NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Androgenic steroids are used for male sex hormone replacement and in the therapy of malignancies. The androgens also have anabolic effects and are used in catabolic or muscle wasting states. In addition, the synthetic anabolic steroids are now widely used illicitly for exercise and athletic performance enhancement. Many synthetic androgenic steroids are capable of causing cholestatic liver injury and long term use of androgens is associated with development of liver tumors including hepatocellular carcinoma, hepatic adenoma and vascular changes (peliosis hepatis).
Background
Testosterone is the major male sex hormone and is produced by the male testes in men and to a lesser extent by the adrenal glands in both men and women. Unmodified testosterone is not orally available, so it must be given intramuscularly, sublingually or by transcutaneous patch. Modifications of testosterone have been developed that are more bioavailable or have a longer duration of action. Modification by C-17β esterification (testosterone cypionate, enanthate and propionate) maintains the virilizing effects of testosterone, but increases potency and duration of action. Alkylation of the C-17α position of testosterone allows for oral administration by inhibiting metabolic deactivation in the liver and often alters the relative anabolic potency in relation to the masculinizing effects. The C-17 alkylated testosterones include methyltestosterone (meth" il tes tos' ter one), methandrostenolone (meth an" droe stene' oh lone), oxymetholone (ox" i meth' oh lone), danazol (dan' a zol), epistane (ep is tane’), fluoxymesterone (floo ox" i mes' ter one), stanozolol (stan oh' zoe lol), norethandrolone (nor eth' an droe lone), and oxandrolone (ox an' droe lone), and have been extensively evaluated as a means of increasing weight gain and muscle development in catabolic states as well as to improve athletic performance. They have also been used to treat aplastic anemia and bone marrow failure of several causes. They are often well tolerated and have limited virilizing activity. However, the C-17α alkylated androgenic steroids have all been implicated in cases of liver injury, including prolonged cholestasis, peliosis hepatis, nodular regeneration, hepatic adenomas and hepatocellular carcinoma. In contrast, the C-17β esterified testosterones have only rarely been implicated in causing cholestasis, although their long term use may increase the risk of hepatic tumors and nodular transformation, but seemingly at a much lower rate than the 17α alkylated testosterones. Currently approved uses of androgenic steroids include androgen deficiency, delayed puberty, breast cancer, postpartum breast engorgement, hereditary angioneurotic edema, endometriosis and fibrocystic breast disease. The androgenic steroids are also used off label and illegally as a means of increasing muscle mass and athletic performance. The abuse of anabolic steroids is particularly common among body builders and young male athletes, although their use has been banned from the Olympics and in major professional and college sports. Recently, anabolic steroids have been found in some nutritional supplements available over-the-counter or via the internet which are advertised as increasing a sense of wellbeing and muscle mass or as an aid to body building.
Hepatotoxicity
Androgenic and anabolic steroids have been implicated in four distinct forms of liver injury: transient serum enzyme elevations, an acute cholestatic syndrome (“bland cholestasis”), chronic vascular injury to the liver (peliosis hepatis) and hepatic tumors including adenomas and hepatocellular carcinoma. These adverse events have been most closely linked with the C-17α alkylated testosterones, although tumors have also been associated with unmodified and esterified testosterone preparations.
Use of androgenic steroids is associated with a variable rate of serum enzyme elevations which are usually asymptomatic and self-limited. Such elevations have been most closely linked to danazol and oxymetholone, but are usually transient and do not require dose adjustment or discontinuation.
More importantly, therapy with anabolic steroids is linked to a distinctive form of acute cholestasis often referred to as “bland cholestasis”. The liver injury generally arises within 1 to 4 months of starting therapy, but may be delayed to as long as 6 to 24 months (Case 1). The onset is usually insidious with development of nausea, fatigue and itching followed by dark urine and jaundice. Jaundice and pruritus can be prolonged even if the anabolic steroids are discontinued promptly. Typically, serum enzyme elevations are quite modest, with ALT and alkaline phosphatase levels that are less than 2 to 3 times elevated and that are sometimes normal despite deep jaundice. Serum ALT levels may be somewhat high early during injury, but then fall to moderate or low levels. Liver biopsy typically shows a bland cholestasis with minimal inflammation and hepatocellular necrosis. Bile duct injury is typically absent or mild and vanishing bile duct syndrome rarely ensues. The frequency of acute cholestasis from androgenic steroids is not well known, but it is likely somewhat dose related and may occur in ~1% of patients treated with methyltestosterone, danazol, stanozolol or oxymetholone. Cholestasis has not been described in patients receiving unmodified testosterone (by injection or transdermal patch). This clinical phenotype of bland cholestasis is so typical of anabolic steroids, that the diagnosis can be suspected in a patient who denies taking anabolic steroids or who is taking an herbal formulation meant to increase muscle strength or energy and that contains an anabolic steroid even though it is not labelled as such.
Use of anabolic steroids has also been linked to vascular changes in the liver referred to as peliosis hepatis. Peliosis hepatis is a rare syndrome in which there are blood filled enlarged sinusoids and cysts focally or throughout the liver. There is usually an accompanying sinusoidal dilatation and loss of the normal endothelial barrier. The liver may be enlarged, deep red in color and fragile. Peliosis hepatis most typically occurs in patients with advanced wasting diseases (tuberculosis, cancer), but has also been associated with long term use of anabolic steroid therapy for aplastic anemia and hypogonadism as well as in body building. Serum enzyme levels are usually normal or are mildly and nonspecifically elevated. Patients may present with right upper quadrant discomfort and hepatomegaly or with sudden abdominal pain and vascular collapse due to hepatic rupture and hemoperitoneum. Peliosis may also be an incidental finding found on imaging of the liver or during abdominal surgery or at autopsy. Peliosis associated with anabolic steroids usually reverses, at least in part, with stopping therapy. Peliosis can involve other organs, most typically the spleen.
The most serious complication of anabolic steroid use is the development of hepatic tumors, either adenoma or hepatocellular carcinoma. The hepatic tumors arise in patients on long term androgenic steroids, usually during therapy of aplastic anemia or hypogonadism, but occasionally in athletes or body builders using anabolic steroids illicitly. Tumors are typically found after 5 to 15 years of use, but onset within 2 years of starting therapy with testosterone esters has been described. Many of the case reports have occurred in patients with other risk factors for cancer, such as Fanconi’s syndrome, iron overload or chronic hepatitis C (from blood transfusions). However, hepatic adenomas and hepatocellular carcinoma have also been described in patients taking androgenic steroids who have no other evidence of liver disease and normal histology in the nontumorous parts of the liver. The pathology of the tumors is usually hepatic adenoma or “well differentiated” hepatocellular carcinoma or hepatic adenoma with areas of malignant transformation. Rare instances of cholangiocarcinoma and angiosarcoma have also been described in patients on long term androgenic steroids. Clinical presentation is generally with right upper quadrant discomfort and a hepatic mass found clinically or on imaging studies. Routine liver tests are often normal unless there is extensive spread or rupture or an accompanying liver disease. Alpha-fetoprotein levels are usually normal. There is often (but not always) spontaneous regression in the tumor when the anabolic steroids are stopped. Hepatocellular carcinoma arising during anabolic steroid therapy is believed to have a better prognosis than that related to cirrhosis or chronic hepatitis B and C; however, deaths from hepatic rupture or tumor spread and metastasis have been reported in patients with anabolic steroid related hepatocellular carcinoma without cirrhosis.
Finally, nodular regenerative hyperplasia of the liver has been described in rare patients on long term anabolic or androgenic steroids. The condition is usually asymptomatic or associated with mild abdominal discomfort due to hepatomegaly. Rarely, marked nodular regenerative hyperplasia with portal hypertension and splenomegaly has been described. This process may also be related with development of hepatic tumors with androgenic steroids as nodular regeneration is sometimes found in the surrounding “normal” liver.
Mechanism of Injury
The androgens act by engagement of intracellular androgenic steroid receptors which are translocated to the nucleus and bind to androgen response elements on DNA inducing a cassette of androgen stimulated genes that are important in cell growth and development. An unregulated growth stimulus to hepatocytes is the likely cause of nodular regeneration and hepatic tumors related to anabolic steroid use. The cause of cholestasis due to the C-17 substituted androgens is not well defined, but high doses cause a similar cholestasis in some animal models. The syndrome is similar to cholestasis of pregnancy and the jaundice associated with high doses of estrogens or birth control pills and may be due to partial lack or variant of bile salt transporter proteins, as reported in some patients with androgenic anabolic steroid associated cholestasis.
Outcome and Management
The severity of liver injury due to anabolic steroids ranges from minor, transient serum enzyme elevations to profound and prolonged cholestasis, as well as hepatic peliosis and benign and malignant liver tumors. The first priority in management should be stopping the androgenic steroid. Unfortunately, athletes and body builders may resist this recommendation. Merely decreasing the dose of androgenic steroid or switching to another formulation is not appropriate and should be specifically discouraged. Patients being treated for hypogonadism may be switched to an unmodified form of testosterone, given by injection or cutaneous patch. Patients with marked cholestasis may be benefitted by symptomatic therapy of pruritus and fat soluble vitamin supplementation. Ursodiol is often used in drug induced cholestasis, but is efficacy has never been shown in a controlled prospective manner. Use of corticosteroids is usually ineffective and should be avoided. The syndrome is usually reversable with stopping therapy, but full recovery is often delayed. In addition, fatalities have been reported, usually due to marked cholestasis complicated by malnutrition, renal failure and associated opportunistic infections.
Representative androgenic steroids include the following: danazol, fluoxymesterone, methandienone, methenolone, methyltestosterone, nandrolone, norethandrolone, oxandrolone, oxymetholone, stanozolol, testosterone (cypionate, enanthate, propionate).
Drug Class: Androgenic Steroids
CASE REPORTS
Case 1. Cholestasis due to anabolic steroid use.(1)
A 24 year old body builder developed pruritus and jaundice having taken various anabolic steroids for one and a half years. He was also taking several herbal products and dietary supplements including Ma Huang (6% ephedrine), carnitine and chromium. He also drank alcohol, estimating his average intake as one case of beer per day for the last year. He developed dark urine and jaundice and stopped all medications and his alcohol intake promptly. Despite this, he remained jaundiced for a month and had worsening nausea and weight loss and eventually sought medical care. He had no history of liver disease or risk factors for viral hepatitis and took no other medications. On examination, he was muscular and physically fit but deeply jaundiced. He had an enlarged liver but no rash, fever or splenomegaly. Laboratory testing showed a total serum bilirubin of 53 mg/dL, but only modest elevations in serum aminotransferase and a normal alkaline phosphatase level (Table). His prothrombin time was normal. Tests for hepatitis A, B and C were negative. Abdominal ultrasound showed no evidence of biliary obstruction. Liver biopsy was not done. He was treated symptomatically for pruritus with antihistamines, cholestyramine and ursodiol. His jaundice gradually improved and pruritus waned. Six months after the onset of jaundice, he was asymptomatic, had regained most of his weight loss (40 pounds), serum bilirubin was 1.5 mg/dL and serum enzymes were normal.
Key Points
| Medication: | Anabolic steroids (nandrolone, stanozolol) |
|---|---|
| Pattern: | Bland cholestasis |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 16 months |
| Recovery: | 0.6 months |
| Other medications: | Various herbal products and dietary supplements |
Laboratory Values
| Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/L) | Comments | |
|---|---|---|---|---|---|
| Anabolic agent use for ~1.5 years | |||||
| 6 weeks | 237 | 129 | 21 | ||
| 8 weeks | 90 | 121 | 53 | ||
| 10 weeks | 203 | 91 | 51 | Ursodiol started | |
| 12 weeks | 119 | 81 | 22 | ||
| 14 weeks | 116 | 67 | 8 | ||
| 4 months | 58 | 50 | 4 | ||
| 5 months | 33 | 75 | 1.5 | Asymptomatic | |
| Normal Values | <56 | <139 | <1.2 | ||
Comment
A very typical case of severe cholestasis due to anabolic steroid use. Because the steroids were being used without medical supervision, the dose and actual duration of use of each preparation was unclear, but cholestasis usually arises within 4 to 12 weeks of starting a C-17 alkylated androgenic steroid. The jaundice can be severe and prolonged and accompanied by severe pruritus and marked weight loss. The serum enzymes are typically minimally elevated except for a short period immediately after stopping therapy. The pattern of enzyme elevations can be hepatocellular, cholestatic or mixed. Liver biopsy reveals a “bland” cholestasis with minimal inflammation and hepatocellular necrosis. Ma Huang has also been implicated in cases of drug induced liver injury, but is associated with an acute hepatocellular pattern of injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Danazol – Generic,
Fluoxymesterone – Androxy®,
Methandienone – Dianabol®
Methenolone – Primobolan®, Nibal®
Methyltestosterone – Generic, Android®, Methitest®, Testred®
Nandrolone – Generic, Deca-Durabolin®
Norethandrolone – Generic, Nilevar®, Pribabol®
Oxandrolone – Generic, Oxandrin®
Oxymetholone – Anadrol®
Stanozolol – Winstrol®
Testosterone – Generic, Depo-Testosterone®
DRUG CLASS
Androgenic Steroids
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
Danazol, Fluoxymesterone, Methandienone, Methenolone, Methyltestosterone, Nandrolone, Norethandrolone, Oxandrolone, Oxymetholone, Stanozolol, Testosterone
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Danazol | 17230-88-5 | C22-H27-N-O2 |
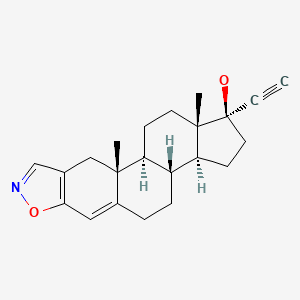
|
| Fluoxymesterone | 76-43-7 | C20-H29-F-O3 |
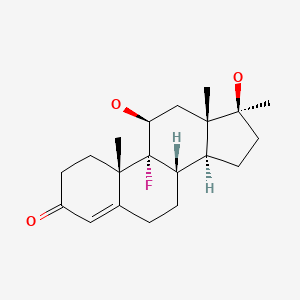
|
| Methandienone | 72-63-9 | C20-H28-O2 |
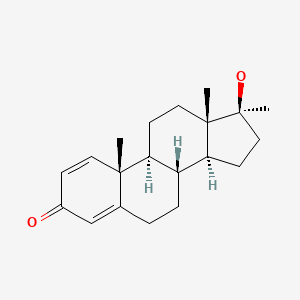
|
| Methenolone | 303-42-4 | C27-H42-O3 |
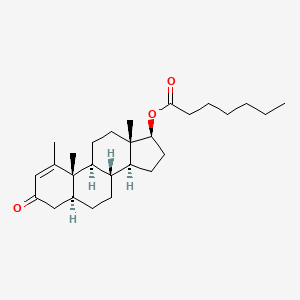
|
| Methyltestosterone | 58-18-4 | C20-H30-O2 |
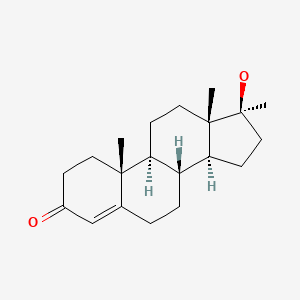
|
| Nandrolone | 360-70-3 | C28-H44-O3 |
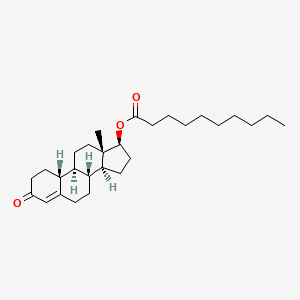
|
| Norethandrolone | 52-78-8 | C20-H30-O2 |
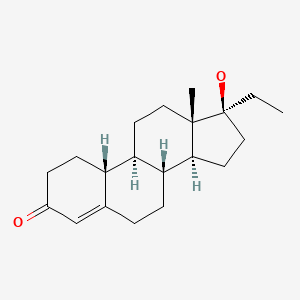
|
| Oxandrolone | 53-39-4 | C19-H30-O3 |
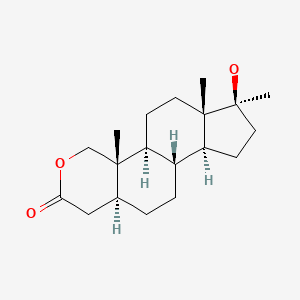
|
| Oxymetholone | 434-07-1 | C21-H32-O3 |
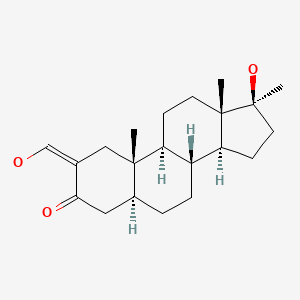
|
| Stanozolol | 10418-03-8 | C21-H32-N2-O |
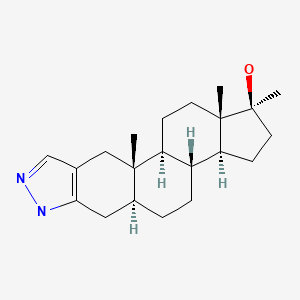
|
| Testosterone | 58-20-8 | C27-H40-O3 |
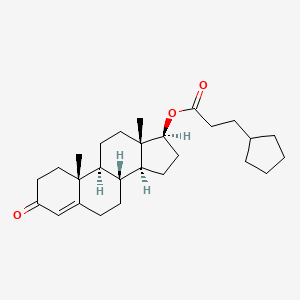
|
CITED REFERENCE
- 1.
