NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pimavanserin is an atypical antipsychotic used in the treatment of hallucinations and delusions in patients with Parkinson disease and psychosis. Use of pimavanserin is associated with a low rate of serum enzyme elevations during therapy but it has not been linked to instances of clinically apparent acute liver injury.
Background
Pimavanserin (pim" a van' ser in) is non-dopaminergic atypical antipsychotic agent that appears to act as a selective inverse agonist of the serotonin (5-HT) 2A receptor. It has little or no activity against the 5-HT2B and 2C receptors which may account for its relative lack of adverse effects. The absence of dopamine receptor activity suggested that pimavanserin would be appropriate for patients with Parkinson disease psychosis which is usually resistant to the atypical antipsychotic medications and can be worsened by inhibition of dopaminergic transmission. Clinical studies demonstrated its effectiveness in Parkinson disease psychosis, and it was approved for this indication in the United States in 2016. Pimavanserin is available in capsules of 34 mg and tablets of 10 mg under the brand name Nuplazid. The typical dose is 34 mg once daily. Common side effects include somnolence, headache, confusion, hallucinations, and peripheral edema. Rare, but potentially serious adverse events include prolongation of the QT interval and increased risk of death in elderly patients with dementia related psychosis.
Hepatotoxicity
Liver test abnormalities are uncommon (<1%) in patients taking pimavanserin, and the frequency of elevations appears to be similar to the rate that occurs with placebo therapy. No liver related serious adverse events or cases of clinically apparent liver injury were reported in the preregistration trials of pimavanserin. Pimavanserin has had limited clinical use since its approval and general availability, and it has not been implicated in published reports of clinically apparent liver injury. Thus, liver injury due to pimavanserin must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The potential mechanism by which pimavanserin might cause liver injury is not apparent. Pimavanserin is metabolized in the liver, primarily via CYP 3A and is susceptible to drug-drug interactions with agents that are potent inducers or inhibitors of CYP 3A activity.
Outcome and Management
Liver test abnormalities during pimavanserin therapy are uncommon and typically transient, mild and not associated with symptoms or jaundice. There have been no reports of hepatitis, acute liver failure, chronic hepatitis or vanishing bile duct syndrome attributed to pimavanserin. It is unlikely that there is cross sensitivity to liver injury between pimavanserin and other atypical antipsychotic medications.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pimavanserin – Nuplazid®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
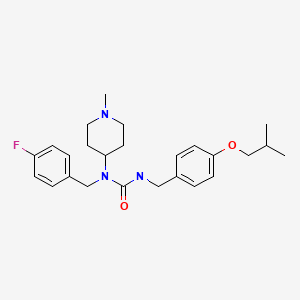
| Pimavanserin | 706779-91-1 | C25-H34-F-N3-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 05 June 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Meltzer HY, Elkis H, Vanover K, Weiner DM, van Kammen DP, Peters P, Hacksell U. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141:144–52. [PubMed: 22954754](Among 423 patients with an acute exacerbation of schizophrenia treated with one of five regimens combining pimavanserin or placebo with either haloperidol or risperidone for 6 weeks, combining low doses of risperidone with pimavanserin yielded better efficacy with lower rates of adverse events [including lower rates of ALT and AST elevations] compared to full doses of risperidone or haloperidol with or without pimavanserin).
- Meltzer HY, Roth BL. Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest. 2013;123:4986–91. [PMC free article: PMC3859385] [PubMed: 24292660](Review of serotonin [5-HT] receptor activity and the advantages of highly selective receptor-subtype directed therapies, examples being lorcaserin, the 5-HT2C agonist [without 5-HT2B agonist activity] used for weight loss, and pimavanserin, the 5-HT2A inverse agonist [without dopaminergic activity] used for Parkinson disease psychosis).
- Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, et al. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–40. [PubMed: 24183563](Among 199 patients with Parkinson disease psychosis treated with pimavanserin [40 mg daily] or placebo for 6 weeks, symptoms improved more with pimavanserin, and adverse event rates were similar; “laboratory assessments were unremarkable, and no safety signals were reported”).
- Fox SH. Pimavanserin as treatment for Parkinson's disease psychosis. Lancet. 2014;383(9916):494–6. [PubMed: 24183566](Editorial in response to Cummings [2014]).
- Markham A. Pimavanserin: first global approval. Drugs. 2016;76:1053–7. [PubMed: 27262680](Review of the development of pimavanserin, its mechanism of action, pharmacodynamics, inverse agonism of 5HT-2A, clinical efficacy, and safety; no mention of ALT elevations or hepatotoxicity).
