment tools, including clinical judgement, are associated with improved clinical and patient outcomes when using a symptom-triggered dose regimen in patients with acute alcohol withdrawal?
No papers were identified for the question.
2.3.3. Clinical Evidence Statements
Symptom-triggered versus fixed-dosing regimen
A summary of the results is presented in the table Table 2-5 below.
Table 2-5
Summary of results.
Overall, symptom-triggered dosing was associated with significantly lower doses of benzodiazepines than fixed-dosing 31 and with a shorter treatment duration and importantly without an increase in the incidence of seizures or delirium tremens 28; 29;30. One study reported that the difference in the amount of medication received between the two regimens was dependent on CIWA-Ar score at day one (the higher the initial score the greater the difference)31.
Level 3
Despite decreased doses of medication with symptom-triggered compared with fixed-dosing, the former were not associated with an increase in the severity of withdrawal during treatment as indicated by the non-significant differences in number and amount of ‘as-needed’ or rescue medication required 28; 29; or co-medication 30.
Level 3
There were no significant differences in the number of patients reporting ‘health concerns’, for example discomfort 29 or depression 28 when comparing symptom-triggered with fixed-dose regimen (not significant). One study reported no significant differences between symptom-triggered with fixed dose regimen on the Medical Outcomes Study Short-Form Health Survey (MOS SF-36) when assessed at day three (physical functioning 91.9 [SD11.32] versus 84.2 [19.04]; p<0.01; vitality (59.6 [19.03] versus 55.2 [21.51]; ns; energy 67.0 [17.37] versus 66.3 [21.94]; ns)
Level 1++
One study reported significantly more protocol errors, for example, dose inconsistent with CIWA-Ar score or a mixture of scheduled doses and those based on assessment in the symptom-triggered group compared to the fixed-schedule dosing (18 versus 8%; p<0.05)31.
Level 2++
Symptom-triggered versus routine hospital practice
In one retrospective case series 15/26 (58%) patients who received symptom-triggered dosing did not reach the threshold required to receive medication and 3/14 (21%) in the non-protocol group (PRN medication ordered by not administered) 33. In the other retrospective case series 88% of patients receiving the symptom-triggered protocol and 82% on the fixed-dose/as-needed protocol were prescribed benzodiazepines 32.
Level 3
Medication
One study reported significant differences in favour of the symptom-triggered compared with the routine hospital practice with respect to mean number of doses of medication (1.7 [SD3.1] versus 10.4 [7.9], MD −8.7;95%CI −11.2 to −6.2; p<0.00001); the total amount of medication (82.7 [153.6] versus 367.5 [98.2] mg, MD −284.8; 95%CI −363.1 to −206.5; p<0.00001); but not the duration of medication use (10.7 [20.7] versus 64.3 [60.4] hours; MD −49.7; 95%CI −101.2 to 1.76; p=0.06) 33.
Level 3
In contrast, the study on medical in-patients reported no significant differences between those patients on symptom-triggered dosing compared with ‘usual care’ (a fixed-dose/as-needed protocol) for the duration of treatment (mean 55.5 [SD54.5] versus 44.9 [49.6] hour; MD10.6; 95%CI −17.9 to 39.1; p=0.47); the proportion of patients prescribed benzodiazepines (74/84 [88%] versus 108/132 [82%]; RR1.08 [0.96 to 1.20]; p=0.20) ; or the mean total amount (mg) of benzodiazepines prescribed (20.1 [SD20.7] versus 20.1 [29.7] MD0.00; 95%CI −6.73 to 6.73; p=1.00) 32.
Level 3
Complications
One study reported that no patient developed DTs or experienced a seizure 33.
Level 3
One study reported that symptom-triggered compared with ‘usual care’ was most effective at reducing the incidence on DTs in those patients without a prior history of DTs (17/84 versus 9/132; RR2.97; 95%CI 1.36 to 6.35; p=0.005). In those with a prior history of DTS the rates were 39% and 40% respectively (p=0.03 for the interaction between the intervention and prior history of DTs) 32.
Level 3
Loading-dose versus fixed-dosing
A summary of the results is presented in the table Table 2-6 below.
Table 2-6
Summary of results.
Three of the studies reported reduced total amounts of medication in patients treated with front-loading compared with fixed-dosing 34; 37; 36, although only one performed statistical analyses 34. Two studies reported no significant differences in severity of alcohol withdrawal measured using the CIWA-Ar 37 and a scoring system developed within the hospital 35
Level 2+
In patients presenting with alcohol dependence with a history of DTs 34 or with alcohol withdrawal syndrome presenting with DTs36, front-loading compared with fixed-dosing was associated with a significantly reduced duration of DTs.
Level 2+
Owing to a low incidence rate of seizures, none of the studies performed statistical analyses on the data. However, all of the reported seizures were in the front-loading groups 34; 37; 36.
Level 2+
Front-loading was not associated with any significant differences on a measure of patient satisfaction 34. Nursing staff reported that patients in the front-loading group were less sedated throughout the detoxification period and this enabled them to participate in psychological group work earlier than those in the fixed-dosing group 34.
Level 1+
Symptom-triggered bolus therapy (bolus group) versus continuous infusion
In the study on surgical intensive care patients who developed alcohol withdrawal, the results indicated that bolus-titrated therapy compared with infusion-titration led to a reduction in medication, incidence of intubation and pneumonia and duration of ITU stay (see table Table 2-7 below) 38.
Table 2-7
Summary of results.
Level 1+
The daily mean CIWA-Ar remaining elevated for a significantly longer period in patients and the duration of AWS was significantly shorted than in the bolus titrated compared with the infusion titrated group (both p 0.01).
Level 1+
Front-loading plus CIWA-Ar compared with front-loading alone
Patients treated with reference to the CIWA-Ar received significantly less diazepam (median total dose 50 mg diazepam equivalent versus 75 mg, p=0.04) and a significantly greater proportion received low dose treatment (< 20 mg diazepam) (44/133 [25%] versus 25/117 [21%], p=0.05) in comparison with those treated without reference to the CIWA-Ar. There was no significant difference between the two groups with respect to mean length of stay (3.9 [SD2.2] versus 4.3 [2.4]; MD −0.40; 95%CI −0.97 to 0.17; p=0.17). One patient in each group developed delirium tremens and two patients in the group treated with reference to the scale developed seizures 39.
Level 3
2.3.4. Health Economic Methodological Introduction
No cost-effectiveness analysis was identified comparing treatment regimen for use in people with acute alcohol withdrawal (AAW).
The clinical evidence review showed that the symptom-triggered dosing regimen of benzodiazepines was associated with significantly lower doses of benzodiazepines31 and shorter treatment duration compared to a fixed-dosing regimen28–30. A quality of life assessment found that a symptom-triggered dosing regimen improved patients’ physical functioning compared to the fixed-dosing regimen (p<0.01)28.
There are different cost implications associated with each type of dosing regimen. In addition to the difference in drug cost, the duration of treatment could have a large impact on the hospital length of stay and related costs. Similarly, each dosing regimen has different training and implementation implications and demands different amount of staff resource (to assess and monitor patients).
We undertook our own economic evaluation of symptom-triggered versus fixed-dose acute alcohol withdrawal (see A.3 for the full analysis).
2.3.5. Health Economic Evidence Statements
The objective of the economic analysis undertaken was to assess the cost-effectiveness of the fixed-schedule dosing regimen of benzodiazepines or clomethiazole, compared to a symptom-triggered dosing regimen, for the in-hospital management of patients with AAW in England and Wales. This economic analysis had mainly considered the experience of implementing and using the symptom-triggered regimen in the Addenbrooke’s Hospital (Cambridge), the Huntercombe Centre (Sunderland), and the Royal Liverpool and Broadgreen University Hospital Trust. Four cost-effectiveness analyses were conducted, each based on a different clinical study comparing the symptom-triggered regimen with the fixed-dosing regimen. Two populations of patients were considered: patients with AAW admitted for the treatment of this condition alone; and patients with AAW admitted for a co-morbid medical condition. The economic modelling of the three clinical studies on patients admitted for AAW only (Deappen 200228, Saitz 199429, Lange-Asschenfeldt 200330) considered the difference in length of hospital stay, which was significantly lower in the symptom-triggered arm of all three studies (see A.3 for details). In the Weaver study31 (where patients were admitted for a co-morbid condition) there was no difference in the length of hospital stay between the trial arms as the co-morbid condition determined the length of hospital stay. The health outcome considered for this analysis was the Quality-Adjusted Life Year (QALY). This analysis was conducted from an England and Wales NHS perspective, with a time horizon extending to the end of the hospital admission.
