NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2010.

How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General.
Show detailsThe Global Tobacco Epidemic
Tobacco use remains the leading preventable cause of premature death in the United States, and the World Health Organization (WHO) has called tobacco use “the single most preventable cause of death in the world today” (WHO 2008, p. 8). Predictions based on large population studies indicate that one-half of all long-term smokers, particularly those who began smoking in adolescence, will eventually die from their use of tobacco. Furthermore, one-half of the deaths caused by smoking will occur in middle age (35 through 69 years), resulting in the loss of 20 to 25 years of normal life expectancy (Peto et al. 1992, 2006; Doll et al. 1994). In the 45 years since the first U.S. Surgeon General’s report on smoking and health was published in 1964 (U.S. Department of Health, Education, and Welfare [USDHEW] 1964), smoking has been the primary underlying cause of more than 12 million U.S. deaths. Each year since 2004, more than 430,000 additional smoking-attributable deaths have been added to the national total (U.S. Department of Health and Human Services [USDHHS] 2004; Bonnie et al. 2007; Centers for Disease Control and Prevention [CDC] 2008a). It has been estimated that worldwide, tobacco use caused 100 million deaths during the twentieth century, and that tobacco use may cause as many as 1 billion deaths in the twenty-first century, unless urgent and effective action is taken (WHO 2008).
Understanding the health consequences and diseases caused by tobacco use has provided the scientific foundation for public health actions aimed at tobacco use prevention, cessation, and protection from secondhand smoke exposure. Since the first Surgeon General’s report, this series has considered research findings on mechanisms of disease production in assessing the biologic plausibility of associations observed in epidemiologic studies. The important contribution of evidence on biologic plausibility and coherence in evaluation of causality has been reviewed in recent reports (USDHHS 2004, 2006). For example, evidence regarding the biologic plausibility of the observed relationship between exposure to secondhand smoke and coronary heart disease (CHD) has been very important in the evaluation of causality (USDHHS 2001, 2006). In initial studies (e.g., Hirayama 1984; Garland et al. 1985), the estimated magnitude of the association between exposure to secondhand smoke and CHD seemed large compared with the association between active smoking and CHD. However, further findings on mechanisms linking tobacco smoke exposure to CHD risk—in particular, the impact of tobacco smoke exposure on platelet aggregation and acute endothelial dysfunction—provided plausible and quantitatively consistent mechanisms for the observed nonlinear relationship with exposure levels to tobacco smoke (Glantz and Parmley 1991, 1995; Law et al. 1997). More recently, the potential effect of active smoking and exposure to secondhand smoke on breast cancer risk is an area for which data on biologic plausibility and mechanisms are critically needed in the evaluation of potential causality (USDHHS 2006; International Agency for Research on Cancer [IARC] 2004; Miller et al. 2007; Phillips and Garte 2008).
Despite the wealth of scientific evidence on the adverse health effects of exposure to tobacco smoke, many gaps remain in our understanding of the molecular and cellular mechanisms of tobacco-induced diseases. It has been suggested that “given the obvious dangers of tobacco and the associated imperative to eliminate it, research undertaken purely to unravel mechanisms of tobacco-related cancer is difficult to justify” (Carlsten and Burke 2006, p. 2481). However, as discussed in Chapter 1, research to further understand the biologic mechanisms by which exposure to tobacco smoke causes disease has several important applications beyond assisting in the determination of causal relationships, including
- developing biomarkers of injury to identify smokers at early stages of disease development;
- providing a basis for preventive therapies that block or reverse the underlying process of injury;
- identifying the contribution of exposure to tobacco smoke to causation of diseases with multiple etiologic factors; and
- assessing tobacco products for their potential to cause injury through a particular mechanism.
Expanding our knowledge of several common molecular and cellular mechanisms underlying seemingly diverse smoking-induced diseases—such as dysregulation of inflammatory and immune processes (including oxidative stress, altered antibody production, endothelial cell dysfunction, suppression of T cells) and the dysregulation of inflammatory cells—could have important implications in the potential development of novel therapeutic targets for various environmentally induced diseases (Wang and Scott 2005). For example, with growing understanding of genetic and epigenetic mechanisms, opportunities are expanding to address the broader applications of disease mechanisms related to exposure to tobacco smoke (Esteller 2008; Herbst et al. 2008; Caporaso et al. 2009; Breton et al. 2009; National Cancer Institute [NCI] 2009). As noted in the 2007–2008 Annual Report of the President’s Cancer Panel, “…even if all current smokers cease using tobacco today and no new smokers take up the habit, the latency of tobacco-caused cancer and other diseases dictates that cancer and other morbidity and mortality from tobacco will still be affecting our population for at least another two decades” (Reuben 2008, p. 57). A list of research priorities identified in this report is provided in Appendix 9.1.
A key feature of tobacco use is the development of nicotine addiction, which often leads to chronic, daily exposure to tobacco that typically persists for many years. As reviewed in Chapter 4 of this report, addiction, or more technically the diseases of dependence and withdrawal, make it essential to ensure that effective behavioral and pharmacological cessation treatments are widely available and accessible to diverse populations of smokers (Zaza et al. 2005; National Institutes of Health [NIH] State-of-the-Science Panel 2006). The various treatments for tobacco use have targeted different aspects of nicotine addiction, such as reinforcement, withdrawal, and cue-associated learning. Pharmacologic treatments have included nicotinic acetylcholine receptor ligands (nicotine replacement, and varenicline, a partial α4β2 agonist) or have involved alterations in signal transduction to stimulate the release of the same neurochemicals that are released by nicotine (e.g., bupropion and nortriptyline). Ideally, those who smoke would find it as easy to access cessation services as they do commercial tobacco products (Fiore et al. 2008).
Effective public health and clinical approaches to increase smoking cessation rates have been developed (USDHHS 2000; Zaza et al. 2005; Bonnie et al. 2007; Reuben 2007; Fiore et al. 2008). These public health strategies and the clinical treatments need to be more fully implemented.
Significant progress has been achieved in the United States during the last 50 years in reducing smoking initiation and increasing smoking cessation (Bonnie et al. 2007; Reuben 2007; Cokkinides et al. 2009). After 30 years of declining rates of smoking, particularly among men, the total U.S. cancer deaths began to decline in the late 1990s, driven largely by a reduction in male lung cancer deaths (Cokkinides et al. 2009). Moreover, the declines in lung cancer death rates among men and women in California declined more rapidly than in the rest of the country after the implementation of a comprehensive and sustained statewide tobacco control program (Barnoya and Glantz 2004; Jemal et al. 2008).
Today, an estimated one-half of all Americans who have ever smoked have quit (CDC 2008a, 2009a), and the benefits of cessation have been documented for smokers of all ages (USDHHS 1990; IARC 2007a). However, as a group, smokers who successfully quit early in life can avoid a large proportion of the excess mortality caused by smoking (USDHHS 1990; IARC 2007a). Data from the British Doctors’ Study have been used to demonstrate the lifetime risks of smoking and the amount of that risk that can be avoided by sustained cessation at various ages (Doll et al. 2004). Figure 9.1 contrasts the cumulative survival curves for all-cause mortality of continuing smokers (blue lines), with never smokers (red lines), and with smokers who quit by various ages (e.g., effect from sustained quitting at ages 35, 40, 50, and 60 years). The survival curves demonstrate that even at older ages, a substantial and important fraction of the excess all-cause mortality due to smoking can be averted by sustained quitting. Nonetheless, smokers who quit after the age of 44 years continue to have excess risk for tobacco-related diseases.
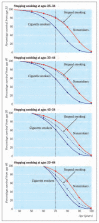
Figure 9.1
Effects on survival of stopping smoking cigarettes at ages 25–34 years (effect from age 35), ages 35–44 years (effect from age 40), ages 45–54 years (effect from age 50), and ages 55–64 years (effect from age 60). Source: (more...)
As noted in Chapter 6, cigarette smoking is a major cause of cardiovascular disease (CVD) and has multiplicative interactions with the other major risk factors for CHD. Importantly, although there is a strong dose-response relationship between the number of cigarettes smoked per day and cardiovascular risk, the relationship is not linear, with those who smoke few cigarettes per day or who do not smoke every day remaining at significantly elevated risk for CVD. It has been estimated that one-fifth of U.S. smokers are intermittent or occasional smokers (CDC 2008a), and this pattern of use is more common among some racial and ethnic groups, including Blacks and Hispanics, and smokers living below the poverty level (Fagan and Rigotti 2009). This emphasizes the importance of increasing our understanding of the process of smoking and quitting and of providing appropriate cessation services to this group of smokers.
The time course of reduction of risk after quitting smoking varies substantially across disease outcomes, with the risk of CHD declining more rapidly than the risk of tobacco-related cancers, particularly among those with a longer duration of smoking before sustained quitting (USDHHS 1990; IARC 2007a). The continued excess risk of lung cancer among former smokers has focused attention on the need to better identify those at greatest risk and to develop effective methods of early detection (Black and Baron 2007; Dubey and Powell 2008; Field and Duffy 2008). More than one-half of all cases of lung cancer are diagnosed at an advanced stage, and the five-year survival rate for lung cancer remains at about 15 percent (Jemal et al. 2008). At present, the efficacy of screening for lung cancer with low-dose computed tomography or other methodologies remains controversial (Black and Baron 2007). A better understanding of the molecular and cellular pathways involved in respiratory carcinogenesis could increase the feasibility of chemoprevention trials or of more cost-effective application of lung cancer screening (Alberg et al. 2005; Dubey and Powell 2008; Field 2008).