- Singh C, Bishop P, Wilson R. Extreme hyperbilirubinemia associated with the use of anabolic steroids, health/nutritional supplements and ethanol: response to ursodeoxycholic acid treatment. Am J Gastroenterol. 1996;91:783–5. [PubMed: 8677950]
ANNOTATED BIBLIOGRAPHY
References updated: 30 May 2020
Abbreviations: HDS, herbal and dietary supplements; BSP, bromosulfophthalein.
- Zimmerman HJ. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 555-88.(Expert review of effects of androgenic steroids on the liver published in 1999; two forms of hepatic injury occur with anabolic steroids: cholestasis that occurs within the first 6 months associated with the synthetic agents that have an alkyl group in the C17 position, and a delayed injury with vascular or neoplastic changes in which natural androgens can play a role).
- Chitturi S, Farrell GC. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 605-20.(Review of hepatotoxicity of androgenic steroids including cholestasis, vascular disorders, benign tumors and hepatocellular carcinoma).
- Synder P. Androgens and the male reproductive tract. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 833-43.(Textbook of pharmacology and therapeutics).
- Werner SC, Hanger FM, Kritzler RA. Jaundice during methyltestosterone therapy. Am J Med. 1950;8:325–31. [PubMed: 15404975](7 patients: 6 men and 1 woman who developed jaundice during oral methyltestosterone therapy, ages 17-67 years, onset after 8 days to 4 months, prodrome of nausea and malaise for 1-2 weeks before jaundice [peak bilirubin 5.1-29 mg/dL, Alk P 1.4 to 4.5 times ULN, albumin normal], lasted 1-3 months; biopsy showed bile stasis with little inflammation; recovery complete; no recurrence upon rechallenge in 3 patients).
- Brick IB, Kyle LH. Jaundice of hepatic origin during the course of methyltestosterone therapy. N Engl J Med. 1952;246:176–9. [PubMed: 14890832](Two men, ages 44 and 63 years, developed jaundice 7 and 12 weeks after switching from long term testosterone injections to sublingual methyltestosterone [bilirubin not given and 6.8 mg/dL, Alk P 4-5 times ULN], with full but slow recovery after stopping).
- Burger RA, Marcuse PM. Peliosis hepatis: report of a case. Am J Clin Pathol. 1952;22:569–73. [PubMed: 14933333](39 year old woman with metastatic colon cancer and emaciation treated with oral testosterone was found to have peliosis hepatis on autopsy, without endothelial lining and with hepatic necrosis).
- Lloyd-Thomas HG, Sherlock S. Testosterone therapy for the pruritus of obstructive jaundice. Br Med J. 1952;2:1289–91. [PMC free article: PMC2022428] [PubMed: 12997743](Experience in treating 7 patients having intractable itching due to liver disease with methyltestosterone, which led to improvement in itching within 4-7 days but worsening jaundice, serum bilirubin rising by 2.0-9.3 mg/dL).
- Almaden PJ, Ross SW. Jaundice due to methyl testosterone therapy. Ann Intern Med. 1954;40:146–52. [PubMed: 13114814](45 year old woman developed jaundice 7 months after starting methyltestosterone [bilirubin 16.7 mg/dL Alk P slightly elevated], biopsy showing intrahepatic cholestasis and resolution of jaundice within 2 months of stopping).
- Kaplan AA. Jaundice due to methyltestosterone therapy. Gastroenterology. 1956;31:384–90. [PubMed: 13365845](58 year old man developed jaundice and pruritus 6 months after starting sublingual methyltestosterone [bilirubin 13.6 mg/dL, Alk P rising to twice normal], resolving 3 months after stopping).
- Koszalka MF. Medical obstructive jaundice: report of a death due to methyltestosterone. J Lancet. 1957;77:51–4. [PubMed: 13398705](60 year old man with colon cancer developed jaundice 3 weeks after starting methyltestosterone which was continued another 3 weeks [bilirubin 10 mg/dL, Alk P rising to twice normal], with progressive jaundice and death from hepatic failure 2 months after presentation, autopsy showing intrahepatic cholestasis).
- Peters JH, Randall AH Jr, Mendeloff J, Peace R, Coberly JC, Hurley MB. Jaundice during administration of methylestrenolone. J Clin Endocrinol Metab. 1958;18:114–5. [PubMed: 13491691](Two of 10 patients receiving 17-methyl-19-nortestosterone for cancer developed jaundice after 4 and 6 weeks of therapy; both died of underlying cancer while still jaundiced).
- Seelen JC. Complications during administration of methylestrenolone. J Clin Endocrinol Metab. 1958;18:1137–8. [PubMed: 13587633](Pregnant woman treated with methylestrenolone for habitual abortion developed jaundice 2 months later [bilirubin 19 mg/dL, Alk P 1.5 times ULN], resolving after stopping therapy).
- Kory RC, Bradley MH, Watson RN, Callahan R, Peters BJ. A six-month evaluation of an anabolic drug, norethandrolone, in underweight persons. II. Bromsulphalein (BSP) retention and liver function. Am J Med. 1959;26:243–8. [PubMed: 13617281](Prolonged BSP retention found in 74% of samples from 47 patients on norethandrolone for weight gain, returned to normal within a few weeks of stopping; only two patients had mild bilirubin elevations [1.7 and 2.2 mg/dL]).
- Schaffner F, Popper H, Chesrow E. Cholestasis produced by administration of norethandrolone. Am J Med. 1959;26:249–54. [PubMed: 13617282](Among 27 patients treated with norethandrolone for nutritional support for 3-5 weeks undergoing liver biopsy before and during therapy, 4 developed cholestasis within 1-5 weeks [bilirubin 32, 1.8, 1.0 and 0.7 mg/dL, AST 190-224 U/L, Alk P 2-20 times ULN], all resolving after stopping).
- Foss GL, Simpson SL. Oral methyltestosterone and jaundice. Br Med J. 1959;1:259–63. [PMC free article: PMC1992426] [PubMed: 13618612](Despite extensive use of methyltestosterone, authors found only one case of jaundice, 60 year old man developed jaundice after 5 weeks of therapy and subsequently died of dehydration and acidosis; summarizes 42 cases from literature; of 5 patients retreated, only one had recurrence).
- Shaw RK, Gold GL. Jaundice associated with norethandrolone(Nilevar) therapy. Ann Intern Med. 1960;52:428–34. [PubMed: 14445654](60 year old with multiple myeloma developed pruritus after 12 weeks and jaundice after 16 weeks of norethandrolone [bilirubin 9.5 mg/dL, AST 25 U/L, Alk P 3 times ULN], biopsy showed intrahepatic cholestasis, resolving on stopping and prednisone therapy).
- Wernze H. Dtsch med Wschr. 1960;85:2237–42. [Clinical investigation of the problem of liver damage by the newer androgenic and anabolic steroids] German. [PubMed: 13784491](Study of BSP retention in 36 volunteers given C-17 alkylated androgenic steroids; retention rose from <5% to 6-23% within 8-30 days and fell to normal or near normal with 8-10 days thereafter, whereas AST, Alk P and bilirubin levels changed minimally if at all).
- Kintzen W, Silny J. Peliosis hepatic after administration of fluoxymesterone. Can Med Assoc J. 1960;83:860–2. [PMC free article: PMC1938415] [PubMed: 13756172](60 year old woman with metastatic breast cancer treated with fluoxymesterone for 2 years was found to have peliosis hepatis on autopsy after death from widespread metastatic disease).
- Schaffner F, Popper H, Perez V. Changes in bile canaliculi produced by norethandrolone: electron microscopic study of human and rat liver. J Lab Clin Med. 1960;56:623–8. [PubMed: 13747218](4 patients treated with norethandrolone for 2 weeks underwent liver biopsy which showed “mild nonspecific changes” by light microscopy, but dilated bile canaliculi and shortening of microvilli by electron microscopy).
- Marquardt GH, Fisher CI, Levy P, Dowben RM. Effects of anabolic steroids on liver function tests and creatine excretion. JAMA. 1961;175:851–3. [PubMed: 13767058](Six anabolic steroids given to 38 healthy controls for 5 weeks; all led to increase in BSP retention [2.3-17.6%], but no change in bilirubin levels).
- Wynn V, Landon J, Kawarau E. Studies of hepatic function during methandienone therapy. Lancet. 1961;1:69–75. [PubMed: 13786996](Followed liver tests in 30 patients treated with methandienone; AST elevations occurred in 27% [60-180 U/L], Alk P [2 times ULN] and bilirubin [2.0] in 1, and BSP retention in 62%; all without symptoms and resolving rapidly, some without stopping drug).
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137–8. [PubMed: 13942388](35 year old woman developed jaundice and abdominal pain 2 years after starting norethindrone for dysmenorrhea [bilirubin 6.5 mg/dL], biopsy showing intrahepatic cholestasis and repeat biopsy 2 months after stopping estrogen showing resolution).
- Wilder EM. Death due to liver failure following the use of methandrostenolone. Can Med Assoc J. 1962;87:768–9. [PMC free article: PMC1849648] [PubMed: 14000685](71 year old woman developed jaundice 8 weeks after starting methandrostenolone for osteoporosis [bilirubin 20.4 mg/dL, AST 200 U/L, Alk P twice normal], with progressive mental obtundation, coagulopathy and death 2 months later, autopsy showing shrunken liver with marked cholestasis).
- Scherb J, Kirschner M, Arias IM. Studies of hepatic excretory function. The effect of 17α-ethyl-19-nortestosterone on sulfobromophthalein sodium (BSP) metabolism in man. J Clin Invest. 1963;42:404–8. [PMC free article: PMC289293] [PubMed: 13991599](Prospective study of BSP metabolism in 8 subjects treated with 19-nortestosterone and 10 controls; BSP transport maximum decreased within 7-10 days of starting androgen therapy and returned to normal 1 week afterwards, BSP storage was normal; best explained as a decrease in excretion of conjugated BSP [similar to defect in Dubin-Johnson syndrome).
- Gilbert EF, DaSilva AQ, Queen DM. Intrahepatic cholestasis with fatal termination following norethandrolone therapy. JAMA. 1963;185:538–9. [PubMed: 13947807](74 year old man developed jaundice 5 months after starting norethandrolone but continued for another month and developed delirium [bilirubin 20 mg/dL, AST 475 U/L, Alk P 3 times ULN], dying in hepatic coma; severe cholestasis on autopsy).
- Marquardt GH, Logan CE, Tomhave WG, Dowben RM. Failure of non-17-alkylated anabolic steroids to produce abnormal liver function tests. J Clin Endocrinol Metab. 1964;24:1334–6. [PubMed: 14243179](Prospective study of 23 patients given methenolone [non-C-17 substituted synthetic androgen] for weight gain found no change in BSP retention or serum bilirubin levels).
- Yanoff M, Rawson AJ. Peliosis hepatis: an anatomic study with demonstration of two varieties. Arch Pathol. 1964;77:159–65. [PubMed: 14088761](Two cases: 73 year old woman with septic arthritis treated with norethandrolone for 6 weeks and 42 year old man with malignant teratoma treated with chemotherapy for 8 months, both found to have peliosis on autopsy; two forms of peliosis, one with and one without endothelial lining, anabolic steroids associated with the latter parenchymal type that also shows bile retention and hepatocyte necrosis).