None of the studies measured utility (health-related quality of life on a zero-one scale) but one study28 employed the SF-36. We therefore derived mean utilities for each regimen by applying the SF-6D algorithm40 to the original patient-level SF-36 data from this study 28. The difference in utility scores between the cohorts was modest (0.0194) and non-significant (95% CI, -0.00972 to 0.4843; p=0.19). The Daeppen study28 assessed health-related quality of life (SF-36) at three days post start of treatment and asked the patients to judge their health-related quality of life over the past three days for both the symptom-triggered and the fixed-dosing cohorts. QALYs were calculated by multiplying the utility score by the three days’ duration for each arm. The Daeppen QALY gain was applied to the other studies.
Four categories of cost were considered in this analysis: drug treatment; hospitalisation; staff time for a nurse monitoring a patient with AAW; and the cost of implementing the symptom-triggered regimen. The cost of staff time was calculated by multiplying the average hourly cost of an NHS nurse by the time a nurse would be in contact with the patient. The amount of time a nurse is in contact with the patient was determined by the assessment schedule used by the nurse monitoring the patient and the number of minutes required to conduct each assessment. The assessment schedule assumptions used to calculate the staff time cost were based on schedules used in the clinical studies and in a selection of hospitals in England and Wales. The implementation cost was calculated considering that the training for staff is conducted in-house.
For the base-case analysis, in addition to a deterministic analysis (where cost and effect variables were analysed as point estimates), a probabilistic analysis was undertaken applying probability distributions to each model parameter and presenting the empirical distribution of the cost-effectiveness results. Deterministic sensitivity analyses were performed to assess the robustness of the results to plausible variations in the model parameters: one-way sensitivity analyses involved varying the treatment cost, the hospitalisation cost, and the staff time cost; scenario sensitivity analyses varied the staff time cost (using alternative scenarios of assessment schedule and also varying the time a nurse is in contact with a patient for one assessment).
Deterministic results of the base-case analysis of the four cost-effectiveness analyses found the symptom-triggered regimen dominates the fixed-dosing regimen (it was more effective and less costly – refer to Table 2-8). The deterministic sensitivity analysis showed the conclusions of the base-case analyses are robust as the symptom-triggered option always remains dominant (cost-saving) or cost-effective (Table 2-8). The probabilistic results of the base-case analysis are in agreement with the deterministic results, showing that using a symptom-triggered regimen is cost-saving for treating patients admitted for AAW and those admitted for a co-morbid condition compared to a fixed-dosing regimen (Table 2-9). However, the probability of cost-effectiveness is quite low, reflecting the lack of significance in the difference in utility scores in the Daeppen trial (p=0.19).
Table 2-8
Deterministic results.
Table 2-9
Probabilistic results.
The results were most sensitive to the assumptions about time spent per assessment. In the Weaver analysis (patients with AAW admitted for treating a co-morbid condition), if nurses spend more time on the symptom-triggered assessments than on the fixed-dosing assessments, then the symptom-triggered dosing regimen is likely to be no longer cost-saving. If the difference is more than 4 minutes per assessment, then symptom-triggered dosing regimen is no longer cost-effective (it costs more than £20,000 per QALY gained).
According to the results presented, the implementation and use of a symptom-triggered dosing regimen in patients with AAW in hospitals in England and Wales is cost-effective for the NHS, in both assessed populations of patients (those patients admitted for AAW treatment and those admitted for a co-morbid condition). The results of the four economic analyses, each based on a different trial, are in agreement, even considering the heterogeneity of trial results (drug dose and duration of treatment).
Results of the analyses conducted on the population of patients admitted for AAW treatment are mainly driven by the hospitalisation cost saved from the reduced length of hospitalisation using the symptom-triggered regimen. Results of the analyses conducted on the population of patients admitted for a co-morbid condition are mainly driven by the staff time cost saved using the symptom-triggered regimen. The sensitivity analysis illustrates the robustness of the results, even considering the small difference in QALYs between the compared regimens.
It was necessary to make some assumptions when developing this economic analysis and these were based on the clinical experience of GDG members with the aim of reflecting current medical practice. The assessment schedule assumptions used to calculate the staff time cost were based on schedules used in the clinical studies and in a selection of hospitals in England and Wales. For the base-case analyses, determining the assessment schedule for fixed-dosing regimen was straight forward as all protocols proposed were similar. As there was variability in the assessment schedules in the symptom-triggered protocols used in the clinical trials, agreeing the frequency of monitoring to use in the base case was more problematic. The commonly used symptom-triggered assessment schedule in the Addenbrooke’s Hospital (Cambridge) is every hour for 6 hours, then every 2 hours for 18 hours, then every four hours; in the Huntercombe Centre (Sunderland), 10 assessments in the first 24 hours and then 4 hourly; and in the Royal Liverpool and Broadgreen University Hospital Trust, every hour for 12 hours then every 4 hours. The latter was used in base-case analyses and is considered to be the most conservative (i.e. least favourable to the symptom-triggered dosing regimen). The Huntercombe Centre regimen was used in the scenario favouring symptom-triggered option in the deterministic sensitivity analysis as this was the least intensive of the symptom-triggered schedules. The scenario favouring the fixed-dosing regimen is a hypothetical scenario that uses an increased number of assessments than what we believe would be usual for current practice. Even in this scenario, the symptom-triggered dosing regimen remains cost-effective.
The results of the analysis conducted on patients admitted for a co-morbid condition are sensitive to how long a health-care worker spends with a patient each assessment. If the health-care worker spends longer than four minutes extra per assessment using the symptom-triggered regimen compared to using the fixed-dosing regimen, then the symptom-triggered option is no longer cost-effective. While it is unlikely that a competent nurse would ever spend longer than five minutes on each assessment, this highlights the need for effective training prior to implementing the symptom-triggered regimen in a service.
The cost of training nurses and implementing the symptom-triggered regimen was marginal and removing this cost did not affect the results of the analyses.
2.3.6. Evidence to recommendations
The clinical evidence for the front-loading versus fixed-schedule dosing studies was of lower quality (particularly with regard to sample size) compared to the evidence examining symptom-triggered versus fixed-schedule dosing. Therefore, the GDG agreed there was insufficient evidence to recommend front-loading dosing regimen at this time.
Overall, symptom triggered dosing is associated with significantly lower doses of benzodiazepines and with a shorter treatment duration without an increase in the incidence of seizures or delirium tremens. Despite decreased doses of medication with symptom-triggered compared with fixed-dosing regimen, the former regimen were not associated with an increase in the severity of withdrawal during treatment as indicated by the non-significant differences in number and amount of ‘as-needed’ or rescue medication required.
Health economic evidence suggests that symptom-triggered regimen is also cost-effective.
The GDG reviewed the evidence and noted that in the two studies comparing symptom-triggered with fixed dosing regimen and the one study comparing front-loading with fixed dosing regimens which also measured patient-reported outcomes (e.g. discomfort and depression), these data were gathered at the end of the treatment. Therefore, these reports may not have been as accurate as if the information was reported during treatment.
The majority of studies were obtained from predominantly male populations admitted to specialist addiction services. There was only one study which reported on the management of withdrawal in a general medical ward setting. The GDG have therefore recommended that further research on the most appropriate regimen is carried out specifically in the acute setting of general hospitals with patients admitted for an unplanned medically assisted withdrawal from alcohol.
The trials reviewed provide evidence from both planned and unplanned medically-assisted alcohol withdrawal episodes. There was debate amongst the members of the GDG as to whether data from planned episodes could be extrapolated to unplanned episodes. It was considered that while the symptoms and signs of withdrawal in the two populations may be similar, the patients admitted in unplanned withdrawal may have a more severe syndrome at presentation than those with planned withdrawal and, as a result, may be more likely to progress to a seizure or the DTs. In addition, the setting of planned and unplanned withdrawal from alcohol is often different. As a result, people presenting for planned withdrawal are more likely to be managed by dedicated alcohol workers with specific sets of skills, while those presenting in withdrawal to a general hospital are more likely to be managed by doctors and nurses with more general skills.
The GDG discussed their concerns about the suitability of recommending a treatment regimen that has been proven to be successful in a certain setting (specialist addition services) and recommending it in another setting where the conditions are likely to be different and the people required to deliver the treatment often do not have the necessary skills (general medical hospital ward). Nevertheless, because of the paucity of studies in the acute setting and the apparent benefits of a symptom-triggered regimen in the controlled setting, it was ultimately decided that the recommendation should reflect this apparent superiority. It was agreed that a caveat regarding the facilities for assessment and monitoring should be included in the recommendation.