- Pimavanserin (Nuplazid) for Parkinson's disease psychosis. Med Lett Drugs Ther. 2016;58(1496):74–5. [PubMed: 27249096](Concise review of the mechanism of action, efficacy, safety and costs of pimavanserin shortly after its approval in Parkinson psychosis in the US; no mention of ALT elevations or hepatotoxicity).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that olanzapine can cause aminotransferase elevations, and that olanzapine and ziprasidone can cause DRESS syndrome, but does not mention ALT elevations or hepatotoxicity for any of agents discussed, including aripiprazole, brexpiprazole, cariprazine, clozapine, quetiapine, risperidone, asenapine, iloperidone, paliperidone, and lurasidone).
- Mathis MV, Muoio BM, Andreason P, Avila AM, Farchione T, Atrakchi A, Temple RJ. The US Food and Drug Administration's perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78:e668–e673. [PubMed: 28493654](Summary of FDA’s review of the efficacy and safety of pimavanserin in Parkinson disease psychosis mentions that symptom improvements occurred in 80.5% of pimavanserin- vs 58% of placebo-treated patients, but concerns remain about the safety of antipsychotic agents in frail, elderly subjects with dementia).
- Ballard C, Banister C, Khan Z, Cummings J, Demos G, Coate B, Youakim JM, Owen R, Stankovic S, Investigators ADP. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer's disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17:213–222. [PubMed: 29452684](Among 181 elderly patients with Alzheimer disease related psychosis treated with pimavanserin [34 mg] or placebo daily for 12 weeks, improvements in psychosis symptoms were more frequent with pimavanserin than placebo at 6 weeks but not at 12 weeks, while adverse event rates were similar; no patient on pimavanserin had ALT or AST elevations).
- Fava M, Dirks B, Freeman MP, Papakostas GI, Shelton RC, Thase ME, Trivedi MH, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80: 19m12928. [PubMed: 31556975](Among 207 patients with major depressive disorder and an inadequate response to antidepressant therapy who were treated with the addition of pimavanserin or placebo for 5 weeks, symptoms of depression improved more in those treated with pimavanserin but adverse events were more frequent [48% vs 27%], but there were no “clinically relevant” changes in the clinical laboratory test results).
- Ballard CG, Kreitzman DL, Isaacson S, Liu IY, Norton JC, Demos G, Fernandez HH, et al. 015 Study Group. Long-term evaluation of open-label pimavanserin safety and tolerability in Parkinson's disease psychosis. Parkinsonism Relat Disord. 2020;77:100–106. [PubMed: 32712560](Among 459 patients with Parkinson disease psychosis treated in an open label extension study with pimavanserin for a median of 454 days, improvements in symptoms of psychosis were sustained and there were no hepatic severe adverse events and “no noteworthy changes from baseline” of ALT, AST or bilirubin).
- Drugs for Parkinson's disease. Med Lett Drugs Ther. 2021;63(1618):25–32. [PubMed: 33647001](Concise review of the mechanism of action, clinical efficacy, safety and costs of drugs approved for therapy of Parkinson disease in the United States mentions that pimavanserin is FDA-approved for the treatment of Parkinson disease psychosis; no mention of ALT elevations or hepatotoxicity).
- Tariot PN, Cummings JL, Soto-Martin ME, Ballard C, Erten-Lyons D, Sultzer DL, Devanand DP, et al. Trial of Pimavanserin in Dementia-Related Psychosis. N Engl J Med. 2021;385:309–319. [PubMed: 34289275](Among 217 patients with dementia-related psychosis who had a response to pimavanserin in a 12 week open label phase who were then continued on therapy or switched to placebo, relapse in psychotic symptoms was less frequent in those on pimavanserin than placebo [13% vs 28%], while adverse events rates were similar, headache, constipation and urinary tract infections being more frequent with treatment; no mention of ALT levels or hepatotoxicity).
