NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Venlafaxine is a serotonin and norepinephrine reuptake inhibitor widely used as an antidepressant. Venlafaxine therapy can be associated with transient asymptomatic elevations in serum aminotransferase levels and has been linked to rare instances of clinically apparent acute liver injury.
Background
Venlafaxine (ven" la fax' een) is a both a serotonin and norepinephrine reuptake inhibitor that acts by blocking the reuptake of these neurotransmitters in CNS synaptic clefts, thus increasing active levels in the brain which is associated with an antidepressant effect. Venlafaxine was approved for use in the United States in 1995 and is still widely used with more than 16 million prescriptions filled yearly. Current indications include major depressive disorder, generalized and social anxiety disorder, panic disorder and bipolar mood disorder. Venlafaxine is available as tablets of 25, 37.5, 50, 75, 100 and 150 mg and as extended release capsules of 37.5, 75 and 150 mg in multiple generic forms and under the brand name of Effexor. The recommended dosage in adults is 75 mg daily in two or three divided doses, increasing based on tolerance and effects to a maximum of 225 mg daily. Common side effects are drowsiness, dyspepsia, nausea, headache, increased sweating, increased appetite, weight gain and sexual dysfunction. Uncommon but potentially severe adverse events include suicidal thoughts and behaviors, serotonin syndrome, activation of mania, hypertension, abnormal bleedings and angle-closure glaucoma.
Desvenlafaxine (des” ven la fax' een) is the major active metabolite of venlafaxine and has been marketed separately as a serotonin and norepinephrine reuptake inhibitor. Desvenlafaxine is believed to act by blocking the reuptake of neurotransmitters in CNS synaptic clefts, thus increasing active levels in the brain which is associated with an antidepressant effect. Desvenlafaxine was approved for use in the United States in 2008 and is still used with more than 1 million prescriptions filled yearly. Current indications are limited to major depressive disorder. There is little evidence to suggest that it is more effective or has a different spectrum or rate of adverse events as venlafaxine. Desvenlafaxine is available as extended release tablets of 25, 50, or 100 mg in generic forms and under the brand name of Pristiq. The recommended dosage in adults is 50 mg once daily; increasing the dose has not been shown to improve efficacy. While believed to be better tolerated than venlafaxine, desvenlafaxine appears to have a similar spectrum and frequency of side effects.
Hepatotoxicity
Liver test abnormalities have been reported to occur in less than 1% of patients on venlafaxine or desvenlafaxine, and elevations are usually modest and usually do not require dose modification or discontinuation. Rare instances of acute, clinically apparent episodes of liver injury with marked liver enzyme elevations with or without jaundice have been reported in patients on both venlafaxine but not desvenlafaxine, perhaps because of less overall use of the more recently approved agent. The onset of injury is usually within 1 to 3 months. The patterns of serum enzyme elevation have varied from cholestatic to hepatocellular. All cases have been self-limiting and resolved within a few months. Autoimmune (autoantibodies) and immunoallergic features (rash, fever, eosinophilia) are uncommon or mild. The estimated frequency is 1 per 5000 patients years of exposure.
Likelihood score (venlafaxine): B (highly likely rare causes of clinically apparent liver injury).
Likelihood score (desvenlafaxine): E* (suspected but unproven cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which venlafaxine and desvenlafaxine cause liver injury is not known. Venlafaxine and desvenlafaxine are metabolized by the liver, mainly via the cytochrome P450 system (predominantly CYP 2D6), and hepatotoxicity may be mediated by toxic intermediates of their metabolism. Both agents can also result in significant drug-drug interactions.
Outcome and Management
The serum aminotransferase elevations that occur on venlafaxine and desvenlafaxine therapy are usually self-limited and do not require dose modification or discontinuation of therapy. Cases of acute and prolonged or severe liver injury have been reported in patients receiving venlafaxine, but there have been no convincing instances of acute liver failure due to venlafaxine or desvenlafaxine therapy in the published literature. Persons with intolerance to venlafaxine or desvenlafaxine may have similar reactions to each other as well as other SSRIs and careful monitoring is warranted if other such agents are used.