All of the evidence for symptom-triggered versus fixed-schedule regimens used the CIWA-Ar to measure the severity of alcohol withdrawal. While this provided consistency between the studies, it did not allow us to compare the CIWA-Ar with other assessment tools. In addition, there were no studies that compared the use of CIWA-Ar to supplement clinical judgement with clinical judgement alone.
The GDG noted that symptom-triggered dosing regimen require people to be closely monitored for changes in the severity of their withdrawal. In addition, specialist expertise is required, that is health care workers with clinical knowledge to identify signs and symptoms that imply a change in severity of withdrawal. The GDG considered that in specialist units this can be achieved through experience, but that the introduction of a symptom-triggered regimen into a general medical setting may need to include training in the use of a valid and reliable tool (for example, the CIWA-Ar) to supplement clinical judgement. This question will be further assessed when discussing the aspects of supportive care required to manage patients with acute alcohol withdrawal.
The cost-effectiveness analysis comparing symptom-triggered and fixed-dosing regimens was assessed by the GDG. In this analysis, the symptom-triggered option was likely to be cost-saving in a majority of scenario. For patients admitted for AAW, the length of hospital stay was the main cost component, this resource use clearly favoring the symptom-triggered option28,29,30. The probabilistic sensitivity analysis showed the robustness of the results, and the relatively low probability of cost-effectiveness was mainly due to the lack of significance in the difference in quality of life from the Daeppen trial28. In the economic assessment based on the Weaver trial31 (patient admitted for a co-morbid condition), the length of stay did not differ between compared regimens, and results were sensitive to the cost related to health-care worker time: if the difference was more than 4 minutes per assessment, then symptom-triggered dosing regimen was no longer cost-effective (it costs more than £20,000 per QALY gained). With regard to this, the GDG questioned the feasibility of implementing the symptom-triggered option and the likelihood that health-care workers would be able to get optimal skills to use it (results of the cost-effectiveness analysis assumed that health-care workers using symptom-triggered regimen are properly trained to dilever it). According to GDG members experience of implementing the symptom-triggered regimen, it was guaranteed that it could be done easily and that health-care workers could get the appropriate skills to deliver it.
2.3.7. Recommendations
- R8.
Follow a symptom-triggered regimene for drug treatment for people in acute alcohol withdrawal who are:
- in hospital or
- in other settings where 24-hour assessment and monitoring are available.
2.3.8. Research Recommendations
- RR3.
What is the clinical and cost effectiveness of interventions delivered in an acute hospital setting by an alcohol specialist nurse compared to those managed through acute care setting with no input from an alcohol nurse specialist?
2.4. Management of Delirium Tremens
2.4.1. Clinical Introduction
Delirium tremens (DT) is an extremely distressing condition, and patients may represent a danger to themselves or others. Untreated, it has a significant mortality associated with severe sympathetic over-activity. DTs occur primarily under two circumstances (i) when a patient with established withdrawal or who is at risk of developing withdrawal receives treatment which is ineffective (break through) or (ii) when a patient presents late with established symptoms having not received treatment. There is no consensus on the best pharmacological agent to manage this condition.
The clinical question asked, and upon which literature searching was undertaken was:
“What is the safety and efficacy of a) neuroleptic agents, promazine hydrochloride, haloperidol, clozapine, risperidone, olanzapine, quetiapine) versus placebo b) other neuroleptic agents c) neurolepetic agents in combination with benzodiazepines (diazepam, chlordiazepoxide, alprazolam, oxazepam, clobazam, lorazepam) for patients with DTs?”
2.4.2. Clinical Methodological Introduction
No relevant papers were identified for this question.
2.4.3. Health economic methodological introduction
No relevant economic evidence was identified that assessed the cost-effectiveness of using benzodiazepines, neuroleptic agents, and other agents as treatment for people with delirium tremens. GDG members received a list of costs for the different drugs assessed by the clinical question, in association with the specific dosages as recommended for use in England and Wales.
2.4.4. Health economic evidence statements
The cost of oral lorazepam, identified by the GDG as potential first-line treatment, is low (few pence per dose27 – Table 2.3). If symptoms are severe or oral medication is declined, parenteral lorazepam, haloperidol or olanzapine are options. Parenteral olanzapine is more expensive than lorazepam and haloperidol (£3.48 per olanzapine dose (10mg), versus few pence per dose for lorazepam and haloperidol27 – Table 2.3).
Table 2-3
Drug treatment for seizures.
2.4.5. GDG discussion
The GDG considered the clinical and cost-effectiveness evidence for the treatment of delirium tremens under circumstances where the treatment for withdrawal prescribed has not been effective (break through) or the patient presents with established symptoms having not received treatment. The clinical evidence review found no papers to inform the discussion so any recommendations are based on experience and consensus.
The GDG noted that people experiencing delirium tremens are often distressed. It is important to provide treatment urgently. As it is unclear when the initial management regimen will become effective, the clinician will need to administer a drug that will work until the point the initial regimen takes over. As there was no clinical evidence showing preference for one agent over another the GDG agreed on consensus that symptoms should be relieved using oral lorazepam in the first instance. If symptoms are severe or oral medication is declined, parenteral lorazepam, haloperidol or olanzapine may be used.
The GDG felt that olanzapine has a better side effect profile than lorazepam and haloperidol, especially in high doses, which is the case here. In spite of the additional cost associated with parenteral olanzapine compared to lorazepam and haloperidol, the overall cost-impact of giving this treatment is likely to be small because this indication often only required a single dose, and the number of patients that may required this treatment are few, especially if used as a second-line treatment for agitation.
2.4.6. Recommendations
- R9.
In people with delirium tremens, offer oral lorazepamf as first-line treatment. If symptoms persist or oral medication is declined, give parenteral lorazepam12, haloperidolg or olanzapineh.
- R10.
If delirium tremens develops in a person during treatment for acute alcohol withdrawal, review their withdrawal drug regimen.
2.5. Treatment of alcohol withdrawal seizures
2.5.1. Clinical Introduction
One of the important goals of treatment in acute alcohol withdrawal is the prevention of seizures. In fact, one of the outcome measures used to determine the success of a treatment regimen is the frequency of seizures in the population treated. Guidelines for the prevention of seizures are therefore the same as the guidelines for the management of acute alcohol withdrawal. Good management will reduce the incidence of seizures, but guidance is still required to manage seizures should they occur. This can happen during a planned or unplanned medically assisted withdrawal from alcohol with the frequency reported as around 8%. Seizures may also be the presenting feature of alcohol withdrawal when a dependent drinker has reduced their alcohol consumption in the community.
The primary goal of treatment is initially to terminate the seizure. Fortunately, alcohol-withdrawal seizures are almost universally self-limiting, and, most commonly, patients present after the event. In this situation the goal is to prevent further seizures and allow the continued management of the other features of alcohol withdrawal as recommended above. This is the most common clinical scenario.
Although several different benzodiazepines and anticonvulsants are in regular clinical use, the optimum management of this common problem is still unclear.
The clinical question asked, and upon which literature searching was undertaken was:
What is the safety and efficacy of benzodiazepines versus a) placebo b) other benzodiazepines c) other anticonvulsants for the prevention of recurrent seizures during acute alcohol withdrawal?
2.5.2. Clinical Methodological Introduction
One meta-analysis (N=4 placebo-controlled randomised trials) was identified addressing the management of recurrent seizures in patients with acute alcohol withdrawal 42.
Level 1+
One trial (N=188) 43 in the meta-analysis compared lorazepam 2mg with saline in patients presenting to the emergency department after a witnessed generalised seizure. Patients were observed for a minimum seizure-free period of 6 hours.
Level 1+
Three trials in the meta-analysis (N=252 patients in total) compared phenytoin with placebo 44; 45; 46. Two of the studies observed patients for a minimum seizure-free period of 6 hours 45; 46 and in the remaining study for 12 hours 44
Level 1+
All of the studies recruited patients who presented to an emergency department with a seizure thought to be related to acute alcohol withdrawal and were therefore not on medication for treatment of this condition. The question addressed here is how to manage patients who have been started on a treatment regimen for acute alcohol withdrawal but who then have a seizure presumed to be withdrawal-related.
2.5.3. Clinical Evidence Statements
Lorazepam but not phenytoin is effective in the management of withdrawal seizures compared with placebo (see table below for details of the individual studies in the meta-analysis) 42. The number of patients needed to be treated with lorazepam to prevent one seizure is five (95%CI 3.2 to 8.5)i. See table 2-10 for a summary of results.
