NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The fixed combination of extended-release naltrexone and bupropion has been developed as a weight loss agent and was approved for use in the United States in 2014. This combination has not been linked to serum enzyme elevations or clinically apparent liver injury, but has had limited general use. Bupropion, approved for depression and as an aid for smoking cessation, has been implicated in rare instances of acute liver injury. Naltrexone, an opioid receptor antagonist, is used for opioid and alcohol dependency and has been shown to cause serum aminotransferase elevations when given in high doses but has not been shown to cause clinically apparent liver injury.
Background
Naltrexone (nal trex’ one) has been available for many years as a therapy for opioid dependency, acting largely as an opiate receptor antagonist. Bupropion (bue proe’ pee on) was developed as an antidepressant and studies of its efficacy in patients with depression noted mild weight loss. Because both agents are fairly well tolerated, the combination was studied as an approach to weight loss. First in small pilot studies and later in sponsored, large randomized controlled trials, these studies showed that fixed combinations of these two agents led to weight loss that was significantly greater than occurred with placebo. The combination of naltrexone and bupropion was approved for use in the United States in 2014 as a weight loss agent for patients with obesity (BMI ≥30) or with BMI ≥27 plus an obesity-related comorbidity. There is only limited information on general clinical use of the combination. Fixed doses of naltrexone (8 mg) with bupropion (90 mg) are available as extended release tablets under the brand name of Contrave. The recommended initial dose is one tablet daily followed by a gradual in increase to two tablets twice daily (total daily dose 36 mg naltrexone and 360 mg of bupropion). Common side effects include paresthesias, dizziness, dry mouth, nausea, constipation, insomnia and change in taste. Uncommon side effects may include seizures (bupropion), vulnerability to opiate overdose and opioid withdrawal (naltrexone), increase in blood pressure, serum enzyme elevations (naltrexone), activation of mania (bupropion) and suicidal ideation and behaviors (bupropion) and hypersensitivity reactions. Bupropion is contraindicated in patients with anorexia nervosa or bulimia nervosa due to increased risk for seizures.
Hepatotoxicity
In premarketing clinical trials, serum aminotransferase elevations were no more common among patients receiving the combination of naltrexone and bupropion than placebo. Clinically apparent liver injury due to this combination has not been reported, but several instances of acute liver injury have been linked to bupropion monotherapy of other conditions. In clinical trials of naltrexone alone, transient serum aminotransferase elevations occurred in approximately 25% of patients who received 300 mg daily, 6 times the recommended dose for opioid dependency. Some of the episodes of ALT elevations were associated with mild symptoms and slight bilirubin elevations, but all resolved rapidly upon stopping naltrexone. Case reports of liver injury attributed to naltrexone given in conventional doses have occurred largely in patients with alcohol or opioid dependency who have a high rate of underlying liver disease (viral hepatitis, alcoholic liver disease) and were characterized as serum aminotransferase elevations only. Thus, instances of clinically apparent liver injury have not been reported with the combination used as a weight loss product although both agents have been linked to liver injury when used alone.
Likelihood score: E* (suspected but unproven rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which naltrexone-bupropion might cause liver injury is not known. Because both agents have been associated with rare instances of liver injury, it is reasonable to assume that similar instances will occur with the combination if it is used as frequent as the individual agents. Bupropion is metabolized by CYP 2B6 and inhibits the activity of cytochrome CYP 2D6 which can result in significant drug-drug interactions.
Outcome and Management
No instances of acute liver failure or chronic liver injury have been linked to naltrexone-bupropion, but it has had limited general clinical use. There is no reason to suspect that persons with hepatic injury attributed to this combination would exhibit cross sensitivity to liver injury from other weight loss agents.
References on the hepatotoxicity and safety of naltrexone and bupropion separately are given in the sections on the individual agents.
Drug Class: Weight Loss Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Naltrexone-Bupropion – Contrave®
DRUG CLASS
Weight Loss Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
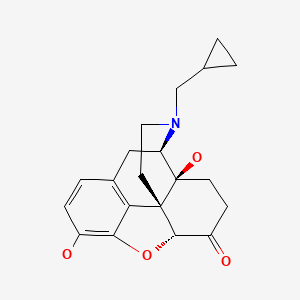
| Naltrexone | 16590-41-3 | C20-H23-N-O4 |
|
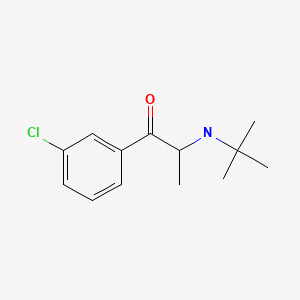
| Bupropion | 34911-55-2 | C13-H18-Cl-N-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 06 June 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999, well before the availability of naltrexone-bupropion).
