NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Atomoxetine is a selective norepinephrine reuptake inhibitor used primarily for therapy of attention deficit hyperactivity disorder. Atomoxetine has been linked to a low rate of serum aminotransferase elevations and to rare cases of acute, clinically apparent liver injury.
Background
Atomoxetine (a" toe mox' e teen) is a selective norepinephrine reuptake inhibitor that blocks the presynaptic norepinephrine transporter leading to an increase in levels of this potent neurotransmitter, predominantly in the central nervous system. Therapy with atomoxetine has been shown to lead to improvements in levels of psychological functioning and performance in children and adults with suspected attention deficit/hyperactivity disorder (ADHD). Atomoxetine was approved for use in adults, adolescents and children above the age of 6 years with ADHD in the United States in 2002. Atomoxetine is available in capsules of 10, 18, 25, 40, 60, 80 and 100 mg in generic forms and under the brand name Strattera. The recommended initial dosage in adults is 40 mg once daily, with subsequent increases to a maintenance dose which averages 60 to 100 mg daily. The dosage in children is based upon body weight. Common side effects include headache, insomnia, irritability, dry mouth, erectile dysfunction, urinary hesitancy, gastrointestinal upset, nausea, constipation and rash. Uncommon but potential severe adverse events include suicidal ideation and behavior, cardiovascular symptoms and events, manic or aggressive behavior, priapism and hypersensitivity reactions.
Hepatotoxicity
Atomoxetine has been linked to serum aminotransferase elevations in a small proportion of patients (~0.5%). More importantly, there have been several reports of clinically apparent acute liver injury due to atomoxetine. The onset of injury was within 3 to 12 weeks of starting the medication. The typical pattern of serum enzyme elevations was hepatocellular with marked increases in serum aminotransferase levels (often >20 times upper limit of normal) and clinical features that resembled acute viral hepatitis. Most cases have been self-limited, but instances of acute liver failure sometimes requiring emergency liver transplantation have been reported. Immunoallergic features were not found, but several patients with acute injury had antinuclear antibody and at least one patient had other features that resembled autoimmune hepatitis (with typical liver histology and high levels of immunoglobulins in serum).
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which atomoxetine might cause liver injury is unknown. Atomoxetine is extensively metabolized in the liver by the cytochrome P450 system, predominantly CYP 2D6 and production of a toxic intermediate or immunogenic byproduct are reasonable explanations. Atomoxetine is susceptible to drug-drug interactions with other substrates that use CYP 2D6 as well as inducers and inhibitors of the enzyme. Patients who have low or absent levels of CYP 2D6 (poor metabolizers) may have high plasma levels of atomoxetine and have more severe adverse events when receiving conventional doses, and those with high CYP 2D6 levels (ultrafast metabolizers) may have low plasma levels and fail to respond to treatment.
Outcome and Management
The liver injury due to atomoxetine varies from minor, transient and asymptomatic elevations in serum aminotransferase levels to clinically apparent hepatitis that can be prolonged and even fatal. Chronic liver injury and vanishing bile duct syndrome due to atomoxetine have not been described. Atomoxetine should be discontinued if jaundice or symptoms of liver injury accompany serum enzyme elevations during treatment or if aminotransferase levels rise to above 5 times the upper limit of normal. There does not seem to be cross reactivity to hepatic injury between atomoxetine and other agents used for ADHD, but there may be cross reactivity with other norepinephrine reuptake inhibitors.
Drug Class: Central Nervous System Stimulants; ADHD Agents
CASE REPORT
Case 1. Acute hepatitis in a child treated with atomoxetine.(1)
A 12 year old girl with ADHD developed abdominal pain, nausea, diarrhea and jaundice three weeks after restarting atomoxetine (40 mg daily). She had no previous history of liver disease or risk factors for viral hepatitis. She had received atomoxetine for approximately one year, but stopped taking it when she ran out of the medication, leading to an interruption of therapy for 6 weeks before restarting. She was not taking any other medications, over-the-counter drugs or herbals. On examination, she was jaundiced and had mild right upper quadrant tenderness without hepatomegaly or other manifestations of chronic liver disease. She had no fever or rash. Laboratory tests showed a total bilirubin of 9.1 mg/dL (direct 5.5 mg/dL) and marked elevations in serum aminotransferase levels (ALT 3264 U/L, AST 2999 U/L), with minimal increase in alkaline phosphatase (231 U/L) and gamma glutamyl transpeptidase (108 U/L) (Table). Tests for hepatitis A, B and C were negative. Antinuclear antibody was positive in a titer of 1:160; smooth muscle antibody was negative. There was no hypergammaglobulinemia, and total IgG levels were normal (769 mg/dL). A liver biopsy showed focal hepatocyte necrosis and marked portal and parenchymal inflammatory infiltrates, with minimal fibrosis and normal bile ducts. Atomoxetine was discontinued and she was monitored on no specific therapy. She improved markedly over the next six weeks, and liver tests were normal when tested six months later.