2-10
Summary of results.
Level 1+
2.5.4. Health Economic methodological introduction
No relevant cost-effectiveness evidence was identified involving patients suffering from recurrent seizures, and the efficacy of anticonvulsant agents and benzodiazepines. GDG members received a list of costs for the different drugs appraised by the clinical literature review, in association with the specific dosages as recommended for use in England and Wales.
2.5.5. Health economic evidence statements
The cost of medications for treating patients with AAW is relatively low27 (see Table 2-3 in Section 2.2.5), and this treatment is given for a short period (mean duration of treatment for AAW was reported to be between 9 hours to 101 hours28–30). The cost-impact related to this therapy is therefore likely to be small.
2.5.6. Evidence to recommendations
The GDG discussed the difference between preventing seizures, treating a patient during a seizure and preventing recurrent seizures. It was noted that effective treatment of acute alcohol withdrawal will result in the prevention of seizures. As such, a seizure in a patient during treatment can be considered as a treatment failure. The GDG therefore agreed that it was important to emphasise the need to review a patient’s treatment regimen if they develop a seizure as this may be due to a sub-optimal level of initial treatment.
Further discussion revolved around the issues of treating an acute seizure and preventing further seizures in those patients who present having had a seizure. The GDG noted that the evidence considered was obtained from people not receiving any treatment for acute alcohol withdrawal but who presented to Accident and Emergency following an initial alcohol withdrawal related seizure. In spite of this, the GDG thought that the evidence could be extrapolated to those patients that have had a seizure on a withdrawal regimen.
It is rare for an alcohol withdrawal seizure not to be self-limiting, so the clinical question had been posed to determine how to manage a patient who has had a seizure. Specifically, it had been posed to determine if benzodiazepines or anticonvulsants were efficacious in this clinical situation.
The evidence included a low quality meta-analysis with no assessment of individual study quality. The evidence did not report any adverse events or complications associated with lorazepam.
The D’Onofrio43 study showed that lorazepam was superior to placebo in preventing further seizures. It was noted that this study excluded people after enrolment if they required treatment for moderate to severe withdrawal. As such, the GDG recognised significant limitations with the study as it does not reflect the population in the UK that usually needs treatment to prevent recurrent seizures.
The GDG considered it important that the three studies comparing phenytoin with placebo reported no significant differences in the incidence of recurrent seizures.
None of the evidence reviewed included people from the young adult and older adult populations.
2.5.7. Recommendations
- R11.
In people with alcohol withdrawal seizures, consider offering a quick-acting benzodiazepine (such as lorazepamj) to reduce the likelihood of further seizures.
- R12.
If alcohol withdrawal seizures develop in a person during treatment for acute alcohol withdrawal, review their withdrawal drug regimen.
- R13.
Do not offer phenytoin to treat alcohol withdrawal seizures.
2.6. Assessment and monitoring
2.6.1. Clinical Introduction
Patients who are alcohol dependent and therefore at risk of developing acute alcohol withdrawal (AAW) may have complex needs. They are likely to have experienced health problems leading to frequent attendance at acute hospitals, particularly accident and emergency departments4. It would seem both sensible and practical to ensure that when such patients present, health professionals in this setting have the necessary skills to manage their condition in an effective and timely manner. Such skills include the ability to detect alcohol dependence at an early stage in a presentation, and to accurately assess the severity of, or the risk of developing AAW.
It is recognised that the management of AAW varies according to the expertise available at the point of assessment. Early detection and prompt initiation of treatment is crucial as untreated AAW may progress to delirium tremens, which can be fatal in untreated patients. Death may result from respiratory and cardiovascular collapse or cardiac arrhythmias. As well as reducing mortality, accurate assessment and optimal treatment results in fewer complications, reduces progression to delirium, reduces the course and duration of AAW, and consequently reduces length of stay in hospital.
The scope of this guidance is to provide recommendations for the medical management of AAW. Thus, we need to determine if tools are available to assist in accurate assessment of the severity of alcohol withdrawal, if these tools are clinically effective, and who is best placed to utilise these tools in the development of effective care pathways.
The dedicated alcohol specialist nurse (ASN) is considered important in assessing patients and enhancing patient compliance and concordance, augmenting medical treatments and co-ordinating aftercare and follow-up. These factors have been demonstrated to be essential components of effective treatment. It is noteworthy that the recently revised version of CIWA-Ar, the CIWA-Ad, has been demonstrated to have good inter-rater reliability for use by nurses, the K-value for the entire AAS scale being 0.6447.
The clinical question asked, and upon which literature searching was undertaken was:
2.6.2. Clinical methodological introduction
What is the accuracy of a tool and/or clinical judgement for the a) assessment b) monitoring of patients who are alcohol dependent and therefore at risk of developing acute alcohol withdrawal?
One paper (N= 203) was identified. The study reported on patients under the care of all specialties, [and of] general and orthopaedic surgeons, who were identified as at risk of alcohol withdrawal within the first 24 hours of admission. The Clinical Institute Withdrawal Assessment (CIWA) score was used to determine frequency of monitoring (range one to four hourly), duration of monitoring and treatment based on a loading dose regimen 48.
Level 3
Does the assessment and monitoring of patients with acute alcohol withdrawal improve patient outcomes?
Papers were included if they compared outcomes before and after the implementation of a protocol, guideline or patient pathway that used a tool, scale or clinical judgement to assess and/or monitor patients with acute alcohol withdrawal.
An important methodological consideration is that the majority of studies changed the treatment regimen whilst simultaneously altering aspects of assessment and monitoring. Some studies also implemented an education/training programme. The large numbers of confounding variables make it impossible to identify precisely which of these different components were associated with changes in outcome. The results are reported as follows:
- One prospective case series (N=539 episodes) reported on factors associated with the incidence of seizures, hallucinations or delirium in patients in a general hospital who experienced alcohol withdrawal (only the factor ‘delayed assessment’ is reported here)49.Level 3
- Four studies reported on patients at risk of, or with, alcohol withdrawal that were treated with reference to a rating scale compared to those that were treated without reference to a scale 50 51 14,52. See table 2-11 below for methodological details.Level 3
- One study of patients with uncomplicated alcohol withdrawal, implemented a change from fixed-dose scheduling to a symptom-triggered regimen 53. See Table 2-11 below for methodological details.Level 3
- One study was included that reported on the inappropriate use of symptom-triggered dosing in medical and surgical patients admitted to a general hospital (N=124) 54.Level 3
- One study reported on patients with acute alcohol withdrawal admitted to intensive care unit 55. See Table 2-11 below for methodological details.Level 3
Table 2-11
Summary of included studies.
2.6.3. Clinical Evidence Statements
Accuracy of a tool for assessing and monitoring
One study reported on the use of a modified CIWA in the management of alcohol withdrawal in a general hospital 48.
Level 3
Incidence of complications
- 110/204 (54%) patients had a score of greater than 15 and received at least one dose of diazepam 20 mg48.Level 3
- 15/93 (16%) of those patients who scored less than 15 received prophylactic treatment with at least diazepam 20 mg 48.Level 3
- 37/204 (18%) patients suffered complicated alcohol withdrawal reactions (N=4 seizures, N=33 confusion with or without hallucinations, N=0 hallucinations alone) 48.Level 3
- Scores were significantly higher in patients who developed complications (confusion, hallucinations or seizures) compared to those patients who did not develop complications (mean highest score 21.8 [SD1.2] versus 15.6 [0.55], MD6.10; 95%CI 5.67 to 6.53; p<0.00001) 48Level 3
Prophylactic effect of treatment on different scores
- Of the 110/204 (54%) patients who had scores greater than 15, 75 were treated, of whom 11 developed severe withdrawal. In the 35 who were not treated, 21 (15% of 204) developed severe withdrawal. The relative risk of severe withdrawal in those remaining untreated was 3.72 (95%CI 2.85 to 4.85) 48
Overall, the scale was reported as valuable at identifying patients in early withdrawal who need drug therapy to avoid complications. Table 2-12 below gives the relative risks for untreated patients according to the score on the modified CIWA 48.
Table 2-12
Relative risks for untreated patients according to CIWA score.
Level 3
Assessment and patient outcomes
Timing of assessment & frequency of monitoring
One prospective case series reported on the incidence of seizures, hallucinations and delirium and the risks associated with these events in patients with acute alcohol withdrawal admitted to a general hospital 49.