- FDA. Information on naltrexone-bupropion (Contrave). 2014. Available at: https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2014/200063Orig1s000MedR.pdf. (FDA website with Medical Review of naltrexone-bupropion for efficacy and safety used in its approval for as a weight loss agent, mentions that ALT elevations arose in 7 of 1515 placebo- [0.5%] vs 8 of 3239 drug-treated [0.6%] subjects which were greater than 3 times ULN in 0.2% vs 0.4%, and no patient developed clinically apparent liver injury with jaundice while cholecystitis was diagnosed in 1 patient on placebo [<0.1%] vs 6 on naltrexone-bupropion [0.3%]). - Malcolm R, O'Neil PM, Sexauer JD, Riddle FE, Currey HS, Counts C. A controlled trial of naltrexone in obese humans. Int J Obes. 1985;9:347–53. [PubMed: 3908352](Controlled trial of naltrexone [200 mg daily] or placebo for 8 weeks in 41 overweight subjects, found similar degrees of weight loss [-1.8 vs -1.5 kg] and adverse events [25% vs 55%], and 3 of 20 naltrexone treated subjects had ALT elevations above twice the ULN during treatment, resolving with stopping in 2 and attributed to alcohol use in one ).
- Pfohl DN, Allen JI, Atkinson RL, Knopman DS, Malcolm RJ, Mitchell JE, Morley JE. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66–72. [PubMed: 3092099](Independent review of the ALT elevations occurring in controlled trials of naltrexone in obesity and dementia, found ALT and AST elevations occurring after 6-8 weeks in those receiving the highest dose [300 mg daily – 6 times recommended dose of substance abuse prevention] with peak levels of 10-15 times ULN rapidly improving with discontinuation only one with symptoms and one with mild bilirubin elevations].
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA. NB-201 Study Group. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94:4898–906. [PubMed: 19846734](Among 419 patients with uncomplicated obesity treated with naltrexone or bupropion or both [in 3 dose regimens] or placebo for 24 weeks, weight loss was greatest with the combination [-4.3% to -5.4%] compared to placebo [-0.8%], naltrexone alone [-1.2%] or bupropion alone [-2.7%], while common adverse events were nausea, headache, dizziness and insomnia and there were “no consistent clinically meaningful changes in laboratory values”).
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, et al. COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. [PubMed: 20673995](Among 1742 obese subjects treated with naltrexone-bupropion [16 or 32 mg/360 mg] or placebo for 56 weeks, weight loss was greatest with the combination [-5.0% and -6.1% vs -1.3%], while common adverse events were nausea, headache, constipation, dizziness and dry mouth and there were “no clinically significant effects…on…safety laboratory measures”).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none to naltrexone or bupropion).
- Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J. 2012;36:13–25. [PMC free article: PMC3283822] [PubMed: 22363917](Review of the safety and efficacy of current and potentially future medications for obesity; mentions that several controlled trials have shown weight loss with naltrexone-bupropion and lists adverse side effects of nausea, headache, insomnia and constipation but does not mention ALT elevations or hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](Population based prospective analysis of cases of drug induced liver injury seen over a two year period in Iceland identified 96 cases, none of which were attributed to naltrexone, bupropion or weight loss agents).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996-2012 identified 176 cases, none of which were attributed to naltrexone or bupropion, separately or together).
- Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, Perri MG, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110–20. [PMC free article: PMC4459776] [PubMed: 20559296](Among 793 overweight or obese subjects treated with naltrexone-bupropion or placebo for 56 weeks, weight loss was greater with the drug combination [-9.3% vs -5.1%] and there were “no consistent effects … on clinical laboratory tests … and no evidence of treatment-related hepatotoxicity”).
- Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, Kim D, Dunayevich E., COR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935–43. [PMC free article: PMC3739931] [PubMed: 23408728](Among 1496 obese or overweight subjects with dyslipidemia or hypertension treated with naltrexone-bupropion or placebo for 56 weeks, weight loss was greater with the drug combination [-6.1%vs -1.2%] and it had “no clinically significant effects … on laboratory measures”).
