NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The corticosteroids are a group of chemically related natural hormones and synthetic agents that resemble the human adrenal hormone cortisol and have potent antiinflammatory and immunosuppressive properties and are widely used in medicine. Corticosteroid therapy is associated with several forms of liver injury, some due to exacerbation of an underlying liver disease and some that appear to be caused directly by corticosteroid therapy. This discussion will cover eight agents: betamethasone, cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisolone, prednisone, and triamcinolone.
Background
The corticosteroids are hormones that have glucocorticoid (cortisol-like) and/or mineralocorticoid (aldosterone-like) activities and which are synthesized predominantly by the adrenal cortex. In clinical practice, the term “corticosteroids” usually refers to the glucocorticoids and are represented by a large group of natural or synthetic steroid compounds that have varying potency, durations of action and relative glucocorticoid (measured by antiinflammatory activity) vs mineralocorticoid (measured by sodium retention) activities. Cortisol and the corticosteroids act by engagement of the intracellular glucocorticoid receptor, which then is translocated to the cell nucleus where the receptor-ligand complex binds to specific glucocorticoid-response elements on DNA, thus activating genes that mediate glucocorticoid responses. The number of genes modulated by corticosteroids are many and the effects are multiple and interactive with other intracellular pathways. Thus, the effects of corticosteroids on inflammation and the immune system cannot be attributed to a single gene or pathway. The potent antiinflammatory and immunosuppressive qualities of the corticosteroids have made them important agents in the therapy of many diseases. Corticosteroids are available in multiple forms, including oral tablets and capsules; powders and solutions for parenteral administration; topical creams and lotions for skin disease; eye, ear and nose liquid drops for local application; aerosol solutions for inhalation and liquids or foams for rectal application. Representative corticosteroids (and the year of their approval for use in the United States) include cortisone (1950), prednisone (1955), prednisolone (1955), methylprednisolone (1957), dexamethasone (1958), betamethasone (1961), and hydrocortisone (1983). All are available in generic forms. In this website, only the oral and intravenous formulations of corticosteroids are described and they are discussed together with common list of references and representative case reports.
The corticosteroids are used widely in medicine largely for their potent antiinflammatory and immunosuppressive activities. The clinical conditions for which corticosteroids are used include, but are not limited to: asthma, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, inflammatory bowel disease, nephritic syndrome, cancer, leukemia, organ transplantation, autoimmune hepatitis, hypersensitivity reactions, cardiogenic and septic shock, severe COVID-19 pneumonia, and, of course, glucocorticoid deficiency diseases such as in Addison’s disease and panhypopituitarism.
Corticosteroids are used in several liver diseases, most commonly in autoimmune hepatitis for which they have been shown to improve outcome and survival. Corticosteroids are also used after liver transplantation to prevent rejection. An important element in managing these liver diseases and conditions is to maintain the dose of corticosteroids at the lowest effective level. The adverse effects of long term corticosteroid therapy (which are rarely hepatic) are still major causes of morbidity and even mortality in these conditions.
Prednisone, prednisolone, methylprednisolone and triamcinolone are the most commonly used oral agents as they are inexpensive, rapid in onset, intermediate in duration of action and have potent glucocorticoid with minimal mineralocorticoid activities, at least as compared to cortisone and hydrocortisone. Betamethasone and dexamethasone have greater glucocorticoid potency and less aldosterone-like activity than prednisone, but have a longer duration of action, and they are mostly used in topical or liquid forms for local application and in injectable forms for severe hypersensitivity reactions and inflammation. Methylprednisolone, dexamethasone, and hydrocortisone are most commonly used for intravenous administration, typically given in emergency or critical situations in which rapid and profound immunosuppression or antiinflammatory activity is needed. Intravenous methylprednisolone is used most commonly in treatment of Graves’ ophthalmopathy and multiple sclerosis. Dexamethasone is used commonly for severe COVID-19 pneumonia.
The table below provides the major forms of corticosteroids and their relative glucocorticoid and mineralocorticoid activity and equivalent daily doses.
| RELATIVE POTENCIES OF CORTICOSTEROIDS | |||
|---|---|---|---|
| AGENT | GLUCOCORTICOID ACTIVITY | MINERALOCORTICOID ACTIVITY | EQUIVALENT ORAL OR INTRAVENOUS DOSE |
| Cortisol | 1 | 1 | 20 |
| Cortisone | 0.8 | 0.8 | 25 |
| Prednisone | 4 | 0.8 | 5 |
| Prednisolone | 4 | 0.8 | 5 |
| Methylprednisolone | 5 | 0.5 | 4 |
| Triamcinolone | 5 | 0 | 4 |
| Betamethasone | 25 | 0 | 0.75 |
| Dexamethasone | 25 | 0 | 0.75 |
Cortisone (kor' ti sone) is a short acting glucocorticoid that is used for therapy of adrenal insufficiency and for treatment of allergic and inflammatory conditions. Cortisone is available in generic forms in tablets of 25 mg, which is considered a daily physiologic dose in adults. Cortisone has both glucocorticoid and mineralocorticoid properties.
Hydrocortisone (hye" droe kor' ti sone) is a rapid and short acting glucocorticoid that is used for therapy of adrenal insufficiency and in treatment of allergic and inflammatory conditions. Hydrocortisone has the same chemical structure as cortisol and thus most closely resembles the human adrenal hormone. Hydrocortisone is available in generic forms in tablets of 5, 10 and 20 mg, with 20 mg being considered a daily physiologic dose in adults. Hydrocortisone is also available in multiple forms in solution for oral, rectal, topical or parenteral administration. A major use of intravenous hydrocortisone is in the acute therapy of severe hypersensitivity reactions and shock. Hydrocortisone has both glucocorticoid and mineralocorticoid properties.
Prednisone (pred' ni sone) is a synthetic, intermediate acting glucocorticoid that is widely used in the therapy of severe inflammation, autoimmune conditions, hypersensitivity reactions and organ rejection. Prednisone is converted to prednisolone, its active form, in the liver. Prednisone is available in multiple generic forms in tablets of 1, 2.5, 5, 10, 20 and 50 mg and as oral solutions. Four times more potent that cortisol, prednisone is used in varying doses, with 5 mg daily being considered physiologic doses in adults.
Prednisolone (pred nis' oh lone) is a synthetic, intermediate acting glucocorticoid that is widely used in the therapy of severe inflammation, autoimmune conditions, hypersensitivity reactions and organ rejection. Prednisolone is available in multiple generic forms in tablets of 5, 10, 15 and 30 mg and in several forms for systemic administration. Four times more potent that cortisol, prednisolone is used in varying doses, with 5 mg daily being considered physiologic doses in adults.
Methylprednisolone (meth" il pred nis' oh lone) is a synthetic, intermediate acting glucocorticoid that is widely used in the therapy of severe inflammation, autoimmune conditions, hypersensitivity reactions and organ rejection. Methylprednisolone is available in multiple forms in tablets of 2, 4, 8, 16 and 32 mg generically and under the brand name of Medrol and in Medrol Dosepaks (21 tablets of 4 mg each). Injectable forms of methylprednisolone are also available generically and under brand names of Solu-Medrol and Depo-Medrol. Five times more potent that cortisol, methylprednisolone is used in varying doses, with 4 mg daily being considered physiologic doses in adults. Methylprednisolone has minimal mineralocorticoid activity.
Triamcinolone (trye" am sin' oh lone) is a synthetic, long acting glucocorticoid that is used in topical solutions and aerosols for therapy of allergic and hypersensitivity reactions and control of inflammation as well as in parenteral formulations for therapy of hypersensitivity reactions, shock and severe inflammation. Oral forms of triamcinolone include tablets of 4 and 8 mg and oral syrups. Parenteral forms for injection are available under various generic and trade names including Aristocort and Kenacort. Triamcinolone is five times more potent than cortisol in its glucocorticoid activity, but has minimal mineralocorticoid activity.