- DeLorimier AA, Gordan GS, Lowe RC, Carbone JV. Methyltestosterone, related steroids, and liver function. Arch Intern Med. 1965;116:289–94. [PubMed: 14315662](66 healthy controls were given 9 different 17α-alkylated androgenic steroids or testosterone for 2 weeks; no change in serum bilirubin or Alk P, but most resulted in increased BSP retention, not occurring with non-alkylated testosterone).
- Ticktin HE, Zimmerman HJ. Effects of synthetic anabolic agent on hepatic function. Am J Med Sci. 1966;251:674–84. [PubMed: 5946368](Prospective analysis of liver tests in 54 patients treated with norbolethone for at least 2 weeks; reversible minor AST elevations in 33% and BSP retention in 66% [both dose related], bilirubin >1.0 mg/dL in 10% and abnormal Alk P in none).
- Orlandi F, Jezequel AM. On the pathogenesis of the cholestasis induced by C-17 alkylated steroids: ultrastructure and functional changes of the liver cells during treatment. Rev Int Hepatol. 1966;16:331–3. [PubMed: 5947852]
- Glober GA, Wilkerson JA. Biliary cirrhosis following the administration of methyltestosterone. JAMA. 1968;204:170–3. [PubMed: 5694548](56 year old woman developed jaundice after taking a nutritional supplement with methyltestosterone and estrone for 4 years with prolonged jaundice [bilirubin 3.2 mg/dL, AST 7 UL, Alk P 54 KA U], evolving into cirrhosis over next 5 years with intractable pruritus and xanthomas; probable primary biliary cirrhosis).
- Androgens and anabolic steroids. Br Med J. 1969;2:165–7. [PMC free article: PMC1982939] [PubMed: 5778942](Review of clinical indications, efficacy and safety of androgenic steroids; mentions that 17α-alkylated androgens can cause jaundice).
- McGiven AR. Peliosis hepatis: case report and review of pathogenesis. J Pathol. 1970;101:283–5. [PubMed: 5483428](75 year old man with rheumatoid arthritis died of an acute myocardial infarction and was found to have peliosis hepatis on autopsy, having received methandienone for the previous year).
- Rozman C, Urbano A, Galera H. Minerva Med. 1971;62:2605–11. [Hepatotoxicity of anabolic steroids] [PubMed: 5566874]
- Sansoy OM, Roy AN, Shields LM. Anabolic action and side effects of oxandrolone in 34 mental patients. Geriatrics. 1971;26:139–43. [PubMed: 5549745](34 patients were treated with oxandrolone for 2 months to promote weight gain after illness; AST levels increased and were abnormal in 53%, BSP increased in 35%; no patient developed jaundice).
- Bernstein MS, Hunter RL, Yachnin S. Hepatoma and peliosis hepatis developing in a patient with Fanconi's anemia. N Engl J Med. 1971;284:1135–6. [PubMed: 4324228](20 year old with Fanconi anemia developed HCC 1 year after starting oxymetholone with peliosis and multiple intrahepatic hematomas found on autopsy).
- Johnson FL, Feagler JR, Lerner KG, Majerus PW, Siegel M, Hartmann JR, Thomas ED. Association of androgenic-anabolic steroid therapy with the development of hepatocellular carcinoma. Lancet. 1972;2:1273–6. [PubMed: 4117807](Description of 4 cases of hepatocellular carcinoma related to C17-alkylated anabolic steroids given for aplastic anemia or Fanconi syndrome, ages 5-27 years taking drugs for 1-7 years; regression of tumor in one with withdrawal; all died within one year).
- Guy JT, Auslander MO. Androgenic steroids and hepatocellular carcinoma. Lancet. 1973;1:148. [PubMed: 4118484](38 year old man with Fanconi’s syndrome had hepatocellular carcinoma on autopsy with mildly abnormal liver tests; had received androgenic steroids for 4 months at age 14).
- Henderson JT, Richmond J, Sumerling MD. Androgenic-anabolic steroid therapy and hepatocellular cancer. Lancet. 1973;1:934. [PubMed: 4123858](12 year old male with aplastic anemia developed hepatocellular carcinoma having received 8 years of intermittent anabolic steroid therapy and many transfusions).
- Liver tumours and steroid hormones. Lancet. 1973;2:1481–2. Editorial. [PubMed: 4129317](Editorial discussing the development of cholestasis, peliosis, adenoma and hepatocellular carcinoma after anabolic and contraceptive steroids).
- Jackson ST, Rallison ML, Buntin WH, Johnson SB, Flynn RR. Use of oxandrolone for growth stimulation in children. Am J Dis Child. 1973;126:481–4. [PubMed: 4361338](9 children with constitutional growth retardation treated with oxandrolone for 2 6-month periods with careful monitoring; no clinical or laboratory evidence of liver injury; one patient had increase in BSP retention).
- Presant CA, Safdar SH. Oxymetholone in myelofibrosis and chronic lymphocytic leukemia. Arch Intern Med. 1973;132:175–8. [PubMed: 4719544](Among 9 patients with refractory anemia, oxymetholone decreased transfusion requirements; 4 developed hepatotoxicity after 3-6 months, mild jaundice in 1 [bilirubin 3.1 mg/dL], mild Alk P or AST elevations in others, most resolved despite continuing medication).
- Ziegenfuss J, Carabasi R. Androgens and hepatocellular carcinoma. Lancet. 1973;1:262. [PubMed: 4119402](68 year old man treated with methyltestosterone for 30 years for impotence presented with abdominal pain and hepatocellular carcinoma which was “almost completely” resected).
- Frasier SD. Androgens and athletes. Am J Dis Child. 1973;125:479–80. [PubMed: 4699885](Editorial criticizing use of anabolic steroids to improve athletic performance).
- Naeim F, Copper PH, Seminion AA. Peliosis hepatis: possible etiologic role of anabolic steroids. Arch Pathol. 1973;95:284–5. [PubMed: 4348726](Two cases of peliosis found on autopsy; a child with Fanconi’s anemia who had developed jaundice after a short course of fluoxymesterone; a 65 year old woman on hormone replacement therapy for 18 months who died of congestive heart failure).
- Bagheri SA, Boyer JL. Peliosis hepatis associated with androgenic-anabolic steroid therapy. A severe form of hepatic injury. Ann Intern Med. 1974;81:610–8. [PubMed: 4423214](Seven patients on anabolic steroids for 2 to 27 months developed peliosis, 2 developing hemorrhage, 3 hepatic failure and 2 renal failure; serum bilirubin levels as high as 34.6 mg/dL, Alk P 1-20 times elevated, AST 30 to 950 U/L).
- Groos G, Arnold OH, Brittinger G. Letter: Peliosis hepatis after long-term administration of oxymetholone. Lancet. 1974;1:874. [PubMed: 4132832](33 year old woman with aplastic anemia developed hepatomegaly [bilirubin 6.6 mg//dL, ALT 243 U/L, Alk P 229 U/L] 1.5 years after starting oxymetholone, liver size decreasing when drug was stopped).
- Cattan D, Vesin P, Wautier J, Kalifat R, Meignan S. Liver tumors and steroid hormones. Lancet. 1974;1:878. [PubMed: 4132843](7 year old girl with Fanconi’s anemia developed hepatocellular carcinoma but had never received androgenic steroids, instead had transfusion-attributed hemosiderosis and cirrhosis).
- Meadows AT, Naiman JL, Valdes-Dapena MV. Hepatoma associated with androgen therapy for aplastic anemia. J Pediatr. 1974;84:109–10. [PubMed: 12119927](6 year old girl with aplastic anemia on androgens for 3.5 years died of intracranial hemorrhage and was found to have hepatocellular carcinoma on autopsy; the liver was enlarged but liver tests were all normal).
- Mulvihill JJ, Ridolfi RL, Schultz FR, Borzy MS, Haughton PB. Hepatic adenoma in Fanconi anemia treated with oxymetholone. J Pediatr. 1975;87:122–4. [PubMed: 168333](12 year old boy with Fanconi’s anemia treated with oxymetholone developed jaundice [bilirubin 23 mg/dL and “liver function abnormalities”], improving when oxymetholone was stopped; on autopsy after intracerebral bleed found to have 9 cm hepatic adenoma “with distant metastases”).
- Sarna G, Tomasulo P, Lotz MJ, Bubinak JF, Shulman NR. Multiple neoplasms in two siblings with a variant form of Fanconi's anemia. Cancer. 1975;36:1029–33. [PubMed: 171048](Two brothers with Fanconi’s anemia on long term androgen therapy both developed malignancies, including leukemia, gingival squamous cell cancer, and hepatocellular carcinoma).
- Kühböck J, Radaszkiewicz T, Walek H. Med Klin. 1975;70:1602–7. [Peliosis hepatis, complicating treatment with anabolic steroids (author's transl)] German. [PubMed: 810650]
- Ball JH, Lowrie EG, Hampers CL, Merrill JP. Testosterone therapy in hemodialysis patients. Clin Nephrol. 1975;4:91–8. [PubMed: 1183099](30 patients on chronic hemodialysis were treated with testosterone injections for anemia; AST elevations occurred in both treated and untreated controls).
- Farrell GC, Joshua DE, Uren RF, Baird PJ, Perkins KW, Kronenberg H. Androgen-induced hepatoma. Lancet. 1975;1:430–2. [PubMed: 48615](Three cases of hepatocellular carcinoma in men on androgenic steroids, ages 28 to 40 years, on oxymetholone or methyltestosterone for 5-8 years, liver and tumor size decreasing on stopping androgenic steroids).
- Anthony PP. Hepatoma associated with androgenic steroids. Lancet. 1975;1:685–6. [PubMed: 47110](Letter in response to Farrell [1975] stressing the poor prognosis of hepatocellular carcinoma; median survival time being 1 month and raising a question of the accuracy of the diagnosis).
- Bakker K, Brouwers TM, Houthoff HJ, Postma A. Ned Tijdschr Geneeskd. 1976;120:2214–20. [Liver lesions due to long-term use of anabolic steroids and oral contraceptives] Dutch. [PubMed: 1012379]
- Kew MC, Van Coller B, Prowse CM, Skikne B, Wolfsdorf JI, Isdale J, Krawitz S, et al. Occurrence of primary hepatocellular cancer and peliosis hepatis after treatment with androgenic steroids. S Afr Med J. 1976;50:1233–7. [PubMed: 184552](3 cases of complications of androgenic steroids: 34 year old woman with Fanconi’s anemia developed hepatocellular carcinoma after 7 years of therapy with methyltestosterone and 2 children [1 girl and 1 boy] developed peliosis hepatis, 5 and 8 years after starting androgenic steroids, both died soon after diagnosis).
- Kessler E, Bar-Meir S, Pinkhas J. Harefuah. 1976;90:521–4. [Focal nodular hyperplasia and spontaneous hepatic rupture in aplastic anemia treated with oxymetholone] Hebrew. [PubMed: 964746]
- Boyer JL, Preisig R, Zbinden G, de Kretser DM, Wang C, Paulsen CA. Guidelines for assessment of potential hepatotoxic effects of synthetic androgens, anabolic agents and progestogens in their use in males as antifertility agents. Contraception. 1976;13:461–8. [PubMed: 767052](Recommendations for monitoring for hepatotoxicity in trials of anabolic steroids).
- Hast R, Skårberg KO, Engstedt L, Jameson S, Killander A, Lundh B, Reizenstein P, et al. Oxymetholone treatment in aregenerative anaemia. II. Remission and survival—a prospective study. Scand J Haematol. 1976;16:90–100. [PubMed: 1257703](53 patients with anemia due to bone marrow insufficiency were treated with oxymetholone for at least 6 months; 3 developed jaundice requiring discontinuation).
- Hervey GR, Hutchinson I, Knibbs AV, Burkinshaw L, Jones PR, Norgan NG, Levell MJ. "Anabolic" effects of methandienone in men undergoing athletic training. Lancet. 1976;2:699–702. [PubMed: 61389](Double-blind crossover study of 6 weeks of methandienone in 11 male athletes; no change in bilirubin, ALT, AST or Alk P levels during drug period).
- Lesna M, Spencer I, Walker W. Liver nodules and androgens. Lancet. 1976;1:1124. [PubMed: 57524](17 year old male with long standing aplastic anemia treated with oxymetholone developed sudden rupture of right lobe of liver and autopsy revealed multiple hepatic nodules and adenomas).
- Slater SD, Davidson JF, Patrick RS. Jaundice induced by stanozolol hypersensitivity. Postgrad Med J. 1976;52:229–32. [PMC free article: PMC2496182] [PubMed: 1273017](66 year old man developed jaundice 7 months after starting stanozolol [bilirubin 8.0 mg/dL, ALT 74 U/L, Alk P 3 times ULN], with biopsy showing cholestasis but conspicuous eosinophils suggesting “hypersensitivity” with slow resolution over ensuing 6 months).
- Sweeney EC, Evans DJ. Hepatic lesions in patients treated with synthetic anabolic steroids. J Clin Pathol. 1976;29:626–33. [PMC free article: PMC476130] [PubMed: 185239](Three cases of liver injury due to anabolic steroids; 8 year old boy with Fanconi’s syndrome developed hepatocellular carcinoma after 3 years of methyltestosterone therapy; 46 year old man with severe, ultimately fatal cholestasis [bilirubin 9.8 mg/dL, AST 102 U/L, Alk P 1510 U/L] 3 months after starting oxymetholone; 31 year old woman with aplastic anemia died of intracerebral bleeding after 3 months of oxymetholone therapy and had regenerative hepatic nodules on autopsy).
- Tso SC, Chan TK, Todd D. Aplastic anaemia: a study of prognosis and the effect of androgen therapy. Q J Med. 1977;46:513–29. [PubMed: 594300](129 cases of aplastic anemia from Hong Kong between 1955-74; 75 received androgen therapy, among whom 12 [16%] had ALT elevations, 7 [10%] jaundice and 2 died with persistent jaundice).