Drug Class: Antidepressant Agents
Other Drugs in the Subclass, SNRIs/SSRIs: Citalopram, Escitalopram, Duloxetine, Fluoxetine, Fluvoxamine, Levomilnacipran, Paroxetine, Sertraline, Vilazodone, Vortioxetine
CASE REPORT
Case 1. Acute hepatitis with jaundice due to venlafaxine.(1)
A 39 year old woman developed nausea, vomiting, pruritus and jaundice having been on oral therapy with venlafaxine for depression for two and half years, but also having recently increased the dose (from 75 to 300 mg daily) 2 months previously. She had had a cholecystectomy 20 years previously, but had no history of chronic liver disease, alcohol use, or exposures to hepatitis. She was mildly jaundiced and laboratory findings showed a serum bilirubin of 2.6 mg/dL, ALT 2063 U/L, and alkaline phosphatase 274 U/L. Tests for hepatitis A, B, C and E were negative, as were tests for HIV, cytomegalovirus and Epstein Barr virus infections. Autoantibodies were not present, and abdominal ultrasound showed no evidence of biliary obstruction. A liver biopsy showed a cholestatic hepatitis compatible with drug-induced liver injury. Of interest, three years previously she had a similar episode of acute liver injury with jaundice that arose 3 to 4 months after increasing her dose of venlafaxine from 75 to 150 mg daily, having been on this agent for 6 years. Upon stopping venlafaxine after the second episode, she improved minimally and serum bilirubin levels rose to 11.2 mg/dL. She was started on a course of methylprednisolone for 7 days which resulted in a prompt and lasting improvement in liver tests.
Key Points
| Medication: | Venlafaxine (300 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=25) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 2.5 years, 2 months after dose increase |
| Recovery: | Unclear |
| Other medications: | None mentioned |
Comment
In this case, the pattern of serum enzyme elevations was hepatocellular, but symptoms and liver biopsy findings suggested a cholestatic hepatitis. Striking in the history was a previous episode arising years after starting venlafaxine but only a few months after an increase in the daily dose. The long latency to onset of an acute hepatitis is unusual, but a history of a change in drug dose or source of the medication is sometimes given. Cases such as this suggest that idiosyncratic acute liver injury is not completely independent of drug dose.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Venlafaxine – Generic, Effexor®
Desvenlafaxine – Generic, Pristiq®
DRUG CLASS
Antidepressant Agents
COMPLETE LABELING (Venlafaxine)
COMPLETE LABELING (Desvenlafaxine)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Venlafaxine | 93413-69-5 | C17-H27-N-O2 |
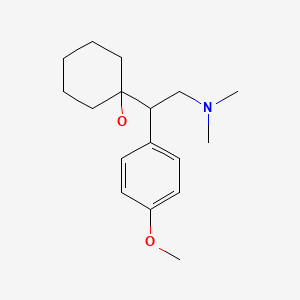
|
| Desvenlafaxine | 93413-62-8 | C16-H25-N-O2 |
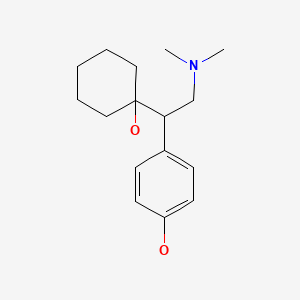
|
CITED REFERENCE
- 1.
- Stadlmann S, Portmann S, Tschopp S, Terracciano LM. Venlafaxine-induced cholestatic hepatitis: case report and review of literature. Am J Surg Pathol. 2012;36:1724–8. [PubMed: 23073329]
ANNOTATED BIBLIOGRAPHY
References updated: 06 March 2020
- Zimmerman HJ. Antidepressants. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 493-8.(Expert review of hepatotoxicity published in 1999; venlafaxine is not mentioned).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of hepatotoxicity of antidepressants mentions that clinically apparent liver injury from the SNRIs is rare, venlafaxine having been implicated in occasional cases of cholestatic injury).
- O'Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 267-78.(Textbook of pharmacology and therapeutics).
