NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Solriamfetol is dopamine and norepinephrine reuptake inhibitor that is used in the therapy of excessive daytime sleepiness and cataplexy in patients with narcolepsy. Solriamfetol has not been associated with serum enzyme elevations during therapy or to instances of idiosyncratic acute liver injury.
Background
Solriamfetol (sol”ri am’ fe tol) is an orally available, small molecule dopamine and norepinephrine reuptake inhibitor (DNRI) that is used to treat excessive daytime sleepiness in adults with narcolepsy or obstructive sleep apnea. Dopamine and norepinephrine are major stimulatory neurotransmitters and inhibition of their synaptic reuptake results in higher levels. Dysregulation of dopaminergic and norepinephrine systems plays at least a partial role in wakefulness and sleep. Narcolepsy is associated with a deficiency in orexin, a major mediator of wakefulness. Modulation of dopamine and norepinephrine signaling by solriamfetol increases wakefulness, independent of orexin and has been shown to improve wakefulness and decrease excessive daytime sleepiness in patients with narcolepsy as well as in patients with obstructive sleep apnea who have difficulty with daytime sleepiness. In several randomized, placebo controlled trials, solriamfetol was found to decrease sleepiness and increase wakefulness scores in the majority of treated subjects. Solriamfetol was approved in the United States in 2019 as therapy for excessive daytime sleepiness associated with narcolepsy and obstructive sleep apnea in adults. Solriamfetol is available tablets of 75 and 150 mg under the brand name Sunosi. The recommended initial dose is 75 mg once daily in adults with narcolepsy and 37.5 mg once daily in obstructive sleep apnea with subsequent titration to a maximum of 150 mg daily. Side effects can include headache, nausea, anxiety, irritability, insomnia, dizziness, palpitations, dry mouth and decreased appetite which are frequent during the titration period but often resolve with maintenance therapy. Nevertheless, up to 10% of patients discontinue therapy because of adverse events. Severe adverse events are rare, but solriamfetol can cause increase in blood pressure and heart rate which should be monitored during therapy. Solriamfetol is a Schedule IV controlled agent, indicating that it has the potential for dependence and abuse.
Hepatotoxicity
In placebo-controlled trials of solriamfetol in patients with narcolepsy, minor serum aminotransferase elevations occurred in a small proportion of patients during therapy, but the rates of enzyme elevations overall were similar to those in placebo recipients. In preregistration trials, there were no instances of clinically apparent liver injury or serum aminotransferase elevations with jaundice attributable to solriamfetol. Since its approval in 2019, there have been no publications describing clinically apparent liver injury due to solriamfetol.
Likelihood score: E (unlikely cause of acute liver injury with jaundice).
Mechanism of Injury
The mechanism by which solriamfetol might cause liver injury is not known. Its hepatic safety is probably due to the fact that it has little metabolism and is excreted largely unchanged (>95%) in the urine. Solriamfetol has minimal drug-drug interactions.
Drug Class: CNS Stimulants
Other Drugs for Narcolepsy: Amphetamines, Modafinil, Armodafinil, Methylphenidate, Oxybate, Pitolisant
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Solriamfetol – Sunosi®
DRUG CLASS
CNS Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
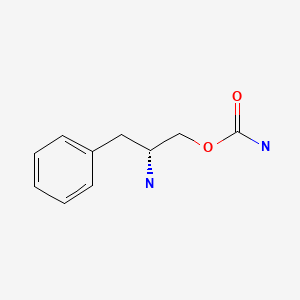
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Solriamfetol | 178429-62-4 | C10-H14-N2-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 18 August 2021
Abbreviations: DNRI, dopamine and norepinephrine reuptake inhibitor.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of solriamfetol).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/211230Orig1Orig2s000RiskR.pdf. (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA multidisciplinary scientific review of the solriamfetol application for safety and efficacy, which reported that the trials of solriamfetol had no fatalities and no treatment related serious hepatic adverse events). - Strollo PJ Jr, Hedner J, Collop N, Lorch DG Jr, Chen D, Carter LP, Lu Y, et al. Tones 4 Study Investigators. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest. 2019;155:364–374. [PubMed: 30471270](Among 174 adults with obstructive sleep apnea and excessive daytime sleepiness treated with a dose titration of solriamfetol followed by randomization to continue therapy or switch to placebo, there was clinical improvement on drug and relapse upon withdrawal, adverse events occurring largely during the titration phase [total rate of 49% decreasing to 10.2% during maintenance]; no mention of ALT elevations or hepatotoxicity).
- Thorpy MJ, Shapiro C, Mayer G, Corser BC, Emsellem H, Plazzi G, Chen D, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019;85:359–370. [PMC free article: PMC6593450] [PubMed: 30694576](Among 231 patients with narcolepsy and excessive daytime sleepiness treated with solriamfetol [75, 150 or 300 mg] or placebo daily for 12 weeks, both higher doses were associated with improvements achieved within 1 week of starting and maintained thereafter, and while adverse events were more frequent with solriamfetol [68% vs 46%] as were discontinuations [5% to 8.5% vs 1.7%], “no drug-related effects were found on clinical laboratory assessments”).
- Markham A. Solriamfetol: first global approval. Drugs. 2019;79:785–790. [PubMed: 31062265](Review of the mechanism of action, history of development, pharmacology, clinical efficacy, and safety of solriamfetol shortly after its approval for use in the US, mentions the most common symptoms but does not mention ALT elevations or hepatotoxicity).