- Young GP, Bhathal PS, Sullivan JR, Wall AJ, Fone DJ, Hurley TH. Fatal hepatic coma complicating oxymetholone therapy in multiple myeloma. Aust N Z J Med. 1977;7:47–51. [PubMed: 266893](Two patients, 56 year old woman and 60 year old man developed severe jaundice 6 and 8 weeks after starting oxymetholone for multiple myeloma [bilirubin 11.7 and 21.9 mg/dL, AST normal, Alk P 286 and 700 U/L], with progressive jaundice, renal failure, hepatic encephalopathy and death within 4-6 weeks of presentation).
- Leong AS, Sage RE. Drug-induced hepatic injury. Aust N Z J Med. 1977;7:537–9. [PubMed: 272174](Letter in response to Young [1977] describing 76 year old woman who developed jaundice while receiving oxymetholone and was continued for 3 months [bilirubin 6.2 mg/dL, ALT 346 U/L, Alk P 116 U/L], followed by hepatic failure but biopsy showed marked cholestasis with little necrosis).
- Bhathal PS, Fone DJ, Hurley TH, Sullivan JR, Wall AJ, Young GP. Drug-induced hepatic injury. Aust N Z J Med. 1977;7:539–40. [PubMed: 272175](Reply to Leong and Sage [1977]; retrospective review of 27 patients with multiple myeloma treated with oxymetholone identified 9 [47%] who developed jaundice).
- Nadell J, Kosek J. Peliosis hepatis. Twelve cases associated with oral androgen therapy. Arch Pathol Lab Med. 1977;101:405–10. [PubMed: 577673](12 patients with peliosis who had received oral androgens for 3 to 24 months from a single institution; 3 died of hepatic failure related to peliosis, one had diagnosis by biopsy and peliosis resolved with stopping androgens, 8 others found incidentally on autopsy; also reviewed 42 cases of peliosis in English literature).
- Bird DR, Vowles KDJ. Liver damage from long-term methyltestosterone. Lancet. 1977;2:400–1. [PubMed: 70606](39 year old transsexual treated with methyltestosterone for 7 years developed sudden hepatic rupture due to peliosis hepatis).
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet. 1977;2:262–3. [PubMed: 69876](Among 60 patients on long term methyltestosterone, 32% had raised AST, 55% abnormal liver scan; liver biopsies in 11 showed sinusoidal dilatation in 9 and cholestasis in 3 [one with bilirubin of 1.7 mg/dL] and one developed adenoma).
- Mokrohisky ST, Ambruso DR, Hathaway WE. Fulminant hepatic neoplasia after androgen therapy. N Engl J Med. 1977;296:1411–2. [PubMed: 193011](6 year old girl with Fanconi’s anemia developed hepatomegaly 2 months after starting oxymetholone with hepatocellular carcinoma in a noncirrhotic liver, dying 5 weeks after diagnosis).
- Boyd PR, Mark GJ. Multiple hepatic adenomas and a hepatocellular carcinoma in a man on oral methyl testosterone for 11 years. Cancer. 1977;40:1765–70. [PubMed: 198106](29 year old man developed multiple hepatic adenomas and hepatocellular carcinoma 12 years after starting oral methyltestosterone for hypopituitarism).
- Sale GE, Lerner KG. Multiple tumors after androgen therapy. Arch Pathol Lab Med. 1977;101:600–3. [PubMed: 199136](37 year old man with aplastic anemia developed hepatocellular carcinoma and peliosis hepatis as well as pancreatic and renal tumors after 5 years of anabolic steroid therapy).
- Hernandez-Nieto L, Bruguera M, Bombi J, Camacho L, Rozman C. Benign liver-cell adenoma associated with long-term administration of an androgenic-anabolic steroid (methandienone). Cancer. 1977;40:1761–4. [PubMed: 198105](19 year old man presented with two hepatic adenomas, one of which ruptured after 3 years of methandienone therapy for paroxysmal nocturnal hemoglobinuria).
- Paradinas FJ, Bull TB, Westaby D, Murray-Lyon IM. Hyperplasia and prolapse of hepatocytes into hepatic veins during longterm methyltestosterone therapy: possible relationships of these changes to the development of peliosis hepatis and liver tumours. Histopathology. 1977;1:225–46. [PubMed: 615837](Liver histology from 11 patients treated with methyltestosterone for up to 3 years [ALT abnormal in 6 patients, range 53-246 U/L], found sinusoidal dilatation in most cases and “microcysts” with hyperplasia of hepatocytes that partially occluded the sinusoidal or hepatic vein lumens).
- Shapiro P, Ikeda RM, Ruebner BH, Connors MH, Halsted CC, Abildgaard CF. Multiple hepatic tumors and peliosis hepatis in Fanconi's anemia treated with androgens. Am J Dis Child. 1977;131:1104–6. [PubMed: 199056](13 year old boy with Fanconi’s anemia, who was treated with oxymetholone for 5-6 years, died of septicemia and was found to have hepatocellular carcinoma and peliosis hepatis on autopsy).
- Coombes GB, Reiser J, Paradinas FJ, Burn I. An androgen-associated hepatic adenoma in a trans-sexual. Br J Surg. 1978;65:869–70. [PubMed: 737424](27 year old woman transsexual treated with methyltestosterone for 3 years presented with abdominal pain and was found to have an hepatic adenoma with hemorrhage; no follow up provided).
- McDonald EC, Speicher CE. Peliosis hepatis associated with administration of oxymetholone. JAMA. 1978;240:243–4. [PubMed: 666906](26 year old man with Hodgkin’s disease developed jaundice 2 months after starting oxymetholone [bilirubin 6.0 mg/dL, AST ~50 U/L, Alk P ~350 U/L], progressing to liver decompensation and death from sepsis, autopsy showing peliosis hepatis).
- Taxy JB. Peliosis: a morphologic curiosity becomes an iatrogenic problem. Hum Pathol. 1978;9:331–40. [PubMed: 658966](Five cases of peliosis hepatis in patients [4 men and 1 woman, ages 35 to 71 years] on anabolic steroids for 3 months to 3 years for cancer or bone marrow disease, several with splenic involvement and one fatal).
- Leblay R, Brissot P, leCalve JL, Ferrand B. Nouv Presse Med. 1978;7:1026. [Peliosis hepatis after treatment with androgens. A recent case] [PubMed: 662612]
- Benjamin DR, Shunk B. A fatal case of peliosis of the liver and spleen. Am J Dis Child. 1978;132:207–8. [PubMed: 626188](15 year old male with aplastic anemia treated with oxymetholone for 5 years developed sudden intraperitoneal bleeding from splenic peliosis and a capsular tear).
- Stromeyer FW, Smith DH, Ishak KG. Anabolic steroid therapy and intrahepatic cholangiocarcinoma. Cancer. 1979;43:440–3. [PubMed: 217519](47 year old man developed an hepatic tumor and progressive hepatic failure 2 years after starting oxymetholone therapy for refractory anemia; autopsy showed cholangiocarcinoma).
- Arnold GL, Kaplan MM. Peliosis hepatis due to oxymetholone—a clinically benign disorder. Am J Gastroenterol. 1979;71:213–6. [PubMed: 433907](67 year old man with sideroblastic anemia treated with oxymetholone for 2 years developed jaundice [bilirubin 4.9 mg/dL, ALT 81 U/L, Alk P normal], biopsy showing cholestasis and peliosis, resolving within 2 months of stopping).
- Karasawa T, Shikata T, Smith RD. Peliosis hepatitis: report of nine cases. Acta Pathol Jpn. 1979;29:457–69. [PubMed: 582233]
- Falk H, Thomas LB, Popper H. Hepatic angiosarcoma associated with androgenic-anabolic steroids. Lancet. 1979;2:1120–3. [PubMed: 91848](Among 168 cases of angiosarcoma identified in a US retrospective study [1964-74], 4 had received anabolic steroids for 1-23 years).
- Scheuer A, Gerdes H, Lehmann FG. Dtsch Med Wochenschr. 1979;104:779–83. [Anabolic agents and liver neoplasms] German. [PubMed: 446297](Review of association of anabolic steroids and liver tumors; animal studies suggest that androgens promote liver tumor growth and in humans the association has been best shown in patients with a predisposition to liver cancer [Fanconi’s anemia and chronic hepatitis]).
- Treuner J, Niethammer D, Flach A, Fischbach H, Schenck W. Med Welt. 1980;31:952–5. [Hepatocellular carcinoma following oxymetholone treatment] [PubMed: 6252413](16 year old boy with aplastic anemia was treated with oxymetholone for 6 years and presented with hepatocellular carcinoma that could not be completely resected but that regressed with stopping androgens).
- Montgomery RR, Ducore JM, Githens JH, August CS, Johnson ML. Regression on oxymetholone-induced hepatic tumors after bone marrow transplantation in aplastic anemia. Transplantation. 1980;30:90–6. [PubMed: 7010713](13 year old boy with aplastic anemia responded to oxymetholone therapy but developed multiple hepatic tumors 3 years later; underwent bone marrow transplant when aplastic anemia relapsed and over next 3 years liver tumors completely regressed).
- Wright JE. Anabolic steroids and athletics. Exerc Sport Sci Rev. 1980;8:149–202. [PubMed: 7016547](Review of literature on effects of anabolic steroids on increasing athletic performance as well as side effects).
- Pearson K, Zimmerman HJ. Danazol and liver damage. Lancet. 1980;1:645–6. [PubMed: 6102641](Five patients, 4 women and 1 man, ages 13 to 34 years, developed abnormal liver tests 1-5 months after starting danazol for endometriosis or angioneurotic edema [ALT 61-126 U/L, AST 81-290 U/L], one had jaundice, but limited details provided).
- Saheb F. Absence of peliosis hepatis in patients receiving testosterone enanthate. Hepatogastroenterology. 1980;27:432–40. [PubMed: 7203375](Among 52 patients with renal failure who had received testosterone enanthate for anemia, none had peliosis or liver tumors on autopsy, suggesting that natural forms of testosterone do not lead to these complications).
- Wilson JD, Griffin JE. The use and misuse of androgens. Metabolism. 1980;29:1278–95. [PubMed: 7005620](Three types of modifications of testosterone molecule improve its duration of action [β-esterification], improve its oral bioavailability [17α-alkylation], or increase its activity [ring modification]; the 17α-alkylated modifications can cause liver disease).
- Pecking A, Lejolly JM, Najean Y. Nouv Rev Fr Hematol. 1980;22:257–65. [Hepatotoxicity of androgens in the course of therapy of aplastic anemia] [PubMed: 6782550](254 patients with aplastic anemia were treated with androgens and monitored for up to 8 years; 17% developed jaundice and another 18% had liver test abnormalities without jaundice; no severe liver injury or cirrhosis recorded but 2 developed hepatic adenomas [after 20-21 months]).
- Zevin D, Turani H, Cohen A, Levi J. Androgen-associated hepatoma in a hemodialysis patient. Nephron. 1981;29:274–6. [PubMed: 6275283](68 year old man on hemodialysis was treated with oxymetholone for 18 months and nandrolone for 18 months when he presented with large hepatic mass, dying two weeks later; hepatocellular carcinoma but no cirrhosis on autopsy).
- Cocks JR. Methyltestosterone-induced liver-cell tumours. Med J Aust. 1981;2:617–9. [PubMed: 6278274](51 year old man on methyltestosterone for 20 years because of infertility developed hepatic rupture due to hepatocellular carcinoma with regression of mass on stopping androgens).
- Ishak KG. Hepatic lesions caused by anabolic and contraceptive steroids. Semin Liver Dis. 1981;1:116–28. [PubMed: 6287645](Review of effects of male and female sex hormones on the liver including biochemical changes, subcellular alternations, cholestasis, vascular disorders, hyperplasia, neoplasia and miscellaneous).
- Stromeyer FW, Ishak KG. Nodular transformation (nodular “regenerative hyperplasia”) of the liver: a clinicopathologic study of 30 cases. Hum Pathol. 1981;12:60–71. [PubMed: 7203455](Analysis of 30 cases of nodular regenerative hyperplasia from the files of the Armed Forces Institute of Pathology; half men, ages 14 to 80 years, variable presentations, 15 died, some of hepatic failure; AST and Alk P mildly elevated; associated diseases included polycythemia vera, rheumatoid arthritis, lymphoproliferative disorders, renal transplantation and autoimmune diseases; associated medications included prednisone, azathioprine, OCCs, and immunosuppressive agents).
- Westaby D, Portmann B, Williams R. Androgen-related primary hepatic tumors in non-Fanconi patients. Cancer. 1983;51:1947–52. [PubMed: 6299502](Three men, ages 28 to 58 years, developed hepatocellular carcinoma after 10-17 years of methyltestosterone therapy for hypogonadism, with some degree of regression upon stopping anabolic steroid).
- Turani H, Levi J, Zevin D, Kessler E. Hepatic lesions in patients on anabolic androgenic therapy. Isr J Med Sci. 1983;19:332–7. [PubMed: 6853130](11 men on long term androgenic steroids had abnormal liver findings; 3 were found to have carcinoma [hepatocellular and cholangiocarcinoma] and 8 peliosis hepatis).
- Cáp J, Ondrus B, Danihel L. Bratisl Lek Listy. 1983;79:73–81. [Focal nodular hyperplasia of the liver and hepatocellular carcinoma of the liver in children with Fanconi's anemia after long-term treatment with androgens] Slovak. [PubMed: 6297692]
- Malt RA, Galdabini JJ, Jeppsson BW. Abnormal sex-steroid milieu in young adults with hepatocellular carcinoma. World J Surg. 1983;7:247–52. [PubMed: 6306934](Among 7 patients ages 20-30 years with hepatocellular carcinoma seen over 15 year period, 2 were men on methyltestosterone in whom the tumor regressed on stopping androgens, and they were alive 2 and 7 years later).
- Schmidt E, Deeg HJ, Storb R. Regression of androgen-related hepatic tumors in patients with Fanconi's anemia following marrow transplantation. Transplantation. 1984;37:452–5. [PubMed: 6375000](Two patients with Fanconi’s anemia [1 girl, 1 boy] developed liver disease and hepatic masses after being treated with oxymetholone for 4 and 11 years; after bone marrow transplantation the masses regressed as documented by serial imaging studies).