- Druschky K, Toto S, Bleich S, Baumgärtner J, Engel RR, Grohmann R, Maier HB, et al. Severe drug-induced liver injury in patients under treatment with antipsychotic drugs: data from the AMSP study. World J Biol Psychiatry. 2021;22:373–386. [PubMed: 32892689](Among 246 cases of severe liver injury due to antipsychotic medications identified in a prospective registry of German psychiatric hospitals between 1993 and 2016, 46 arose in 38,349 patients [0.12%] who received clozapine [34 as a single antipsychotic agent]; other commonly implicated agents being olanzapine [n=90 of 54,822: 0.16%], quetiapine [34 of 66,209: 0.05%] and risperidone [27 of 51,683: 0.05%]; two fatal cases occurred in olanzapine-treated patients; low rates were found for ziprasidone [no cases among 3568 patients treated] and aripiprazole [6 cases of 15,988 patients treated: 0.01%], pimavanserin not listed).
- Drugs for cognitive loss and dementia. Med Lett Drugs Ther. 2022;64(1657):129–136. [PubMed: 35984642](Concise review of the mechanism of action, clinical efficacy, safety, and costs of drugs available for therapy of cognitive loss and dementia in the US mentions that “Pimavanserin does not cause somnolence, gait changes, or extrapyramidal effects but does prolong the QT interval should not be used in patients with hepatic or severe renal impairment”).
- Bugarski-Kirola D, Arango C, Fava M, Nasrallah H, Liu IY, Abbs B, Stankovic S. Pimavanserin for negative symptoms of schizophrenia: results from the ADVANCE phase 2 randomised, placebo-controlled trial in North America and Europe. Lancet Psychiatry. 2022;9:46–58. [PubMed: 34861170](Among 403 patients with schizophrenia with negative symptoms treated with pimavanserin or placebo added to ongoing antipsychotic medications, symptoms improved more with pimavanserin, and adverse events were similar in the two groups, most commonly headache and somnolence; no mention of ALT elevations or hepatotoxicity).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine, but none for risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine; pimavanserin is not discussed).
- Gunther M, Dopheide JA. Antipsychotic safety in liver disease: a narrative review and practical guide for the clinician. J Acad Consult Liaison Psychiatry. 2023;64:73–82. [PubMed: 36180017](Review of the literature on hepatotoxicity of antipsychotic medications and guidance on their use in patients with liver disease characterizes chlorpromazine, clozapine, and olanzapine as having the greatest risk for causing liver injury, quetiapine and risperidone as having moderate risk, haloperidol as having low risk and paliperidone, aripiprazole, lurasidone, and loxapine as having low risk; pimavanserin is not discussed).
- Layton JB, Forns J, McQuay LJ, Danysh HE, Dempsey C, Anthony MS, Turner ME. Mortality in patients with Parkinson’s disease-related psychosis treated with pimavanserin compared with other atypical antipsychotics: a cohort study. Drug Saf. 2023;46:195–208. [PMC free article: PMC9883317] [PubMed: 36517664](In a retrospective matched cohort of patients with Parkinson disease psychosis treated with pimavanserin compared to a group taking other atypical antipsychotics, overall mortality was slightly less in those on pimavanserin; no discussion of cause of death or liver disease).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review The US Food and Drug Administration's Perspective on the New Antipsychotic Pimavanserin.[J Clin Psychiatry. 2017]Review The US Food and Drug Administration's Perspective on the New Antipsychotic Pimavanserin.Mathis MV, Muoio BM, Andreason P, Avila AM, Farchione T, Atrakchi A, Temple RJ. J Clin Psychiatry. 2017 Jun; 78(6):e668-e673.
- Pimavanserin (Nuplazid™) for the treatment of Parkinson disease psychosis: A review of the literature.[Ment Health Clin. 2017]Pimavanserin (Nuplazid™) for the treatment of Parkinson disease psychosis: A review of the literature.Touma KTB, Touma DC. Ment Health Clin. 2017 Sep; 7(5):230-234. Epub 2018 Mar 23.
- Review Pimavanserin: A Novel Antipsychotic for Parkinson's Disease Psychosis.[Ann Pharmacother. 2017]Review Pimavanserin: A Novel Antipsychotic for Parkinson's Disease Psychosis.Bozymski KM, Lowe DK, Pasternak KM, Gatesman TL, Crouse EL. Ann Pharmacother. 2017 Jun; 51(6):479-487. Epub 2017 Feb 1.
- Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist.[Schizophr Res. 2019]Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist.Nasrallah HA, Fedora R, Morton R. Schizophr Res. 2019 Jun; 208:217-220. Epub 2019 Mar 2.
- Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson's disease psychosis.[Neuropsychopharmacology. 2010]Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson's disease psychosis.Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH. Neuropsychopharmacology. 2010 Mar; 35(4):881-92. Epub 2009 Nov 11.
- Pimavanserin - LiverToxPimavanserin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...