Key Points
| Medication: | Atomoxetine (40 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=39) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 3 weeks (reexposure) |
| Recovery: | ~6 weeks |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 0 | Atomoxetine (40 mg daily) started | ||||
| 3 weeks | 0 | 1979 | 363 | 8.0 | Atomoxetine stopped |
| 5 weeks | 2 weeks | 9.8 | Liver biopsy | ||
| 7 weeks | 4 weeks | 1438 | 177 | 2.6 | |
| 9 weeks | 6 weeks | 107 | 182 | 1.6 | |
| 7 months | 6 months | <15 | 176 | 0.3 | |
| Normal Values | <50 | <397 | <1.2 | ||
Comment
A young girl who had previously taken atomoxetine without difficulty developed an acute, self-limited hepatitis 3 weeks after restarting it. The hepatitis was accompanied by autoantibody formation, but without elevations in globulins or IgG levels. She improved upon stopping atomoxetine and corticosteroids were not used. Drug induced liver injury due to atomoxetine is rare, but several instances of an acute hepatitis-like syndrome arising within 3 to 12 weeks of starting or restarting the medication have been reported in patients without any other obvious reason for acute liver injury. The injury is usually hepatocellular with markedly elevated serum aminotransferase levels (as in this case). Recurrence with reexposure has been reported and should be avoided.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Atomoxetine – Generic, Strattera®
DRUG CLASS
Central Nervous System Stimulants
Product labeling at DailyMed, National Library of Medicine, NIH
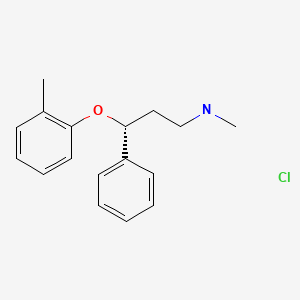
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Atomoxetine | 82248-59-7 | C17-H21-N-O.Cl-H |
|
CITED REFERENCE
- 1.
- Lim JR, Faught PR, Chalasani NP, Molleston JP. Severe liver injury after initiating therapy with atomoxetine in two children. J Pediatr. 2006;148:831–4. [PubMed: 16769398]
ANNOTATED BIBLIOGRAPHY
References updated: 25 August 2021
Abbreviations: ADHD, attention deficit/hyperactivity disorder.
- Zimmerman HJ. Psychotropic and anticonvulsant agents. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-516.(Expert review of hepatotoxicity published in 1999; atomoxetine is not mentioned).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of hepatotoxicity of antidepressants; atomoxetine is not discussed).
- O'Donnell JM, Shelton RC. Pharmacotherapy of depression and anxiety disorders. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 397-416.(Textbook of pharmacology and therapeutics).
- Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs. 2004;64:205–22. [PubMed: 14717619](Review of pharmacology, clinical efficacy and safety of atomoxetine in attention deficit hyperactivity disorder [ADHD]; common side effects are dry mouth, insomnia, nausea, poor appetite, constipation, dizziness, sweating, sexual dysfunction and palpitations; no mention of ALT elevations or hepatotoxicity).
- Drugs for treatment of ADHD. Treat Guidel Med Lett. 2006;4:77–82. [PubMed: 17039210](Drugs approved for use in ADHD in the US include atomoxetine, amphetamines and methylphenidate; common side effects of atomoxetine include somnolence, nausea and decreased appetite; “Hepatic toxicity has been reported rarely”).
- Lim JR, Faught PR, Chalasani NP, Molleston JP. Severe liver injury after initiating therapy with atomoxetine in two children. J Pediatr. 2006;148:831–4. [PubMed: 16769398](Two cases; 12 year old girl developed jaundice 3 weeks after restarting atomoxetine [bilirubin 9.1 mg/dL, ALT 3264 U/L, Alk P 231 U/L, ANA 1:160, IgG 769 mg/dL], resolving in 8 weeks of stopping [Case 1]; 11 year old girl developed fatigue 3 months after starting atomoxetine [bilirubin 0.5 mg/dL, ALT 675 U/L, Alk P 96 U/L, ANA 1:320, IgG 7,390 mg/dL] and biopsy suggesting chronic hepatitis, hepatitis improving within a few weeks of starting prednisone).