Dexamethasone (dex" a meth' a sone) is a synthetic, long acting glucocorticoid that is used parenterally as therapy of severe hypersensitivity reactions, shock and control of severe inflammation as well as in topical, otic, ophthalmologic solutions, aerosols and lotions or creams for local therapy of allergic reactions and inflammation. Most recently, intravenous dexamethasone has been found to be beneficial in severe COVID-19 pneumonia with respiratory failure in patients requiring high flow oxygen supplementation or invasive mechanical ventilation. Dexamethasone is available in multiple forms for injection under various generic and trade names including Decadron. Dexamethasone is 25 times more potent than cortisol in its glucocorticoid activity, but has minimal mineralocorticoid activity.
Betamethasone (bay" ta meth' a sone) is a synthetic, long acting glucocorticoid that is used in parenteral forms for therapy of allergic and hypersensitivity reactions and control of severe inflammation. Betamethasone is available in solution for injection under the trade name of Celestone and in multiple generic forms as syrups and effervescent tablets for oral use, edemas and foams for rectal use, aerosols for nasal and respiratory use, and creams and lotions for topical use. Betamethasone is 25 times more potent than cortisol in glucocorticoid activity, but has minimal mineralocorticoid activity.
Hepatotoxicity, Mechanism of Injury
Corticosteroids have multiple adverse side effects, due to their multiplicity of actions affecting virtually all organs. Long term use has very profound effects on growth and can lead to cataracts, glaucoma, opportunistic infections, thinning of the skin, weight gain and redistribution of fat, insulin resistance and diabetes, hypertension, headache, psychiatric problems, sodium retention and peripheral edema; all of the clinical features of Cushing syndrome.
Corticosteroids also have major effects on the liver, particularly when given long term and in higher than physiologic doses. Glucocorticoid use can result in hepatic enlargement and steatosis or glycogenosis. Corticosteroids can trigger or worsen nonalcoholic steatohepatitis. Long term use can also exacerbate chronic viral hepatitis. Importantly, treatment with corticosteroids followed by withdrawal or pulse therapy can cause reactivation of hepatitis B and worsening or de novo induction of autoimmune hepatitis, both of which can be fatal. Finally, high doses of intravenous corticosteroids, largely methylprednisolone, have been associated with acute liver injury which can result in acute liver failure and death. Thus, the hepatic complications of corticosteroids are mostly associated with high intravenous dosing usually represent the worsening or triggering of an underlying liver disease and rarely are the result of drug hepatotoxicity.
Corticosteroid therapy can cause hepatic steatosis and hepatic enlargement, but this is often not clinically apparent, particularly in adults. This effect can occur quite rapidly and is rapidly reversed with discontinuation. High doses and long term use has been associated with the development or exacerbation of nonalcoholic steatohepatitis with elevations in serum aminotransferase levels and liver histology resembling alcoholic hepatitis with steatosis, chronic inflammation, centrolobular ballooning degeneration and Mallory bodies (Case 1). However, symptomatic or progressive liver injury from corticosteroid induced steatohepatitis is uncommon. Furthermore, corticosteroids may act to worsen an underlying nonalcoholic fatty liver disease rather than causing the condition de novo. The worsening may be due to direct effects of glucocorticoids on insulin resistance or fatty acid metabolism or may be the result of weight gain which is common with long term corticosteroid therapy. While simple steatosis induced by corticosteroids is rapidly reversible, steatohepatitis can be slow to resolve upon withdrawal of corticosteroids.
Corticosteroids in high doses can also cause hepatic glycogenosis, in which liver cells exhibit a homogenous appearance and stain strongly for glycogen (using PAS staining with and without diastase). Glycogenosis can also be associated with hepatomegaly (in children) and elevations in serum aminotransferase levels with minimal or no change in alkaline phosphatase or bilirubin levels. Glycogenosis is usually asymptomatic and does not appear to progress to chronic liver injury, cirrhosis or acute liver failure. While glycogenosis has been described largely in patients with poorly controlled type 1 diabetes, it also can occur acutely in patients started on high dose corticosteroids.
An important complication of corticosteroid therapy is the worsening of an underlying chronic viral hepatitis. In chronic hepatitis B, corticosteroids can induce increases in viral replication and serum hepatitis B virus (HBV) DNA levels while decreasing serum aminotransferase levels. Eventually, however, the increase in viral replication can worsen the underlying liver disease. Exacerbation of hepatitis becomes particularly evident when corticosteroids are withdrawn or lowered to physiological levels. As the immune system recovers, hepatitis worsens and serum aminotransferase levels can rise to greater than 10- to 20-fold elevated usually accompanied by a prompt decrease in HBV DNA levels. This flare of disease following withdrawal of corticosteroids can be severe and result in acute liver failure or significant worsening of chronic hepatitis and development of cirrhosis (Case 2). Indeed, even patients with the “inactive carrier states” (as shown by the presence of HBsAg in serum without HBeAg or detectable HBV DNA or any elevation in serum aminotransferase levels) can suffer severe reactivation of disease and acute liver failure as a result of a short course of high dose corticosteroids as occurs with cancer chemotherapy or with treatment of severe autoimmune conditions or even asthma, hay fever or allergic dermatitis. Reactivation of hepatitis B can be prevented by prophylactic use of antiviral therapy during the period of immunosuppression, but even this may not prevent some degree of liver injury.
Corticosteroids also appear to worsen the course of chronic hepatitis C, although in a less dramatic fashion than in chronic hepatitis B. Corticosteroid therapy leads to a rise in hepatitis C virus (HCV) RNA levels which may eventually cause worsening of the underlying liver disease. Chronic hepatitis C appears to be more severe and is particularly difficult to manage in patients receiving chemotherapy or immunosuppression, and corticosteroids are believed to be a major factor in this effect. Thus, corticosteroids should be avoided if possible in patients with underlying chronic viral hepatitis.
Corticosteroids are used in the therapy of autoimmune hepatitis and, therefore, are likely to be beneficial rather than harmful in patients with this disease. The difficulty arises when corticosteroids are stopped, which can cause a rebound exacerbation of the autoimmune hepatitis that is often severe and can be fatal. Importantly, there have been multiple reported instances of de novo appearance of severe autoimmune hepatitis in patients who received a short course or pulse of corticosteroids for another, unrelated condition (such as asthma or allergic reactions). In these situations, a mild and subclinical autoimmune hepatitis was likely present before corticosteroids were started, and the suppression of the disease followed by immune rebound caused the clinical presentation of the condition. These patients generally respond to restarting corticosteroids, but may require long term if not lifelong immunosuppressive treatment thereafter.
Finally, there have been several reports of an acute hepatitis-like liver injury arising after a short, high dose course of intravenous methylprednisolone that can be severe and even fatal, and in which viral hepatitis and autoimmune hepatitis cannot be clearly implicated (Case 3). The cause of this apparent hepatotoxicity is not known, but it may represent severe autoimmune hepatitis triggered by the sudden profound immunosuppression and subsequent immune reconstitution. Importantly, symptoms and jaundice develop 1 to 6 weeks after stopping methylprednisolone and the pattern of serum enzyme elevations is typically hepatocellular. These episodes can be symptomatic and severe. Immunoallergic manifestations are uncommon and autoantibodies may not be present. Several instances have resulted in acute liver failure resulting in death or need for emergency liver transplantation. But transient injury without symptoms can also occur accompanied by serum aminotransferase elevations that are 10 to 40 times the ULN. Restarting corticosteroids may be appropriate for this syndrome particularly if symptomatic, but this approach has not been evaluated systematically and many instances have resolved spontaneously. Recurrence of injury, often in a more rapid and severe form, arises upon reexposure to high dose pulse methylprednisolone. Strangely, it is not clear whether this syndrome can be induced by high dose intravenous prednisone or dexamethasone.
Likelihood score: A[HD] (well established cause of liver injury when given in high doses, either the result of reactivation of hepatitis B, or an acute hepatocellular injury after high dose intravenous treatment, particularly with methylprednisolone).