Recent advances in the understanding of the molecular origins of lung cancer have focused attention on the possibility that molecular profiling of genes and proteins could lead to the development of biomarkers for defining cancer risk, prognosis, and potentially improved treatment for some of the even more difficult to manage types of lung cancer (Herbst et al. 2008). The evidence reviewed in Chapter 5 on the major established pathways of cancer causation by cigarette smoking identifies important steps along these mechanistic pathways that could be used in potential biomarkers for defining cancer risk, prognosis, and potentially improved treatment. Figure 9.2 graphically presents how molecular profiling and assessments of genes and proteins could influence decisions regarding lung cancer treatment options for individual patients.
Figure 9.2
Molecular-profiling approaches to the development of personalized therapy. Source:Herbst et al. 2008. Reprinted with permission from Massachusetts Medical Society, © 2008. Note: Host profiling involves innate characteristics of the cancer patient. (more...)
Chronic obstructive pulmonary disease (COPD) remains a leading cause of death in the United States, and in 2005, approximately 1 in 20 deaths in the United States had COPD as the underlying cause, with an estimated 75 percent of these COPD deaths attributable to smoking (CDC 2008b). While the U.S. trend in COPD deaths has remained fairly stable from 2000 to 2005 (CDC 2008b), the global burden of COPD is increasing (Mannino and Buist 2007; Barnes 2007). Importantly, the evidence indicates that in the United States, COPD could be almost completely prevented by the elimination of smoking (USDHHS 2004). Although the risks for COPD morbidity and mortality decline with smoking cessation, they may not return to the levels of nonsmokers (USDHHS 2004). The U.S. Lung Health Study documented the benefits of substantially reduced mortality among individuals with asymptomatic airway obstruction who quit smoking (Anthonisen et al. 2005). The U.S. Lung Health Study also documented the benefits of providing an intensive 10-week smoking cessation program to this at-risk population; nearly 22 percent of intervention participants succeeded in quitting smoking compared with only 5.4 percent of participants who received usual care (Anthonisen et al. 2005). These results emphasize that rates of smoking cessation among patients most at risk of COPD could be increased up to fourfold if current available smoking cessation treatment options were delivered more routinely.
Numerous studies have shown that COPD is associated with lung cancer risk; this association may be attributed to shared exposure to cigarette smoke, to shared genetic susceptibility, and/or to facilitation of tumor initiation and promotion by inflammation (Dubey and Powell 2008). The growing global burden of COPD emphasizes the need for research to develop biomarkers of injury to identify smokers at early stages of disease development and to provide a basis for preventive therapies that block or reverse the underlying process of injury, particularly among former smokers. Research in this area may be guided by the evidence reviewed in Chapter 7, which identifies the two major mechanisms underlying the causation of COPD by cigarette smoking, oxidative stress (injury) and protease-antiprotease imbalance, and the strong association between COPD occurrence and a specific genetic disorder: AAT deficiency.
As noted in Chapter 8, health professionals have long known that exposure to tobacco smoke during pregnancy poses serious risks to fetal development. Despite this, approximately 19 percent of women of reproductive age smoke cigarettes, and based on birth certificate data, more than 1 in 10 (11.4 percent) women reported smoking during pregnancy (CDC 2004, 2008a). However, birth certificate data often underreport smoking during pregnancy. In survey data from 2002 to 2005, 17.3 percent of pregnant women reported smoking cigarettes in the month preceding the survey (NSDUH Report 2007). In addition, many pregnant women who do not smoke are exposed to secondhand smoke in workplaces, public places, and in their own homes. Recommendations have been made regarding the types of smoking cessation services that should be provided to all pregnant smokers (Fiore et al. 2008). Despite the documented costs of poor infant outcomes caused by smoking during pregnancy, and the higher prevalence of prenatal tobacco use found among lower income women, in 2006 only 27 states covered tobacco cessation counseling services for pregnant women in their Medicaid populations (CDC 2008e).
Reducing the Risks from Smoking
The benefits of quitting have been shown for smokers of all ages (USDHHS 1990, 2004; IARC 2007a). Smokers who quit completely and permanently early in life have a risk of premature death very similar to lifetime nonsmokers (Figure 9.1) (USDHHS 1990, 2004; IARC 2007a). However, for lung cancer, there is a persistent elevated risk in former smokers compared with lifetime nonsmokers of the same age even after a long abstinence (IARC 2007a). Evidence indicates that lung cancer risk increases far more strongly with each additional year of smoking than it increases for a higher average number of cigarettes smoked per day (Flanders et al. 2003; IARC 2007a). Although sustained smoking cessation at any age produces substantial reductions in risk, significant health benefits (other than for the fetus during pregnancy) from reducing the amount smoked or from short-term cessation have not been demonstrated (Benhamou et al. 1989; Godtfredsen et al. 2002; Anthonisen et al. 2005; Tverdal and Bjartveit 2006; IARC 2007a; Bjartveit and Tverdal 2009). The evidence presented in this report on the biologic mechanisms by which exposure to tobacco smoke causes cancers, cardiovascular and chronic obstructive pulmonary diseases, and reproductive and developmental effects document the importance of smokers quitting completely early in life and avoiding even occasional or infrequent smoking.
As reviewed in Chapter 2, in recent years a range of new products have been introduced and marketed to smokers as an alternative to conventional cigarettes, sometimes accompanied by messages, explicit or implied, that they offer reduced exposure to toxic substances or risk of disease. Evidence reviewed in this and previous reports indicates that five decades of evolving cigarette design have not reduced overall disease risk among smokers, and new designs can be used to undermine prevention and cessation efforts. It is now recognized that substantial risks may be associated with new tobacco products:
- Smokers who might have otherwise stopped smoking may continue to smoke because of perceived reduction in risk with use of new products.
- Former smokers may resume smoking because of perceived reduction in risk with use of new products.
- Nonsmokers, particularly youth, may start to use new products because of their perceived safety.
The evidence reviewed in this report highlights many of the scientific challenges that will be faced in evaluating new cigarette products presented as alternatives to conventional cigarettes, because of the diversity of such products, the multitude of smoking-related diseases, the impact of these products on nonusers, and the dearth of empirical data on their effects. The Institute of Medicine (IOM) Committee to Assess the Science Base for Tobacco Harm Reduction (Stratton et al. 2001) and more recent reviews (Gray et al. 2005; Royal College of Physicians of London 2007; European Commission 2008) have discussed the potential role in tobacco control of a wider range of alternatives to the current cigarette. Several questions need to be carefully considered when proposing novel tobacco products as strategies to reducing smoking-attributable mortality: Do these products decrease individual risk? Do they increase initiation of tobacco use or promote relapse? Do they delay cessation? Do they lead to dual product use? How does their use compare to cessation? As discussed in Chapter 2, in the absence of a sound science base to support such alternative strategies, the primary public health approaches remain prevention and cessation of all forms of tobacco products.
Chapter 4 in this report documents the importance of nicotine as the drug causing the addiction that is the fundamental reason that individuals persist in using tobacco products. However, other constituents in tobacco and tobacco smoke may also be reinforcing or may facilitate the reinforcing effects of nicotine. The factors contributing to the high addiction potential of tobacco products are multiple and complex. Understanding these relationships is critical in developing better treatments for cessation and for determining appropriate strategies to reduce use of tobacco products (Benowitz and Henning-field 1994; Henningfield et al. 1998, 2004; Gray et al. 2005; Benowitz et al. 2006; Royal College of Physicians of London 2007; Benowitz 2008; Zeller et al. 2009).
The evidence on the CVD risks of low levels of tobacco smoke exposure in Chapter 6 of this report clearly demonstrate that the dose response for CVD is not linear, with risk rising rapidly at low doses and then plateauing at relatively low levels of exposure. The data on CVD also demonstrates the potential for dual tobacco product use resulting in a greater risk of disease than either product alone (Teo et al. 2006). Additional research to identify those toxicants in tobacco products that are most responsible for acute cardiac risks is critically needed (Boffetta and Straif 2009).
As reviewed in this and prior reports, the risk of lung and other cancers, as well as COPD, increases dramatically with greater duration of smoking. In addition, dual use of cigarettes along with other tobacco products could not only result in delays in sustained smoking cessation but may also increase the risk of disease more than cigarette smoking alone. If the use of alternative tobacco products hinders tobacco prevention and cessation efforts and results in longer durations of smoking among some smokers, the population burden of tobacco-related morbidity and mortality would be higher than for an approach focused on helping these smokers quit completely. In addition, tobacco products used in places where smoking is not allowed may defeat public health efforts to reduce smoking rates. Thus, there are continuing concerns about the population health impact of tobacco product modification or alternatives to cigarettes (Stratton et al. 2001; European Commission 2008).
Ending the Tobacco Epidemic
Since the health risks of tobacco use were first identified, the public health response has focused on preventing initiation of tobacco use, encouraging cessation among existing users, and more recently, protecting non-smokers from exposure to secondhand smoke. Although the primary focus of previous Surgeon General’s reports has been a review of scientific evidence related to health effects of tobacco use, numerous reports have provided specific recommendations to reduce the use of tobacco and exposure to secondhand smoke (Lynch and Bonnie 1994; USDHHS 2000; Zaza et al. 2005; NIH State-of-the-Science Panel 2006; Bonnie et al. 2007; Reuben 2007; Fiore et al. 2008). Effective public health and clinical approaches to increase smoking cessation rates have been developed and need to be more fully implemented (USDHHS 2000; Zaza et al. 2005; Bonnie et al. 2007; Reuben 2007; Fiore et al. 2008).
