NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
General Concepts
General Biology of Human Herpesviruses
Of the more than 100 known herpesviruses, 8 routinely infect only humans: herpes simplex virus types 1 and 2, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, human herpesvirus 6 (variants A and B), human herpesvirus 7, and Kaposi's sarcoma virus or human herpesvirus 8. A simian virus, called B virus, occasionally infects humans. All herpesviruses can establish latent infection within specific tissues, which are characteristic for each virus.
Structure
Herpesviruses have a unique four-layered structure: a core containing the large, double-stranded DNA genome is enclosed by an icosapentahedral capsid which is composed of capsomers. The capsid is surrounded by an amorphous protein coat called the tegument. It is encased in a glycoprotein-bearing lipid bilayer envelope.
Classification
Herpesviruses are divided into three groups: The α herpesviruses, herpes simplex virus types 1 and 2, and varicella-zoster virus, have a short replicative cycle, induce cytopathology in monolayer cell cultures, and have a broad host range; β herpesviruses, cytomegalovirus, and human herpesviruses 6 and 7, with a long replicative cycle and restricted host range; and γ herpesviruses, Epstein-Barr virus and human herpesvirus 8, with a very restricted host range.
Multiplication
Transcription, genome replication, and capsid assembly occur in the host cell nucleus. Genes are replicated in a specific order: (1) immediate-early genes, which encode regulatory proteins; (2) early genes, which encode enzymes for replicating viral DNA; and (3) late genes, which encode structural proteins. The tegument and envelope are acquired as the virion buds out through the nuclear membrane or endoplasmic reticulum. Virions are transported to the cell membrane via the Golgi complex, and the host cell dies as mature virions are released. Alternatively, in selected cell types, the virus may be maintained in a latent state. The latent viral genome may reactivate at any time; the mechanism of reactivation is not known.
Diagnosis
Cytomegalovirus retinitis is diagnosed clinically. Diagnosis of all other herpesvirus infection relies on isolation of the virus through culturing and/or on detection of viral genes or gene products, particularly using polymerase chain reaction technology.
Control of Herpesvirus Infections
Prevention: A vaccine to prevent varicella-zoster virus infections was recently licensed in the United States. Vaccines against herpes simplex virus 2, and cytomegalovirus are undergoing extensive evaluations in field trials. Passive immunization with immunoglobulin or hyperimmune globulin is used either to prevent infection or as an adjunct to antiviral therapy.
Treatment: Infections with herpes simplex virus 1 and 2 and varicella-zoster virus are currently the most amenable to therapy; acyclovir, valaciclovir and famciclovir are all licensed therapeutics. Ganciclovir is used to treat cytomegalovirus retinitis. B virus appears to respond to either of these drugs. There is as yet no treatment for Epstein-Barr virus or human herpesvirus 6,7 or 8 infections.
Herpes Simplex Viruses
Clinical Manifestations
Herpes simplex viruses 1 and 2 have only about 50 percent genomic homology. However, they share most other characteristics. Mucocutaneous manifestations of herpes simplex virus infection include gingivostomatitis, herpes genitalis, herpetic keratitis, and dermal whitlows. Neonatal herpes simplex virus infection and herpes simplex virus encephalitis also occur.
Pathogenesis
The virus replicates initially in epithelial cells, producing a characteristic vesicle on an erythematous base. It then ascends sensory nerves to the dorsal root ganglia, where, after an initial period of replication, it establishes latency. During reactivated infection, the virus spreads distally from the ganglion to initiate new cutaneous and/or mucosal lesions.
Host Defenses
Interferon and humoral, mucosal, and cellular immunity are important defenses. Herpes simplex virus infections are more severe in immunocompromised hosts.
Epidemiology
Herpes simplex virus 1 transmission is primarily oral, and herpes simplex virus 2 primarily genital. Transmission requires intimate contact.
Varicella-Zoster Virus
Clinical Manifestations
Primary varicella-zoster virus infection causes varicella (chickenpox). Reactivation of latent virus (usually in adults) causes herpes zoster (shingles), manifesting as vesicular rash with a dermatomal distribution and acute neuritis.
Pathogenesis
Varicella-zoster virus is usually transmitted by droplets and replicates initially in the nasopharynx. In seronegative individuals, viremia and chickenpox ensue. Latency is established in dorsal root ganglia, and virus reactivation results in virion transport down sensory nerves.
Host Defenses
As with herpes simplex virus, interferon and cellular and humoral immunity are important defenses. Reactivated virus can cause disseminated disease in immunocompromised individuals.
Epidemiology
Varicella-zoster virus is highly contagious; about 95 percent of adults show serologic evidence of infection.
Cytomegalovirus
Clinical Manifestations
Cytomegalovirus causes three clinical syndromes. (1) Congenital cytomegalovirus infection (when symptomatic) causes hepatosplenomegaly, retinitis, rash, and central nervous system involvement. (2) In about 10 per cent of older children and adults, primary cytomegalovirus infection causes a mononucleosis syndrome with fever, malaise, atypical lymphocytosis, and pharyngitis. (3) Immunocompromised hosts (transplant recipients and human immunodeficiency virus [HIV]-infected individuals) may develop life-threatening disseminated disease involving the lungs, gastrointestinal tract, liver, retina, and central nervous system.
Pathogenesis
Cytomegalovirus replicates mainly in the salivary glands and kidneys and is shed in saliva and urine. Replication is slow, and the virus induces characteristic giant cells with intranuclear inclusions.