Level 3
A delay of greater than 24 hours before the first assessment was significantly associated with:
- any complication (25/52 [48%], OR [adj.] 4.0; 95%CI 2.7 to 7.6)
- delirium (20/52 [38%], OR [adj.] 8.1; 95%CI 3.7 to 17.7)
Level 3
Patients (excluding those with complications on admission) whose monitoring was delayed were:
Level 3
Studies implementing protocols using fixed-dose regimen
Timing of assessment & frequency of monitoring
One study reported that the implementation of a pathway was associated with a non significant increase in:
Level 3
Medication dose
The results of the studies varied with respect to changes in medication before and after the implementation of a ‘fixed dose’ pathway are presented in Table 2-13:
Table 2-13
Summary of results.
To summarise, fixed dose regimen pathways compared to hospital practice prior to the implementation of the pathway were associated with
- significantly fewer patients being treated with diazepam 52
- a significantly lower proportion of benzodiazepines administered as a standing dose, days one to three 50
- significantly more patients receiving drug therapy but with significantly lower doses of lorazepam and clonidine 51
- significantly fewer patients discharged on tapered benzodiazepine therapy 51
- significantly fewer patients receiving clomethiazole and at a lower mean dose per patient 56
Length of stay/duration of treatment
Pre versus post-implementation:
- a significant increase in the length of stay when comparing pre and post implementation of pathway (median 3 [2 to 6] versus 4 [2 to 7] days [OR adj. 0% or percent increase 18% [95%CI0.9 to 37%]) and a similar finding was reported when comparing pre-pathway with a two year follow-up (median 3 versus 4 days; OR [adj) −3% (−14% to 8%) 52.Level 3
- a significant decrease in the duration of treatment (mean 3.8 [SD1.6] versus 2.7 [2.5] days; MD1.10; [95%CI 0.28 to 1.92; p=0.009]) 56.Level 3
One study reported:
Complications
Pre- versus post-implementation:
- a significant increase in the proportion of patients who died (2.7 versus 3.5%); OR (adj) 2.1 (95%CI 1.0 to 4.6). A similar finding was reported when comparing pre-pathway with two years after pathway implementation (2.2 versus 3.3%; OR [adj] 1.2 [95%CI 0.6 to 2.4])/ 52. Note: no explanation for this finding was identified.Level 3
- a significant decrease in the proportion of patients transferred to a higher level of care after the implementation of a pathway (22 versus 17%; OR [adj] 0.6 [95%CI 0.3 to 1.0])52Level 3
- a significant decrease in the incidence of delirium tremens (adjusted 52% versus 40%; p<0.05) 50;Level 3
There was no significant difference when comparing pre and post implementation of pathway for:
- Level 3
Protocol changing from a fixed-dose schedule to symptom-triggered prescribing in patients with ‘uncomplicated alcohol withdrawal’
Medication dose
One study reported that following the initiation of the pathway changing from a fixed-dose regimen to a symptom-triggered regimen (with no prescribing regime) followed by a symptom-triggered regimen with prescribing based on the CIWA-Ar score (‘one year’ after) there was:
- a significant decrease in the mean dose of benzodiazepine per episode as scheduled medication (diazepam equivalents) (74.6 [SD 92.7] mg to 31.4 [SD 47.5] mg after [RR43.20; 95%CI 17.6 to 68.8; p=0.009]), and to 9.9 (SD 32.2) 1 year after (RR64.7; 95%CI 41.2 to 88.2; p<0.00001) 53.Level 3
- Mean milligrams of benzodiazepine per episode-total (diazepam equivalents) significantly decreased from 95.3 (SD 100.2) diazepam equivalents (mg) to 47.5 (SD 56.6) after pathway initiated (RR47.8; 95CI 19.4 to 76.2; p=0.0010), and dropped further to 31.4 (SD 41.9) 1 year after (RR63.9;95%CI 37.9 to 89.9; p<0.00001) 53.Level 3
Length of stay/duration of treatment
The implementation of a clinical pathway for uncomplicated alcohol withdrawal incorporating the use of the CIWA-Ar to ‘encourage’ symptom-triggered dosing (after) and in a follow-up with a more prescriptive protocol for benzodiazepine dosing based on the CIWA-Ar resulted in:
ITU setting
Medication dose
One prospective case series looked at outcomes in patients with alcohol withdrawal delirium in patients admitted to ITU when treated with a symptom-driven benzodiazepine protocol versus non-protocol benzodiazepine infusions 55
Level 3
The symptom-triggered protocol compared to the pre-protocol was associated with significantly:
- Less time to reach a Minnesota Detoxification Scale MINDS score of less than 20 (symptom control) (mean 7.7 [4.9] versus 19.4 [9.7]; MD −11.70;95%CI 16.26 to −7.14; p=<0.00001)
- Lower cumulative mean benzodiazepine dose (1044 [SD534] versus 1677 (937) lorazepam equivalent; MD-633; 95%CI −113.9 to −126.6; p=0.01).
- Less time receiving continuous-infusion benzodiazepine (52 [35] versus 122 [64] hours; MD −70; 95CI −104.34 to −35.66; p<0.0001) 55.Level 3
Length of stay/duration of treatment
Complications
Pre-protocol group:
There were 7 treatment-related complications (44%):
- N=3 intubations (N=2 due to over sedation)
- N=2 aspiration pneumonia
- N=2 diazepam IV extravasations.
Symptom-triggered group:
There were 6 treatment-related complications (25%) including
- N=2 intubations for acute respiratory failure
- N=2 propylene glycol toxicity in patients receiving high infusion rates of lorazepam.
Inappropriate use of symptom-triggered therapy
One study reported on the inappropriate use of symptom-triggered therapy in medical and surgical patients. Symptom-triggered therapy was deemed appropriate if the person has a history of recent alcohol abuse and has intact verbal communication (symptoms of withdrawal were monitored using the CIWA-Ar that depends on the ability to communicate) 54.
Level 3
- 60/124 (48%) patients met both inclusion criteria (drinking history and communication) for symptom-triggered therapy. Of the remaining 64, nine patients (14%) were heavy drinkers but had been unable to communicate; 35 patients (55%) did not have a recent history of heavy drinking but were able to communicate; 20 (31%) fulfilled neither criteria 54.Level 3
- A multivariate analysis reported that liver disease (OR 0.25; 95%CI 0.20 to 0.80; p=0.02) and postoperative status (OR 3.10; 95%CI 1.35 to 7.09; p=0.008) were associated with inappropriate placement on the CIWA-Ar protocol, with the former less likely and the latter more likely to experience inappropriate placement 54.Level 3
- There was no significant difference between those patients who received appropriate and those that received inappropriate therapy with respect the incidence of adverse events (not significant) 54.Level 3
2.6.4. Health economic methodological introduction
No relevant economic analysis related to the assessment and monitoring of patients with AAW was identified by the economic review.
The economic analysis developed for this guideline assessing the cost-effectiveness of the fixed-schedule dosing regimen of benzodiazepines or clomethiazole, compared to a symptom-triggered dosing regimen, for the in-hospital management of patients with AAW, considered the use of a monitoring tool when managing patients using a symptom-triggered dosing regimen. The CIWA-Ar scale was used in the four clinical studies on which the economic analysis was based on (Daeppen 200228, Saitz 199429, Lange-Asschenfeldt 200330, Weaver 200631). In addition, the CIWA-Ar and the CIWA-AD scales are used in England and Wales where the symptom-triggered regimen forms part of the AAW management protocol, and experience from current practice was considered when developing the economic analysis. The full analysis is presented in Section A.3.
2.6.5. Evidence to recommendations
The GDG noted that the majority of studies are representative of people admitted to general hospitals under the care of a number of different specialties rather than dedicated alcohol services.
The majority of studies involved a change in treatment regimen (for example, from fixed schedule to symptom-triggered dosing) whilst concurrently changing methods of assessment and monitoring. Education and training also form a component of a number of the studies. It is therefore impossible to identify the specific aspect of care that was associated with any change in patient outcomes.
It was noted that all of the protocol-based studies used an assessment scale to quantify and monitor symptoms of withdrawal. In some studies this was also used to guide pharmacological intervention. In clinical practice, the severity of withdrawal can be assessed by an experienced clinician. An ideal assessment tool will be rapid to perform and will give a validated score that can act as an adjunct to clinical experience. In some circumstances assessment tools may be useful when there is less experience in managing patients with withdrawal. One prospective case series reported that the CIWA-Ar was valuable at identifying patients in early withdrawal who required drug therapy to avoid complications.