The IOM report, Ending the Tobacco Problem: A Blueprint for the Nation, concluded that the ultimate goal is “to reduce smoking so substantially that it is no longer a significant public health problem” (Bonnie et al. 2007, p. 1). The report proposed a two-pronged strategy to accomplish this goal: first, to strengthen and fully implement traditional tobacco control measures known to be effective, and second, to change the regulatory landscape to permit policy innovations such as strong federal regulation of tobacco products and their marketing and distribution (Bonnie et al. 2007). A complete list of the 42 recommendations made by the IOM report is provided in Appendix 9.2. In addition, the President’s Cancer Panel has highlighted the critical importance of reducing tobacco use stating that “ridding the nation of tobacco is the single most important action needed to dramatically reduce cancer mortality and morbidity” (Reuben 2008, p. iii) and that “if the population ceased smoking, this single behavior change would be tantamount to a vaccine against one-third of cancer deaths” (Reuben 2007, p. vi).
In its 2006–2007 Annual Report, the President’s Cancer Panel provided a detailed review of the status of tobacco control efforts in this country to address tobacco use prevention and treatment (Chapter 4) and environmental tobacco smoke exposure (Chapter 5). In this review, the President’s Cancer Panel outlined the important roles in reducing tobacco-caused death and disease that could be undertaken by federal, state, and local governments; nongovernmental organizations and other partners; the educational system; employers, insurance, and the health care system; and individuals and families (Reuben 2007). Many of these actions are very consistent with the 42 recommendations made by IOM (Appendix 9.2) and emphasize the evidence-based methods identified by the 2006 NIH State-of-the-Science consensus conference (NIH State-of-the-Science Panel 2006). The 15 recommendations from the President’s Cancer Panel are provided in Appendix 9.3.
Since the release of the recommendations from IOM (Bonnie et al. 2007) and the President’s Cancer Panel review of the status of tobacco control in this country (Reuben 2007), additional actions have been taken at the federal, state, and local levels. Below is a summary of the status of tobacco control efforts in this country within the WHO MPOWER framework (WHO 2008):
- Monitor tobacco use and prevention policies.
- Protect people from tobacco smoke.
- Offer help to quit tobacco use.
- Warn about the dangers of tobacco use.
- Enforce comprehensive restrictions on tobacco advertising, promotion, and sponsorship.
- Raise taxes on tobacco.
MONITOR. The monitoring of the population pattern of tobacco use and the status of prevention and control policies and programs has been defined as essential in national efforts to counter the tobacco epidemic (WHO 2008; Giovino et al. 2009). Current efforts to monitor the tobacco use epidemic and identify additional steps to optimize measurement of tobacco use and factors influencing use in the United States have been reviewed (Cruz 2009; Delnevo and Bauer 2009; Farrelly 2009; Giovino et al. 2009; Stellman and Djordjevic 2009). These papers provide detailed analysis of national tobacco monitoring, research, and evaluation under the classic Agent, Host, Vector, and Environment framework. These reviews indicate that many of the most important basic elements of a national monitoring system are in place but that several key elements are needed to improve the system.
PROTECT. This report provides additional scientific evidence that there is no risk-free level of exposure to tobacco smoke. Although progress has been made to increase protection of nonsmokers in the United States from secondhand smoke exposure since the release of the 2006 Surgeon General’s report on the health consequences of involuntary exposure to tobacco smoke (CDC 2008f), biomonitoring of exposure indicates that almost one-half of nonsmokers, and more than 60 percent of young children, continue to be exposed (CDC 2008c). Wide geographic, occupational, and demographic disparities remain (CDC 2008c,f). It has been estimated that only about one in three residents of the United States live under state or local laws that make worksites, restaurants, and bars completely smoke-free (CDC 2008f).
OFFER. The U.S. Tobacco Use and Dependence Guideline Panel has identified the most effective interventions to assist tobacco users to successfully quit (Fiore et al. 2008). Moreover, a systematic review and analysis of recommended clinical preventive services has identified clinical smoking cessation services as one of the most successful and cost-effective recommendations (Maciosek et al. 2006). However, data indicate that less than 30 percent of smokers are offered assistance in quitting annually (Partnership for Prevention 2008). Both IOM and the President’s Cancer Panel address this issue in their recommendations (Appendices 9.2 and 9.3).
WARN. WHO recommends that national efforts to warn about the dangers of tobacco should create high levels of awareness of the health risks of tobacco use across age groups, genders, and places of residence so all people understand that the result of tobacco use is often suffering, disfigurement, and early death (WHO 2008). The President’s Cancer Panel noted that the warning labels on tobacco products in many other countries are larger and more graphic than those on U.S. cigarette packages (see figure 17, page 74, Reuben 2007). The Federal Cigarette Labeling and Advertising Act of 1965 (the Cigarette Act) and the Comprehensive Smokeless Tobacco Health Education Act of 1986 (the Smokeless Act), as amended by the Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act) (2009), require new, stronger warning statements on cigarette and smokeless tobacco packages and advertising and require color graphics depicting the negative health consequences of smoking on cigarette packages and advertising. The Tobacco Control Act further amends the Cigarette Act and the Smokeless Act to give the U.S. Food and Drug Administration the authority to revise the warning label statements and the color graphics for both cigarettes and smokeless tobacco through rulemaking. In addition to potential changes in package warning labels, evidence supports the effectiveness of health communication and countermarketing strategies employing a wide range of efforts, including paid television, radio, billboard, print, and Web-based advertising at the national, state, and local levels; media advocacy through public relations efforts; and efforts to reduce or replace tobacco industry sponsorship and promotions (CDC 2007; NCI 2008). Recommendations 15 and 16 from IOM address the need for a national, youth-oriented, countermarketing campaign as well as increased mass media and other general and targeted public education programs to promote effective cessation programs and activities (Appendix 9.2) (Bonnie et al. 2007).
ENFORCE. Both the 2007 IOM and 2006–2007 President’s Cancer Panel reports have identified the sophisticated strategies used by the tobacco industry to counter policies and programs to reduce tobacco consumption (Bonnie et al. 2007; Reuben 2007). Cigarettes remain one of the most heavily marketed products in the United States, with more than $250 billion (in 2006 dollars) expended between 1940 and 2005 on cigarette advertising and promotion (NCI 2008). The influence of these tobacco industry efforts in shaping tobacco-related knowledge, opinions, attitudes, and behavior was reviewed in detail, and it was concluded that the total weight of evidence demonstrated a causal relationship between tobacco advertising and promotion and increased tobacco use (NCI 2008). The Tobacco Control Act will enable new actions to be taken at the federal, state, and local levels to counteract the influence of tobacco advertising, promotions, and sponsorship.
RAISE. With the increase in the federal excise tax on cigarettes from $0.24 to $1.01 on April 1, 2009, the combined federal and average state cigarette excise tax increased to $2.21 per pack (CDC 2009b). Evidence-based reviews have concluded that increases in the price of cigarettes through excise taxes or other strategies are an effective policy intervention to prevent smoking initiation among adolescents and young adults, reduce cigarette consumption, and increase the number of smokers who quit (USDHHS 2000; Zaza et al. 2005; NIH State-of-the-Science 2006; Bonnie et al. 2007; CDC 2007; Reuben 2007). Additionally, the WHO MPOWER recommendations emphasize the importance of ensuring that the tax rates on tobacco products are adjusted periodically to keep pace with inflation and rise faster than consumer purchasing power. WHO also stresses that implementation of effective strategies to limit smuggling and the availability of untaxed tobacco products is essential to maximizing the effectiveness of higher taxes in reducing tobacco use (WHO 2008).
Concluding Remarks
In 1964, the Surgeon General’s Advisory Committee concluded: “Cigarette smoking is a health hazard of sufficient importance in the United States to warrant appropriate remedial action” (USDHEW 1964, p. 33). Evidence-based recommendations have helped to define appropriate remedial actions, including those from the 2000 Surgeon General’s report on reducing tobacco use (USDHHS 2000), NIH (NIH State-of-the-Science Panel 2006), the Task Force on Community Preventive Services (Zaza et al. 2005), IOM reports (Stratton et al. 2001; Bonnie et al. 2007), and President’s Cancer Panel reports (Reuben 2007, 2008). The specific scientific conclusions from this report may provide further guidance for developing additional remedial actions.
Despite significant progress, tobacco use remains the single most preventable cause of death and disease in the United States. It is worth noting that lung cancer was once a very rare disease. Primary Malignant Growths of the Lungs and Bronchi, published in 1912, reviewed the worldwide scientific literature and was able to identify only 374 verified cases of lung cancer (Adler 1912; Spiro and Silvestri 2005). In stark contrast, lung cancer is today the nation’s leading cause of cancer death among both men and women, killing an estimated 160,000 people in the United States, and an estimated 1.34 million people worldwide each year (Jemal et al. 2008; WHO 2008). An estimated 90 percent of U.S. lung cancer deaths in men and 80 percent of U.S. lung cancer deaths in women are caused by smoking and exposure to secondhand smoke (CDC 2009c). In addition, more than 100,000 deaths from pulmonary diseases and more than 140,000 deaths from heart disease and stroke in the United States are caused each year by active smoking and exposure to secondhand smoke (CDC 2008d).
Since the publication of the 1964 Surgeon General’s report on smoking and health, this series of reports has provided an incontrovertible body of research evidence documenting the burden of sickness and death caused by tobacco use. Faced with these facts, it is appropriate to restate the challenge issued by a former Director-General of WHO, Dr. Gro Harlem Brundtland, at the start of the international negotiations that led to the landmark Framework Convention on Tobacco Control:
If we do not act decisively today, a hundred years from now our grandchildren and their children will look back and seriously question how people claiming to be committed to public health and social justice allowed the tobacco epidemic to unfold unchecked (Brundtland 1999).