- Schweizer E, Thielen RJ, Frazer A. Venlafaxine: a novel antidepressant compound. Expert Opin Investig Drugs. 1997;6:65–78. [PubMed: 15989562](Review of the structure, pharmacology, mechanism of action, efficacy and side effects of venlafaxine; no mention of hepatotoxicity or ALT elevations).
- Mourilhe P, Stokes PE. Risks and benefits of selective serotonin reuptake inhibitors in the treatment of depression. Drug Saf. 1998;18:57–82. [PubMed: 9466088](Review of pharmacology, efficacy and safety of SSRIs; no mention of ALT elevations or hepatotoxicity).
- Kim KY, Hwang W, Narendran R. Acute liver damage possibly related to sertraline and venlafaxine ingestion. Ann Pharmacother. 1999;33:381–2. [PubMed: 10200868](27 year old man took overdose of sertraline and cephalexin and had elevations of ALT to 1247 U/L, bilirubin 2.1 mg/dL; switched to venlafaxine but 1 week later developed abdominal pain [bilirubin 1.6 mg/dL, ALT 814 U/L], improving on stopping but relapsing 3 days after restarting sertraline; recovered fully on stopping all SSRIs).
- Grohmann R, Rüther E, Engel RR, Hippius H. Assessment of adverse drug reactions in psychiatric inpatients with the AMSP drug safety program: methods and first results for tricyclic antidepressants and SSRI. Pharmacopsychiatry. 1999;32:21–8. [PubMed: 10071179](Analysis of reporting of adverse events among inpatients in 29 German hospitals between 1993 to 1997; 896 severe adverse events among 48,564 patients [1.8%], both total and hepatic events were more common with tricyclics than SSRIs).
- Horsmans Y, De Clercq M, Sernpoux C. Venlafaxine-associated hepatitis. Ann Intern Med. 1999;130:944. [PubMed: 10375350](44 year old woman developed fatigue 6 months after starting venlafaxine [ALT 1082 U/L], improving over 6 months after stopping venlafaxine while continuing trazodone; no mention of bilirubin or Alk P levels).
- Cardona X, Avila A, Castellanos P. Venlafaxine-associated hepatitis. Ann Intern Med. 2000;132(5):417. [PubMed: 10691596](78 year old man developed jaundice 6 weeks after starting venlafaxine [bilirubin 5.1 mg/dL, ALT 238 U/L, Alk P 680 U/L], resolving within 5 weeks of stopping).
- Carvajal García-Pando A, García del Pozo J, Sánchez AS, Velasco MA, Rueda de Castro AM, Lucena MI. Hepatotoxicity associated with the new antidepressants. J Clin Psychiatry. 2002;63:135–7. [PubMed: 11874214](Analysis of cases of hepatotoxicity from antidepressants in Spanish Pharmacovigilance System from 1989-1999, identified 99 cases; among SSRIs, 26 due to fluoxetine, 14 paroxetine, 6 fluvoxamine, 5 sertraline, 3 venlafaxine and 2 citalopram; among tricyclics, 16 clomipramine 7 amitriptyline, 6 imipramine; among miscellaneous, 3 nefazodone and 1 trazodone; but all similar in rate ~1-3 per 100,000 patient-years of exposure, except for nefazodone=29/100,000).
- Maroy B. Hepatite aigue cytolytique apres de venlafaxine. Gastroenterol Clin Biol. 2002;26:804. [Acute cytolytic hepatitis after venlafaxine therapy] French. [PubMed: 12434090](51 year old man developed jaundice 2 months after starting venlafaxine with mild rash [bilirubin 5.8 mg/dL, ALT 73 times ULN, Alk P 2 times ULN], resolving within 2 months of stopping).
- Lucena M, Carvajal A, Andrade R, Velasco A. Antidepressant-induced hepatotoxicity. Expert Opin Drug Saf. 2003;2:249–62. [PubMed: 12904104](Review of hepatotoxicity of antidepressants; antidepressant use has increased markedly between 1992 and 2002, accounting for 5% of cases of hepatotoxicity; SSRIs are less likely to cause injury than tricyclics and MAO inhibitors but SSRI liver injury has a range of presentations, although typically self-limited and with rapid recovery and without hallmarks of hypersensitivity).