Drug Class: Corticosteroids, COVID-19 Drugs
Selected Drugs in the Class: Betamethasone, Cortisone, Dexamethasone, Hydrocortisone, Methylprednisolone, Prednisolone, Prednisone, Triamcinolone. Budesonide is also a corticosteroid but has not been linked to liver injury and is discussed separately in LiverTox.
CASE REPORTS
Case 1. Nonalcoholic steatohepatitis after long term corticosteroid therapy.(1)
A 34 year old woman with systemic lupus erythematosus was treated with betamethasone with good clinical response with improvements in rash, fatigue and laboratory tests. Over a 6 month period, the daily dosage was gradually decreased from 5 to 1.25 mg daily. Initially, her liver tests were normal, but with continued corticosteroid therapy, ALT and AST became mildly elevated (Table). However, after 16 months of therapy, serum aminotransferase levels were more than 5-fold elevated (ALT 256 U/L, AST 272 U/L) and she was readmitted for evaluation. Her weight had risen by 11 kilograms and she had firm hepatomegaly. Laboratory tests showed elevations in serum aminotransferase levels, but normal serum bilirubin, albumin, and prothrombin time. She had an abnormal glucose tolerance test (fasting glucose 118 mg/dL; 2 hour postprandial glucose 248 mg/dL). Testing for HBsAg was negative. She was known to be positive for antinuclear antibody (1:128). She denied alcohol use, which was confirmed by family members and friends. A liver biopsy showed marked steatosis with inflammation including neutrophils, occasional Mallory bodies and mild central sinusoidal and portal fibrosis. Weight loss led to slight decreases in serum ALT levels.
Key Points
| Medication: | Betamethasone (1.25 mg daily) |
|---|---|
| Pattern: | Hepatocellular or mixed (R=~4.6) |
| Severity: | Mild (serum enzyme elevations only) |
| Latency: | Several months |
| Recovery: | Not mentioned |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Body Weight (kg) | ALT (U/L) | Alk P (U/L) | Albumin (g/dL) | Other |
|---|---|---|---|---|---|
| 0 | 42.7 | 18 | 95 | 3.4 | |
| 6 months | 52.2 | 58 | 62 | 3.9 | |
| 16 months | 58.2 | 256 | 131 | 4.0 | Protime 10.4 sec |
| 17 months | 55.5 | 134 | 76 | 4.2 | |
| Normal Values | <40 | <85 | <3.5 | ||
Comment
This is an early, but well documented report of nonalcoholic steatohepatitis arising during corticosteroid therapy. The patient was evidently asymptomatic of liver disease, but the height of the serum aminotransferase elevations led to a hospital admission and liver biopsy. An issue is whether the liver disease was due to corticosteroid therapy directly or was the result of weight gain and insulin resistance caused by the therapy. Betamethasone is a synthetic, high potency glucocorticoid; 1.25 mg of betamethasone is roughly equivalent to 15 mg of prednisone.
Case 2. Reactivation of chronic hepatitis B by corticosteroids.(2)
A 69 year old man with ulcerative colitis and the HBsAg carrier state developed jaundice and hepatitis after 9 months of continuous prednisolone therapy and shortly after intravenous pulse treatment with methylprednisolone. He was known to be a carrier of HBsAg with normal serum aminotransferase levels and no detectable HBeAg or HBV DNA in serum for several years. Because of relapsing ulcerative colitis, he was started on prednisolone therapy in tapering doses (60 mg daily down to 5 mg daily). Approximately 8 months into therapy, he received a 7 day course of intravenous methylprednisolone. One week later, while still on low doses of oral prednisolone, he developed fatigue and nausea. Liver tests, which had been normal, were markedly elevated with ALT 517 U/L, Alk P 356 U/L and bilirubin 3.98 mg/dL (Table). He was admitted. On examination, he was jaundiced and had mild mental dullness and asterixis. At this point, he tested positive for both HBsAg and HBeAg, and HBV DNA levels (which had been undetectable in the past) were markedly elevated (>100 million copies/mL). In addition he had IgM anti-HBc. Despite initiation of lamivudine therapy for hepatitis B and intensive medical management, he developed progressive liver failure, coagulopathy, hepatic coma and died 25 days after admission. Autopsy was refused.
Key Points
| Medication: | Prednisolone, methylprednisolone |
|---|---|
| Pattern: | Hepatocellular (R=6.1) |
| Severity: | 5+ (fatal) |
| Latency: | 8 months of oral prednisone |
| Recovery: | None |
| Other medications: | 5-Aminosalicylic acid, isosorbide dinitrate, theophylline, insulin |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|
| Pre | <30 | <1.0 | HBV DNA <100 copies/mL | |
| 7 months | 60 | <1.0 | Methylprednisolone bolus | |
| 8 months | 180 | <1.0 | Prednisone: 5 mg daily | |
| 9 months | 0 | 517 | <1.0 | Alk P 356 |
| 30 days | 600 | 4 | Admission; HBeAg positive | |
| 32 days | 700 | 14 | ||
| 34 days | 830 | 20 | ||
| 10 months | 36 days | 1140 | 24 | |
| 6 weeks | 980 | 34 | ||
| 7 weeks | 220 | 36 | ||
| 8 weeks | 50 | 41 | Died | |
| Normal Values | <4.5 | <1.2 | ||
- *
Results and time sequence estimated from Figure 1.
Comment
Reactivation of hepatitis B in an HBsAg carrier can be followed by a severe episode of hepatitis as immunosuppression is withdrawn. The clinical syndrome is similar to acute hepatitis B and IgM anti-HBc may become detectable. The diagnosis can be made based upon reappearance and/or marked rise in HBV DNA levels. Many cases present as "acute-on-chronic" liver failure rather than classical acute liver failure. The prognosis is poor and antiviral therapy appears to have little effect once hepatic failure is present. HBsAg carriers who undergo immunosuppressive therapy with prednisone should be given prophylaxis during the immunosuppression with an oral nucleoside analogue such as tenofovir or entecavir or monitored carefully and started on antiviral therapy if HBV DNA levels appear de novo or rise significantly (generally by more than 1-2 log10 IU/mL.
Case 3. Severe acute hepatitis following bolus, high dose methylprednisone therapy.(3)
A 43 year old woman with Graves disease developed worsening ophthalmopathy despite adequate control of thyroid function. She received orbital radiotherapy and was started on cycles of intravenous methylprednisolone (15/mg/kg) given over two days every two weeks. After the sixth infusion (6 weeks after starting), she was found to have elevations in serum ALT (264 U/L) and AST (120 U/L) and the infusions were held. Over the next six weeks, serum aminotransferase levels rose further (Table), with no or only minor elevations in serum alkaline phosphatase, gamma glutamyltranspeptidase and bilirubin. However, the prothrombin index worsened (61%) and platelet count fell to 89,000/µL. Tests of hepatitis A, B and C were negative. Immunoglobulin levels were normal and tests for autoantibodies, including antinuclear antibody, smooth muscle antibody and liver-kidney microsomal antibody, were negative. A liver biopsy showed changes suggestive of chronic aggressive hepatitis with marked lymphocytic inflammation, interface hepatitis, and both focal and bridging necrosis. Because the histological and clinical features supported the diagnosis of severe autoimmune hepatitis, oral prednisone was initiated, with prompt improvements in serum aminotransferase levels. Prednisone doses were gradually reduced and ultimately withdrawn. Serum enzymes were normal within 2 months of starting prednisone and the dosage was gradually reduced and then withdrawn 3 months after initiation.