- Stojanovski SD, Casavant MJ, Mousa HM, Baker P, Nahata MC. Atomoxetine-induced hepatitis in a child. Clin Toxicol (Phila). 2007;45:51–5. [PubMed: 17357382](8 year old female developed abdominal pain 1 month after starting atomoxetine [bilirubin 10.8 mg/dL, ALT 5182 U/L, Alk P 528 U/L, ANA negative], resolving within 2 months of stopping; also describes 2 cases reported to FDA: 14 year old boy and 31 year old woman developed symptoms 2.5-3.5 months after starting atomoxetine [bilirubin 1.2 and 16 mg/dL, ALT 31-36 times ULN, Alk P normal], resolving in 2-4 months).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, atomoxetine accounted for 3 cases [1%], 2 of which had been previously reported [Lim 2006]).
- Bangs ME, Jin L, Zhang S, Desaiah D, Allen AJ, Read HA, Regev A, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31:345–54. [PubMed: 18366245](Review of hepatic adverse events of atomoxetine; of 7961 patients treated in clinical trials, 41 had hepatobiliary events, but most were mild increases in ALT and none had jaundice with hepatitis; since marketing, there have been 351 spontaneous reports of liver adverse events, but only 3 were considered probable, one of which had a positive rechallenge).
- Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11:203–26. [PubMed: 19445548](Review of mechanism of action, pharmacology, clinical efficacy and safety of atomoxetine as treatment of ADHD in children; common side effects are headache, abdominal pain, decreased appetite, nausea, somnolence, fatigue, irritability and dizziness; mild elevations in ALT or AST occur in 0.5% of subjects, and the sponsor has received reports of 351 hepatic adverse events from an estimated 4.3 million recipients [<0.01%]).
- Johnson M, Cederlund M, Råstam M, Areskoug B, Gillberg C. Open-label trial of atomoxetine hydrochloride in adults with ADHD. J Atten Disord. 2010;13:539–45. [PubMed: 19458384](Open label trial of atomoxetine in 20 adults with ADHD; one patient discontinued drug because of raised liver enzymes; no details given).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, 2 agents used for ADHD were among the top 41 causes; methylphenidate [11th, 96 cases] and atomoxetine [14th, 64 cases]).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen between 1997 and 2008 at a large hospital in Bangalore, India, one case was attributed to atomoxetine).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including one attributed to cocaine and one to ecstasy but none to atomoxetine).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N. Drug-induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with suspected drug induced liver injury, 3 were attributed to atomoxetine and one to methylphenidate).
- Erdogan A, Ozcay F, Piskin E, Karaman MG, Bilezikci B, Calik M, Tekin I, et al. Idiosyncratic liver failure probably associated with atomoxetine: a case report. J Child Adolesc Psychopharmacol. 2011;21:295–7. [PubMed: 21663435](10 year old boy developed fatigue 2 days and jaundice 5 days after starting atomoxetine [bilirubin 5.2 rising to 23.0 mg/dL, ALT 942 to 2832 U/L, Alk P 400 U/L, INR 4.1], with progressive liver failure, encephalopathy and emergency living donor liver transplantation).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, Presentation and Outcomes in Patients with Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to atomoxetine).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to atomoxetine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 4 [0.5%] were attributed to atomoxetine).
- Cortese S, Panei P, Arcieri R, Germinario EA, Capuano A, Margari L, Chiarotti F, et al. Safety of methylphenidate and atomoxetine in children with attention-deficit/ hyperactivity disorder (ADHD): data from the Italian National ADHD Registry. CNS Drugs. 2015;29:865–77. [PubMed: 26293742](The Italian ADHD Registry of children treated with methylphenidate [n=1350] and atomoxetine [n=753] included adverse events reports in 27% of cases and serious events in 4%, more frequently with atomoxetine among which were 3 cases of hyperbilirubinemia which were considered possibly life-threatening).
- Camporeale A, Porsdal V, De Bruyckere K, Tanaka Y, Upadhyaya H, Deix C, Deberdt W. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29:3–14. [PubMed: 25424623](Among 4829 patients enrolled in 15 placebo controlled trials of atomoxetine in adults with ADHD, rates of serious adverse events were similar in the two groups and there were no differences in changes in ALT or in numbers of liver-related serious adverse events).
- Drugs for ADHD. Med Lett Drugs Ther. 2015;57(1464):37–40. [PubMed: 25758544](Concise review of the mechanism of action, clinical efficacy, safety and costs of drugs approved for use in ADHD, including atomoxetine and methylphenidate; no mention of ALT elevations or hepatotoxicity of either drug).
