By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsAs mentioned throughout this book, the expression of Hox genes provides the basis for anterior-posterior axis specification throughout the animal kingdom. This means that the enormous variation of morphological form in the animal kingdom is underlain by a common set of instructions. Indeed, one of the most remarkable pieces of evidence for deep homologies among all the animals of the world is provided by the Hox genes. As mentioned in Chapter 11, not only are the Hox genes themselves homologous, but they are in the same order on their respective chromsomes. The expression patterns are also remarkably similar between the Hox genes of different phyla: the genes at the 3′ end are expressed anteriorly, while those at the 5′ end are expressed more posteriorly (Figure 22.2). As if this evidence of homology were not enough, Malicki and colleagues (1992) demonstrated that the human HOXB4 gene could mimic the function of its Drosophila homologue, deformed, when introduced into Dfd-deficient Drosophila embryos. Slack and his colleagues (1993) postulated that the Hox gene expression pattern defines the development of all animals, and that the pattern of Hox gene expression is constant for all phyla.*
If the underlying Hox gene expression is uniform, how did the differences among the phyla emerge? It is thought that they arose from differences in how the Hox genes are regulated and what target genes the Hox-encoded proteins regulate. Gellon and McGinnis (1998) have catalogued four critical ways in which variation in Hox expression patterns might lead to evolutionary change (Figure 22.3):

Figure 22.3
Changes in Hox genes correlate with evolutionary changes in animal morphology. (A) Changes in the number of Hox genes correlate with changes at the level of phyla, and increasing the number of Hox genes may allow increased complexity. (B) Broad changes (more...)
- Changes in the Hox protein-responsive elements of downstream genes
- Changes in Hox gene transcription patterns within a portion of the body
- Changes in Hox gene transcription patterns between portions of the body
- Changes in the number of Hox genes
Changes in Hox-responsive elements of downstream genes
One of the most obvious differences between a fruit fly and a butterfly is that the fly has two halteres where the butterfly has a pair of hindwings (Figure 22.4). The expression pattern of the Hox genes, however, does not differ between a butterfly larva and a fly larva. In both cases, the Ultrabithorax (Ubx) gene is expressed throughout the imaginal discs of the third thoracic segment (from which the hindwing and haltere are derived). What distinguishes a haltere from a wing is the response of the target genes. The Ubx protein downregulates several genes in the Drosophila imaginal discs. Many of these same genes are not regulated by Ubx in butterflies. Moreover, some other genes regulated by Ubx in Drosophila are regulated differently in butterflies (Carroll et al. 1995; Weatherbee et al. 1999). Thus, the wing differences between dipterans (two-winged insects such as flies) and lepidopterans (butterflies and moths) can be attributed to the different ways in which potential target genes in the imaginal discs respond to the Ubx protein.