- Spigset O, Hägg S, Bate A. Hepatic injury and pancreatitis during treatment with serotonin reuptake inhibitors: data from the World Health Organization (WHO) database of adverse drug reactions. Int Clin Psychopharmacol. 2003;18:157–61. [PubMed: 12702895](Among 27,542 reports of hepatic injury in WHO database, 786 were related to SSRIs [3%], including citalopram 42, fluoxetine 222, fluvoxamine 54, paroxetine 191, sertraline 112, nefazodone 91 and venlafaxine 74; only nefazodone has an excess of hepatic reports in relationship to total reports).
- Sencan I, Sahin I, Ozcetin A. Low-dose venlafaxine-associated liver toxicity in chronic hepatitis. Ann Pharmacother. 2004;38:352–3. [PubMed: 14742779](30 year old woman with chronic hepatitis B developed ALT elevations [689 U/L] without jaundice 2 months after stopping interferon and 6 weeks after starting venlafaxine, resolving in 6 weeks; unclear whether hepatotoxicity or posttreatment flare of hepatitis B).
- Degner D, Grohmann R, Kropp S, Rüther E, Bender S, Engel RR, Schmidt LG. Severe adverse drug reactions of antidepressants: results of the German multicenter drug surveillance program AMSP. Pharmacopsychiatry. 2004;37 Suppl 1:S39–45. [PubMed: 15052513](Analysis of adverse drug reactions reported from 1993-2000 in 35 psychiatric hospitals; 0.7% of SSRI recipients had a severe adverse event; hepatic in 0.05%).
- Pinzani V, Peyriere H, Hillaire-Buys D, Pageaux GP, Blayac BP, Larrey D. Specific serotonin recapture inhibitor (SSRI) antidepressants: hepatoxicity assessment in a large cohort in France. J Hepatol. 2006;44:S256.(Abstract; Analysis of French Pharmacovigilance data on SSRIs found 63 cases of hepatotoxicity from paroxetine, 45 fluoxetine, 30 citalopram, 18 sertraline, and 2 fluvoxamine).
- Phillips BB, Digmann RR, Beck MG. Hepatitis associated with low-dose venlafaxine for postmenopausal vasomotor symptoms. Ann Pharmacother. 2006;40:323–7. [PubMed: 16418323](60 year old woman developed abdominal pain 1 month after starting venlafaxine [bilirubin normal, ALT 372 U/L, Alk P 758 U/L], with rapid resolution upon stopping and relapse within 6 days of restarting).
- Christensen RC, Garces LK. Hepatotoxic effects with high-dose venlafaxine. Psychiatry (Edgmont). 2006;3:10–1. [PMC free article: PMC2958863] [PubMed: 20975814](33 year old man with bipolar disorder developed fatigue and abnormal liver tests 6 months after increasing his daily venlafaxine dose from 150 to 225 mg daily [ALT 188 U/L, bilirubin and Alk P not given], which fell to normal within 2 weeks of reducing the dose back to 150 mg daily).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 3 were attributed to paroxetine and 3 to fluoxetine, with a relative risk of injury to rate of use in the population of 3.0 and 1.8 respectively).
- DeSanty KP, Amabile CM. Antidepressant-induced liver injury. Ann Pharmacother. 2007;41:1201–11. [PubMed: 17609231](Review of drug induced liver injury and reports of injury from MAO inhibitors, SSRIs, tricyclics and atypical agents).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 6 were attributed to duloxetine, 3 to atomoxetine, 2 to fluoxetine, 2 to bupropion, and 1 to sertraline as single agents; venlafaxine not mentioned).
- Yildirim B, Tuncer C, Ergun M, Unal S. Venlafaxine-induced hepatotoxicity in a patient with ulcerative colitis. Ann Hepatol. 2009;8:271–2. [PubMed: 19841512](55 year old man with ulcerative colitis developed fatigue and abdominal pain 2 months after starting venlafaxine [bilirubin normal, ALT 192 U/L, Alk P 419 rising to 696 U/L], resolving within 2 months of stopping).