The GDG discussed the study which reported that a delay in assessment (greater than 24 hours) was associated with alcohol withdrawal complications. This reflects the group’s experience that the late recognition of withdrawal leads to a more severe syndrome, and promotes the concept that hazardous and harmful alcohol misusers should be assessed as soon as possible after presentation for dependence (and therefore risk of withdrawal)(see ‘Alcohol use disorders: diagnosis and clinical management of harmful drinking and alcohol dependence’ [NICE clinical guideline in development]). Those patients in alcohol withdrawal should be assessed by an appropriately skilled health worker for the severity of AAW and the need for pharmacotherapy.
One study reported that some medical and surgical patients were inappropriately started on symptom-triggered dosing. This was deemed inappropriate if they were either unable to communicate or did not have a recent history of alcohol misuse, or both. Although this was not associated with adverse events, it further highlighted to the GDG the need for adequate training in those managing the syndrome. Some group members have had experience of symptom-triggered regimen being effective when in the hands of well-trained staff and ineffective when the staff are not appropriately trained.
One of the studies reported that changing from fixed to symptom-triggered regimen resulted in a decrease in the amount of medication prescribed and length of stay; compatible with recommendations made elsewhere in this guideline. A reduction in medication was reported in another study on patients with alcohol-related delirium admitted to the intensive care unit.
It was noted that none of the studies reported on patient experience.
Results of the cost-effectiveness analysis comparing fixed-dosing and symptom-triggered regimens concluded that the use of symptom-triggered was likely to be cost saving (reducing the hospitalization cost when the patient was admitted for treating AAW; and reducing the staff time cost when the patient treated for AAW was admitted for a co-morbid condition). The GDG recognized that these results are consequential to the proper use of the CIWA-Ar with symptom-triggered.
2.6.6. Recommendations
- R14.
Healthcare professionals who care for people in acute alcohol withdrawal should be skilled in the assessment and monitoring of withdrawal symptoms and signs.
- R15.
Follow locally specified protocols to assess and monitor patients in acute alcohol withdrawal. Consider using a tool (such as the Clinical Institute Withdrawal Assessment –Alcohol, revised [CIWA–Ar] scalek) as an adjunct to clinical judgement.
- R16.
People in acute alcohol withdrawal should be assessed immediately on admission to hospital by a healthcare professional skilled in the management of alcohol withdrawal.
2.7. Wernicke’s encephalopathy
2.7.1. Clinical Introduction
The Wernicke-Korsakoff syndrome develops in problem drinkers who are thiamine deficient. However, other as yet unidentified factors must be important in its genesis as thiamine deficiency is not invariably associated with the development of this syndrome. Wernicke's encephalopathy comprises a triad of global confusion, eye signs and ataxia; the confusional state is accompanied by apathy, disorientation and disturbed memory, but drowsiness and stupor are uncommon. The ocular abnormalities include nystagmus, gaze palsies and ophthalmoplegia, while the ataxia affects the trunk and lower extremities. The clinical abnormalities may develop acutely or evolve over several days. The cerebral lesion is characterized by degenerative changes in the structures surrounding the third ventricle and aqueduct, particularly the mammilliary bodies. Korsakoff's psychosis is an amnesic state in which there is profound impairment of both retrograde and anterograde memory but relative preservation of other intellectual abilities; confabulation may be a feature. The cerebral lesion is characterized by changes in the dorsomedial thalamus. Korsakoff's psychosis generally develops after an acute episode of Wernicke's encephalopathy. However, some patients develop a combined syndrome, from the outset, with memory loss, eye signs and unsteadiness but without confusion; others do not develop either the eye signs or ataxia.
Post-mortem analysis has demonstrated that Wernicke’s encephalopathy may occur in as many as 12.5% of chronic alcohol misusers 57, although Wernicke’s encephalopathy or Korsakoff’s psychosis (characterised by a chronic amnesic syndrome and short-term memory loss) has historically been diagnosed during life in only 5–20%57–60). The discrepancy between the pathological findings and the clinical recognition of the syndrome may be explained by the fact that the classical presentation is seen in only 10% of patients 60.A presumptive diagnosis of the Wernicke-Korsakoff syndrome should therefore be made in patients with a history of harzardous or harmful drinking and one or more of the following otherwise unexplained symptoms: ataxia, ophthalmoplegia, nystagmus, confusion, memory disturbance, comatosed/unconscious, hypotension, and or hypothermia.
The pathogenesis is most likely linked to inadequate dietary intake and poor thiamine absorption. Oral thiamine absorption is limited by an active transport process, a single 10mg-30mg oral dose seeming to maximise absorption. No additional benefit is apparent from higher oral doses as passive diffusion does not occur61. Absorption of thiamine appears to be independently affected by both alcohol and malnutrition. Absorption is reduced by around 70% in abstinent malnourished previous alcohol misusers and the remaining absorption is reduced by a further 50% in a third of patients by the concomitant administration of alcohol62 . Other factors commonly seen in alcohol misusers such as poor diet, diarrhoea and vomiting may additionally affect absorption63,64. Once alcohol is stopped, oral thiamine absorption may take six weeks to return to normal63. As thiamine requirements are linked to carbohydrate intake it is very important that intravenous dextrose is not given to a thiamine deficient patient without concomitant thiamine.
It is now common practice to give patients with Wernicke’s encephalopathy (and those with a presumptive diagnosis) intravenous thiamine but the dose and length of treatment required is unclear and there is variation in prescribing practices across the UK65. It is also common practice to give prophylactic thiamine to hospitalised malnourished harmful drinkers but there are no routinely used evidence-based recommendations for the route of administration, dose and length of treatment. It is also not clear which patients are most at risk of Wernicke’s encephalopathy and which require long term prophylaxis or the dose or form that this prophylaxis should take.
The GDG searched the literature around the following clinical questions:
2.7.2. Clinical methodological introduction
Studies were included that reported on the safety, efficacy, dosing or treatment duration of Pabrinex, oral b vitamin, oral thiamine, multivitamins, placebo or any combinations or comparison of these for the prevention and/or treatment of Wernicke’s encephalopathy. Outcomes included mortality and morbidity.
Studies comparing the safety and efficacy of intravenous (i.v.) or intramuscular (i.m.) thiamine or multivitamins compared with oral preparations reporting on tissue thiamine levels as an outcome were also included.
Five studies were included in the review66–70.
One randomised-control trial reported on the use of thiamine in the prevention of Wernicke’s encephalopathy 68. See Table 2-14 below for study details.
Table 2-14
Summary of included study details.
Level 1+
Two case series reported on the use of thiamine for the treatment of Wernicke’s encephalopathy 66,67. These two studies used the same cohort of patients, with the more recent publication reporting on different outcomes. See Table 2-15 below for study details.
Table 2-15
Summary of study details.
Level 3
One RCT compared treatment with thiamine i.m. with oral thiamine and a control group on no vitamins 70. See Table 2-16 below for study details.
Table 2-16
Summary of study details.
Level 1+
One non-randomized trial 69 compared treatment with i.v. thiamine with oral thiamine and a control group given placebo 69. See Table 2-16 below for study details.
Level 2+
One case-control study was excluded due to low quality methodology with no statistical analysis of results, no consideration of potential confounders and no clear differentiation made between cases and controls. 71.
Level 2−
No studies were found that directly answered the question ‘Which patients are at risk of developing Wernicke’s encephalopathy and therefore require prophylactic treatment?’
2.7.3. Clinical evidence statements
Prevention of Wernicke’s encephalopathy
Test of working memory (delayed alternation task):
- There was a significant difference between dosage groups in the number of trials taken to reach the alternation task criterion, p=0.047, with 50 mg thiamine treatment group needing the fewest trials (38) to reach the criterion and the 20mg treatment group needing the most (56).
- Although the 50mg treatment group appeared to require fewer trials, post-hoc comparisons made between the 50mg group and the other treatment groups were non-significant (5 versus 50 mg p=0.166; 20 versus 50mg p=0.043; 100 versus 50mg p=0.090; 200 versus 50mg p=0.561; critical alpha for all comparisons 0.013)
Treatment of Wernicke’s encephalopathy
The initial study by Wood et al.66 reported on change in clinical characteristics between admission and follow-up after treatment with thiamine hydrochloride. See Table 2-17 and Table 2-18 below.
Table 2-17
On admission and discharge (N=32).
Table 2-18
At discharge and at last visit (N=27).
Level 3
A significant reduction was seen in:
- Ophthalmoplegia
- Long-term memory deficit
- Short-term memory deficit
- Muscle weakness66.Level 3
Mortality
- At long term follow up (5 lost) 2/27 (7%) patients died and three others could not be located.66.Level 3
The second publication from the same cohort of patients reported further details on ophthalmoplegia, nystagmus, global confusion state and global severity of Wernicke’s encephalopathy, see below 67.