Appendix 9.1. Recommendations for Future Research
The evidence reviewed within this report identified important gaps in our scientific knowledge that merit greater attention in future research. Below is a listing of areas of research that can substantially contribute to a better understanding of how tobacco use causes disease.
Nicotine Addiction
With the emerging science base for understanding the physiological, behavioral, and cognitive bases for addiction and for identifying the genetics and other host factors that may moderate the effects of nicotine, new types of interventions are being developed that are expanding the treatment armamentarium, thus providing more effective interventions for those who continue to have difficulty in quitting smoking. For example, pharmacologic treatments are being marketed that target specific nicotinic receptors responsible for the reinforcing effects of nicotine, such as varenicline (Gonzales et al. 2006; Jorenby et al. 2006; Nides et al. 2006; Oncken et al. 2006). Nicotine immunotherapies (vaccines) under development also offer potential treatments for nicotine addiction. Nicotine immunotherapies stimulate the immune system to develop antibodies to nicotine that reduce the level and speed of nicotine entering the brain, potentially changing the pharmacokinetics of nicotine and thereby reducing the reinforcing effects of nicotine (Pentel 2004; Hatsukami et al. 2005). Another area of major development in the treatment of smokers, as with the treatment of other diseases, involves tailoring treatments to the phenotype and genotype of the individuals to select the most efficacious treatments (Lerman and Niaura 2002). All of these treatment developments have been aided by greatly expanded understanding of the mechanisms of dependence and withdrawal during the past two decades.
Valid indicators and biomarkers of addiction are needed to assess future “less addictive” or “less toxic” tobacco products and treatments and to provide a better understanding of the addictive process. Many subjective and behavioral measures and some cognitive measures have been developed and used to test addiction to tobacco. However, fundamental gaps in knowledge exist, and other areas need to be more vigorously pursued.
Biomarkers for Addiction Potential
Table 9.1 summarizes the methods used for animal and human testing to assess the addiction potential of a nicotine product. Most of these methods have been referred to in Chapter 4 of this report. Although there are many subjective and behavioral methods, researchers have devoted little attention to cognitive and neurophysiological measures of addiction through the use of functional magnetic resonance imaging or positron emission tomography. Using more precise tools to assess and better understand learning processes, decision making, and brain changes associated with addiction will lead to the development of measurements in these areas. The limitations and questions associated with the measures listed in Table 9.1 are similar to those concerning the diagnosis of tobacco addiction or, for that matter, biomarkers for disease in general. That is, other than relative terms (e.g., product A has a greater abuse potential than does product B) or the occurrence of addiction, the threshold or criterion that determines the extent of abuse potential is unknown.
Table 9.1
Behavioral indicators of addiction potential of a drug or addiction to a drug.
Future Directions in Understanding Addiction
Several areas of research that can substantially contribute to a better understanding of nicotine addiction include—but are not limited to—the following:
- Improve understanding of the criteria for and measures of nicotine addiction or dependence and how they might differ across various populations, including youth and ethnic minorities.
- Adapt commonly used measures of assessing addiction to cigarettes to other tobacco products as demonstrated by preliminary efforts to develop and validate scales for smokeless tobacco dependence and, most recently, to waterpipe smoking.
- Develop a better understanding of the contribution of the design features of tobacco products and of constituents other than nicotine that play an important role in all aspects of nicotine addiction, including initiation, maintenance, withdrawal, and relapse.
- Use current knowledge of the neurosystems associated with the reinforcement, withdrawal, and conditioning effects of nicotine addiction to develop a strategic road map for future discoveries in this area.
- Move toward a better understanding of the role and neurobiology of associative learning and cognitive processing in the development of and recovery from nicotine addiction.
- Foster an interdisciplinary effort to develop links among genotypes, endophenotypes, phenotypes, and the neurobiologic effects of nicotine.
- Explore the differences between adolescents and adults in their sensitivity to nicotine and to other factors associated with tobacco use, and find out which factors contribute to these differences (e.g., stage of neurodevelopment, sex hormones).
- Increase understanding of the relationship between comorbid disorders and nicotine addiction, including the common neural pathways and psychosocial vulnerabilities, and the mechanisms associated with an increased risk of nicotine addiction.
Cancer
Even though the evidence has long been sufficient to infer that both active and involuntary smoking cause at least 15 types of cancer, the long latency of tobacco-caused cancers emphasizes the need for further research on the mechanisms by which exposure to tobacco causes cancer. Several areas of research that can substantially contribute to a better understanding of these mechanisms include—but are not limited to—the following:
- Investigate genetic polymorphisms and phenotypic variations among smokers in critical aspects of the carcinogenic process that may lead to variations in susceptibility to the carcinogens in tobacco smoke. Examples include differences in carcinogen- and nicotine-metabolizing enzymes and their products, DNA repair genes, and cell cycle genes. This research could lead to the identification of individuals who are particularly susceptible to the effects of tobacco smoke and who could be targeted for preventive interventions.
- Develop a panel of quantitative biomarkers of carcinogens or their metabolites in blood or urine. Apply this panel of biomarkers to determine carcinogen dose in smokers and its relationship to cancer. Such a panel could be extremely useful in determining individual risk of tobacco-induced cancer and potentially useful for regulation.
- Develop quantitative reproducible and reliable methods for assessing levels of DNA adducts specific to all major carcinogens in tobacco smoke, and carry out biomarker studies to investigate the relationship between DNA adducts and cancer in smokers. This approach could potentially identify highly susceptible smokers and further define mechanisms of cancer induction in general, beyond the effects of tobacco products.
- Further study the role of nicotine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, and other toxicants in tobacco smoke in the activation of nicotinic acetylcholine receptors in lung epithelial cells, as well as similar key intracellular proteins and related epigenetic events that can lead to tumor promotion, cocarcinogenesis, progression, and maintenance of cancer.
- Conduct additional studies of the potential mechanisms by which carcinogens in tobacco smoke affect breast tissue, and how various other effects from tobacco smoke exposure, including possible antiestrogenic effects, could modulate or reduce the carcinogenic effects of tobacco smoke exposure.
- Develop a predictive algorithm including tobacco carcinogen and DNA biomarker data and related parameters such as polymorphisms in DNA repair genes—to identify those smokers most susceptible to cancer induction by cigarette smoke. This algorithm would be analogous to the Gail model for breast cancer susceptibility.
- Investigate the mechanisms by which alcohol consumption and asbestos exposure enhance the risk for tobacco-related cancers.
- Study the major pathway by which tobacco smoke induces cancer through DNA adduct formation by tobacco smoke carcinogens and other contributing factors such as tumor promotion, cocarcinogenesis, direct receptor binding effects of nicotine and tobacco-specific nitrosamines, and hypermethylation of tumor suppressor gene promoter regions that clearly contribute. Further research is necessary to elucidate the relevant mechanisms involved in these pathways.
Cardiovascular Diseases
Even though the evidence has long been sufficient to infer that both active and involuntary smoking cause coronary heart disease, the observed risks from exposure to toxicants in combustible and noncombustible tobacco products emphasize the need for further research on the mechanisms by which exposure to tobacco adversely affects the cardiovascular system. Several areas of research that can substantially contribute to a better understanding of these mechanisms include—but are not limited to—the following:
- Conduct further study of the role of oxidizing chemicals, nicotine, or other toxicants in tobacco smoke in the development of endothelial dysfunction.
- Promote additional study of the role of specific toxicants in tobacco smoke in the development of acute and chronic inflammatory reactions and the development of reliable biomarkers of these reactions predictive of acute cardiovascular events and atherosclerosis.
- Identify the toxicants in tobacco smoke most responsible for the nonlinear dose response between exposure dose to tobacco smoke (including secondhand smoke) and indicators of acute cardiovascular risk and related cardiovascular events.
- Identify the toxicants in tobacco smoke most responsible for platelet activation effects.
- Analyze the toxicants in various forms of smokeless tobacco products that could produce acute or chronic changes in mechanisms related to cardiovascular risk.
- Conduct further study of the differential effects of alveolar deposition of particulate constituents from tobacco smoke and other ambient air sources on biochemical and physiological acute and chronic reactions related to cardiovascular risk and related cardiovascular events.
- Further explore the role of nicotine and other toxicants in tobacco smoke in the development of insulin resistance.
Pulmonary Diseases
Chronic obstructive pulmonary disease (COPD) remains a major public health problem that is increasing, but evidence indicates that COPD could be almost completely prevented with the elimination of smoking (U.S. Department of Health and Human Services 2004). Although there are substantial and rapid benefits to lung function after smoking cessation, evidence indicates that morbidity related to COPD persists long after cessation of smoking (International Agency for Research on Cancer 2007a). Several areas of research that can substantially contribute to a better understanding of the mechanisms by which exposure to tobacco smoke increase the risk of COPD include—but are not limited to—the following:
- Promote further research in characterizing the genetic basis of susceptibility to tobacco smoke in the causation of COPD.
- Further explore the role of oxidative stress in the pathogenesis of COPD and the potential to modulate this mechanism of disease production.
- Investigate more deeply the role of protease-anti-protease imbalance in the pathogenesis of COPD and the potential to modulate this mechanism of disease production.
Reproductive and Developmental Effects
Epidemiologic Studies
Numerous adverse pregnancy outcomes or maternal complications have been causally associated with maternal smoking. Research to better define dose- response relationships, especially for preeclampsia, pre-term delivery, and premature rupture of membranes, would be informative. This information could be used to establish more accurate estimates of individual risk and of population-attributable risk percentage. In general, research is needed to better define the effects of smoking cessation (before or during pregnancy) on risk of pregnancy complications or outcomes such as spontaneous abortion, placenta previa, placental abruption, preterm delivery, and premature rupture of membranes. This information could be used to help refine public health strategies for decreasing the contribution of maternal smoking to adverse pregnancy outcomes.