- Detry O, Delwaide J, De Roover A, Hans MF, Delbouille MH, Monard J, Honore P. Fulminant hepatic failure induced by venlafaxine and trazodone therapy: a case report. Transplant Proc. 2009;41:3435–6. [PubMed: 19857765](48 year old woman developed jaundice and liver failure 4 months after starting venlafaxine and trazodone [bilirubin 30 mg/dL, ALT 990 U/L, INR 4.6], undergoing liver transplantation 3 days after admission).
- Arroyo VC, Hallal H, Agudo JL, Aniorte JP. Gastroenterol Hepatol. 2009;32:382–3. [Venlafaxine-induced cholestatic hepatitis] Spanish. [PubMed: 19442411](39 year old man developed abdominal pain 10 days after starting venlafaxine [bilirubin rising to 18.6 mg/dL, ALT 103 U/L, Alk P 880 U/L], resolving within 5 months of stopping).
- Tourian KA, Padmanabhan SK, Groark J, Brisard C, Farrington D. Desvenlafaxine 50 and 100 mg/d in the treatment of major depressive disorder: an 8-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial and a post hoc pooled analysis of three studies. Clin Ther. 2009;31(Pt 1):1405–23. [PubMed: 19698901](Among 615 adults with major depression treated with desvenlafaxine [50 or 100 mg], duloxetine [60 mg] or placebo once daily for 8 weeks, there were minimal changes in mean levels of ALT and AST during treatment and significant isolated elevations of ALT or AST occurred in only 2 patients on desvenlafaxine, 1 on duloxetine and 2 on placebo).
- Clayton AH, Kornstein SG, Rosas G, Guico-Pabia C, Tourian KA. An integrated analysis of the safety and tolerability of desvenlafaxine compared with placebo in the treatment of major depressive disorder. CNS Spectr. 2009;14:183–95. [PubMed: 19407730](Analysis of safety from 9 placebo controlled trials of desvenlafaxine [50 to 400 mg daily] for major depression found that discontinuation due to adverse events was dose related, but rates were similar for the recommended 50 of 50 mg daily to placebo [4.1% vs 3.9%], and that the proportion with “clinically important” changes in liver enzymes were similar between venlafaxine and placebo [1% vs 2%] and there were no serious hepatic adverse events).
- Perry R, Cassagnol M. Desvenlafaxine: a new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder. Clin Ther. 2009;31(Pt 1):1374–404. [PubMed: 19698900](Systematic review of 8 controlled trials of desvenlafaxine for major depression found efficacy with 50 mg daily and no additional effect with higher doses, while adverse events included nausea, suicidal ideation, weight change and increase in blood pressure; no mention of ALT levels or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 1 due to venlafaxine and 1 to fluoxetine but none to other SSRIs).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N. Drug-induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with suspected drug induced liver injury, half [n=15] were due to antimicrobials [minocycline 4, INH 3, azithromycin 3] and the rest largely due to CNS agents and anticonvulsants; one case was attributed to amitriptyline, but no other antidepressant was listed).
- Stadlmann S, Portmann S, Tschopp S, Terracciano LM. Venlafaxine-induced cholestatic hepatitis: case report and review of literature. Am J Surg Pathol. 2012;36:1724–8. [PubMed: 23073329](39 year old woman developed jaundice 2.5 years after starting and 3 months after dose increase of venlafaxine [bilirubin 4.6 mg/dL, ALT 2064 U/L, Alk P 274 U/L], with a history of a similar episode when dose was increased in the past).
- Coleman KA, Xavier VY, Palmer TL, Meaney JV, Radalj LM, Canny LM. An indirect comparison of the efficacy and safety of desvenlafaxine and venlafaxine using placebo as the common comparator. CNS Spectr. 2012;17:131–41. [PubMed: 22883424](Metaanalysis of controlled trials of desvenlafaxine and venlafaxine as therapy for major depression found similar rates of efficacy as measured by depression rating scales and slightly lower rates of nausea and dropout because of adverse events for desvenlafaxine).