- Chandra RS, Kapur SP, Kelleher J Jr, Luban N, Patterson K. Benign hepatocellular tumors in the young. A clinicopathologic spectrum. Arch Pathol Lab Med. 1984;108:168–71. [PubMed: 6320760](Seven children with hepatocellular carcinoma without cirrhosis [some had fibrosis] included 4 with aplastic or Fanconi’s anemia who had received oxymetholone for 3 to 8 years; other 3 were found to have cancer incidentally on autopsy, two with thalassemia and one post-renal transplant).
- Peces R, Ablanedo P, Alvarez J. Peliosis hepatis after renal transplantation. Arch Intern Med. 1984;144:1505. [PubMed: 6375618](Patient on hemodialysis received androgenic steroids for 2 years developed peliosis hepatis with portal hypertension).
- Overly WL, Dankoff JA, Wang BK, Singh ND. Androgens and hepatocellular carcinoma in an athlete. Ann Intern Med. 1984;100:158–9. [PubMed: 6318626](26 year old on multiple anabolic steroids for body building for 4 years developed weakness and weight loss and found to have hepatocellular carcinoma).
- Lyon J, Bookstein JL, Cartwright CA, Romano A, Heeney DJ. Peliosis hepatis: diagnosis by magnification wedged hepatic venography. Radiology. 1984;150:647–9. [PubMed: 6695062](29 year old man with aplastic anemia on oxymetholone for 4 years developed abdominal pain [bilirubin 1.7 mg/dL, ALT 1000 U/L, Alk P 188 U/L], venogram showing changes of peliosis and liver biopsy showing diffuse peliosis and hemorrhage and small adenomas).
- Mays ET, Christopherson W. Hepatic tumors induced by sex steroids. Semin Liver Dis. 1984;4:147–57. [PubMed: 6087460](Review of experience with 201 benign tumors of liver in women, ages 14 to 57; 98 focal nodular hyperplasia, 2 adenoma and 23 hepatocellular carcinoma, 9 unclassified; oral contraceptive use in 82% regardless of type).
- Pelletier G, Frija J, Szekely AM, Clauvel JP. Adenoma of the liver in man. Gastroenterol Clin Biol. 1984;8:269–72. [PubMed: 6325285](26 year old man with solitary hepatic adenoma; review of literature found only 31 cases of adenomas in men, 8 of whom had received anabolic steroids).
- Zafrani ES, Casier A, Baudelot A-M, Feldmann G. Ultrastructural lesions of the liver in human peliosis: a report of 12 cases. Am J Pathol. 1984;114:349–59. [PMC free article: PMC1900425] [PubMed: 6696047](Electron microscopy of liver biopsies from 12 patients with peliosis, 5 with jaundice, 4 with portal hypertension, ALT elevated in 6, Alk P in 9 and bilirubin in 7: dilation of sinusoids and space of Disse; one case attributed to anabolic steroids).
- Goldman B. Liver carcinoma in an athlete taking anabolic steroids. J Am Osteopath Assoc. 1985;85:56. [PubMed: 2984149](37 year old male athlete taking oxymetholone for 5 years developed 4 pound “malignant tumor”).
- McCaughan GW, Bilous MJ, Gallagher ND. Long-term survival with tumor regression in androgen-induced liver tumors. Cancer. 1985;56:2622–6. [PubMed: 2996742](Two patients with hepatocellular carcinoma arising after 5 and 6 years of therapy with oxymetholone and methyltestosterone had tumor regression on stopping anabolic steroids and survival for more than 10 years).
- Lowdell CP, Murray-Lyon IM. Reversal of liver damage due to long term methyltestosterone and safety of non-17 alpha-alkylated androgens. Br Med J (Clin Res Ed). 1985;291:637. [PMC free article: PMC1417521] [PubMed: 3928062](1 to 6 year follow up of 42 patients with liver injury from methyltestosterone [Westaby 1977]; all recovered and those on sublingual testosterone had normal liver tests and liver scans).
- Nuzzo JL, Manz HJ, Maxted WC. Peliosis hepatis after long-term androgen therapy. Urology. 1985;25:518–9. [PubMed: 3992778](After 13 years of testosterone and 16 months of fluoxymesterone replacement therapy, patient presented with jaundice that resolved on reducing dose; on autopsy 5 years later, liver histology showed peliosis).
- Buamah PK. An apparent danazol induced primary hepatocellular carcinoma. J Surg Oncol. 1985;28:114–6. [PubMed: 2578590](49 year old woman treated with danazol for 2 years for endometriosis presented with jaundice and pain, laparotomy showing hepatocellular carcinoma in noncirrhotic liver).
- Carrasco D, Prieto M, Pallardo L, Moll JL, Cruz JM, Monoz C, Berenguer J. Multiple hepatic adenomas after long-term therapy with testosterone enanthate. J Hepatol. 1985;1:573–8. [PubMed: 2997324](32 year old man with renal transplant treated with testosterone enanthate for 12 years developed hepatic masses found to be adenomas).
- Nesher G, Dolberg L, Zimran A, Hershko A. Hepatosplenic peliosis after danazol and glucocorticoids for ITP. N Engl J Med. 1985;312:242–3. [PubMed: 4038421](74 year old woman with immune thrombocytopenia treated with danazol for 3 months was found to have peliosis hepatis during splenectomy).
- Nordsten M. Ugeskr Laeger. 1985;147:2615–6. [Hemangiosarcoma of the liver associated with administration of androgenic steroids] Danish. [PubMed: 4071695]
- Garrigues Gil V, Berenguer Lapuerta J, Ponce García J, Rayón Martín M. A non-C17 alkylated steroid and long-term cholestasis. Ann Intern Med. 1986;104:135–6. [PubMed: 3940497](27 year old woman developed jaundice 4 months after starting 19-norandrostenolone with prolonged jaundice [bilirubin 10.8 m/dL, ALT 310 U/L, Alk P 1560 U/L], lasting 14 months and complete resolution at 21 months).
- Serke S, Dienemann D, Speck B, Zimmermann R, Baer U, Huhn D. Hepatocellular carcinoma and focal nodular hyperplasia associated with norethandrolone-therapy: a case report. Blut. 1986;52:111–6. [PubMed: 3004620](33 year old woman with aplastic anemia treated with norethandrolone for 4 years presented with abdominal pain and hepatic masses, one being focal nodular hyperplasia and one hepatocellular carcinoma; no follow up given).
- Király CL. Androgen-anabolic steroid effects on serum and skin surface lipids, on red cells, and on liver enzymes. Int J Sports Med. 1988;9:249–52. [PubMed: 2972634]
- Boue F, Coffin B, Delfraissy JF. Danazol and cholestatic hepatitis. Ann Intern Med. 1986;105:139–40. [PubMed: 3717792](73 year old woman with thrombocytopenic purpura developed pruritus and abnormal liver tests 4 weeks after starting danazol but without jaundice [ALT 57 U/L, peak Alk P 619 U/L], pruritus persisted for 3 weeks, liver test abnormalities for 3 months).
- Lucey MR, Moseley RH. Severe cholestasis associated with methyltestosterone: a case report. Am J Gastroenterol. 1987;82:461–2. [PubMed: 3578226](62 year old man developed fatigue followed by jaundice 8 months after starting methyltestosterone [bilirubin 29 mg/dL, AST 243 I/L, Alk P 290 U/L], resolving within 2 months of stopping).
- Ishak KG, Zimmerman HJ. Hepatotoxic effects of the anabolic/androgenic steroids. Semin Liver Dis. 1987;7:230–6. [PubMed: 3317860](Thorough review of the hepatic complications of androgenic steroid use, including cholestasis, peliosis, hyperplastic nodules, nodular regeneration, adenomas, hepatocellular carcinoma and other hepatic malignancies).
- Evely RS, Triger DR, Milnes JP, Low-Beer TS, Williams R. Severe cholestasis associated with stanozolol. Br Med J (Clin Res Ed). 1987;294:612–3. [PMC free article: PMC1245653] [PubMed: 3103828](Three patients, ages 62 to 65 years, developed jaundice and pruritus 1-5 months after starting stanozolol [bilirubin 38.6-46.7 mg/dL, AST 56-69 U/L, Alk P 284-732 U/L], resolving 3-6 months after drug withdrawal).
- Oda K, Oguma N, Kawano M, Kimura A, Kuramoto A, Tokumo K. Hepatocellular carcinoma associated with long-term anabolic steroid therapy in two patients with aplastic anemia. Nippon Ketsueki Gakkai Zasshi. 1987;50:29–36. [PubMed: 3035855](Two patients with aplastic anemia treated with oxymetholone for 12-15 years developed multiple liver tumors and had hepatocellular carcinoma without cirrhosis on autopsy).
- Király CL. Androgenic-anabolic steroid effects on serum and skin surface lipids, on red cells, and on liver enzymes. Int J Sports Med. 1988;9:249–52. [PubMed: 2972634](Prospective study of 7 power athletes taking multiple anabolic steroids during 6 weeks of training; ALT levels rose [~28 to 52 U/L] and HDL cholesterol levels decreased).
- Creagh TM, Rubin A, Evans DJ. Hepatic tumors induced by anabolic steroids in an athlete. J Clin Pathol. 1988;41:441–3. [PMC free article: PMC1141472] [PubMed: 2835401](27 year old male body builder on anabolic steroids for 3 years presented with rupture of large hepatocellular carcinoma).
- Lenders JW, Demacker PN, Vos JA, Jansen AJ, Hoitsma AJ. van ‘t Laar, Thien T. Deleterious effects of anabolic steroids on serum lipoproteins, blood pressure, liver function in amateur body builders. Int J Sports Med. 1988;9:19–23. [PubMed: 3366514](Among 58 body builders, ALT and AST levels were higher among those currently on anabolic steroids [mean ALT=57 U/L] compared to previous users [36 U/L] and never users [19 U/L], and levels rose during a course of anabolic steroids [29 to 67 U/L]).
- Veneri RJ, Gordon SC. Anabolic steroid-induced cholestasis: choleretic response to corticosteroids. J Clin Gastroenterol. 1988;10:467–8. [PubMed: 3418094](29 year old male body builder with a history of pruritus and jaundice after starting anabolic steroids developed prolonged pruritus and jaundice 4 months after a one month course of testosterone enanthate injections [bilirubin 16.4 mg/dL, ALT 47 U/L, Alk P 205 U/L], with apparent response to prednisone therapy).
- Middleton C, McCaughan GW, Painter DM, Stephen MS, Beale M, Fraser I. Danazol and hepatic neoplasia: a case report. Aust N Z J Med. 1989;19:733–5. [PubMed: 2561047](35 year old woman presented with a large hepatocellular carcinoma after several years of intermittent danazol therapy for endometriosis with improvement on stopping danazol and resection).
- Winwood PJ, Robertson DA, Wright R. Bleeding oesophageal varices associated with anabolic steroid use in an athlete. Postgrad Med J. 1990;66:864–5. [PMC free article: PMC2429716] [PubMed: 2099434](30 year old male body builder on anabolic steroids for 18 months developed variceal hemorrhage; liver biopsy was read as normal and varices were no longer detected after steroids were stopped).
- Gleeson D, Newbould MJ, Taylor P, McMahon RF, Leahy BC, Warnes TW. Androgen associated hepatocellular carcinoma with an aggressive course. Gut. 1991;32:1084–6. [PMC free article: PMC1379057] [PubMed: 1655591](48 year old man on methyltestosterone for primary hypogonadism for 24 years presented with abdominal pain and hepatocellular carcinoma; withdrawal of androgens led to regression, but 2 years later he had progressive metastatic disease and died; he was also anti-HCV positive).
- Kuipers H, Wijnen J, Hartgen F, Willems S. Influence of anabolic steroids on body composition, blood pressure, lipid profile and liver function in body builders. Int J Sports Med. 1991;12:413–8. [PubMed: 1917227](Prospective study of testosterone vs nandrolone vs placebo intramuscular injections for 8 weeks in 50 male and female body builders; androgens led to increase in muscle mass, but also a 25% decrease in HDL cholesterol and increase in diastolic blood pressure; but “all enzyme values remained within the normal range”).
- Søe KL, Søe M, Gluud C. Liver pathology associated with the use of anabolic-androgenic steroids. Liver. 1992;12:73–9. [PubMed: 1535676](Review of adverse effects of anabolic steroids on the liver with a focus on histologic changes).
- Touraine RL, Bertrand Y, Foray P, Gilly J, Philippe N. Hepatic tumours during androgen therapy in Fanconi anaemia. Eur J Pediatr. 1993;152:691–3. [PubMed: 8404976](12 year old boy with Fanconi’s anemia developed jaundice after 4.5 years of norethandrolone therapy with three hepatic masses, 4, 8 and 12 cm in size and diagnosed as hepatic adenomas; there was partial resolution on stopping androgen therapy as shown by 50% shrinkage after 16 months).
- Cabasso A. Peliosis hepatis in a young adult bodybuilder. Med Sci Sports Exerc. 1994;26:2–4. [PubMed: 8133732](27 year old male body builder taking various anabolic steroids for 8 years presented with abdominal pain and hepatic mass compatible with peliosis by CT scan, which regressed on stopping androgens).
- Gurakar A, Caraceni P, Fagiuoli S, Van Thiel DH. Androgenic/anabolic steroid-induced intrahepatic cholestasis: a review with four additional case reports. J Okla State Med Assoc. 1994;87:399–404. [PubMed: 7996313](4 cases of jaundice due to anabolic steroid use; all men, ages 63, 33, 53 and 26 years, taking anabolic steroids [methyltestosterone, stanozolol or oral testosterone], for 1-5 months, developed jaundice [bilirubin 13.2, 44.3, 47.8, and 19.5 mg/dL, ALT 52, 12, 27 and 33 U/L, Alk P 337, 189, 294, and 137 U/L], with slow resolution 5-12 months after stopping androgens).
- Wood P, Yin JA. Oxymetholone hepatotoxicity enhanced by concomitant use of cyclosporin A in a bone marrow transplant patient. Clin Lab Haematol. 1994;16:201–4. [PubMed: 7955931](16 year old boy with acute leukemia and bone marrow transplant developed jaundice 2 months after starting oxymetholone [bilirubin 4.0 mg/dL, ALT 488 U/L, Alk P 442 U/L], abnormalities persisting for 3 months after stopping).