Smoking should continue to be examined in studies of birth defects, particularly in geographic areas in which smoking during pregnancy remains prevalent, as the evidence so far is suggestive, but not conclusive. These studies would be most beneficial if they also examine interactions with genetic polymorphisms (see below), requiring additional subjects and funding. Animal studies that better simulate smoke exposure would also be very useful in this regard.
Evidence is increasing that exposures during pregnancy may have long-lasting effects on offspring. Furthermore, developmental delays and disabilities are increasing in the population, so additional studies of smoking effects (in utero and postnatal exposure) on neurobehavioral endpoints, including cognition and behavior, are needed, perhaps as large cohort studies. In the context of some suggestive or early studies, it is important to pursue examination of in utero exposure to smoking and later reproductive effects in the offspring, including pubertal development, sperm quality, and female fertility. Studies of in utero exposure in offspring, either as children or adults, are difficult to carry out logistically because of long follow-up times or lack of retrospective data on parental tobacco use or secondhand smoke exposure during pregnancy if adults are studied. Such studies should also consider postnatal secondhand smoke exposure. Studies in adult offspring would need to be limited to nonsmokers, while considering secondhand smoke exposure in adults as well, requiring larger numbers of study subjects for stratification. In addition, numerous other factors may affect these endpoints after birth. Because of some of these methodological difficulties, further studies on mechanisms related to endocrine function would help support causal relationships (see below).
Research on humans would be improved with measurement of a biomarker of exposure, such as cotinine. Studies tend to show higher cotinine levels in young children exposed to secondhand smoke relative to older children and adults, so studying the pharmacokinetics of cotinine in the very young (birth to five years) would be of interest to determine whether this is attributable to slower metabolism of nicotine and cotinine or to greater exposure to secondhand smoke.
Pathophysiological and Cellular/Molecular Mechanisms
Effects on Organ Systems
- Smoking has often been considered antiestrogenic, but studies measuring hormone levels in nonpregnant women or in men do not support this hypothesis. Therefore, studies of effects of smoking and secondhand smoke exposure on levels of other hormones in males and females would help elucidate the mechanism of effects of smoking on reproductive function and some pregnancy outcomes.
- Research is needed to better understand mechanisms underlying causal relationships between maternal smoking and placental damage such as placenta previa, placental abruption, preeclampsia, preterm delivery, and premature rupture of membranes. Specifically needed are studies
- to better characterize the effects of maternal smoking on physiological transformation and on the development of the villous capillary system of the placenta;
- of the effects of maternal smoking on the balance between pro- and antiangiogenic factors; and
- of the effects of maternal smoking on nutrient transport in uteroplacental circulation and the potential consequences for fetal growth and development.
- The possible mechanisms of smoke exposure affecting organogenesis that may lead to birth defects require more research, particularly on the histopathologic changes in the brain and lung.
- Research is needed to better define effects of active smoking on immunoregulation in general. In addition, research is needed to better understand the contribution of tobacco-related dysregulation of the immune response to adverse pregnancy outcomes, such as preterm delivery and preterm premature rupture of membranes, and to determine what mechanisms are involved, such as increased risk of infection of the upper genital tract or modification of the inflammatory response.
Molecular Mechanisms and Specific Toxicants
- Cigarette smoke contains thousands of toxicants, but more data documenting exposure to toxicants in the fetus of maternal active and involuntary smokers are necessary to link effects of smoking to specific toxic mechanisms or models. Some of the primary toxicants of interest are heavy metals (e.g., lead, mercury, cadmium, arsenic), polycyclic aromatic hydrocarbons, solvents, and other less studied compounds such as pyrazines and phenols. Additional media could be used as substrate, including placenta, umbilical cord, amniotic fluid, urine excretion in the first days, and meconium for metals only (atomic absorption).
- In addition, the bioavailability and bioaccumulation of these compounds (particularly metals) from inhalation of smoke in adults or children should be studied, including animal studies to interpret toxicologic data on these compounds.
- Research is needed to establish whether deficiencies of micronutrients such as vitamin C and zinc contribute to adverse pregnancy outcomes and if mechanisms exist to compensate for the deficiencies, leading to better pregnancy outcomes.
- Research on the effects of smoking and its components on DNA damage should be conducted.
- Further studies that include information on genetic polymorphisms affecting drug- and carcinogen- metabolizing enzymes are critical to uncovering mechanisms of smoking effects in many areas, including birth defects, other adverse pregnancy outcomes, and developmental effects.
- In addition, a potential genetic basis for the population of women who have difficulty quitting smoking during pregnancy, including polymorphisms for nicotine-metabolizing enzymes or for central nervous system receptors, should be investigated to develop new pharmacologic treatments.
Appendix 9.2. Ending the Tobacco Problem: A Blueprint for the Nation
Contributors
Committee on Reducing Tobacco Use: Strategies, Barriers, and Consequences Institute of Medicine, 2007; Editors: Richard J Bonnie, Kathleen Stratton, and Robert B Wallace.Complete List of Recommendations
Strengthening Traditional Tobacco Control Measures
Recommendation 1: Each state should fund state tobacco control activities at the level recommended by the CDC [Centers for Disease Control and Prevention]. A reasonable target for each state is in the range of $15 to $20 per capita, depending on the state’s population, demography, and prevalence of tobacco use. If it is constitutionally permissible, states should use a statutorily prescribed portion of their tobacco excise tax revenues to fund tobacco control programs.
Recommendation 2: States with excise tax rates below the level imposed by the top quintile of states should also substantially increase their own rates to reduce smuggling and tax evasion. State excise tax rates should be indexed to inflation.
Recommendation 3: The federal government should substantially raise federal tobacco excise taxes, currently set at 39 cents a pack. Federal excise tax rates should be indexed to inflation.
Recommendation 4: States and localities should enact complete bans on smoking in all nonresidential indoor locations, including workplaces, malls, restaurants, and bars. States should not preempt local governments from enacting bans more restrictive than the state ban.
Recommendation 5: All health care facilities, including nursing homes, psychiatric hospitals, and medical units in correctional facilities, should meet or exceed JCAHO [Joint Commission on the Accreditation of Healthcare Organizations] standards in banning smoking in all indoor areas.
Recommendation 6: The American Correctional Association should require through its accreditation standards that all correctional facilities (prisons, jails, and juvenile detention facilities) implement bans on indoor smoking.
Recommendation 7: States should enact legislation requiring leases for multiunit apartment buildings and condominium sales agreements to include the terms governing smoking in common areas and residential units. States and localities should also encourage the owners of multiunit apartment buildings and condominium developers to include nonsmoking clauses in these leases and sales agreements and to enforce them.
Recommendation 8: Colleges and universities should ban smoking in indoor locations, including dormitories, and should consider setting a smoke-free campus as a goal. Further, colleges and universities should ban the promotion of tobacco products on campus and at all campus-sponsored events. Such policies should be monitored and evaluated by oversight committees, such as those associated with the American College Health Association.
Recommendation 9: State health agencies, health care professionals, and other interested organizations should undertake strong efforts to encourage parents to make their homes and vehicles smoke free.
Recommendation 10: States should not preempt local governments from restricting smoking in outdoor public spaces, such as parks and beaches.
Recommendation 11: All states should license retail sales outlets that sell tobacco products. Licensees should be required to (1) verify the date of birth, by means of photographic identification, of any purchaser appearing to be 25 years of age or younger; (2) place cigarettes exclusively behind the counter and sell cigarettes only in a direct face-to-face exchange; and (3) ban the use of self-service displays and vending machines. Repeat violations of laws restricting youth access should be subject to license suspension or revocation. States should not preempt local governments from licensing retail outlets that sell tobacco products.
Recommendation 12: All states should ban the sale and shipment of tobacco products directly to consumers through mail order or the Internet or other electronic systems. Shipments of tobacco products should be permitted only to licensed wholesale or retail outlets.
Recommendation 13: School boards should require all middle schools and high schools to adopt evidence-based smoking prevention programs and implement them with fidelity. They should coordinate these in-school programs with public activities or mass media programming, or both. Such prevention programs should be conducted annually. State funding for these programs should be supplemented with funding from the U.S. Department of Education under the Safe and Drug-Free School Act or by an independent body administering funds collected from the tobacco industry through excise taxes, court orders, or litigation agreements.
Recommendation 14: All physicians, dentists, and other health care providers should screen and educate youth about tobacco use during their annual health care visits and any other visit in which a health screening occurs. Physicians should refer youth who smoke to counseling services or smoking cessation programs available in the community. Physicians should also urge parents to keep a smoke-free home and vehicles, to discuss tobacco use with their children, to convey that they expect their children to not use tobacco, and to monitor their children’s tobacco use. Professional societies, including the American Medical Association, the American Nursing Association, the American Academy of Family Physicians, the American College of Physicians, and the American Academy of Pediatrics, should encourage physicians to adopt these practices.
Recommendation 15: A national, youth-oriented media campaign should be funded on an ongoing basis as a permanent component of the nation’s strategy to reduce tobacco use. State and community tobacco control programs should supplement the national media campaign with coordinated youth prevention activities. The campaign should be implemented by an established public health organization with funds provided by the federal government, public-private partnerships, or the tobacco industry (voluntarily or under litigation settlement agreements or court orders) for media development, testing, and purchases of advertising time and space.
Recommendation 16: State tobacco control agencies should work with health care partners to increase the demand for effective cessation programs and activities through mass media and other general and targeted public education programs.
Recommendation 17: Congress should ensure that stable funding is continuously provided to the national quit- line network.