Epidemiology
Transmission is via intimate contact with infected secretions. Cytomegalovirus infections are among the most prevalent viral infections worldwide.
Epstein-Barr Virus
Clinical Manifestations
Epstein-Barr virus causes classic mononucleosis. In immunocompromised hosts, the virus causes a lymphoproliferative syndrome. In some families, Epstein Barr virus causes Duncan's syndrome.
Pathogenesis
Epstein Barr virus replicates the epithelial cells of the oropharynx and in β lymphocytes.
Epidemiology
Epstein Barr virus is transmitted by intimate contact, particularly via the exchange of saliva.
Human Herpesvirus 6 and 7
Clinical Manifestations
Human herpes viruses 6 and 7 are associated with exanthem subitem (roseola) and with rejection of transplanted kidneys.
Pathogenesis
The pathogenesis is poorly understood.
Epidemiology
Antibodies to this virus are present in almost everyone by age 5.
Human Herpesvirus 8
Clinical Manifestations
Human herpesvirus 8 has been found associated with Kaposi's sarcoma in AIDS patients as well as intra-abdominal solid tumors.
Pathogenesis and Epidemiology
Virtually nothing is known about the pathogenesis and epidemiology of this newly described herpesvirus.
B Virus
Clinical Manifestations
In humans, B virus causes an encephalitis that is usually fatal; survivors have brain damage.
Pathogenesis
B virus is transmitted to humans by the bite of infected rhesus monkeys and is transported up neurons to the brain.
Epidemiology
The reservoir for the disease is latent infection in rhesus monkeys, particularly those from Southeast Asia and India. In stressed or unhealthy animals, the virus may reactivate and appear in saliva.
Introduction
In nature, herpesviruses infect both vertebrate and non-vertebrate species, and over a hundred have been at least partially characterized. Only eight of these have been isolated routinely from humans and are discussed here. They are known as the human herpesviruses and are herpes simplex virus type 1, herpes simplex virus type 2, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, human herpesvirus 6, human herpesvirus 7 and, most recently, Kaposi's Sarcoma herpesvirus. A primate herpesvirus, namely B virus, is an uncommon human pathogen that may cause life-threatening disease.
General Biologic Properties
The human herpesviruses share four significant biologic properties. First, all of the herpesviruses code for unique enzymes involved in the biosynthesis of viral nucleic acids. These enzymes are structurally diverse and parenthetically provide unique sites for inhibition by antiviral agents. Secondly, the synthesis and assembly of viral DNA is initiated in the nucleus. Assembly of the capsid is also initiated in the nucleus. Third, release of progeny virus from the infected cell is accompanied by cell death. Finally, all herpesviruses establish latent infection within tissues that are characteristic for each virus, reflecting the unique tissue trophism of each member of this family.
Structure
Membership in the family Herpesviridae is based on the structure of the virion. These viruses contain double-stranded DNA which is located at the central core. The precise arrangement of the DNA within the core is not known. Herpesvirus DNA varies in molecular weight from approximately 80 to 150 million, or 120 to 250 kilobase pairs, depending on the virus. This DNA core is surrounded by a capsid which consists of 162 capsomers, arranged in icosapentahedral symmetry. The capsid is approximately 100 to 110 nanometers in diameter. Tightly adherent to the capsid is the tegument, which appears to consist of amorphous material. Loosely surrounding the capsid and tegument is a lipid bilayer envelope derived from host cell membranes. The envelope consists of polyamines, lipids, and glycoproteins. These glycoproteins confer distinctive properties to each virus and provide unique antigens to which the host is capable of responding.
A fascinating feature of herpesvirus DNA is its genomic sequence arrangement. Herpesviruses can be divided into six groups arbitrarily classified A to F. For those herpesviruses which infect humans (group C, group D, and group E) unique structures are demonstrable. In the group C genomes, as exemplified by Epstein-Barr virus and the newly identified Kaposi's sarcoma herpesvirus, the number of terminal reiterations divides the genome into several well-delineated domains. The group D genomes, such as varicella-zoster virus, have sequences from one terminus repeated in an inverted orientation internally. Thus, the DNA extracted from these virions consist of two equal molar populations. For group E viral genomes, such as herpes simplex virus and cytomegalovirus, the genomes are divided into internal unique sequences whereby both termini are repeated in an inverted orientation. Thus, the genomes can form four equimolar populations which differ in relative orientation of the two unique segments.
Classification
The grouping of herpesviruses into sub-families serves the purpose of identifying evolutionary relatedness as well as summarizing unique properties of each member.
Alpha herpesviruses
The members of the alpha herpesvirus sub-family are characterized by an extremely short reproductive cycle (hours), prompt destruction of the host cell, and the ability to replicate in a wide variety of host tissues. They characteristically establish latent infection in sensory nerve ganglia. This sub-family consists of herpes simplex virus 1 and 2 and varicella-zoster virus.
Beta herpesviruses
In contrast to the alpha herpesviruses, beta herpesviruses have a restricted host range. Their reproductive life cycle is long (days), with infection progressing slowly in cell culture systems. A characteristic of these viruses is their ability to form enlarged cells, as exemplified by human cytomegalovirus infection. These viruses can establish latent infection in secretory glands, cells of the reticuloendothelial system, and the kidneys.