Figure 22.4
Differences in larval and adult morphology due to Hox gene differences. (A, B) Larva and adult of Drosophila, a dipteran. An arrow points to one of the halteres of the adult. The larva lacks prolegs; its anterior end is at the left. (C, D) Larva and adult (more...)
Changes in Hox gene transcription patterns within a body portion
In Chapter 16, we saw that changes in Hox gene expression are correlated with the change in morphology from the fish fin to the tetrapod limb. Among arthropods, differences in limb morphology may also be caused by Hox gene expression differences. Insects have six legs as adults, a pair arising from each of the three thoracic segments. In Drosophila, the Distal-less (Dll) gene is critical for providing the proximal-distal axis of the appendages (see Figure 18.15). Distal-less expression occurs in the cephalic and thoracic limb-forming discs, but it is excluded in the abdomen by the abdA and Ubx proteins. Thus, the appendages grow into legs and wings in the thorax and into jaws in the head. The Drosophila larva never develops limbs in its abdomen.
Butterfly and moth larvae, however, are characterized by rudimentary abdominal legs called prolegs (see Figure 22.4). Panganiban and her colleagues (1994) cloned the Distal-less homologue from the buckeye (Precis) butterfly and mapped its expression during development. During the early portion of Precis embryogenesis, Dll expression is the same as it is in Drosophila. During gastrulation, Dll expression is seen first in the head regions and in the thoracic regions that will give rise to the leg imaginal discs. However, as development proceeds, the Dll gene of Precis becomes expressed in the third through sixth abdominal segments (Figure 22.5). Whereas Dll expression is seen in both the proximal ring and the “socks” of the true thoracic legs, the expression of Dll in the abdomen is restricted to the proximal ring. Thus, the lepidopteran prolegs appear to be homologous to the proximal portion of the thoracic legs. The expression of Dll in the maxilla and labial segments in both Drosophila and Precis is interesting because it is consistent with recent paleontological evidence (Kukalova-Peck 1992) that, although these jaw structures originated from limb primordia, distal limb elements have been lost from all arthropod jaws.
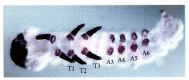
Figure 22.5
Distal-less gene expression in the larva of the buckeye butterfly Precis. By 40% of the way through embryonic development, Dll expression in Precis has diverged significantly from that of Drosophila in that Dll expression is also seen in abdominal segments (more...)
The presence of larval prolegs and Dll expression in the Precis abdominal segments suggests that Dll is regulated differently in dipterans and lepidopterans. Two possibilities come to the fore: first, that the Dll genes of Precis are not repressed by the abdA and Ubx homeodomain proteins, and second, that the expression of the repressing homeodomain genes is somehow abrogated in the abdominal regions of Precis. Warren and co-workers (1994) showed that the Drosophila and Precis embryos have the same initial pattern of abdA and Ubx gene expression. However, at about 20% of the way through Precis embryogenesis, the expression of these genes becomes downregulated in small patches of segments A3-A6, the abdominal segments that give rise to the prolegs (Figure 22.6). Shortly thereafter, the Dll and Antennapedia (Antp) genes are expressed in those “holes.” It is not known what molecules are used to downregulate abdA and Ubx gene expression in the regions of Dll expression. The Polycomb group genes are the best suspects, since they can repress both genes in Drosophila.
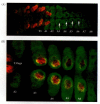
Figure 22.6
“Holes” in the expression of abdA and Ubx in the abdomen of the larval butterfly Precis. The abdA and Ubx proteins have been stained green. The Distal-less protein is stained red, and areas of overlap appear yellow. (A) In the early caterpillar, (more...)
Changes in Hox gene expression between body segments
The origins of maxillipeds in crustaceans
There are substantial differences in Hox gene expression patterns between insects and crustaceans, and there are also significant differences in Hox gene expression patterns within the different crustacean groups. Crustaceans are characterized by a pre-gnathal head (similar to the insect acron), gnathal (jawed) head segments, six thoracic segments, genital segments, abdominal segments, and a telson (Figure 22.7). Each of the thoracic segments of the crustacean expresses Antp, Ubx, and abdA, and these genes appear to be interchangeable in the crustaceans. Indeed, the thoracic segments all look alike, and there is no specialization between these segments. In the arthropod lineage that gave rise to the insects, each gene would take on different (but sometimes overlapping) functions (Averof and Akam 1995).
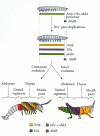
Figure 22.7
Hox gene expression and morphological change in crustaceans. At the bottom are the structures of a brine shrimp (crustacean) and a grasshopper (insect). The domains of Hox gene expression specifying the various structures are coded in color. Whereas the (more...)
But within the crustacean lineages, there are interesting variations on this theme. Averof and Patel (1997) have shown that if a thoracic segment does not express Ubx and abdA, it converts its anterior locomotor limb into a feeding appendage called a maxilliped. Thus, brine shrimp such as Artemia have a uniform expression of Ubx and abdA in their thoracic segments, and they lack maxillipeds. Lobsters such as Homaris lack Ubx and abdA expression in their first and second thoracic segments, and these segments have paired maxillipeds (Figure 22.8). The fossil record suggests that the earliest crustaceans lacked maxillipeds and had uniform thoracic segments. This would mean that the presence of maxillipeds is a derived characteristic that evolved in several crustacean lineages.

Figure 22.8
Schematic representation of the expression of Ubx and abdA (green) in the thoracic segments of different types of crustaceans. The generation of maxillipeds occurs in the thoracic segments that do not express either of these homeodomain proteins. (After (more...)
Why snakes don't have legs
As shown in Chapter 11, the expression patterns of Hox genes in vertebrates determines the type of vertebral structure formed. Thoracic vertebrae, for instance, have ribs, while cervical (neck) vertebrae and lumbar vertebrae do not. The type of vertebra produced is specified by the Hox genes expressed in the somite.
One of the most radical alterations of the vertebrate body plan is seen in the snakes. Snakes evolved from lizards, and they appear to have lost their legs in a two-step process. Both paleontological and embryological evidence supports the view that snakes first lost their forelimbs and later lost their hindlimbs (Caldwell and Lee 1997; Graham and McGonnell 1999). Fossil snakes with hindlimbs, but no forelimbs, have been found. Moreover, while the most derived snakes (such as vipers) are completely limbless, more primitive snakes (such as boas and pythons) have pelvic girdles and rudimentary femurs.
The missing forelimbs can be explained by the Hox expression pattern in the anterior portion of the snake. In most vertebrates, the forelimb forms just anterior to the most anterior expression domain of Hoxc-6 (Gaunt 1994; Burke et al. 1995). Caudal to that point, Hoxc-6, in combination with Hoxc-8, helps specify vertebrae to be thoracic. During early python development, Hoxc-6 is not expressed in the absence of Hoxc-8, so the forelimbs do not form. Rather, the combination of Hoxc-6 and Hoxc-8 is expressed for most of the length of the organism, telling the vertebrae to form ribs throughout most of the body (Figure 22.9; Cohn and Tickle 1999).