Recommendation 18: The Secretary of the U.S. Department of Health and Human Services [HHS], through the National Cancer Institute, the Centers for Disease Control and Prevention, and other relevant federal health agencies, should fund a program of developmental research and demonstration projects combining media techniques, other social marketing methods, and innovative approaches to disseminating smoking cessation technologies.
Recommendation 19: Public and private health care systems should organize and provide access to comprehensive smoking cessation programs by using a variety of successful cessation methods and a staged disease management model (i.e. stepped care), and should specify the successful delivery of these programs as one criterion for quality assurance within those systems.
Recommendation 20: All insurance, managed care, and employee benefit plans, including Medicaid and Medicare, should cover reimbursement for effective smoking cessation programs as a lifetime benefit.
Recommendation 21: While sustaining their own valuable tobacco control activities, state tobacco control programs, CDC, philanthropic foundations, and voluntary organizations should continue to support the efforts of community coalitions promoting, disseminating, and advocating for tobacco use prevention and cessation, smoke-free environments, and other policies and programs for reducing tobacco use.
Recommendation 22: Tobacco control programs should consider populations disproportionately affected by tobacco addiction and tobacco-related morbidity and mortality when designing and implementing prevention and treatment programs. Particular attention should be paid to ensuring that health communications and other materials are culturally-appropriate and that special outreach efforts target all high-risk populations. Standard prevention or treatment programs that are modified to reach high-risk populations should be evaluated for effectiveness.
Changing the Regulatory Landscape
Recommendation 23: Congress should repeal the existing statute preempting state tobacco regulation of advertising and promotion “based on smoking and health” and should enact a new provision that precludes all direct state regulation only in relation to tobacco product characteristics and packaging while allowing complementary state regulation in all other domains of tobacco regulation, including marketing and distribution. Under this approach, federal regulation sets a floor while allowing states to be more restrictive.
Recommendation 24: Congress should confer upon the FDA [U. S. Food and Drug Administration] broad regulatory authority over the manufacture, distribution, marketing, and use of tobacco products.
Recommendation 25: Congress should empower the FDA to regulate the design and characteristics of tobacco products to promote the public health. Specific authority should be conferred
- to require tobacco manufacturers to disclose to the agency all chemical compounds found in both product and the product’s smoke, whether added or occurring naturally, by quantity; to disclose to the public the amount of nicotine in the product and the amount delivered to the consumer based on standards established by the agency; to disclose to the public research on their product, as well as behavioral aspects of its use; and to notify the agency whenever there is a change in a product;
- to prescribe cigarette testing methods, including how the cigarettes are tested and which smoke constituents must be measured;
- to promulgate tobacco product standards, including reduction of nicotine yields and reduction or elimination of other constituents, wherever such a standard is found to be appropriate for protection of the public health, taking into consideration the risks and benefits to the population as a whole, including users and non-users of tobacco products; and
- to develop specific standards for evaluating novel products that companies intend to promote as reduced-exposure or reduced-risk products, and to regulate reduced-exposure and reduced-risk health claims, assuring that there is a scientific basis for claims that are permitted.
Recommendation 26: Congress should strengthen the federally mandated warning labels for tobacco products immediately and should delegate authority to the FDA to update and revise these warnings on a regular basis upon finding that doing so would promote greater public understanding of the risks of using tobacco products or reduce tobacco consumption. Congress should require or authorize the FDA to require rotating color graphic warnings covering 50 percent of the package equivalent to those required in Canada.
Recommendation 27: Congress should empower the FDA to require manufacturers to include in or on tobacco packages information about the health effects of tobacco use and about products that can be used to help people quit.
Recommendation 28: Congress should ban, or empower the FDA to ban, terms such as “mild,” “lights,” “ultra-lights,” and other misleading terms mistakenly interpreted by consumers to imply reduced risk, as well as other techniques, such as color codes, that have the purpose or effect of conveying false or misleading impressions about the relative harmfulness of the product.
Recommendation 29: Whenever a court or administrative agency has found that a tobacco company has made false or misleading communications regarding the effects of tobacco products, or has engaged in conduct promoting tobacco use among youth or discouraging cessation by tobacco users of any age, the court or agency should consider using its remedial authority to require manufacturers to include corrective communications on or with the tobacco package as well as at the point of sale.
Recommendation 30: Congress and state legislatures should enact legislation regulating the retail point of sale of tobacco products for the purpose of discouraging consumption of these products and encouraging cessation. Specifically:
- All retail outlets choosing to carry tobacco products should be licensed and monitored. (See also youth access section in Chapter 5.)
- Commercial displays or other activity promoting tobacco use by or in retail outlets should be banned, although text-only informational displays (e.g., price or health-related product characteristics) may be permitted within prescribed regulatory constraints.
- Retail outlets choosing to carry tobacco products should be required to display and distribute prescribed warnings about the health consequences of tobacco use, information regarding products and services for cessation, and corrective messages designed to offset misstatements or implied claims regarding the health effects of tobacco use (e.g., that “light” cigarettes are less harmful than other cigarettes).
- Retail outlets choosing to carry tobacco products should be required to allocate a proportionate amount of space to cessation aids and nicotine replacement products and, after regulatory clearance by the FDA or a designated state agency, to “qualifying” exposure-reduction products. (The FDA or a suitable state health agency should promulgate a list of “qualifying” exposure-reducing products.)
Recommendation 31: Congress should explicitly and unmistakably include production, marketing, and distribution of tobacco products on Indian reservations by Indian tribes within the regulatory jurisdiction of FDA. Authority to investigate and enforce the Jenkins Act should be transferred to the Bureau of Alcohol, Tobacco, Firearms and Explosives. State restrictions on retail outlets should apply to all outlets on Indian reservations.
Recommendation 32: State governments should develop and, if feasible, implement and evaluate legal mechanisms for restructuring retail tobacco sales and restricting the number of tobacco outlets.
Recommendation 33: Congress should empower the FDA to restrict outlets in order to limit access and facilitate regulation of the retail environment, and thereby protect the public health.
Recommendation 34: If most states fail to increase tobacco control funding and reduce variations in tobacco excise tax rates as proposed in Recommendations 1 and 2, Congress should enact a National Tobacco Control Funding Plan raising funds through a per-pack remedial assessment on cigarettes sold in the United States. Part of the proceeds should be used to support national tobacco control programs and the remainder of the funds should be distributed to the states to subsidize state tobacco control programs according to a formula based on the level of state tobacco control expenditures and state tobacco excise rates. The plan should be designed to give states an incentive, not only to increase state spending on tobacco control, but also to raise cigarette taxes, especially in low-tax states. Congress should assure that any federal coordination mechanism affecting the coverage and collection of state tobacco excise taxes applies to Indian tribes.
Recommendation 35: Congress and state legislatures should enact legislation limiting visually displayed tobacco advertising in all venues, including mass media and at the point-of-sale, to a text-only, black-and-white format.
Recommendation 36: Congress and state legislatures should prohibit tobacco companies from targeting youth under 18 for any purpose, including dissemination of messages about smoking (whether ostensibly to promote or discourage it) or to survey youth opinions, attitudes and behaviors of any kind. If a tobacco company wishes to support youth prevention programs, the company should contribute funds to an independent non-profit organization with expertise in the prevention field. The independent organization should have exclusive responsibility for designing, executing, and evaluating the program.
Recommendation 37: The Motion Picture Association of America (MPAA) should encourage and facilitate the showing of anti-smoking advertisements before any film in which smoking is depicted in more than an incidental manner. The film rating board of the MPAA should consider the use of tobacco in the movies as a factor in assigning mature film ratings (e.g., an R-rating indicating Restricted: no one under age 17 admitted without parent or guardian) to films that depict tobacco use.
Recommendation 38: Congress should appropriate the necessary funds to enable the U.S. Department of Health and Human Services to conduct a periodic review of a representative sample of movies, television programs, and videos that are offered at times or in venues in which there is likely to be a significant youth audience (e.g., 15 percent) in order to ascertain the nature and frequency of images portraying tobacco use. The results of these reviews should be reported to Congress and to the public.
Recommendation 39: State tobacco control agencies should conduct surveillance of tobacco sales and use and the effects of tobacco control interventions in order to assess local trends in usage patterns; identify special groups at high risk for tobacco use; determine compliance with state and local tobacco-related laws, policies, and ordinances; and evaluate overall programmatic success.
Recommendation 40: The Secretary of HHS, through FDA or other agencies, should establish a national comprehensive tobacco surveillance system to collect information on a broad range of elements needed to understand and track the population impact of all tobacco products and the effects of national interventions (such as attitudes, beliefs, product characteristics, product distribution and usage patterns, and marketing messages and exposures to them).
New Frontiers in Tobacco Control
Recommendation 41: Congress should direct the Centers for Disease Control and Prevention to undertake a major program of tobacco control policy analysis and development and should provide sufficient funding to support the program. This program should develop the next generation of macro-level simulation models to project the likely effects of various policy innovations, taking into account the possible initiatives and responses of the tobacco industry as well as the impacts of the innovations on consumers.
Recommendation 42: Upon being empowered to regulate tobacco products, the FDA should give priority to exploring the potential effectiveness of a long-term strategy for reducing the amount of nicotine in cigarettes and should commission the studies needed to assess the feasibility of implementing such an approach. If such a strategy appears to be feasible, the agency should develop a long-term plan for implementing the strategy as part of a comprehensive plan for reducing tobacco use.