- Liebowitz MR, Tourian KA, Hwang E, Mele L., Study 3362 Investigators. A double-blind, randomized, placebo-controlled study assessing the efficacy and tolerability of desvenlafaxine 10 and 50 mg/day in adult outpatients with major depressive disorder. BMC Psychiatry. 2013;13:94. [PMC free article: PMC3763843] [PubMed: 23517291](Among 673 adults with major depression treated with desvenlafaxine [10 or 50 mg] or placebo once daily for 8 weeks, there were no differences among treatment groups in response or adverse event rates and no liver related severe adverse events and no mention of ALT elevations).
- Rosenthal JZ, Boyer P, Vialet C, Hwang E, Tourian KA. Efficacy and safety of desvenlafaxine 50 mg/d for prevention of relapse in major depressive disorder: a randomized controlled trial. J Clin Psychiatry. 2013;74:158–66. [PubMed: 23473348](Among 548 adults with a history of major depression treated with desvenlafaxine [50 mg] or placebo once daily for 8 months, the probability of relapse was less with desvenlafaxine [14% vs 30%] and adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to venlafaxine despite it being one of the 50 most prescribed drugs in Iceland).
- Park SH, Ishino R. Liver injury associated with antidepressants. Curr Drug Saf. 2013;8:207–23. [PubMed: 23914755](Review of hepatotoxicity of modern antidepressants).
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–15. [PubMed: 24362450](Review of hepatotoxicity of antidepressants, mentions 9 case reports from venlafaxine with latency of 10 days to 6 months, both hepatocellular and cholestatic injury, with liver transplant in 1 and with recovery in 9).
- Findling RL, Groark J, Chiles D, Ramaker S, Yang L, Tourian KA. Safety and tolerability of desvenlafaxine in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol. 2014;24:201–9. [PMC free article: PMC4026302] [PubMed: 24611442](Among 40 children and adolescents with major depression enrolled in a 6 month, open label extension trial of desvenlafaxine, 21 discontinued therapy early but none because of liver disease or ALT elevations; mean levels of ALT and AST did not change).
- Boyer P, Vialet C, Hwang E, Tourian KA. Efficacy of desvenlafaxine 50 mg/d versus placebo in the long-term treatment of major depressive disorder: a randomized, double-blind trial. Prim Care Companion CNS Disord. 2015;17(4) [PMC free article: PMC4664561] [PubMed: 26693033] [CrossRef](Among 548 patients with major depression who responded to an open label course of desvenlafaxine and then were randomized to continue therapy or be withdrawn [placebo], 74% on venlafaxine maintained a remission vs 52% on placebo; adverse event rates and ALT results were not reported ).
- Clayton AH, Tourian KA, Focht K, Hwang E, Cheng RF, Thase ME. Desvenlafaxine 50 and 100 mg/d versus placebo or the treatment of major depressive disorder: a phase 4, randomized controlled trial. J Clin Psychiatry. 2015;76:562–9. [PubMed: 25375652](Among 909 patients with major depression treated with desvenlafaxine [50 or 100 mg] or placebo for 8 weeks, improvements in depression rating scores were greater for desvenlafaxine while rates of adverse events were similar and there were no serious hepatic adverse events).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 20 cases [2%] were attributed to antidepressants including 9 due to SNRIs including duloxetine [n=7], nefazodone [n=1], trazodone [n=1], and 5 to SSRIs including escitalopram [n=3], fluoxetine [n=1] and sertraline [n=1], but none were attributed to venlafaxine).
- Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, Toto S, et al. Drug-induced liver injury during antidepressant treatment: results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol. 2016;19:pyv126. pii. [PMC free article: PMC4851269] [PubMed: 26721950](Among 184,234 psychiatric inpatients from 80 hospitals, 149 cases [0.08%] of drug induced liver injury were reported including 25 [0.09%] attributed to venlafaxine, most cases being asymptomatic and anicteric).