- Yoshida EM, Karim MA, Shaikh JF, Soos JG, Erb SR. At what price, glory? Severe cholestasis and acute renal failure in an athlete abusing stanozolol. CMAJ. 1994;151:791–3. [PMC free article: PMC1337134] [PubMed: 8087755](26 year old male power lifter developed jaundice one month after starting stanozolol [bilirubin 19.6 rising to 51.2 mg/dL, AST 33 U/L, Alk P 137 U/L], complicated by acute renal failure, biopsy showing intrahepatic cholestasis, resolving slowly over the following 5 months).
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. [PubMed: 8637535](Controlled trial of testosterone enanthate vs placebo for 10 weeks in 43 normal men who were also randomized to exercise or not; testosterone increased muscle size and strength; no change in liver enzymes).
- Singh C, Bishop P, Wilson R. Extreme hyperbilirubinemia associated with the use of anabolic steroids, health/nutritional supplements and ethanol: response to ursodeoxycholic acid treatment. Am J Gastroenterol. 1996;91:783–5. [PubMed: 8677950](24 year old male body builder developed jaundice after 3 month use of several anabolic steroids [stanozolol and nandrolone] and Ma Huang [bilirubin 21 rising to 53 mg/dL, ALT 237 U/L, Alk P 129 U/L], with prolonged jaundice and pruritus resolving 5 months after onset: Case 1).
- Staub PG, Leibowitz CB. Peliosis hepatis associated with oral contraceptive use. Australas Radiol. 1996;40:172–4. [PubMed: 8687355](35 year old woman on oral contraceptives for several years developed abdominal pain and abnormal imaging studies with multiple lesions of varying size [2-5 cm], biopsy showing peliosis with decrease in size of lesions 6 months after stopping).
- Kosaka A, Takahashi H, Yajima Y, Tanaka M, Okamura K, Mizumoto R, Katsuta K. Hepatocellular carcinoma associated with anabolic steroid therapy: report of a case and review of the Japanese literature. J Gastroenterol. 1996;31:450–4. [PubMed: 8726841](35 year old woman with aplastic anemia developed hepatocellular carcinoma 3 years after starting oxymetholone therapy, with long term survival after resection and stopping androgens; review of 12 cases reported from Japan).
- Mörk H, al-Taie O, Klinge O, Scheurlen M. Z Gastroenterol. 1997;35:1087–91. [Successful therapy of persistent androgen-induced cholestasis with ursodeoxycholic acid] German. [PubMed: 9487641](55 year old man developed jaundice 12 months after starting methyltestosterone [bilirubin 5.1 mg/dL, ALT 177 U/L, Alk P normal]; when bilirubin rose to 8.0 mg/dL, ursodiol was started with resolution in 3 months, whereupon ursodiol was stopped without recurrence).
- Dourakis SP, Tolis G. Sex hormonal preparations and the liver. Eur J Contracept Reprod Health Care. 1998;3:7–16. [PubMed: 9678067](Review of sex hormones and liver injury; for estrogens complications include cholestasis, cholelithiasis, Budd-Chiari syndrome, peliosis, adenomas and focal nodular hyperplasia and hepatocellular carcinoma; androgenic steroids are not discussed).
- Schumacher J, Muller G, Klotz K-F. Large hepatic hematoma and intraabdominal hemorrhage associated with abuse of anabolic steroids. N Engl J Med. 1999;340:1123–4. [PubMed: 10206841](24 year old male body builder developed hepatic hemorrhage and rupture having used anabolic steroids for 2 years [bilirubin normal, ALT 927 U/L, Alk P normal], biopsy showing extensive hepatic necrosis without malignancy).
- Habscheid W, Abele U, Dahm HH. Dtsch Med Wochenschr. 1999;124:1029–32. [Severe cholestasis with kidney failure from anabolic steroids in a body builder] German. [PubMed: 10506840](28 year old male body builder developed jaundice 3 months after starting anabolic steroids [initial bilirubin 4.5 mg/dL, ALT 38 U/L, Alk P 94 U/L], with worsening jaundice and renal failure despite stopping androgens [peak bilirubin 77.9 mg/dL], jaundice lasting 4 months).
- Bork K, Pitton M, Harten P, Koch P. Hepatocellular adenomas in patients taking danazol for hereditary angio-oedema. Lancet. 1999;353:1066–7. [PubMed: 10199359](Among 87 patients with hereditary angioneurotic edema, 41 were treated with danazol, 11 for more than 10 years, 3 of whom developed hepatocellular adenomas at ages 29, 39 and 67 which were resected and did not show carcinoma).
- Bagia S, Hewitt PM, Morris DL. Anabolic steroid-induced hepatic adenomas with spontaneous haemorrhage in a bodybuilder. Aust N Z J Surg. 2000;70:686–7. [PubMed: 10976903](31 year old male body builder on long term anabolic steroids presented with hemorrhage into a hepatic adenoma ultimately requiring resection).
- Simon JA. Safety of estrogen/androgen regimens. J Reprod Med. 2001;46(3) Suppl:281–90. [PubMed: 11304876](Combinations of estrogen with low doses of methyltestosterone [1.25-2.5 mg] have been used to treat vasomotor symptoms in menopausal women with no serious adverse events, including no hepatic events or liver-enzyme elevations, in 641 women followed prospectively in controlled trials).
- Stimac D, Milić S, Dintinjana RD, Kovac D, Ristić S. Androgenic/Anabolic steroid-induced toxic hepatitis. J Clin Gastroenterol. 2002;35:350–2. [PubMed: 12352300](26 year old male body builder developed nausea and jaundice 5 weeks after starting anabolic steroids [bilirubin 27.5 mg/dL, ALT 10,580 U/L, Alk P 152 U/L] which he had taken intermittently for 9 years; gradual improvement over 3 months).
- Hengge UR, Stocks K, Wiehler H, Faulkner S, Esser S, Lorenz C, Jentzen W, et al. Double-blind, randomized, placebo-controlled phase III trial of oxymetholone for the treatment of HIV wasting. AIDS. 2003;17:699–710. [PubMed: 12646793](Controlled trial of 16 weeks of oxymetholone vs placebo is 89 HIV-infected patients; ALT levels rose above 5 times ULN in 31% of oxymetholone, but none of placebo treated patients; one developed jaundice and cholestasis).
- Tan RS, Salazar JA. Risks of testosterone replacement therapy in ageing men. Expert Opin Drug Saf. 2004;3:599–606. [PubMed: 15500418](Review of use of androgens in aging males for loss of libido and inability to focus mentally; “to our knowledge, there has been no reported hepatotoxicity from testosterone delivered via topical gel, transdermal patch, or by intramuscular injections”).
- Orr R, Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64:725–50. [PubMed: 15025546](Review of clinical efficacy and side effects of anabolic steroids and oxandrolone which is approved for use to promote weight gain and decrease catabolism after surgery or severe trauma).
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–54. [PubMed: 15248788](Review of effects and toxicity of anabolic steroids used to improve athletic performance; “Injectable testosterone cypionate and enanthate preparations do not appear to affect liver function enzymes”).
- Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol. 2004;77:257–67. [PubMed: 15495253](Systematic review of published literature on association of androgen therapy and liver tumors identified 133 cases, 36 in patients with Fanconi’s anemia, others with aplastic anemia, endocrine and gynecological disorders and body builders; most due to oxymetholone, methyltestosterone and danazol, but 6 associated with intramuscular testosterone injections; hepatocellular carcinoma 53%, adenomas 37%, others included cholangiocarcinoma and angiosarcoma).
- Maravelias C, Dona A, Stefanidou M, Spiliopoulou C. Adverse effects of anabolic steroids in athletes. A constant threat. Toxicol Lett. 2005;158:167–75. [PubMed: 16005168](Review of adverse effects of anabolic steroids in athletes; hepatic effects include serum enzyme elevations, cholestatic jaundice, hepatic tumors and peliosis hepatis).
- Socas L, Zumbado M, Pérez-Luzardo O, Ramos A, Pérez C, Hernández J R, Boada L D. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39:e27. [PMC free article: PMC1725213] [PubMed: 15849280](Two cases of hepatic adenomas in male body builders abusing anabolic steroids; ages 35 and 23 years old on anabolic steroids for 6 months and 15 years presented with hepatic masses which appeared to be benign and regressed on stopping anabolic steroids).
- Capra F, Nicolini N, Morana G, Guglielmi A, Capelli P, Vantini I. Vanishing bile duct syndrome and inflammatory pseudotumor associated with a case of anabolic steroid abuse. Dig Dis Sci. 2005;50:1535–7. [PubMed: 16110850](32 year old treated for 3 years with testosterone undecanoate for hypogonadism developed jaundice [bilirubin 37 mg/dL, ALT 182 U/L, Alk P 212 U/L], with inflammatory pseudo tumor and biopsy showing paucity of bile ducts, although patient ultimately recovered).
- Clark BM, Schofield RS. Dilated cardiomyopathy and acute liver injury associated with combined use of ephedra, gamma-hydroxybutyrate, and anabolic steroids. Pharmacotherapy. 2005;25:756–61. [PubMed: 15899737](40 year old man developed shortness of breath and jaundice starting 2 months after initiating therapy with androgens and ephedra to promote muscle development [bilirubin 3.9 mg/dL, ALT 2173 U/L, Alk P normal], requiring therapy for congestive cardiomyopathy, but ultimately recovering over next 18 months).
- Jasiurkowski B, Raj J, Wisinger D, Carlson R, Zou L, Nadir A. Cholestatic jaundice and IgA nephropathy induced by OTC muscle building agent Superdrol. Am J Gastroenterol. 2006;101:2659–62. [PubMed: 16952289](23 year old body builder developed abdominal pain and jaundice two months after starting an over-the-counter supplement called “Superdrol” [containing methasteron] [bilirubin 36.2 mg/dL, ALT 93 U/L, Alk P 224 U/L], with protracted jaundice and itching and biopsy showing marked cholestasis with minimal inflammation).
- Kafrouni MI, Anders RA, Verma S. Hepatotoxicity associated with dietary supplements containing anabolic steroids. Clin Gastroenterol Hepatol. 2007;5:809–12. [PubMed: 17509944](Two cases; 31 and 40 year old body builders developed prolonged jaundice and pruritus beginning 4 and 6 weeks after starting “Superdrol” [bilirubin 37.5 and 49.7 mg/dL, ALT 59 and 301 U/L, Alk P 375 and 416 U/L], with slow recovery over ensuing 2-3 months).
- McCullough MC, Namias N, Schulman C, Gomez E, Manning R, Goldberg S, Pizano L, et al. Incidence of hepatic dysfunction is equivalent in burn patients receiving oxandrolone and controls. J Burn Care Res. 2007;28:412–20. [PubMed: 17438485](Retrospective analysis of liver test results during oxandrolone therapy of patients with major burns; some evidence of hepatic dysfunction found in 43% of oxandrolone and 43% of 61 control subjects).
- Patil JJ, O'Donohoe B, Loyden CF, Shanahan D. Near-fatal spontaneous hepatic rupture associated with anabolic androgenic steroid use: a case report. Br J Sports Med. 2007;41:462–3. [PMC free article: PMC2465371] [PubMed: 17224443](43 year old professional body builder developed abdominal pain and hepatic rupture having used anabolic steroids for 25 years; cause of rupture not explained [peliosis vs adenoma vs carcinoma]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, et al. Drug Induced Liver Injury Network(DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, at least 6 were due to anabolic steroids being used for body building or for improving athletic performance, but none were attributed to physician-prescribed androgenic steroids).
- Sánchez-Osorio M, Duarte-Rojo A, Martínez-Benítez B, Torre A, Uribe M. Anabolic-androgenic steroids and liver injury. Liver Int. 2008;28:278–82. [PubMed: 17900246](29 year old man developed progressive fatigue, nausea and then jaundice and pruritis 2-3 months after starting high dose anabolic steroids [bilirubin 29 mg/dL, ALT 54 U/L, Alk P 694 U/L], jaundice persisting for 2 months, but resolving completely by 5 months after stopping).
- Gorayski P, Thompson CH, Subhash HS, Thomas AC. Hepatocellular carcinoma associated with recreational anabolic steroid use. Br J Sports Med. 2008;42:74–5. [PubMed: 18178686](35 year old male body builder presented with large hepatocellular carcinoma having used anabolic steroids for several years [bilirubin 0.6 mg/dL, ALT 364 U/L, Alk P 70 U/L, alpha-fetoprotein normal], no follow up provided).
- Martin NM, Abu Dayyeh BK, Chung RT. Anabolic steroid abuse causing recurrent hepatic adenomas and hemorrhage. World J Gastroenterol. 2008;14:4573–5. [PMC free article: PMC2731289] [PubMed: 18680242](27 year old male body builder having used anabolic steroids for 5 years had spontaneous hepatic bleeding from 10 cm hepatic adenoma [bilirubin 2.2 mg/dL, ALT 2457 U/L, Alk P 275 U/L], treated with resection and had recurrence 3 years later having restarted anabolic steroid use).
- Shah NL, Zacharias I, Khettry U, Afdhal N, Gordon FD. Methasteron-associated cholestatic liver injury: clinicopathologic findings in 5 cases. Clin Gastroenterol Hepatol. 2008;6:255–8. [PubMed: 18187367](5 cases of jaundice due to methasteron use by male body builders, ages 20 to 33 years, with onset of symptoms 1-2 months after starting the nutritional supplement [initial bilirubin 7.6-41.8 mg/dL, ALT 59-151 U/L, Alk P 85-189 U/L], all resolving with stopping the 17-alkylated androgenic steroid).
- Singh V, Rudraraju M, Carey EJ, Byrne TJ, Vargas HE, Williams JE, Balan V, et al. Severe hepatotoxicity caused by a methasteron-containing performance-enhancing supplement. J Clin Gastroenterol. 2009;43:287. [PubMed: 18813027](3 men, ages 25, 33, and 51, developed jaundice and pruritus 1-2 months after starting "Superdrol", a form of methasteron [bilirubin 8.3, 17.3 and 22 mg/dL, ALT 69, 236 and 1025 U/L, Alk P 106, 111 and unknown U/L] associated with hepatic encephalopathy and peak INR 1.5, in one patient, but resolving spontaneously in all within 6-12 weeks of onset).