Key Points
| Medication: | Methylprednisolone |
|---|---|
| Pattern: | Hepatocellular |
| Severity: | 1+ (serum enzyme elevations without jaundice) |
| Latency: | 6 weeks |
| Recovery: | 3 months |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | GGT (U/L) | Other |
|---|---|---|---|---|
| Pre | Normal | Normal | ||
| 3 cycles of two days of pulse methylprednisolone given 2 weeks apart | ||||
| 8 weeks | 2 weeks | 264 | 40 | |
| 12 weeks | 6 weeks | 320 | 40 | |
| 13 weeks | 7 weeks | 610 | 80 | Bilirubin and Alk P normal |
| 14 weeks | 8 weeks | 1050 | 100 | ANA negative, Protime 61% |
| 15 weeks | 9 weeks | 1419 | 183 | im prednisolone started |
| 4 months | 10 weeks | 600 | Oral prednisone | |
| 11 weeks | 260 | 100 | ||
| 5 months | 15 weeks | 65 | 50 | |
| 6 months | 20 weeks | 40 | 40 | |
| Normal Values | <45 | <50 | ||
- *
Values and time sequence estimated from Figure 1.
Comment
An example of an acute hepatitis-like syndrome arising after pulse methylprednisolone therapy. These episodes arise typically 2 to 4 weeks after an early cycle of pulse therapy, and range in severity from an asymptomatic and transient rise in serum aminotransferase levels to an acute hepatitis and even fulminant hepatic failure. In this instance, the marked and persistent rise in serum enzymes coupled with liver histology suggesting chronic hepatitis led to a diagnosis of new-onset autoimmune hepatitis, despite the absence of serum autoantibodies or hypergammaglobulinemia. Autoimmune hepatitis may initially present in this fashion, without the typical pattern of serum autoantibodies during the early, anicteric phase. The diagnosis was further supported by the prompt improvements in serum enzymes with prednisone therapy. The acute hepatitis-like syndrome that can occur after pulses of methylprednisolone is best explained as a triggering of an underlying chronic autoimmune hepatitis caused by the sudden and profound immunosuppression followed by rapid withdrawal. This syndrome can be severe, and fatal instances have been reported. Whether reinitiation of corticosteroid therapy with gradual tapering and withdrawal is effective in ameliorating the course of illness is unclear, but anecdotal reports suggest that they are beneficial and should be initiated promptly. Long term follow up of such cases is also necessary to document that the autoimmune hepatitis does not relapse once corticosteroids are ultimately withdrawn again.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Betamethasone – Generic, Celestone®
Cortisone – Generic
Dexamethasone – Generic, Decadron®
Hydrocortisone – Generic, Cortef®
Methylprednisolone – Generic, Medrol®
Prednisolone – Generic
Prednisone – Generic
Triamcinolone – Generic, Aristocort®, Kenacort®, Kenalog®
DRUG CLASS
Glucocorticoids, Synthetic
COMPLETE LABELING (Betamethasone)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Betamethasone | 378-44-9 | C22-H29-F-O5 |
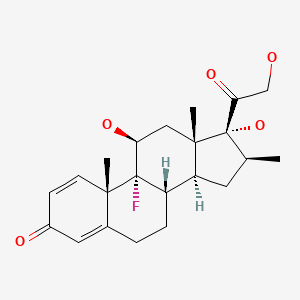
|
| Cortisone | 53-06-5 | C21-H28-O5 |
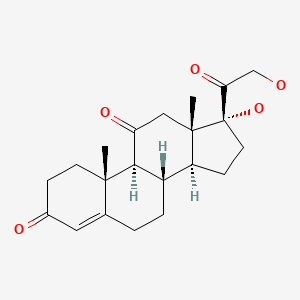
|
| Dexamethasone | 50-02-2 | C22-H29-F-O5 |
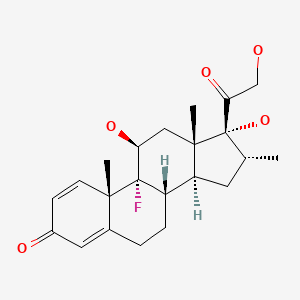
|
| Hydrocortisone | 50-23-7 | C21-H30-O5 |
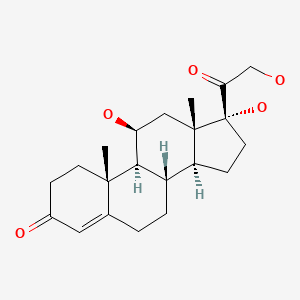
|
| Methylprednisolone | 83-43-2 | C22-H30-O5 |
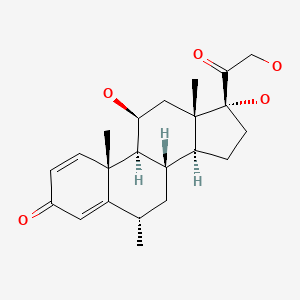
|
| Prednisolone | 50-24-8 | C21-H28-O5 |
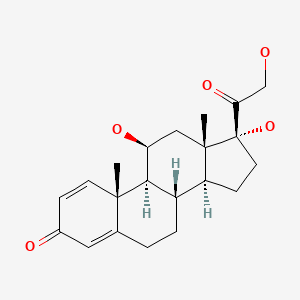
|
| Prednisone | 53-03-2 | C21-H26-O5 |
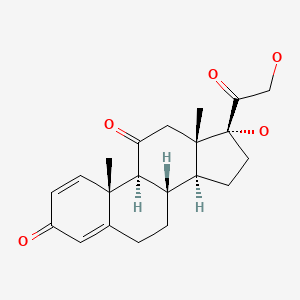
|
| Triamcinolone | 124-94-7 | C21-H27-F-O6 |
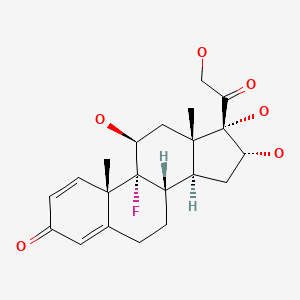
|
CITED REFERENCES
- 1.
- Itoh S, Igarashi M, Tsukada Y, Ichinoe A. Nonalcoholic fatty liver with alcoholic hyaline after long-term glucocorticoid therapy. Acta Hepato-Gastroenterologica. 1977;24:415–8. [PubMed: 74931]
- 2.
- Hammond A, Ramersdorfer C, Palitzsch KD, Schölmerich J, Lock G. Dtsch Med Wochenschr. 1999;124:687–90. [Fatal liver failure after corticosteroid treatment of a hepatitis B virus carrier] German. [PubMed: 10394348]
- 3.
- Marinò M, Morabito E, Altea MA, Ambrogini E, Oliveri F, Brunetto MR, Pollina LE, et al. Autoimmune hepatitis during intravenous glucocorticoid pulse therapy for Graves' ophthalmopathy treated successfully with glucocorticoids themselves. J Endocrinol Invest. 2005;28:280–4. [PubMed: 15952415]
ANNOTATED BIBLIOGRAPHY
References updated: 07 May 2021
- Zimmerman HJ. Corticosteroids. Drugs to treat rheumatic/musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 541.(Expert review of hepatotoxicity of corticosteroids, focusing upon fatty liver and alcoholic hyaline-like changes with high doses or long term low doses).
- Chitturi S, Farrell GC. Corticosteroids. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 613-4.(Review of liver injury from corticosteroids mentions hepatic steatosis and acute liver injury following high dose methylprednisolone).
- Schimmer BP, Funder JW. Adrenocortical steroids. ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1215-33.(Textbook of pharmacology and therapeutics).
- Steinberg H, Webb WM, Rafsky HA. Hepatomegaly with fatty infiltration secondary to cortisone therapy: case report. Gastroenterology. 1952;21:304–9. [PubMed: 14937220](14 year old boy treated for rheumatic fever with cortisone developed marked hepatomegaly beginning on day 6, and by day 43 liver biopsy showed fat and inflammation, which resolved rapidly upon stopping therapy; liver biopsy 20 days later showed normal liver without steatosis).
- Hill RB Jr, Droke WA. Production of fatty liver in the rat by cortisone. Proc Soc Exp Biol Med. 1963;114:766–9. [PubMed: 14120343](Rats treated with cortisone developed fatty liver and increases in hepatic triglyceride levels).