Gamma herpesviruses
Finally, the gamma herpesviruses have the most limited host range. They replicate in lymphoblastoid cells in vitro and can cause lytic infections in certain targeted cells. Latent virus has been demonstrated in lymphoid tissue. Epstein-Barr virus is a member of this sub-family. In addition, human herpesvirus 6 and 7 are probably best classified as a gamma herpesvirus. However, the latter has host range properties of the beta sub-family. Further studies will need to clarify the most appropriate classification of this virus. Kaposi's sarcoma herpesvirus is most closely related genetically to Epstein-Barr virus.
Replication and Latency
Replication of all herpesviruses is a multi-step process. Following the onset of infection, DNA is uncoated and transported to the nucleus of the host cell. This is followed by transcription of immediate-early genes, which encode for the regulatory proteins. Expression of immediate-early gene products is followed by the expression of proteins encoded by early and then late genes.
Assembly of the viral core and capsid takes place within the nucleus. This is followed by envelopment at the nuclear membrane and transport out of the nucleus through the endoplasmic reticulum and the Golgi apparatus. Glycosylation of the viral membrane occurs in the Golgi apparatus. Mature virions are transported to the outer membrane of the host cell inside vesicles. Release of progeny virus is accompanied by cell death. Replication for all herpesviruses is considered inefficient, with a high ratio of non-infectious to infectious viral particles.
A unique characteristic of the herpesviruses is their ability to establish latent infection. Each virus within the family has the potential to establish latency in specific host cells, and the latent viral genome may be either extra-chromosomal or integrated into host cell DNA. Herpes simplex virus 1 and 2 and varicella-zoster virus all establish latency in the dorsal root ganglia. Epstein-Barr virus can maintain latency within B lymphocytes and salivary glands. Cytomegalovirus, human herpesvirus 6 and 7, Kaposi's sarcoma herpesvirus and B virus have unknown sites of latency.
Latent virus may be reactivated and enter a replicative cycle at any point in time. The reactivation of latent virus is a well-recognized biologic phenomenon, but not one that is understood from a biochemical or genetic standpoint. It should be noted here that an anti-sense message to one of the immediate-early genes (alpha-O) may be involved in the maintenance of latent virus. Stimuli that have been observed to be associated with the reactivation of latent herpes simplex virus have included stress, menstruation, and exposure to ultraviolet light. Precisely how these factors interact at the level of the ganglia remains to be defined. It should be noted that reactivation of herpesviruses may be clinically asymptomatic, or it may produce life-threatening disease.
Diagnosis
With the exception of cytomegalovirus retinitis, the definitive diagnosis of a herpesvirus infection requires either isolation of virus or detection of viral gene products. For virus isolation, swabs of clinical specimens or other body fluids can be inoculated into susceptible cell lines and observed for the development of characteristic cytopathic effects. This technique is most useful for the diagnosis of infection due to herpes simplex virus 1 and 2 or varicella-zoster virus because of their relatively short replicative cycles. The identification of cytomegalovirus by cell culture requires a longer period of time due to its prolonged period of replication. Epstein-Barr virus does not induce cytopathic changes in cell culture systems and, therefore, can only be identified in culture by transformation of cord blood lymphocytes. Similarly, human herpes virus 6 and 7 have unique growth characteristics which make identification in cell culture systems difficult.
Newer and more rapid diagnostic techniques involve the detection of viral gene products. This can be done by applying fluorescence antibody directed against immediate-early or late gene products to tissue cultures after 24 to 72 hours of incubation. A positive result is the appearance of intranuclear fluorescence. A method which utilizes monoclonal antibodies to an immediate-early gene has been most useful for the identification of CMV. Alternatively, fluorescence antibodies may be applied directly to cell monolayers or scrapings of clinical lesions, with intranuclear fluorescence again indicating a positive result.
Recently developed diagnostic techniques that have clinical utility include in situ and dot-blot hybridization and, importantly, polymerase chain reaction DNA amplification. This latter technique has proved most successful in the diagnosis of herpes simplex virus infections of the central nervous system, particularly when applied to cerebrospinal fluid. Importantly, this tool has been utilized to study the natural history of genital herpes simplex virus infections as well as identify new herpesvirus infections (i.e. Kaposi's sarcoma herpesvirus).
In addition to new tests for virus gene products and viral DNA, improved serologic assays are also becoming available, particularly the application of immunoblot technology to distinguishing herpes simplex virus 1 from 2 infections. However, these tests are only useful for making a diagnosis in retrospect.
Finally, the diagnosis of cytomegalovirus retinitis deserves special mention because it is made clinically by the presence of characteristic retinal changes. The diagnosis is further supported by the presence of cytomegalovirus viruria or viremia, but this is not an absolute requirement.
Herpes Simplex Viruses
Of all the herpesviruses, herpes simplex virus type 1 and herpes simplex virus type 2 are the most closely related, with nearly 70 per cent genomic homology. These two viruses can be distinguished most reliably by DNA composition; however, differences in antigen expression and biologic properties also serve as methods for differentiation.