- Nasr J, Ahmad J. Severe cholestasis and renal failure associated with the use of the designer steroid Superdrol (methasteron): a case report and literature review. Dig Dis Sci. 2009;54:1144–6. [PubMed: 18720005](42 year old man developed jaundice and pruritus 7 weeks after starting "Superdrol" with methasteron [bilirubin 41.2 mg/dL, ALT 98 U/L, Alk P 353 U/L, creatinine 3.5 mg/dL], both renal and liver injury resolving slowly over the following 4 months).
- Choi SK, Jin JS, Cho SG, Choi SJ, Kim CS, Choe YM, Lee KY. Spontaneous liver rupture in a patient with peliosis hepatis: a case report. World J Gastroenterol. 2009;15:5493–7. [PMC free article: PMC2778108] [PubMed: 19916182](20 year old man with aplastic anemia treated with oxymetholone for 8 years developed spontaneous rupture of liver due to peliosis hepatis, responding to hemi-hepatectomy and stopping androgenic steroids).
- Bispo M, Valente A, Maldonado R, Palma R, Glória H, Nóbrega J, Alexandrino P. Anabolic steroid-induced cardiomyopathy underlying acute liver failure in a young bodybuilder. World J Gastroenterol. 2009;15:2920–2. [PMC free article: PMC2699014] [PubMed: 19533818](40 year old body builder developed fatigue followed by jaundice having used large doses of anabolic steroids for 10 years [bilirubin 6.8 mg/dL, ALT 7125 U/L, INR 3.3, LDH 7140 U/L], acute liver failure being due to ischemic hepatitis caused by cardiomyopathy, responding rapidly to treatment of right heart failure).
- Masumori N, Ikeda H, Endo T. Acute hepatitis induced by replacement oral testosterone product in a female-to-male patient with gender identity disorder. Int J Urol. 2009 May;16(5):530–1. [PubMed: 19467123](27 year old female-to-male transgender woman developed fatigue 3 months after switching from fluoxymesterone to methyltestosterone [both oral 17-alpha methylated androgenic steroids] [bilirubin 1.2 mg/dL, peak ALT 1418 U/L, Alk P not given], resolving within 6 weeks of stopping).
- Krishnan PV, Feng ZZ, Gordon SC. Prolonged intrahepatic cholestasis and renal failure secondary to anabolic androgenic steroid-enriched dietary supplements. J Clin Gastroenterol. 2009;43:672–5. [PubMed: 19238093](Three cases of anabolic steroid induced hepatotoxicity; 21, 30 and 38 year old men developed jaundice and pruritus 4 to 8 weeks after starting a nutritional supplement with an oral anabolic steroid [bilirubin 8.0-53 mg/dL, ALT 200-467 U/L, Alk P 104-494 U/L], with slow recovery after stopping).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were linked to anabolic steroid use).
- Woerdeman J, de Hon O, Levi M, de Ronde WP. Ned Tijdschr Geneeskd. 2010;154:A2004. [Anabolic androgenic steroids in amateur sports in the Netherlands] Dutch. [PubMed: 20858322](Review of anabolic steroid use in the Netherlands and their complications including cholestasis).
- Wingert N, Tavakoli H, Yoder E. Acute hepatitis and personality change in a 31-year-old man taking prohormone supplement SUS500. Psychosomatics. 2010;51:340–4. [PubMed: 20587764](31 year man developed fatigue and personality change having used an prohormone nutritional supplement for one year [ALT 1754 U/L, bilirubin and Alk P not given], resolving rapidly after stopping the supplement).
- van Amsterdam J, Opperhuizen A, Hartgens F. Adverse health effects of anabolic-androgenic steroids. Regul Toxicol Pharmacol. 2010;57:117–23. [PubMed: 20153798](Review of the health consequences of anabolic steroid use including cholestasis, hepatic adenoma and peliosis).
- Büttner A, Thieme D. Side effects of anabolic androgenic steroids: pathological findings and structure-activity relationships. Handb Exp Pharmacol. 2010;(195):459–84. [PubMed: 20020376](Review of the side effects of anabolic steroids based upon structure-activity relationships).
- Tornai I. Orv Hetil. 2010;151:1132–6. [Role of environmental factors in the etiology of hepatocellular carcinoma] [PubMed: 20570793](Review of risk factors for hepatocellular carcinoma mentions that oral contraceptives may play a role in some cases).
- Schwingel PA, Cotrim HP, Salles BR, Almeida CE, dos Santos CR Jr, Nachef B, Andrade AR, et al. Anabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver disease. Liver Int. 2011;31:348–53. [PubMed: 21040407](Among 180 recreational body builders, mean aminotransferase levels were higher [ALT: 43 vs 31 U/L and AST: 55 vs 32 U/L] and more likely to be elevated [33% vs 7%] in those who admitted to use of injectable anabolic steroids, who were also more likely to have steatosis by ultrasound evaluation).
- Machado MV, Cortez-Pinto H. The dark side of sports: using steroids may harm your liver. Liver Int. 2011;31:280–1. [PubMed: 21281427](Editorial in response to Schwingel [2011]).
- Yurci A, Yucesoy M, Unluhizarci K, Torun E, Gursoy S, Baskol M, Guven K, Ozbakir O. Effects of testosterone gel treatment in hypogonadal men with liver cirrhosis. Clin Res Hepatol Gastroenterol. 2011;35:845–54. [PubMed: 22074639](Among 10 hypogonadal men with cirrhosis treated with transdermal testosterone, there was no change in ALT or bilirubin and none developed clinically apparent liver injury).
- Rosenfeld GA, Chang A, Poulin M, Kwan P, Yoshida E. Cholestatic jaundice, acute kidney injury and acute pancreatitis secondary to the recreational use of methandrostenolone: a case report. J Med Case Rep. 2011;5:138. [PMC free article: PMC3079674] [PubMed: 21470406](50 year old man with chronic hepatitis C developed anorexia, weight loss and jaundice 8 weeks after starting methandrostenolone [bilirubin 53.9 mg/dL, ALT 56 U/L, Alk P 154 U/L] accompanied by mild pancreatitis and renal insufficiency, resolving slowly after stopping anabolic steroids).
- Neri M, Bello S, Bonsignore A, Cantatore S, Riezzo I, Turillazzi E, Fineschi V. Anabolic androgenic steroids abuse and liver toxicity. Mini Rev Med Chem. 2011;11:430–7. [PubMed: 21443508](Review of the hepatic complications of anabolic steroid use).
- Stickel F, Kessebohm K, Weimann R, Seitz HK. Review of liver injury associated with dietary supplements. Liver Int. 2011;31:595–605. [PubMed: 21457433](Review of current understanding of liver injury from herbals and dietary supplements focusing upon herbalife and hydroxycut products, green tea, usnic acid, Noni juice, Chinese herbs, vitamin A and anabolic steroids).
- Avelar-Escobar G, Méndez-Navarro J, Ortiz-Olvera NX, Castellanos G, Ramos R, Gallardo-Cabrera VE, Vargas-Alemán Jde J, et al. Hepatotoxicity associated with dietary energy supplements: use and abuse by young athletes. Ann Hepatol. 2012;11:564–9. [PubMed: 22700641](17 year old boy developed jaundice and pruritus 3 months after starting dietary supplements [N.O.Xplode, Growth Factor ATN and SimiCaritina] for body building [bilirubin 17.5 rising to 31.5 mg/dL, ALT 640 U/L, Alk P 850 U/L, GGT normal], with resolution within 6-8 weeks of stopping).
- Kou T, Watanabe M, Yazumi S. Hepatic failure during anabolic steroid therapy. Gastroenterology. 2012;143:e11–2. [PubMed: 23085354](78 year old man developed jaundice and fatigue 2 years after starting danazol for thrombocytopenic purpura [bilirubin 11.7 mg/dL, ALT 185 U/L, Alk P 677 U/L] and died 26 days later, autopsy showing peliosis hepatis).
- Timcheh-Hariri A, Balali-Mood M, Aryan E, Sadeghi M, Riahi-Zanjani B. Toxic hepatitis in a group of 20 male body-builders taking dietary supplements. Food Chem Toxicol. 2012;50:3826–32. [PubMed: 22809474](20 male body builders, ages 24 to 32 years, presented with jaundice and pruritus having been taking 3 supplements for a year [bilirubin 3.9-8.0 mg/dL, ALT 225-600 U/L, Alk P 325-701 U/L], the responsible agent causing the injury being unclear from the list of ingredients).
- Elsharkawy AM, McPherson S, Masson S, Burt AD, Dawson RT, Hudson M. Cholestasis secondary to anabolic steroid use in young men. BMJ. 2012;344:e468. [PubMed: 22302781](Two cases, 16 and 32 year old men developed jaundice and pruritus 5 days and 2 months after starting an anabolic steroid "methandrostenolone" [bilirubin 25.8 and 38.0 mg/dL, ALT 156 and 76 U/L, Alk P 203 and 262 U/L], requiring 4 months to resolve after stopping).
- Elsharkawy AM, McPherson S, Masson S, Burt A, Dawson RT, Hudson M. [Cholestasis in young men after taking anabolic steroids]. Praxis (Bern 1994) 2012; 101: 661-4. German. [PubMed: 22565557](Same cases as described by the authors in BMJ [2012]).
- Porro LJ, Herndon DN, Rodriguez NA, Jennings K, Klein GL, Mlcak RP, Meyer WJ, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;214:489–502. [PMC free article: PMC3412530] [PubMed: 22463890](Among children with severe burns, 70 received oxandrolone for one year and 152 served as standard of care controls; ALT levels were elevated to a similar degree in both groups during the acute period, returning to normal levels 3-6 months after the burns).
- Pais-Costa SR, Lima OA, Soares AF. Giant hepatic adenoma associated with anabolic-androgenic steroid abuse: case report. Arq Bras Cir Dig. 2012;25:180–2. [PubMed: 23411809](28 year old male body builder developed abdominal pain having been on anabolic steroids for 6 years, surgery revealing two hepatic adenomas, one being 10 cm in diameter).
- Vilella AL, Limsuwat C, Williams DR, Seifert CF. Cholestatic jaundice as a result of combination designer supplement ingestion. Ann Pharmacother. 2013;47:e33. [PubMed: 23757384](50 year old man developed jaundice and pruritus 2 months after starting body building supplements [bilirubin 29.4 mg/dL, ALT 125 U/L, Alk P 160 U/L], with slow recovery upon stopping).
- El Sherrif Y, Potts JR, Howard MR, Barnardo A, Cairns S, Knisely AS, Verma S. Hepatotoxicity from anabolic androgenic steroids marketed as dietary supplements: contribution from ATP8B1/ABCB11 mutations? Liver Int. 2013;33:1266–70. [PubMed: 23750872](Two men, ages 25 and 45 years, developed jaundice 3 and 6 weeks after starting an anabolic steroid "Mass-Drol" [bilirubin 10.8 and 6.0 mg/dL, ALT 207 and 152 U/L, Alk P 137 and 259 U/L, GGT 72 and 59 U/L = normal], sequencing of ABC B11 [the gene that is abnormal in FIC1] was normal).
- Hymel BM, Victor DW, Alvarez L, Shores NJ, Balart LA. Mastabol induced acute cholestasis: A case report. World J Hepatol. 2013;5:133–6. [PMC free article: PMC3612572] [PubMed: 23556046](26 year old man developed jaundice and pruritus 10 days after starting daily injections of the anabolic steroid "Mastabol" [peak bilirubin 23.6 mg/dL, ALT 117 U/L, Alk P 441 U/L], improving slowly on withdrawal).
- Archer JR, Dargan PI, Hudson S, Wood DM. Analysis of anonymous pooled urine from portable urinals in central London confirms the significant use of novel psychoactive substances. QJM. 2013;106:147–52. [PubMed: 23178933](Analysis of pooled urine samples collected from 12 public urinals in central London identified cocaine, cannabis and MDMA from 11 sites, amphetamines from 10 and anabolic steroids from 4).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to herbals and dietary supplements, including one each attributed to Dianabol [methandrostenolone], "Mega men heart" and "Serious Mass").
- Supasyndh O, Satirapoj B, Aramwit P, Viroonudomphol D, Chaiprasert A, Thanachatwej V, Vanichakarn S, et al. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:271–9. [PMC free article: PMC3562853] [PubMed: 23124786](Among 43 hemodialysis patients treated with oxymetholone or placebo for 24 weeks, fat free mass and handgrip strength increased with the androgenic steroid, but liver enzymes rose from a mean serum ALT of 15 to 70 U/L and direct bilirubin from 0.09 to 0.51 mg/dL and one patient stopped therapy early because of “cholestatic hepatitis”).
- Hoedebecke K, Rerucha C, Maxwell K, Butler J. Drug-induced liver injury secondary to testosterone prohormone dietary supplement use. J Spec Oper Med. 2013;13:1–5. [PubMed: 24227554](28 year old Special Services member developed pruritus and jaundice 4 weeks after starting a testosterone prohormone supplement [bilirubin 13.6 mg/dL rising to 27.2 ten days later, ALT 693 U/L, Alk P not given], with slow recovery).
- Ding NS, De Cruz P, Lim L, Thompson A, Desmond P. Androgenic-anabolic steroid drug-induced liver injury. Intern Med J. 2013;43:215–6. [PubMed: 23402488](46 year old man took two anabolic steroids for two months and developed jaundice several months later [bilirubin 17.8 mg/dL, ALT 125 U/L, Alk P 295 U/L, GGT 114 U/L], resolving on no therapy within 7 weeks).
- Bauman WA, Spungen AM, Collins JF, Raisch DW, Ho C, Deitrick GA, Nemchausky BA, et al. The effect of oxandrolone on the healing of chronic pressure ulcers in persons with spinal cord injury: a randomized trial. Ann Intern Med. 2013;158:718–26. [PubMed: 23689765](Among 41 patients with pressure ulcers treated with oxandrolone or placebo for 24 weeks, ulcer healing was not affected but asymptomatic ALT elevations arose in 32% on androgenic steroids vs 2.9% on placebo, and 4.6% of patients stopped therapy early because of liver test abnormalities, all of which resolved within 4 weeks of stopping).
- Luciano RL, Castano E, Moeckel G, Perazella MA. Bile acid nephropathy in a bodybuilder abusing an anabolic androgenic steroid. Am J Kidney Dis. 2014;64:473–6. [PubMed: 24953892](41 year old man developed weight loss, pruritus and jaundice 6 weeks after starting nandrolone twice weekly, testosterone once weekly and daily methandrostenolone [bilirubin 11.8 rising to 47.9 mg/dL, ALT 267 U/L, Alk P 162 U/L], with worsening renal function [creatinine rising to 2.9 mg/dL] but ultimate full recovery).