- Itoh S, Igarashi M, Tsukada Y, Ichinoe A. Nonalcoholic fatty liver with alcoholic hyaline after long-term glucocorticoid therapy. Acta Hepato-Gastroenterologica. 1977;24:415–8. [PubMed: 74931](56 year old woman with systemic lupus treated with betamethasone for 16 months gained 24 pounds and developed insulin resistance, moderate increases in ALT [from 18 to 272 U/L] and Alk P [95 to 121 U/L] levels without jaundice; biopsy showed steatohepatitis and Mallory bodies: Case 1).
- Iancu TC, Shiloh H, Dembo L. Hepatomegaly following short-term high-dose steroid therapy. J Pediatr Gastroenterol Nutr. 1986;5:41–6. [PubMed: 3944744](Among 140 children treated with prednisone [2-4 mg/kg/day], 19 developed hepatomegaly within 3-6 days, regressing with stopping treatment; liver biopsies showed glycogenosis and some degree of steatosis; no mention of ALT levels).
- Hoofnagle JH, Davis GL, Pappas SC, Hanson RG, Peters M, Avigan MI, Waggoner JG, et al. A short course of prednisolone in chronic type B hepatitis. Report of a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1986;104:12–7. [PubMed: 3940480](Clinical trial of 4 week course of prednisolone [60 mg/day for 2 weeks, 30 mg/day for 2 weeks] in 15 patients with chronic hepatitis B found flares of hepatitis occurring in most patients 1-2 months after withdrawal that could be prolonged and lead to worsening of liver histology).
- Wald JA, Farr RS. Abnormal liver-function tests associated with long-term systemic corticosteroid use in subjects with asthma. J Allergy Clin Immunol. 1991;88:277–8. [PubMed: 1880328](Retrospective analysis of 80 patients with asthma found mean ALT and Alk P levels were higher among 25 patients on “high dose” corticosteroids compared to those on no or low doses; 80% on high doses had at least one liver test abnormality compared to 23% on low doses and 5% on no corticosteroid therapy).
- Koga Y, Kumashiro R, Yasumoto K, Shakado S, Ono N, Noguchi H, Nagata K, et al. Two fatal cases of hepatitis B virus carriers after corticosteroid therapy for bronchial asthma. Intern Med. 1992;31:208–13. [PubMed: 1600269](53 year old woman with asthma and HBsAg developed acute hepatitis 2-3 weeks after 1 month course of intravenous hydrocortisone [200-300 mg/day], with jaundice, hepatic failure and death 6 weeks later; 68 year old man with asthma and HBsAg developed symptoms and jaundice 1 month after 8 day course of intravenous hydrocortisone, with progression to acute liver failure and death).
- Fong TL, Valinluck B, Govindarajan S, Charboneau F, Adkins RH, Redeker AG. Short-term prednisone therapy affects aminotransferase activity and hepatitis C virus RNA levels in chronic hepatitis C. Gastroenterology. 1994;107:196–9. [PubMed: 8020662](Among 10 patients with chronic hepatitis C treated with 7 week course of prednisone, most had rise in HCV RNA levels during therapy with fall in ALT and rebound afterwards, but without clinically apparent flares).
- Gerolami R, Mambrini P, Barthet M, Jean-Pastor MJ, Salducci J, Grimaud JC. Gastroenterol Clin Biol. 1997;21:236–7. [Acute hepatitis caused by Solupred in a patient with Crohn disease] French. [PubMed: 9161506](27 year old woman with Crohn disease received methylprednisolone [50 mg/day] followed by intravenous prednisone [60 mg/day] and developed abnormal liver tests 6 days later, ALT levels rising from normal to 11 times ULN, Alk P to 3.2 times ULN, ANA negative, resolving within 1 week of starting oral corticosteroids; elevations possibly due to acute glycogenosis).
- Hammond A, Ramersdorfer C, Palitzsch KD, Schölmerich J, Lock G. Dtsch Med Wochenschr. 1999;124:687–90. [Fatal liver failure after corticosteroid treatment of a hepatitis B virus carrier] German. [PubMed: 10394348](69 year old man with HBsAg carrier state and ulcerative colitis developed jaundice and progressive hepatic failure starting at the end of a 9 month course of prednisone [60 mg down to 5 mg/day], with appearance of HBV DNA, ALT 1140 U/L, and death 30 days later: Case 2).
- Nanki T, Koike R, Miyasaka N. Subacute severe steatohepatitis during prednisolone therapy for systemic lupus erythematosis. Am J Gastroenterol. 1999;94:3379. [PubMed: 10566758](53 year old woman with systemic lupus had rise of ALT [144 rising to 658 U/L] arising 38 days after starting oral prednisolone therapy [20 mg/day], liver biopsy showing steatohepatitis; patient reported to have died of liver failure subsequently, but no clinical details given).
- Shiota G, Harada K, Oyama K, Udagawa A, Nomi T, Tanaka K, Tsutsumi A, et al. Severe exacerbation of hepatitis after short-term corticosteroid therapy in a patients with "latent" chronic hepatitis B. Liver. 2000;20:415–20. [PubMed: 11092261](46 year old woman with polyneuropathy developed hepatitis after each of 3 pulses of methylprednisolone [1 g/day for 3 days], twice with jaundice [bilirubin normal, rising to 20 mg/dL, ALT 500-1500 U/L, no detectable HBsAg, but “occult” HBV DNA identified in liver).
- Weissel M, Hauff W. Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid. 2000;10:521. [PubMed: 10907999](71 year old woman with Graves disease developed hepatitis after fifth monthly cycle of high dose methylprednisolone [1 g/day for 3 days], with acute liver failure, death and massive necrosis on autopsy; patient also on methimazole and few clinical details given).
- Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell'Unto E, Rocchi R, Barbesino G, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves' ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86:3562–7. [PubMed: 11502779](Controlled trial of intravenous methylprednisolone [15 mg/kg in 4 cycles] vs oral prednisone [starting at 100 mg/day] in 82 patients with Graves’ ophthalmopathy; one methylprednisolone treated patient had marked, asymptomatic increase in ALT at end of treatment, with recovery in 2 months; authors also mention an unpublished fatal case that they had seen before).
- Dourakis SP, Sevastianos VA, Kaliopi P. Acute severe steatohepatitis related to prednisolone therapy. Am J Gastroenterol. 2002;97:1074–5. [PubMed: 12003403](67 year old woman with dermatomyositis developed jaundice after 8 days of intravenous prednisolone [75 mg/day] [bilirubin 10.8 mg/dL, ALT 545 U/L, Alk P 467 U/L, negative ANA], resolving on decreasing dose, but patient died of pneumonia shortly thereafter, liver histology showing severe fat without fibrosis).
- Stravitz RT, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2003;7:435–51. [PubMed: 12879993](Review of drugs that can cause steatohepatitis including glucocorticoids; mechanism unknown).
- Candelli M, Nista EC, Pignataro G, Zannoni G, de Pascalis B, Gasbarrini G, Gasbarrini A. Steatohepatitis during methylprednisolone therapy for ulcerative colitis exacerbation. J Intern Med. 2003;253:391–2. [PubMed: 12603510](25 year old man with ulcerative colitis developed elevated ALT [106 U/L], fatty liver by ultrasound, and steatohepatitis on liver biopsy 40 days after starting oral methylprednisolone, ALT elevations and ultrasound changes resolving within 6 months of stopping).
- Marinó M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves' ophthalmopathy. Thyroid. 2004;14:403–6. [PubMed: 15186621](Among 800 patients with Graves ophthalmopathy treated with intravenous methylprednisolone, 7 [~1%] developed acute hepatitis-like illness following 3-6 courses, ages 30-63 years, arising 3-17 weeks after starting, 3 were jaundiced and died, 4 were asymptomatic [ALT 179-2490 U/L, Alk P 100-498 U/L], autoantibodies uncommon, resolving spontaneously in 9-22 weeks).