Clinical Manifestations and Pathogenesis
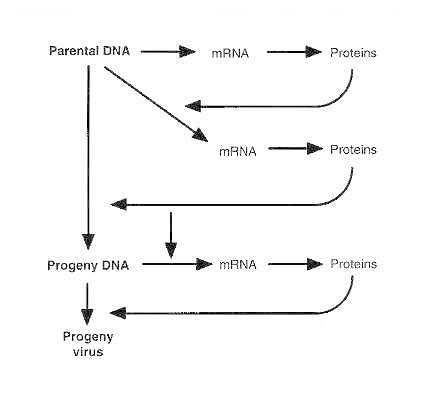
A critical factor for transmission of herpes simplex viruses, regardless of virus type, is the requirement for intimate contact between a person who is shedding virus and a susceptible host. After inoculation onto the skin or mucous membrane and an incubation period of four to six days, herpes simplex virus replicates in epithelial cells (Figure 68-1). As replication continues, cell lysis and local inflammation ensue, resulting in characteristic vesicles on an erythematous base. Regional lymphatics and lymph nodes become involved: viremia and visceral dissemination may develop depending upon the immunologic competence of the host. In all hosts, the virus generally ascends the peripheral sensory nerves to reach the dorsal root ganglia. Replication of herpes simplex virus within neural tissue is followed by retrograde axonal spread of the virus back to other mucosal and skin surfaces via the peripheral sensory nerves. Virus replicates further in epithelial cells, reproducing the lesions of the initial infection, until infection is contained through both systemic and mucosal immunity.
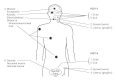
Figure 68-1
Pathogenesis of HSV infections.
Latency is established when herpes simplex virus reaches the dorsal root ganglia after anterograde transmission via sensory nerve pathways. In its latent form, intracellular herpes simplex virus DNA cannot be detected routinely unless specific molecular probes are utilized.
Mucocutaneous Infections
Gingivostomatitis
Mucocutaneous infections are the most common clinical manifestations of herpes simplex virus 1 and 2. Gingivostomatitis, which is usually caused by herpes simplex virus 1, occurs most frequently in children less than five years of age. Gingivostomatitis is characterized by fever, sore throat, pharyngeal edema and erythema, followed by the development of vesicular or ulcerative lesions on the oral and pharyngeal mucosa. Recurrent herpes simplex virus 1 infections of the oropharynx most frequently manifest as herpes simplex labialis (cold sores), and usually appear on the vermillion border of the lip. Intraoral lesions as a manifestation of recurrent disease are uncommon in the normal host but do occur frequently in immunocompromised individuals.
Genital Herpes
Genital herpes is most frequently caused by herpes simplex virus 2 but an ever increasing number of cases are attributed to herpes simplex virus 1. Primary infection in women usually involves the vulva, vagina, and cervix (Figure 68-2). In men, initial infection is most often associated with lesions on the glans penis, prepuce or penile shaft. In individuals of either sex, primary disease is associated with fever, malaise, anorexia, and bilateral inguinal adenopathy. Women frequently have dysuria and urinary retention due to urethral involvement. It is estimated that as many as 10 per cent of individuals will develop an aseptic meningitis with primary infection. Sacral radiculomyelitis may occur in both men and women, resulting in neuralgias, urinary retention, or obstipation. The complete healing of primary infection may take several weeks. It has been recognized that the first episode of genital infection is less severe in individuals who have had previous herpes simplex virus infections at other sites, such as herpes simplex labialis.
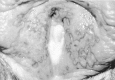
Figure 68-2
Genital herpes lesions of the vulva.
Recurrent genital infections in either men or women can be particularly distressing. The frequency of recurrence varies significantly from one individual to another. It has been estimated that one-third of individuals with genital herpes have virtually no recurrences, one-third have approximately three recurrences per year, and another one-third greater than three per year. Recent seroepidemiologic studies have found that between 25 percent and 65 percent of individuals in the United States in 1988 had antibodies to herpes simplex virus 2, and that seroprevalence is dependent upon the number of sexual partners. If genital swabs from women with a history of recurrent genital herpes are subjected to polymerase chain reaction, virus DNA can be detected in the absence of culture proof of infection. This finding suggests the chronicity of genital herpes as opposed to a recurrent infection.
Herpetic Keratitis
Herpes simplex keratitis is usually caused by herpes simplex virus 1 and is accompanied by conjunctivitis in many cases. It is considered the most common infectious cause of blindness in the United States. The characteristic lesions of herpes simplex keratoconjunctivitis are dendritic ulcers best detected by fluorescein staining. Deep stromal involvement has also been reported and may result in visual impairment.
Other Skin Manifestations
Herpes simplex virus infections can manifest at any skin site. Common among health care workers are lesions on abraded skin of the fingers, known as herpetic whitlows (Figure 68-3). Similarly, wrestlers, because of physical contact may develop disseminated cutaneous lesions known as herpes gladiatorum.
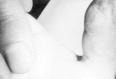
Figure 68-3
Herpetic whitlow involving the thumb.
Neonatal Herpes Simplex Virus Infection
Neonatal herpes simplex virus infection is estimated to occur in approximately one in 3000 deliveries in the United States annually. Approximately 70 percent of the cases are caused by herpes simplex virus 2 and usually result from contact of the fetus with infected maternal genital secretions at the time of delivery. Manifestations of neonatal herpes simplex virus infection can be divided into three categories: 1) skin, eye and mouth disease; 2) encephalitis; and 3) disseminated infection. As the name implies, skin, eye and mouth disease consists of cutaneous lesions and does not involve other organ systems (Figure 68-4). Involvement of the central nervous system may occur with encephalitis or disseminated infection, and generally results in a diffuse encephalitis. The cerebrospinal fluid formula characteristically reveals an elevated protein and a mononuclear pleocytosis. Disseminated infection involves multiple organ systems and can produce disseminated intravascular coagulation, hemorrhagic pneumonitis, encephalitis, and cutaneous lesions. Diagnosis can be particularly difficult in the absence of skin lesions. The mortality rate for each disease classification varies from zero for skin, eye and mouth disease to 15 per cent for encephalitis and 60 percent for neonates with disseminated infection. In addition to the high mortality associated with these infections, morbidity is significant in that children with encephalitis or disseminated disease develop normally in only approximately 40 per cent of cases, even with the administration of appropriate antiviral therapy.