- Awai HI, Yu EL, Ellis LS, Schwimmer JB. Liver toxicity of anabolic androgenic steroid use in an adolescent with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2014;59:e32–3. [PMC free article: PMC3772995] [PubMed: 23568051](18 year old male with history of NASH developed jaundice 2 weeks after starting “Beastrdrol” [bilirubin 11 rising to 28 mg/dL, ALT 229 U/L, GGT 65 U/L], resolving within 3 months of stopping).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, only one was attributed to an androgenic steroid [nandrolone]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 185 were attributed to HDS but only 31 [3.5%] to anabolic steroids).
- Abbate V, Kicman AT, Evans-Brown M, McVeigh J, Cowan DA, Wilson C, Coles SJ, et al. Anabolic steroids detected in bodybuilding dietary supplements - a significant risk to public health. Drug Test Anal. 2015;7(7):609–18. [PubMed: 25284752](24 products sold at fitness store were analyzed and 23 were found to have anabolic steroids, with 16 different than package label and one without steroids. Of 13 different anabolic steroids identified, 12 were controlled substance emphasizing the availability of anabolic steroids in herbal supplements).
- Robles-Diaz M, Gonzalez-Jimenez A, Medina-Caliz I, Stephens C, García-Cortes M, García-Muñoz B, Ortega-Alonso A, et al. Spanish DILI Registry; SLatinDILI Network. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment Pharmacol Ther. 2015;41:116–25. [PubMed: 25394890](Combination of Spanish and Latin American databases included 25 cases of anabolic steroid jaundice [8% of cases from 2010-2013], all men, mean age 32 [bilirubin 12 times, ALT 14 times, and Alk P 1.3 times ULN], renal dysfunction developing in 6 [24%] all with bilirubin levels above 35 mg/dL, but ultimate recovery in all and no mortality).
- Schwingel PA, Cotrim HP, Santos CR Jr, Santos AO, Andrade AR, Carruego MV, Zoppi CC. Recreational anabolic-androgenic steroid use associated with liver injuries among Brazilian young men. Subst Use Misuse. 2015;50:1490–8. [PubMed: 26549387](Among 923 Brazilian recreational body builders, 182 [20%] admitted to anabolic steroid use [long term but often intermittent], of whom 38.5% had elevated liver enzymes and 12% had hepatic steatosis by ultrasound; one had focal nodular hyperplasia and one a hepatic adenoma but none evidently had jaundice or clinically apparent liver injury).
- Brazeau MJ, Castaneda JL, Huitron SS, Wang J. A case report of supplement-induced hepatitis in an active duty service member. Mil Med. 2015;180:e844–6. [PubMed: 26126259](26 year old Army soldier developed jaundice 4 months after starting “Mutant Plexx” for body building being unaware that it contained anabolic steroids [bilirubin 4.9 mg/dL, ALT 223 U/L], resolving within 3 months of stopping).
- Tabatabaee SM, Elahi R, Savaj S. Bile cast nephropathy due to cholestatic jaundice after using stanozolol in 2 amateur bodybuilders. Iran J Kidney Dis. 2015;9:331–4. [PubMed: 26174462](Two men, ages 30 and 43 years, developed jaundice and renal dysfunction 3 and 6 weeks after starting oral and parenteral stanozolol [bilirubin 48 and 49 mg/dL, ALT 33 and 66 U/L, Alk P 535 and 690 U/L, creatinine 8.7 and 1.9 mg/dL], both ultimately requiring dialysis but eventually recovering).
- Flores A, Nustas R, Nguyen HL, Rahimi RS. Severe cholestasis and bile acid nephropathy from anabolic steroids successfully treated with plasmapheresis. ACG Case Rep J. 2016;3:133–5. [PMC free article: PMC4748206] [PubMed: 26958570](31 year old man developed jaundice 16 weeks after starting a regimen that included 2 anabolic steroids [bilirubin 53 mg/dL] with severe pruritus, weight loss, hepatic encephalopathy and renal dysfunction treated with plasmapheresis with subsequent improvement).
- Bourgeois AL, Auriche P, Palmaro A, Montastruc JL, Bagheri H. Risk of hormonotherapy in transgender people: Literature review and data from the French Database of Pharmacovigilance. Ann Endocrinol (Paris). 2016;77:14–21. [PubMed: 26830952](Review of the literature and adverse event registries on medical regimens for transgender individuals, trans-males receiving antiestrogens [GnRH analogues and antigonadotropins] and testosterone, which can be associated with serum enzyme elevations but has not been linked to clinically apparent liver injury).
- Alkhunaizi AM, ElTigani MA, Rabah RS, Nasr SH. Acute bile nephropathy secondary to anabolic steroids. Clin Nephrol. 2016;85:121–6. [PubMed: 26587777](28 year old man developed jaundice 6 months after starting a body building regimen that included several anabolic steroids [bilirubin 5.6 rising to 44 mg/dL, ALT and Alk P not provided], with accompanying bilirubin nephropathy and bile stained urinary granular casts and renal tubular epithelial cells).
- Bond P, Llewellyn W, Van Mol P. Anabolic androgenic steroid-induced hepatotoxicity. Med Hypotheses. 2016;93:150–3. [PubMed: 27372877](Hypothesis suggesting that potent engagement of the androgen receptor by synthetic anabolic steroids would up-regulate carnitine palmitoyltransferase 1 [CPT1], which controls mitochondrial fatty acid β-oxidation and would result in generation of reactive oxygen species that would damage hepatocytes).
- Magee CD, Witte S, Kwok RM, Deuster PA. Mission compromised? drug-induced liver injury from prohormone supplements containing anabolic-androgenic steroids in two deployed U.S. service members. Mil Med. 2016;181:e1169–71. [PubMed: 27612377](Two actively deployed service members developed liver injury 3-4 weeks after starting combinations of products said to be prohormone supplements [one with jaundice and one with serum ALT elevations], and testing of the products [“TANK”, “DMZ” and “Epi-10”] revealed several anabolic steroids including stanozolol, methyltestosterone and methasteron among others).
- Cabb E, Baltar S, Powers DW, Mohan K, Martinez A, Pitts E. The diagnosis and manifestations of liver injury secondary to off-label androgenic anabolic steroid use. Case Rep Gastroenterol. 2016;10:499–505. [PMC free article: PMC5043289] [PubMed: 27721739](45 year old man developed jaundice and pruritus 3 months after starting two anabolic steroids [bilirubin 4.9 rising to 6.4 mg/dL, ALT 102 U/L, Alk P 262 U/L], resolving in follow up).
- Boks MN, Tiebosch AT, van der Waaij LA. A jaundiced bodybuilder; cholestatic hepatitis as side effect of injectable anabolic-androgenic steroids. J Sports Sci. 2017;35:2262–4. [PubMed: 27937337](24 year old man developed pruritus and jaundice several weeks after finishing an 8 week cycle of trenbolone enanthate, a C-19 and C-17β substituted androgenic steroid used in cattle and used by body builders under the popular name “Tren”, [bilirubin 9.3 rising to 31.5 mg/dL, ALT 115 U/L, Alk P 70 U/L, GGT 36 U/L], worsening for 6 weeks but ultimately resolving).
- Solimini R, Rotolo MC, Mastrobattista L, Mortali C, Minutillo A, Pichini S, Pacifici R, et al. Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping. Eur Rev Med Pharmacol Sci. 2017;21(1) Suppl:7–16. [PubMed: 28379599](Review of the mechanism of action of androgenic steroids and their synthetic derivatives, usually with either 17-α alkylation, which creates an orally available, anabolic product that is capable of causing cholestasis, or 17-β esterification that creates an hydrophobic and long acting form of testosterone and is not likely to cause cholestasis).
- Velho I, Fighera TM, Ziegelmann PK, Spritzer PM. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology. 2017;5:881–8. [PubMed: 28709177]
- Eapen J, Ayoola R, Subramanian RM. 'The efficacy of extracorporeal liver support with molecular adsorbent recirculating system in severe drug-induced liver injury'. Oxf Med Case Reports. 2018;2018:omx077. [PMC free article: PMC5751031] [PubMed: 29308212](Systematic review of the literature on studies of adverse effects of testosterone [parenteral undecanoate, cypionate, enanthate, propionate or topical gel or patch] therapy for transgender men identified six studies that included tests of liver function which changed minimally or not at all, no levels rising above the upper limits of normal for men).
- Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, Cabello MR, Robles-Diaz M, Sanabria-Cabrera J, Sanjuan-Jimenez R, et al. Spanish DILI Registry. Herbal and dietary supplement-induced liver injuries in the Spanish DILI Registry. Clin Gastroenterol Hepatol. 2018;16:1495–502. [PubMed: 29307848](Among 856 cases of drug induced liver injury enrolled in a Spanish registry between 1994 and 2016, 32 [3.7%] were due to HDS products and another 20 [2.3%] to anabolic steroids; only in men, mean age 31 years, 95% with jaundice, none fatal, and mean bilirubin 17 times, ALT 14 times, GGT 1.8 times and Alk P 1.3 times ULN).
- Hassan A, Fontana RJ. Liver injury associated with sporting activities. Semin Liver Dis. 2018;38:357–65. [PubMed: 30357773](Review of liver injury associated with athletic activities discusses heat stroke, anabolic steroid jaundice and HDS induced liver injury from body building supplements).
- Kiracofe B, Coffey R, Jones LM, Bailey JK, Thomas S, Porter K, Murphy CV. Incidence of oxandrolone induced hepatic transaminitis in patients with burn injury. Burns. 2019;45:891–7. [PubMed: 30545697](Among 66 patients hospitalized for significant body burns treated with oxandrolone with frequent monitoring, 28 [42%] developed elevations in ALT or AST, but abnormalities were asymptomatic and transient and there was no mention of clinically apparent liver injury).
- Stolz A, Navarro V, Hayashi PH, Fontana RJ, Barnhart HX, Gu J, Chalasani NP, et al. DILIN Investigators. Severe and protracted cholestasis in 44 young men taking bodybuilding supplements: assessment of genetic, clinical and chemical risk factors. Aliment Pharmacol Ther. 2019;49:1195–204. [PMC free article: PMC6682544] [PubMed: 30934130](Among 44 patients with anabolic steroid jaundice enrolled in a prospective database between 2004 and 2013, all were men, aged 21 to 59 years presenting with jaundice an average of 2.5 months after starting the steroid [bilirubin 9.8 rising to 25.8 mg/dL, ALT 169 U/L, Alk P 111 U/L], jaundice persisting for an average of 3 months, but ultimately resolving with no mortality or liver transplantation; various rare and function affecting variants in the bile acid transporter genes were found but none consistently).
- Zheng E, Sandhu N, Navarro V. Drug-induced liver injury secondary to herbal and dietary supplements. Clin Liver Dis. 2020;24:141–55. [PubMed: 31753247](Review of HDS associated liver injury including that caused by anabolic steroids which has increase in the last 2 decades both in US and European registries, the typical case marked by intense and prolonged cholestasis arising 1-4 months after starting an anabolic steroid, rarely fatal, liver histology showing marked canalicular cholestasis with minimal inflammation and necrosis, referred to as bland cholestasis).
- Petrov PD, Fernández-Murga L, Conde I, Martínez-Sena T, Guzmán C, Castell JV, Jover R. Epistane, an anabolic steroid used for recreational purposes, causes cholestasis with elevated levels of cholic acid conjugates, by upregulating bile acid synthesis (CYP8B1) and cross-talking with nuclear receptors in human hepatocytes. Arch Toxicol. 2020;94:589–607. [PubMed: 31894354](Among 3 patients with bland cholestasis due to epistane, all had marked increases in serum bile acids, largely primary conjugated forms; while in vitro studies demonstrated that epistane increases bile acid synthesis in hepatocytes, with no change in SHP and decrease in FGF-19 with accompanying increases in bile acid synthetic genes and increases in transporters; epistane also activates the androgen receptor and increases bile acid levels via CYP8B1).
- Flores JE, Chitturi S, Walker S. Drug-induced liver injury by selective androgenic receptor modulators. Hepatol Commun. 2020;4:450–2. [PMC free article: PMC7049679] [PubMed: 32140660](Two patients, 24 and 49 year old men, developed jaundice 10 weeks and 4 months after starting over-the-counter selective androgen receptor modulators [SARMs] called Ligandrol and RAD-140 for body building [bilirubin 6.8 and 17.1 mg/dL, ALT 273 and 54 U/L, Alk P 289 and 327 U/L], resolving in follow up at 4 and 12 months after stopping the products).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Benign hepatic tumors and oral contraceptive pills.[Am J Med. 1976]Benign hepatic tumors and oral contraceptive pills.Kalra TM, Mangla JC, DePapp EW. Am J Med. 1976 Dec; 61(6):871-7.
- Anabolic androgenic steroid-induced hepatotoxicity.[Med Hypotheses. 2016]Anabolic androgenic steroid-induced hepatotoxicity.Bond P, Llewellyn W, Van Mol P. Med Hypotheses. 2016 Aug; 93:150-3. Epub 2016 Jun 5.
- Review [Liver tumors caused by androgenic steroids].[Orv Hetil. 1990]Review [Liver tumors caused by androgenic steroids].Balázs M, Faller J. Orv Hetil. 1990 Nov 11; 131(45):2485-8.
- Review Anabolic androgenic steroid-induced liver injury: An update.[World J Gastroenterol. 2022]Review Anabolic androgenic steroid-induced liver injury: An update.Petrovic A, Vukadin S, Sikora R, Bojanic K, Smolic R, Plavec D, Wu GY, Smolic M. World J Gastroenterol. 2022 Jul 14; 28(26):3071-3080.
- Versatility of Anabolic Androgenic Steroid-Induced Hepatotoxicity.[J Clin Exp Hepatol. 2022]Versatility of Anabolic Androgenic Steroid-Induced Hepatotoxicity.Patil V, Jothimani D, Harika K, Hakeem AR, Sachan D, Vij M, Rela M. J Clin Exp Hepatol. 2022 Jan-Feb; 12(1):216-221. Epub 2021 Mar 11.
- Androgenic Steroids - LiverToxAndrogenic Steroids - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...