NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Resveratrol is a plant polyphenol found in high concentrations in red grapes that has been proposed as a treatment for hyperlipidemia and to prevent fatty liver, diabetes, atherosclerosis and aging. Resveratrol use has not been associated with serum enzyme elevations or with clinically apparent liver injury.
Background
Resveratrol is a natural plant polyphenol (3,5,4’-trihydroxystilbene) that is found in highest concentrations in the skin of red grapes and other fruits (mulberries, blueberries, blackberries). In cell culture, resveratrol has antiinflammatory, cytoprotective, and antineoplastic properties which can be reproduced in several animal models. In model systems such as yeast (S. cerevisiae), worms (C. elegans) and fruit flies (Drosophilia), chronic administration of resveratrol extends lifespan in a manner similar to caloric restriction. These results were somewhat controversial, but subsequent studies in several mammalian species supported the finding to some extent. Thus, resveratrol extended lifespan in mice fed a high fat diet (but not in normal mice), seemingly by counteracting the detrimental effects of the diet, and improving insulin sensitivity and mitochondrial function. The bases for the beneficial effects of resveratrol are unclear. Resveratrol has direct antioxidant effects, but also stimulates expression of antioxidant enzymes and the activity of sirtuin 1 (Sirt-1) and adenosine monophosphate activated protein kinase (AMP-K), both of which have major effects on glucose and fat metabolism and may play a role in aging. Resveratrol is available without prescription as a nutritional supplement in multiple preparations and doses. In human trials, doses of resveratrol have ranged from 20 mg to 5 g daily; but a typical over-the-counter recommended dose is 500 mg twice daily. Importantly, the purity of commercial products is rarely well defined, its oral bioavailability is poor and the form responsible for its activity is not known. Thus, resveratrol exists in both trans and cis configuration and the major form found in plasma is a sulfated or glucuronidal conjugate rather than free resveratrol. At present, there is no conclusive evidence that resveratrol has beneficial effects in humans. On the other hand, it has few if any side effects. Side effects may include minor gastrointestinal upset, nausea, headache and fatigue and possible supplement-drug interactions with estrogens and anticoagulants.
Hepatotoxicity
Liver injury attributable to resveratrol has not been reported. In trials of resveratrol in human subjects, there have been no reports of serum enzyme elevations or clinically apparent liver injury. Thus, hepatotoxicity due to resveratrol must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Herbal and Dietary Supplements
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Resveratrol – Generic
DRUG CLASS
Herbal and Dietary Supplements
SUMMARY INFORMATION
Fact Sheet at MedlinePlus, NLM
Fact Sheet at National Center for Complementary and Integrative Health, NIH
Product labeling at DailyMed, National Library of Medicine, NIH
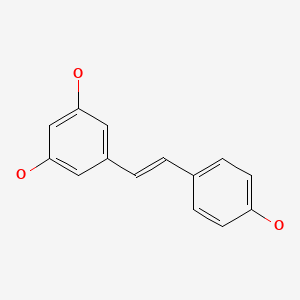
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Resveratrol | 501-36-0 | C14-H12-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 21 May 2018
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 731-4.(Expert review of hepatotoxicity published in 1999; several herbal medications are discussed, but not resveratrol).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 631-58.(Review of hepatotoxicity of herbal and dietary supplements [HDS]; no mention of resveratrol).
- Resveratrol. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007: pp. 19-26.(Compilation of short monographs on herbal medications and dietary supplements).
- Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195–206. [PubMed: 12016550](Review and description of patterns of liver injury, including discussion of potential risk factors, and herb-drug interactions).
- Schiano TD. Hepatotoxicity and complementary and alternative medicines. Clin Liver Dis. 2003;7:453–73. [PubMed: 12879994](Comprehensive review of herbal associated hepatotoxicity, including common patterns of presentation).
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. [PMC free article: PMC4990206] [PubMed: 17086191](In mice fed a high caloric diet long term, resveratrol [22 mg/kg/day] supplementation increased life span, improved insulin sensitivity, and decreased hepatic fat).
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–8. [PMC free article: PMC2474520] [PubMed: 18599449](Mice with overexpression of Sirt1 were protected from the metabolic effects of a high fat diet, and had improved insulin sensitivity and less hepatic fat and inflammation with lower levels of inflammatory cytokines and higher antioxidant enzymes).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network(DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 9% of cases were attributed to herbal medications, but no case attributed to resveratrol alone or in combination with other agents).