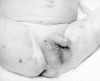
Figure 68-4
Cutaneous lesions caused by HSV in a neonate.
Herpes Simplex Encephalitis
Herpes simplex encephalitis is characterized by hemorrhagic necrosis of the inferiomedial portion of the temporal lobe (Figure 68-5). Disease begins unilaterally, then spreads to the contralateral temporal lobe. It is the most common cause of focal, sporadic encephalitis in the United States today, and occurs in approximately 1 in 150,000 individuals. Most cases are caused by herpes simplex virus 1. The actual pathogenesis of herpes simplex encephalitis requires further clarification, although it has been speculated that primary or recurrent virus can reach the temporal lobe by ascending neural pathways, such as the trigeminal tracts or the olfactory nerves.
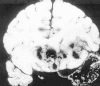
Figure 68-5
Hemorrhagic necrosis of the temporal lobe due to HSV encephalitis.
Clinical manifestations of herpes simplex encephalitis include headache, fever, altered consciousness, and abnormalities of speech and behavior. Focal seizures may also occur. The cerebrospinal fluid formula for these patients is variable, but usually consists of a pleocytosis with both polymorphonuclear leukocytes and monocytes present. The protein concentration is characteristically elevated and glucose is usually normal. Historically, a definitive diagnosis could only be achieved by brain biopsy, since other pathogens may produce a clinically similar illness. However, the application of polymerase chain reaction for detection of virus DNA has replaced brain biopsy as the standard for diagnosis. The mortality and morbidity are high, even when appropriate antiviral therapy is administered. At present, the mortality rate is approximately 30 per cent one year after treatment. In addition, approximately 70 per cent of survivors will have significant neurologic sequelae.
Herpes Simplex Virus Infections in the Immunocompromised Host
Herpes simplex virus infections in the immunocompromised host are clinically more severe, may be progressive, and require more time for healing. Manifestations of herpes simplex virus infections in this patient population include pneumonitis, esophagitis, hepatitis, colitis, and disseminated cutaneous disease. Individuals suffering from human immunodeficiency virus infection may have extensive perineal or orofacial ulcerations. Herpes simplex virus infections are also noted to be of increased severity in individuals who are burned.
Epidemiology
Transmission of herpes simplex virus is dependent upon intimate contact. Thus, herpes simplex virus 1 is usually transmitted by kissing or other contact with saliva, while herpes simplex virus 2 is usually a consequence of sexual contact. Nosocomial spread of herpes simplex virus 2 has been documented, particularly in newborn intensive care units.
Varicella-Zoster Virus
Clinical Manifestations and Pathogenesis
Varicella-zoster virus is one of the most common viruses encountered by humans. Varicella-zoster virus is usually transmitted by airborne routes (droplet spread) with initial replication in the oropharynx (Figure 68-6). In the susceptible or seronegative individual, replication of virus in the oropharynx leads to primary viremia, with subsequent development of a vesicular rash. The replication of varicella-zoster virus in vitro is similar to that for herpes simplex virus, although the period of replication is somewhat prolonged.
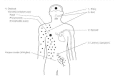
Figure 68-6
Pathogenesis of VZV infections.
Varicella
Varicella, or chickenpox, is the manifestation of primary varicella-zoster virus infection. This infection occurs most commonly in young children of preschool age and has a characteristic disseminated vesicular rash which appears after an incubation period of 14 to 17 days. The rash begins on the face and trunk and spreads to the extremities. The lesions of chickenpox are initially vesicles which become pustular, crusted, and then scabbed prior to healing. The average duration of lesion formation is three to five days in the normal child; however, it is usually longer in adolescents and adults and certainly in the immunocompromised. At the time of primary infection, varicella-zoster virus may establish latency in dorsal root ganglia.
Herpes Zoster
The recurrent form of varicella-zoster virus is herpes zoster or shingles. This form of infection, which is a reactivation of latent virus, typically manifests as a localized vesicular rash with a dermatomal distribution. The rash initially appears within the dermatome as erythema, which is soon followed by the development of vesicles (Figure 68-7). Some individuals will have coalescence of vesicles into bullous lesions. New vesicles may form for five to seven days, then evolve through the sequence of healing described for the lesions of varicella. The average time to healing for individuals with shingles ranges from 10 to 21 days, depending upon the age and immune status of the individual.
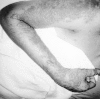
Figure 68-7
The vesicular rash of herpes zoster.
Characteristic of herpes zoster is the appearance of both acute neuritis and post-herpetic neuralgia. Acute neuritis is present in most individuals with localized zoster, the exception being young children. Post-herpetic neuralgia will develop in as many as 50 per cent of adults, depending upon the age of the individual. The treatment of acute neuritis and post-herpetic neuralgia can be problematic for individual patients.
Varicella-Zoster Virus Infections in the Immunocompromised Host
Serious complications of chickenpox in the non-immunocompromised child are rare, but secondary bacterial infection can be problematic. Adults and immunocompromised children have a higher incidence of visceral disease. Immunocompromised children, particularly those with acute lymphoblastic leukemia, are at increased risk for progressive disease, specifically resulting in pneumonitis and/or hepatitis. It is estimated that as many as one out of three of immunocompromised children suffer visceral disease, with a mortality of 15 per cent in the absence of antiviral therapy.