Figure 22.9
Loss of limbs in snakes. (A) Skeleton of the garter snake, Thamnophis, stained with alcian blue. Ribbed vertebrae are seen from the head to the tail. (B, C) Hox expression patterns in chick (B) and python (C). (Photograph courtesy of A. C. Burke; B, C (more...)
The hindlimb buds do begin to form in pythons, but they do not make anything more than a femur. This appears to be due to the lack of sonic hedgehog expression by the limb bud mesenchyme. Sonic hedgehog is needed both for the polarity of the limb and for maintenance of the apical ectodermal ridge (AER). Python hindlimb buds lack the AER. Interestingly, the phenotype of the python hindlimb resembles that of mouse embryos with loss-of-function mutations of sonic hedgehog (Chiang et al. 1996).
Hox genes and atavisms
As we have seen, disruptions of the Hox genes can change one type of vertebra into another type. In some instances, the mutation of a Hox gene can produce “atavistic” conditions, wherein the organism resembles an earlier evolutionary stage. Deletion or misregulation of the Hoxa-2 genes in mice, for instance, results in a partial transformation of the second pharyngeal arch into a copy of the first pharyngeal arch. The mutant fetuses lack the stapes and styloid bones formed from the second arch, but have extra malleus, incus, tympanic, and squamosal bones. They also possess a rodlike cartilage that has no counterpart in normal mice, but looks like the pterygoquadrate cartilage thought to have been present in therapsids, the reptilian ancestors that gave rise to the mammals (Figure 22.10; Rijli et al. 1993; Lohnes et al. 1994; Mark et al. 1995). Thus, major evolutionary changes can be correlated with the alteration of Hox gene expression in different parts of the embryo.

Figure 22.10
Representation of skeletal elements derived from the first pharyngeal arch (in gray) and the second pharyngeal arch (in black). (AS, alisphenoid; I, incus; I2, duplicated incus; P and P2, normal and duplicated pteroid cartilage; PQ, pterygoquadrate cartilage; (more...)
Changes in Hox gene number
The number of Hox genes may play a role in permitting the evolution of complex structures. All invertebrates have a single Hox complex per haploid genome. In the most simple invertebrates—such as sponges—there appear to be only one or two Hox genes in this complex (Degnan et al. 1995; Schierwater and Kuhn 1998). In the more complex invertebrates, such as insects, there are numerous Hox genes in this complex. Comparing the Hox genes of chordates, arthropods, and molluscs suggests that there was a common set of seven Hox genes in the Urbilaterian ancestor of the protostomes and deuterostomes. Indeed, in invertebrate deuterostomes (echinoderms and amphioxus, an invertebrate chordate), there is only one Hox complex, which looks very much like that of the insects (Figure 22.11; Holland and Garcia-Fernández 1996). By the time the earliest vertebrates (agnathan fishes) evolved, there were at least four Hox complexes. The transition from amphioxus to early fish is believed to be one of the major leaps in complexity during evolution (Amores et al. 1998; Holland 1998). This transition involved the evolution of the head, the neural crest, new cell types (such as osteoblasts and odontoblasts), the brain, and the spinal cord. As we saw in Chapter 11, the regionalization of the brain and spinal cord is dependent upon Hox genes, and the regional specification of the somitic segments depends upon the paralogous members of the different Hox clusters. For instance, deletions of Hoxa-3 (from the A cluster) affect the neural crest-derived glands of the neck; deletions of Hoxd-3 (its paralogue from the D cluster) affect the somite-derived skeleton of the neck. This distinction may be due to the different levels of expression of these genes within the same tissues (Greer et al. 2000). Holland (1998) speculates that the generation of these new structures was allowed by the fourfold duplication of the Hox gene complex.
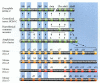
Figure 22.11
Postulated ancestry of the homeotic genes from a hypothetical ancestor of both deuterostomes and prototomes. Amphioxus has only one cluster, similar to that of insects. Vertebrates have four clusters, none of which is complete. (After Holland and Garcia-Fernández (more...)
Box
Assessing Homologies through Regulatory Gene Expression Patterns.
Footnotes
- *
The reason for this remarkable conservation of structure in the Hox gene complex is thought to be the sharing of cis-regulatory regions by neighboring genes. If a Hox gene is moved to a different region within the complex, its regulation is altered. The critical regulatory regimes might be the binding sites for the Polycomb proteins. These proteins are also conserved throughout evolution, and they silence the Hox genes at specific times and places. Here, then, we see a “phyletic constraint” at the molecular level (Chiang et al. 1995; Müller et al. 1995; Kmita et al. 2000).
- Hox Genes: Descent with Modification - Developmental BiologyHox Genes: Descent with Modification - Developmental Biology
Your browsing activity is empty.
Activity recording is turned off.
See more...