Level 3
Ophthalmoplegia
- The participants of improvement was affected by the severity of liver disease, p<0.001 and by the severity of fatty liver, p<0.001
- Participants with no fatty liver had the fastest improvement in ophthalmoplegia to treatment, but all participants reached the same level by the end of 14 days. 67Level 3
Nystagmus
- Scores for individual tests of nystagmus all showed improvement, p<0.01 At discharge only six participants were completely free of nystagmus67.Level 3
Global confusion state
(see Table 2-19 below)
Table 2-19
Global severity of acute Wernicke’s.
- The state of consciousness rapidly improved within hours of thiamine treatment, p<0.001 and continued to improve slowly, p<0.02
- The severity of disorientation in time improved over time, p<0.001, but improvement slowed by 7 days, p<0.05, and thereafter, p<0.01.
- By discharge, most participants were still disorientated in time and 18 patients still did not know the day of the week67.Level 3
Limitations:
- The study did not report the dose of thiamine given. It is also possible that the dose of thiamine that they gave was too small and/or the treatment period too short.
Parenteral versus oral thiamine
The response of Erythrocyte thiamine diphosphate (TDP) level
One study reported on the response of erythrocyte TDP level when giving oral compared to i.m. (parental) preparations of thiamine 70. See Table 2-20 below for results.
Table 2-20
(Normal reference range for TDP level 165–286 nmol/l).
Level 1+
Limitations:
- There is some debate over the most accurate measure of tissue thiamine level, with previous studies reporting erythrocyte enzyme transketolase (ETKA) rather than TDP. This may affect the final results.
- This study excluded patients with vitamin deficiencies, which may be an important group of patients in which thiamine is used. Also there was no explanation of what defined a patient as vitamin deficient..
- Short-term follow up of only 7 days may have not been a sufficient time to see results.
Response of erythrocyte transketolase (ETK) activity
One study reported on the response of ETK to treatment with intravenous and oral thiamine compared with placebo 69.
- intravenous thiamine (n=26) versus placebo (n=23) at day 2:
- Mean ± SD: 68.7*± 14.0 versus 68.4 ± 13.8; MD 0.30 (−7.50, 8.10), p=0.94
- intravenous thiamine (n=26) versus placebo (n=23) at day 5:
- Mean ± SD: 75.5**±12.9 versus 75.8**± 15.2; MD −0.30 (−8.25, 7.65), p=0.94
- Oral thiamine (n=24) versus placebo (n=23) at day 2:
- Mean ± SD: 70.0* ±12.5 versus 68.4 ± 13.8; MD 1.60 (−5.94, 9.14), p=0.68
- Oral thiamine (n=24) versus placebo (n=23) at day 5:
- Mean ± SD: 76.8**± 11.4 versus 75.8**± 15.2; MD 1.00 (−6.71, 8.71), p=0.8069
Level 2+
Note: the significant differences (within each group) from the previous mean are indicated at the 95% (*) and 99.9% (**) confidence levels.
Response of ETK activity to vitamin supplementation in patients originally deficient
- intravenous thiamine (n=16) versus placebo (n=15) at day 2:
- Mean ± SD: 59.5* ± 7.8 versus 60.6 ± 9.9; MD −1.10 (−7.40, 5.20), p=0.73
- intravenous thiamine (n=16) versus placebo (n=15) at day 5:
- Mean ± SD: 66.8**± 6.1 versus 67.9** ± 12.1 ; MD −1.10 (−7.91, 5.71), p=0.75
- Oral thiamine (n=16) versus placebo (n=15) at day 2:
- Mean ± SD: 64.4* ± 8.5 versus 60.6 ± 9.9 ; MD 3.80 (−2.72, 10.32), p=0.25
- Oral thiamine (n=16) versus placebo (n=15) at day 5:
- Mean ± SD: 71.8** ± 8.2 versus 67.9** ± 12.1 ; MD 3.90 (−3.42, 11.22), p=0.3069
Level 2+
Note: the significant differences (within each group) from the previous mean are indicated at the 95% (*) and 99.9% (**) confidence levels.
Limitations:
- The measure ETK may not be the most accurate measure of tissue thiamine levels.
- The doses of oral and parenteral thiamine given were not equal, and may not have been given at an adequate dose.
- Both groups were given i.v. thiamine at the start, which may have affected the final results.
- Short term follow up of only five days may not have been sufficient.
2.7.4. Health economic methodological introduction
No relevant economic analysis was identified assessing the cost-effectiveness of vitamin supplementation for the treatment/prevention of Wernicke’s encephalopathy. Costs and resource use information associated with the use of vitamin supplementation for the treatment/prevention of Wernicke’s encephalopathy were presented to the GDG.
2.7.5. Health economic evidence statements
Vitamin-supplementation options used for the treatment/prevention of Wernicke’s encephalopathy have a low-drug cost (especially oral preparations). Pabrinex is the only treatment given parenterally for rapid correction of acute vitamin depletion and is more costly than oral preparations (few pence for high dose of oral preparations versus £1.96 for Pabrinex intravenous preparation [10 ml in 2 ampoules] and for Pabrinex intramuscular preparation [7 ml in 2 ampoules]72,73). Parenteral treatment is normally given to patients when hospitalized for a co-morbidity and therefore use of Pabrinex does not affect the length of hospital stay in its current use. Nevertheless, additional staff time is associated with giving parenteral preparations.
The use of parenteral thiamine (Pabrinex) is associated with a potentially serious allergic adverse reaction that may rarely occur during, or shortly after administration. Since the January 1989 UK Committee on Safety of Medicines warning, 0.5 to 1 million pairs of ampoules of each preparation of Parentrovite were sold annually in the UK. There were four reports of an anaphylactoid reaction for every 1 million pairs of intravenous ampoules and one report per five million intramuscular ampoules sold74.
This reaction may incur extra treatment costs in addition to morbidity. However, allergic reactions from the use of parenteral thiamine are extremely rare and the extra cost associated to it is likely to be marginal. The BNF72 recommends that the potential serious allergic adverse reaction should not preclude the use of parenteral thiamine in patients where this route of administration is required. This is crucial in patients at risk of Wernicke-Korsakoff syndrome where treatment with thiamine is essential considering the serious long-term implications of developing this syndrome and the high cost related to it (supported accommodation for example). In light of the above, the treatment/prevention of Wernicke’s encephalopathy with vitamin-supplementation is likely to be highly cost-effective.
2.7.6. Evidence to recommendations
The GDG noted that the absence of RCTs on this subject would mean any recommendations would need to be by consensus. Due to this lack of RCTs and the potentially catastrophic long term effects of acute thiamine deficiency some of the evidence that was presented was based on clinical studies of thiamine absorption and metabolism.
The GDG first considered evidence on prevention of Wernicke’s encephalopathy with thiamine prophylaxis. It then considered treatment where there was a presumptive or actual diagnosis.
Prophylaxis
In order to determine which patients should receive prophylaxis and how, the risk factors for thiamine deficiency and the absorption of oral thiamine were discussed. Malnourishment is a key pre-disposing factor to thiamine deficiency and the risk factors for malnourishment are dietary intake reduction, nausea and vomiting. Alcohol intake and liver dysfunction also predispose to thiamine deficiency. It was emphasised that patients who are malnourished are not only more likely to be thiamine deficient, but also likely to have impaired absorption of oral thiamine.
When deciding which patients should receive prophylaxis certain other factors were felt to be important. These were; compliance, the treatment for the underlying malnutrition, cost and the inconvenience of daily tablets or parenteral thiamine. We divided patients into low and high risk of developing Werniecke’s encephalopathy.
‘Low risk’ group
This was defined as people who are alcohol-dependent but otherwise eating a normal diet and with no other alcohol-related problem. This will tend to be people with mild or moderate dependence as those with more severe dependence will start to neglect their diet. It was not felt that there was evidence to recommend thiamine to this group. The sub-group of younger people was discussed because nutritional requirements are higher and they may be more susceptible to alcohol-induced neuro-degeneration. It was decided not to make a separate recommendation about thiamine use in this group because of a lack of evidence.
In conclusion, the GDG noted that it could not recommend widespread use of thiamine in this low risk group.