Herpes zoster in the immunocompromised host may be associated with cutaneous dissemination and visceral complications. In the absence of antiviral therapy, as many as 25 per cent of individuals with lymphoproliferative malignancies will have cutaneous dissemination and 10 per cent will develop visceral complications with an overall mortality rate of approximately 8 per cent.
Epidemiology
The spread of varicella-zoster virus depends upon airborne droplet transmission from a person who is shedding virus to a susceptible host. By adulthood, as many as 90 to 95 per cent of individuals have serologic evidence of infection with varicella-zoster virus.
The epidemiology of herpes zoster is more complicated. It does not appear that herpes zoster can be transmitted from one individual to another. However, spread of virus from the vesicles of herpes zoster may lead to the development of varicella in a susceptible host. Individuals over the age of 50 experience zoster at a frequency of approximately 1 per cent.
Cytomegalovirus
Clinical Manifestations
Cytomegalovirus infection can result in one of three distinct clinical syndromes. Congenital cytomegalovirus infection is a common occurrence in the United States today, occurring in approximately one per cent of all live births. Approximately 10 per cent of congenitally infected children will have severe symptomatic congenital cytomegalic inclusion disease, as evidenced by hepatosplenomegaly, retinitis, a petechial/purpuric skin rash, and involvement of the central nervous system (ventriculomegaly, intracranial calcifications, etc) (Figure 68-8). Some children who excrete the virus at birth, but have no other symptoms, may later have impaired hearing. An additional 10 to 25 per cent of children acquire cytomegalovirus infection early in life, either through contact with infected maternal genital secretions, blood transfusions (in the premature) or by acquisition from breast milk. Symptomatic congenital disease is most frequent when the mother has a primary cytomegalovirus infection during gestation and is extremely uncommon when the infection is acquired after the neonatal period.
Figure 68-8
Pathogenesis of CMV infections.
The second manifestation of cytomegalovirus infection is that of a mononucleosis syndrome. This occurs in approximately 10 per cent of primary cytomegalovirus infections in older children and adults; the remaining 90 per cent have asymptomatic primary infection. Mononucleosis in these patients is heterophile negative, but otherwise similar to classic Epstein-Barr virus mononucleosis. Patients characteristically have fever, malaise, atypical lymphocytosis, pharyngitis and, rarely, cervical adenopathy or hepatitis. Cytomegalovirus mononucleosis can be distinguished from Epstein-Barr virus mononucleosis by the absence of specific antibodies to either nuclear or viral capsid antigens of Epstein-Barr virus.
The third clinical entity is cytomegalovirus infection in severely immunocompromised individuals. In contrast to symptomatic congenital and mononucleosis infections, which are most commonly manifestations of primary cytomegalovirus infection, immunocompromised hosts may experience life-threatening disease from either primary or reactivated cytomegalovirus infection. In these patients, infection can involve the lungs, gastrointestinal tract, liver, retina, and central nervous system (Figure 68-9). Individuals at high risk for severe disease due to cytomegalovirus infection include organ transplant recipients, particularly bone marrow transplant recipients, and individuals with human immunodeficiency virus infection. Patients with human immunodeficiency virus infection and bone marrow transplant recipients seem particularly at risk for the development of CMV pneumonia.
Figure 68-9
Cytomegalovirus retinitis.
Pathogenesis
Replication of cytomegalovirus is most prominent in cells of glandular origin, particularly in the salivary glands and the kidneys. As a result, large quantities of virus can be shed in saliva and urine. The replicative cycle of cytomegalovirus in these organs is more prolonged than that of other herpesviruses and produces characteristic multi-nucleated giant cells with Cowdry type A intranuclear inclusions. Intracytoplasmic inclusion bodies may also be present, but are less easily demonstrated. These giant cells can be found in the parotid gland, and similar cells can be seen excreted in the urine.
Cytomegalovirus can cause persistent infection in various tissues, including those of the salivary glands, breasts, kidneys, endocervix, seminal vesicles and peripheral blood leukocytes. This persistent infection leads to chronic viral excretion by the involved organ. Transmission of virus is through contact with infected secretions. The average incubation period is four to six weeks. It should also be noted that the kidneys of organ donors can be a source of cytomegalovirus for the recipient, and that peripheral blood leukocytes have been implicated in the transmission of cytomegalovirus via blood transfusion.
Epidemiology
Cytomegalovirus infections are among the most prevalent virus infections worldwide. As with other herpesviruses, transmission is by intimate contact. Large quantities of virus can be excreted in saliva and urine for prolonged periods of time. Transmission of virus from mother to child can occur by one of several routes, including infected breast milk, cervical secretions, and saliva. Conversely, a child can transmit infection to the mother through infected secretions or urine.
Moreover, transmission of cytomegalovirus by children in the day care environment has introduced new occupational risks, particularly for seronegative women of child-bearing age. Hence these susceptible women are at risk for developing primary infection during gestation and delivering a child with symptomatic congenital cytomegalovirus infection.
Reactivation of cytomegalovirus infection in immunosuppressed individuals can be particularly problematic, as noted above. The extent of immunosuppression is a major determinant for severity of disease. In addition, those seronegative individuals who receive organs from persons seropositive for CMV can develop a life-threatening primary CMV infection.