- Contrave--a combination of bupropion and naltrexone for weight loss. Med Lett Drugs Ther. 2014;56(1455):112–4. [PubMed: 25372849](Concise review of the mechanism of action clinical efficacy, safety and costs of naltrexone-bupropion [32 mg/360 mg per day] shortly after its approval as a weight loss agent in the US, mentions that aminotransferase elevations and hepatotoxicity have been reported with use of naltrexone alone).
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. [PMC free article: PMC3928674] [PubMed: 24231879](Systematic review of the literature on the efficacy of long term use of drugs for obesity that were FDA approved [at the time of the analysis] mentions that phentermine, diethylpropion and phendimetrazine are approved for short term use only, but that orlistat, lorcaserin and phentermine/topiramate are approved for long term use although their efficacy is modest; no discussion of hepatotoxicity).
- Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA. 2015;313:1213–4. [PMC free article: PMC4993523] [PubMed: 25803343](Review of the mechanism of action, clinical efficacy and safety of naltrexone-bupropion, the 4th agent approved in the US for long term therapy of obesity mentions that the mean weight loss across studies was -6.8% [-7.3 kg] at one year which was more than that reported in studies of orlistat and lorcaserin but less than with topiramate-phentermine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 were attributed to bupropion but none to naltrexone or to the combination).
- Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, Perez A, Smith SR. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315:990–1004. [PubMed: 26954408](Postmarketing study of naltrexone-bupropion therapy in 8910 overweight or obese subjects with cardiovascular risk factors found that major adverse cardiovascular events arose in 102 patients on placebo [2.3%] and in 90 on the drug combination [2.0%], while adverse events were more common with active therapy, gastrointestinal effects in 14% vs 2% and CNS symptoms in 5.1% vs 1.2% while weight change averaged -3.9 kg [-3.6%] vs -1.2 kg [-1.1%]; no mention of ALT elevations or hepatotoxicity).
- Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315:2424–34. [PMC free article: PMC5617638] [PubMed: 27299618](Systematic review of 28 randomized controlled trials of weight loss drugs in 29,018 patients found significant weight loss in 23% of placebo recipients compared to 55% treated with naltrexone-bupropion, 75% with topiramate-phentermine, 63% with liraglutide, 49% with lorcaserin and 44% with orlistat, but with similar overall rates of adverse events; no mention of hepatotoxicity).
- Mooney ME, Schmitz JM, Allen S, Grabowski J, Pentel P, Oliver A, Hatsukami DK. Bupropion and naltrexone for smoking cessation: A double-blind randomized placebo-controlled clinical trial. Clin Pharmacol Ther. 2016;100:344–52. [PMC free article: PMC5017904] [PubMed: 27213949](Among 121 treatment-seeking smokers treated with bupropion [300 mg] and placebo vs naltrexone and bupropion [50 mg/300 mg], abstinence was achieved in 54% on the combination compared to 33% on bupropion alone, while side effects were more frequent with the combination including nausea, lightheadedness, shakiness and stomach and muscle ache; no mention of ALT elevations or hepatotoxicity).
- Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016;17:2235–42. [PubMed: 27700187](Review of the structure, mechanism of action, pharmacology, clinical efficacy and safety of naltrexone-bupropion mentions that studies identified “no clinically meaningful” changes in safety laboratory measures during therapy).
- Aagaard L, Hallgreen CE, Hansen EH. Serious adverse events reported for antiobesity medicines: postmarketing experiences from the EU adverse event reporting system EudraVigilance. Int J Obes (Lond). 2016;40:1742–7. [PubMed: 27478924](Analysis of adverse event reports from antiobesity medications to the European pharmacovigilance database [EudraVigilance] between 2007 and 2013 identified 4941 reports detailing 13,957 individual adverse events, 90% serious, only 15 attributed to phentermine with 2 deaths, but none were classified as hepatobiliary; naltrexone-bupropion was not included in the analysis).
- Halpern B, Mancini MC. Safety assessment of combination therapies in the treatment of obesity: focus on naltrexone/ bupropion extended release and phentermine-topiramate extended release. Expert Opin Drug Saf. 2017;16:27–39. [PubMed: 27732121](Review of the mechanism of action, clinical efficacy and safety of fixed combination therapies for obesity discusses the common adverse events of naltrexone-bupropion including nausea [30% vs 9% with placebo], constipation [14% vs 6%], vomiting [10% vs 2.5%], dizziness [9% vs 3%] and headache [14% vs 9%], while serious adverse events were rare; no mention of ALT elevations or hepatotoxicity).