- Salvi M, Vannucchi G, Sbrozzi F, Del Castello AB, Carnevali A, Fargion S, Beck-Peccoz P. Onset of autoimmune hepatitis during intravenous steroid therapy for thyroid-associated ophthalmopathy in a patient with Hashimoto's thyroiditis: case report. Thyroid. 2004;14:631–4. [PubMed: 15320978](43 year old woman with ophthalmopathy developed hepatitis after fourth course of methylprednisolone [ALT rising to 1152 U/L, ANA positive], responding to oral prednisone in tapering doses, but still on low doses of prednisone [2.5 mg/day] 6 years later).
- Marinò M, Morabito E, Altea MA, Ambrogini E, Oliveri F, Brunetto MR, Pollina LE, et al. Autoimmune hepatitis during intravenous glucocorticoid pulse therapy for Graves' ophthalmopathy treated successfully with glucocorticoids themselves. J Endocrinol Invest. 2005;28:280–4. [PubMed: 15952415](43 year old woman with ophthalmopathy who developed marked ALT elevations [1419 U/L] with normal bilirubin and Alk P levels after 3 pulse cycles of methylprednisolone, ANA negative, but biopsy showed chronic aggressive hepatitis which responded to oral prednisone therapy).
- Hofstee HM, Nanayakkara PW, Stehouwer CD. Acute hepatitis related to prednisolone. Eur J Intern Med. 2005;16:209–10. [PubMed: 15967341](46 year old woman with multiple sclerosis developed marked ALT elevations [peak levels 1095, ~1550 and ~2300 U/L with Alk P 140 U/L, LDH 473 U/L, ANA negative] 4-6 weeks after methylprednisolone pulse therapy on multiple occasions, always without jaundice and resolving rapidly each time).
- Das D, Graham I, Rose J. Recurrent acute hepatitis in patient receiving pulsed methylprednisolone for multiple sclerosis. Indian J Gastroenterol. 2006;25:314–6. [PubMed: 17264438](48 year old woman developed nausea and jaundice 6 weeks after a third course of high dose pulse methylprednisolone for multiple sclerosis [bilirubin ~12 rising to ~35 mg/dL, ALT 1625 U/L, Alk P 210 U/L, ANA negative], resolving in 1-2 months and recurring 1 year later after a fourth course).
- Topal F, Ozaslan E, Akbulut S, Küçükazman M, Yüksel O, Altiparmak E. Methylprednisolone-induced toxic hepatitis. Ann Pharmacother. 2006;40:1868–71. [PubMed: 16926305](47 year old woman developed jaundice one week after finishing 7 day course of methylprednisolone [32 mg/day] [bilirubin rising to 15 mg/dL, ALT 2478 U/L, Alk P 138 U/L, ANA negative], resolution in 8 weeks, also on topiramate which was also stopped, but restarted briefly).
- Reuß R, Retzlaff K, Vogel S, Franke FE, Oschmann P. Autoimmune hepatitis after high-dose intravenous methylprednisolone pulse in RR-MS. CEJ Med. 2007;2:356–9.(42 year old woman with multiple sclerosis developed elevations in ALT [1082 U/L] without jaundice 3 weeks after second pulse of intravenous methylprednisolone; liver biopsy showed necrosis, collapse and fibrosis, levels slowly improved; no mention of ANA or bilirubin levels).
- Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves' ophthalmopathy. Thyroid. 2007;17:357–62. [PubMed: 17465867](Among 27 patients with Graves ophthalmopathy receiving 1-4 pulses of methylprednisolone, 7 had transient ALT elevations [peak level 120 U/L] shortly after infusion, all resolved and none were jaundiced).
- Takahashi A, Kanno Y, Takahashi Y, Sakamoto N, Monoe K, Saito H, Abe K, et al. Development of autoimmune hepatitis type 1 after pulsed methylprednisolone therapy for multiple sclerosis: a case report. World J Gastroenterol. 2008;14:5474–7. [PMC free article: PMC2744174] [PubMed: 18803363](43 year old woman with multiple sclerosis developed acute hepatitis 1 month after third course of pulse methylprednisolone [1 g/day for 3 days] [bilirubin 3.4 mg/dL, ALT 1067 U/L, Alk P 377 U/L, IgG 1370 mg/dL, ANA negative] with bilirubin rising to 19.1 mg/dL, attributed to beta interferon; but developed hepatitis again 2 weeks after a subsequent pulse of methylprednisolone [bilirubin 1.7 mg/dL, ALT 875 U/L, Alk P 214 U/L, IgG 1785 mg/dL and ANA 1:80], resolving with oral prednisolone therapy).
- Rivero Fernández M, Riesco JM, Moreira VF, Moreno A, López San Román A, Arranz G, Ruiz Del Arbol L. Rev Esp Enferm Dig. 2008;100:720–3. [Recurrent acute liver toxicity from intravenous methylprednisolone] Spanish. [PubMed: 19159178](57 year old woman with multiple sclerosis developed ALT elevations after each of 3 pulse cycles of methylprednisolone [peak ALT 1223, 833 and 2685 U/L, Alk P 113 and 115 U/L, ANA negative] without jaundice and with rapid resolution, biopsy showing acute hepatitis with “lytic” necrosis).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to corticosteroid therapy or methylprednisolone).
- Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5) Suppl:S156–65. [PubMed: 19399803](Review of reactivation of hepatitis B as caused by chemotherapy or immunosuppression).
- Loraschi A, Banfi P, Mauri M, Sessa F, Bono G, Cosentino M. Hepatotoxicity after high-dose methylprednisolone for demyelinating disease. Clin Neuropharmacol. 2010;33:52–4. [PubMed: 19935411](One man and one woman, ages 33 and 27 years with demyelinating disease developed abnormal ALT levels [1042 U/L and 122 U/L] 5 and 1 week after starting high dose methylprednisolone therapy [4 and 6 days of 2.5 and 4.5 g], resolving rapidly; no mention of symptoms or jaundice).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to methylprednisolone or other corticosteroids).
- Gutkowski K, Chwist A, Hartleb M. Liver injury induced by high-dose methylprednisolone therapy: a case report and brief review of the literature. Hepat Mon. 2011;11:656–61. [PMC free article: PMC3227489] [PubMed: 22140391](24 year old woman with multiple sclerosis developed jaundice 4 weeks after a pulse of methylprednisolone and 1 week after 2 doses of interferon beta [bilirubin 17.9 mg/dL, ALT 740 U/L, Alk P 186 U/L, prothrombin index 35%, SMA >1:320], resolving spontaneously, but recurring after another pulse of methylprednisolone with interferon beta [bilirubin 7.3 mg/dL, ALT 1129 U/L, Alk P 164 U/L], again resolving spontaneously).
- Furutama D, Kimura F, Shinoda K, Maeda T, Tanaka T, Ohsawa N. Recurrent high-dose intravenous methylprednisolone succinate pulse therapy-induced hepatopathy in a patient with multiple sclerosis. Med Princ Pract. 2011;20:291–3. [PubMed: 21455003](11 year old girl with multiple sclerosis had 3 episodes of marked serum ALT elevations [peak levels ~700-1400 U/L] 1-2 months after bolus therapy with methylprednisolone, with minimal symptoms and no jaundice or bilirubin elevations).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, of which none were attributed to corticosteroids or methylprednisolone).
- Carrier P, Godet B, Crepin S, Magy L, Debette-Gratien M, Pillegand B, Jacques J, et al. Acute liver toxicity due to methylprednisolone: consider this diagnosis in the context of autoimmunity. Clin Res Hepatol Gastroenterol. 2013;37:100–4. [PubMed: 23318289](30 year old woman with multiple sclerosis developed jaundice 3 weeks after a second bolus dose of methylprednisolone [bilirubin 25.8 mg/dL, ALT 547 U/L, Alk P 141 U/L, ANA negative], with rapid resolution on stopping and recurrence 2 and 5 years later with subsequent single bolus doses of methylprednisone [peak bilirubin 6.6 and 9.6 mg/dL, ALT 415 and 2826 U/L and Alk P 60 and 148 U/L, latency 10-20 days]).