Epstein-Barr Virus
Clinical Manifestations
The most significant clinical manifestations of Epstein-Barr virus infection are those associated with classic mononucleosis. Epstein-Barr virus mononucleosis is the most common that occurs in humans. The predominant findings are malaise, myalgia, pharyngitis, cervical adenopathy, splenomegaly, and atypical lymphocytosis. The diagnosis is confirmed by demonstrating heterophile antibodies or type-specific antibodies to nuclear antigen and viral capsid antigen to Epstein-Barr virus.
Pathogenesis
Epstein-Barr virus is trophic for B-lymphocytes. Replication has been documented in the parotid gland, as well as other lymphatic tissues. Evidence of lytic disease, as evidenced by the formation of multinucleated giant cells, is not apparent with infection caused by Epstein-Barr virus.
Epstein-Barr Virus Infections in the Immunocompromised Host
Epstein-Barr virus has also been incriminated as a cause of lymphoproliferative disease in highly immunocompromised individuals. The development of lymphoproliferative malignancy in heart and bone marrow transplant recipients has been documented, and is felt to be associated with the presence of virus.
Epidemiology
Epstein-Barr virus is transmitted by intimate contact. Exchange of saliva provides a major route for horizontal transmission of infection. Excretion of virus from other sites does occur, but does not appear to be a major vector for transmission of infection.
Human Herpesvirus 6 and 7
Clinical Manifestations
Human herpesvirus 6 and 7 have recently been isolated. Human herpes virus 6 (as has human herpes virus 7, but to a lesser extent), has been associated with exanthem subitum, or roseola. This illness is characterized by 3 – 5 days of fever, followed by the appearance of a maculopapular “slapped cheek” rash. In addition, there has been an association between human herpesvirus 6 and rejection of transplanted kidneys, fulminant hepatitis and infections of the central nervous system.
Pathogenesis
The reservoir and mode of transmission of human herpesvirus 6 and 7 are not well understood at the present time. It should be noted that high prevalence of antibodies early in life would implicate transmission within the home from oropharyngeal secretions; however, this has not yet been documented.
Epidemiology
The epidemiology of human herpesvirus 6 and 7 is poorly understood at present. Loss of transplacental antibodies, followed by acquisition of antibodies early in life, implies horizontal transmission within the home environment. By the age of 5, antibodies are present in virtually 100 per cent of the population to both of these viruses. Human herpesvirus 6 exists as type A and B. The type A variant was the original isolate being retrieved from an immunocompromised host. The type B variant is associated with roseola. Some investigators suggest that the genetic differences in these two types warrant distinct names; thus, it is conceivable that the International Herpesvirus Nomenclature Committee may designate these agents as distinct.
Kaposi's Sarcoma Herpesvirus
Recently, a new herpesvirus has been associated with Kaposi's sarcoma and AIDS-related lymphomas of organ cavities. The DNA of this virus is partially homologous to the DNA of Epstein-Barr virus and that of herpesvirus saimiri. This virus immortalizes B lymphocytes. Isolation of the virus has yet to be achieved.
B-Virus
Clinical Manifestations
A major concern following exposure to B virus is the development of an almost uniformly fatal encephalitis in most individuals. The total number of cases reported in the world's literature is under 30, with a mortality of approximately 75 percent. Survivors of B virus infection of the central nervous system have been left with a broad spectrum of neurologic impairment. Recurrent cutaneous disease has been noted, but generally only in patients who initially had a severe encephalitis.
Pathogenesis
Fortunately, B virus infections in humans are uncommon, because humans are not the natural reservoir of this infection. Instead, the virus is found routinely in rhesus monkey colonies. Infection is transmitted to humans by the bite of an infected animal. Virus replicates locally in a fashion very similar to that of HSV infection. An important difference, however, is that there is a predisposition for rapid neuronal transport of virus to the central nervous system, with ensuing encephalitis in most cases.
Epidemiology
B virus is resident in rhesus monkeys, particularly those obtained from Southeast Asia and India. As is the case with human herpesvirus infections, crowding and stress of monkeys lead to virus reactivation and excretion in saliva. Improper animal handling techniques can cause personnel to be exposed to this virus. Strict adherence to guidelines for the handling of rhesus monkeys is advised.
Control of Herpesvirus Infection
Prevention
At present, only one vaccine is licensed for the prevention of a herpesvirus infection; it is directed against varicella-zoster virus. This live, attenuated vaccine is intended for use in the normal child, and not in immunocompromised individuals. Experimental vaccines for herpes simplex virus 1 and 2 and cytomegalovirus are in various stages of clinical trials. Vaccines engineered for the control of Epstein-Barr virus are in early stages of development.
Passive immunization with immune or hyperimmune serum, including monoclonal antibodies, has been used either to prevent infection or as an adjunct to therapy. The administration of varicella-zoster virus immune globulin to the immunocompromised child exposed to this virus is routinely used to prevent, or at least attenuate, chickenpox in these high-risk individuals. More recently, cytomegalovirus immune globulin has been utilized along with antiviral drugs to treat life-threatening infection in immunocompromised patients, with reported success.
Treatment
Infections due to herpes simplex virus, varicella-zoster virus and, to a lesser extent, cytomegalovirus are the most amenable to therapy with antiviral drugs. Acyclovir has proved useful for the management of specific infections caused by herpes simplex and varicella-zoster viruses. At present, acyclovir is the treatment of choice for mucocutaneous HSV infections in the immunocompromised host, herpes simplex encephalitis, neonatal herpes simplex virus infections, and varicella-zoster virus infections in the immunocompromised host. Intravenous administration is preferred for therapy against life-threatening disease. Immunocompromised individuals with mucocutaneous herpes simplex virus infections that are not life-threatening may be given oral acyclovir. Caution must be exercised when acyclovir is used intravenously, because it may crystallize in the renal tubules when given too rapidly or to dehydrated patients.