- Dean L. Atomoxetine Therapy and CYP2D6 Genotype. 2015 Sep 10 [updated 2020 Jun 29]. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kane MS, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–.(Atomoxetine is metabolized by CYP 2D6 and is sensitive to the effects of various variants that can create ultrafast, normal, intermediate or poor metabolizers, which can create low or high plasma levels with conventional dosages).
- Goto T, Hirata Y, Takita Y, Trzepacz PT, Allen AJ, Song DH, Gau SS, et al. Efficacy and safety of atomoxetine hydrochloride in Asian adults with ADHD. J Atten Disord. 2017;21:100–109. [PubMed: 24203774](Among 391 Asian adults with ADHD treated with atomoxetine or placebo for 10 weeks, improvements in ADHD rating scores were greater with atomoxetine and adverse events included nausea, decreased appetite, drug mouth, thirst, vomiting, weight loss, dizziness and dysuria, discontinuations occurring in 5% vs 1.5% of placebo recipients; no mention of ALT elevations or hepatotoxicity).
- Tumuluru RV, Corbett-Dick P, Aman MG, Smith T, Arnold LE, Pan X, Buchan-Page KA, et al. Adverse events of atomoxetine in a double-blind placebo-controlled study in children with autism. J Child Adolesc Psychopharmacol. 2017;27:708–14. [PMC free article: PMC5651962] [PubMed: 28509573](Among 128 children [ages 5 to 14 years] with ADHD treated with atomoxetine alone or placebo with or without parent training, adverse events linked to atomoxetine included decrease in appetite and fatigue, and "no significant difference of changes in laboratory tests was noted" between the groups).
- Hinson VK, Delambo A, Elm J, Turner T. A randomized clinical trial of atomoxetine for mild cognitive impairment in Parkinson's disease. Mov Disord Clin Pract. 2016;4:416–423. [PMC free article: PMC6174374] [PubMed: 30363371](Among 130 adults with cognitive impairment due to Parkinson disease treated with atomoxetine or placebo for 10 weeks, global scores of attention, working memory and processing speed did not improve with atomoxetine, but adverse events were more frequent, while laboratory monitoring of “liver function did not reveal any clinically significant changes”).
- Padilha SCOS, Virtuoso S, Tonin FS, Borba HHL, Pontarolo R. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry. 2018;27:1335–45. [PubMed: 29460165](Systematic review of efficacy and safety of drugs for ADHD based upon 48 trials [4169 participants]; makes no mention of ALT elevations or hepatotoxicity of any of the agents studied, including guanfacine).
- Cortese S, Adamo N, Mohr-Jensen C, Hayes AJ, Bhatti S, Carucci S, Del Giovane C, et al. European ADHD Guidelines Group (EAGG). Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open. 2017;7:e013967. [PMC free article: PMC5253538] [PubMed: 28073796](Review of the pharmacological therapy of ADHD with discussion of amphetamines, methylphenidate, atomoxetine, guanfacine and clonidine; no mention of ALT elevations during therapy or hepatotoxicity).
- Schoretsanitis G, de Leon J, Eap CB, Kane JM, Paulzen M. Clinically significant drug-drug interactions with agents for attention-deficit/hyperactivity disorder. CNS Drugs. 2019;33:1201–1222. [PubMed: 31776871](Review of drug-drug interactions of agents used to treat ADHD mentions that there is little information on the metabolism of clonidine and possible drug-drug interactions).
- Drugs for ADHD. Med Lett Drugs Ther. 2020;62(1590):9–15. [PubMed: 31999670](Concise review of drugs for attention deficit/hyperactivity disorder mentions that atomoxetine is a nonstimulatory agent that is less effective but sometimes better tolerated than methylphenidate, rare but severe adverse events include hepatitis [rarely] growth delay, increase in blood pressure and pulse and priapism).
- Abrams M, Hasan S, Mehta A. liver enzyme elevation from atomoxetine use–a case report. J Child Adolesc Psychopharmacol. 2021;31:322–323. [PubMed: 33970028](17 year old transgender male with ADHD on long term atomoxetine was found to have mild serum aminotransferase elevations without symptoms [ALT 71 U/L initially and then 52 U/L 3 months and 19 U/L 6 months after stopping]; no information on body weight, ultrasound findings, or hepatitis serology).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Atomoxetine - LiverToxAtomoxetine - LiverTox
- Borreliella burgdorferi B31Borreliella burgdorferi B31Causes Lyme diseaseBioProject
- eggc.vipv3n (0)BioProject
- 3 eggc.vipvIA (1)BioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...