- D'Agnolo HM, Drenth JP. High-dose methylprednisolone-induced hepatitis in a patient with multiple sclerosis: a case report and brief review of literature. Neth J Med. 2013;71:199–202. [PubMed: 23723114](48 year old woman developed abdominal pain and nausea 19 days after high dose methylprednisolone for multiple sclerosis [bilirubin 1.7 mg/dL, ALT 3028 U/L, GGT 182 U/L], resolving within the following few weeks; by history, she had a similar event 14 years earlier, but did not develop clinically apparent liver injury after 3 subsequent courses of oral dexamethasone [200 mg/day]).
- Melamud B, Lurie Y, Goldin E, Levi I, Esayag Y. Methylprednisolone-induced liver injury: a diagnostic challenge. Isr Med Assoc J. 2014;16:180–1. [PubMed: 24761710](52 year old man with Graves disease developed liver injury within a few weeks of intravenous methylprednisolone, which recurred when he received a second course [bilirubin 3.4 mg/dL, ALT 465 U/L, Alk P 80 U/L], resolving on no therapy within 7 months).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to budesonide or methylprednisolone or other corticosteroids).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 2 were attributed to high dose methylprednisolone, but none to budesonide or other corticosteroids).
- Juan J, Feld JJ. Hepatitis B virus and hepatitis C virus treatment and management in patients receiving immune-modifying agents. Curr Opin Rheumatol. 2014;26:395–403. [PubMed: 24841230](Review of reactivation of hepatitis B and C by chemotherapeutic and immune-modifying agents including corticosteroid based therapies).
- Ferraro D, Mirante VG, Losi L, Villa E, Simone AM, Vitetta F, Federzoni L, et al. Methylprednisolone-induced toxic hepatitis after intravenous pulsed therapy for multiple sclerosis relapses. Neurologist. 2015;19:153–4. [PubMed: 26075468](Short summaries of 4 cases of liver injury after high dose intravenous methylprednisolone therapy of multiple sclerosis, all 4 were women, ages 24 to 50 years, usually with onset within 1 month, peak ALT levels of 260-494 U/L, evidently without jaundice, and resolving spontaneously, but recurring with reexposure).
- High-dose intravenous methylprednisolone: liver injury. Prescrire Int. 2015;24:270. [PubMed: 26688906](Summary of a recent Canadian drug regulatory agency review of 32 cases [4 new] of acute liver injury after intravenous methylprednisolone therapy which were accompanied by jaundice in 10, recurred with rechallenge in 11 and resulted in death in 4).
- Davidov Y, Har-Noy O, Pappo O, Achiron A, Dolev M, Ben-Ari Z. Methylprednisolone-induced liver injury: Case report and literature review. J Dig Dis. 2016;17:55–62. [PubMed: 26676833](23 year old Israel woman with multiple sclerosis developed jaundice three weeks after receiving a 3 day course of high dose methylprednisolone [bilirubin 6.9 mg/dL, ALT 2011 U/L, Alk P 148 U/L, INR 1.18], biopsy showing zone 3 necrosis and inflammation, resolving rapidly; had a previous history of similar episode 3 years earlier).
- Moleti M, Giuffrida G, Sturniolo G, Squadrito G, Campennì A, Morelli S, Puxeddu E, et al. Acute liver damage following intravenous glucocorticoid treatment for Graves' ophthalmopathy. Endocrine. 2016;54:259–68. [PubMed: 27003434](2 patients with Graves’ ophthalmopathy developed liver enzyme elevations during high dose methylprednisolone therapy, 58 year old man and 50 year old women, arising after 5th and 3rd weekly infusions [ALT 809 and 822 U/L, GGT 112 and normal, bilirubin normal or not given], with spontaneous resolution 12 and 16 weeks after stopping).
- Sayin R, Gokgul A, Ebinc S, Dulger AC, Tombul T. Clinical overlap of multiple sclerosis and autoimmune hepatitis: three cases. J Coll Physicians Surg Pak. 2016;26(6) Suppl:S45–7. [PubMed: 27376220](Description of 3 patients with multiple sclerosis and features of autoimmune hepatitis, two of whom were receiving interferon beta and the third with preexisting autoimmune hepatitis found to have brain imaging changes suggestive of multiple sclerosis).
- Dumortier J, Cottin J, Lavie C, Guillaud O, Hervieu V, Chambon-Augoyard C, Scoazec JY, et al. Methylprednisolone liver toxicity: A new case and a French regional pharmacovigilance survey. Clin Res Hepatol Gastroenterol. 2017;41:497–501. [PubMed: 28438569](27 year old woman with multiple sclerosis treated with multiple agents had repeated bouts of acute liver injury arising within a month of pulse methylprednisolone therapy that was sometimes clinically apparent [bilirubin 3.7 mg/dL, ALT 1704 U/L, GGT 124 U/L] and resolved within a few months of stopping, later tolerating fingolimod; review of a French pharmacovigilance registry identified 4 other cases of severe injury attributed to high dose, pulses of methylprednisolone).
- Abramavicius S, Velickiene D, Kadusevicius E. Methimazole-induced liver injury overshadowed by methylprednisolone pulse therapy: Case report. Medicine (Baltimore). 2017;96:e8159. [PMC free article: PMC5626305] [PubMed: 28953662](74 year old woman with Graves’ ophthalmopathy was treated with methimazole and pulses of methylprednisolone and developed mild-to-moderate ALT elevations after the 4th pulse that continued until the 9th pulse and eventually resolved despite continuing both drugs).
- Bresteau C, Prevot S, Perlemuter G, Voican C. Methylprednisolone-induced acute liver injury in a patient treated for multiple sclerosis relapse. BMJ Case Rep. 2018;2018:bcr2017223670. [PMC free article: PMC5847962] [PubMed: 29507031](35 year old woman with 13 year history of multiple sclerosis developed acute liver injury 2 months after a 5 day course of high dose methylprednisolone [bilirubin 2.9 mg/dL, ALT 1512 U/L, Alk P 86 U/L, GGT 109 U/L], resolving within 6 weeks).
- Nociti V, Biolato M, De Fino C, Bianco A, Losavio FA, Lucchini M, Marrone G, et al. Liver injury after pulsed methylprednisolone therapy in multiple sclerosis patients. Brain Behav. 2018;8:e00968. [PMC free article: PMC5991562] [PubMed: 29729087](Among 175 patients with multiple sclerosis receiving 251 cycles of high dose intravenous methylprednisolone, 21 [8.6%] developed de novo ALT elevations after therapy, which were transient and mild in 15 but severe in 6 [3.4%: bilirubin 1.3 to 10.8 mg/dL, ALT 778 to 2956 U/L, Alk P 91 to 350 U/L, hepatocellular in all], resolving in 4 subjects but evolving into chronic hepatitis suspected to be autoimmune in 2 both of whom required long term immunosuppressive therapy).
- Adamec I, Pavlović I, Pavičić T, Ruška B, Habek M. Toxic liver injury after high-dose methylprednisolone in people with multiple sclerosis. Mult Scler Relat Disord. 2018;25:43–5. [PubMed: 30032042](Three women, ages 37 to 46 years, with multiple sclerosis developed acute liver injury after infusions of high dose, intravenous methylprednisolone [ALT 2259, 395 and 1340 U/L], recurring with milder ALT elevations upon reexposure [ALT 140, 134, and 85 U/L], and with no evidence of injury with lower doses).
- Rotondo E, Graziosi A, Di Stefano V, Mohn AA. Methylprednisolone-induced hepatotoxicity in a 16-year-old girl with multiple sclerosis. BMJ Case Rep. 2018;11:e226687. [PMC free article: PMC6301543] [PubMed: 30567201](16 year old girl with recent onset of multiple sclerosis developed an acute hepatitis a month after a 5 day course of intravenous methylprednisolone [bilirubin 2.1 mg/dL, ALT 2438 U/L, GGT 68 U/L], resolving spontaneously within 2 months of onset).