- Schweitzer PK, Rosenberg R, Zammit GK, Gotfried M, Chen D, Carter LP, Wang H, et al. TONES 3 Study Investigators. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3). A randomized controlled trial. Am J Respir Crit Care Med. 2019;199:1421–1431. [PMC free article: PMC6835071] [PubMed: 30521757](Among 459 adults with obstructive sleep apnea and excessive daytime sleepiness treated with solriamfetol [37.5, 75, 150 or 300 mg daily] or placebo for 12 weeks, sleepiness scores decreased and wakefulness scores increased, and while total adverse events were more common with solriamfetol, severe adverse events were rare [0.8% vs 1.7% in controls] and “laboratory evaluations did not indicate effects of clinical relevance related to solriamfetol”).
- Solriamfetol (Sunosi) for excessive daytime sleepiness. Med Lett Drugs Ther. 2019;61(1579):132–134. [PubMed: 31581157](Concise review of the mechanism of action, clinical efficacy, safety and cost of solriamfetol for excess sleepiness associated with narcolepsy and obstructive sleep apnea mentions adverse events of headache, nausea, anorexia, dry mouth, anxiety, and insomnia but does not discuss ALT elevations of hepatotoxicity).
- Malhotra A, Shapiro C, Pepin JL, Hedner J, Ahmed M, Foldvary-Schaefer N, Strollo PJ, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020;43:zsz220. [PMC free article: PMC7315408] [PubMed: 31691827](1 year extension study of solriamfetol in 643 patients who completed participation in 12-week placebo controlled trials found that the effects on excessive sleepiness were maintained, and adverse events being headache [11%], nausea [9%], insomnia [8%], dry mouth [7%], anxiety [7%], and anorexia [5%], with 9% discontinuing therapy because of adverse events; no mention of ALT elevations or hepatotoxicity).
- Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34:9–27. [PMC free article: PMC6982634] [PubMed: 31953791](Review of the mechanism of action, pharmacology, drug-drug interactions, clinical efficacy and safety of newly approved medications for narcolepsy including pitolisant and solriamfetol; no mention of ALT elevations or hepatotoxicity).
- Videnovic A, Amara AW, Comella C, Schweitzer PK, Emsellem H, Liu K, Sterkel AL, et al. Solriamfetol for Excessive Daytime Sleepiness in Parkinson's Disease: Phase 2 Proof-of-Concept Trial. Mov Disord. 2021 Jun 30; Epub ahead of print. [PMC free article: PMC8596433] [PubMed: 34191352](Among 66 patients with Parkinson disease and excessive daytime sleepiness treated with 4 one-week courses of solriamfetol [75, 150, or 300 mg] or placebo once daily, “there were minor or no clinically meaningful changes in…clinical laboratory findings”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Solriamfetol for the Treatment of Excessive Daytime Sleepiness in Participants with Narcolepsy with and without Cataplexy: Subgroup Analysis of Efficacy and Safety Data by Cataplexy Status in a Randomized Controlled Trial.[CNS Drugs. 2020]Solriamfetol for the Treatment of Excessive Daytime Sleepiness in Participants with Narcolepsy with and without Cataplexy: Subgroup Analysis of Efficacy and Safety Data by Cataplexy Status in a Randomized Controlled Trial.Dauvilliers Y, Shapiro C, Mayer G, Lammers GJ, Emsellem H, Plazzi G, Chen D, Carter LP, Lee L, Black J, et al. CNS Drugs. 2020 Jul; 34(7):773-784.
- Review Solriamfetol for the treatment of excessive daytime sleepiness associated with narcolepsy.[Expert Rev Clin Pharmacol. 2019]Review Solriamfetol for the treatment of excessive daytime sleepiness associated with narcolepsy.Yang J, Gao J. Expert Rev Clin Pharmacol. 2019 Aug; 12(8):723-728. Epub 2019 Jun 19.
- Review Solriamfetol: A Review in Excessive Daytime Sleepiness Associated with Narcolepsy and Obstructive Sleep Apnoea.[CNS Drugs. 2023]Review Solriamfetol: A Review in Excessive Daytime Sleepiness Associated with Narcolepsy and Obstructive Sleep Apnoea.Hoy SM. CNS Drugs. 2023 Nov; 37(11):1009-1020. Epub 2023 Oct 17.
- Review Profile of Solriamfetol in the Management of Excessive Daytime Sleepiness Associated with Narcolepsy or Obstructive Sleep Apnea: Focus on Patient Selection and Perspectives.[Nat Sci Sleep. 2021]Review Profile of Solriamfetol in the Management of Excessive Daytime Sleepiness Associated with Narcolepsy or Obstructive Sleep Apnea: Focus on Patient Selection and Perspectives.Abad VC. Nat Sci Sleep. 2021; 13:75-91. Epub 2021 Jan 25.
- Review A Comprehensive Review of Solriamfetol to Treat Excessive Daytime Sleepiness.[Psychopharmacol Bull. 2024]Review A Comprehensive Review of Solriamfetol to Treat Excessive Daytime Sleepiness.Fuller MC, Carlson S, Pysick H, Berry V, Tondryk A, Swartz H, Cornett EM, Kaye AM, Viswanath O, Urits I, et al. Psychopharmacol Bull. 2024 Mar 4; 54(1):65-86.
- Solriamfetol - LiverToxSolriamfetol - LiverTox
- Licorice - LiverToxLicorice - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...