Appendix 9.3. Promoting Healthy Lifestyles: Policy, Program, and Personal Recommendations for Reducing Cancer Risk
Authors
President’s Cancer Panel.2006–2007 Annual Report
Recommendations Addressing Tobacco Use Prevention and Treatment; Environmental Tobacco Smoke Exposure
| 1. Ratify and fully implement the Framework Convention for Tobacco Control. Key provisions include: comprehensive bans on tobacco advertising, promotion, and sponsorship, larger and stronger warning labels on tobacco product packaging, provision of tobacco addiction treatment, disclosure of tobacco product ingredients, and public protection against environ- mental tobacco smoke exposure. |
|
| 2. Authorize the Food and Drug Administration (FDA) to strictly regulate tobacco products and product marketing. FDA must receive sufficient funding and personnel to carry out this crucial role. |
|
| 3. Increase the Federal excise tax on tobacco products. |
|
| 4. Require all Federal facilities to be smoke-free. |
|
| 5. Reallocate existing National Cancer Institute, Centers for Disease Control and Prevention, and other Federal resources to better mirror the tobacco-related disease burden and capitalize on opportunities for progress. |
|
| 6. Add the conduct of meaningful tobacco-related activities to the evaluation criteria for NCI-designated Cancer Centers. |
|
7. Reduce the influence of the tobacco industry:
|
|
8. Strengthen anti-tobacco efforts at the state and local levels:
|
|
| 9. Develop and provide evidence-based multimedia curricula and educational materials in grades K-12 on the dangers of tobacco use and tobacco smoke exposure and the role of the tobacco industry in promoting tobacco use. Encourage colleges and universities to disseminate tested anti-tobacco messages for the 18 to 24 year-old age group through campus radio and television stations, Web sites, and print publications. |
|
| 10. Cease including images of smoking in movies, television, music videos, video games, and other visual media with child, adolescent, and young adult audiences. |
|
| 11. Prohibit smoking in and around the workplace. Support worker efforts to quit smoking; provide incentives for cessation. |
|
| 12. Make coverage of tobacco use cessation services and medications a standard benefit in all comprehensive health benefit packages. |
|
| 13. Incorporate smoking cessation services into the comprehensive care of cancer patients, survivors, and their family members. |
|
| 14. Adopt the Agency for Healthcare Research and Quality Guidelines for Clinicians Treating Tobacco Use and Dependence as part of the standard of care for all health care providers. |
|
| 15. Quit smoking and use of any smokeless tobacco products. Prohibit smoking in the home and car. Protect children from exposure to smoking in movies and smoking role models. Patronize only smoke-free restaurants and other businesses. |
|
References
- Adler I. Primary Malignant Growths of the Lungs and Bronchi: A Pathological and Clinical Study. New York: Longmans, Green, and Company; 1912.
- Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. Journal of Clinical Oncology. 2005;23(14):3175–85. [PubMed: 15886304]
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Annals of Internal Medicine. 2005;142(4):233–9. [PubMed: 15710956]
- Barnes PJ. Chronic obstructive pulmonary disease: a growing but neglected global epidemic. PLoS Medicine. 2007;4(5):e112. [PMC free article: PMC1865560] [PubMed: 17503959] [CrossRef]
- Barnoya J, Glantz S. Association of the California tobacco control program with declines in lung cancer incidence. Cancer Causes and Control. 2004;15(7):689–95. [PubMed: 15280627]
- Benhamou E, Benhamou S, Auquier A, Flamant R. Changes in patterns of cigarette smoking and lung cancer risk: results of a case-control study. British Journal of Cancer. 1989;60(4):601–4. [PMC free article: PMC2247103] [PubMed: 2803931]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clinical Pharmacology & Therapeutics. 2008;83(4):531–41. [PubMed: 18305452]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction: the implications for tobacco regulation. New England Journal of Medicine. 1994;331(2):123–5. [PubMed: 7818638]
- Benowitz NL, Jacob P III, Herrera B. Nicotine intake and dose response when smoking reduced–nicotine content cigarettes. Clinical Pharmacology & Therapeutics. 2006;80(6):703–14. [PubMed: 17178270]
- Bjartveit K, Tverdal A. Health consequences of sustained smoking cessation. Tobacco Control. 2009;18(3):197–205. [PubMed: 19228666]
- Black WC, Baron JA. CT screening for lung cancer: spiraling into confusion? JAMA: the Journal of the American Medical Association. 2007;297(9):995–7. [PubMed: 17341714]
- Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ (British Medical Journal). 2009;339:b3060. [PMC free article: PMC2728803] [PubMed: 19690343] [CrossRef]
- Bonnie RJ, Stratton K, Wallace RB, editors. Ending the Tobacco Problem: A Blueprint for the Nation. Washington: National Academies Press; 2007.
- Breton CV, Byun H-M, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. American Journal of Respiratory and Critical Care Medicine. 2009;180(5):462–7. [PMC free article: PMC2742762] [PubMed: 19498054]
- Brundtland GH. Speech to the WHO International Conference on Tobacco and Health; November 15, 1999; Kobe, Japan. [accessed: March 25, 2009]. < http://www
.who.int/director-general /speeches /1999/english/19991115_kobe.html>; - Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, Chen C, Jacobs K, Wheeler W, Landi MT, et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS ONE. 2009;4(2):e4653. [PMC free article: PMC2644817] [PubMed: 19247474] [CrossRef]
- Carlsten C, Burke W. Potential for genetics to promote public health: genetics research on smoking suggests caution about expectations. JAMA: the Journal of the American Medical Association. 2006;296(20):2480–2. [PubMed: 17119145]
- Centers for Disease Control and Prevention. Smoking during pregnancy—United States, 1990–2002. Morbidity and Mortality Weekly Report. 2004;53(39):911–5. [PubMed: 15470322]
- Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2007. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2007.
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2007. Morbidity and Mortality Weekly Report. 2008a;57(45):1221–6. [PubMed: 19008790]
- Centers for Disease Control and Prevention. Deaths from chronic obstructive lung disease—United States, 2000–2005. Morbidity and Mortality Weekly Report. 2008b;57(45):1229–32. [PubMed: 19008792]
- Centers for Disease Control and Prevention. Disparities in secondhand smoke exposure—United States, 1988–1994 and 1999–2004. Morbidity and Mortality Weekly Report. 2008c;57(27):744–7. [PubMed: 18614993]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008d;57(45):1226–48. [PubMed: 19008791]
- Centers for Disease Control and Prevention. State Medicaid coverage for tobacco-dependence treatments—United States, 2006. Morbidity and Mortality Weekly Report. 2008e;57(5):117–22. [PubMed: 18256583]
- Centers for Disease Control and Prevention. State smoking restrictions for private-sector worksites, restaurants, and bars—United States, 2004 and 2007. Morbidity and Mortality Weekly Report. 2008f;57(20):549–52. [PubMed: 18496503]
- Centers for Disease Control and Prevention. Cigarette smoking among Adults and trends in smoking cessation—United States, 2008. Morbidity and Mortality Weekly Report. 2009a;58(44):1227–32. [PubMed: 19910909]
- Centers for Disease Control and Prevention. Federal and state excise taxes—United States, 1995–2009. Morbidity and Mortality Weekly Report. 2009b;58(19):524–7. [PubMed: 19478719]
- Centers for Disease Control and Prevention. November is Lung Cancer Awareness Month. Jul, 2009c. [accessed: July 17, 2009]. < http://www
.cdc.gov/features/lungcancer/>. - Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States—recent progress and opportunities. CA A Cancer Journal for Clinicians. 2009;59(6):352–65. [PubMed: 19897839]
- Comprehensive Smokeless Tobacco Health Education Act of 1986, Public Law 99-252, US Statutes at Large 100 (1986):30
- Cruz TB. Monitoring the tobacco use epidemic IV. The vector: tobacco industry data sources and recommendations for research and evaluation. Preventive Medicine. 2009;48(1 Suppl):S24–S34. [PubMed: 18976685]
- Delnevo CD, Bauer UE. Monitoring the tobacco use epidemic III. The host: data sources and methodological challenges. Preventive Medicine. 2009;48(1 Suppl):S16–S23. [PubMed: 18851990]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ (British Medical Journal). 2004;328(7455):1519–28. [PMC free article: PMC437139] [PubMed: 15213107]
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ (British Medical Journal). 1994;309(6959):901–11. [PMC free article: PMC2541142] [PubMed: 7755693]
- Dubey S, Powell CA. Update in lung cancer 2007. American Journal of Respiratory and Critical Care Medicine. 2008;177(9):941–6. [PMC free article: PMC2720127] [PubMed: 18434333]
- Esteller M. Molecular origins of cancer: epigenetics in cancer. New England Journal of Medicine. 2008;358(11):1148–59. [PubMed: 18337604]
- European Commission. Health Effects of Smokeless Tobacco Products. Brussels: European Commission, Scientific Committee on Emerging and Newly Identified Health Risks; 2008.
- Fagan P, Rigotti NA. Light and intermittent smoking: the road less traveled. Nicotine & Tobacco Research. 2009;11(2):107–10. [PMC free article: PMC2658903] [PubMed: 19264864]
- Family Smoking Prevention and Tobacco Control Act, Public Law 111-31. US Statutes at Large. 2009;12:3. 1776.
- Farrelly MC. Monitoring the tobacco use epidemic V. The environment: factors that influence tobacco use. Preventive Medicine. 2009;48(1 Suppl):S35–S43. [PubMed: 19022281]
- Federal Cigarette Labeling and Advertising Act of 1965, Public Law 89-92. US Statutes at Large. 1965;79:281.
- Field JK. Lung cancer risk models come of age. Cancer Prevention Research. 2008;1(4):226–8. [PubMed: 19138964]
- Field JK, Duffy SW. Lung cancer screening: the way forward. British Journal of Cancer. 2008;99(4):557–62. [PMC free article: PMC2527832] [PubMed: 18665179]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, et al. Treating Tobacco Use and Dependence: 2008 Update, Clinical Practice Guideline. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service; 2008.