- Zoubek ME, Pinazo-Bandera J, Ortega-Alonso A, Hernández N, Crespo J, Contreras F, Medina-Cáliz I, et al. Liver injury after methylprednisolone pulses: A disputable cause of hepatotoxicity. A case series and literature review. United European Gastroenterol J. 2019;7:825–37. [PMC free article: PMC6620870] [PubMed: 31316787](Three women, ages 31 to 36 years, with multiple sclerosis developed acute liver injury 2-6 weeks after receiving a 3-7 day course of intravenous methylprednisolone [bilirubin 3.6, 0.8 and 13.9 mg/dL, ALT 2194, 630 and 2737 U/L, Alk P 229, 193 and 169 U/L], resolving spontaneously and recurring with reexposure in two patients).
- Nguyen N, Reddy YK, Jain N, Patel V, James D, Sistani B, Steinberg H. Challenges in management of autoimmune hepatitis with concurrent Graves thyrotoxicosis. ACG Case Rep J. 2019;6:e00277. [PMC free article: PMC7145210] [PubMed: 32309475](37 year old African American woman with Graves’ disease developed jaundice [bilirubin 9.4 mg/dL, direct 13 mg/dL, ALT 2725 U/L, Alk P 314 U/L, IgG 1910, ANA negative] and was treated with iv methylprednisolone followed by oral prednisone, with remission in disease on long term immunosuppression).
- Cottin J, Pierre S, Pizzoglio V, Simon C, Durrieu G, Bleyzac N, Gouraud A, et al. Methylprednisolone-related liver injury: A descriptive study using the French pharmacovigilance database. Clin Res Hepatol Gastroenterol. 2020;44:662–73. [PubMed: 31948782](Analysis of all cases of liver injury after exposure to methylprednisolone [MP] from the French pharmacovigilance database [2016] identified 414 persons exposed, 97 being judged due to MP; 59% women, mean age 46 years; used for autoimmune disease in 48%, multiple sclerosis in 27%, given iv in 79%, mean time to onset 9.5 days but as long as 9 months after starting; hepatocellular injury in 73%, mild course in 45%, moderate 45%, fatal in 3, but usually with rapid recovery, rechallenge in 13 cases led to recurrence in 10 [77%]).
- Milovanovic T, Jankovic K, Boricic I, Dragasevic S, Stojkovic Lalosevic M, Dumic I. Methylprednisolone induced liver injury in a patient with multiple sclerosis. J Gastrointestin Liver Dis. 2020;29:119–20. [PubMed: 32176748](28 year old woman with multiple sclerosis developed evidence of liver injury after a second monthly course of methylprednisolone [3 gm over 3 days] without jaundice [ALT 459 U/L, bilirubin and Alk P normal, ANA negative], resolving after discontinuation).
- Kimura H, Takeda A, Kikukawa T, Hasegawa I, Mino T, Uchida-Kobayashi S, Ohsawa M, et al. Liver injury after methylprednisolone pulse therapy in multiple sclerosis is usually due to idiosyncratic drug-induced toxicity rather than autoimmune hepatitis. Mult Scler Relat Disord. 2020;42:102065. [PubMed: 32259746](Between 2005 and 2016, 8 patients with multiple sclerosis developed liver injury after pulse methylprednisolone therapy, all women, ages 28 to 49 years, developing hepatocellular injury 4-90 days after starting MP, peak ALT 170 to 2720 U/L, with mild jaundice only, spontaneous recovery in 1-7 months, and liver biopsies in 6 showing acute centrilobular necrosis in 5 with no fibrosis and possible autoimmune hepatitis in one).
- RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. [PMC free article: PMC7383595] [PubMed: 32678530](Among 6425 hospitalized patients with COVID 19 enrolled in a large multicenter pragmatic trial, the 28 day mortality was lower among those who received dexamethasone [6 mg daily for up to 10 days] versus those receiving usual care alone [22.9% vs 25.7%], the decrease in mortality being seen in those on mechanical ventilation [29.3% vs 41.4%] or receiving oxygen [23.3% vs 26.2%], but not in those receiving no respiratory support [17.8% vs 14%]; there were no serious hepatic adverse events or deaths from liver injury).
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, et al. COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324:1307–16. [PMC free article: PMC7489411] [PubMed: 32876695](Among 299 hospitalized patients with COVID-19 pneumonia and moderate-to-severe respiratory distress syndrome admitted to 41 ICUs in Brazil, those treated with dexamethasone for up to 10 days had more ventilator free days during the first 28 days than those receiving standard care [6 vs 4.4 days], although overall mortality was similar [56.3% vs 61.5%] as were severe adverse events including infections and hyperglycemia; no hepatic severe adverse events reported).
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–41. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, et al. [PMC free article: PMC7489434] [PubMed: 32876694](Metaanalysis of efficacy and safety of systemic corticosteroids in critically ill patients with COVID-19 identified 7 randomized controlled trials with a total of 1703 patients and concluded that systemic corticosteroid therapy [dexamethasone, methylprednisolone, or hydrocortisone] was associated with a lower all-cause mortality at 28 days with a summary odds ratio of 0.70, while severe adverse event rates were similar in frequency).
- Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, Bassett J. COVID-19 presenting as fulminant hepatic failure: A case report. Medicine (Baltimore). 2020;99:e22818. [PMC free article: PMC7581048] [PubMed: 33120805](35 year old woman with lupus erythematosus developed abdominal pain and jaundice and upon admission was found to be positive for SARS-CoV-2 RNA [bilirubin 7.0 2 days later rising to 10.5 mg/dL, ALT 278 rising to 5,524 stable at 171 U/L, Alk P 200 U/L and INR 1.5 rising to 4.8] with rapid onset of hepatic encephalopathy, but subsequent spontaneous recovery while developing only mild symptoms of COVID-19).
- Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, Moniri A, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. [PMC free article: PMC7885705] [PubMed: 33607104](Among 50 hospitalized patients with mild-to-moderate COVID 19 treated with dexamethasone or standard care, overall mortality was similar in the two groups [64% vs 60%] as was need for mechanical ventilation [52% vs 44%] and duration of hospital stay [11 vs 6 days]; no discussion of adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- [PRINCIPLES OF CORTISONE THERAPY].[Urologe. 1963][PRINCIPLES OF CORTISONE THERAPY].KAISER H. Urologe. 1963 Jul 15; 2:203-10.
- [FEVER IN CHILDREN. (3)].[Chiryo. 1963][FEVER IN CHILDREN. (3)].SATOMI M. Chiryo. 1963; 45:2223-7.
- [PROBLEMS IN THE SIDE-EFFECTS OF ADRENAL CORTEX HORMONE THERAPY OF SKIN DISEASES].[Nisshin Igaku Jpn J Med Prog. ...][PROBLEMS IN THE SIDE-EFFECTS OF ADRENAL CORTEX HORMONE THERAPY OF SKIN DISEASES].NOHARA N, TSUKINOKI K, TODA M. Nisshin Igaku Jpn J Med Prog. 1963 Mar; 50:139-58.
- Review Intramuscular versus oral corticosteroids to reduce relapses following discharge from the emergency department for acute asthma.[Cochrane Database Syst Rev. 2018]Review Intramuscular versus oral corticosteroids to reduce relapses following discharge from the emergency department for acute asthma.Kirkland SW, Cross E, Campbell S, Villa-Roel C, Rowe BH. Cochrane Database Syst Rev. 2018 Jun 2; 6(6):CD012629. Epub 2018 Jun 2.
- Review [Side-effects of cortisone derivatives].[Internist (Berl). 1967]Review [Side-effects of cortisone derivatives].Begemann H, Kaboth W. Internist (Berl). 1967 Mar; 8(3):85-94.
- Corticosteroids - LiverToxCorticosteroids - LiverTox
- eggc.vippXL (0)BioProject
- eggcsite.combbC (0)BioProject
- Treponema pallidum subsp. pallidum str. NicholsTreponema pallidum subsp. pallidum str. NicholsCausative agent of syphilisBioProject
- 5 3eggc.viptsb (1)BioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...