Recently, two prodrugs have been licensed for the treatment of herpes zoster in the elderly. Valaciclovir, the prodrug of acyclovir, and famciclovir, the prodrug of penciclovir, provide high plasma levels of the parent compounds and offer added efficacy as well as decreased dosing frequency in the management of shingles.
Ganciclovir and foscarnet are licensed for the treatment of cytomegalovirus retinitis in immunocompromised individuals. Treatment with ganciclovir is associated with potential hematologic toxicity, notably neutropenia and thrombocytopenia. Dose reductions are required if evidence of toxicity appears. Foscarnet is associated with electrolyte imbalances, particularly hypocalcemia.
B virus infections of humans have been treated both with acyclovir and ganciclovir with some reports of success; however, no controlled studies have been performed.
There is no form of therapy for infection due to Epstein-Barr virus, human herpesvirus 6 or 7 or Kaposi's sarcoma virus at this time.
References
- Bloom JN, Palestine AG: The diagnosis of cytomegalovirus retinitis. Ann Intern Med . [PubMed: 2848436]
- Chang Y, Cesarman E, Pessin MS. et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865. [PubMed: 7997879]
- Corey L, Spear P. Infections with herpes simplex viruses. N Engl J Med. 1986;314:686. [PubMed: 3005858]
- Corey L, Spear P. Infections with herpes simplex viruses. N Engl J Med. 1986;314:749. [PubMed: 3005859]
- Drew WL. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988;158:449. [PubMed: 2841381]
- Ho M: Cytomegalovirus. p.1351. In Mandell GL, Bennett JE, Dolin R (eds): Principles and Practice of Infectious Diseases, 4th Ed., Churchill Livingstone, New York, 1995 .
- Nalesnik NA. Pathology of posttransplant lymphoproliferative disorders occurrig in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173. [PMC free article: PMC1880655] [PubMed: 2845789]
- Roizman B: Herpesviridae: A brief introduction. In press. In Fields BN, Knipe DM,
- Chanock E, Hirsch M, Melnick J, Monath T, Roizman B (eds): Virology, 3rd Ed., Raven Press, New York, 1995 .
- Roizman BR. New Viral Footprints in Kaposi's Sarcoma. N Eng J Med. 1995; 332:1227- [PubMed: 7700319]
- Schooley RT: Epstein-Barr virus (Infectious Mononucleosis). p. 1364. In: Mandell GL,
- Bennett JE, Dolin R (eds): Principles and Practice of Infectious Diseases, 4th Edition, Churchill Livingstone, New York, 1995 .
- Straus S: Introduction to herpesviridae. p. 1330. In Mandell GL, Bennett JE, Dolin R (eds): Principles and Practice of Infectious Diseases, 4th Ed., Churchill Livingstone, New York, 1995 .
- Whitley RJ. Cercopithecine Herpes Virus 1 (B Virus). in press. In Fields BN, Knipe DM,
- Chanock E, Hirsch M, Melnick J, Monath T, Roizman B (eds): Virology, 3rd Edition, Raven Press, New York, 1995 .
- Whitley RJ, Gnann JW: p. 329. Antiviral therapy. In Roizman BR, Whitley RJ, Lopez C .
- Whitley RJ, Schlitt M: Encephalitis caused by herpesviruses, including B virus. p. 41. In Scheld WM, Whitley RJ, Durack DT (eds). Infections of the Central Nervous System. Raven Press, New York. 1991 .
- Contribution of carbohydrate-related metabolism in Herpesvirus infections.[Curr Res Microb Sci. 2023]Contribution of carbohydrate-related metabolism in Herpesvirus infections.Ma F, Fa C, Aj N, Aa S, Ia PF, Lj C, Pa G. Curr Res Microb Sci. 2023; 4:100192. Epub 2023 May 17.
- Review Herpesvirus infections in persons infected with human immunodeficiency virus.[Clin Infect Dis. 1995]Review Herpesvirus infections in persons infected with human immunodeficiency virus.Stewart JA, Reef SE, Pellett PE, Corey L, Whitley RJ. Clin Infect Dis. 1995 Aug; 21 Suppl 1:S114-20.
- Review Herpesviruses and the microbiome.[J Allergy Clin Immunol. 2013]Review Herpesviruses and the microbiome.Dreyfus DH. J Allergy Clin Immunol. 2013 Dec; 132(6):1278-86. Epub 2013 Apr 20.
- Animal herpesviruses and their zoonotic potential for cross-species infection.[Ann Agric Environ Med. 2015]Animal herpesviruses and their zoonotic potential for cross-species infection.Woźniakowski G, Samorek-Salamonowicz E. Ann Agric Environ Med. 2015; 22(2):191-4.
- Usefulness of Herpes Consensus PCR methodology to routine diagnostic testing for herpesviruses infections in clinical specimens.[Virol J. 2007]Usefulness of Herpes Consensus PCR methodology to routine diagnostic testing for herpesviruses infections in clinical specimens.Vrioni G, Kalogeropoulos C, Gartzonika C, Priavali E, Levidiotou S. Virol J. 2007 Jun 12; 4:59. Epub 2007 Jun 12.
- Herpesviruses - Medical MicrobiologyHerpesviruses - Medical Microbiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