- Diet, drugs, devices, and surgery for weight management. Med Lett Drugs Ther. 2018;60(1548):91–8. [PubMed: 29913463](Concise review of the medical and surgical therapies for obesity mentions that for orlistat “severe liver injury has been reported rarely, but no cause-and-effect relationship has been established”; discussions of adverse events due to other weight loss agents [phentermine/ topiramate, naltrexone/bupropion, lorcaserin and liraglutide] do not mention ALT elevations or hepatotoxicity).
- Comparison table: some FDA-approved drugs for weight management. Med Lett Drugs Ther. 2018;60(1548):e98–e100. [PubMed: 29913464](Table comparing nine agents with FDA approval for use in weight management lists under the adverse events that “naltrexone can cause aminotransferase elevations and hepatotoxicity”).
- Saunders KH, Umashanker D, Igel LI, Kumar RB, Aronne LJ. Obesity pharmacotherapy. Med Clin North Am. 2018;102:135–48. [PubMed: 29156182](Review of the pharmacotherapy of obesity focusing upon the 6 most commonly used medications, discusses the common side effects of naltrexone-bupropion, but does not discuss hepatotoxicity).
- Patel DK, Stanford FC. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med. 2018;130:173–82. [PMC free article: PMC6261426] [PubMed: 29388462](Review of the history of approval and indications, efficacy and long term safety of major currently available weight loss agents including orlistat, phentermine/topiramate, lorcaserin, liraglutide and naltrexone/bupropion; does not discuss hepatotoxicity).
- Bello NT. Update on drug safety evaluation of naltrexone/bupropion for the treatment of obesity. Expert Opin Drug Saf. 2019;18:549–52. [PubMed: 31092063](Review of the efficacy and safety of naltrexone-bupropion mentions that naltrexone was approved in doses of 50 mg daily for opioid dependence in 1984 and alcohol dependence in 1994 and, when evaluated for obesity, had little effect despite high doses [up to 300 mg daily] which were associated with ALT elevations).
- Onakpoya IJ, Lee JJ, Mahtani KR, Aronson JK, Heneghan CJ. Naltrexone-bupropion (Mysimba) in management of obesity: A systematic review and meta-analysis of unpublished clinical study reports. Br J Clin Pharmacol 2020; 86(4): 646-67. [PMC free article: PMC7098870] [PubMed: 31918448](Analysis of reports to US and European agencies on the efficacy and safety of naltrexone-bupropion found that therapy was associated with greater weight loss than was placebo, but a higher rate of adverse events; no mention of ALT elevations or hepatotoxicity).
- Tak YJ, Lee SY. Anti-obesity drugs: long-term efficacy and safety: an updated review. World J Mens Health. 2020;38:e14. [PMC free article: PMC7994651] [PubMed: 32202085](Review of currently available agents for long term therapy of obesity with specific discussion of mechanism of action, dosing regimen, efficacy and safety, mentions that naltrexone-bupropion therapy is associated with nausea, headache, dizziness, drug mouth, and gastrointestinal discomfort; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Drug safety evaluation of naltrexone/bupropion for the treatment of obesity.[Expert Opin Drug Saf. 2014]Review Drug safety evaluation of naltrexone/bupropion for the treatment of obesity.Verpeut JL, Bello NT. Expert Opin Drug Saf. 2014 Jun; 13(6):831-41. Epub 2014 Apr 28.
- Naltrexone + bupropion (Mysimba). Too risky for only modest weight loss.[Prescrire Int. 2015]Naltrexone + bupropion (Mysimba). Too risky for only modest weight loss.. Prescrire Int. 2015 Oct; 24(164):229-33.
- Review Bupropion.[LiverTox: Clinical and Researc...]Review Bupropion.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Phentermine-Topiramate.[LiverTox: Clinical and Researc...]Review Phentermine-Topiramate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Other Antidepressants.[Handb Exp Pharmacol. 2019]Other Antidepressants.Schwasinger-Schmidt TE, Macaluso M. Handb Exp Pharmacol. 2019; 250:325-355.
- Naltrexone-Bupropion - LiverToxNaltrexone-Bupropion - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...