- Iragavarapu C, Gupta T, Chugh SS, Aronow WS, Frishman WH. Type B lactic acidosis associated with venlafaxine overdose. Am J Ther. 2016;23:e1082–4. [PubMed: 25405896](55 year old man developed nausea, tachycardia and high lactate levels within hours of taking an overdose of venlafaxine [lactate 8.6 mmol/L, pH 7.39] with normal liver tests, resolving within 24 hours with hydration and medical support).
- Poitras V, Visintini S. Desvenlafaxine versus venlafaxine for the treatment of adult patients with major depressive disorder: a review of the comparative clinical and cost-effectiveness [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017 Oct 25. Available from http://www
.ncbi.nlm.nih .gov/books/NBK507133/ [PubMed: 29889482] (Review of systematic reviews on the comparison of the efficacy of desvenlafaxine and venlafaxine concluded that the two agents were similar in effectiveness). - Ferrajolo C, Scavone C, Donati M, Bortolami O, Stoppa G, Motola D, Vannacci A, et al. DILI-IT Study Group. Antidepressant-induced acute liver injury: a case-control study in an Italian inpatient population. Drug Saf. 2018;41:95–102. [PubMed: 28770534](Among 179 cases of hospitalizations for unexplained acute liver injury enrolled in an Italian prospective study between 2010 and 2014, 17 had been exposed to antidepressants including citalopram [n=4], sertraline [n=3], amitriptyline [n=3] and paroxetine [n=2], but venlafaxine was not implicated in any case).
- Billioti de Gage S, Collin C, Le-Tri T, Pariente A, Bégaud B, Verdoux H, Dray-Spira R, et al. Antidepressants and hepatotoxicity: A cohort study among 5 million individuals registered in the French National Health Insurance Database. CNS Drugs. 2018;32:673–84. [PMC free article: PMC6061298] [PubMed: 29959758](Among 5 million persons identified in a national French health insurance database who started an antidepressant between 2010 and 2015, 382 developed serious liver injury resulting in hospitalization, rates per 100,0000 persons-years being 19 for SSRIs, 22 venlafaxine, 13 duloxetine, and 33 mirtazapine).
- Atkinson S, Lubaczewski S, Ramaker S, England RD, Wajsbrot DB, Abbas R, Findling RL. Desvenlafaxine versus placebo in the treatment of children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol. 2018;28:55–65. [PMC free article: PMC5771531] [PubMed: 29185786](Among 363 children with major depression treated with desvenlafaxine in two doses or placebo for 8 weeks, there were no changes in depression rating scores and side effect rates were similar in all groups, mean ALT, AST and Alk P levels not changing significantly).
- Atkinson S, Thurman L, Ramaker S, Buckley G, Jones SR, England R, Wajsbrot D. Safety, tolerability, and efficacy of desvenlafaxine in children and adolescents with major depressive disorder: results from two open-label extension trials. CNS Spectr. 2019;24:496–506. [PubMed: 30419989](Analysis and comparison of two 6-month open-label trials of desvenlafaxine in 552 children and adolescents with major depression found no new safety signals; no discussion of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Serotonin and Norepinephrine Reuptake Inhibitors.[Handb Exp Pharmacol. 2019]Serotonin and Norepinephrine Reuptake Inhibitors.Shelton RC. Handb Exp Pharmacol. 2019; 250:145-180.
- Review Duloxetine.[LiverTox: Clinical and Researc...]Review Duloxetine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fluoxetine.[LiverTox: Clinical and Researc...]Review Fluoxetine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Desvenlafaxine: another "me too" drug?[Ann Pharmacother. 2008]Review Desvenlafaxine: another "me too" drug?Sopko MA Jr, Ehret MJ, Grgas M. Ann Pharmacother. 2008 Oct; 42(10):1439-46. Epub 2008 Aug 12.
- Review Fluvoxamine.[LiverTox: Clinical and Researc...]Review Fluvoxamine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Venlafaxine, Desvenlafaxine - LiverToxVenlafaxine, Desvenlafaxine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...