- García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, Martínez-Sierra MC, et al. Rev Esp Enferm Dig. 2008;100:688–95. [Liver injury induced by "natural remedies": an analysis of cases submitted to the Spanish Liver Toxicity Registry] Spanish. [PubMed: 19159172](Among 521 cases of drug induced liver injury submitted to Spanish registry, 13 [2%] were due to herbals; none were due to resveratrol).
- Jacobsson I, Jönsson AK, Gerdén B, Hägg S. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039–47. [PubMed: 19650152](Review of 778 spontaneous reports of adverse reactions to herbals to Swedish Registry; no instance was linked to resveratrol).
- Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009 May;53 Suppl 1:S7–15. [PubMed: 19194969](Pharmacokinetic study of 4 doses of resveratrol vs placebo given every 4 hours for 48 hours in 40 volunteers; side effects included headache, dyspepsia and dizziness; "no clinically significant abnormalities were found in the laboratory parameters").
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–11. [PMC free article: PMC2982884] [PubMed: 20935227](Pharmacokinetic study of 4 doses of resveratrol [0.5-4 g daily] for 29 days in 40 volunteers found occasional minor abdominal discomfort, nausea, diarrhea, flatulence and dizziness with higher doses, but no serious adverse events).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury, of which 12 [9%] were due to herbals, including several herbal mixtures, usnic acid, Ma Huang, black cohosh, and Hydroxycut, but not resveratrol).
- Bishayee A, Darvesh AS, Politis T, McGory R. Resveratrol and liver disease: from bench to bedside and community. Liver Int. 2010;30:1103–14. [PubMed: 20557453](Extensive review of the in vitro and in vivo studies of resveratrol as a hepatoprotective agent against several forms of experimental liver injury, and the challenges of clinical studies of resveratrol in humans).
- Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health.a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–41. [PubMed: 21688389](Review of the literature on pharmacokinetics, efficacy and safety of resveratrol in humans; few studies have been done, but none have reported severe adverse events or significant toxicities).
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, et al. Phase I randomized, double-blind pilot study of micronized resveratrol(SRT501) in patients with hepatic metastases.safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res(Phila). 2011;4:1419–25. [PMC free article: PMC3173869] [PubMed: 21680702](9 subjects scheduled to undergo hepatectomy for cancer metastases were given a micronized resveratrol [5 g/day] or placebo for 14 days; peak plasma levels were variable and achieved in 2-4 hours with plasma half-life of 1 hour, and detectable levels in tissue at surgery).
- Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. [PubMed: 21261636](Resveratrol exists as trans and cis isomers and is well absorbed orally, but is rapidly metabolized by intestine and liver to sulfate and glucuronide conjugates).
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–64. [PMC free article: PMC3496026] [PubMed: 23102619](Controlled study of resveratrol [75 mg/day] vs placebo in 29 normal volunteers, found no change in weight, insulin sensitivity, serum enzyme levels or in Sirt-1 expression [in muscle and adipose tissue] or AMP-K activity [in muscle tissue] after 12 weeks of therapy).
- Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int. 2012;32:1543–56. [PubMed: 22928722](A systematic compilation of all publications on the hepatotoxicity of specific herbals identified 185 publications on 60 different herbs, herbal drugs and supplements; none implicated resveratrol).
- Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, et al. Alzheimer's Disease Cooperative Study. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–91. [PMC free article: PMC4626244] [PubMed: 26362286](Among 119 patients with Alzheimer disease treated with resveratrol or placebo for 52 weeks, there was no improvement in clinical or radiologic features and adverse event rates were similar between the 2 groups; no mention of ALT levels except that "routine laboratory tests were normal").
- Yiu EM, Tai G, Peverill RE, Lee KJ, Croft KD, Mori TA, Scheiber-Mojdehkar B, et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J Neurol. 2015;262:1344–53. [PubMed: 25845763](Among 24 patients with Friedreich ataxia treated with resveratrol [1 g vs 5 g daily] for 12 weeks, adverse events more frequent with the higher dose included diarrhea [71% vs 8%], nausea [36% vs 8%] and skin rash [29% vs none], and 1 subject developed liver test abnormalites 4 weeks after starting high dose resveratrol [bilirubin not provided, ALT <2 times ULN, Alk P 4 times ULN], resolving 8 weeks after stopping).
- Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, Shu F, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis. 2015;47:226–32. [PubMed: 25577300](Among 60 patients with nonalcoholic fatty liver disease treated with resveratrol vs placebo for 3 months, median ALT levels decreased by 7 vs 1 U/L, while ultrasound graded hepatic fat did not change).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:537. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products, does not list resveratrol).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Resveratrol - LiverToxResveratrol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...