‘High risk’ group
The GDG discussed features that might necessitate thiamine use in hazardous, harmful or dependent drinkers to prevent Wernicke’s. The GDG highlighted the following:
- Alcohol-related liver disease
- medically-assisted withdrawal from alcohol (planned or unplanned)
- malnourishment or risk of malnourishment; this may include;
- weight loss in past year
- reduced BMI
- loss of appetite
- nausea and vomiting
- a general impression of malnourishment
- hospitalised for acute illness
- hospitalised for co-morbidity or another alcohol issue.
The GDG decided that any of these risk factors were enough to recommend prophylactic thiamine. These patients do not have Wernicke’s but are at risk, so it is important to increase the patient’s thiamine stores but this does not need to be done emergently. It was recognised that an adequate diet would likely suffice in many situations, but it was felt that additional prophylaxis should be provided. Although absorption is inhibited in some of these situations, it was felt that oral thiamine would be adequate prophylaxis. Evidence for a specific dose was lacking. It was decided by consensus that the dosing should be at the upper limit of the BNF recommendations as the lower end (10–25mg/day) may not be adequate in this higher risk group.
Concerns were raised about patients with severe withdrawal or with co-morbid conditions that may mask the neurological signs of Wernicke’s such as encephalopathy. These concerns arise from evidence showing that some patients develop Wernicke’s during withdrawal of alcohol. It was felt that parenteral therapy should be used in malnourished patients if withdrawal is severe enough to warrant hospital attendance or admission. This recommendation was then extended to cover harmful and hazardous drinkers that are at risk of malnutrition if they attend hospital for any reason. This was done so that the opportunity to give intravenous thiamine would not be lost in these patients. This may be a single dose followed up by oral thiamine, or intravenous treatment for several days followed up by oral thiamine. It is accepted that formal nutritional assessment is rarely available or practical in this setting. The recommendation is written with the assumption that malnourishment will be assessed during the routine examination, and that risk of malnourishment can be assessed based on a good clinical history – recent dietary intake, vomiting and unintentional weight loss being examples of risk factors.
It was also emphasised that patients with comorbid conditions that may mask the features of Wernicke’s should be managed cautiously. The index of suspicion for considering Wernicke’s in these patients should be high and the threshold for considering following the treatment recommendations should be low.
Diagnosis and treatment
The GDG discussed the issue of treatment of Wernicke’s encephalopathy. The main themes of the discussion were the difficulty in making the diagnosis and the catastrophic nature of a missed diagnosis. Most patients do not present with the classical triad of symptoms so there needs to be a high index of clinical suspicion. The GDG discussed the difficulty in making a diagnosis in the confused patient who misuses alcohol and emphasised the importance of confusion in a patient with a blood alcohol concentration of zero.
Due to the need for rapid absorption of thiamine in patients that are suspected of having Wernicke’s encephalopathy the oral route of administration was felt to be inadequate. It was noted that blood thiamine levels fall rapidly after administration so the treatment should be given more than once a day. Due to the concern of long term brain injury, it was felt that patients with even a low index of suspicion for Wernicke’s encephalopathy should be treated with parenteral thiamine. With no evidence to guide the period of treatment, the recommendation was based on the group’s expert consensus.
Finally, the GDG accepted that the use of vitamin-supplementation for the treatment/prevention of Wernicke’s encephalopathy is likely to be highly cost-effective, especially given the considerable clinical and economic impact related to the development of Wernicke-Korsakoff syndrome.
2.7.7. Recommendations
- R17.
Offer thiamine to people at high risk of developing, or with suspected, Wernicke’s encephalopathy. Thiamine should be given in doses toward the upper end of the ‘British national formulary’ range. It should be given orally or parenterally as described in recommendations R18 to R20.
- R18.
Offer prophylactic oral thiamine to harmful or dependent drinkers:
- if they are malnourished or at risk of malnourishment or
- if they have decompensated liver disease or
- if they are in acute withdrawal or
- before and during a planned medically assisted alcohol withdrawal.
- R19.
Offer prophylactic parenteral thiamine followed by oral thiamine to harmful or dependent drinkers:
- if they are malnourished or at risk of malnourishment or
- if they have decompensated liver disease and in addition
- they attend an emergency department or
- are admitted to hospital with an acute illness or injury.
- R20.
Offer parenteral thiamine to people with suspected Wernicke’s encephalopathy. Maintain a high level of suspicion for the possibility of Wernicke’s encephalopathy, particularly if the person is intoxicated. Parenteral treatment should be given for a minimum of 5 days, unless Wernicke’s encephalopathy is excluded. Oral thiamine treatment should follow parenteral therapy.
2.7.8. Research Recommendations
- RR4.
What is the clinical and cost effectiveness of the use of parenteral versus oral thiamine in preventing the first onset of Wernicke’s encephalopathy in people undergoing medically assisted alcohol withdrawal?
Footnotes
- a
While abstinence is the goal, a sudden reduction in alcohol intake can result in severe withdrawal in dependent drinkers.
- b
Benzodiazepines are used in UK clinical practice in the management of alcohol-related withdrawal symptoms. Diazepam and chlordiazepoxide have UK marketing authorisation for the management of acute alcohol withdrawal symptoms. However, at the time of writing (May 2010), alprazolam, clobazam and lorazepam did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented. In addition, the summary of product characteristics (SPC) for alprazolam advises that benzodiazepines should be used with extreme caution in patients with a history of alcohol abuse. The SPC for clobazam states that it must not be used in patients with any history of alcohol dependence (due to increased risk of dependence). The SPC for lorazepam advises that use in individuals with a history of alcoholism should be avoided (due to increased risk of dependence).
- c
Carbamazepine is used in UK clinical practice in the management of alcohol-related withdrawal symptoms. At the time of writing (May 2010), carbamazepine did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented.
- d
Clomethiazole has UK marketing authorisation for the treatment of alcohol withdrawal symptoms where close hospital supervision is also provided. However, at the time of writing (May 2010), the SPC advises caution in prescribing clomethiazole for individuals known to be addiction-prone and to outpatient alcoholics. It also advises against prescribing it to patients who continue to drink or abuse alcohol. Alcohol combined with clomethiazole, particularly in alcoholics with cirrhosis, can lead to fatal respiratory depression even with short-term use. Clomethiazole should only be used in hospital under close supervision or, in exceptional circumstances, on an outpatient basis by specialist units when the daily dosage must be monitored closely.
- e
A symptom-triggered regimen involves treatment tailored to the person’s individual needs. These are determined by the severity of withdrawal signs and symptoms. The patient is regularly assessed and monitored, either using clinical experience and questioning alone or with the help of a designated questionnaire such as the CIWA–Ar. Drug treatment is provided if the patient needs it and treatment is withheld if there are no symptoms of withdrawal.
- f
Lorazepam is used in UK clinical practice in the management of delirium tremens. At the time of writing (May 2010), lorazepam did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented. In addition, the SPC advises that use in individuals with a history of alcoholism should be avoided (due to increased risk of dependence).
- g
Haloperidol is used in UK clinical practice in the management of delirium tremens. At the time of writing (May 2010), haloperidol did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented. In addition, the SPC advises caution in patients suffering from conditions predisposing to convulsions, such as alcohol withdrawal.
- h
Olanzapine is used in UK clinical practice in the management of delirium tremens. At the time of writing (May 2010), olanzapine did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented. In addition, the SPC advises that the safety and efficacy of intramuscular olanzapine has not been evaluated in patients with alcohol intoxication.
- i
The meta-analysis reports the NNT as −150 (95%CI 10 to −1)
- j
Lorazepam is used in UK clinical practice in the management of alcohol withdrawal seizures. At the time of writing (May 2010), lorazepam did not have UK marketing authorisation for this indication. Informed consent should be obtained and documented. In addition, the SPC advises that use in individuals with a history of alcoholism should be avoided (due to increased risk of dependence).
- k
Sullivan JT, Sykora K, Schneiderman J et al. (1989) Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar). British Journal of Addiction 84:1353–1357
- Addendum to NICE guideline 100, Alcohol-use disorders: diagnosis and management of physical complications
- Alcohol-use disorders: physical complications: Evidence Update March 2012: A summary of selected new evidence relevant to NICE clinical guideline 100 'Diagnosis and management of alcohol-related physical complications' (2010)
- Surveillance report 2016 - Alcohol-use disorders: diagnosis and management of physical complications (2010) NICE guideline CG100
- 2019 surveillance of alcohol-use disorders: diagnosis and management of physical complications (NICE guideline CG100)
- Acute Alcohol Withdrawal - Alcohol Use DisordersAcute Alcohol Withdrawal - Alcohol Use Disorders
Your browsing activity is empty.
Activity recording is turned off.
See more...