- Flanders WD, Lally CA, Zhu B-P, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Research. 2003;63(19):6556–62. [PubMed: 14559851]
- Garland C, Barrett-Connor E, Suarez L, Criqui MH, Wingard DL. Effects of passive smoking on ischemic heart disease mortality of nonsmokers: a prospective study. American Journal of Epidemiology. 1985;121(5):645–50. [PubMed: 4014156]
- Giovino GA, Biener L, Hartman AM, Marcus SE, Schooley MW, Pechacek TF, Vallone E. Monitoring the tobacco use epidemic. I. Overview: optimizing measurement to facilitate change. Preventive Medicine. 2009;48( 1 Suppl):S4–S10. [PubMed: 18809429]
- Glantz SA, Parmley WW. Passive smoking and heart disease: epidemiology, physiology, and bio-chemistry. Circulation. 1991;83(1):1–12. [PubMed: 1984876]
- Glantz SA, Parmley WW. Passive smoking and heart disease: mechanisms and risk. JAMA: the Journal of the American Medical Association. 1995;273(13):1047–53. [PubMed: 7897790]
- Godtfredsen NS, Holst C, Prescott E, Vestbo J, Osler M. Smoking reduction, smoking cessation, and mortality: a 16-year follow-up of 19,732 men and women from The Copenhagen Centre for Prospective Population Studies. American Journal of Epidemiology. 2002;156(11):994–1001. [PubMed: 12446255]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2006;296(1):47–55. [PubMed: 16820546]
- Gray N, Henningfield JE, Benowitz NL, Connolly GN, Dresler C, Fagerström K, Jarvis MJ, Boyle P. Toward a comprehensive long term nicotine policy. Tobacco Control. 2005;14(3):161–5. [PMC free article: PMC1748036] [PubMed: 15923465]
- Hatsukami DK, Giovino GA, Eissenberg T, Clark PI, Lawrence D, Leischow S. Methods to assess potential reduced exposure products. Nicotine & Tobacco Research. 2005;7(6):827–44. [PubMed: 16298718]
- Henningfield JE, Benowitz NL, Connolly GN, Davis RM, Gray N, Myers ML, Zeller M. Reducing tobacco addiction through tobacco product regulation. Tobacco Control. 2004;13(2):132–5. [PMC free article: PMC1747873] [PubMed: 15175528]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Tobacco Control. 1998;7(3):281–93. [PMC free article: PMC1763900] [PubMed: 9825424]
- Herbst RS, Heymach JV, Lippman SM. Molecular origins of lung cancer: lung cancer. New England Journal of Medicine. 2008;359(13):1367–80. [PMC free article: PMC10662965] [PubMed: 18815398]
- Hirayama T. Lung cancer in Japan: effects of nutrition and passive smoking. Lung Cancer: Causes and Prevention. Mizell M, Correa P, editors. Deerfield Beach (MA): Verlag Chemie International; 1984. pp. 175–95.
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking. Vol. 83. Lyon (France): International Agency for Research on Cancer; 2004. [PMC free article: PMC4781536] [PubMed: 15285078]
- International Agency for Research on Cancer. Tobacco Control: Reversal of Risk After Quitting Smoking, IARC Handbooks of Cancer Prevention. Vol. 11. Lyon (France): International Agency for Research on Cancer; 2007a.
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. Vol. 89. Lyon (France): International Agency for Research on Cancer; 2007b. [PMC free article: PMC4781254] [PubMed: 18335640]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer Journal for Clinicians. 2008;58(2):71–96. [PubMed: 18287387]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Varenicline Phase 3 Study Group. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2006;296(1):56–63. [PubMed: 16820547]
- Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ (British Medical Journal). 1997;315(7114):973–80. [PMC free article: PMC2127675] [PubMed: 9365294]
- Lerman C, Niaura R. Applying genetic approaches to the treatment of nicotine dependence. Oncogene. 2002;21(48):7412–20. [PubMed: 12379882]
- Lynch BS, Bonnie RJ, editors. Growing Up Tobacco Free: Preventing Nicotine Addiction in Children and Youths. Washington: National Academy Press; 1994. [PubMed: 25144107]
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–73. [PubMed: 17765526]
- Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. American Journal of Preventive Medicine. 2006;31(1):52–61. [PubMed: 16777543]
- Miller MD, Marty MA, Broadwin R, Johnson KC, Salmon AG, Winder B, Steinmaus C. The association between exposure to environmental tobacco smoke and breast cancer: a review by the California Environmental Protection Agency. Preventive Medicine. 2007;44(2):93–106. [PubMed: 17027075]
- National Cancer Institute. The Role of the Media in Promoting and Reducing Tobacco Use, Tobacco Control Monograph No 19. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2008. NIH Publication No 07-6242.
- National Cancer Institute. Phenotypes and Endopheno-types: Foundations for Genetic Studies of Nicotine Use and Dependence, Tobacco Control Monograph No 20. Bethesda (MD): U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2009. NIH Publication No 08-6366.
- NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference statement: tobacco use: prevention, cessation, and control. Annals of Internal Medicine. 2006;145(11):839–44. [PubMed: 16954353]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Varenicline Study Group. Smoking cessation with varenicline, a selective α4β2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Archives of Internal Medicine. 2006;166(15):1561–8. [PubMed: 16908788]
- NSDUH Report. Cigarette use among pregnant women and recent mothers, NSDUH Report. Feb 9, 2007.
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine. 2006;166(15):1571–7. [PubMed: 16908789]
- Partnership for Prevention, A Call for ACTTION: Access for Cessation Treatment for Tobacco in Our Nation. Washington: Partnership for Prevention; 2008.
- Pentel PR. Vaccines and depot medications for drug addiction: rationale, mechanisms of action, and treatment implications. New Treatments for Addiction: Behavioral, Ethical, Legal, and Social Questions. Harwood HJ, Myers TG, editors. Washington: National Academies Press; 2004. pp. 63–97.
- Peto R, Lopez AD, Boreham J, Thun M. Mortality from Smoking in Developed Countries 1950–2000. 2nd ed., revised. Jun, 2006. [accessed: March 13, 2008]. < http://www
.ctsu.ox.ac .uk/~tobacco/C4308.pdf>. - Peto R, Lopez AD, Boreham J, Thun M, Heath C Jr. Mortality from smoking in developed countries: indirect estimates from national vital statistics. Lancet. 1992;339(8804):1268–78. [PubMed: 1349675]
- Phillips DH, Garte S. Smoking and breast cancer: is there really a link? Cancer Epidemiology, Biomarkers & Prevention. 2008;17(1):1–2. [PubMed: 18187387]
- Reuben SH. Promoting Healthy Lifestyles: Policy, Program, and Personal Recommendations for Reducing Cancer Risk, 2006–2007 Annual Report: President’s Cancer Panel. Rockville (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2007.
- Reuben SH. Maximizing Our Nation’s Investment in Cancer: Three Crucial Actions for America’s Health. President’s Cancer Panel 2007–2008, Annual Report. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2008.
- Royal College of Physicians of London. Harm Reduction in Nicotine Addiction: Helping People Who Can’t Quit A Report by the Tobacco Advisory Group of the Royal College of Physicians of London. London: Royal College of Physicians of London; 2007.
- Spiro SG, Silvestri GA. One hundred years of lung cancer. American Journal of Respiratory and Critical Care Medicine. 2005;172(5):523–9. [PubMed: 15961694]
- Stellman SD, Djordjevic MV. Monitoring the tobacco use epidemic. II. The agent: current and emerging tobacco products. Preventive Medicine. 2009;48(1 Suppl):S11–S15. [PMC free article: PMC2667905] [PubMed: 18848577]
- Stratton K, Shetty P, Wallace R, Bondurant S, editors. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington: National Academy Press; 2001. [PubMed: 25057541]
- Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647–58. [PubMed: 16920470]
- Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tobacco Control. 2006;15(6):472–80. [PMC free article: PMC2563668] [PubMed: 17130377]
- US Department of Health and Human Services. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990.
- US Department of Health and Human Services. Reducing Tobacco Use: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2000.
- US Department of Health and Human Services. Women and Smoking A Report of the Surgeon Generaz. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001.
- US Department of Health and Human Services. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; The Health Consequences of Smoking: A Report of the Surgeon General. 2004
- US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006.
- US Department of Health, Education, and Welfare. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1964. PHS Publication No 1103.
- Wang XL, Scott DA, editors. Molecular Mechanisms of Tobacco-Induced Diseases. New York: Nova Biomedical Books; 2005.
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva: World Health Organization; 2008.
- Zaza S, Briss PA, Harris KW, editors. The Guide to Community Preventive Services: What Works to Promote Health? New York: Oxford University Press; 2005.
- Zeller M, Hatsukami D, Backinger C, Benowitz N, Biener L, Burns D, Clark P, Connolly G, Djordjevic M, Eissenberg T, et al. The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the United States. Tobacco Control. 2009:tc. [PMC free article: PMC4915216] [PubMed: 19240228] [CrossRef]
- The Global Tobacco Epidemic
- Reducing the Risks from Smoking
- Ending the Tobacco Epidemic
- Concluding Remarks
- Recommendations for Future Research
- Ending the Tobacco Problem: A Blueprint for the Nation
- Promoting Healthy Lifestyles: Policy, Program, and Personal Recommendations for Reducing Cancer Risk
- References
- A Vision for the Future - How Tobacco Smoke Causes Disease: The Biology and Beha...A Vision for the Future - How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease
Your browsing activity is empty.
Activity recording is turned off.
See more...